Abstract
CXC chemokine receptor 4 (CXCR4) is overexpressed on most breast cancer cell surfaces including triple negative breast cancer (TNBC) which lacks traditional receptor overexpression. We targeted gold nanoparticles (GNPs) to this receptor via conjugation to anti-CXCR4 antibody (cGNPs). Irradiation of cells treated with cGNPs compared to PEGylated GNPs (pGNPs) resulted in more prominent radiosensitization of MDA-MB-231 cells with abundant CXCR4 overexpression than HTB-123 cells with moderate and MCF-7 cells with minimal CXCR4 overexpression. Overexpression of CXCR4 facilitated improved cellular internalization of cGNPs and irradiation of internalized cGNPs resulted in more unrepaired DNA double strand breaks and increased the production of oxygen free radicals compared to irradiation with non-internalized pGNPs. In a murine TNBC xenograft model, CXCR4 targeting potently increased tumor regrowth delay following radiation compared to radiation in the presence of pGNPs or vehicle alone. CXCR4 targeted GNPs enhance the efficacy of TNBC radiotherapy by increasing oxidative stress and DNA damage.
Introduction
Breast cancer is the most commonly diagnosed cancer in females and is the second most common cause of death from cancer accounting for 11.6% of total cases. The incidence of breast cancer has shown a steady upward trend due to better detection techniques and increased awareness of the disease1. Four distinct molecular subtypes of breast cancer have now been characterized - luminal A (estrogen-receptor-positive [ER+] and/or progesterone-receptor-positive [PR+] and human epidermal growth factor receptor 2-negative [HER2−]), luminal B (ER+ and/or PR+/HER2+), HER2-enriched (ER− and PR−/HER2+) and triple-negative (ER− and PR−/HER2−). Amongst the molecular subtypes, luminal A has the best survival outcomes and triple negative breast cancer (TNBC) has the worst survival outcomes across all stages. TNBC accounts for 10-20% of all breast cancers and is the most aggressive; characterized by a propensity for increased Ki-67, higher mitosis, more frequent BRCA1 mutations, and increased risk of brain or lung dissemination2, 3. Lack of expression of well-known druggable receptors like ER, PR or HER2 has limited the therapeutic arsenal and led to worse breast cancer specific survival as well as poorer overall survival.
CXCR4 chemokine receptor is expressed in breast cancers across all molecular subtypes. CXCR4 orchestrates the homing of lymphocytes to sites of inflammation. CXCR4 cell surface expression is prognostic for disease recurrence and survival in breast cancer4. In addition, increased expression of CXCR4 has been correlated with intense desmoplasia, reduced T-lymphocyte infiltration into the tumor, and increased incidence of lymph node metastasis5. Dissemination of cancer to distant organs such as the lungs, liver, bones and brain, the most common sites of metastatic disease from breast cancer, is also mediated in part by secretion of high levels of the CXCR4 ligand, CXCL12, by these organs6. Crosstalk between the mTOR pathway and CXCL12/CXCR4 axis has been postulated to mediate epithelial mesenchymal transition7. Hence, targeting the CXCR4 receptor in TNBC is an attractive option to enhance treatment outcomes. Inhibition of CXCR4 receptor has been shown to benefit HER2 positive cancer patient, however, CXCR4 inhibition does not have advantage in triple-negative breast cancer patients (REF).
Several therapeutic strategies for managing TNBC are promising: a) cell proliferating agents (e.g., anthracyclines); b) DNA repair blockators (e.g., platinum compounds and polyADP ribose polymerase inhibitors, PARP); c) microtubule function disruptors (e.g., taxanes); d) immune activation reducers (e.g., atezolizumab) (REF). However, postoperative radiation therapy remained the gold standard for treating TNBC patients.
Postoperative radiation therapy (RT) enhances local and locoregional control and improves survival in breast cancer patients. Locoregional recurrence occurs in roughly 5-10% of patients with TNBC after standard-of-care treatment. The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) reported that RT reduced the 10-year risk of any recurrence from 35% to 19% (p<0·00001) and reduced the 15-year risk of breast cancer death from 25% to 21% (p=0·00005)8. While RT forms an important component of the multimodal arsenal in the management of breast cancer, there is room for further improvement of outcomes, especially in TNBC.
Multiple studies confirm that irradiation of tumors laden with gold nanoparticles (GNPs) results in improved outcomes due to microscopic radiation dose enhancement via increased secondary electron showers emanating from the GNP after interaction with ionizing radiation. GNPs are stable (not chemically or thermally denatured), biologically compatible (used in clinical practice for over a century), relatively non-immunogenic, and readily synthesized with great size uniformity and with dimensions that are small enough to capitalize on the enhanced permeability and retention (EPR) effect of tumors with leaky vasculature. In addition, they are easily modifiable by decoration with polyethylene glycol to render them stealth properties for evasion of reticuloendothelial capture and peptides or antibodies for cell-specific docking9. We investigated the potential to improve RT outcomes in TNBC by using GNPs that bind to CXCR4 receptor.
Materials and Methods
Cell lines and reagents
We tested three human breast cancer cell lines with differential expression of CXCR4: MCF-7, HTB-123, and MDA-MB-231, which were obtained from the American Type Culture Collection (Manassas, VA) and from the Characterized Cell Line Core Facility at MD Anderson Cancer Center. All cell lines were cultured in media and treated under conditions recommended by the supplier. All reagents were of analytical grade. 20 nm GNPs were purchased from NanoHybrids, product number 23988. Polyethylene glycols (PEGs: HS-PEG5k-OMe and HS-PEG5k-CO2H) were from Laysan Bio. CXCR4 monoclonal antibody (12G5, lot number UB280543) was from Thermo Fisher Scientific.
The ζ-potential values and average hydrodynamic particle size (dh) of GNP formulations were determined using a ZEN3600 Malvern Zetasizer (Worcestershire, UK). Particles were suspended in 0.005 M sodium MES buffer, pH=6.5, placed into the DTS1070 cuvette and each sample was measured 3–6 times at 25 °C, with data presented as averages with standard deviations. Optical spectra ranging from 200 to 900 nm were collected using a Varian CARY 300Bio UV-VIS Spectrophotometer and 10 mm quartz cells (Starna, Inc.) thermostatted to 25 °C, with DI water used as the reference. Infrared spectroscopic measurements were performed with a Nicolet 6700 FTIR spectrometer (Thermo Electron Corporation, USA; DTGS TEC detector; KBr beamsplitter). Spectra were collected in the absorbance mode with each spectrum being a result of 512 scans (resolution 2 cm−1) against the ZnSe disc background by applying a <0.5 μL droplet of nanoparticle suspension on the disc and allowing it to dry under nitrogen gas multiple times to obtain a sufficient amount for better signal-to-noise ratio. Spectra were background-corrected with the OMNIC software, version 8.3.103. The FTIR spectroscopic measurements were reproducible and performed in triplicate.
Cellular toxicity assay
MDA-MB-231 cells (0.5·104) were seeded separately in 96-well plates in 250 μl of culture media. After 24-h incubation to allow adherence of cells, cell were subjected to varying concentrations (0-3.5 OD) of CXCR4 monoclonal antibody-conjugated GNPs (cGNPs) in triplicates for 24 h. Cell viability was assessed by the colorimetric MTS assay using Cell Titer 96 Aqueous One Solution Proliferation Assay System (Promega, Madison, WI, USA).
Classical Clonogenic assay
Human breast cancer cells (MCF-7, HTB-123, and MDA-MB-231) were seeded in 60 mm petri dishes at 50,000 cells/5 ml and cultured at 37 °C in a 5% CO2 incubator. When cells reached 60-70% confluence, fresh media was added with or without cGNPs (0.5 OD) or PEGylated GNPs (pGNPs, 0.5 OD) and incubated for 24 h. All dishes were washed with PBS twice to remove the un-internalized GNPs present in the media. Fresh media was added before cells were exposed to 0, 2, 4, or 6 Gy of ionizing radiation (XRAD 320 orthovoltage irradiator, Precision X-Ray, North Bradford, CT). Cells were trypsinized and known numbers of cells were plated in sextuplicate in six-well plates and further incubated for 12-14 days. When colonies were visible to the naked eye, plates were fixed and stained with 0.5% crystal violet diluted in 95% ethanol. The resulting colonies were counted on a high-resolution Oxford optotronix gel counter (Oxford, UK). The number of surviving colonies (>50 cells per colony) were plotted against radiation dose, and the dose enhancement factors at surviving fractions of 10% (DEF10) were calculated using Sigma plot. Experimental conditions were (a) radiation only, (b) cGNP + radiation, (c) pGNP + radiation; each experiment was performed in triplicate.
Dark field microscopic evaluation of cellular uptake of cGNPs.
Cellular internalization of GNPs can easily be detected by dark field microscopy. There were three experimental groups: (a) untreated control, (b) cGNP treatment, and (c) pGNP treatment. Cells were seeded in wells of a chamber slide and allowed to grow at optimal culture conditions (5% CO2 at 37 °C). When cells reached 60-70% confluence, old media was aspirated and replaced with fresh media containing (a) no additional material, (b) cGNPs, and (c) pGNPs, respectively, and incubated for 24 h. Later, slides were fixed in 4% paraformaldehyde for 15 min at room temperature, washed in PBS, and counterstained with a nuclear stain (4’,6-diamidino-2-phenylindole, DAPI; 1 μg/mL in PBS) for 5 min. Slides were washed in PBS, air dried, and mounted in mounting media (ProLong gold antifade agent, Life Technologies) and covered with a coverslip. Dark field images were captured using a Leica microscope with a 100× objective.
Gamma-H2AX immunofluorescence
Ionizing radiation causes double strand breaks in DNA. We evaluated the effect of cGNP internalization of ionizing radiation-induced DNA double strands break by Gamma-H2AX staining assay where phosphorylated histone 2AX protein that binds to unrepaired DNA strand breaks are visualized by immunofluorescence as foci of fluorescence that can be counted. MDA-MB-231 breast cancer cells were cultured on 8-well microscope slides (Corning, NY), and were allowed to reach 60-75% confluency. We assessed the DNA damage in the following groups: (a) control (media), (b) radiation alone, (c) radiation + 0.5 OD pGNP, (d) radiation + 0.5 OD cGNP. After treatment, all slides were incubated in a 5% CO2 incubator at 37 °C for 24 h. Then, all 8-well slides were washed twice in PBS, re-incubated with fresh media, and exposed to 2 Gy of ionizing radiation using an XRAD 320 orthovoltage irradiator (Precision X-Ray, North Bradford, CT). Ninety minutes after radiation exposure, the experimental arms were assessed for phosphorylated histone 2AX (g-H2AX) foci. Briefly, cells were washed with PBS, fixed with 1% paraformaldehyde (15 min) and 70% ethanol (15 min) at room temperature. Cells were then treated with 1% NP-40 (30 min), blocked with 5% bovine serum albumin (BSA; 30 min), and incubated with anti-g-H2AX antibody (Millipore, Billerica, MA) in 5% BSA for 2 h. Subsequently, cells were washed with PBS, labeled with Alexa-Fluor 488-conjugated secondary antibody (Life Technologies) for 30 min and counterstained with DAPI for 5 min. Coverslips were mounted with ProLong gold antifade agent and examined under a fluorescent microscope (Leica, Bannockburn, IL). On the images captured, nuclear g-H2AX foci were then counted manually from at least 50 cells for each treatment condition by an investigator blinded to treatment conditions.
Reactive oxygen species estimation
Ionizing radiation is known to produce reactive oxygen species (ROS) in a cellular milieu. We evaluated the effect of cGNP internalization on radiation-induced intracellular ROS generation. The production of intracellular ROS was assessed using cell permeant, fluorogenic CellROX green reagent (Life Technologies) according to the manufacturer’s instructions. We quantified the ROS in the following groups: (a) radiation alone, (b) radiation + 0.5 OD of pGNP, (c) radiation + 0.5 OD of cGNP, and (d) 50 μM of N-acetyl cysteine. All plates were incubated in a 5% CO2 incubator at 37 °C for 24 h. Un-internalized GNPs were removed by washing with PBS. All treatment groups received fresh media. CellROX green reagent (final concentration 5 micromol/L in medium) was added to each well and the cells were incubated at 37°C for 1.5 h before exposure to 0 or 2 Gy of ionizing radiation. Post-treatment, the cell culture media was aspirated, cells were washed with PBS, re-incubated with PBS and fluorescence was measured on a multi-mode fluorescence plate reader (BioTek, Winooski, VT; Ex/Em 485/528 nm). The mean signal intensity (in relative fluorescence units [RFU]) for each sample (triplicates) was calculated and averaged.
In vivo experiments
All animal experiments were performed in compliance with the relevant laws and to the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources, National Research Council and US National Academy of Sciences, and the MD Anderson Cancer Center Institutional Animal Care and Use Committee (IACUC). IACUC, MD Anderson approved the animal experiments; and the informed consent was obtained for any experimentation with animal subjects from IACUC, MD Anderson Cancer Center. Mice were purchased from MD Anderson’s Experimental Radiation Oncology Animal Facility and housed in an air-conditioned facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Mice were housed (no more than 5 per cage) in individually ventilated Tecniplast cages in a room with a HEPA-filtered air supply at relative humidity of 60% ± 10% on a 12 h light/dark cycle. All mice were quarantined for 1 week before any experiments were begun. Food (Purina PicoLab Rodent Diet 5053, Harlan Teklad, WI, USA) and water (reverse osmosis chlorinated or acidified water, pH 2.5–2.8) were provided ad libitum.
Tumor regrowth delay assay
cGNP- and pGNP-mediated radiosensitization of MDA-MB-231 breast cancer cells was evaluated in an orthotopic murine xenograft model as described below. Swiss nude mice (nu/nu) were injected subcutaneously in the right fourth mammary breast bad with 2·106 MDA-MB-231 cells in 50 μL of 1:1 media/matrigel. When the resulting tumors reached 5-8 mm in diameter, the mice were randomly assigned to one of the following treatment groups (at least 6 mice per group): (a) control; (b) cGNP only; (c) radiation only; (d) radiation + pGNP, and (e) radiation + cGNP. The control and radiation groups received 100 μl of PBS. Groups (b), (d), and (e) received either 10 OD of cGNP or 10 OD pGNP intravenously 24 h before radiation. A single dose of 10 Gy of radiation was delivered to the mouse tumors using an orthovoltage irradiator. Mice were monitored and tumors measured three times a week until the tumors reached 2 cm or up to 30 days. Tumor volume was measured as (where a = long axis and b = short axis dimension). Normalized tumor volumes were calculated by dividing current tumor volume by tumor volume at the start of treatment for each individual mouse and plotted with Graph Pad. The mean tumor doubling time (in days) was calculated for each treatment group.
Statistical analysis
All experiments were carried out in triplicate unless otherwise specified. Results are presented as means and standard deviations (SD). Statistically significant differences were calculated by using two-tailed unpaired Student’s t tests or by one-way analysis of variance. Results with a P value of <0.05 were considered significant.
Results
Synthesis and characterization of GNPs conjugated to anti-CXCR4 antibody
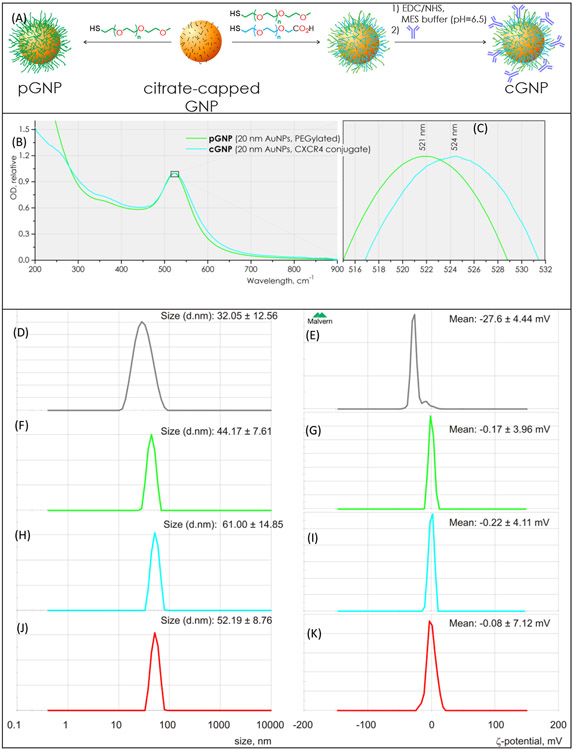
Citrate-stabilized 20 ± 1.5 nm spherical GNPs with a surface plasmon resonance peak of 519 nm were PEGylated by adding 2 mg of 5kDa thiol-terminated methoxy polyethylene glycol (HS-PEG5k-OMe) dissolved in 500 μL of deionized (DI) water to 2 mL of 25 OD citrate-stabilized GNPs with stirring. The resulting pGNP suspension was purified using an 8-10 kDa molecular weight cut-off (MWCO) cellulose ester dialysis membrane (Spectra-Por, Spectrum Laboratories, Inc.) for 4 days using DI water to remove unbound substances Figure 1A.
Figure 1. Synthesis and characterization of PEGylated (pGNP) and anti-CXCR4 antibody conjugated gold nanoparticles (cGNPs).
(a) Schematic diagram of synthetic steps in cGNP fabrication. (b, c) Normalized UV-vis absorption spectra of the PEGylated GNPs (pGNP, green) and conjugated GNPs (cGNPs, cyan) suspensions. (d, f, h, j) Hydrodynamic particle size distribution of citrate-capped GNP, pGNP, cGNP and isotypeGNP suspensions, determined using dynamic light scattering. (e, f, i, k) Surface charge measurement of citrate-capped GNP, pGNP, cGNP and isotypeGNP by ζ-potentiometry. ζ-Potential distribution of pGNP and cGNP in suspensions, determined by particle electrophoresis..
CXCR4 monoclonal antibody-conjugated GNPs (cGNP) were prepared as shown in Figure 1A. 3 mL of 25 OD suspension of 20 ± 1.5 nm citrate-coated GNPs were incubated with 1 mL of PEGs solution, prepared of 9 mg of HS-PEG-OMe, 1 mg of HS-PEG-CO2H (both 5 kDa, Laysan Bio) and 0.005 M sodium MES buffer, pH=6.5. The carboxy-PEGylated GNP suspension was subjected to dialysis using 5-8 kDa biotech grade cellulose ester membrane (Spectrum, Inc) in 18 MΩ DI water (Millipore) until MES salt was completely removed, which was traced by measuring the electrical conductivity. After the conductivity stopped decreasing, the material was additionally dialyzed for 48 h at room temperature to ensure the complete removal of unbound PEGs. 0.5 mg of EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, TCI America) and 1 mg of N-hydroxysulfosuccinimide sodium salt (sulfo-NHS, Sigma-Aldrich Co) were dissolved in 100 μL of 0.1 M MES buffer solution (pH = 6.5, Jena Bioscience) and the solution was added to 2 mL of prepared carboxy-PEGylated GNP suspension (OD≈20), and stirred at room temperature for 20 min. The resulting solution was centrifuged for 30 min at 2,800 g at 4 °C. Supernatant was carefully discarded and the fluid pellet was resuspended in 1 ml of 0.02M MES buffer. 0.25 mL of 0.5 mg/mL solution of CXCR4 antibody was added with stirring, incubated for 1 h at room temperature under dark, and then subjected to purification by centrifugation, as was explained above. The obtained colloidal cGNP solution was preserved in the dark at 4 °C.
The UV-vis absorption spectra of the pGNP and cGNP suspensions showed typical characteristics of GNPs (Figure 1 B), i.e., a single peak at 521 nm and no significant differences in the collected spectra except for a minor 2–3 nm red-shift of the surface plasmon resonance for cGNP (Figure 1 C).
Surface charges and particle sizes in water were measured using a ZEN3600 Malvern Zetasizer (Worcestershire, UK). The average sizes of the pGNP and cGNP conjugates were determined to be 41.6 ± 15.6 nm (Figure 1 D) and 52.8 ± 22.0 nm (Figure 1 E), respectively. The surface charge of pGNP had a mean value of −4.7 ± 9.4 mV (Figure 1 F) and cGNP showed the surface potential of −0.5 ± 5.3 mV. (Figure 1 G).
FTIR spectra of pGNP and cGNP conjugates showed several distinct features attributed to PEG (Figure 2), including two main peaks characteristic of C-O-C bond vibrational modes observed around 1112 (asym) and 842 cm−1 (sym), and the frequency mode around 2880 cm−1 in all spectra attributed to symmetric CH2 stretching. The bands at 1665 and 1656 cm−1 in the spectrum of cGNPs are due to polypeptide amide carbonyl (hydrogen bonded) asymmetric stretching. A very broad elevation in the spectrum of cGNPs in the region between 3100 and 3600 cm−1 indicates the presence of exchangeable protons from amide groups of polypeptide and associated water molecules, which also indicates the presence of antibody attached to the nanoparticle (Figure 2 A).
Figure 2. FTIR spectra of the cGNP and pGNP conjugates.
a) Infrared absorption peaks attributed to the amide bands in proteins are highlighted with cyan. b) Certain distinct infrared absorption bands typically observed in PEGs are highlighted in green.
Estimation of antibodies per GNP
The number of CXCR4 antibody molecules attached per cGNP particle was determined by colorimetric reaction with 2,4,6-trinitrobenzenesulfonic acid (TNBSA, TCI), following the procedure, outlined in10, 11. Briefly, 10 μL of the cGNP with OD=1 was added to 10 μL of 0.1M KCN (VWR) solution, followed by addition of 0.48 mL of 0.1M carbonate-hydrocarbonate buffer (pH=8.5). 0.5 mL of 0.01% TNBSA solution was added and the resulting solution was incubated at 40 °C for 2 h under dark. The absorbance was determined at 420 nm using a quartz cuvette, and compared to a standard curve generated by use of the CXCR4 antibody diluted at a series of known concentrations in the bicarbonate buffer and assayed under identical conditions.
The analysis was performed in triplicate and the number of CXCR4 antibodies per GNP was calculated to be 36 ± 8, assuming CXCR4 has a molecular weight of 39746 Da, and 20 nm GNP suspension with OD=1 contains 4.3·1013 particles/mL.
cGNPs are not toxic to breast cancer cells.
To assess the optimal concentration of cGNPs to be used for in vitro experiments we first evaluated the possible cytotoxicity of cGNPs on breast cancer cell lines using the MTT assay. Two breast cancer cell lines (MDA-MB-231 and HTB-123) expressing high CXCR4, and one cell line (MCF-7) expressing low CXCR4 were selected to evaluate the toxicity of cGNPs. MCF-7, MDA-MB-231, and HTB-123 cells were seeded (0.5·104 cells/well) separately in 96-well plates in 250 μl of culture media. Cells were treated with 0-3.5 OD concentrations of cGNPs in triplicate for 24 h and 48 h. No conspicuous cGNP-mediated growth inhibition was observed in any cell line at any concentration of cGNP tested in our experiment (Figure 3 A). The lowest concentration (0.5 OD) was selected for further studies.
Figure 3. Cytotoxicity of cGNPs and clonogenic survival of breast cancer cell lines.
(a) MTT assay showing no appreciable toxicity of MCF-7, HTB-123 and MDA-MB-231 cells treated with up to 3.5 OD of pGNPs and cGNPs for 24 and 48 h. Clonogenic survival following irradiation of (b) MCF-7, (c) MDA-MB-231, and (d) HTB-123 cells pretreated for 24 h with 0.5 OD pGNPs or cGNPs.
Higher CXCR4 expression levels are associated with greater cGNP-mediated radiosensitization
We assessed the radiosensitization potential of pGNPs and cGNPs through a classical clonogenic assay in MDA-MB-231 and HTB-123 expressing high CXCR4 levels and MCF-7 expressing low CXCR4 levels. cGNPs enhanced the efficacy of radiation in HTB-123 and MDA-MB-231 (Figure 3 B,C) cells which have higher expression of CXCR4 whereas no augmentation was seen in MCF-7 cell line (Figure 3D). The Dose Enhancement Factor at 10% cell survival (DEF10) of PEGylated GNPs (pGNPs) for MDA-MB-231, HTB-123, and MCF-7 were 1.02 (4.82/4.71), 1.03 (4.75/4.58) and 1.02 (4.92/4.81), respectively and the DEF10 of CXCR4-targeted GNPs (cGNPs) for MDA-MB-231, HTB-123, and MCF-7 cells were 1.20 (4.82/4.01), 1.20 (4.75/3.94), and 1.03 (4.92/4.75), respectively. Notably, the low CXCR4 overexpressing cell line (MCF-7) showed no appreciable difference in radiosensitization between pGNPs and cGNPs whereas the high CXCR4 overexpressing cell lines (HTB-123 and MDA-MB-231) were radiosensitized potently by cGNPs but not by pGNPs (Figure 3B, C, D).
Uptake of cGNPs by MDA-MB-231 breast cancer cells
To explain the differential sensitization of pGNPs and cGNPs in CXCR4 overexpressing cell lines, we evaluated their cellular internalization using dark field microscopy. MDA-MB-231 breast cancer cells were seeded in three groups: (A-i) untreated control, (A-ii) cGNP treated group, and (A-iii) pGNP treated group. 5000 MDA-MB-231 cells per well were seeded in 8-well chamber slides and cultured in optimal culture conditions (5% CO2 at 37 °C). When MDA-MB-231 breast cancer cells reached 60-70% confluence, old media was aspirated and replaced with (A-i) fresh media alone, (A-ii) media with 0.5 OD cGNPs, and (A-iii) media with 0.5 OD pGNPs, respectively, for 24 h to assess the uptake kinetics in tumor cells. Qualitative assessment using dark field and fluorescence microscopy showed that the cGNPs have superior uptake kinetics compared to pGNPs as shown in Figure 4A. Active targeting of the CXCR4 receptor might be responsible for enhanced uptake kinetics of cGNP.
Figure 4.
cGNPs internalization and consequent generation of radiation-induced reactive oxygen species and DNA double strand breaks. (A-i) MDA-MB-231 breast cancer cells incubated with PBS, (A-ii) pGNP (0.5 OD), or (A-iii) cGNP (0.5 OD) and imaged by dark field (to visualize GNPs) and fluorescence (to visualize DAPI-stained nuclei) microscopy demonstrate internalization and predominantly cytoplasmic localization of GNPs in cells treated with cGNPs. (B) MDA-MB-231 breast cancer cells incubated with PBS, pGNP (0.5 OD), or cGNP (0.5 OD), or 50 μM N-acetyl cysteine 24 h before 0 or 2 Gy of radiation. Following irradiation, cGNP (0.5 OD) pretreatment induces significantly (p < 0.05) higher ROS production than pGNP pretreatment or vehicle alone pretreatment; this increase is inhibited by N-acetyl cysteine treatment. ** PBS + 2 Gy vs. cGNP + 2 Gy (p<0.001), and * pGNP + 2 Gy vs. cGNP + 2 Gy (p<0.001). (C) Immunofluorescence staining for gamma-H2AX foci documents more unrepaired DNA double strand breaks following pre-treatment with cGNPs than vehicle alone or pGNPs. (D) Quantitative analysis of gamma-H2AX foci following irradiation. ** PBS + 2 Gy vs. cGNP + 2 Gy (p<0.001), and * pGNP + 2 Gy vs. cGNP + 2 Gy (p<0.005)
cGNPs enhance radiation-induced reactive oxygen species production
Ionizing radiation mediates its anti-tumor effects via ionization of molecules including oxygen to create free radicals or reactive oxygen species (ROS), which in turn cause DNA damage. MDA-MB-231 breast cancer cells were treated with PBS, pGNP (0.5 OD), cGNP (0.5 OD), or 50 μM Nac 24 h before 2 Gy of radiation. We evaluated the production of intracellular ROS using fluorogenic CellROX green reagent (Life Technologies) according to the manufacturer’s instructions. The treatment of cGNP (0.5 OD) 24 h before irradiation significantly (p < 0.05) enhanced the induction ROS as compared to PBS treated and irradiated group. The ROS of cells treated with cGNP was significantly higher (p < 0.05) as compared to cells treated with pGNP indicating that cGNP internalization in MDA-MB-231 cells enhances ROS generation (Figure 4B-1).
GNPs mediate radiosensitization via increased DNA double strand breaks
We evaluated DNA double strands break by immunofluorescence staining for Gamma-H2AX foci. MDA-MB-231 breast cancer cells were cultured on 8-well microscope slides (Corning, NY) and treated with (a) control (media), (b) radiation alone, (c) radiation + pGNP, or (d) radiation + cGNP (Figure 4 C- i to iv). The control group and radiation alone group received 200 μl of fresh media. Groups (c) and (d) received 0.5 OD of pGNP or cGNP in media, respectively. Significantly higher number of foci per cell was observed in the cGNP and radiation treatment group as compared to the radiation alone group or the group pretreated with 0.5 OD of pGNP before radiation. No significant difference in the number of foci/cells was observed in the radiation alone group vs. the pGNP + radiation group (Figure 4 C- v).
Intravenous administration of cGNPs radiosensitizes murine breast cancer xenografts to radiation
Two million TNBC cell line MDA-MB-231 cells in 50 μL 1:1 media/matrigel were implanted into the right 4th mammary fat pad of female Swiss nu/nu nude mice. Once tumors reached ~7 mm in size, the mice were randomized into the following groups (a) control, (b) radiation alone, (c) pGNP + radiation, (d) cGNP alone, and (e) cGNP + radiation. Mice received PBS, pGNP (100 μL; 10 OD via tail vein) or cGNP (100 μL; 10 OD via tail vein) 24 h before irradiation. Radiation was administered as a single 10 Gy dose locally to the tumor. The normalized tumor volumes were plotted as shown in Figure 5. (a) Tumor continued to grow rapidly in the un-irradiated control and cGNP alone treatment groups. Radiation delayed tumor regrowth significantly; the time to tumor volume tripling (~32 days) was ~18 days longer than for untreated controls (~14 days). pGNP treatment did not cause any further delays in tumor regrowth. However, cGNP treatment resulted in tumors shrinking to baseline levels and not regrowing even after 50 days (Figure 5a). On day 32, the cGNP + radiation group had the smallest tumor of all the groups, again confirming potent radiosensitization mirroring the in vitro findings (Figure 5b). We also measured the body weight of different treatment group of mice to evaluate the safety or side effect in mice. No significant difference in mice weight was observed in any treatment groups (Figure S2).
Figure 5. cGNPs enhance biological effectiveness of radiation in vivo.
(A) Normalized tumor volumes were tracked longitudinally over time after orthotopic right 4th mammary fat pad MDA-MB-231 xenografts in nude mice were treated with vehicle alone, cGNP alone, radiation (10 Gy single dose) alone, pGNP + radiation, or cGNP + radiation 24 h before irradiation. GNPs were administered intravenously (100 μL of 10 OD) 24 h before irradiation. Whereas pGNP treatment did not delay tumor regrowth following radiation any more than radiation alone, cGNP treatment significantly reduced tumor regrowth. (B) Quantitative analysis of average normalized tumor volume of all groups on day 32 following treatment. ** cGNP vs. cGNP + RT (p<0.001), and * RT vs. cGNP + RT (p<0.005)
Discussion
CXCR4 is a member of the G protein-coupled cell surface receptor family with little or no expression on normal breast epithelial cells but progressively higher expression as one advances from breast atypia to breast carcinoma12. Our experimental results suggest that GNPs can be decorated with anti-CXCR4 antibodies for tumor-specific targeting to enhance nanoparticle uptake and internalization across a spectrum of breast cancer cells. This uptake is higher in cells with greater CXCR4 expression. Correspondingly, greater internalization leads to greater oxidative stress and greater DNA damage, resulting in greater radiosensitization in vitro. While PEGylated GNPs tend to accumulate in tumor cells via the EPR effect, active targeting leads to increased accumulation and internalization which translates to potent radiosensitization in vivo.
The 100 μL of 10 OD concentration used per mouse in vivo translates to ~2 mg/Kg body weight, a concentration that makes this approach economically viable, clinically relevant and translatable. The 24 h interval between intravenous administration and irradiation rather than intravenous administration followed immediately by irradiation also makes clinical translation less logistically challenging in a clinical environment. The other advantages of our experimental design are that we used an intravenous route of administration of GNP formulations for mouse studies rather than intratumoral injection, tumor-specific targeting via a receptor that is differentially expressed in tumor cells compared to normal cells, relatively low concentrations of cGNPs, irradiation 24 h after intravenous administration, and orthotopic models of breast cancer. Furthermore, the differential expression of CXCR4 in TNBC vs. normal breast epithelial cells significantly enhances tumor selectivity and, thereby, the therapeutic ratio.
It has been reported that the activation of CAFs supports tumor growth and metastasis. The de novo activation of CAFs favors the establishment of macrometastases via production of tenascin and periostin that provide supporting signals to the cancer cells13, 14. CAFs mediated alterations in matrix production increases tissue stiffness that triggers prosurvival and proproliferation signalling in cancer cells15. Further increased mechanical stress can collapse blood vessels, leading to hypoxia, promoting more aggressive cancer phenotypes, and reducing drug delivery16. CAFs are also a substantial source of growth factors, cytokines and exosomes that can promote tumour growth and modulate therapy responses17. From a mechanistic standpoint, the in vitro data suggest that CXCR4 targeting localizes and internalizes the GNPs in TNBC cells leading to increased oxygen free radical production and indirect DNA damage in the form of double strand breaks. The primary driver of radiosensitization is the increased concentration of GNPs inside the tumor cells. Indeed, the clonogenic assay results confirm that greater CXCR4 expression results in greater radiosensitization. Breast cancer cells with higher CXCR4 overexpression than normal cells consequently receive a focal escalated dose of radiation which may provide an avenue for eradication of radioresistant tumor cells and/or mesenchymal stem cells thereby reducing the probability of recurrence in TNBC18.
CXCL12 docking to CXCR4 has been implicated in metastatic spread to a number of distant sites such as bones, liver, lungs, brain, lymph nodes, and kidneys. In addition, the CXCR4/CXCL12 axis modulates primary tumor growth in an autocrine and paracrine manner6, 12, 19, 20. There is increased recognition that stereotactic RT can be used in the treatment of oligometastatic disease21. In TNBC, the sites of metastasis also express CXCR4 receptor. One of the biggest pitfalls of using RT in metastatic sites is the normal tissue complication probability, which limits the dose to metastatic disease sites and/or number of metastases that can be treated safely. CXCR4 targeted GNPs may facilitate reduction of the radiation dose required at metastatic sites and/or safe treatment of more sites of metastatic disease.
Given the profound reduction in tumor volumes, we noted with cGNP + radiation in vivo, we speculate that such a construct might have broad applicability across the diagnostic and/or therapeutic spectrum beyond TNBC. In principle, such a construct could serve as a radiosensitizer of all breast cancer types, an enhancer of contrast in mammography, and an aid to intraoperative recognition of tumor margins.
There are distinct limitations to our study. We have not fully optimized the decoration ratio of antibodies to PEG on our GNPs. Titrating this ratio could improve (or may be reduce) efficacy. We used a nude mouse xenograft model in our studies. This limits the ability to interrogate the immune system’s role in shaping anti-tumor response. Tagging GNPs to CXCR4 has the potential to reduce desmoplasia and increase the infiltration of T lymphocytes into the tumor microenvironment5, 22. Such analyses were not performed in our study and are warranted in future studies. Though our work focused on docking GNPs to CXCR4 receptors on breast cancer cells, CXCR4 can be tagged to doxorubicin, docetaxel, liposome, siRNA, oligonucleotides to further increase the reach of targeted delivery in TNBC23-25.
Conclusions
TNBC has poor outcomes in terms of local recurrence and breast cancer specific survival. CXCR4 is one of the few druggable cell surface receptors expressed by TNBC. Anti-CXCR4 antibody decorated GNPs facilitate tumor-specific accumulation and internalization of GNPs, resulting in greater radiosensitization via increased oxidative stress and DNA damage. This decoration also enables greater tumor accumulation upon intravenous administration in vivo and thereby more pronounced tumor regression after irradiation. Collectively, these results suggest that CXCR4 affords a means to achieve selective and focal radiation boost doses to TNBC tumors to increase radiocurability of this treatment-refractory tumor.
Supplementary Material
Acknowledgements
We acknowledge Mr. Keith Sanders and Ms. Kathryn Aziz for measuring tumor and helping the irradiation treatment.
Supported in part by Cancer Center Support (Core) Grant (P30 CA16672) from the US National Cancer Institute, National Institutes of Health to The University of Texas MD Anderson Cancer and the Center for Radiation Oncology Research Seed Grant to P.K.S. and S.B
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A, CA Cancer J Clin, 2018, 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Fallahpour S, Navaneelan T, De P and Borgo A, CMAJ Open, 2017, 5, E734–e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Cronin KA, Kurian AW and Andridge R, Cancer Epidemiol Biomarkers Prev, 2018, 27, 619–626. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Guo L, Zhao H, Zhao J, Weng H and Zhao B, Oncotarget, 2015, 6, 5022–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R and Jain RK, Proc Natl Acad Sci U S A, 2019, DOI: 10.1073/pnas.1815515116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee D and Zhao J, Am J Cancer Res, 2013, 3, 46–57. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Chen SM, Wang X, Ding XF, Ding J and Meng LH, J Biol Chem, 2012, 287, 12132–12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y and Peto R, Lancet, 2011, 378, 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V and Mijakovic I, Int J Mol Sci, 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satake K, Okuyama T, and T OM. Shinoda, The Journal of Biochemistry, 1960, 47, 654–660. [Google Scholar]
- 11.Snyder SL and Sobocinski PZ, Anal Biochem, 1975, 64, 284–288. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Zhao H, Chen H and Yao Q, Drug Des Devel Ther, 2015, 9, 4953–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF and Huelsken J, Cancer Research, 2012, 72. [DOI] [PubMed] [Google Scholar]
- 14.Oskarsson T, Acharyya S, Zhang XHF, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E and Massague J, Nature Medicine, 2011, 17, 867–U256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA and Weaver VM, Cancer Cell, 2005, 8, 241–254. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han XX, Adstamongkonkul P, Popovic Z, Huang PG, Bawendi MG, Boucher Y and Jain RK, Nature Communications, 2013, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and Wrana JL, Cell, 2012, 151, 1542–1556. [DOI] [PubMed] [Google Scholar]
- 18.Shima H, Yamada A, Ishikawa T and Endo I, Gland Surg, 2017, 6, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M and Yamamoto N, Biomed Pharmacother, 2006, 60, 273–276. [DOI] [PubMed] [Google Scholar]
- 20.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J and Ratajczak MZ, Stem Cells, 2005, 23, 879–894. [DOI] [PubMed] [Google Scholar]
- 21.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A and Senan S, Lancet, 2019, 393, 2051–2058. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Chatterjee DK, Lee MH and Krishnan S, Cancer Lett, 2014, 347, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chittasupho C, Lirdprapamongkol K, Kewsuwan P and Sarisuta N, Eur J Pharm Biopharm, 2014, 88, 529–538. [DOI] [PubMed] [Google Scholar]
- 24.Wang RT, Zhi XY, Yao SY and Zhang Y, Colloids Surf B Biointerfaces, 2015, 133, 43–50. [DOI] [PubMed] [Google Scholar]
- 25.Guo P, You JO, Yang J, Moses MA and Auguste DT, Biomaterials, 2012, 33, 8104–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.