Abstract
There is considerable evidence correlating the production of increased proportions of membrane unsaturated fatty acids (UFAs) with bacterial growth at low temperatures or high pressures. In order to assess the importance of UFAs to microbial growth under these conditions, the effects of conditions altering UFA levels in the psychrotolerant piezophilic deep-sea bacterium Photobacterium profundum SS9 were investigated. The fatty acids produced by P. profundum SS9 grown at various temperatures and pressures were characterized, and differences in fatty acid composition as a function of phase growth, and between inner and outer membranes, were noted. P. profundum SS9 was found to exhibit enhanced proportions of both monounsaturated (MUFAs) and polyunsaturated (PUFAs) fatty acids when grown at a decreased temperature or elevated pressure. Treatment of cells with cerulenin inhibited MUFA but not PUFA synthesis and led to a decreased growth rate and yield at low temperature and high pressure. In addition, oleic acid-auxotrophic mutants were isolated. One of these mutants, strain EA3, was deficient in the production of MUFAs and was both low-temperature sensitive and high-pressure sensitive in the absence of exogenous 18:1 fatty acid. Another mutant, strain EA2, produced little MUFA but elevated levels of the PUFA species eicosapentaenoic acid (EPA; 20:5n-3). This mutant grew slowly but was not low-temperature sensitive or high-pressure sensitive. Finally, reverse genetics was employed to construct a mutant unable to produce EPA. This mutant, strain EA10, was also not low-temperature sensitive or high-pressure sensitive. The significance of these results to the understanding of the role of UFAs in growth under low-temperature or high-pressure conditions is discussed.
One of the characteristics believed critical for life at low temperatures or high pressures is the maintenance of appropriate membrane fluidity or phase. Reduced temperature and increased hydrostatic pressure exert profound physical influences on biological membranes, resulting in supraoptimal membrane viscosity or phase transition caused primarily by the tighter packing of the fatty acyl chains (9, 17, 26). Thus, at elevated pressures and/or low temperatures, acyl chains assume a closely packed, ordered array in which molecular motion is highly restricted. Such membrane gelling effects are predicted to provide a strong inducement for adaptive membrane restructuring in order to circumvent the deleterious consequences of altered membrane function. The maintenance of biological membranes in a narrow range of viscosity (homeoviscous response) (49) or within a liquid-crystalline phase (homeophasic response) (28) may be key to an organism’s growth ability and survival. Membrane transport, intracellular signaling and gene regulation, membrane protein dispersion and protein-protein interactions within the lipid bilayer, and metabolic electron transport are reliant on an appropriate membrane physical structure (20).
Perhaps the most pervasive cellular response to a temperature change entails the retailoring of the membrane’s fatty acid composition. It is well documented that the biological response of numerous bacteria to decreases in temperature results in substantial increases in the proportion of unsaturated fatty acids (UFAs) within membrane phospholipids (30). Likewise, many deep-sea bacteria display a high ratio of UFAs to saturated fatty acids (SFAs) in their membrane phospholipids, which in many instances increases with increasing growth pressure (11, 12, 21, 54). Fatty acyl chains containing one or more double bonds adopt a more expanded conformation, pack less compactly, and possess lower melting temperatures than their saturated counterparts, allowing for their less orderly alignment within the membrane phospholipids (17). Consequently, increases in membrane unsaturation may be important for the homeostatic maintenance of an appropriate physical structure of the membrane in response to environmental variables which elicit membrane gelling effects, such as reduced temperature and elevated pressure.
One particularly remarkable characteristic shared by many low-temperature-adapted (psychrophilic or psychrotolerant) and high-pressure-adapted (piezophilic or piezotolerant, previously termed barophilic and barotolerant [56]) bacteria is the production of the omega-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA; 20:5) and docosahexanenoic acid (DHA; 22:6). There is a preponderance of PUFA-producing bacteria associated with cold (or permanently cold) and high-pressure environments compared to tropical and shallow-water environments (12, 37, 60, 61). By virtue of their extremely low melting temperatures, such PUFAs would be expected to exert disproportionately large effects on membrane structure, effectively reducing the melting temperature and increasing the melting pressure of a lipid bilayer. Consequently, PUFA production and their temperature- and pressure-dependent regulation by deep-sea bacteria have been considered potentially important adaptations to the low temperatures and high hydrostatic pressures of the deep sea (12, 15, 38, 54, 55). The deep sea is characterized by a temperature of 2°C in most habitats and by hydrostatic pressures ranging from 10 MPa at a depth of 1,000 m to approximately 110 MPa in the Challenger Deep of the Mariana Trench at 10,898 m.
Whereas correlations have been found to exist between the degree of membrane unsaturation and growth under low-temperature and high-pressure conditions, confirmatory evidence that UFAs are indeed required for growth under these conditions is lacking. Prior studies have remained nearly exclusively descriptive and phenomenological, reporting the phenotypic changes which ensue in response to temperature and pressure variables. In the present study, we have addressed the relative importance of UFAs for growth of the psychrotolerant piezophilic deep-sea bacterium Photobacterium profundum SS9 at low temperatures and high pressures. P. profundum SS9 is a genetically tractable model system for studies of low-temperature and high-pressure adaptation (1). Isolated from an amphipod homogenate enrichment in the Sulu Sea at a depth of 2,551 m and an ambient temperature of approximately 9°C, P. profundum SS9 is capable of growth at temperatures of less than 2°C to greater than 20°C (optimal temperature, 15°C) and from 0.1 MPa (0.101 MPa = 1 atm = 1.01 bar) to nearly 70 MPa (optimal pressure, 28 MPa) (10). In addition, P. profundum SS9 produces the PUFA EPA (39). To begin assessment of the importance of fatty acid composition in vivo, the effects of altered UFA levels on the growth properties of P. profundum SS9 have been analyzed through UFA inhibitor analyses, the generation of mutants exhibiting altered fatty acid profiles, and the engineering of a P. profundum SS9 mutant defective in EPA production.
MATERIALS AND METHODS
Strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. P. profundum strains were routinely cultured at 15°C in 2216 marine medium (28 g/liter; Difco Laboratories, Detroit, Mich.). All temperature experiments (15, 9, and 4°C) were conducted aerobically in 2216 medium. For solid media, agar (Difco Laboratories) was added at 17 g/liter. The antibiotics kanamycin (50 μg/ml for Escherichia coli; 200 μg/ml for P. profundum strains), rifampin (100 μg/ml), and ampicillin (100 μg/ml) were added to the medium when required. The antibiotic cerulenin (2,3-epoxy-4-oxo-7,10-dodecadienamide) was added at 12 μg/ml when used in inhibition studies. All antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.). When required, media were supplemented with oleic acid (18:1) in the form of Tween 80 (polyoxyethylenesorbitan-monooleate) at a final concentration of 0.025%. Tween 20 (polyoxyethylenesorbitan-monolaurate), Tween 40 (polyoxyethylenesorbitan-monopalmitate), and Tween 80 were obtained from Sigma Chemical Co.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| P. profundum | ||
| SS9 | Wild type | 10 |
| DB110 | Lac− Rifr SS9 derivative | 6 |
| EA2 | Oleic acid-auxotrophic mutant; DB110 derivative | This study |
| EA3 | Oleic acid-auxotrophic mutant; DB110 derivative | This study |
| EA10 | EPA-deficient mutant; DB110 derivative | This study |
| E. coli | ||
| DH5α | recA strain used in maintaining plasmids | 16 |
| ED8654 | Strain in which pRK2073 is maintained | 33 |
| Plasmids | ||
| pRK2073 | Helper plasmid carrying the tra genes necessary for conjugal transfer | 3 |
| pAMP1 | Plasmid containing an 885-bp internal fragment of SS9 EPA ORF3/4; Ampr | This study |
| pMUT100 | Mobilizable suicide plasmid; Kmr | 5 |
| pEA30 | 710-bp internal fragment of SS9 ORF3/4 in pMUT100; Kmr | This study |
High-pressure growth studies.
High-pressure cultivation of P. profundum strains for growth studies or fatty acid analysis was conducted as previously described (6). Each culture was grown to stationary phase in 2216 marine medium at 1 atm (1 atm = 0.101 MPa) and 15°C. Stationary-phase cultures were diluted 1/200 into 2216 medium buffered with HEPES (100 mM, pH 7.5; Sigma Chemical Co.) containing 22 mM glucose (Sigma Chemical Co.). The diluted culture was used to fill 4.5- or 15-ml polyethylene transfer pipettes (Samco, San Fernando, Calif.). The pipettes were filled completely and then heat sealed with a hand-held heat sealing clamp (Nalgene, Rochester, N.Y.). Cells were incubated at a hydrostatic pressure of 0.1, 28, or 50 MPa (1, 280, or 500 atm, respectively) at 9°C (unless otherwise stated) in stainless steel vessels which could be pressurized by using water and a hydraulic pump and which were equipped with quick-connect fittings for rapid decompression and recompression as described by Yayanos and Van Boxtel (57).
Chemical mutagenesis.
P. profundum DB110, a Lac−, rifampin-resistant derivative of wild-type P. profundum SS9 (6), served as the parental strain for mutagenesis experiments. N-Methyl-N′-nitro-N-nitrosoguanidine (NG) (Sigma Chemical Co.) mutagenesis experiments were performed according to the protocols of Miller (31). Streptozotocin (Sigma Chemical Co.) enrichment and selection were performed similarly to the procedures of Jacobson et al. (18). Prior to mutagenesis, kill curves were obtained for strain DB110, using various concentrations of the mutagen NG and the antibiotic streptozotocin in order to determine the effectiveness of killing. The conditions used here represent concentrations and times of exposure that resulted in 50% killing. Strain DB110 was grown in 25-ml batches in 2216 marine medium supplemented with 0.4% N-acetylglucosamine (NAG) (Sigma Chemical Co.) at 15°C in an environmental shaker to an optical density at 600 nm of 1.0, corresponding to a density of approximately 108 cells ml−1. NAG supplementation was performed to preinduce cells for uptake of the antibiotic streptozotocin for later antibiotic selection. Streptozotocin is an analogue of NAG which is transported into the cell by the same phosphotransferase system proteins (23). Hence, preinduction with NAG facilitates uptake of streptozotocin. The 25-ml batches were divided into 5 equal volumes and centrifuged at 5,000 × g for 5 min. The cell pellets were washed once in 0.1 M citrate buffer (pH 7.2) supplemented with sea salts (32 g/liter; Sigma Chemical Co.) and resuspended in an equal volume of citrate buffer. NG was added to the cultures to a final concentration of 100 μg/ml. The cultures were allowed to incubate at 15°C for 4 h without shaking. After 4 h, the cultures were washed twice in 0.1 M phosphate buffer to remove the NG and resuspended in an equal volume of 2216 marine medium. The cultures were then allowed a 6-h outgrowth recovery period at 15°C. To enrich for fatty acid-requiring auxotrophs, the antibiotic streptozotocin was then added to each culture to a final concentration of 50 μg/ml and the cultures were allowed to incubate at 15°C with shaking for an additional 2 h. At 30-min intervals, 1 ml from each culture was removed and 100 μl was plated onto each of five antibiotic-free 2216 agar plates containing 0.005% oleic acid-Na+ salt and 0.05% Tween 40 (Sigma Chemical Co.) as a solubilizing agent for the oleic acid. The plates were incubated at 15°C in the dark for approximately 5 to 7 days to allow growth of mutagenized cells. Colonies were then replica plated onto 2216 agar supplemented with oleic acid, as well as onto unsupplemented 2216 agar. Of the 6,345 colonies screened, 5 displayed an auxotrophic requirement for oleic acid, showing no growth on unsupplemented 2216 agar. These oleic acid auxotrophs were designated P. profundum EA1 to EA5.
Due to the extreme insolubility of the oleic acid-Na+ salt alone in a marine medium, a result of the presence of a high concentration of divalent cations, a variety of liquid media were prepared for growth of these mutants. 2216 marine medium was first supplemented with 0.005% (wt/vol) oleic acid-Na+ salt and 0.05% (vol/vol) Tween 40 (as a solubilizing agent); however, this was not an ideal medium due to the presence of large amounts of insoluble oleic acid. Alternatively, a modified medium was prepared according to the recipe for 2216 marine medium (Difco Laboratories), except for the omission of all divalent-cation salts, and supplemented with 0.005% oleic acid-Na+ salt. This medium provided moderate growth of strain DB110 and the mutant strains. Finally, 2216 marine medium supplemented with Tween 80 (18:1) as a source of oleic acid was prepared. Tween 80 is an oleate ester of sorbitol and its anhydrides copolymerized with approximately 20 mol of ethylene oxide per mol of sorbitol and its anhydrides. Such Tween products are highly soluble in water due to their hydrophilic character supplied by the free hydroxyl and oxyethylene groups, while the lipophile portion is found in the esterified fatty acid chains. This medium provided excellent growth of P. profundum strains and was used for routine culturing of the mutants.
Gene disruption mutagenesis.
Construction of an EPA-deficient strain of P. profundum SS9 was performed via gene disruption mutagenesis with the mobilizable suicide plasmid pMUT100 (encoding kanamycin resistance) (5). An 885-bp internal fragment of an SS9 EPA biosynthetic open reading frame (designated ORF3/4) was PCR amplified from strain DB110 genomic DNA with the following primers, designed from the known EPA ORF3/4 gene sequence of Shewanella sp. strain SCRC-2738 (59): 5′-CUACUACUACUAACAGCGAAATGCTTATCAAG-3′ (primer 1) and 5′-CAUCAUCAUCAUGCCACCAAAACCAAATGAGCTAATAC-3′ (primer 2). The PCR product was first cloned into pAMP1 by using a Gibco BRL CloneAmp system (Life Technologies, Gaithersburg, Md.) and subsequently subcloned into pMUT100 as an EcoRI-BamHI fragment, yielding pEA30. Due to an internal EcoRI site within the SS9 ORF3/4 PCR product, pEA30 contains a 710-bp fragment. Bacterial conjugations were used to transfer plasmid pEA30 from E. coli into P. profundum DB110 as described by Chi and Bartlett (6). Kanr exconjugants arose from integration of the pMUT100 plasmid into the genome of P. profundum SS9. These experiments yielded the EPA-deficient strain P. profundum EA10, containing a disruption in EPA ORF3/4 which was confirmed by Southern blot analysis (45). Genomic DNA from P. profundum EA10 and DB110 was digested with restriction enzyme PstI, HindIII, BglII, HpaI, or KpnI and probed with the internal fragment of ORF3/4 harbored on pEA30, labelled with [α-32P]dCTP by random priming (Life Technologies). Evidence for gene disruption was revealed by the replacement of discrete fragments in the strain DB110 genome by fragments 6.2 kb larger in the case of strain EA10. Killing experiments were conducted at an extremely high pressure (100 MPa) and an extremely low temperature (−20°C) to assess the susceptibility of strain EA10 to such extremes compared to the parental strain, DB110. Stationary-phase cultures of strains EA10 and DB110 were diluted to a density of approximately 107 cells ml−1 and incubated either at 100 MPa (9°C) or −20°C (0.1 MPa) for up to 6 h. Every 30 min for 100-MPa incubations and every 45 min for −20°C incubations, CFU of both strains per milliliter were determined at 15°C (0.1 MPa) following culture dilution and plating onto 2216 marine agar.
Fatty acid analyses and lipid extracts.
Cells grown at various hydrostatic pressures or temperatures were harvested in late exponential phase via centrifugation at 5,000 × g, washed in an equal volume of 50% artificial seawater (16 g of Sigma sea salts per liter; Sigma Chemical Co.), frozen at −70°C, and lyophilized prior to fatty acid methyl ester (FAME) derivatization. Whole-cell methanolysates were used throughout the study for FAME preparation and analysis. FAMEs were prepared by reacting 10 mg (dry weight) of a lyophilized cell sample with 5% H2SO4 in anhydrous methanol at 90°C for 90 min in 1.5-ml sample vials with Teflon-lined caps (Wheaton, Millville, N.J.). Samples were allowed to cool, and FAMEs were extracted twice with hexane and nonesterified fatty acids saponified with 10% NaCl. Following FAME derivatization, the hexane layer was removed and evaporated completely under a gentle stream of N2. The FAME residue was then redissolved in 25 μl (final volume) of hexane containing a known concentration of 19:0 methyl ester as an internal standard and stored at −70°C until analysis.
Analyses of the FAME preparations were performed with a Hewlett-Packard model 5890 gas chromatograph equipped with an Econo-Cap EC-Wax (Carbowax) capillary column (30 m by 0.25 mm [internal diameter] by 0.25 μm; Alltech Associates Inc., Deerfield, Ill.) connected to a Hewlett-Packard model 5988A mass spectrometer (MS). Samples were injected in the split mode with a split ratio of 25:1. After 1 min at 165°C, the oven was temperature programmed to increase from 165 to 260°C at a rate of 6°C min−1. Helium was used as the carrier gas, and the injector was maintained at 250°C. Peak areas were quantified, and mass spectra were acquired and processed with Hewlett-Packard G1034C MS ChemStation software operated in the scan acquisition mode. MS operating conditions were as follows: electron multiplier, 1,800 V; transfer line, 250°C; electron impact energy, 70 eV; scan threshold, 50; 1.3 scans s−1 with a mass range of 50 to 550 atomic mass units; and solvent delay, 2.35 min. Compounds were identified by comparison of their retention times with those of known standards, and sample mass spectra data were compared to the mass spectra data of 75,000 compounds in the Chemstation NBS75K library. EPA production was confirmed by comparison of mass spectra data of the EPA methyl ester standard (Sigma Chemical Co.) with that of the corresponding peak from P. profundum SS9. Fatty acids are denoted as ratios of the number of carbon atoms to the number of double bonds.
IM and OM separation and analysis.
Isolations of inner (IM) and outer (OM) membrane fractions were performed with P. profundum DB110 grown at 15°C in 2216 marine medium at atmospheric pressure according to the methods of Schnaitman (47). Cells (500 ml) were harvested in late exponential phase at a density of approximately 108 ml−1 by centrifugation at 5,000 × g for 10 min. Cell pellets were weighed and resuspended in a volume of ice-cold sucrose buffer (200 mM Tris [pH 7.8], 5 mM EDTA, 0.25 M sucrose, and 0.5 mg of lysozyme ml−1) such that 30% of the total volume was cells. The cells were then transferred to an Erlenmeyer flask and lysed by being subjected to six cycles of alternating freezing (in a dry ice-ethanol bath) and thawing (in a room-temperature water bath). The cell lysate was then poured into 20 volumes of cold 200 mM Tris, pH 7.8, containing 0.5 mM MgCl2 and 0.1 mg of DNase I (Calbiochem, La Jolla, Calif.) ml−1. This material was then passed through an 18-gauge needle for 10 min to shear the DNA and disperse the cell debris. Unbroken cells were removed by centrifugation twice at 7,000 × g, with the supernatant being decanted between clearing spins. The envelope material, consisting of IM and OM, was recovered by centrifugation at 27,000 × g for 30 min. The membrane pellet was dried under vacuum without heat and resuspended in 1 ml of 25% (wt/wt) sucrose containing 5 mM EDTA. Sucrose gradients, each containing 5 mM EDTA, were prepared as follows in SW41 ultracentrifuge tubes (Beckman Instruments, Fullerton, Calif.): 0.5 ml of 55% (wt/wt) sucrose, 2.1 ml of 50% sucrose, 2.1 ml of 45% sucrose, 2.1 ml of 40% sucrose, 2.1 ml of 35% sucrose, and 2.1 ml of 30% sucrose. The 1-ml membrane suspension in 25% sucrose was layered onto the gradient, and the gradient was spun for 14 h, in an SW41 ultracentrifuge rotor at 92,000 × g and 4°C. The OM appeared as a pair of similar opalescent bands at about 50% (wt/wt) sucrose. The IM was a translucent, yellowish band at about 36% (wt/wt) sucrose. Fractions were removed from the gradients and analyzed for purity of composition by (i) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and (ii) enzyme assays for both d-lactate dehydrogenase activity and succinate dehydrogenase activity. SDS-PAGE analysis was used for comparison of the OM fractions to OM proteins isolated by a Triton X-114 detergent extraction method (4) modified as described by Chi and Bartlett (6). OM fractions appeared nearly identical to the Triton X-114 extraction OM preparations. Bicinchoninic acid protein assays (Pierce, Rockford, Ill.) were performed to quantitate the total protein present in the membrane fractions, and d-lactate and succinate dehydrogenase activity assays were used as markers for the cytoplasmic membrane fractions as described by Osborn et al. (42). Fractions identified as IM had d-lactate and succinate dehydrogenase specific activities (in micromoles per minute per milligram of protein) of 1.2 and 0.7, respectively. Fractions identified as OM had d-lactate and succinate dehydrogenase activities of 0.09 and 0.01, respectively, indicating that the level of contamination of the OM with IM was very low. Once membrane fractions were confirmed and their purity was assessed, fatty acid analysis was performed as described above.
RESULTS
Fatty acid composition of P. profundum SS9.
The major fatty acids produced by P. profundum SS9 include (shown as the systematic name followed by the common name) 12:0 (dodecanoic acid; lauric acid), 14:0 (tetradecanoic acid; myristic acid), 14:1 (cis-7-tetradecanoic acid; myrisoleic acid), iso-16:0 (14-methyl-pentadecanoic acid), 16:0 (hexadecanoic acid; palmitic acid), 16:1 (cis-9-hexadecanoic acid; palmitoleic acid), 12-OH (3-hydroxydodecanoic acid; β-hydroxylauric acid), 18:0 (octadecanoic acid; stearic acid), 18:1 (cis-11-octadecanoic acid; cis-vaccenic acid), and 20:5 (all-cis-5, 8, 11, 14, 17-eicosapentaenoic acid; EPA). The fatty acid profile of P. profundum SS9 cultivated aerobically at 15°C (0.1 MPa) was similar to that recently reported by Nogi et al. (39) under similar conditions. Included in Tables 2 and 3 and Fig. 1 are the unsaturation index values (sums of the percentages [by weight] of UFA species multiplied by the numbers of double bonds) (12) and the UFA/SFA ratios for the various strains and conditions used in this study in order to display the relative degree of membrane unsaturation.
TABLE 2.
Fatty acid compositions of P. profundum strains as a function of growth pressure and temperaturea
| Strain | Culture conditionsb | Mean % (by wt) of fatty acid ± SD
|
Unsaturation indexc | UFA/SFA ratiod | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12:0 | 14:0 | 14:1 | iso-16:0 | 16:0 | 16:1 | 12-OH | 18:0 | 18:1 | 20:5 | ||||
| DB110 | |||||||||||||
| Untreated | 15°C | 1.8 ± 0.9 | 4.9 ± 1.4 | 1.2 ± 0.8 | 9.8 ± 3.1 | 21.7 ± 2.8 | 41.3 ± 8.3 | 3.0 ± 1.2 | 1.1 ± 0.4 | 9.9 ± 2.6 | 5.3 ± 1.1 | 79 ± 5.1 | 1.95 |
| 4°C | 2.7 ± 1.0 | 3.3 ± 0.8 | 2.5 ± 0.5 | 0.2 ± 0.1 | 20.2 ± 3.8 | 48.8 ± 6.8 | 3.4 ± 1.3 | 0.2 ± 0.1 | 10.3 ± 1.7 | 8.4 ± 1.1 | 104 ± 6.6 | 2.65 | |
| 0.1 MPa | 1.5 ± 0.1 | 5.0 ± 0.3 | 0.8 ± 0.1 | 21.4 ± 1.5 | 64.1 ± 1.5 | 0.6 ± 0.1 | 0.2 ± 0.0 | 3.6 ± 0.1 | 2.7 ± 0.1 | 82 ± 2.4 | 2.53 | ||
| 28 MPa | 2.2 ± 0.6 | 1.6 ± 0.7 | 0.7 ± 0.1 | 17.5 ± 3.2 | 48.7 ± 9.6 | 2.0 ± 0.8 | 0.1 ± 0.1 | 16.2 ± 1.6 | 11.0 ± 0.8 | 121 ± 3.3 | 3.57 | ||
| 50 MPa | 1.4 ± 0.8 | 0.8 ± 0.5 | 0.5 ± 0.1 | 12.5 ± 1.2 | 45.9 ± 7.0 | 2.3 ± 0.5 | 0.1 ± 0.1 | 25.1 ± 4.1 | 11.4 ± 0.2 | 129 ± 7.2 | 5.60 | ||
| Cerulenin treatede | 15°C | 4.9 ± 0.8 | 21.7 ± 2.7 | 4.5 ± 1.6 | 0.3 ± 0.3 | 28.3 ± 4.3 | 27.3 ± 4.2 | 3.8 ± 2.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 8.7 ± 2.6 | 76 ± 8.9 | 0.79 |
| 4°C | 9.3 ± 0.0 | 17.9 ± 0.8 | 9.8 ± 2.3 | 19.0 ± 0.4 | 22.4 ± 0.6 | 8.3 ± 0.1 | 0.3 ± 0.1 | 13.0 ± 0.9 | 98 ± 2.7 | 0.98 | |||
| 0.1 MPa | 9.5 ± 0.3 | 24.8 ± 3.2 | 5.6 ± 1.0 | 30.0 ± 2.9 | 10.8 ± 0.8 | 6.2 ± 0.7 | 0.2 ± 0.0 | 0.3 ± 0.0 | 12.6 ± 1.1 | 80 ± 1.9 | 0.45 | ||
| 28 MPa | 16.1 ± 2.3 | 35.1 ± 3.7 | 5.3 ± 0.9 | 17.2 ± 2.0 | 7.1 ± 1.1 | 6.3 ± 0.9 | 12.9 ± 1.2 | 77 ± 1.7 | 0.37 | ||||
| EA3 | |||||||||||||
| 15°C | 8.2 ± 1.3 | 19.7 ± 2.3 | 11.7 ± 2.8 | 21.5 ± 1.4 | 27.9 ± 2.9 | 5.0 ± 2.6 | 3.5 ± 0.5 | 0.9 ± 0.5 | 1.6 ± 0.3 | 49 ± 6.6 | 0.80 | ||
| 4°C | 9.2 ± 1.2 | 24.0 ± 0.6 | 10.9 ± 1.2 | 22.0 ± 0.7 | 20.9 ± 1.9 | 4.3 ± 1.6 | 0.3 ± 0.1 | 0.1 ± 0.0 | 8.3 ± 0.6 | 73 ± 3.9 | 0.72 | ||
| 0.1 MPa | 3.9 ± 0.2 | 19.3 ± 1.7 | 3.6 ± 0.2 | 53.4 ± 0.5 | 11.9 ± 1.1 | 3.5 ± 0.2 | 0.8 ± 0.1 | 0.5 ± 0.1 | 3.1 ± 0.2 | 32 ± 2.6 | 0.25 | ||
| 28 MPa | 3.6 ± 0.1 | 17.9 ± 0.4 | 3.1 ± 0.1 | 53.2 ± 0.1 | 15.3 ± 0.6 | 2.7 ± 0.3 | 1.2 ± 0.1 | 0.7 ± 0.1 | 2.3 ± 0.2 | 31 ± 0.5 | 0.28 | ||
| EA2 | |||||||||||||
| 15°C | 3.5 ± 0.3 | 16.5 ± 1.2 | 1.1 ± 0.1 | 35.9 ± 0.5 | 10.1 ± 0.3 | 3.7 ± 2.0 | 2.6 ± 0.7 | 0.7 ± 0.1 | 25.8 ± 1.2 | 141 ± 6.0 | 0.64 | ||
| 4°C | 4.9 ± 0.2 | 24.4 ± 3.2 | 1.7 ± 0.6 | 26.9 ± 1.0 | 7.1 ± 0.5 | 4.7 ± 1.5 | 0.4 ± 0.1 | 0.2 ± 0.1 | 29.5 ± 1.9 | 157 ± 3.6 | 0.68 | ||
| 0.1 MPa | 4.5 ± 0.1 | 25.2 ± 0.1 | 0.5 ± 0.5 | 35.8 ± 2.2 | 3.9 ± 0.1 | 4.5 ± 1.1 | 0.4 ± 0.2 | 0.2 ± 0.1 | 24.8 ± 0.9 | 129 ± 4.5 | 0.45 | ||
| 28 MPa | 4.3 ± 0.6 | 15.5 ± 3.1 | 1.8 ± 0.3 | 29.7 ± 7.0 | 9.1 ± 0.1 | 3.9 ± 0.9 | 0.2 ± 0.1 | 0.8 ± 0.4 | 32.7 ± 4.5 | 176 ± 21.0 | 0.89 | ||
| 50 MPa | 4.0 ± 0.1 | 5.0 ± 0.1 | 0.4 ± 0.1 | 35.6 ± 1.3 | 18.3 ± 1.0 | 5.1 ± 0.6 | 0.3 ± 0.0 | 2.8 ± 0.1 | 28.3 ± 2.7 | 163 ± 12.4 | 1.11 | ||
| EA10 | |||||||||||||
| 15°C | 2.5 ± 1.0 | 6.5 ± 2.2 | 4.7 ± 0.5 | 5.0 ± 1.5 | 16.7 ± 2.8 | 46.3 ± 3.8 | 3.3 ± 0.7 | 1.0 ± 0.2 | 14.0 ± 1.4 | 65 ± 3.0 | 2.43 | ||
| 4°C | 3.0 ± 0.2 | 3.2 ± 0.7 | 4.1 ± 0.7 | 2.1 ± 1.0 | 10.6 ± 0.9 | 55.2 ± 3.9 | 3.9 ± 0.2 | 0.2 ± 0.1 | 17.7 ± 3.3 | 77 ± 1.3 | 4.53 | ||
| 0.1 MPa | 3.1 ± 0.1 | 3.7 ± 0.3 | 2.2 ± 0.3 | 20.4 ± 0.5 | 52.9 ± 1.0 | 4.6 ± 0.4 | 0.4 ± 0.1 | 12.7 ± 0.1 | 68 ± 0.8 | 2.46 | |||
| 28 MPa | 2.8 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.0 | 12.2 ± 2.0 | 52.3 ± 0.3 | 4.2 ± 0.1 | 0.1 ± 0.1 | 25.4 ± 2.6 | 79 ± 2.4 | 4.81 | |||
| 50 MPa | 2.6 ± 1.0 | 1.6 ± 0.3 | 1.1 ± 0.2 | 13.5 ± 0.3 | 51.6 ± 3.6 | 5.5 ± 1.2 | 0.1 ± 0.1 | 24.0 ± 3.8 | 77 ± 0.2 | 4.31 | |||
Data represent values derived from triplicate samples harvested in the late exponential phase of growth.
15 and 4°C cultures were grown aerobically in 2216 marine medium at 0.1 MPa; 0.1-, 28-, and 50-MPa cultures were grown at 9°C in 2216 marine medium containing 22 mM glucose buffered with 100 mM HEPES.
Unsaturation index was calculated as the sum of the mean percentages (by weight) of the UFA species multiplied by the number of double bonds (12).
UFAs, 14:1, 16:1, 18:1, and 20:5; SFAs, 12:0, 14:0, 16:0, and 18:0.
Cerulenin was used at 12 μg/ml.
TABLE 3.
Fatty acid profiles corresponding to harvest times indicated on growth curve in Fig. 1
| Time point | Mean % (by wt) of fatty acid species
|
Unsaturation indexa | UFA/SFA ratiob | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12:0 | 14.0 | 14.1 | iso-16 | 16:0 | 16:1 | 12-OH | 18:0 | 18:1 | 20:5 | |||
| 1 | 3.6 | 12.9 | 7.1 | 21.3 | 46.9 | 5.6 | 2.1 | 0.5 | 58.1 | 1.5 | ||
| 2 | 3.1 | 7.3 | 3.9 | 19.5 | 45.3 | 4.5 | 1.7 | 9.3 | 5.4 | 85.5 | 1.8 | |
| 3 | 3.0 | 5.4 | 2.7 | 21.0 | 47.0 | 3.8 | 1.5 | 10.4 | 5.2 | 86.1 | 2.1 | |
| 4 | 2.8 | 5.0 | 2.1 | 1.9 | 22.0 | 44.8 | 3.9 | 1.5 | 10.2 | 5.8 | 86.1 | 2.0 |
| 5 | 2.6 | 5.2 | 2.2 | 4.9 | 22.5 | 42.9 | 4.8 | 1.4 | 8.9 | 4.6 | 77.0 | 1.8 |
| 6 | 2.8 | 4.7 | 2.0 | 7.3 | 19.3 | 44.1 | 5.3 | 1.3 | 8.6 | 4.6 | 77.7 | 2.1 |
| 7 | 2.5 | 5.0 | 1.6 | 10.9 | 19.5 | 43.7 | 4.8 | 1.5 | 7.5 | 3.0 | 67.8 | 1.9 |
Unsaturation index was calculated as the sum of the mean percentages (by weight) of the UFAs multiplied by the number of double bonds (12).
UFAs, 14:1, 16:1, 18:1, and 20:5; SFAs, 12.0, 14.0, 16.0, and 18.0.
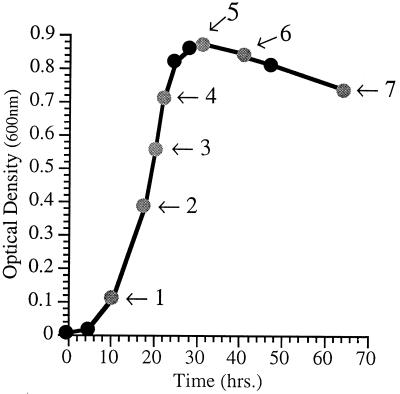
FIG. 1.
Dependence of cellular fatty acid composition in P. profundum DB110 on phase of growth. Shown is the growth curve for DB110 at 15°C and 0.1 MPa in 2216 marine medium. Arrows denote times at which cells were harvested for fatty acid analysis (see Table 3 for corresponding fatty acid profiles).
Dependence of fatty acid composition on growth phase.
The percentages of the major fatty acid species were found to vary depending on the phase of growth. This was characterized for P. profundum DB110 grown aerobically at 15°C and 0.1 MPa (Fig. 1). Across the spectrum of time periods examined, the most dramatic regulation involved the progressive increase in the branched-chain fatty acid iso-16:0 as a function of culture age. iso-16:0 levels were consistently undetected until late exponential phase and then increased throughout stationary phase, reaching nearly 11% of the total fatty acid (by weight) in advanced stationary-phase cultures. 18:1 and EPA levels increased from early to late log phase, whereas 14:0 decreased from early log phase to later growth stages. The unsaturation index values and UFA/SFA ratios were lowest during early exponential phase and highest during late log phase. In light of these results, care was taken to analyze the fatty acid contents of all P. profundum strains within a particular growth phase, specifically late exponential phase (corresponding to an optical density at 600 nm of approximately 0.7), so that the data would not be skewed as a result of growth phase differences.
Analysis of IM and OM composition.
Fatty acid composition differences as a function of cellular location were also investigated (Table 4). IM and OM were isolated by density gradient centrifugation from strain DB110 grown at 15°C. Compared to the IM fraction, the OM fraction was enriched with the hydroxylated fatty acid 12-OH (3.2% versus 12.3%, respectively). The specific enrichment of the OM with 12-OH is likely to derive from the lipid A component of the lipopolysaccharide layer (44). In addition, the OM contained a higher percentage of the shorter-chain SFAs, specifically 12:0 (7.6% in OM versus 2.9% in IM) and, to a lesser extent, 14:0 (7.2% in OM versus 5.1% in IM). This higher percentage of SFAs in the OM is consistent with findings in E. coli (24). UFA types exhibited no dramatic differential localization between the membrane fractions.
TABLE 4.
Fatty acid compositions of P. profundum SS9 IM and OM fractions
| Fatty acid species | Mean % (by wt) ± SD (n = 3)
|
|
|---|---|---|
| IM | OM | |
| 12:0 | 2.9 ± 0.1 | 7.6 ± 0.3 |
| 14:0 | 5.1 ± 0.8 | 7.2 ± 0.6 |
| 14:1 | 2.5 ± 0.6 | 2.5 ± 0.6 |
| iso-16:0 | 4.3 ± 1.9 | 2.1 ± 1.6 |
| 16:0 | 25.0 ± 1.5 | 20.1 ± 1.2 |
| 16:1 | 31.7 ± 5.0 | 31.1 ± 0.7 |
| 12-OH | 3.2 ± 2.1 | 12.3 ± 1.3 |
| 18:0 | 6.6 ± 2.8 | 2.6 ± 1.0 |
| 18:1 | 12.1 ± 3.7 | 9.7 ± 1.1 |
| 20:5 | 6.5 ± 0.2 | 4.7 ± 1.2 |
Temperature- and pressure-dependent regulation of fatty acid composition.
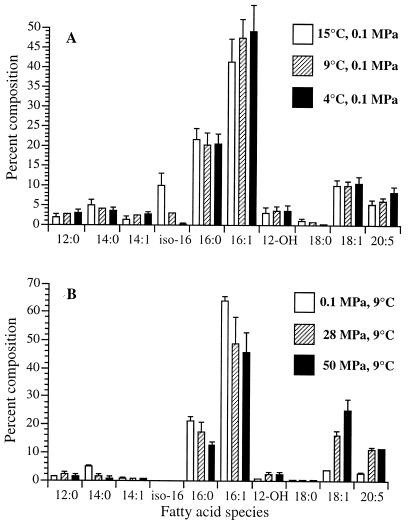
Comparison of the fatty acid profiles of P. profundum DB110 grown under different conditions indicated that low temperature and high pressure both enhanced UFA levels, but to different extents and with different selective effects (Fig. 2). It should be noted that the 15, 9, and 4°C cultures were grown aerobically, whereas all experiments in pressurizable bulbs necessitated growth under fermentative conditions with glucose and buffer added to the cultures. With decreased temperature, moderate to slight increases in the proportions of 16:1 and 18:1 occurred along with a significant increase in EPA and a dramatic reduction in the proportion of iso-16:0. Changes in pressure resulted in an even more dramatic effect on the fatty acid composition. The proportion of 18:1 increased from 3.6 to 16.2% of total fatty acids as cells were pressurized from 0.1 MPa (9°C) to 28 MPa (9°C), with an additional increase to 25.1% upon progression from 28 MPa (9°C) to 50 MPa (9°C). Over the pressure range tested, the proportion of EPA also increased, from 2.7% at 0.1 MPa (9°C) to 11.4% at 50 MPa (9°C). No iso-16:0 was produced in any of the cultures grown in the pressurizable bulbs regardless of the growth phase. Akin to observations made for other piezophilic species (12, 21), the relative degree of fatty acid unsaturation in P. profundum SS9 increased in response to decreased temperature or increased hydrostatic pressure, as indicated by the general increase in unsaturation index values and UFA/SFA ratios as a function of decreased temperature or increased pressure (Table 2).
FIG. 2.
Effect of growth temperature (A) and growth pressure (B) on cellular fatty acid composition in P. profundum DB110 (1 MPa = 10 bar ≈ 9.87 atm). Data represents mean percentages (by weight) of fatty acid species, ± standard deviations, derived from triplicate samples harvested in the late exponential phase of growth. See Materials and Methods for cultivation conditions.
Effect of cerulenin treatment on growth at high pressures and low temperatures.
As a first step in assessing the role of UFAs in the growth of P. profundum SS9, the effect of the antibiotic cerulenin was tested. Cerulenin irreversibly inhibits fatty acid biosynthesis enzymes β-ketoacyl-acyl carrier protein synthases I and II in a variety of bacteria and fungi, perturbing the formation of UFAs (41). Cerulenin treatment allowed us to specifically determine the effect of diminished monounsaturated fatty acid (MUFA) levels (specifically 16:1 and 18:1) on growth of P. profundum SS9. As shown in Table 2, analysis of the fatty acid composition of cerulenin-treated strain DB110 under the various temperature and pressure growth conditions revealed a nearly complete inhibition of 18:1 production and moderate reductions in 16:1. In addition, cerulenin elicited dramatic increases in the proportions of 12:0, 14:0, and 14:1 as well as moderate increases in EPA. The fact that EPA levels rose while 18:1 values declined is consistent with separate pathways directing the biosynthesis of the two UFAs, with the pathway responsible for 18:1 production being far more sensitive to the antibiotic than that of the EPA pathway.
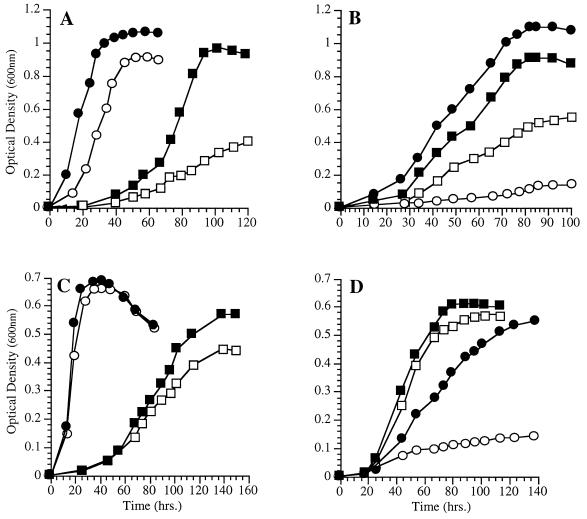
The effect of cerulenin treatment on growth was particularly inhibitory at low temperature and at high pressure (Fig. 3A and B). However, if cerulenin-treated cultures are supplemented with exogenous 18:1, in the form of 0.025% Tween 80, a reversal of the cerulenin inhibition was observed to various degrees (Fig. 3C and D). Cells grown in the presence of both cerulenin and 18:1 exhibited a modest enhancement of growth at low temperature and a marked enhancement of growth at high pressure (28 MPa) compared to cerulenin-treated cells grown in the absence of the added fatty acid supplement. These results suggest that 18:1 is important for low-temperature and high-pressure growth.
FIG. 3.
Effect of cerulenin (12 μg/ml) on the growth of P. profundum DB110 at various temperatures (A and C) and pressures (B and D) with or without exogenous 18:1 in the form of 0.025% Tween 80. (A) ●, 15°C, without cerulenin; ○, 15°C, with cerulenin; ■, 4°C, without cerulenin; □, 4°C, with cerulenin. (B) ●, 28 MPa, without cerulenin; ○, 28 MPa, with cerulenin; ■, 0.1 MPa, without cerulenin; □, 0.1 MPa, with cerulenin. (C) ●, 15°C, with cerulenin and 18:1; ○, 15°C, with cerulenin but without 18:1; ■, 4°C, with cerulenin and 18:1; □, 4°C, with cerulenin but without 18:1. (D) ●, 28 MPa, with cerulenin and 18:1; ○, 28 MPa, with cerulenin but without 18:1; ■, 0.1 MPa, with cerulenin and 18:1; □, 0.1 MPa, with cerulenin but without 18:1.
Growth characteristics and fatty acid analyses of oleic acid-auxotrophic mutants.
A second line of experimentation which was performed to address the importance of particular UFAs for growth at low temperature or high pressure involved the generation of a collection of chemical mutants exhibiting an auxotrophic requirement for oleic acid (18:1). We predicted that many of these mutants would be altered in the production of certain UFAs. Of the 6,345 NG-mutagenized colonies screened, 5 displayed a requirement for oleic acid, exhibiting negligible or complete lack of growth on unsupplemented 2216 agar. These mutant strains were designated P. profundum EA1 to EA5. All of these mutants were verified to be derivatives of P. profundum SS9 based on Coomassie blue R staining of whole-cell proteins and SDS-PAGE analysis with comparison to strain DB110 proteins. All of these mutants exhibited a specific enhancement of growth in the presence of UFAs, since SFAs such as palmitic acid (16:0) and lauric acid (12:0) failed to compensate for their growth defects. Only mutant EA5 exhibited an absolute requirement for oleic acid for growth.
The fatty acid profiles of mutants EA1 to EA4 were obtained. However, mutant EA5 could not be accurately analyzed due to its obligate requirement for exogenous 18:1. Of the five mutants isolated, mutants EA1 and EA4 exhibited the least-stringent requirements for exogenous 18:1 and displayed wild-type levels of all fatty acids. However, mutants EA2 and EA3 exhibited markedly altered fatty acid profiles relative to strain DB110 and were therefore selected for further analysis.
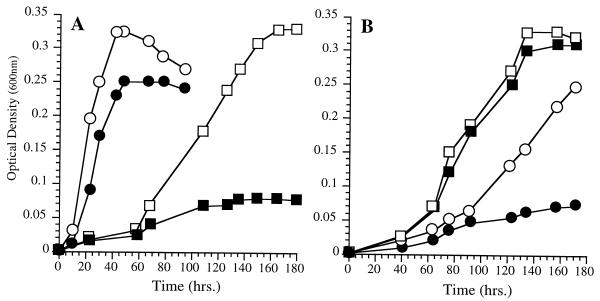
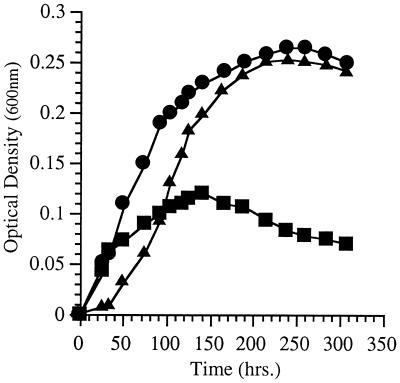
The fatty acid compositions of strains EA2 and EA3 grown at various temperatures and pressures are listed in Table 2. Strain EA3 exhibited severely diminished 16:1, 18:1, and EPA levels as well as elevated proportions of 12:0, 14:0, and 14:1 under routine culture conditions of 15°C (0.1 MPa). At low temperature, strain EA3 produced fivefold-higher EPA levels, but little difference in fatty acid composition was evident in cells grown at low and high pressures. Figure 4 shows the growth characteristics of strain EA3 at various pressures and temperatures in the presence or absence of exogenous 18:1 in the form of Tween 80 at a concentration of 0.025%. Without 18:1 supplementation, strain EA3 exhibited dramatic low-temperature and high-pressure sensitivities, exhibiting virtually no growth at 4°C (0.1 MPa) or 28 MPa (9°C). However, strain EA3 displayed dramatically enhanced growth at a low temperature (4°C) or an elevated pressure (28 MPa) in the presence of Tween 80. These results are qualitatively consistent with the cerulenin studies, although the influence of exogenous 18:1 on growth at low temperature was substantially more dramatic for strain EA3 than for cerulenin-treated cells. The growth characteristics of strain EA3 were also similar to those previously reported for strain EA5 (2).
FIG. 4.
Growth characteristics of P. profundum SS9 oleic acid-auxotrophic chemical mutant EA3 at various temperatures (A) and pressures (B) in the absence or presence of exogenous 18:1 in the form of 0.025% Tween 80. (A) ●, 15°C, without 18:1; ○, 15°C, with 18:1; ■, 4°C, without 18:1; □, 4°C, with 18:1. (B) ●, 28 MPa, without 18:1; ○, 28 MPa, with 18:1; ■, 0.1 MPa, without 18:1; □, 0.1 MPa, with 18:1.
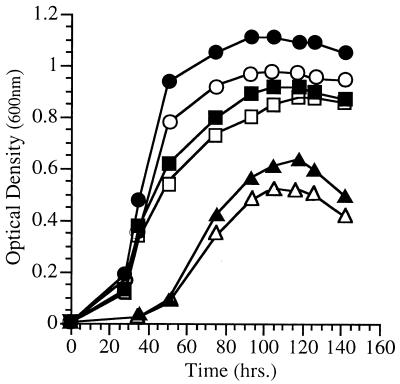
The fatty acid profile of strain EA2 was similar to that of strain EA3 in that it produced diminished levels of MUFAs 16:1 and 18:1 and elevated levels of 14:0. However, strain EA2 produced substantially less 16:1, and it constitutively overproduced EPA to a level nearly fivefold higher than strain DB110 at 15°C (approximately 28% of the total fatty acids [Table 2]). Also unlike strain EA3, the growth of strain EA2 was not low-temperature or high-pressure sensitive. Although the growth rate and yield of strain EA2 were poor under all conditions, it was not particularly low-temperature or high-pressure sensitive whether it was grown with or without exogenous 18:1. The growth characteristics of strain EA2 at various pressures without 18:1 supplementation are shown in Fig. 5. Remarkably, strain EA2 grew at 50 MPa (9°C) nearly the same as it did at 28 MPa (9°C), and it exhibited a dramatic growth reduction at 0.1 MPa (9°C). Expressed as growth yield ratios, strain EA2 exhibited 50/0.1 and 28/0.1 MPa ratios of 2.2 and 2.3, respectively. In contrast, the corresponding growth yield ratios for strain DB110 under identical conditions were 0.64 (50/0.1 MPa) and 1.25 (28/0.1 MPa). Possibly the overproduction of EPA by strain EA2 enhanced its growth at a low temperature and high pressure and, conversely, inhibited its growth at an elevated temperature or decreased pressure.
FIG. 5.
Growth characteristics of P. profundum SS9 chemical mutant EA2 at various pressures. Cultures were grown at the corresponding pressure (9°C) without exogenous 18:1 supplementation. (Refer to Fig. 6 for comparison to DB110 under identical pressure conditions.) ▴, 50 MPa; ●, 28 MPa; ■, 0.1 MPa.
Growth characteristics and fatty acid analysis of strain EA10, a P. profundum SS9 mutant defective in EPA production.
The results presented above indicate a role for MUFAs in the growth of P. profundum SS9 at low temperatures and at elevated pressures (cerulenin and strain EA3 data), as well as a possible role for EPA under similar conditions, at least when the proportions of certain other fatty acids have been altered by mutation (strain EA2 data). To assess the role of EPA more directly, a reverse-genetics methodology was employed to construct a mutant unable to produce EPA. This first entailed the cloning of an internal fragment of a P. profundum SS9 EPA biosynthesis gene by making use of the previously published sequence of a cluster of genes required for EPA biosynthesis from Shewanella sp. strain SCRC-2738 (59) and sequence information obtained from Vibrio marinus of genes involved in the production of DHA (22:6n-3) (22). From these reports seven open reading frames (ORFs) were determined to be necessary for imparting the ability to produce PUFAs to recombinant E. coli strains.
An internal fragment of a homologue of Shewanella ORF3/4 from P. profundum SS9 was amplified and cloned by PCR with primers derived from SCRC-2738 ORF3/4 positions 542 to 561 and 1403 to 1428. This amplification yielded a product of the expected size whose sequence possessed a high degree of relatedness, 83 and 87% identity at the DNA and deduced protein levels, respectively, to ORF3/4 from Shewanella sp. strain SCRC-2738. Construction of a P. profundum SS9 EPA mutant, designated strain EA10, followed the introduction of the SS9 sequence into a suicide plasmid and its delivery into P. profundum DB110 by conjugal transfer (described in Materials and Methods). Kanamycin-resistant exconjugants were screened initially by examining their fatty acid profiles at 15°C (0.1 MPa) for the lack of EPA production. One of these mutants was subsequently confirmed by Southern blot analysis to be a gene disruption mutant and designated P. profundum EA10 (data not shown).
The fatty acid profile of strain EA10 grown under various conditions is listed in Table 2. By comparison with strain DB110, under identical conditions strain EA10 considerably upregulated its proportion of MUFAs (18:1, 16:1, and 14:1) while 16:0 levels tended to remain lower than those of strain DB110. At a low temperature (4°C), strain EA10 exhibited markedly reduced SFA content, with significant increases in 16:1 and 18:1, relative to the level obtained by a 15°C cultivation. The most dramatic effect of high pressure (28 MPa, 9°C) on strain EA10 was to increase the proportion of 18:1 fatty acid from 12.7 to 25.4%.
Surprisingly, when the growth of strain EA10 was examined at a low temperature and high pressure, no significant deviations from wild-type growth were evident, except for a modest reduction in growth yield under all conditions tested. The growth characteristics of strain EA10 versus strain DB110 at various pressures are shown in Fig. 6. Strains EA10 and DB110 exhibited nearly identical growth rates at 4 and 15°C (0.1 MPa) or at 0.1, 28, and 50 MPa (9°C). Moreover, the combined effects of increased pressure and decreased temperature (28 MPa, 4°C) resulted in nearly identical growth abilities for the two strains. Under these conditions (28 MPa, 4°C), strain EA10 displayed a UFA/SFA ratio of 8.38, with 18:1 and 16:1 comprising 27.2 and 58% of the cellular fatty acids, respectively. This is in comparison to strain DB110, which, under identical conditions, exhibited a UFA/SFA ratio of 3.1, with 18:1, 16:1, and EPA comprising 20, 37, and 14.8% of the total cellular fatty acids, respectively. These results indicate that under the laboratory conditions used in this study, EPA is not vital to the growth of P. profundum SS9 over the course of many generations, even under low-temperature or high-pressure conditions, situations in which its levels are typically upregulated. Likewise, no differences between the two strains with regard to survival at extremes of temperature (−20°C, 0.1 MPa) or pressure (100 MPa, 9°C) were identified (data not shown).
FIG. 6.
Growth characteristics of EPA-deficient mutant EA10 versus strain DB110 at various pressures (9°C). ▴, DB110, 50 MPa; ●, DB110, 28 MPa; ■, DB110, 0.1 MPa; ▵, EA10, 50 MPa; ○, EA10, 28 MPa; □, EA10, 0.1 MPa.
DISCUSSION
The present study has evaluated the types of fatty acids produced by the psychrotolerant, piezophile P. profundum SS9, their distribution between the IM and OM, and their regulation as a function of growth phase, temperature, and hydrostatic pressure. In addition, the significance of UFAs in growth at low temperatures and elevated pressures was explored through the use of a fatty acid synthesis inhibitor and by mutant analysis.
In general, the cellular distribution of fatty acids in P. profundum SS9 is similar to that reported for other gram-negative bacteria. For example, the high proportion of the hydroxylated fatty acid 12-OH, derived from the lipid A component of the lipopolysaccharide layer, and the elevated levels of shorter-chain SFAs present in the OM are consistent with findings for other gram-negative bacteria (24, 44). In addition, MUFAs and EPA are present in large amounts in both membranes, revealing no dramatic differential localization.
P. profundum SS9 was found to exhibit markedly increased levels of the branched-chain fatty acid iso-16:0 concomitant with entry into stationary phase. The nature of growth phase regulation of fatty acid production depends on the microorganism being studied (14, 25, 29, 36, 40). Our results are similar to those obtained in studies of another gram-negative marine isolate, which exhibited progressive increases in branched-chain fatty acids with culture age (40), and are also similar to the observed induction of cyclopropane fatty acids with the onset of stationary phase in E. coli (25, 29).
Modulation of membrane lipids by temperature or pressure is well established among many poikilothermic organisms. Such adjustments most notably include changes at the level of fatty acyl chain composition. The responses of bacteria to a reduced temperature or elevated pressure frequently entail the increased incorporation into membrane phospholipids of UFAs, which include PUFAs in those organisms capable of their production (11, 12, 15, 25, 35, 36, 54). Fatty acid profiling of P. profundum SS9 revealed a pronounced regulation of cellular fatty acid composition in response to changes in temperature or pressure, most notably a greater proportion of 16:1 and EPA at low temperatures and an increased proportion of 18:1 and EPA at elevated pressures.
Many deep-sea isolates exhibit substantial increases in MUFAs in response to increased cultivation pressure. The piezophilic bacterium CNPT3, which grows optimally at hydrostatic pressures of 30 to 50 MPa, does not produce PUFAs yet is capable of growth at pressures up to nearly 70 MPa (11). As a function of increasing pressure, CNPT3 exhibits higher proportions of 16:1 and 18:1 while the relative amounts of 14:1, 16:0, and 14:0 decrease. Likewise, it was suggested that the pressure-induced increases in the MUFA iso-17:1 contributed to the piezotolerance of the deep-sea bacterium RS103 (21).
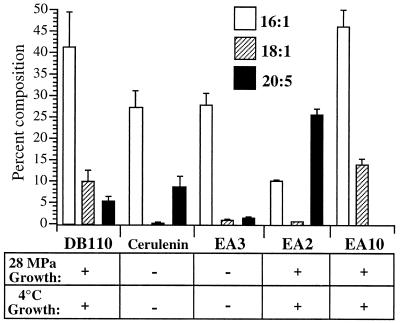
Thus, a correlation between the degree of fatty acid unsaturation and the cultivation temperature or pressure has frequently been drawn. However, to address the possible adaptive role of UFAs in membrane function in vivo at low temperatures or high pressures, physiological and genetic experiments are required. Here the functional significance of UFAs was examined by using the β-ketoacyl-acyl carrier protein synthase I and II inhibitor cerulenin to preferentially inhibit MUFA synthesis and by obtaining mutants altered in UFA synthesis. Figure 7 summarizes the 16:1, 18:1, and EPA levels of the various P. profundum strains (DB110, EA3, EA2, and EA10) and cerulenin-treated strain DB110 at 15°C (0.1 MPa) along with their low-temperature (4°C) and high-pressure (28 MPa) growth phenotypes. Those strain-treatment combinations which resulted in reduced MUFA (18:1 and 16:1) levels without dramatic compensatory increases in the proportion of EPA (i.e., strain EA2) exhibited both low-temperature and elevated-pressure sensitivity (cerulenin treatment and strain EA3); conversely, those strain-treatment combinations which exhibited wild-type (or higher) 16:1 and 18:1 levels (strains DB110 and EA10) displayed low-temperature- and elevated-pressure-adapted growth. These results, in conjunction with the fact that 18:1 supplementation was able to complement the ability of strain EA3 to grow at low temperature and high pressure and when cells were treated with cerulenin, suggests that MUFAs are particularly important for growth of P. profundum SS9 under low-temperature or high-pressure conditions. Furthermore, the fact that a P. profundum SS9 mutant defective in EPA production retained both elevated-pressure- and reduced-temperature-adapted growth via modulations solely in MUFAs is consistent with the primary importance of MUFAs under these conditions.
FIG. 7.
16:1, 18:1, and EPA (20:5) levels, ± standard deviations, of strain DB110, cerulenin-treated strain DB110 (12 μg/ml), strain EA3, strain EA2, and strain EA10 grown at 15°C (0.1 MPa) (n = 3) and their corresponding growth phenotypes at 28 MPa (9°C) and at 4°C (0.1 MPa).
A decrease in MUFA levels may be partially compensated for by EPA overproduction. Strain EA2 overproduces EPA while underproducing MUFAs. This strain is fascinating because of the shift in its growth ability at higher pressures. However, because the growth rate and yield of this strain are so reduced compared with those of wild-type P. profundum SS9, it would appear that EPA is a poor substitute for MUFAs.
What is the explanation for the need for MUFAs during growth at low temperatures and high pressures? Two potential hypotheses can be proposed. Foremost, it is possible that these fatty acids are required to maintain membrane fluidity or phase within an acceptable range for optimal growth. Studies of E. coli and Acholeplasma laidlawii (27, 43) have suggested that the ability of microorganisms which are unable to effectively regulate their membrane lipid fatty acid composition to grow at various temperatures may be determined by the phase state of their membrane lipids, as evidenced by the severe impairment of growth when cells exhibit more than about half of their lipids in the gel state (29). Indeed, it has been shown that membrane proteins of highly ordered gel-phase membranes are inactive or are excluded (50). To address this hypothesis, it will be necessary to directly measure physical properties of membranes of different P. profundum strains at various temperatures and pressures.
Alternatively, the critical nature of MUFAs could be based on a role in specific membrane protein interactions which, if disrupted, result in altered growth ability at reduced temperatures or elevated pressures. This suggests a need for MUFAs in local—as opposed to global—membrane processes. This latter hypothesis is consistent with the notion of a lipid annulus surrounding individual membrane proteins (34, 53). However, despite evidence that many membrane-associated proteins have an absolute lipid requirement for activity (46), we are unaware of any such proteins exhibiting a strict functional requirement for phospholipids with particular fatty acyl chains.
A major result of this work was the discovery that neither low-temperature- nor high-pressure-adapted growth mandates EPA production in P. profundum SS9, at least under the culture conditions employed. The ability of EPA-deficient strain EA10 to grow at a reduced temperature or an elevated pressure (Fig. 6) was essentially the same as that of the parental strain. This result was surprising. The few previous studies which have examined phenotypic effects associated with altered PUFA production, the majority of which having been conducted in cyanobacteria, plants, and fungi, have implicated PUFA production as being a necessary component for growth at low temperatures (19, 32, 48, 51, 52).
The distribution of EPA-producing bacteria in the environment has also been taken as evidence of a need for EPA for growth at low temperatures or high pressures. The discovery by DeLong and Yayanos (12) that numerous deep-sea bacterial isolates contain substantial quantities of omega-3 PUFAs, namely, EPA and DHA (22:6), led to the speculation that such polyenoic fatty acids are specifically involved in the adaptation of piezophilic bacteria to the high-pressure, low-temperature conditions prevalent in the deep-sea environment. Since then, it has been confirmed that PUFA production occurs in numerous bacterial species isolated from Antarctic regions as well as temperate marine environments. Nichols et al. (37) have compiled data regarding the percentage of EPA producers isolated from various environments. Analyses revealed that only 1.5% of temperate marine isolates (60, 61), approximately 14% of Antarctic isolates (37), and 27% of deep-sea isolates (12) produce EPA. More recently, Yano et al. (55) investigated the distributions of bacteria containing PUFAs (both EPA and DHA) in the intestines of deep-sea fish and shallow-sea poikilothermic animals. Not only did the intestinal microflora of deep-sea fish contain a higher proportion of PUFA producers, but the percentage of PUFAs within these isolates was also greater than that of shallow-water animals. These results all suggest that there is a preponderance of PUFA producers associated with high-pressure and low-temperature environments.
What then is the explanation for EPA-deficient strain EA10’s apparent lack of low-temperature or elevated-pressure sensitivity? Foremost, its increased MUFA content may be capable of compensating for the absence of EPA. Increased unsaturation of a membrane phospholipid-bound fatty acid does not result in a linear decrease in the membrane phase transition temperature. Biophysical studies employing synthetic mixed acid phosphotidylcholines (PC) have shown that the introduction of a double bond into 18:0/18:0-PC, yielding 18:0/18:1-PC, lowers the gel–to–liquid-crystalline phase transition temperature by nearly 50°C, whereas incorporation of the PUFA 20:4n-3 to yield 18:0/20:4-PC lowers the phase transition temperature by only an additional 19°C (8). Thus, in the case of strain EA10, the increased levels of 16:1 and 18:1 may provide adequate compensation for the loss of EPA. Alternatively, EPA may be required only under certain physiological conditions not evaluated in our work.
A final possibility is that EPA (and possibly DHA) is not required for psychrotolerant or piezotolerant bacterial growth but is needed as a nutritional source by higher organisms with which the EPA-producing microorganisms have established symbiotic associations. DeLong and Yayanos have previously suggested a specific role for in situ secondary production of PUFAs by piezophilic bacteria (12). Indeed, many of the PUFA-producing microorganisms that have been discovered have been isolated from vertebrate or invertebrate sources (10, 12, 13, 55, 58, 59).
ACKNOWLEDGMENTS
We thank Stu Brody of the University of California, San Diego, Biology Department for advice on fatty acid analysis.
This work was supported by grant MCB96-30546 from the National Science Foundation to D.H.B.
REFERENCES
- 1.Bartlett D, Wright M, Yayanos A A, Silverman M. Isolation of a gene regulated by hydrostatic pressure in a deep-sea bacterium. Nature. 1989;342:572–574. doi: 10.1038/342572a0. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett D H, Allen E E. Role of unsaturated fatty acids in growth at high pressure and low temperature in the deep-sea bacterium Photobacterium species strain SS9. In: Bennett P B, Demchenko I, Marquis R E, editors. High pressure biology and medicine. Rochester, N.Y: University of Rochester Press; 1998. pp. 32–37. [Google Scholar]
- 3.Better M, Helinski D R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983;155:311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 5.Brahamsha B. A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl Environ Microbiol. 1996;62:1747–1751. doi: 10.1128/aem.62.5.1747-1751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi E, Bartlett D H. Use of a reporter gene to follow high-pressure signal transduction in the deep-sea bacterium Photobacterium sp. strain SS9. J Bacteriol. 1993;175:7533–7540. doi: 10.1128/jb.175.23.7533-7540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi E, Bartlett D H. An rpoE-like locus controls outer membrane protein synthesis and growth at cold temperatures and high pressures in the deep-sea bacterium Photobacterium sp. strain SS9. Mol Microbiol. 1995;17:713–726. doi: 10.1111/j.1365-2958.1995.mmi_17040713.x. [DOI] [PubMed] [Google Scholar]
- 8.Coolbear K P, Bearde C B, Keough K M W. Gel to liquid-crystalline phase transitions of aqueous dispersions of polyunsaturated mixed-acid phosphatidylcholines. Biochemistry. 1983;22:1466–1473. doi: 10.1021/bi00275a022. [DOI] [PubMed] [Google Scholar]
- 9.Cossins A R, MacDonald A G. Homeoviscous theory under pressure. II. The molecular order of membranes from deep-sea fish. Biocheim Biophys Acta. 1984;776:144–150. [Google Scholar]
- 10.DeLong E F. Adaptation of deep-sea bacteria to the abyssal environment. Ph.D. thesis. University of California, San Diego; 1986. [Google Scholar]
- 11.DeLong E F, Yayanos A A. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science. 1985;228:1101–1103. doi: 10.1126/science.3992247. [DOI] [PubMed] [Google Scholar]
- 12.DeLong E F, Yayanos A A. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl Environ Microbiol. 1986;51:730–737. doi: 10.1128/aem.51.4.730-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deming J W, Hada H, Colwell R R, Luehrsen K R, Fox G E. The ribonucleotide sequence of 5S rRNA from two strains of deep-sea barophilic bacteria. J Gen Microbiol. 1984;130:1911–1920. doi: 10.1099/00221287-130-8-1911. [DOI] [PubMed] [Google Scholar]
- 14.Hamamoto T, Takata N, Kudo T, Horikoshi K. Effect of temperature and growth phase on fatty acid composition of the psychrophilic Vibrio sp. strain no. 5710. FEMS Microbiol Lett. 1994;119:77–82. [Google Scholar]
- 15.Hamamoto T, Takata N, Kudo T, Horikoshi K. Characteristic presence of polyunsaturated fatty acids in marine psychrophilic vibrios. FEMS Microbiol Lett. 1995;129:51–56. [Google Scholar]
- 16.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 17.Hazel J R, Williams E E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990;29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson G R, Poy F, Lengeler J W. Inhibition of Streptococcus mutans by the antibiotic streptozotocin: mechanisms of uptake and the selection of carbohydrate-negative mutants. Infect Immun. 1990;58:543–549. doi: 10.1128/iai.58.2.543-549.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jareonkitmongkol S, Shimizu S, Yamada H. Fatty acid desaturation-defective mutants of an arachidonic-acid-producing fungus, Mortierella alpina 1S-4. J Gen Microbiol. 1992;138:997–1002. [Google Scholar]
- 20.Kadner R J. Cytoplasmic membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 58–87. [Google Scholar]
- 21.Kamimura K, Fuse H, Takimura O, Yamaoka Y. Effects of growth pressure and temperature on fatty acid composition of a barotolerant deep-sea bacterium. Appl Environ Microbiol. 1993;59:924–926. doi: 10.1128/aem.59.3.924-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knauf, V. C., D. Facciotti, R. Valentine, W. Schreckengost, K. Lardizabal, and J. G. Metz. Polyunsaturated fatty acid biosynthesis in marine bacteria. Submitted for publication.
- 23.Lengeler J. Streptozotocin, an antibiotic superior to penicillin in the selection of rare bacterial mutations. FEMS Microbiol Lett. 1979;5:417–419. [Google Scholar]
- 24.Lugtenberg E J J, Peters R. Distribution of lipids in cytoplasmic and outer membranes of Escherichia coli K12. Biochim Biophys Acta. 1976;441:38–47. doi: 10.1016/0005-2760(76)90279-4. [DOI] [PubMed] [Google Scholar]
- 25.Marr A G, Ingraham J L. Effect of temperature on the composition of fatty acids in Escherichia coli. J Bacteriol. 1962;84:1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald A G. The role of membrane fluidity in complex processes under high pressure. In: Marquis R E, Zimmerman A M, Jannasch H W, editors. Current perspectives in high pressure biology. London, England: Academic Press; 1987. pp. 207–223. [Google Scholar]
- 27.McElhaney R N. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J Mol Biol. 1974;84:145–157. doi: 10.1016/0022-2836(74)90218-6. [DOI] [PubMed] [Google Scholar]
- 28.McElhaney R N. Effects of membrane lipids on transport and enzymic activities. In: Razin S, Rottem S, editors. Current topics in membranes and transport. New York, N.Y: Academic Press; 1982. pp. 317–380. [Google Scholar]
- 29.McGarrity J T, Armstrong J B. The effect of temperature and other growth conditions on the fatty acid composition of Escherichia coli. Can J Microbiol. 1981;27:835–840. doi: 10.1139/m81-128. [DOI] [PubMed] [Google Scholar]
- 30.Melchior D L. Lipid phase transitions and regulation of membrane fluidity in prokaryotes. In: Razin S, Rottem S, editors. Current topics in membranes and transport. New York, N.Y: Academic Press; 1982. pp. 263–307. [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 32.Miquel M, James D, Jr, Dooner H, Browse J. Arabidopsis requires polyunsaturated lipids for low temperature survival. Proc Natl Acad Sci USA. 1993;90:6208–6212. doi: 10.1073/pnas.90.13.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray N E, Brammar W J, Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977;150:53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M, Ohnishi S. Organization of lipids in sarcoplasmic reticulum membrane and Ca2+-dependent ATPase activity. J Biochem (Tokyo) 1975;78:1039–1045. doi: 10.1093/oxfordjournals.jbchem.a130981. [DOI] [PubMed] [Google Scholar]
- 35.Nichols D S, Brown J L, Nichols P D, McMeekin T A. Production of eicosapentaenoic acid and arachidonic acids by an Antarctic bacterium: response to growth temperature. FEMS Microbiol Lett. 1997;152:349–354. [Google Scholar]
- 36.Nichols D S, McMeekin T A, Nichols P D. Manipulation of polyunsaturated, branched-chain and trans-fatty acid production in Shewanella putrefaciens strain ACAM 342. Microbiology. 1994;140:577–584. [Google Scholar]
- 37.Nichols D S, Nichols P D, McMeekin T A. Polyunsaturated fatty acids in Antarctic bacteria. Antarct Sci. 1993;2:149–160. [Google Scholar]
- 38.Nichols D S, Nichols P D, McMeekin T A. Ecology and physiology of psychrophilic bacteria from Antarctic saline lakes and sea ice. Sci Prog. 1995;78:311–347. [Google Scholar]
- 39.Nogi Y, Masui N, Kato C. Photobacterium profundum sp. nov., a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extremophiles. 1998;2:1–7. doi: 10.1007/s007920050036. [DOI] [PubMed] [Google Scholar]
- 40.Oliver J D, Colwell R R. Extractable lipids of gram-negative marine bacteria: fatty-acid composition. Int J Syst Bacteriol. 1973;23:442–458. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omura S. Cerulenin. Methods Enzymol. 1981;72:520–532. [PubMed] [Google Scholar]
- 42.Osborn M J, Gander J E, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J Biol Chem. 1972;247:3962–3972. [PubMed] [Google Scholar]
- 43.Overath P, Schairer H U, Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids of Escherichia coli. Proc Natl Acad Sci USA. 1970;67:606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rietschel E T, Gottert H, Luderitz O, Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972;28:166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Sandermann H. Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978;515:209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- 47.Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general Bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 52–61. [Google Scholar]
- 48.Schneider J C, Livne A, Sukenik A, Roessler P G. A mutant of Nannochloropsis deficient in eicosapentaenoic acid production. Phytochemistry. 1995;40:807–814. [Google Scholar]
- 49.Sinensky M. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thilo L, Trauble H, Overath P. Mechanistic interpretation of the influence of lipid phase transitions on transport functions. Biochemistry. 1977;16:1283–1290. doi: 10.1021/bi00626a007. [DOI] [PubMed] [Google Scholar]
- 51.Wada H, Gombos Z, Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature. 1990;347:200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- 52.Wada H, Murata N. Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol. 1989;30:971–978. [Google Scholar]
- 53.Warren G B, Toon P A, Birdsall M J M, Lee A G, Metcalf J C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci USA. 1974;71:622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wirsen C O, Jannasch H W, Wakeham S G, Canuel E A. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr Microbiol. 1987;14:319–322. [Google Scholar]
- 55.Yano Y, Nakayama A, Yoshida K. Distribution of polyunsaturated fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Appl Environ Microbiol. 1997;63:2572–2577. doi: 10.1128/aem.63.7.2572-2577.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yayanos A A. Microbiology to 10,500 meters in the deep sea. Annu Rev Microbiol. 1995;49:777–805. doi: 10.1146/annurev.mi.49.100195.004021. [DOI] [PubMed] [Google Scholar]
- 57.Yayanos A A, Van Boxtel R. Coupling device for quick high pressure connection to 1000 MPa. Rev Sci Instrum. 1982;53:704–705. [Google Scholar]
- 58.Yayanos A A, Dietz S, Van Boxtel R. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science. 1979;205:808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- 59.Yazawa K. Production of eicosapentaenoic acid from marine bacteria. Lipids. 1996;31:S297–S300. doi: 10.1007/BF02637095. [DOI] [PubMed] [Google Scholar]
- 60.Yazawa K, Araki K, Okazaki N, Watanabe K, Ishikawa C, Inoue A, Numao N, Kondo K. Production of eicosapentaenoic acid by marine bacteria. J Biochem. 1988;103:5–7. doi: 10.1093/oxfordjournals.jbchem.a122238. [DOI] [PubMed] [Google Scholar]
- 61.Yazawa K, Araki K, Watanabe K, Ishikawa C, Inoue A, Kondo K, Watabe S, Hashimoto K. Eicosapentaenoic acid productivity of the bacteria isolated from fish intestines. Nippon Suisan Gakkaishi. 1988;54:1835–1838. [Google Scholar]