Abstract
Introduction
Periodontitis is characterized by the destruction of tooth-supporting tissues including the alveolar bone. Barrier membranes are used in dentistry for tissue regenerative therapy. Nevertheless, conventional membranes have issues related to membrane stability and direct induction of bone mineralization. Amelotin (AMTN), an enamel matrix protein, regulates hydroxyapatite crystal nucleation and growth. To apply an AMTN membrane in clinical practice, we investigated the mineralizing and adhesive effects of recombinant human (rh) AMTN in vitro using a collagen-based system.
Methods
Collagen hydrogel incorporated with rhAMTN (AMTN gel) and rhAMTN-coated dentin slices were prepared. AMTN gel was then applied on a commercial membrane (AMTN membrane). Samples were incubated for up to 24 h in mineralization buffer, and the structures were observed. The peak adhesive tensile strength between the dentin and AMTN membrane was measured. Using an enzyme-linked immunosorbent assay, the release kinetics of rhAMTN from the membrane were investigated.
Results
The AMTN gel resulted in the formation of hydroxyapatite deposits both onto and within the collagen matrix. Furthermore, coating the dentin surface with rhAMTN promoted the precipitation of mineral deposits on the surface. Interestingly, site-specific mineralization was observed in the AMTN membrane. Only 1% of rhAMTN was released from the membrane. Hence, the AMTN membrane adhered to the dentin surface with more than twofold greater tensile strength than that detected for a rhAMTN-free barrier membrane.
Conclusions
RhAMTN can accelerate mineralization and adhesion in collagen-based systems. Furthermore, the AMTN membrane could inform the optimal design of calcified tissue regenerative materials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-022-00722-2.
Keywords: Bioengineering, Dentin, Enamel biomineralization/formation, Guided tissue regeneration, Hydroxyapatite
Introduction
Amelotin (AMTN) is an enamel protein that is specifically expressed by enamel-forming cells, known as ameloblasts, during the maturation stage of amelogenesis.18 AMTN is expressed during the late stage of enamel formation and localized on the enamel surface at the cell–mineral interface during the maturation stage.30 In mice overexpressing AMTN, the enamel microstructure is disrupted because of the induction of premature mineralization during enamel formation.19 By contrast, AMTN deficiency in mice leads to delayed enamel mineralization, which results in hypomineralization/maturation.23 Whole exome sequencing revealed mutations to human AMTN at exons 3–6 in nonsyndromic amelogenesis imperfecta.29 Hence, AMTN plays an important role in enamel mineralization.
Besides dental enamel, AMTN is expressed in the gingival junctional epithelium (JE) at relatively low levels, and it is located de novo at the JE–tooth interface during the late stage of wound healing following gingivectomy.25 Specifically, enamel proteins (including AMTN) are secreted into the dental cuticle, i.e., the interposed matrix layer between the tooth and JE.3 AMTN forms aggregates with itself and other proteins, including odontogenic ameloblast-associated protein and secretory calcium-binding phosphoprotein proline-glutamine rich 1.8,14,17 In a previous study, we found that AMTN binds to hydroxyapatite (HA), a calcium phosphate mineral, and promotes HA mineralization in vitro.1 HA is the main component of hard tissues such as bone, dental enamel, and dentin. Thus, the mineral-promoting properties of AMTN make it an attractive molecule for use in strategies associated with attaching biomaterials to mineralized tissues. However, the adhesive capacity of AMTN has yet to be thoroughly investigated.
Periodontitis is the result of bacteria-induced degradation of the dentogingival junction and connective tissue as well as the resorption of alveolar bone and cementum, which eventually leads to tooth loss.16,35 Surgical flap debridement of the periodontal pocket is often conducted during the clinical treatment of moderate to severe periodontitis to restore the attachment of the gingiva connective tissue. Nevertheless, detailed wound healing studies have found that extension of the gingival epithelium along the tooth root and the internal surface of the gingival flap can inhibit the attachment of connective tissue onto the tooth surface2,24; and new attachment is the result of repair followed by long stretches of JE with limited connective tissue attachment and cementum formation.4 To overcome this issue, guided tissue regeneration (GTR) was introduced in the 1980s11,24; although the clinical procedure has remained largely unchanged since then, the materials have been refined over the subsequent decades.28 Instead of directly promoting the sequence of biological processes that leads to new bone formation, a barrier membrane is used in the GTR procedure to prevent downgrowth of epithelial cells and gingival tissue before new bone is formed.7 Several materials have been proposed in which the membrane is impregnated with drugs or growth factors to play an additional role as a drug delivery system.15 Membrane stability is vital to the success of GTR because it helps create and maintain space.36 The GTR membrane is usually fixed around the tooth surface using sling sutures. This method has the advantage of simplicity and accurate membrane placement; however, it has the disadvantage of low stability because only one anchorage method is used, and the presence of the suture around the tooth cervical area causes plaque accumulation and gingival inflammation. Previous studies found that epithelial cells can penetrate between the membrane and the regeneration site and interfere with periodontal tissue regeneration when a GTR membrane is applied,27,33 suggesting that sufficient closure is not achieved by a GTR membrane and suture. To overcome this problem, methods for fixing membranes with adhesives have been proposed.6,34 For example, in the dog model, the formation of new bone at the experimental site where the membrane was fixed with 4-acryloyloxyethyl trimellitate anhydride/methyl methacrylate-tri-n-butylborane resin was found to be significantly greater than that at the control sites where the membrane was fixed with sutures.34 However, concerns regarding plaque accumulation and inflammation due to residual adhesive remain. To address these issues simultaneously, we applied recombinant human (rh) AMTN to commercial GTR membranes, and tested whether its presence promoted mineralization and thereby strengthened the adhesive force between the membrane and mineralized tissue.
Thus, the present study aimed to the mineralizing and adhesion-promoting effects of rhAMTN in vitro as a first step toward new strategies that can combat gingival attachment loss and promote tissue regeneration.
Materials and Methods
Production of rhAMTN
RhAMTN was prepared as previously described.1 Briefly, N-terminal 6 × His-tagged rhAMTN was expressed in Escherichia coli cells using Overnight Express™ Instant LB Medium for high-level protein production (Merck KGaA, Darmstadt, Germany) and affinity-purified on a Ni-nitrilotriacetic acid agarose column (Qiagen, Inc., Valencia, CA, USA). The N-terminal 6 × His tag was removed using a Thrombin CleanCleave Kit (Sigma-Aldrich, St. Louis, MO, USA). The protein was then dialyzed against water to remove calcium ions, after which it was freeze-dried, and stored at – 80 °C until its use in the following experiments.
Preparation of Collagen Hydrogels Impregnated with rhAMTN
Polymerized collagen hydrogel was produced by mixing 6 mg mL−1 of bovine collagen solution type I (Nutragen; #5010-D; Advanced BioMatrix, Carlsbad, CA, USA), 0.1 M sodium hydroxide, 20 mM genipin, a cross-linking agent (G4796; Sigma-Aldrich), and phosphate-buffered saline (PBS; #311-425-CL; Wisent, Inc., St-Bruno, QC, Canada) on ice to a final collagen hydrogel concentration of 3.4 mg mL−1; this was incubated at 37 °C overnight. To produce rhAMTN-impregnated collagen hydrogel, rhAMTN was dissolved in PBS to a final concentration of 530 µg mL−1 in the gel product (Fig. 1a).
Figure 1.
Mineralization of rhAMTN-impregnated collagen hydrogel incubated in SBF buffer. (a) Schematic diagram illustrating the production of rhAMTN-impregnated collagen hydrogel. Collagen solution with rhAMTN was plated on a glass slide and incubated overnight in a humidified incubator. (b)–(d) SEM images. No precipitation was observed in the rhAMTN-impregnated collagen hydrogel before incubation (b). Calcium phosphate precipitates were observed on the surface and deeper into and embedded within the rhAMTN-impregnated collagen hydrogel incubated in SBF buffer for 5 h (c). Control gels without any rhAMTN incorporation contained no minerals (d). (e) and (f) TEM images of collagen samples incubated in SBF buffer for 5 h. TEM and SAED analyses revealed that these precipitates had a HA crystalline structure.
Membranes
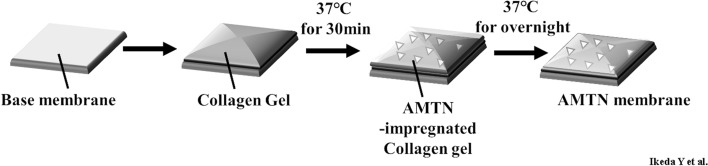
A Cytoplast™ RTM Collagen membrane (Collagen Matrix Inc., Oakland, NJ, USA) and a Vicryl (polyglactin 910) woven mesh membrane (Ethicon, Inc., Somerville, NJ, USA) served as base membranes. AMTN membranes (comprising a base membrane with rhAMTN-impregnated collagen hydrogel) and base membranes with or without collagen hydrogel were used in this study. Figure 2 shows a schematic diagram detailing the production of the AMTN membranes. The collagen hydrogel described above was applied to a 5 × 4 mm base membrane and incubated at 37 °C for 30 min in a humidified incubator. Additionally, the collagen hydrogel solution with/without rhAMTN (8.0 µg; final concentration of 530 µg mL−1) was applied to the top of the hydrogel/membrane and dried overnight at 37 °C in a humidified incubator.
Figure 2.
Schematic diagram illustrating AMTN membrane production. Collagen hydrogel was applied to the base membrane and incubated at 37 °C for 30 min in a humidified incubator. The collagen hydrogel solution with rhAMTN was overlapped on the top and allowed to dry at 37 °C overnight in an incubator. A Cytoplast and Vicryl mesh membranes were used as the base membranes, respectively.
Preparation of Dentin Slices
A warthog tusk (Chichester Canada Inc., Toronto, ON, Canada) was cut with a slow-speed diamond saw into 7 × 7 × 1 mm dentin slices, which were sterilized using gamma irradiation, etched with 30% phosphoric acid for 5 s, rinsed with water, and air-dried. Subsequently, the dentin surface was incubated with 4.84 µL of PBS with or without 9.7 µg of rhAMTN at 37 °C for 2 h in a humidified incubator. After incubation, the solution was aspirated.
In Vitro Mineralization Assay
A previously described modified simulated body fluid (SBF) buffer,1,26 was used in this assay. Samples were placed into the wells of a sterile nontissue culture plate containing SBF and incubated at 37 °C in a humidified incubator for 5 or 24 h. Subsequently, the samples were fixed in 4% paraformaldehyde in PBS (#P6148; Sigma-Aldrich), spray-coated with gold, and assessed using scanning electron microscopy (SEM; FEI XL30 Environmental Scanning Microscope; FEI Company, Hillsboro, OR, USA) and transmission electron microscopy (TEM; FEI Company). TEM images were obtained at 200 kV using the bright field and selected area electron diffraction (SAED) modes.
Enzyme-Linked Immunosorbent Assay (ELISA)
The release kinetics of rhAMTN from the membrane were investigated using an indirect enzyme-linked immunosorbent assay (ELISA). AMTN membranes with Cytoplast were incubated in 1 mL of PBS at 37 °C for 0 and 5 s, 1, 5, 10, and 30 min, and 1, 3, 6, 12, 24, and 48 h. Subsequently, the supernatant was collected and stored at 4 °C until use (test sample). The test samples and 100 mL of rhAMTN solution in PBS (350 ng to 5.34 pg; standard solution) were added to a 96 well plate (96 well EIA/RIA plate; Corning, Corning, NY, USA) and the plate was kept overnight at 4 °C. The following day, the solution was removed, and the wells were washed once with PBS containing 0.1% Tween® 20 (PBST; Sigma-Aldrich Corporation). After washing, 100 μL of a fourfold dilution of Block Ace (KAC, Hyogo, Japan) was added, and the plate was incubated for 2 h at 25 °C. After incubation, the wells were washed three times with PBST. After washing, 100 μL of human AMTN polyclonal antibody (A62078; EpiGentek, Farmingdale, NY, USA; 1:4000 concentration using a tenfold dilution of Block Ace) was added to each well and incubated for 1 h at 37 °C. The wells were washed with PBST thrice and 100 μL of anti-rabbit IgG antibody (Goat Anti-rabbit IgG HRP, HAF008, Bio-Techne, Minneapolis, MN) (1:10,000 concentration using a tenfold dilution of Block Ace) was added to each well and incubated for 1 h at 37 °C. The wells were washed again with PBST, and 100 μL of 3,3′,5,5′-tetramethylbenzidine solution (Thermo Fisher Scientific) was added to each well and incubated for 15 min 37 °C. Finally, 100 μL of 1 M sulfuric acid (Wako, Osaka, Japan) was added to stop the reaction, and the absorbance was taken at 450 and 650 nm using a 96-well plate reader (VMax® Microplate Reader, Molecular Devices, San Jose, CA, USA). The value at 450–650 nm was used for analysis. A standard curve (R2 = 0.99) was generated using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Pull-Off Test
A membrane was set on a dentin slice and incubated in SBF buffer for 5 or 24 h. Peak adhesive tensile strength between the dentin and membrane was measured using a digital force gauge (series DFG55; Omega Engineering, Inc., Stamford, CT, USA). Pull-off tests were performed in triplicate.
Statistical Analysis
All statistical analyses were performed using EZR statistical software (Saitama Medical Center, Jichi Medical University, Saitama, Japan). The results of the pull-off tests were assessed using a one-way analysis of variance with a Bonferroni multiple comparison test. In the figures, error bars represent standard deviations; p values of < 0.05 were considered statistically significant.
Results
RhAMTN Induces Mineralization of Collagen Hydrogel
No precipitation was observed on the collagen hydrogel before the start of the incubation period (Fig. 1b). After 5 h of incubation in SBF buffer, calcium phosphate precipitates were observed on the surface of the rhAMTN-impregnated collagen hydrogel (Fig. 1c). The minerals were also observed within a depth of several micrometers in the gel and were embedded within the collagen fibers. TEM and SAED analyses revealed that these minerals were needle-like structures (Figs. 1e and 1f). No mineralization was observed on the control collagen samples at 5 h (Fig. 1d) or even at 24 h (data not shown) of incubation. The buffers containing the collagen samples contained no mineral deposits, indicating that the observed minerals on the surfaces of the rhAMTN-impregnated collagen hydrogel did not result from precipitation from the buffer.
RhAMTN Precipitates Calcium Phosphate Particles on the Surface of Dentin
After 5 h of incubation in SBF buffer, calcium phosphate precipitates were selectively observed on the rhAMTN-treated surface of the dentin (Figs. 3a–3c and Supplementary Figs. 1A–C). By contrast, no precipitate was observed on the PBS-treated dentin (Figs. 3d–3f and Supplementary Figs. 1D–F) or the untreated dentin (Figs. 3g–3i and Supplementary Figs. 1G–I) even after 24 h of incubation.
Figure 3.
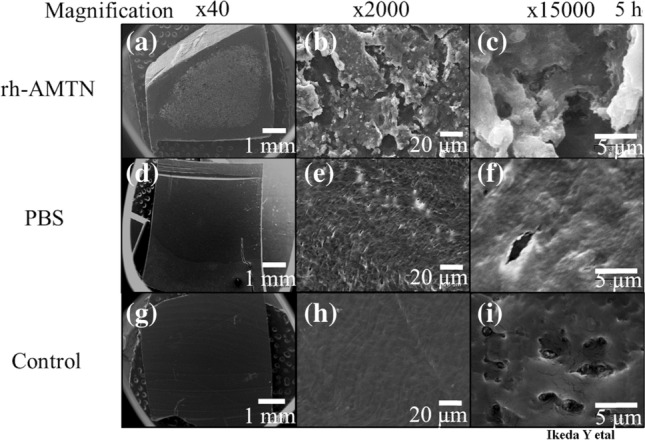
SEM images of rhAMTN-treated dentin incubated in SBF buffer at 37 °C for 5 h. Calcium phosphate precipitates were observed on the surface of rhAMTN-treated dentin (a)–(c). No precipitate was observed in PBS-treated slices (d)–(f) or control slices (g)–(i). Scale bars = 1000, 20, or 5 µm.
Release Kinetics of rhAMTN on the AMTN Membrane
Figure 4 shows the in vitro release profiles of rhAMTN from an AMTN membrane with a Cytoplast membrane. A rapid release of rhAMTN was observed within 5 min, but the release had almost stopped after 30 min. The amount was equivalent to approximately 1% of the total rhAMTN mounted on the AMTN membrane.
Figure 4.
Release kinetics of rhAMTN from the AMTN membrane. AMTN membrane with a Cytoplast RTM membrane was incubated in PBS at 37 °C for 0 s, 5 s, 1 min, 5 min, 10 min, 30 min, 1, 3, 6, 12, 24, and 48 h. The amount of rhAMTN released from the AMTN membrane was measured using an indirect ELISA. Values for each sample are represented by black dots, and the average value is represented by a gray line (n = 3 experiments).
Site-Specific Mineralization of the AMTN Membranes
First, a Cytoplast collagen membrane was used as the base membrane for the AMTN membrane (Figs. 2 and 5a). Calcium phosphate precipitates were observed only on the rhAMTN-containing surface of the membrane after 5 h of incubation (Figs. 5b and 5c). By contrast, no precipitate was observed on the AMTN-free surface of the membrane (Figs. 5d and 5e). After 24 h of incubation, precipitates were also observed only on one side (Supplementary Fig. 2A–D). The Cytoplast collagen membranes with and without the collagen hydrogel were not mineralized even after 24 h of incubation (Figs. 5f–5m and Supplementary Fig. 2E–L). Similar results were obtained using an the AMTN membrane using Vicryl as the base membrane (Supplementary Fig. 3). TEM analysis revealed that these minerals were located only on the rhAMTN-containing surface (Figs. 5n–5p) and that the minerals formed needle-like structures (Fig. 5q). Nevertheless, no clear diffraction pattern was observed using SAED analysis (Fig. 5r).
Figure 5.
Mineralization of the AMTN membrane incubated in SBF buffer for 5 h. (a) AMTN membrane. (b)–(m) SEM images of an AMTN membrane incubated in SBF buffer for 5 h. Calcium phosphate precipitates were observed on the top surface of the AMTN membrane (b) and (c). No precipitate was observed on the bottom surface of the AMTN membrane (d) and (e), on both surfaces of the base membrane with collagen hydrogel (f)–(i), and on both surfaces of the base membrane (j)–(m). Scale bars = 10 and 2 µm. (n)–(r) TEM images of an AMTN membrane incubated in SBF buffer for 5 h. Calcium phosphate precipitated only on the top surface (n). Black arrows indicate calcium phosphate precipitates, which had needle-like structures (q) and (r). No precipitate was observed in the middle part or bottom side of the membrane (o) and (p). Scale bars = 1 µm and 200 nm. A Cytoplast RTM membrane was used as the base membrane.
Improved Adhesion Strength of the AMTN Membranes to Dentin
The AMTN membrane with Cytoplast had significantly stronger adherence to the dentin than that of the Cytoplast membrane alone at 5 h (26.00 ± 3.75 vs. 13.43 ± 5.10 kPa, respectively; p = 0.045) and 24 h (31.17 ± 7.93 vs. 10.67 ± 2.04 kPa, respectively; p = 0.014) (Figs. 6a and 6b). Interestingly, pretreatment of dentin with the rhAMTN solution did not improve the adhesive strength of the Cytoplast membrane to the dentin surface at 5 h (12.33 ± 2.76 kPa) or 24 h (10.5 ± 2.40 kPa). The same experiment was conducted using Vicryl with collagen hydrogel as a control because Vicryl completely failed to adhere to the dentin. The AMTN membrane with Vicryl had significantly stronger adherence to the dentin surface compared with that of the Vicryl with the collagen hydrogel at 5 h (20.25 ± 2.65 vs. 13.10 ± 3.23 kPa; respectively, p = 0.032) and 24 h (27.38 ± 3.83 vs. 14.7 ± 3.97 kPa, respectively; p = 0.044) (Supplementary Figs. 4A and B). The rhAMTN pretreatment of dentin also failed to improve the adhesive strength of the Vicryl membrane (9.9 ± 2.57 and 11.93 ± 3.39 kPa at 5 and 24 h, respectively).
Figure 6.
Peak adhesive tensile strength and histological and immunohistochemical analyses of an AMTN membrane with dentin. (a) and (b) Membranes were incubated in SBF buffer at 37 °C for 5 h (a) or 24 h (b) and the peak adhesive tensile strength between the dentin and membrane was measured using a digital force gauge (white, base membrane; black, AMTN membrane; gray, base membrane with rhAMTN-treated dentin). Data are presented as the means ± standard deviation. * indicates a significant difference (p < 0.05) between groups according to one-way ANOVA with the Bonferroni multiple comparison test (n = 3 experiments).
Discussion
Collagen-based materials are increasingly being used for hard-to-soft tissue attachment applications because of their biocompatibility, ease of use, and biodegradability. These materials have been widely tested alone and in combination with osteoinductive biologics for use in tissue engineering of bone and cartilage, as well as for periodontal regeneration.9,15,31
AMTN is a unique enamel protein that can mineralize and adhere to in vitro experimental models; hence, it presents a promising material for use in tissue regeneration therapy. To the best of our knowledge, this study is the first to demonstrate the ability of rhAMTN to adhere to a membrane, regardless of the membrane components. Additionally, the AMTN membrane developed in this study could potentially be applied in the regeneration of hard tissues.
Intriguingly, collagen mineralization was observed within only a few hours of incubation in SBF buffer when rhAMTN was embedded within the collagen gel matrix (Fig. 1c). Interestingly, the minerals were localized only in the surface layer and not inside the gel. In the natural environment of bone, dentin, and cementum, collagen mineralization is regulated by various noncollagenous proteins that are members of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family. These proteins interact with collagen and cells as well as with calcium and phosphate ions in the extracellular matrix, to mediate mineralization.10 Some SIBLING proteins, such as dentin phosphophoryn and dentin matrix protein 1 (DMP1), promote collagen mineralization when immobilized on the collagen surface in vitro.12,21 Similarly, as a mineral-promoting site, rhAMTN has a conserved SSEEL motif that mediates much of the mineral-promoting activity in vitro.1 Phosphorylation of the serine residues in the SSEEL motif promotes the HA precipitation significantly. Notably, the HA-mineralizing ability of rhAMTN is significantly reduced when it lacks the SSEEL motif. Thus, rhAMTN trapped in the collagen matrix may locally concentrate and precipitate calcium and phosphate ions onto and within the collagen meshwork.
Calcium and phosphate ions were precipitated in the form of droplets on the surface of rhAMTN-treated dentin (Figs. 3a–3c), which indicates that nucleation occurred on the dentin surface, but not in the buffer and that rhAMTN was immobilized on the dentin surface. Although the substance in dentin that binds to rhAMTN remains unknown, the key components could include HA and type I collagen, which are the principal elements in dentin. Six to eight consecutive negatively charged amino acid residues reportedly exhibit remarkable affinity to HA.22 Ten of 12 negatively charged amino acids were identified in the second half of the rhAMTN sequence, although there was no consecutive aspartic acid or glutamic acid residues. The sequences DSESSEEDR and SEENRDSDSQDSSR of DMP1 have a strong affinity to the N-telopeptide located in the gap region of type I collagen.13 DMP1-derived peptide, which contains the DSESSEEDR domain, binds to type I collagen fibrils and promotes HA precipitation on demineralized human dentin.26 The sequence around the SSEEL domain of rhAMTN may also play a role in adhesion to collagen.
In the present study, the characteristics of the AMTN membrane were evaluated in vitro. According to our ELISA results, only approximately 1% of rhAMTN is released from the AMTN membrane; thus, the membrane retains most of the rhAMTN (Fig. 4). This may be because rhAMTN and collagen are cross-linked by amino groups via genipin. The membrane retention of rhAMTN allowed us to develop a membrane with two important features. First, the membrane was mineralized only on one side within a few hours of incubation in SBF buffer (Fig. 5). In AMTN membranes, mineralization was observed only in the surface layer, as in gel experiments (Figs. 5b and 5c), and needle-like structures were also observed in the surface layer using TEM (Fig. 5q). However, a clear diffraction pattern could not be obtained using SAED analysis (Fig. 5r). One reason for this was that the needle-like structures precipitated on the membrane were smaller than the structures observed in the gel experiments; therefore, the beam diameter of the SAED may not have been suitable for analysis of the structures on the membrane. Second, the AMTN membrane adhered to the dentin surface with a tensile strength that was more than twofold greater than that of the conventional membrane (Fig. 6). RhAMTN was immobilized on the dentin surface (as suggested in Fig. 3), and the collagen fibers connecting the membrane to the dentin were mineralized (data not shown), which may have increased the tensile strength. In previous studies, collagen gels and membranes impregnated with drugs, e.g., transforming growth factor β, bone morphogenetic protein (BMP) -2 and protein-9, platelet-derived growth factor, fibroblast growth factor-2 and factor-18 and vascular endothelial growth factor, have been tested to construct drug delivery systems15; however, more than half of such studies have focused on BMP-2.15 In BMP-2-impregnated membranes, a burst release of 5–20% BMP-2 has been observed in the first few hours; this is typically followed by a slow-release until a plateau is reached.5,20 These membranes were designed to deliver drugs to bone defects by releasing them from the membrane; the AMTN membrane differs from these membranes as it retains (and functions with) almost all rhAMTN on the membrane.
AMTN overexpression has been reported to promote the formation of mineralized bone nodules in MC3T3-E1 osteoblast cells.1 Using our proposed AMTN membrane, the healing of calvarial defects was significantly accelerated in a mouse model.32 The data indicated that rhAMTN cross-linked on the membrane had stimulated new mineral formation. Thus, rhAMTN may function on or inside the membrane, promoting membrane stability and mineralization, and inducing bone matrix protein synthesis and mineralization in osteoblasts.
In conclusion, this study showed that rhAMTN can accelerate mineralization and adhesion in collagen-based systems in vitro. The promoting effect of rhAMTN on collagen mineralization opens up new possibilities for the optimal design of calcified tissue regeneration techniques. Moreover, current findings could be applicable to other medical fields such as orthopedics. RhAMTN was adsorbed to the collagen hydrogel and dentin, whereas HA was deposited on the surface; the use of the novel layered AMTN membrane led to the formation of HA deposits on only one side of the membrane, i.e., the one which had adhered well to the dentin surface. The proposed AMTN membrane could be used for regeneration therapy, because rhAMTN would retain its characteristics not only in a membrane but also in a gel or solution. We plan to continue this line of research to develop clinical applications for the AMTN membrane.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC, Operating grant #490975) to BG, the Canadian Institutes of Health Research (CIHR, Operating Grant MOP-492418) to BG and JSPS KAKENHI Grant (Grant-in-Aid for Early-Career Scientists 18K17040) to YI. We would like to thank Douglas Holmyard (Advanced Bioimaging Centre, Mount Sinai Hospital, Toronto, Ontario, Canada) for his expert assistance with the TEM imaging. The authors also would like to thank Enago (www.enago.jp) for the English language review.
Author Contributions
YI, JH and BG designed the experiments. YI conducted the experiments. YI analyzed the data. YI, JH, EI and BG interpreted the data. YI, JH, EI and BG drafted the final version of the manuscript. YI takes responsibility for the integrity of the data analysis.
Conflict of interest
YI and BG declare a Canadian Patent (#CA2,968,134 C) and a US Patent (#10,596,301), which are relevant to this study. JH and EI state that they have no conflicts of interest.
Ethical Approval
No human studies nor animal studies were carried out by the authors for this article.
Citation Diversity Statement
Recent work in several fields of science has identified a bias in citation practices such that papers from women and other minority scholars are undercited relative to the number of papers in the field. We recognize this bias and have worked diligently to ensure that we are referencing appropriate papers with fair gender and racial author inclusion.
Abbreviations
- AMTN
Amelotin
- BMP
Bone morphogenetic protein
- DMP1
Dentin matrix protein 1
- ELISA
Enzyme-linked immunosorbent assay
- GTR
Guided tissue regeneration
- HA
Hydroxyapatite
- JE
Junctional epithelium
- PBS
Phosphate-buffered saline
- PBST
Phosphate-buffered saline containing 0.1% Tween® 20
- Rh
Recombinant human
- SAED
Selected area electron diffraction
- SBF
Modified simulated body fluid
- SEM
Scanning electron microscopy
- SIBLING
Small integrin-binding ligand N-linked glycoprotein
- TEM
Transmission electron microscopy
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbarin N, San Miguel S, Holcroft J, Iwasaki K, Ganss B. The enamel protein amelotin is a promoter of hydroxyapatite mineralization. J. Bone Miner. Res. 2015;30:775–785. doi: 10.1002/jbmr.2411. [DOI] [PubMed] [Google Scholar]
- 2.Bogle G, Adams D, Crigger M, Klinge B, Egelberg J. New attachment after surgical treatment and acid conditioning of roots in naturally occurring periodontal disease in dogs. J. Periodontal Res. 1981;16:130–133. doi: 10.1111/j.1600-0765.1981.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 3.Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J. Dent. Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 4.Bowers GM, et al. Histologic evaluation of new attachment apparatus formation in humans. J. Periodontol. 1989;60:664–674. doi: 10.1902/jop.1989.60.12.664. [DOI] [PubMed] [Google Scholar]
- 5.Caridade SG, et al. Myoconductive and osteoinductive free-standing polysaccharide membranes. Acta Biomater. 2015;15:139–149. doi: 10.1016/j.actbio.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rezende MLR, Cunha PO, Damante CA, Santana ACP, Greghi SLA, Zangrando MSR. Cyanoacrylate adhesive as an alternative tool for membrane fixation in guided tissue regeneration. J. Contemp. Dent. Pract. 2015;16:512–518. doi: 10.5005/jp-journals-10024-1714. [DOI] [PubMed] [Google Scholar]
- 7.Elgali I, Omar O, Dahlin C, Thomsen P. Guided bone regeneration: materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017;125:315–337. doi: 10.1111/eos.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouillen A, et al. Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral. Sci. Rep. 2017;7:46683. doi: 10.1038/srep46683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 10.George A, Veis A. Phosphorylated proteins and control over apatite nulcleation, crystal growth and inhibition. Chem. Rev. 2008;108:4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in the human periodontium by guided tissue regeneration case reports. J. Clin. Periodontol. 1986;13:604–616. doi: 10.1111/j.1600-051X.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 12.He G, et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003;2:552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 14.Holcroft J, Ganss B. Identification of Amelotin- and ODAM-interacting enamel matrix proteins using the yeast two-hybrid system. Eur. J. Oral Sci. 2011;119:301–306. doi: 10.1111/j.1600-0722.2011.00870.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, Huang X, Gu L. Periodontal bifunctional biomaterials: progress and perspectives. Materials. 2021;14:7588. doi: 10.3390/ma14247588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda E, et al. Japanese subgingival microbiota in health vs disease and their roles in predicted functions associated with periodontitis. Odontology. 2020;108:280–291. doi: 10.1007/s10266-019-00452-4. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Neshatian M, Holcroft J, Ganss B. The enamel protein ODAM promotes mineralization in a collagen matrix. Connect. Tissue Res. 2018;59:62–66. doi: 10.1080/03008207.2017.1408603. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki K, et al. Amelotin—a novel secreted, ameloblast-specific protein. J. Dent. Res. 2005;84:1127–1132. doi: 10.1177/154405910508401207. [DOI] [PubMed] [Google Scholar]
- 19.Lacruz RS, et al. Targeted overexpression of amelotin disrupts the microstructure of dental enamel. PLoS ONE. 2012;7:e35200. doi: 10.1371/journal.pone.0035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E-J, Kim H-E. Accelerated bony defect healing by chitosan/silica hybrid membrane with localized bone morphogenetic protein-2 delivery. Mater. Sci. Eng. C. 2016;59:339–345. doi: 10.1016/j.msec.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Milan AM, Sugars RV, Embery G, Waddington RJ. Adsorption and interactions of dentine phosphoprotein with hydroxyapatite and collagen. Eur. J. Oral Sci. 2006;114:223–231. doi: 10.1111/j.1600-0722.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MB, Hartgerink JD, Goepferich A, Mikos AG. Synthesis and in vitro hydroxyapatite binding of peptides conjugated to calcium-binding moieties. Biomacromolecules. 2007;8:2237–2243. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama Y, Holcroft J, Ganss B. Enamel hypomineralization and structural defects in amelotin-deficient mice. J. Dent. Res. 2015;94:697–705. doi: 10.1177/0022034514566214. [DOI] [PubMed] [Google Scholar]
- 24.Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J. Clin. Periodontol. 1982;9:257–265. doi: 10.1111/j.1600-051X.1982.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 25.Oyane A, Kim H-M, Furuya T, Kokubo T, Miyazaki T, Nakamura T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A. 2003;65A:188–195. doi: 10.1002/jbm.a.10482. [DOI] [PubMed] [Google Scholar]
- 26.Padovano JD, Ravindran S, Snee PT, Ramachandran A, Bedran-Russo AK, George A. DMP1-derived peptides promote remineralization of human dentin. J. Dent. Res. 2015;94:608–614. doi: 10.1177/0022034515572441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvig KA, Nilveus RE, Fitzmorris L, Kersten B, Khorsandi SS. Scanning electron microscopic observations of cell population and bacterial contamination of membranes used for guided periodontal tissue regeneration in humans. J. Periodontol. 1990;61:515–520. doi: 10.1902/jop.1990.61.8.515. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh Z, et al. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology. 2017;105:1–12. doi: 10.1007/s10266-016-0267-0. [DOI] [PubMed] [Google Scholar]
- 29.Smith CEL, et al. Deletion of amelotin exons 3–6 is associated with amelogenesis imperfecta. Hum. Mol. Genet. 2016;25:3578–3587. doi: 10.1093/hmg/ddw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somogyi-Ganss E, et al. Comparative temporospatial expression profiling of murine amelotin protein during amelogenesis. Cells Tissues Organs. 2012;195:535–549. doi: 10.1159/000329255. [DOI] [PubMed] [Google Scholar]
- 31.Stoecklin-Wasmer C, Rutjes AWS, da Costa BR, Salvi GE, Jüni P, Sculean A. Absorbable collagen membranes for periodontal regeneration: a systematic review. J. Dent. Res. 2013;92:773–781. doi: 10.1177/0022034513496428. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka D, Ikeda Y, Ikeda E, Yokose M, Ganss B, Iwata T. Effect of amelotin on bone growth in the murine calvarial defect model. Ann. Biomed. Eng. 2021;49:3676–3684. doi: 10.1007/s10439-021-02867-z. [DOI] [PubMed] [Google Scholar]
- 33.Tanner MG, Solt CW, Vuddhakanok S. An evaluation of new attachment formation using a microfibhllar collagen barrier. J. Periodontol. 1988;59:524–530. doi: 10.1902/jop.1988.59.8.524. [DOI] [PubMed] [Google Scholar]
- 34.Tomita S, et al. Application of 4-META/MMA-TBB resin for fixation of membrane to tooth in guided tissue regeneration in dog. Dent. Mater. J. 2010;29:690–696. doi: 10.4012/dmj.2010-021. [DOI] [PubMed] [Google Scholar]
- 35.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J. Periodontol. 2018;89:S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 36.Tonetti MS, Prato GP, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J. Clin. Periodontol. 1996;23:548–556. doi: 10.1111/j.1600-051X.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.