Abstract
Introduction
Late initiation (LI) of combination antiretroviral therapy (cART)—defined as having a CD4+ count of < 200 cells/μL or an AIDS-defining disease at cART initiation—has detrimental outcomes but remains prevalent worldwide, with LI trends and etiologies following the implementation of various HIV policies remaining underinvestigated. We assessed key concerns, characterized the determinants of various statuses at cART initiation, and evaluated the effects of those statuses on all-cause mortality after cART initiation.
Methods
This multicenter retrospective cohort study enrolled 1198 patients with newly diagnosed HIV infection during 2009–2019 who were grouped by status at cART initiation: those without LI (non-LI group, 56.01%); those with LI but without late presentation (LP) of HIV (LP: a CD4 + count of < 200 cells/μL at HIV presentation or AIDS events ≤ 3 months of HIV diagnosis) [LILP(−) group, 4.51%]; and those with LI and with LP of HIV [LILP(+) group, 39.48%]. Joinpoint regression was used to identify changes in LI proportion.
Results
The median CD4+ count at cART initiation increased significantly between 2009 (98 cells/μL) and 2015 (325 cells/μL) and stabilized thereafter (P for trend < 0.001). For LI, we identified one joinpoint in 2015: a substantial decrease from 77.14% in 2009 to 34.45% in 2015, followed by a nonsignificant increase to 39.1% in 2019. Overall, LILP(+) explained 89.8% of LI, without significant changes (92.59% in 2009 to 94.23% in 2019). In addition to HIV diagnosis during 2009–2012, multinomial logistic regression identified an age over 30 years and acute HIV infection as risk factors for LILP(+) and LILP(−), respectively. LILP(−) and LILP(+) were associated with a higher all-cause mortality risk.
Conclusion
Given the rise in LI from 2015 in the era of treat-all and rapid cART initiation, strategic interventions to increase earlier cART initiation must be intensified in Taiwan, especially among populations with delayed access to HIV testing services.
Keywords: Human immunodeficiency virus, Late initiation, Late presentation, Mortality, Retroviral therapy
Key Summary Points
| Evolving trends and etiologies of late initiation (LI) of combination antiretroviral therapy (cART), despite the implementation of various HIV policies, have not been thoroughly examined. |
| This multicenter retrospective cohort study investigated the trends, joinpoints, and etiologies of LI of cART during 2009−2019, explored the determinants of various statuses at cART initiation, and evaluated the effects of these statuses on all-cause mortality among newly diagnosed patients living with HIV. |
| Although the LI proportion improved significantly from 2009 to 2015, we identified a slow increasing trend between 2015 and 2019. Moreover, we identified late presentation (LP) of HIV as the consistent main etiology of LI during the 11-year study period. |
| In addition to HIV diagnosis during 2009–2012, we identified acute HIV infection and an age over 30 years as risk factors for LI without LP and LI with LP, respectively. |
| Policymakers should implement strategies to facilitate earlier HIV diagnosis among older populations and improve access to cART among people with newly diagnosed acute HIV infection. |
Introduction
Potent combination antiretroviral therapy (cART) can suppress HIV loads to an undetectable level and restore CD4+ T-cell counts in people living with HIV (PLWH). cART has considerably decreased AIDS-related morbidity and mortality in PLWH [1–5]. Several strategies have been implemented sequentially to upgrade HIV treatment outcomes (including the following recommended CD4+ count thresholds at cART initiation: ≤ 350 cells/µL in 2008, ≤ 500 cells/µL in 2010 [6], and all patients with HIV regardless of CD4+ count in 2015 [7]) to increase global cART coverage (48% in 2015, 53% in 2016, and 59% in 2017) [8]. Nevertheless, a substantial proportion of patients begin cART with an advanced HIV status [9–11].
Among PLWH, late initiation (LI) of cART is defined as having a CD4+ count of < 200 cells/μL or an AIDS-defining disease at cART initiation [9]. LI has been associated with inflammation and metabolic abnormalities [12], increased 48-week mortality [11, 13–15], HIV transmission to sexual partners [16, 17], virological failure-induced regimen modification [11], and decreased life expectancy [18]. Although LI is prevalent in the Asia–Pacific region [9, 19, 20], Canada [21], South America [22, 23], and sub-Saharan Africa [10, 14, 24], how the causes of LI have changed in response to the implementation of various HIV policies has not been comprehensively explored [25–27]. LI may be due to late HIV diagnosis or late cART initiation [25–27], and an assessment of LI causes and barriers to early initiation is necessary. Moreover, the currently reported risk factors for LI, including socioeconomic inequalities [22], male sex [9, 28], older age [28], cART initiation early in the calendar year [9, 21], and HIV exposure risk [9], have primarily been reported by studies that have analyzed the risks for all participants with LI rather than by those that have analyzed the risks for participants classified by various LI etiologies [25, 27, 29]. This may explain the limited applicability of the study findings and the interventions thereof.
In Taiwan, cART has been fully covered by the National Health Insurance program since 1997. Physicians treat PLWH according to the recommendations of evolving, revised guidelines for HIV/AIDS diagnosis and treatment in Taiwan [30–32], and the CD4+ count threshold for cART initiation has been increasing (≤ 200 cells/μL in 2006, ≤ 350 cells/μL in 2010, ≤ 500 cells/μL in 2013, and all patients with HIV in 2016) [33]. A retrospective study in Taiwan concluded that the overall proportion of late cART initiators had decreased significantly, from 49.1% in 2012 to 29.0% in 2016 [11], but the study was limited by a short observation period (2012–2016) and ambiguity about the extent to which LI was induced by late HIV diagnosis. The Taiwanese government has adopted policies promoting the implementation of a “treat-all” scheme since 2016 [33] and the rapid initiation of cART (i.e., within 7 days of HIV diagnosis) since 2018 [34], and, since 2018, the government has made provisions for HIV at-risk populations to be given free access to HIV blood test kits from health bureaux or nongovernmental organizations or to oral fluid self-test kits from vending machines [34]. However, the treat-all and rapid cART policies are not mandatory in Taiwan, and some newly diagnosed PLWH will not receive cART as recommended due to feeling healthy or fear of stigma and discrimination [26]. Moreover, pre-exposure prophylaxis (PrEP) among HIV-negative at-risk population has been shown to reduce the risk of HIV infection by 44–86% [35]. In Taiwan, the PrEP project started in 2016, and 36 HIV-designated hospitals and 2 sexual health clinics provide the service in 2022 [36, 37]. In 2019, 8.9% of men who have sex with men (MSM) were on the Taiwan PrEP project [38]. Currently, the yearly number of new HIV cases has decreased substantially since 2018 [39]. Therefore, understanding LI trends and etiologies after the implementation of HIV prevention policies since 2016 is necessary.
Accordingly, this study investigated the trends, joinpoints, and etiologies of LI during 2009–2019, explored the determinants of various statuses at cART initiation, and evaluated the effects of these statuses on all-cause mortality after cART initiation among newly diagnosed PLWH.
Methods
Study Design and Setting
In Taiwan, only hospitals qualified and designated by Taiwan Centers for Disease Control can provide care for PLWH. To date, 82 hospitals have been listed as HIV-designated hospitals, and, based on the medical care provision levels, these hospitals are further classified as follows: 21 medical centers, 45 regional hospitals, and 16 district hospitals [40]. This multicenter retrospective cohort included patients with newly diagnosed HIV at Kaohsiung Medical University Chung-Ho Memorial Hospital (the largest referral center for HIV treatment in southern Taiwan) and at Kaohsiung Municipal Siaogang Hospital and Kaohsiung Municipal Ta-Tung Hospital (two regional hospitals in southern Taiwan) from January 1, 2009, to December 31, 2019. Health-care staff members at these hospitals have extensive experience in treating PLWH. They measure each patient’s CD4+ count and viral load at baseline and at 0.5–1.5 months after cART initiation, every 3 months for the first year, and every 3–6 months thereafter. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-SV(I)-20210092). The requirement for informed consent was waived. The study was carried out according to the principles expressed in the Declaration of Helsinki of 1964 and its later amendments.
Patients and Study Procedure
A trained research assistant screened the patients between January 1, 2009, and December 31, 2019, from outpatient and inpatient departments. All patients were followed up until death, loss to follow-up, or September 30, 2020, whichever occurred first. Patients without a cART prescription, CD4+ count data within 6 months of HIV presentation, or cART initiation were excluded.
We classified the patients who had a CD4+ count of < 200 cells/μL at cART initiation or an AIDS-defining opportunistic illness before cART initiation as the LI group and those who did not meet either threshold as the non-LI (NLI) group [11, 28]. The LI group was further categorized into two subgroups by the late presentation (LP) of HIV: (1) the LILP(−) group included patients with LI but without LP of HIV, and (2) the LILP(+) group included those with LI and with LP of HIV [25]. LP was defined as having a CD4+ count of < 200 cells/μL at HIV presentation or AIDS events ≤ 3 months of their HIV diagnosis [41].
Data Collection
Information on baseline demographic and clinical characteristics (age, sex, comorbidities, education level, occupation, marital status, HIV diagnosis date, and HIV exposure mode), antiretroviral treatment history, AIDS-defining opportunistic illness events, and date and cause of death was collected. Laboratory test results were collected at baseline [hepatitis A virus antibody (Ab), hepatitis B virus (HBV) surface antigen (HBsAg), and hepatitis C virus Ab levels]. In addition, we collected data on CD4+ counts and plasma viral loads at HIV presentation, cART initiation, and serial follow-up visits. The data were anonymized prior to analysis.
Working Definitions
We defined the HIV infection stage at presentation on a 0–3 scale according to the US CDC 2014 case definition of HIV infection, with stage 0 representing acute HIV infection [42].
Baseline laboratory tests were conducted as soon as possible or within 6 months of HIV diagnosis [43]. Initiation of first-line cART was defined as the first recorded instance of concurrent use of the standard three antiretrovirals for 7 days [44]. The pre-cART CD4+ count and HIV viral load before and nearest to the cART initiation date were considered [41].
Calendar-year periods were classified according to major changes in HIV treatment management guidelines (CD4+ count of ≤ 350 cells/μL in 2010, CD4+ count of ≤ 500 cells/μL in 2013, and all patients in 2016) into periods 1 (2009–2012), 2 (2013–2015), and 3 (2016–2019), respectively.
LI etiologies were dichotomized as follows: LILP(−) and LILP(+).
Statistical Analysis
First, characteristics of patients stratified by their status at cART initiation [NLI, LILP(−), and LILP(+)] were tabulated. We used the χ2 or Fisher’s exact test for categorical variables and the Kruskal–Wallis test followed by Dunn’s post hoc test for continuous variables that did not follow a normal distribution.
The trends of various CD4+ count thresholds at cART initiation, LI, LILP(−), and LILP(+) during 2009–2019 were evaluated using the Cochran–Armitage trend test with modified ridit scores. The trend of the median CD4+ count at cART initiation was analyzed using a generalized linear model.
Joinpoint regression has been used in numerous domains to identify the best-fitting points if a statistically significant change occurs in time series data [45]. Therefore, to identify changes in the proportion of CD4+ count thresholds at cART initiation and LI, joinpoint regression analysis was performed using the Joinpoint Regression Program (v.4.9.0.0; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD, USA). In brief, by using the proportion of patients with various CD4+ count thresholds at cART initiation and LI as input in the analysis, we identified the calendar year associated with changes in the threshold trends at cART initiation and LI. We also calculated the annual percentage change (APC) between trend change points.
To identify NLI, LILP(−), and LILP(+) determinants at cART initiation, multinomial logistic regression (reference: NLI) was used. All covariates of sociodemographic characteristics and laboratory data were selected for the subsequent multivariable logistic regression.
Finally, the probability of survival after cART initiation stratified by various statuses of cART initiation was estimated using the Kaplan–Meier survival curves and log-rank testing. We used Cox proportional hazard models to evaluate the determinants of all-cause mortality after cART initiation. In this model, all variables (sociodemographic characteristics, laboratory data, prophylaxis with trimethoprim/sulfamethoxazole, status at cART initiation, days from HIV diagnosis to cART initiation, and classification of the antiretroviral agent) used in the bivariable analysis were selected for the subsequent Cox regression analysis.
All tests were two-tailed, and statistical significance was set at P < 0.05. All statistical analyses were performed using SAS (v.9.4; SAS Institute, Cary, NC, USA).
Results
Patient Characteristics at HIV Diagnosis
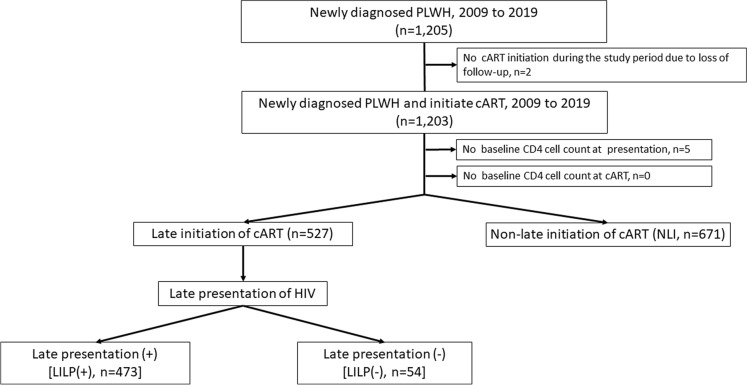
Of the 1198 patients enrolled, 671 (56.01%), 54 (4.51%), and 473 (39.48%) were assigned to the NLI, LILP(−), and LILP(+) groups, respectively (Fig. 1; Table 1). Most patients were men (98.25%), MSM (88.06%), employed (75.21%), and unmarried (93.91%). The median baseline CD4+ count (interquartile range, IQR) was 267.37 (86.51–404.57). The median duration from HIV diagnosis to the first CD4+ count examination and that from the first CD4+ count examination to cART initiation was 6 (1–11) and 14 (6–28) days, respectively. Regarding cART prescription components, zidovudine was most commonly used as the backbone drug (35.06%), and nonnucleoside reverse-transcriptase inhibitors were most commonly used as the third drug (60.08%).
Fig. 1.
Study flowchart. cART combination antiretroviral therapy, HIV human immunodeficiency virus, LI late initiation, LP late presentation, LILP(+) patients with LI and with LP, LILP(−) patients with LI but without LP, NLI non-late initiation, PLWH patients living with HIV
Table 1.
Sociodemographic characteristics of the 1,198 PLWH stratified by cART initiation
| All n = 1198 |
NLI n = 671 |
LILP(−) n = 54 |
LILP(+) n = 473 |
P value | |
|---|---|---|---|---|---|
| Sociodemographic variables | |||||
| Male, n (%) | 1177 (98.25) | 662 (98.66) | 54 (100.00) | 461 (97.46) | 0.191 |
| Median age at HIV presentation, years (IQR) | 29.14 (24.54–35.20) | 27.28 (23.51–33.35)a | 29.75 (22.83–32.24)b | 31.43 (26.66–39.18)a,b | < 0.001 |
| Subgroup of age at HIV presentation, n (%) | < 0.001 | ||||
| ≤ 30 years | 711 (59.35) | 446 (66.47) | 36 (66.67) | 229 (48.41) | |
| 31–40 years | 321 (26.79) | 165 (24.59) | 13 (24.07) | 143 (3.23) | |
| ≥ 41 years | 166 (13.86) | 60 (894) | 5 (9.26) | 101 (21.35) | |
| Period of HIV diagnosis | < 0.001 | ||||
| 2009–2012 | 365 (30.47) | 162 (24.14) | 34 (62.96) | 169 (35.73) | |
| 2013–2015 | 342 (28.55) | 208 (31.0) | 8 (14.81) | 126 (26.64) | |
| 2016–2019 | 491 (40.98) | 301 (44.86) | 12 (22.22) | 178 (37.63) | |
| HIV transmission route, n (%) | 0.005 | ||||
| MSM | 1055 (88.06) | 604 (90.01) | 49 (90.74) | 402 (84.99) | |
| Heterosexual contact | 125 (10.43) | 53 (7.90) | 5 (9.26) | 67 (14.16) | |
| IDU | 18 (1.5) | 14 (2.09) | 0 (0.00) | 4 (0.85) | |
| HIV stage at presentation, according to the 2014 CDC criteria, n (%) | < 0.001 | ||||
| Stage 0 (Acute HIV) | 97 (8.10) | 68 (10.13) | 29 (53.70) | 0 (0.00) | |
| Stage 1 (CD4 + count ≥ 500 cells/μL) | 149 (12.44) | 142 (21.16) | 7 (12.96) | 0 (0.00) | |
| Stage 2 (CD4 + count 200–499 cells/μL) | 479 (39.98) | 461 (68.70) | 18 (33.33) | 0 (0.00) | |
| Stage 3 (AIDS) | 473 (39.48) | 0 (0.00) | 0 (0.00) | 473 (100.00) | |
| Acute HIV, n (%) | 97 (8.10) | 68 (10.13) | 29 (53.70) | 0 (0.00) | < 0.001 |
| Education level above college, n (%) | |||||
| Yes | 683 (57.01) | 392 (58.42) | 32 (59.26) | 259 (54.76) | 0.441 |
| Occupation, n (%) | 0.075 | ||||
| Unemployed | 183 (15.28) | 94 (14.01) | 5 (9.26) | 84 (17.76) | |
| Employed | 901 (75.21) | 503 (74.96) | 43 (79.63) | 355 (75.05) | |
| Student | 114 (9.52) | 74 (11.03) | 6 (11.11) | 34 (7.19) | |
| Marital status | 0.159 | ||||
| Unmarried | 1125 (93.91) | 639 (95.23) | 51 (94.44) | 435 (91.97) | |
| Married | 49 (4.09) | 21 (3.13) | 3 (5.56) | 25 (5.29) | |
| Divorced | 24 (2.00) | 11 (1.64) | 0 (0.00) | 13 (2.75) | |
| Co-morbidities and opportunistic illness, n (%) | |||||
| Co-morbidities | |||||
| Diabetes mellitus | 12 (1.00) | 6 (0.89) | 0 (0.00) | 6 (1.27) | 0.618 |
| Hypertension | 31 (2.59) | 15 (2.24) | 1 (1.85) | 15 (3.17) | 0.581 |
| Chronic kidney disease | 9 (0.75) | 4 (0.6) | 1 (1.85) | 4 (0.85) | 0.563 |
| Dyslipidemia | 21 (1.75) | 11 (1.64) | 1 (1.85) | 9 (1.90) | 0.944 |
| Cerebral vascular accident | 8 (0.67) | 1 (0.15) | 0 (0.00) | 7 (1.48) | 0.020 |
| Opportunistic illness | |||||
| Pneumocystis jiroveci pneumonia | 195 (16.28) | 0 (0.00) | 1 (1.85) | 194 (41.01) | |
| Disseminated mycobacterium avium-intracellulare complex | 19 (1.59) | 0 (0.00) | 0 (0.00) | 19 (4.02) | |
| Mycobacterium tuberculosis infection | 19 (1.59) | 0 (0.00) | 1 (1.85) | 18 (3.81) | |
| Cryptococcosis | 16 (1.34) | 0 (0.00) | 0 (0.00) | 16 (3.38) | |
| Kaposi’s sarcoma | 12 (1.00) | 0 (0.00) | 0 (0.00) | 12 (2.54) | |
| Lymphoma | 10 (0.83) | 0 (0.00) | 0 (0.00) | 10 (2.11) | |
| Leukemia | 1 (0.08) | 0 (0.00) | 0 (0.00) | 1 (0.21) | |
| CMV disease | 50 (4.17) | 0 (0.00) | 0 (0.00) | 50 (10.57) | |
| Laboratory examination | |||||
| Median baseline CD4+cell count, cells/μL (IQR) | 267.37 (86.51–404.57) | 374.54 (290.02–491.70)a,c | 219.71 (143.09–383.27)b,c | 56.84 (24.62–129.64)a,b | < 0.001 |
| Median baseline VL (log) (IQR) | 4.83 (4.35–5.34) | 4.61 (4.16–4.94)a,c | 5.03 (4.52–6.12)c | 5.24 (4.83–5.62)a | < 0.001 |
| Baseline VL > 100,000 copies/mL, n (%) | 489 (40.82) | 146 (21.76) | 29 (53.70) | 314 (66.38) | < 0.001 |
| HAV antibody seropositivity, n (%) (n = 1147) | 218 (19.01)) | 100 (15.11) | 11 (21.15) | 107 (2471) | < 0.001 |
| HBsAg seropositivity, n (%) | 126 (10.66) | 55 (8.26) | 3 (5.55) | 68 (14.69) | 0.001 |
| HCV antibody seropositivity, n (%) | 52 (4.41) | 31 (4.66) | 0 (0.00) | 21 (4.54) | 0.291 |
| VDRL ≥ 1:8 (n = 1190) | 305 (25.63) | 189 (28.25) | 12 (22.64) | 104 (22.22) | 0.064 |
| CMV antibody seropositivity, n (%) | 1161 (96.91) | 648 (96.57) | 53 (98.15) | 460 (97.25) | 0.700 |
| Class of first line of cART at initiation, n (%) | |||||
| Backbone regimen, n (%) | |||||
| Zidovudine-based | 420 (35.06) | 228 (33.98) | 22 (40.74) | 170 (35.98) | 0.530 |
| Abacavir-based | 401 (33.47) | 201 (29.96) | 20 (37.04) | 180 (38.05) | 0.014 |
| TDF/TAF-based | 375 (31.30) | 242 (36.07) | 12 (22.22) | 121 (25.58) | < 0.001 |
| Third regimen, n (%) | |||||
| nNRTI-based | 727 (60.08) | 411 (61.25) | 36 (66.67) | 280 (59.20) | 0.512 |
| PI-based | 92 (7.68) | 38 (5.66) | 5 (9.26) | 49 (10.36) | 0.012 |
| II-based | 379 (31.64) | 222 (33.08) | 13 (24.07) | 144 (30.44) | 0.303 |
| Median duration along the HIV care continuum, days (IQR) | |||||
| From HIV diagnosis to first CD4+ count examination | 6 (1–11) | 7 (2–13)a | 4.5 (0–9) | 5 (1–10)a | 0.402 |
| > 14 days, n (%) | 208 (17.36) | 131 (19.52) | 9 (16.67) | 68 (14.38) | 0.077 |
| From first CD4+ count to cART initiation | 14 (6–28) | 14 (7–77)a,c | 236.5 (15–900) b,c | 10 (5–17) a,b | < 0.001 |
| From HIV diagnosis to cART initiation | 21 (10–49) | 25 (13–105)a,c | 246 (19–965)b,c | 17 (8–28) a,b | < 0.001 |
cART combination antiretroviral therapy, CDC Centers for Disease Control and Prevention, CMV cytomegalovirus, HCV hepatitis C virus, HAV hepatitis A virus, HBsAg hepatitis B surface antigen, HIV human immunodeficiency virus, IDU injecting drug use, II integrase inhibitors, IQR interquartile range, LILP(−) late initiators of cART without late presentation, LILP(+) late initiators of cART with late presentation, MSM men who have sex with men, NLI nonlate initiators of cART, nNRTI nonnucleoside reverse transcriptase inhibitors, PI protease inhibitor, SD standard deviation, TAF tenofovir alafenamide, TDF tenofovir disoproxil fumarate, VDRL Venereal Disease Research Laboratory, VL viral load
aDunn’s post hoc test between group 1 and group 3 < 0.05
bDunn’s post hoc test between group 2 and group 3 < 0.05
cDunn’s post hoc test between group 1 and group 2 < 0.05
The three groups differed significantly in sociodemographic characteristics and laboratory examination results, except for sex, education level, occupation, marital status, comorbidities, and mean duration from HIV diagnosis to the first CD4+ count examination (Table 1).
Trends of Median CD4+ Counts at cART Initiation and Proportions of Various CD4+ Count Thresholds at cART Initiation
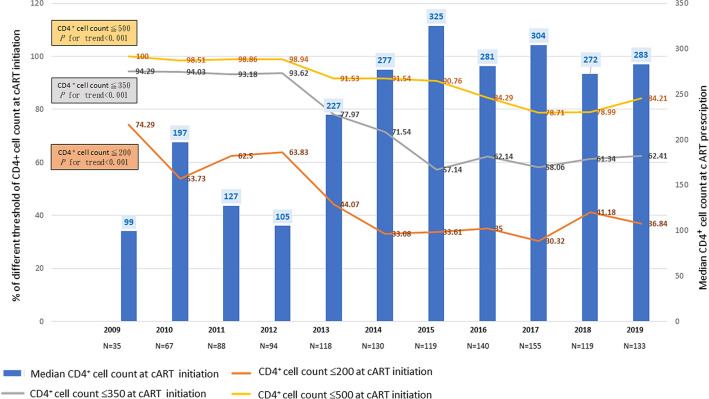
Overall, 42.24%, 71.12%, and 88.65% of the enrolled patients had CD4+ counts of ≤ 200, ≤ 350, and ≤ 500 cells/μL, respectively, at cART initiation.
The median CD4+ count at cART initiation increased significantly from 98 (23–211) cells/μL in 2009 to 325 (127–406) cells/μL in 2015 and stabilized thereafter at 272–304 cells/μL (P for trend < 0.001; Fig. 2). The proportion of patients with CD4+ counts of ≤ 200, ≤ 350, and ≤ 500 cells/mm3 at cART initiation decreased significantly from 2009 (74.29%, 94.29%, and 100%, respectively) to 2019 (36.8%, 62.41%, and 84.21%, respectively; all P for trend < 0.001; Fig. 2).
Fig. 2.
Distribution and median CD4+ counts at cART initiation in Taiwan from 2009–2019. Abbreviation: cART, combination antiretroviral therapy
Changes in the Trends of the Proportions of Various CD4+ Count Thresholds at cART Initiation
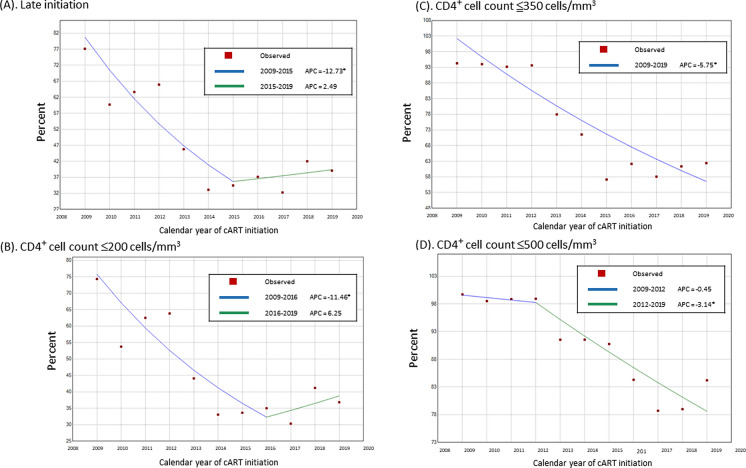
For the CD4+ count threshold of ≤ 200 cells/μL at cART initiation, one joinpoint was identified in 2016 (Fig. 3B), leading to two periods with distinct trends: a decreasing trend between 2009 and 2016 [− 11.46% annual percentage change (APC), P < 0.05], where the proportion of patients with this CD4+ count decreased from 74.29% in 2009 to 35% in 2016 (Fig. 2), and an increasing trend between 2016 and 2019 (6.28% APC, nonsignificant), where the proportion of patients with this CD4+ count increased from 35% in 2016 to 36.84% in 2019.
Fig. 3.
A Joinpoint models for late initiation, B for CD4+ count ≤ 200, C ≤ 350, and D ≤ 500 cells/μL. One joinpoint was identified in 2015 for the late initiation (A). One joinpoint was identified in 2016 and 2012 for the CD4+ count threshold of ≤ 200 cells/μL (B) and ≤ 500 cells/μL (D) at cART initiation, respectively. No joinpoint was identified for the CD4+ count threshold of ≤ 350 cells/μL at cART initiation (C)
For the CD4+ count threshold of ≤ 350 cells/μL at cART initiation, no joinpoint was identified (Fig. 3C), leading to a decreasing trend between 2009 and 2019 (− 5.75% APC, P < 0.05), accompanied by a decrease in the proportion of those with a CD4+ count of ≤ 350 cells/mm3 from 94.29% in 2009 to 62.41% in 2019.
For the CD4+ count threshold of ≤ 500 cells/μL at cART initiation, one joinpoint was identified in 2012 (Fig. 3D), leading to two periods with dissimilar trends: a slow decreasing trend between 2009 and 2012 (− 0.45% APC, nonsignificant) and a rapid decreasing trend between 2012 and 2019 (− 3.14% APC, P < 0.05).
Trends in LI, Identified Joinpoints, and Dichotomous Etiologies of LI
Overall, the proportion of patients in the LI group decreased from 77.14% in 2009 to 39.1% in 2019 (P for trend < 0.001; Fig. 4). One joinpoint was identified in 2015, leading to two periods with unique trends: a decreasing trend between 2009 and 2015 (− 12.73% APC, P < 0.05; Fig. 3A), where the proportion of patients decreased from 77.14% in 2009 to 34.45% in 2015, and a slow increasing trend between 2015 and 2019 (2.49% APC, nonsignificant), where the proportion of patients increased from 34.45% in 2015 to 39.1% in 2019.
Fig. 4.
Trend of late initiation and the associated dichotomized etiologies from 2009 to 2019. cART combination antiretroviral therapy, LI late initiation, LP late presentation, LILP(+) patients with LI and with LP, LILP(−) patients with LI but without LP
Overall, 89.8% of the patients in the LI group were assigned to the LILP(+) subgroup. During the study period, LILP(+) remained the main etiology of LI (92.59% in 2009 to 94.23% in 2019, P for trend = 0.649), whereas LILP(−) remained the minor etiology (7.41% in 2009 to 5.77% in 2019) (Fig. 4).
Determinants of Various Status Groups at cART Initiation
With the NLI group as the control group, we observed that LILP(−)and LILP(+) determinants differed. Positive LILP(−) determinants were acute HIV at presentation [adjusted odds ratio (aOR), 18.00; 95% confidence interval (CI), 8.33–38.89; P < 0.001] and HIV diagnosis during the first calendar-year period (period 1 vs. period 3; 2009–2012: aOR, 11.51; 95% CI, 4.66–28.46; P < 0.001; Table 2).
Table 2.
Multinomial logistic regression modeling results of 1,198 PLWH stratified by status at cART initiation
| LILP(+) vs. NLI, adjusted OR (95% CI) | P value | LILP(−) vs. NLI, adjusted OR (95% CI) | P value | |
|---|---|---|---|---|
| Gender | ||||
| Female | Reference | Reference | ||
| Male | 0.84 (0.28–2.48) | 0.748 | 67.46 (0.01–999.99) | 0.811 |
| Subgroup of age at HIV diagnosis (years) | ||||
| ≤ 30 | Reference | Reference | ||
| 31–40 | 1.53 (1.12–2.08) | 0.007 | 1.79 (0.80–4.04) | 0.158 |
| ≥ 41 | 2.49 (1.53–4.03) | < 0.001 | 1.68 (0.39–7.21) | 0.488 |
| Period of HIV diagnosis | ||||
| 2016–2019 | Reference | Reference | ||
| 2013–2015 | 0.90 (0.66–1.24) | 0.511 | 2.15 (0.75–6.18) | 0.155 |
| 2009–2012 | 1.39 (1.02–1.91) | 0.037 | 11.51 (4.66–28.46) | < 0.001 |
| HIV transmission route | ||||
| MSM | Reference | Reference | ||
| Heterosexual contact | 1.30 (0.79–2.14) | 0.301 | 1.42 (0.40–5.12) | 0.589 |
| IDU | 0.48 (0.13–1.70) | 0.255 | 0.04 (0.01–999.99) | 0.743 |
| Acute HIV | ||||
| No | Reference | Reference | ||
| Yes | 0.01 (0.01–999.99) | 0.955 | 18.00 (8.33–38.89) | < 0.001 |
| Educational level above college | ||||
| No | Reference | Reference | ||
| Yes | 1.09 (0.83–1.43) | 0.553 | 1.19 (0.58–2.45) | 0.628 |
| Occupation | ||||
| Unemployed | Reference | Reference | ||
| Employed | 0.80 (0.55–1.16) | 0.228 | 2.17 (0.69–6.81) | 0.187 |
| Student | 0.73 (0.42–1.28) | 0.276 | 1.08 (0.20–5.83) | 0.926 |
| Marital status | ||||
| Unmarried | Reference | Reference | ||
| Married | 0.78 (0.36–1.67) | 0.51 | 0.76 (0.09–6.65) | 0.804 |
| Divorced | 0.54 (0.19–1.52) | 0.24 | 0.01 (0.01–999.99) | 0.636 |
| Diabetes mellitus | ||||
| No | Reference | Reference | ||
| Yes | 1.47 (0.32–6.88) | 0.621 | 80.51 (0.01–999.99) | 00 |
| Hypertension | ||||
| No | Reference | Reference | ||
| Yes | 0.83 (0.32–2.17) | 0.709 | 56.41 (0.01–999.99) | 0.647 |
| Chronic kidney disease | ||||
| No | Reference | Reference | ||
| Yes | 1.27 (0.26–6.21) | 0.764 | 0.20 (0.01–5.10) | 70.32 |
| Dyslipidemia | 5 | |||
| No | Reference | Reference | ||
| Yes | 1.45 (0.53–3.96) | 0.472 | 0.80 (0.06–10.70) | 0.686 |
| Cerebral vascular accident | ||||
| No | Reference | Reference | ||
| Yes | 0.14 (0.02–1.31) | 0.08 | 40.91 (0.01–999.99) | 0.913 |
| VDRL ≥ 1:8 | ||||
| No | Reference | Reference | ||
| Yes | 0.68 (0.50–0.92) | 0.013 | 0.96 (0.44–2.13) | 0.926 |
| HAV antibody | ||||
| No | Reference | Reference | ||
| Yes | 1.41 (0.97–2.05) | 0.070 | 2.03 (0.80–5.18) | 0.137 |
| HBsAg | ||||
| No | Reference | Reference | ||
| Yes | 1.34 (0.87–2.05) | 0.180 | 0.52 (0.14–1.97) | 0.337 |
| HCV antibody | ||||
| No | Reference | Reference | ||
| Yes | 0.90 (0.45–1.76) | 0.748 | 0.01 (0.01–999.99) | 0.515 |
cART combination antiretroviral therapy, CDC Centers for Disease Control and Prevention, CI confidence interval, CMV cytomegalovirus, HCV hepatitis C virus, HAV hepatitis A virus, HBsAg hepatitis B surface antigen, HIV human immunodeficiency virus, IDU injecting drug use, NLI nonlate initiators of cART, LILP(−) late initiators of cART without late presentation, LILP(+) late initiators of cART with late presentation, MSM men who have sex with men, N/A not available, OR odds ratio, VDRL Venereal Disease Research Laboratory
Positive LILP(+) determinants were age (31–40 and ≥ 41 years vs. ≤ 30 years; 31–40 years: aOR, 1.53; 95% CI, 1.12–2.08; P = 0.007; ≥ 41 years: aOR, 2.49; 95% CI, 1.53–4.03; P < 0.001) and HIV diagnosis during the first calendar-year period (period 1 vs. period 3; 2009–2012: aOR, 1.39; 95% CI, 1.02–1.91; P = 0.037), whereas its negative determinant was a Venereal Disease Research Laboratory (VDRL) titer of ≥ 1:8 (aOR, 0.68; 95% CI, 0.50–0.92; P = 0.013; Table 2).
Determinants of All-Cause Mortality After cART Initiation
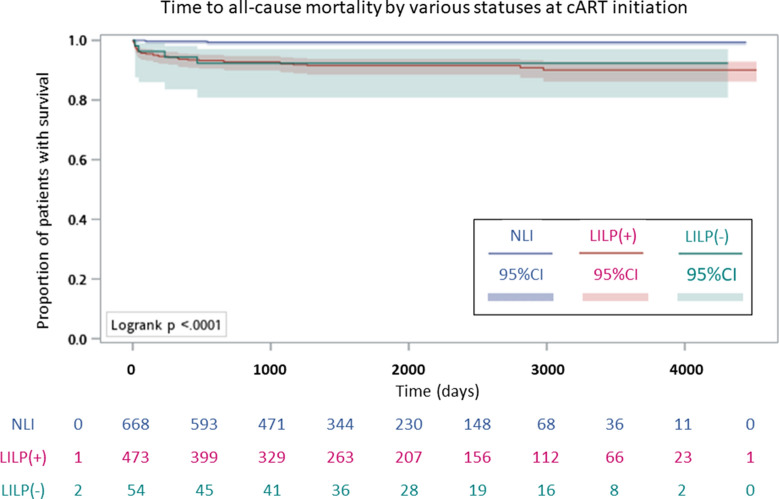
The probability of survival after cART initiation differed significantly throughout the three statuses of cART initiation (log-rank test, P < 0.001) (Fig. 5).
Fig. 5.
Analysis of survival after cART initiation stratified by the various statuses of cART initiation
In Cox regression analyses spanning the entire study period, all-cause mortality after cART initiation was positively associated with age (vs. ≤ 30 years) at HIV diagnosis [31–40 years: adjusted hazard ratio (aHR), 5.79; 95% CI, 1.63–20.58; ≥ 41 years: aHR, 9.24; 95% CI; 2.21–38.67), HBsAg seropositivity (aHR, 3.99; 95% CI, 1.48–10.73), and status (vs. NLI) at cART initiation [LILP(+): aHR, 17.46; 95% CI, 3.46–88.05; LILP(−): aHR, 9.38; 95% CI, 1.44–60.93; Table 3].
Table 3.
Cox regression analysis of all-cause mortality after cART initiation among 1198 PLWH
| Number of patients, n | Number of deaths, n (%) | P value | Bivariable analysis, crude HR (95% CI) | Multivariable analysis, adjusted HR (95% CI) | |
|---|---|---|---|---|---|
| Gender | 0.842 | ||||
| Female | 20 | 1 (2.13) | Reference | Reference | |
| Male | 1177 | 46 (3.91) | N/A | N/A | |
| Subgroup of age at HIV diagnosis (years) | < 0.001 | ||||
| ≤ 30 | 711 | 15 (2.11) | Reference | Reference | |
| 31–40 | 321 | 17 (5.30) | 6.00 (1.91–18.84)** | 5.79 (1.63–20.58)** | |
| ≥ 41 | 166 | 15 (9.04) | 12.31 (3.92–38.68)*** | 9.24 (2.21–38.67)** | |
| Period of HIV diagnosis | 0.014 | ||||
| 2009–2012 | 365 | 23 (6.30) | Reference | Reference | |
| 2013–2015 | 342 | 12 (3.51) | 0.49 (0.17–1.39) | 0.69 (0.20–2.43) | |
| 2016–2019 | 491 | 12 (2.44) | 0.66 (0.26–1.68) | 0.86 (0.16–4.70) | |
| HIV transmission route | 0.002 | ||||
| MSM | 1055 | 34 (3.22) | Reference | Reference | |
| Heterosexual contact | 125 | 12 (9.60) | 1.18 (0.35–3.93) | 0.85 (0.18–3.98) | |
| IDU | 18 | 1 (5.56) | 3.11 (0.42–23.07) | 9.26 (0.76–113.47) | |
| Acute HIV | |||||
| No | 1101 | 44 (4.00) | 0.660 | Reference | Reference |
| Yes | 97 | 3 (3.09) | 1.022 (0.24–4.33) | 5.62 (0.75–42.01) | |
| Educational level above college | 0.019 | ||||
| No | 515 | 28 (5.44) | Reference | Reference | |
| Yes | 683 | 19 (2.78) | 0.63 (0.29–1.37) | 0.90 (0.37–2.18) | |
| Occupation | < 0.001 | ||||
| Unemployed | 183 | 17 (9.29) | Reference | Reference | |
| Employed | 901 | 29 (3.22) | 0.38 (0.17–0.89)* | 0.40 (0.15–1.05) | |
| Student | 114 | 1 (0.88) | 0.19 (0.02–1.53) | 1.03 (0.10–10.19) | |
| Marital status | 0.149 | ||||
| Unmarried | 1125 | 41 (3.64) | Reference | Reference | |
| Married | 49 | 4 (8.16) | 3.96 (1.36–11.50)* | 2.37 (0.55–10.29) | |
| Divorced | 24 | 2 (8.33) | N/A | N/A | |
| Diabetes mellitus | 0.482 | ||||
| No | 1186 | 47 (3.96) | Reference | Reference | |
| Yes | 12 | 0 (0.00) | N/A | N/A | |
| Hypertension | 0.839 | ||||
| No | 1,167 | 46 (3.94) | Reference | Reference | |
| Yes | 31 | 1 (3.23) | 0.64 (0.09–4.71) | 2.12 (0.23–19.12) | |
| Chronic kidney disease | 0.543 | ||||
| No | 1189 | 47 (3.95) | Reference | Reference | |
| Yes | 9 | 0 (0.00) | N/A | N/A | |
| Dyslipidemia | 0.350 | ||||
| No | 1177 | 47 (3.99) | Reference | Reference | |
| Yes | 21 | 0 (0.00) | N/A | N/A | |
| Cerebral vascular accident | 0.566 | ||||
| No | 1190 | 47 (3.95) | Reference | Reference | |
| Yes | 8 | 0 (0.00) | N/A | N/A | |
| Baseline VL > 100,000 copies/mL | 0.039 | ||||
| No | 709 | 21 (2.96) | Reference | Reference | |
| Yes | 489 | 26 (5.32) | 1.55 (0.72–3.34) | 0.61 (0.25–1.54) | |
| VDRL ≥ 1:8 | < 0.001 | ||||
| No | 885 | 36 (4.07) | Reference | Reference | |
| Yes | 305 | 7 (2.30) | 0.55 (0.19–1.59) | 0.57 (0.19–1.76) | |
| HBsAg | < 0.001 | ||||
| No | 1056 | 28 (2.65) | Reference | Reference | |
| Yes | 126 | 13 (10.32) | 5.25 (2.38–11.59)*** | 3.99 (1.48–10.73)** | |
| HCV antibody | < 0.001 | ||||
| No | 1127 | 39 (3.46) | Reference | Reference | |
| Yes | 52 | 3 (5.77) | 2.00 (0.47–8.47) | 1.30 (0.24–6.89) | |
| Prophylaxis with TMP/SMX | 0.018 | ||||
| No | 1032 | 35 (3.39) | Reference | Reference | |
| Yes | 166 | 12 (7.23) | 1.85 (0.74–4.60) | 0.62 (0.23–1.69) | |
| Status at cART initiation | < 0.001 | ||||
| NLI | 671 | 4 (0.60) | Reference | Reference | |
| LILP(+) | 473 | 39 (8.25) | 9.86 (2.93–33.19)*** | 17.46 (3.46–88.05)*** | |
| LILP(−) | 54 | 4 (7.41) | 11.40 (2.29–56.70)** | 9.38 (1.44–60.93)* | |
| Days from HIV diagnosis to cART initiation] | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | |||
| Classification of antiretroviral agent | |||||
| Zidovudine | 0.085 | ||||
| No | 778 | 25 (3.21) | Reference | Reference | |
| Yes | 420 | 22 (1.84) | 0.84 (0.37–1.91) | N/A | |
| Abcavir | 0.817 | ||||
| No | 797 | 32 (4.02) | Reference | ||
| Yes | 401 | 15 (3.74) | 1.09 (0.49–2.45) | N/A | |
| TDF/TAF | 0.067 | ||||
| No | 819 | 39 (4.76) | Reference | ||
| Yes | 379 | 8 (2.11) | 0.91 (0.38–2.18) | N/A | |
| II-based regimen | 0.028 | ||||
| No | 819 | 39 (4.76) | Reference | ||
| Yes | 379 | 8 (2.11) | 0.66 (0.25–1.80) | 1.12 (0.15–8.06) | |
| nNRTI-based regimen | 0.095 | ||||
| No | 471 | 13 (2.76) | Reference | ||
| Yes | 727 | 34 (4.68) | 1.02 (0.45–2.30) | 1.25 (0.31–5.06) | |
| PI-based regimen | 0.437 | ||||
| No | 1106 | 42 (3.8) | Reference | ||
| Yes | 92 | 5 (5.43) | 1.87 (0.64–5.49) | N/A | |
cART combination antiretroviral therapy, CDC Centers for Disease Control and Prevention, CI confidence interval, CMV cytomegalovirus, HCV hepatitis C virus, HAV hepatitis A virus, HBsAg hepatitis B surface antigen, HIV human immunodeficiency virus, HR hazard ratio, IDU injecting drug use, II integrase inhibitors, IQR interquartile range, LILP(−) late initiators of cART without late presentation, LILP(+) late initiators of cART with late presentation, MSM, men who have sex with men, N/A not available, NLI nonlate initiators of cART, nNRTI nonnucleoside reverse transcriptase inhibitors, PI protease inhibitor, SD standard deviation, TAF tenofovir alafenamide, TDF tenofovir disoproxil fumarate, TMP/SMX trimethoprim/sulfamethoxazole, VDRL Venereal Disease Research Laboratory, VL viral load
*P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Our data revealed a shift toward a higher CD4+ count at initiation from 2009 to 2015, as demonstrated by a 228% increase in the median pre-cART CD4+ count from 99 cells/μL in 2009 to 325 cells/μL in 2015. Unexpectedly, this increase stabilized thereafter. Overall, 33.61% − 36.84% of the patients from 2015 to 2019 had a CD4+ count of ≤ 200 cells/μL at cART initiation. These findings indicated that patients in our cohorts continued receiving cART late, even after the implementation of the PrEP project in 2016 and the policies to promote treatment of PLWH with increased CD4+ count thresholds in 2016 [33] and rapid cART initiation in 2018 [34] in Taiwan. We also determined that LP was the consistent driver of LI throughout the study period (89%), with a higher percentage (70%) than that reported by a Canadian study conducted in 2003–2012 [25]. Accordingly, our findings indicate the necessity of intensifying early HIV diagnosis to further improve pre-cART CD4+ in the era of treat-all and rapid cART initiation.
Although the proportion of patients with a CD4+ count of ≤ 350 or ≤ 500 cells/μL at cART initiation decreased from 2009 to 2019, a rising trend was observed in the proportion of patients with a count of ≤ 200 cells/μL since 2016, reflecting a rising trend in the proportion of late initiators since 2015. Notably, a Brazilian study reported a similar trend: the proportion of patients with LI decreased from > 50% in 2004 to nearly 25% in 2014 and then increased until 2018 [22]. In the present study, although the reason for the increase in LI was unclear, it might be due to the nonsignificant increase in the proportion of late presenters from 33.03% in 2015 to 37.80% in 2019 (P for trend = 0.267; data not shown). Accordingly, additional clinical observations and further studies are required to characterize the recent changes in the characteristics of late initiators and inform the adoption of relevant interventions.
This study also identified various determinants of the dichotomized LI etiologies. These findings can provide information useful for tailoring interventions to specific etiologies to optimize cART initiation. Above all, exploring the factors associated with the increased risk of LP should be prioritized in Taiwan, where LP remains the main cause of LI, as shown in this study, and the proportion of late presenters among newly diagnosed PLWH has remained stable despite the nationwide expansion of HIV programs over the past decade [46, 47].
Three factors were found to be associated with LP; earlier year of cART initiation, an age of > 30 years, and VDRL titers of ≥ 1:8. In the years leading up to the intensified HIV screening of gay, bisexual, and homosexual (gbMSM) individuals [48], anonymous voluntary counseling and testing (aVCT) for HIV among gbMSM individuals, the establishment of gbMSM community health centers, and the advocacy of online opinion leaders have reduced LP over time, but these interventions have not consistently reached the nontraditional at-risk population. Therefore, implementing measures to further expand targeted HIV testing is critical not only for typical sexually at-risk populations but also for neglected at-risk populations, such as older individuals. Older populations have been associated with LP [47, 49, 50], probably due to changes in risk-related behaviors (i.e., decreased condom use), a lower perception of HIV risk [51, 52], the presence of multiple comorbidities [53], and an underestimation of the risk of HIV transmission behavior [53]. Additional strategies to mitigate the fear of the negative social consequences of HIV infection, such as discrimination and stigmatization, can also help increase people’s engagement in aVCT [43].
Indicator condition (IC)-guided HIV testing refers to HIV tests conducted under AIDS-defining conditions, as well as under conditions where the prevalence of undiagnosed HIV is > 0.1% or conditions where not identifying the presence of HIV infection may be detrimental for the individual’s clinical management [54]. Sexually transmitted infections (STIs) were listed as ICs associated with an HIV prevalence of > 0.1% [54]. Since 2008, Taiwan’s Ministry of Health has recommended HIV testing for patients with STIs. Our finding that VDRL titers of ≥ 1:8 constitute a protective factor for LILP(+) supports this recommendation [55]. Currently, IC-guided HIV testing is recommended for patients with a new diagnosis of STIs or patients aged 15–49 years with a diagnosis of Mycobacterium tuberculosis complex in Taiwan. Further expanding IC-guided HIV testing for other ICs, as recommended in European guidelines [54], may improve the rates of early HIV diagnosis in Taiwan [56].
Although the LILP(−) group constituted a relatively small proportion of late initiators during 2009–2019, it had a higher all-cause mortality risk, similar to the LILP(+) group. Therefore, identifying patients who start cART late because of delayed HIV care is also crucial. We identified acute HIV at HIV diagnosis as a risk factor for LILP(−), suggesting that PLWH with newly diagnosed acute HIV may not seek treatment in time because of reluctance and denial, perceived health status, fear of stigma and discrimination, or avoidance of linkage to care [26, 57]. This is a critical finding because acute HIV infection is characterized by an extremely high HIV viral load, which is associated with a high risk of onward transmission to uninfected partners in the absence of early cART initiation [58]. Therefore, interventions to improve access to cART should be implemented among PLWH with acute HIV diagnosis. The rapid cART initiation strategy may help overcome these barriers of delayed linkage [59]. This strategy was launched in 2018 in Taiwan [34]. However, the policy is not mandatory, and its influence on the adherence to HIV care continuum among PLWH with acute HIV diagnosis needs further follow-up.
LI of cART is a risk factor for all-cause mortality [9, 11, 13, 14]. In the present study, both etiologies of LI of cART were associated with a high all-cause mortality rate, and this effect was not related to the antiretroviral agent class at initiation. The association between LI of cART and all-cause mortality may be attributed to poor immune recovery after cART in patients with advanced HIV [1, 60, 61], resulting in a higher rate of AIDS-defining illnesses [1] or clinical progression (AIDS events and death) [62].
Other predictors for all-cause mortality after cART initiation were older age at baseline and HBsAg seropositivity. Older age at baseline was associated with less robust CD4+ response to cART [63, 64]. Compared with HBV monoinfection, HBS and HIV coinfection is associated with faster progression of HBV-related liver disease, including cirrhosis, end-stage liver disease, hepatocellular carcinoma, and fatal hepatic failure [65].
Our study has several strengths. We provide direct evidence that LP is the main driver of LI. Additionally, our analyses included data covering the lengthy period between 2009 and 2019—a period during which the eligibility criteria for cART initiation in Taiwan substantially changed and the era when treat-all and rapid cART initiation began—thus providing a clearer delineation of the LI trend and potentially guiding future treatment decisions regarding the prioritization of interventions.
Our study also has some limitations. First, the enrolled participants, accounting for 5.23% (1198/22,921) of newly diagnosed PLWH from 2009 to 2019 in Taiwan [39], were predominantly from southern Taiwan. To generalize our findings, we enrolled three HIV-designated hospitals from different medical care provision levels (one medical center and two regional hospitals) to represent the entire HIV-positive population in Taiwan. However, geographic differences in accessibility to medical resources, educational status, country income, and cultural grounds may prevent the direct generalization of the HIV care continuum in the present study to other countries [9, 22, 27, 66]. Second, we analyzed neither the effect of fear of negative consequences (discrimination or stigmatization) [43] from a positive HIV diagnosis nor the effect of a history of aVCT [43] on LI because of the unavailability of relevant data. This may have affected the analysis of LI determinants.
Conclusions
Although the median CD4+ count at cART initiation increased after the implementation of HIV prevention strategies in Taiwan, initiation at higher CD4+ cell counts remains a challenge. Considering the rising LI trend from 2015 in the era of treat-all and rapid cART initiation, strategic interventions for increasing earlier cART initiation must be intensified, particularly prioritizing strategies to expand HIV testing to target the population at risk of LP (age > 30 years) and other ICs, as recommended in European guidelines. Although the proportion of the LILP(−) group was relatively small, intensified interventions to improve access to cART should be implemented among PLWH with acute HIV diagnosis to prevent the onward HIV transmission to uninfected partners.
Acknowledgements
We thank the participants of the study.
Funding
The design of the study, editorial writing support, the collection, analysis, interpretation of data, and the Rapid Service Fee, were supported by the Ministry of Science and Technology, Taiwan, R.O.C. under grant no. MOST 108-2314-B-037-050, Kaohsiung Municipal Siaogang Hospital, Taiwan, R.O.C. under grant no. H-108-006 and H109-009, and Kaohsiung Medical University Hospital, Taiwan, R.O.C. under grant no. KMUH110-0M22.
Medical Writing, Editorial, and Other Assistance
The authors thank Wallace Academic Editing for editing the manuscript. This study is supported partially by Kaohsiung Medical University Research Center Grant (KMU-TC109B05).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Concept and study design: C-YL, Y-PL, S-FW, P-LL. Data acquisition: all authors. Analyses of the data: C-YL, Y-PL. Statistics: C-YL, Y-PL. Supervision: S-FW, P-LL. Manuscript preparation: C-YL, Y-PL. Review and approval: all authors.
Disclosures
Chun-Yuan Lee, Yi-Pei Lin, Sheng-Fan Wang, and Po-Liang Lu all have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-SV(I)-20210092). The requirement for informed consent was waived. The study was carried out according to the principles expressed in the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
All data containing relevant information to support the study findings are provided in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun-Yuan Lee, Email: leecy8801131@gmail.com.
Yi-Pei Lin, Email: scps950328@gmail.com.
Sheng-Fan Wang, Email: wasf1234@gmail.com.
Po-Liang Lu, Email: d830166@gmail.com.
References
- 1.Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥ 200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57:1038–1047. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- 2.Zhang F, Dou Z, Ma Y, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11:516–524. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 3.Cobucci RN, Lima PH, de Souza PC, et al. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health. 2015;8:1–10. doi: 10.1016/j.jiph.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Poorolajal J, Hooshmand E, Mahjub H, Esmailnasab N, Jenabi E. Survival rate of AIDS disease and mortality in HIV-infected patients: a meta-analysis. Public Health. 2016;139:3–12. doi: 10.1016/j.puhe.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 7.WHO: guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV; 2015. [PubMed]
- 8.2018 Global AIDS Update. https://www.unaids.org/en/resources/documents/2018/2018-global-aids-update-slides-part1. Accessed on February 25, 2022.
- 9.Kiertiburanakul S, Boettiger D, Lee MP, et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;17:18804. doi: 10.7448/IAS.17.1.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24:359–383. doi: 10.1353/hpu.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin KY, Cheng CY, Li CW, et al. Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up. PLos ONE. 2017;12:e0179870. doi: 10.1371/journal.pone.0179870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghislain M, Bastard JP, Meyer L, et al. Late antiretroviral therapy (ART) initiation is associated with long-term persistence of systemic inflammation and metabolic abnormalities. PLoS ONE. 2015;10:e0144317. doi: 10.1371/journal.pone.0144317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkhof MW, Boulle A, Weigel R, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siika A, McCabe L, Bwakura-Dangarembizi M, et al. Late presentation with HIV in Africa: phenotypes, risk, and risk stratification in the REALITY Trial. Clin Infect Dis. 2018;66:S140–s146. doi: 10.1093/cid/cix1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Molina JA, Díaz-Menéndez M, Plana MN, Zamora J, López-Vélez R, Moreno S. Very late initiation of HAART impairs treatment response at 48 and 96 weeks: results from a meta-analysis of randomized clinical trials. J Antimicrob Chemother. 2012;67:312–321. doi: 10.1093/jac/dkr478. [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Tang Z, Lan G, et al. Early antiretroviral therapy on reducing HIV transmission in China: strengths, weaknesses and next focus of the program. Sci Rep. 2018;8:3431. doi: 10.1038/s41598-018-21791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May M, Gompels M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ. 2011;343:d6016. doi: 10.1136/bmj.d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De La Mata NL, Ly PS, Ng OT, et al. Trends in CD4 cell count response to first-line antiretroviral treatment in HIV-positive patients from Asia, 2003–2013: TREAT Asia HIV observational database low intensity transfer. Int J STD AIDS. 2017;28:1282–1291. doi: 10.1177/0956462417699538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupasinghe D, Kiertiburanakul S, Kamarulzaman A, et al. Early mortality after late initiation of antiretroviral therapy in the TREAT Asia HIV Observational Database (TAHOD) of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) Asia-Pacific. HIV Med. 2020;21:397–402. doi: 10.1111/hiv.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cescon A, Patterson S, Davey C, et al. Late initiation of combination antiretroviral therapy in Canada: a call for a national public health strategy to improve engagement in HIV care. J Int AIDS Soc. 2015;18:20024. doi: 10.7448/IAS.18.1.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues A, Struchiner CJ, Coelho LE, Veloso VG, Grinsztejn B, Luz PM. Late initiation of antiretroviral therapy: inequalities by educational level despite universal access to care and treatment. BMC Public Health. 2021;21:389. doi: 10.1186/s12889-021-10421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacheco PRG, Zara A, Silva ESLC, Turchi MD. Late onset of antiretroviral therapy in adults living with HIV in an urban area in Brazil: prevalence and risk factors. J Trop Med. 2019:5165313. [DOI] [PMC free article] [PubMed]
- 24.Belay GM, Engeda EH, Ayele AD. Late antiretroviral therapy initiation and associated factors among children on antiretroviral therapy at University of Gondar Comprehensive Specialized Hospital, Gondar, Northwest Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12:255. doi: 10.1186/s13104-019-4279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lourenço L, Samji H, Nohpal A, et al. Declines in highly active antiretroviral therapy initiation at CD4 cell counts ≤ 200 cells/μL and the contribution of diagnosis of HIV at CD4 cell counts ≤ 200 cells/μL in British Columbia, Canada. HIV Med. 2015;16:337–345. doi: 10.1111/hiv.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali H, Zakar R, Junaid K, Khan A, Fischer F. Frequency and reasons for delayed treatment initiation after HIV diagnosis: cross-sectional study in Lahore, Pakistan. BMC Public Health. 2021;21:1000. doi: 10.1186/s12889-021-11031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Sönnerborg A, Gao L, Wang P, Bouey JZH, Cheng F. Delayed treatment for people living with HIV in China, 2004–2016: an analysis of an observational cohort. Int J Environ Res Public Health. 2020;17:1809. doi: 10.3390/ijerph17051809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutimura E, Addison D, Anastos K, et al. Trends in and correlates of CD4+ cell count at antiretroviral therapy initiation after changes in national ART guidelines in Rwanda. AIDS. 2015;29:67–76. doi: 10.1097/QAD.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Liu L, Shen J, Chen P, Lu H. Trends in baseline CD4 cell counts and risk factors for late antiretroviral therapy initiation among HIV-positive patients in Shanghai, a retrospective cross-sectional study. BMC Infect Dis. 2017;17:285. doi: 10.1186/s12879-017-2398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guidelines for Diagnosis and Treatment of HIV/AIDS (4E). https://www.cdc.gov.tw/En/InfectionReport/Info/9YUAXbFsmorP5T10V8qvMA?infoId=pCFKw_1zRgX2jry-S28vjg. Accessed on February 25, 2022.
- 31.Guidelines for diagnosis and treatment of HIV/AIDS (3E). https://www.cdc.gov.tw/En/InfectionReport/Info/9YUAXbFsmorP5T10V8qvMA?infoId=JL8NlHp_m2jX7HhOPXXE4Q. Accessed on February 25, 2022.
- 32.Guidelines for diagnosis and treatment of HIV/AIDS, 6th edn. http://www.aids-care.org.tw/journal/treatment.asp. Accessed on February 25, 2022.
- 33.Guidelines for diagnosis and treatment of HIV/AIDS. http://www.cdc.gov.tw/uploads/files/201310/1e64c227-32fc-49ab-8bfc-9484ec50c4f3.pdf. Accessed on February 25, 2022.
- 34.Beat AIDS and be Healthy Together—Taiwan CDC urges the public to create an open and friendly environment for HIV testing. https://www.cdc.gov.tw/En/Bulletin/Detail/0afA_nz1r1QpyJCDxNKxaw?typeid=158. Accessed on February 25, 2022.
- 35.Spinner CD, Boesecke C, Zink A, et al. HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection. 2016;44:151–158. doi: 10.1007/s15010-015-0850-2. [DOI] [PubMed] [Google Scholar]
- 36.Wu HJ, Wen-Wei KuS, Chang HH, Li CW, Ko NY, Strong C. Imperfect adherence in real life: a prevention-effective perspective on adherence to daily and event-driven HIV pre-exposure prophylaxis among men who have sex with men—a prospective cohort study in Taiwan. J Int AIDS Soc. 2021;24:e25733. doi: 10.1002/jia2.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lists of hospitals which provide the PrEP service. https://www.cdc.gov.tw/File/Get/0XuQWEpfD6zneUPjXBVWpA. Accessed on February 25, 2022.
- 38.Ku SW‐WLCW, Huang P, Strong C, Garner A, Howell S. Factors associated with uptake of event‐driven and daily regimen of pre‐exposure prophylaxis among gay, bisexual and other men who have sex with men (GBMSM) in Taiwan: 2019 Hornet PrEP survey. In: 23rd International AIDS Conference. San Francisco, USA.
- 39.Statistics of HIV/AIDS. https://www.cdc.gov.tw/En/Category/MPage/kt6yIoEGURtMQubQ3nQ7pA. Accessed on February 25, 2022.
- 40.The list of designated hospitals in Taiwan. https://www.cdc.gov.tw/File/Get/1r1ci3LMQA4X7h1-Ygmykw. Accessed on February 25, 2022.
- 41.Jeong SJ, Italiano C, Chaiwarith R, et al. Late presentation into care of HIV disease and its associated factors in Asia: results of TAHOD. AIDS Res Hum Retroviruses. 2016;32:255–261. doi: 10.1089/aid.2015.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revised Surveillance Case Definition for HIV Infection—United States, 2014. [PubMed]
- 43.Lee CY, Wu PH, Tsai JJ, Chen TC, Chang K, Lu PL. Cascade analysis of anonymous voluntary HIV counseling and testing among patients with HIV infection in Taiwan. AIDS Patient Care STDS. 2020;34:303–315. doi: 10.1089/apc.2020.0044. [DOI] [PubMed] [Google Scholar]
- 44.Wright ST, Law MG, Cooper DA, et al. Temporal trends of time to antiretroviral treatment initiation, interruption and modification: examination of patients diagnosed with advanced HIV in Australia. J Int AIDS Soc. 2015;18:19463. doi: 10.7448/IAS.18.1.19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillis D, Edwards BPM. The utility of joinpoint regression for estimating population parameters given changes in population structure. Heliyon. 2019;5:e02515. doi: 10.1016/j.heliyon.2019.e02515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CY, Wu PH, Lu PL, Tsai HC. Changing spectrum of opportunistic illnesses among HIV-infected Taiwanese patients in response to a 10-year national anti-TB programme. J Clin Med. 2019;8:163. doi: 10.3390/jcm8020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CY, Jen IA, Lan YC, et al. AIDS incidence trends at presentation and during follow-up among HIV-at-risk populations: a 15-year nationwide cohort study in Taiwan. BMC Public Health. 2018;18:589. doi: 10.1186/s12889-018-5500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control Annual Report. http://www.cdc.gov.tw/infectionreport.aspx?nowtreeid=61AB37D12031B95A&treeid=075874DC882A5BFD. Accessed on February 25, 2022.
- 49.Diaz A, del Romero J, Rodriguez C, et al. Effects of region of birth, educational level and age on late presentation among men who have sex with men newly diagnosed with HIV in a network of STI/HIV counselling and testing clinics in Spain. Euro Surveill. 2015;20:21088. doi: 10.2807/1560-7917.ES2015.20.14.21088. [DOI] [PubMed] [Google Scholar]
- 50.Yasin F, Rizk C, Taylor B, Barakat LA. Substantial gap in primary care: older adults with HIV presenting late to care. BMC Geriatr. 2020;20(1):438. doi: 10.1186/s12877-020-01842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward EG, Disch WB, Schensul JJ, Levy JA. Understanding low-income, minority older adult self-perceptions of HIV risk. J Assoc Nurses AIDS Care. 2011;22:26–37. doi: 10.1016/j.jana.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiao EY, Ries KM, Sande MA. AIDS and the elderly. Clin Infect Dis. 1999;28:740–745. doi: 10.1086/515219. [DOI] [PubMed] [Google Scholar]
- 53.Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav. 2008;12:935–942. doi: 10.1007/s10461-008-9370-8. [DOI] [PubMed] [Google Scholar]
- 54.HIV indicator conditions: guidance for implementing HIV testing in adults in health care settings. https://www.eurotest.org/Portals/0/Documents/Guidance.pdf.pdf?ver=2014-01-29-113626-000. Accessed on February 25, 2022.
- 55.Joore IK, Twisk DE, Vanrolleghem AM, et al. The need to scale up HIV indicator condition-guided testing for early case-finding: a case-control study in primary care. BMC Fam Pract. 2016;17:161–161. doi: 10.1186/s12875-016-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen YH, Fang CT, Shih MC, et al. Routine HIV testing and outcomes: a population-based cohort study in Taiwan. Am J Prev Med. 2022;62:234–242. doi: 10.1016/j.amepre.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Stroumpouki T, Perrett S, Kasdovasilis P, Papatheodorou P, Paparizos V, Stavropoulou A. A journey towards acceptance: the process of adapting to life with HIV in Greece. A qualitative study. Appl Nurs Res. 2020;53:151249. doi: 10.1016/j.apnr.2020.151249. [DOI] [PubMed] [Google Scholar]
- 58.García-Deltoro M. Rapid Initiation of antiretroviral therapy after HIV diagnosis. AIDS Rev. 2019;21:55–64. doi: 10.24875/AIDSRev.M19000027. [DOI] [PubMed] [Google Scholar]
- 59.Perez-Patrigeon S, Camiro-Zúñiga A, Jaramillo-Jante MR, et al. Immediate treatment of acute HIV in a tertiary healthcare center: bridging gaps in communication using smartphones. HIV Med. 2019;20:308–316. doi: 10.1111/hiv.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 61.Rava M, Bisbal O, Domínguez-Domínguez L, et al. Late presentation for HIV impairs immunological but not virological response to antiretroviral treatment. AIDS. 2021;35:1283–1293. doi: 10.1097/QAD.0000000000002891. [DOI] [PubMed] [Google Scholar]
- 62.Bonnet F, Thiébaut R, Chêne G, et al. Determinants of clinical progression in antiretroviral-naive HIV-infected patients starting highly active antiretroviral therapy. Aquitaine Cohort, France 1996-2002. HIV Med. 2005;6:198–205. doi: 10.1111/j.1468-1293.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 63.Althoff KN, Justice AC, Gange SJ, et al. Virologic and immunologic response to HAART, by age and regimen class. AIDS. 2010;24:2469–2479. doi: 10.1097/QAD.0b013e32833e6d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Q, Yu X, Wu T, Shang H, Jiang Y. Immunological and virological responses in older HIV-infected adults receiving antiretroviral therapy: an evidence-based meta-analysis. J Acquir Immune Defic Syndr. 2020;83:323–333. doi: 10.1097/QAI.0000000000002266. [DOI] [PubMed] [Google Scholar]
- 65.Parvez MK. HBV and HIV co-infection: impact on liver pathobiology and therapeutic approaches. World J Hepatol. 2015;7:121–126. doi: 10.4254/wjh.v7.i1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Health inequalities in Taiwan. file:///D:/download/%E8%87%BA%E7%81%A3%E5%81%A5%E5%BA%B7%E4%B8%8D%E5%B9%B3%E7%AD%89%E5%A0%B1%E5%91%8A-%E5%85%A8%E6%96%87%E9%9B%BB%E5%AD%90%E6%AA%94.pdf. Accessed on February 25, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data containing relevant information to support the study findings are provided in the manuscript.