Abstract
International clinical practice guidelines have progressively endorsed direct oral anticoagulants (DOACs) as an alternative to low-molecular-weight heparins (LMWHs) monotherapy for the initial and long-term treatment of cancer-associated thrombosis (CAT). Several new randomized controlled trials (RCTs) have recently reported additional results on the safety and efficacy of DOACs in this setting. We performed an updated meta-analysis of all publicly available data from RCTs comparing DOACs with LMWHs for the treatment of CAT. Six RCTs enrolling 3690 patients with CAT were included. Compared with LMWHs, DOACs significantly decreased the risk of CAT recurrence (RR, 0.67; 95%CI, 0.52–0.85), with a non-significant increase in the risk of major bleeding (RR, 1.17; 95%CI, 0.82–1.67), a significant increase in the risk of clinically relevant nonmajor bleeding (RR 1.66; 95%CI, 1.31–2.09) and no difference in all-cause mortality rates. These results increase the level of certainty of available evidence supporting the use of DOACs as an effective and safe option for the treatment of CAT in selected cancer patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01289-1.
Keywords: Cancer, Venous thromboembolism, Direct oral anticoagulant, Low-molecular-weight heparin
To the editor
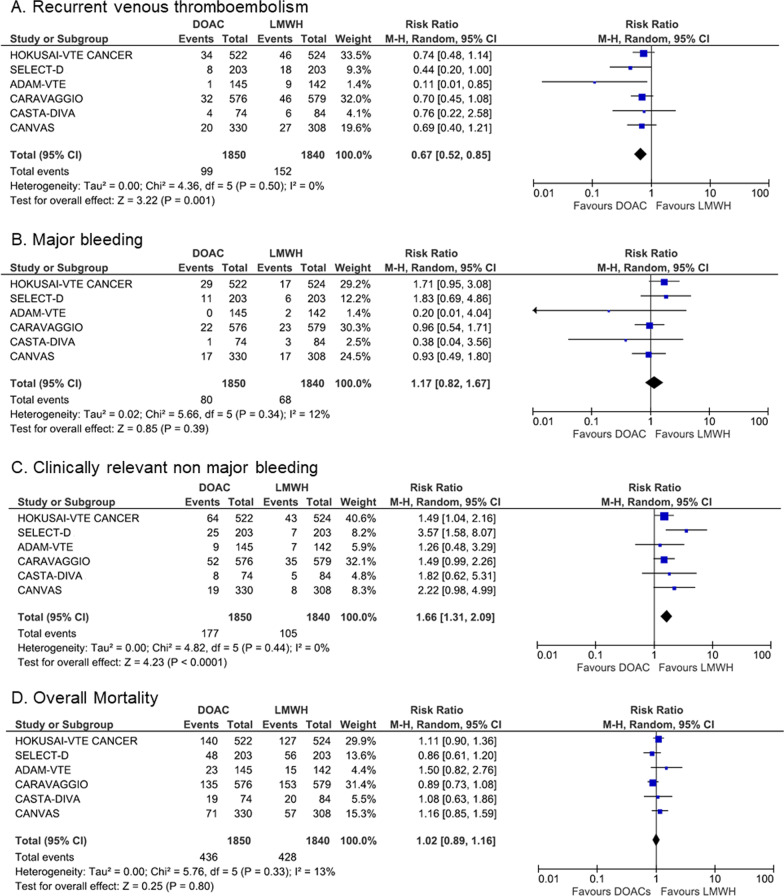
International evidence-based clinical practice guidelines (CPGs), which provide recommendations for the best available care options and guide clinical decision-making, have progressively endorsed direct oral anticoagulants (DOACs) as an alternative to monotherapy with low-molecular-weight heparins (LMWHs) for the initial and long-term treatment of cancer-associated thrombosis (CAT) [1–4]. Several new randomized controlled trials (RCTs) have recently reported additional results on the safety and efficacy of DOACs in this setting. Here, we perform an updated study-level meta-analysis of all publicly available results from RCTs comparing DOACs with LMWHs for the treatment of CAT. The literature search and selection process identified 6 RCTs meeting the inclusion criteria [5–10], which were further included in the pooled-analyses (Additional File 1). Together, these trials enrolled a total of 3690 patients with acute CAT (1850 randomized to the DOACs arms and 1840 randomized to the LMWHs arms). Study characteristics are depicted in Table 1. All studies were open label, used a blinded central outcome adjudication design and were estimated to have low risk for performance and detection bias (Additional File 1). During a 3–6 months follow-up under anticoagulant treatment (intention-to-treat population), recurrent venous thromboembolism (VTE) occurred in 99 of 1850 patients receiving DOACs vs. 152 of 1840 patients receiving LMWHs. The risk of recurrent VTE was significantly lower with DOACs compared to LMWHs (RR, 0.67; 95%CI, 0.52–0.85; p = 0.001; I2 = 0%; Fig. 1). With a rate of VTE recurrence of 8.3% in patients receiving LMWHs, the absolute risk reduction with DOACs was 2.7% (95%CI, –4 to –1.2; high certainty of evidence). Major bleeding occurred in 80 of 1850 patients receiving DOACs vs. 68 of 1840 patients receiving LMWHs. Although the risk of major bleeding was numerically higher with DOACs, this difference did not reach statistical significance (RR, 1.17, 95%CI, 0.82–1.67; p = 0.39; I2 = 12%; Fig. 1). With a risk of major bleeding of 3.7% in the LMWHs group, the absolute risk increase with DOACs was 0.6% (95%CI, –0.7 to 2.5; high certainty of evidence). Clinically relevant nonmajor bleeding (CRNMB) occurred more frequently in patients receiving DOACs compared to those receiving LMWHs (RR, 1.66, 95%CI, 1.31–2.09; p < 0.0001; I2 = 0%, Fig. 1). With a risk of CRNMB of 5.7% in patients receiving LMWHs, the absolute risk increase with DOACs was 3.8% (95% CI, 1.8–6.2). Finally, the rate of all-cause mortality did not differ between the 2 groups (23.3% in the DOACs arms vs. 23.5% in the LMWHs arms; RR, 1.02, 95%CI, 0.89–1.16; p = 0.80; I2 = 13%, Fig. 1). Per Grading of Recommendations Assessment, Development and Evaluation criteria, the quality of evidence was judged to be high for all outcomes.
Table 1.
Main characteristics of randomized controlled trials included in the pooled analysis
| HOKUSAI-VTE CANCER | SELECT-D | ADAM-VTE | CARAVAGGIO | CASTA-DIVA | CANVAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Non inferiority Randomized, open label, noninferiority trial with blinded central outcome adjudication | Randomized, open-label, pilot trial with blinded central outcome adjudication | Randomized, open label, superiority trial with blinded central outcome adjudication | Randomized, open label, noninferiority trial with blinded central outcome adjudication | Randomized, open label, noninferiority trial with blinded central outcome adjudication | Randomized cohort of an unblinded hybrid comparative effectiveness non-inferiority trial | ||||||
| Number of randomized patients | 1050 | 406 | 300 | 1170 | 158 | 671 | ||||||
| Type of patients included | Patients with active cancer and symptomatic or incidental popliteal, femoral or iliac or IVC DVT, symptomatic or incidental PE | Patients with active cancer and symptomatic DVT, symptomatic PE, or incidental PE | Active cancer patients with acute DVT (including upper extremity), PE, splanchnic or cerebral vein thrombosis | Patients with active or recent cancer and acute DVT or PE | Patients with active cancer and acute DVT or PE at high risk of recurrent VTE | Patients with cancer and acute VTE | ||||||
| Mean Age (years) | 64 | 67 | 64 | 67 | 69 | Not reported | ||||||
| Male sex | 52% | 53% | 48% | 49% | 49% | Not reported | ||||||
| Type of cancers included |
Colorectal: 15% Lung: 15% Breast: 12% Genitourinary: 13% Gynecologic: 11% Pancreatic or hepatobiliary: 9% Upper gastrointestinal: 5% Hematological malignancies: 11% Other: 10% |
Colorectal: 25% Lung: 12% Breast: 10% Genitourinary: 17% Gynecologic: 10% Pancreatic or hepatobiliary: 8% Upper gastrointestinal: 10% Hematological malignancies: 8% Other: 10% |
Colorectal: 16% Lung: 17% Breast: 9% Genitourinary: 9% Gynecologic: 10% Pancreatic or hepatobiliary: 16% Upper gastrointestinal: 4% Hematological malignancies: 8% Other: 11% |
Colorectal: 20% Lung: 17% Breast: 13% Genitourinary: 9% Gynecologic: 10% Pancreatic or hepatobiliary: 8% Upper gastrointestinal: 5% Hematological malignancies: 7% Other: 11% |
Gastro-intestinal: 20% Lung: 18% Breast: 12% Genitourinary: 13% Gynecologic: 8% Hematological malignancies: 8% Other: 21% |
Not reported | ||||||
| Metastatic disease | 52.9% | 58.0% | 64.3% | 67.9% | 72.8% | Not reported | ||||||
| Treatment allocation | Intervention (edoxaban) |
Control (dalteparin) |
Intervention (rivaroxaban) |
Control (dalteparin) |
Intervention (apixaban) |
Control (dalteparin) |
Intervention (apixaban) |
Control (dalteparin) |
Intervention (rivaroxaban) |
Control (dalteparin) |
Intervention (DOAC) |
Control (LMWH) |
| Therapeutic dose of LMWH for at least 5 days followed by edoxaban 60 or 30 mg once daily | Dalteparin 200 IU/kg once daily for 1 month followed by 150 IU/kg once daily | Rivaroxaban 15 mg twice daily for 21 days, followed by 20 mg once daily | Dalteparin 200 IU/kg once daily for 1 month followed by 150 IU/kg once daily | Apixaban 10 mg twice daily for 7 days, followed by 5 mg twice daily | Dalteparin 200 IU/kg once daily for 1 month followed by 150 IU/kg once daily | Apixaban 10 mg twice daily for 7 days, followed by 5 mg twice daily | Dalteparin 200 IU/kg once daily for 1 month followed by 150 IU/kg once daily | Rivaroxaban 15 mg twice daily for 21 days, followed by 20 mg once daily | Dalteparin 200 IU/kg once daily for 1 month followed by 150 IU/kg once daily | Any DOAC at the discretion of the treating investigator in accordance with the drug's FDA package insert | Any approved LMWH at the discretion of the treating investigator in accordance with the drug's FDA package insert | |
| Duration of follow-up | 12 months | 6 months | 6 months | 6 months | 3 months | 6 months | ||||||
| Primary outcome | Composite of recurrent VTE or major bleeding | Recurrent VTE | Major bleeding including fatal bleeding |
Efficacy: Recurrent VTE Safety: Major bleeding |
Efficacy: Composite of recurrent VTE and worsening of pulmonary vascular or venous obstruction on systematic examinations Safety: Major bleeding |
Efficacy: Recurrent VTE Safety: Major bleeding |
||||||
| Secondary outcomes |
Recurrent VTE Major bleeding CRNMB Mortality |
Major bleeding CRNMB Mortality |
Recurrent VTE CRNMB Mortality |
CRNMB Mortality |
CRNMB Mortality |
|||||||
| Recurrent VTE | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control |
| 7.9% | 11.3% | 4% | 11% | 0.7% | 6.3% | 5.6% | 7.9% | 6.4% | 10.1% | 6.1% | 8.8% | |
| HR (95% CI) for recurrent VTE | 0.71 (95% CI 0.48–1.06) | 0.43 (95% CI 0.19–0.99) | 0.099 (95% CI 0.013–0.780) | 0.63 (95% CI 0.37–1.07) | 0.75 (95% CI 0.21–2.66) | Not reported | ||||||
| Major bleeding | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control |
| 6.9% | 4% | 6% | 4% | 0% | 1.4% | 3.8% | 4% | 1.4% | 3.7% | 5.2% | 5.6% | |
| HR (95% CI) for Major bleeding | 1.77 (95% CI 1.03–3.04) | 1.83 (95% CI 0.68–4.96) | Not estimable | 0.82 (95% CI 0.40–1.69) | 0.36 (95% CI 0.04–3.43) | Not reported | ||||||
| CRNMB | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control |
| 14.6% | 11.1% | 13% | 4% | 6.2% | 4.9% | 9% | 6% | 10.8% | 6.1% | 5.8% | 2.6% | |
| HR (95% CI) for CRNMB | 1.38 (95% CI 0.98–1.94) | 3.76 (95% CI 1.63–8.69) | – | 1.42 (95% CI 0.88–2.30) | – | Not reported | ||||||
| Mortality | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control |
| 39.5% | 36.6% | 23.6% | 27.6% | 16% | 11% | 23.4% | 26.4% | 25.7% | 23.8% | 21.5% | 18.4% | |
| HR (95% CI) for mortality | 1.12 (95% CI 0.92–1.37) | 0.82 (95% CI 0.62–1.09) | 1.05 (95% CI 0.56–1.97) | Not reported | ||||||||
CI confidence interval, CRNMB clinically relevant nonmajor bleeding, DOAC direct oral anticoagulant, DVT deep vein thrombosis, LMWH low-molecular-weight heparin, HR hazard ratio, PE pulmonary embolism, VTE venous thromboembolism
Fig. 1.
Forest plots of Risk Ratios for Venous Thromboembolism (A), Major Bleeding (B), Clinically Relevant NonMajor Bleeding (C) and Overall Mortality (D)
By pooling the results from 6 high quality RCTs, the present study provides more precise estimates of the anticipated treatment effects. Our findings indicate that in cancer patients, DOACs confer a slight reduction in the risk of recurrent VTE. The proportion of patients discontinuing treatment was lower in those randomized to receive a DOAC compared to those randomized to receive a LMWH, which may explain, in part, the superior efficacy of DOACs. The exclusion criteria used in most RCTs (ECOG Performance Status > 2, brain tumors, platelet count < 50–75 G.L−1, Cockroft Clairance < 30 ml.min−1) may have limited the generalizability of the findings. Importantly, bleeding was more common in patients with gastrointestinal (GI) malignancies receiving edoxaban or rivaroxaban compared with LMWHs [5, 6], while apixaban was not associated with an increased risk of bleeding in these patients [7, 8].
In conclusion, there is growing evidence supporting DOACs as an effective and safe treatment option for VTE in selected cancer patients. Results from the present study increase the level of confidence on available evidence supporting the safety and efficacy of DOACs for the treatment of CAT. LMWHs remain the preferred treatment option in cancer patients at high risk of bleeding, such as GI cancer patients, those who require frequent dose adjustments with chemotherapy-induced thrombocytopenia, those who receive ongoing anticancer therapies with potential drug-drug interactions, as well as those with brain metastases. Dedicated tools, such as the ITAC-CME multi-language web-based mobile application downloadable for free at www.itaccme.com will help to improve the care and quality of life of cancer patients and to further decrease the burden of CAT.
Supplementary Information
Additional file 1. Methods, literature search, summary of finding for pooled analysis.
Author contributions
C.F. and D.F contributed equally to study design, data extraction, statistical analysis, and drafted the manuscript. D.S. and P.H.P provided critical revision of the manuscript. J.M.C was responsible for the study conception and provided key revisions to the manuscript. All authors had full access to all study data and take responsibility for their integrity and for the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
The current study was supported by the International Initiative on Thrombosis and Cancer-Continuous Medical Education (ITAC-CME, www.itaccme.com) without funding from industry.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declarations
Ethics approval and consent to participate
The study was conducted according to the principles of the Declaration of Helsinki and registered on PROSPERO (CRD42021266069).
Consent for publication
Not applicable.
Competing interests
C.F. reported receiving honoraria for participating as a speaker at satellite symposia organized by Bayer, Bristol Myers Squibb, and LEO Pharma; D.S. reported receiving compensation for speaking at a Pfizer satellite symposium in 2019, receiving services for editorial work for JAMA, and research funding from the AACR for project GENIE; J.M.C. reported receiving personal fees from Bristol Meyers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. D.F. and P.H.C have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corinne Frere and Dominique Farge have contributed equally to this work.
References
- 1.Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, Brenner B, Kakkar A, Rafii H, Solymoss S, Brilhante D, Monreal M, Bounameaux H, Pabinger I, Douketis J, International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566–81. [DOI] [PubMed]
- 2.Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, Gates LE, Kakkar AK, Levine MN, Liebman HA, Tempero MA, Lyman GH, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Guideline on Cancer-Associated Venous Thromboembolic Disease. Version 1. 2022. Available at https://www.nccn.org/ professionals/physician_gls/pdf/vte.pdf. Accessed March, 2022.
- 5.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang T-F, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR, Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med 2018; 378: 615–24
- 6.Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 7.McBane RD, Wysokinski WE, Le-Rademacher JG, Zemla T, Ashrani A, Tafur A, Perepu U, Anderson D, Gundabolu K, Kuzma C, Perez Botero J, Leon Ferre RA, Henkin S, Lenz CJ, Houghton DE, Vishnu P, Loprinzi CL. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. 2020;18:411–421. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 8.Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, Cohen A, Bauersachs R, Brenner B, Torbicki A, Sueiro MR, Lambert C, Gussoni G, Campanini M, Fontanella A, Vescovo G, Verso M, Caravaggio Investigators. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. 2020;382:1599–607 [DOI] [PubMed]
- 9.Planquette B, Bertoletti L, Charles-Nelson A, Laporte S, Grange C, Mahé I, Pernod G, Elias A, Couturaud F, Falvo N, Sevestre MA, Ray V, Burnod A, Brebion N, Roy P-M, Timar-David M, Aquilanti S, Constans J, Bura-Riviere A, Brisot D, et al. Rivaroxaban versus dalteparin in cancer-associated thromboembolism: a randomized trial. Chest. 2021;S0012-3692(21):04079–4084. doi: 10.1016/j.chest.2021.09.037. [DOI] [PubMed] [Google Scholar]
- 10.Schrag D, Uno H, Rosovsky RPG, Rutherford C, Sanfilippo KM, Villano JL, Drescher MR, Jayaram NH, Holmes CE, Feldman LE, Zattra O, Cronin C, Basch EM, Weiss A, Connors JM. The comparative effectiveness of direct oral anti-coagulants and low molecular weight heparins for prevention of recurrent venous thromboembolism in cancer: The CANVAS pragmatic randomized trial. JCO Wolters Kluwer. 2021;39:12020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Methods, literature search, summary of finding for pooled analysis.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.