Abstract
Objective:
This study compared the benefits of Cognitive Behavioral Therapy for Insomnia (CBT-I) for sleep, mental health symptoms, and quality of life (QoL) in a sample of women veterans with and without probable posttraumatic stress disorder (PTSD) comorbid with insomnia disorder.
Methods:
Seventy-three women veterans (30 with probable PTSD) received a manual-based 5-week CBT-I treatment as part of a behavioral sleep intervention study. Measures were completed at baseline, posttreatment, and 3-month follow-up. Sleep measures included the Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), sleep efficiency measured by actigraphy, and sleep efficiency and total sleep time (TST) measured by sleep diary. Mental health measures included the PTSD Checklist-5 (PCL-5), nightmares per week, Patient Health Questionnaire-9 (PHQ-9), and Generalized Anxiety Disorder-7 (GAD-7) scale. QoL was measured with the Short Form-12 (SF-12). Linear mixed models compared changes over time across groups. Independent t-tests examined PTSD symptom changes in women veterans with probable PTSD.
Results:
Both groups demonstrated improvements across sleep (ps<0.001-0.04), mental health symptoms (ps<0.001), and QoL measures (ps<0.001). The probable PTSD group reported greater improvements in diary sleep efficiency (p=0.046) and nightmares per week (p=0.001) at post-treatment and in TST (p=0.029) and nightmares per week (p=0.006) at follow-up. Most participants with probable PTSD experienced clinically significant reductions in PTSD symptoms at post-treatment (66.7%) and follow-up (60.0%). Significant reductions in intrusive and arousal/reactivity symptoms were maintained at follow-up.
Conclusions:
CBT-I improves insomnia, mental health symptoms and QoL among women veterans, with greater improvement in those with probable PTSD.
Keywords: insomnia, CBT-I, women, veterans, PTSD
Introduction
Cognitive Behavioral Therapy for Insomnia (CBT-I) is the recommended first-line treatment for insomnia disorder, including among individuals with comorbid psychiatric conditions (Edinger et al., 2020; Mysliwiec et al., 2020; Qaseem, Kansagara, Forciea, Cooke, & Denberg, 2016; Wu, Appleman, Salazar, & Ong, 2015). Studies have examined the impact of CBT-I in veterans with posttraumatic stress disorder (PTSD), and the results support the efficacy of CBT-I for improving sleep and mental health symptoms (DeViva et al., 2018; Gellis & Gehrman, 2011; Talbot et al., 2014). However, with the exception of Talbot et al. (2014), these studies included predominantly male patients. No studies have examined the impact of CBT-I on sleep and mental health symptoms in a sample of exclusively women veterans.
Women veterans with insomnia symptoms linked to a traumatic event demonstrate more severe insomnia and mental health symptoms and go without insomnia treatment longer than women veterans who experience insomnia that is not linked to trauma (Carlson et al., 2020). Given these differences between men and women veterans, it is not clear whether findings from previous CBT-I intervention studies can be generalized from men veterans to women veterans.
A quarter of women veterans receiving Veterans Affairs (VA) healthcare meet diagnostic criteria for both insomnia disorder and PTSD (Hughes, Jouldjian, Washington, Alessi, & Martin, 2013; Martin et al., 2017), with poor sleep being one of the most commonly reported symptoms associated with PTSD. Furthermore, PTSD treatment does not often result in insomnia remission, (Zayfert & DeViva, 2004), yet mental health symptoms, particularly depression symptoms (Tsuno, Besset, & Ritchie, 2005; Wagley, Rybarczyk, Nay, Danish, & Lund, 2013), do improve following engagement in CBT-I. Research suggests that insomnia is a risk factor for PTSD development and severity (Miller, Brownlow, & Gehrman, 2020). CBT-I may reduce PTSD symptoms, similar to CBT-I's secondary impact on depression symptoms Wagley et al., 2013); however, this has not been evaluated in women veterans.
This paper reports secondary analysis of participants who received CBT-I within a behavioral sleep intervention trial for women veterans. The primary goals of the current analyses were to: 1) compare changes in sleep from pre- to post-treatment and 3-month follow-up among women veterans with and without probable PTSD, 2) compare changes in other mental health symptoms and QoL from pre- to post-treatment and 3-month follow-up among women veterans with and without probable PTSD, and 3), examine changes in PTSD symptoms from pre- to post-treatment and 3-month follow-up among women veterans with probable PTSD. We hypothesized there would be significant improvement in sleep, mental health and QoL measures from pre- to post-treatment and 3-month follow-up among women veterans with and without probable PTSD. We also hypothesized there would be significant improvement in PTSD symptoms from pre- to post-treatment and 3-month follow-up among women veterans with probable PTSD.
Methods
Recruitment and Participants
The current study is a secondary data analysis of 73 women veterans assigned to the CBT-I arm of a behavioral sleep intervention trial (NCT02076165). The sample for that study was drawn from the population of women veterans who receive care at one large urban VA healthcare system. Women veterans were recruited through a three-step process. Women veterans with insomnia symptoms were identified using a postal survey (sent to women veterans registered with the healthcare system), a telephone screen, and a baseline assessment. Exclusion criteria included: those who self-described as too ill to participate, did not have access to transportation to the medical center, were unable to provide self-consent for participation, or were without stable housing. All other women veterans who endorsed insomnia symptoms, defined as one or more symptoms of poor sleep and daytime consequences at least three times per week, were invited to enroll in the study, and those meeting diagnostic criteria for insomnia disorder were randomized to one of the two study interventions. The diagnosis of insomnia disorder was based on Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5; American Psychiatric Association, 2013) criteria using a medical chart review and information collected during the baseline assessment (see Measures section; e.g., sleep diary, Insomnia Severity Index). Diagnosis was confirmed by both a psychologist specializing in behavioral sleep medicine and board-certified sleep medicine physician.
Procedure
The study was approved by the Institutional Review Board at VA Greater Los Angeles Healthcare System. Participants completed a 9-day baseline assessment consisting of three visits to the study site. Written informed consent was obtained and all of the questionnaires were administered in an interview format to minimize cognitive burden and accommodate sensory/physical limitations. Participants were presented visual response options pertaining to each item as the item was read aloud. Not all research staff who administered questionnaires were clinicians; however, the measure of PTSD symptoms (see below) was always administered by a psychologist or social worker on the research study team. Between Visits 1 and 2, participants completed overnight home sleep apnea testing with the WatchPAT device (Itamar Medical, Inc.). Women with moderate-severe sleep apnea (defined as AHI ≥30 or AHI ≥15 with daytime sleepiness) were excluded and referred for treatment. Between visits 2 and 3, participants wore a wrist actigraph (described below) and completed a daily sleep diary at home for one week. Participants who completed the baseline assessment and met all inclusion/exclusion criteria were randomly assigned to CBT-I (n=75) or a novel Acceptance Commitment Therapy (ACT)-based Insomnia Treatment (n=74; not reported in the current manuscript). Randomization procedures followed the CONSORT criteria for randomized trials (Schulz, Altman, & Moher, 2010).
The CBT-I program included 5 weekly 60-minute sessions with a clinical psychologist trained to deliver the study intervention. Sessions incorporated the key components of CBT-I: sleep restriction, stimulus control, cognitive therapy exercises, targeted sleep hygiene recommendations, and relaxation strategies (see Figure 1). Fidelity ratings based on 10% of the CBT-I session recordings showed high adherence to the treatment protocol (97.5%).
Figure 1.
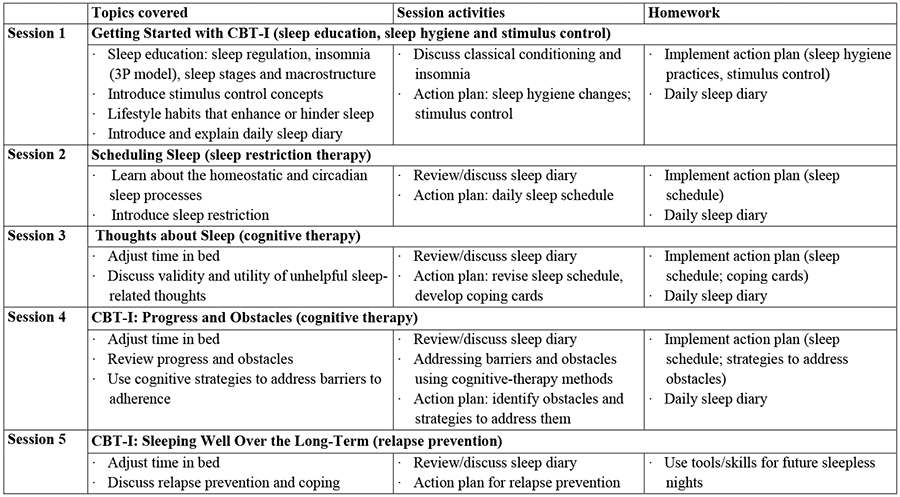
Cognitive Behavioral Therapy for Insomnia (CBT-I) Protocol.
At the end of the final treatment session, participants wore the wrist actigraph, completed the sleep diary for one week, and repeated select measures from the baseline assessment (post-treatment visit). Three months after the last treatment session, participants again wore the wrist actigraph, kept a sleep diary, and repeated select measures from the baseline assessment (3-month follow-up visit).
Measures
Sociodemographic variables.
Age (years) was calculated using each participant’s consent date minus birth date. Participants reported their race/ethnicity, years of education, annual household income, sexual orientation, relationship status, and employment status (see Table 1).
Table 1.
Sociodemographic, sleep, and mental health variables at baseline.
| Total Sample M (SD) or n (%) n=73 |
Without Probable PTSD Group M (SD) or n (%) n=43 |
With Probable PTSD Group M (SD) or n (%) n=30 |
P | |
|---|---|---|---|---|
| Age (Years) | 48.01 (13.46) | 50.23 (14.15) | 44.83 (11.93) | 0.092 |
| Race/Ethnicity † | ||||
| Non-Hispanic/Latina White | 29 (41.10%) | 19 (44.19%) | 10 (36.67%) | 0.47 |
| Non-Hispanic/Latina Black/African American | 26 (36.99%) | 15 (34.88%) | 11 (40.00%) | 1.00 |
| Hispanic/Latina | 17 (23.29%) | 9 (20.93%) | 8 (26.75%) | 0.59 |
| Non-Hispanic/Latina American Indian/Alaska Native | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - |
| Non-Hispanic/Latina Asian American or Asian | 2 (2.74%) | 1 (2.33%) | 1 (3.33%) | 1.00 |
| Non-Hispanic/Latina Native Hawaiian/Pacific Islander | 2 (2.74%) | 2 (4.65%) | 0 (0.00%) | 0.51 |
| Education (Years) | 16.42 (2.87) | 16.42 (2.75) | 16.43 (3.08) | 0.98 |
| Income | 0.057 | |||
| <$10,000 | 4 (5.63%) | 4 (9.52%) | 0 (0.00%) | |
| $10,000-20,000 | 8 (11.27%) | 2 (4.76%) | 6 (20.69%) | |
| $20,000-30,000 | 9 (12.68%) | 4 (9.52%) | 5 (17.24%) | |
| $30,000-40,000 | 9 (12.68%) | 3 (7.14%) | 6 (20.69%) | |
| $40,000-50,000 | 11 (15.59%) | 7 (16.67%) | 4 (13.79%) | |
| $50,000-100,000 | 15 (21.13%) | 11 (26.19%) | 4 (13.79%) | |
| >$100,000 | 15 (21.13%) | 11 (26.19%) | 4 (13.79%) | |
| Sexual Orientation | 0.14 | |||
| Heterosexual/Straight | 52 (76.47%) | 33 (82.50%) | 19 (67.86%) | |
| Gay/Lesbian | 14 (20.59%) | 7 (17.50%) | 7 (25.00%) | |
| Bisexual | 2 (2.94%) | 0 (0.00%) | 2 (7.14%) | |
| Relationship Status | 0.74 | |||
| Married | 30 (44.12%) | 15 (37.50%) | 15 (53.57%) | |
| Divorced | 11 (16.18%) | 7 (17.50%) | 4 (14.29%) | |
| Separated | 4 (5.88%) | 3 (7.50%) | 1 (3.57%) | |
| Widowed | 2 (2.94%) | 1 (2.50%) | 1 (3.57%) | |
| Single/Never Married | 21 (30.88%) | 14 (35.00%) | 7 (25.00%) | |
| Employment | 0.16 | |||
| Unemployed | 34 (46.58%) | 17 (39.53%) | 17 (56.67%) | |
| Employed for wages | 39 (53.42%) | 26 (60.47%) | 13 (43.33%) | |
| Insomnia (ISI Total Score) | 14.52 (5.18) | 12.56 (4.65) | 17.33 (4.61) | <0.001 |
| Sleep Quality (PSQI Total Score) | 10.97 (3.85) | 9.86 (3.42) | 12.57 (3.92) | 0.003 |
| Diary Sleep Efficiency (%) | 78.00 (13.52) | 81.14 (10.82) | 73.34 (15.83) | 0.015 |
| Diary TST (minutes) | 367.49 (81.32 | 376.28 (70.77) | 354.45 (94.67) | 0.27 |
| Objective Sleep Efficiency (%) | 81.41 (7.18) | 82.31 (7.42) | 80.12 (6.72) | 0.20 |
| Nightmares Per Week | 1.88(3.24) | 0.81 (1.55) | 3.40 (4.31) | <0.001 |
| Depression (PHQ-9 Total Score) | 10.12 (5.41) | 7.44 (3.94) | 13.97 (4.91) | <0.001 |
| Anxiety (GAD-7 Total Score) | 10.15 (5.49) | 7.72 (4.76) | 13.63 (4.55) | <0.001 |
| PTSD (PCL-5 Total Score) | 24.41 (20.57) | 9.53 (10.64) | 45.73 (9.40) | <0.001 |
| Mental QoL (SF-12 Mental) | 42.06 (11.34) | 46.24 (11.56) | 36.07 (7.93) | 0.001 |
| Physical QoL (SF-12 Physical) | 42.05 (12.87) | 45.14 (12.30) | 37.63 (12.56) | 0.01 |
Note.
Multiple response options can be selected, and thus percentages do not sum to 100%; ISI=Insomnia Severity Index, PSQI=Pittsburgh Sleep Quality Index, PHQ-9=Patient Health Questionnaire-9, GAD-7=Generalized Anxiety Disorder-7 Scale, PCL-5=PTSD Checklist 5, and SF-12= 12-Item Short Form Health Survey; TST=total sleep time; Lower values indicate poorer sleep efficiency and greater PSQI scores indicate poorer sleep quality; p<0.10 reported as 3 digits after the decimal point, p=0.100-.99 reported as 2 digits after decimal point, and values of .000 and 1.0 reported as <0.001 and >0.99; Valid N varies according to variable, from n=72 to n=73. p values from independent samples t-test for continuous variables; Fisher's exact test for categorical variables.
PTSD symptoms.
Participants were administered the PTSD Checklist for the DSM-5 (PCL-5) and the Life Events Checklist (LEC-5) for the DSM-5 (Weathers et al., 2013a). The PCL-5 is a 20-item self-report measure of PTSD symptoms (Bovin et al., 2016) administered by a study clinician. Individual items were summed to calculate a total score (range 0-80, higher scores indicate greater severity of PTSD symptoms). The PCL-5 contains 4 subscales that correspond to DSM-5 PTSD symptom clusters: intrusive symptoms (Criterion B), avoidance symptoms (Criterion C), negative changes in cognition and mood (Criterion D), and changes in arousal and reactivity (Criterion E; American Psychiatric Association, 2013). The PCL-5 has demonstrated strong internal reliability (α=0.94), test-retest reliability (r=0.82), and convergent validity (r’s=0.74 to 0.85) (Blevins, Weathers, Davis, Witte, & Domino, 2015). A cutoff score of ≥33 is indicative of clinically significant PTSD symptoms. In the current study, participants who endorsed a Criterion A event on the LEC-5 and had a baseline total PCL-5 score of ≥33 were categorized as having probable PTSD (n=30) and participants with either no Criterion A event or a Criterion A event with a baseline total PCL-5 ≤32 were categorized has not having PTSD (n=43). Based on the recommended definition of treatment response by the VA’s National Center for PTSD (National Center for PTSD, n. d.), we used a reduction in PCL-5 score of ≥10 points to define clinically meaningful change (Weathers et al., 2013b).
Insomnia.
The Insomnia Severity Index (ISI) (Bastien, Vallières, & Morin, 2001) is a 7-item instrument using Likert-type scales that measure perceived severity of insomnia symptoms from 0 (not at all) to 4 (very much). Individual items are summed to calculate a total score for each participant (range 0-28, higher scores indicate greater insomnia severity). The ISI correlates well with scores on the Pittsburg Sleep Quality Index (r=0.67) and with sleep diary measures (r’s=0.32-0.91) (Bastien et al., 2001).
Sleep quality.
The Pittsburgh Sleep Quality Index (PSQI) is a widely used 18-item questionnaire that assesses sleep quality and sleep disturbances over the last month (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989). Given the short duration of the study intervention (5 weeks), we used a 1-week time frame for the PSQI. Subscale scores are calculated from individual items, then summed to calculate the PSQI global score (range 0-21, higher scores indicate worse sleep quality). PSQI score greater than 5 has a sensitivity for distinguishing between normal and abnormal sleepers of 89.6% and a specificity of 86.5% (kappa=0.75, P<.001) (Buysse et al., 1989).
Total sleep time (TST).
Participants completed a one-week sleep diary that was based on the Consensus Sleep Diary (Carney et al., 2012). TST was computed by subtracting awake times (time to fall asleep, time awake during the night, and time awake prior to rising in the morning) from the total time in bed. TST represents the calculated mean number of minutes women veterans slept at night across the week of diary monitoring.
Sleep efficiency.
Diary sleep efficiency was calculated by dividing total sleep time (as described above) by total time in bed (minutes from bedtime to rise time) and converting the quotient to a percentage.
Objective sleep efficiency data was calculated from data collected by a wrist actigraph (Actiwatch 2; Philips/Respironics, Bend, OR), which was worn on the nondominant wrist for 7 consecutive days and nights and measured activity levels in 1-minute epochs. Using standard data cleaning processes (Ancoli-Israel et al., 2015), the daily sleep diary nighttime periods were identified and used to estimate sleep parameters, including sleep efficiency. Automated scoring and default algorithms (medium threshold settings) were used in combination with manual scoring of bedtimes and rise times based on sleep diary values. Philips Respironics Actiware software (v. 6.0.8) calculated sleep efficiency as the percentage of epochs scored as sleep within the bedtime and rise time intervals.
Nightmares per week.
A single item from the Disturbing Dream and Nightmare Severity Index (DDNSI), a 5-item version of the Nightmare Frequency Questionnaire (Krakow et al., 2000; Krakow et al., 2002), was used to assess prior week nightmares (i.e. total number of nightmares in the past 7 nights).
Depression.
The Patient Health Questionnaire-9 (PHQ-9) is a 9-item depression module within the Patient Health Questionnaire, a self-report diagnostic instrument for common mental health disorders (Kroenke, Spitzer, Williams, & Löwe, 2010). Individual items are summed to calculate a total score (range 0-27, higher scores indicate greater depression symptoms). The PHQ-9 has strong internal consistency (α=0.87) and good convergent and discriminant validity (Beard, Hsu, Rifkin, Busch, & Björgvinsson, 2016).
Anxiety.
The Generalized Anxiety Disorder-7 (GAD-7) scale is a 7-item self-report measure of anxiety symptoms (Spitzer, Kroenke, Williams, & Löwe, 2006). Individual items are summed to calculate a total score (range 0-21, higher scores indicate greater anxiety symptoms). The GAD-7 has strong internal consistency (α=0.89) and construct validity (Löwe et al., 2008).
Quality of life.
The 12-Item Short Form Health Survey (SF-12) is a self-report measure of health-related quality of life (QoL). The SF-12 consists of two subscales: the physical component summary score and the mental component summary score (range for each subscale is 0-100, higher scores indicate better functioning [Ware et al., 1994]). Test-retest reliability is 0.80 for the physical health component score and 0.76 for the mental health component score (Ware Jr, Kosinski, & Keller, 1996).
Data Analysis
Descriptive statistics were computed for sociodemographic variables, sleep measures, mental health measures, and mental and physical QoL at baseline for the total sample, participants with probable PTSD (PCL-5 total score of ≥33 and a history of an index traumatic event), and participants without probable PTSD (PCL-5 total of <33 or no index traumatic event). In the current study, two participants with a PCL-5 total of score ≥33 were removed from the probable PTSD group because these participants declined to briefly describe an event or described an event that did not correspond to any items on the LEC-5. To examine changes from baseline to post-treatment and baseline to 3-month follow-up in women veterans with and without probable PTSD, linear mixed models were conducted to compare changes in sleep efficiency, TST, ISI, PSQI, nightmares per week, PHQ-9, GAD-7, and SF-12. These outcome variables were selected in advance, based on a theoretical link between these metrics and CBT-I outcomes in the context of PTSD.
Among women veterans with probable PTSD, descriptive statistics were calculated to identify the number of women veterans who experienced a decrease of ≥10 points on the PCL-5 and/or remission of symptoms (PCL-5 total <33) from baseline to post-treatment and baseline to 3-month follow-up. Follow-up analyses examined changes in PTSD symptom clusters in women veterans with probable PTSD. Independent t-tests were performed to compare change scores on the PCL-5 subscales from baseline to post-treatment and 3-month follow-up. For all comparisons, we used p<0.05 to define statistical significance. The analyses were also performed with the sleep items removed from the PHQ-9 (item 3) and the PCL-5 (item 20). No significant differences were observed so, for clarity, here we report the results of the PHQ-9 and the PCL-C with sleep items included. Bivariate correlations were conducted for all outcome variables at baseline (Table S1). See Table S2 for descriptive statistics of outcomes at post-treatment and 3-month follow-up. We computed power to detect a medium-sized effect (Cohen's d=0.50 [Cohen, 1992]; alpha=0.05) for outcome variables. Power to detect a medium-sized effect ranged from 0.56-0.86 at post-treatment and from 0.50-0.82 at 3-month follow-up (Table S3).
Results
Changes in Sleep Measures
Women veterans with and without probable PTSD reported significant improvement in insomnia symptoms (ISI total score) and sleep quality (PSQI total score) at post-treatment and 3-month follow-up (see Table 2). Women veterans with and without probable PTSD reported significant improvements in diary sleep efficiency and TST at post-treatment and 3-month follow-up. Women veterans with probable PTSD reported significantly greater improvements in diary sleep efficiency at post-treatment and TST at 3-month follow-up than those without probable PTSD (see Table 3). Neither women veterans with or without probable PTSD demonstrated significant improvements in objective sleep efficiency (actigraphy) at post-treatment or 3-month follow-up.
Table 2.
Analysis of change scores (baseline to post-treatment, and baseline to 3-month follow-up) among those with and without probable PTSD.
| Change (Baseline to Post-Treatment) | Change (Baseline to 3-Month Follow-up) | |||||||
|---|---|---|---|---|---|---|---|---|
| Without Probable PTSD | With Probable PTSD | Without Probable PTSD | With Probable PTSD | |||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Insomnia (ISI Total Score) | −8.23 (−9.80, −6.66) |
<0.001 | −10.53 (−12.40, −8.67) |
<0.001 | −6.81 (−8.90, −4.73) |
<0.001 | −8.95 (−11.47, −6.44) |
<0.001 |
| Sleep Quality (PSQI Total Score) | −5.63 (−6.90, −4.37) |
<0.001 | −6.37 (−7.86, −4.87) |
<0.001 | −4.37 (−5.83, −2.90) |
<0.001 | −4.94 (−6.71, −2.17) |
<0.001 |
| Diary Sleep Efficiency (%) | 10.84 (7.23, 14.44) |
<0.001 | 16.63 (12.24, 21.02) |
<0.001 | 9.44 (5.75, 13.13) |
<0.001 | 14.89 (10.40, 19.37) |
<0.001 |
| Diary TST (Minutes) | 21.56 (2.38, 40.75) |
0.028 | 34.67 (11.31, 58.03) |
0.004 | 28.01 (7.01, 49.00) |
0.009 | 64.80 (39.31, 90.29) |
<0.001 |
| Objective Sleep Efficiency (%) | 1.41 (−0.55, 3.37) |
0.16 | 2.15 (−0.17, 4.48) |
0.069 | −0.50 (−2.75, 1.76) |
0.66 | 1.42 (−1.32, 4.15) |
0.31 |
| Nightmares Per Week | −0.23 (−0.92, 0.45) |
0.51 | −2.07 (−2.88, −1.25) |
<0.001 | −0.36 (−1.14, 0.43) |
0.37 | −2.08 (−3.02, −1.14) |
<0.001 |
| Depression (PHQ-9 Total Score) | −4.79 (−6.24, −3.33) |
<0.001 | −6.67 (−8.40, −4.94 |
<0.001 | −4.10 (−5.60, −2.61) |
<0.001 | −5.65 (−7.44, −3.86) |
<0.001 |
| Anxiety (GAD-7 Total Score) | −5.26 (−6.92, −3.59) |
<0.001 | −5.5 (−7.48, −3.52) |
<0.001 | −3.86 (5.70, −2.02) |
<0.001 | −4.41 (−6.61, −2.21) |
<0.001 |
| Mental QoL (SF-12 Mental) | 7.00 (3.81, 10.18) |
<0.001 | 6.31 (2.53, 10.09) |
0.001 | 4.32 (1.17, 7.48) |
0.007 | 6.97 (3.15, 10.80) |
<0.001 |
| Physical QoL (SF-12 Physical) | 2.28 (−0.46, 5.49) |
0.10 | 2.01 (−1.23, 5.25) |
0.22 | 2.72 (−0.19, 5.62) |
0.067 | 2.51 (−1.01, 6.03) |
0.16 |
Note. Tabled values show differences in means with 95% confidence interval for the difference in brackets; p<0.10 reported as 3 digits after the decimal point, p=0.100-.99 reported as 2 digits after decimal point, and values of .000 and 1.0 reported as <0.001 and >0.99; Number of observations vary according to variable, from n=65 to n=69.
Table 3.
Change (baseline to post-treatment; and baseline to 3-month follow-up) compared by group.
| Change (Baseline to Post-Treatment) With vs. Without Probable PTSD |
Change (Baseline to 3-Month Follow-Up) With vs. Without Probable PTSD |
|||
|---|---|---|---|---|
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Insomnia (ISI Total Score) | −2.30 (−4.94, 0.14) |
0.064 | −2.14 (−5.41, 1.13) |
0.20 |
| Sleep Quality (PSQI Total Score) | −0.73 (−2.69, 1.23) |
0.47 | −0.57 (−2.87, 1.73) |
0.63 |
| Diary Sleep Efficiency (%) | 5.79 (0.11, 11.47) |
0.046 | 5.54 (−0.36, 11.26) |
0.066 |
| Diary TST (Minutes) | 13.11 (−17.12, 43.33) |
0.40 | 36.79 (3.77, 69.81) |
0.029 |
| Objective Sleep Efficiency (%) | 0.74 (−2.29, 3.78) |
0.63 | 1.91 (−1.63, 5.46) |
0.29 |
| Nightmares Per Week | −1.83 (−2.90, −0.77) |
0.001 | −1.72 (−2.95, −0.49) |
0.006 |
| Depression (PHQ-9 Total Score) | −1.88 (−4.14, 0.38) |
0.10 | −1.55 (−3.87, 0.78) |
0.19 |
| Anxiety (GAD-7 Total Score) | −0.24 | 0.85 (−2.83, 2.35) |
−0.54 (−3.41, 2.32) |
0.71 |
| Mental QoL (SF-12 Mental) | −0.69 (−5.63, 4.25) |
0.79 | 2.65 (−2.31, 7.60) |
0.30 |
| Physical QoL (SF-12 Physical) | −0.27 (−4.50, 3.97) |
0.90 | −0.21 (−4.78, 4.35) |
0.93 |
Note. Tabled values show differences in means with 95% confidence interval for the difference in brackets; p<0.10 reported as 3 digits after the decimal point, p=0.100-.99 reported as 2 digits after decimal point, and values of .000 and 1.0 reported as <0.001 and >0.99.
Changes in Mental Health Symptoms and QoL
Participants with probable PTSD reported significantly greater reductions in nightmares per week at post-treatment and 3-month follow-up than those without probable PTSD (see Table 3). Women veterans with and without probable PTSD reported significant improvements in depression symptoms (PHQ-9 total score), anxiety symptoms (GAD-7 total score), and mental health QoL (SF-12 mental health component summary) at post-treatment and 3-month follow-up. Neither women with or without probable PTSD symptoms reported significant improvement in physical health QoL (SF-12 physical health component summary) at post-treatment or 3-month follow-up.
Changes in PTSD Symptoms among Women Veterans with Probable PTSD
Among the 30 women veterans with probable PTSD at baseline, 20 (66.7%) reported a clinically significant reduction in PTSD symptoms (≥10-point reduction on PCL-5; see Table 4) and 18 (60.0%) demonstrated PTSD symptom remission (PCL-5 total score <33) at post-treatment, and 17 (56.7%) demonstrated both a clinically significant reduction in and remission of PTSD symptoms at post-treatment.
Table 4.
PTSD symptom cluster changes among women veterans with probable PTSD from baseline to post-treatment and baseline to 3-month follow-up.
| Baseline M |
Post- Treatment M |
P | 3-Month Follow-up M |
P | |
|---|---|---|---|---|---|
| PCL-5 Subscale: Items 1-5 Intrusion Symptoms (PTSD Criterion B) Score Range: 0-20 |
12.13 | 8.90 | <0.001 | 9.00 | <0.001 |
| PCL-5 Subscale: Items 6-7 Avoidance Symptoms (PTSD Criterion C) Score Range: 0-8 |
5.00 | 3.67 | 0.011 | 3.77 | 0.079 |
| PCL-5 Subscale: Items 8-14 Negative changes to Cognition and Mood (PTSD Criterion D) Score Range: 0-28 |
15.17 | 10.17 | <0.001 | 11.65 | 0.066 |
| PCL-5 Subscale: Items 15-20 Changes to Arousal and Reactivity (PTSD Criterion E) Score Range: 0-24 |
13.43 | 7.07 | <0.001 | 7.58 | <0.001 |
Note. p<0.10 reported as 3 digits after the decimal point, p=0.100-.99 reported as 2 digits after decimal point, and values of .000 and 1.0 reported as <0.001 and >0.99; Number of observations vary according to variable, from n=26 to n=30.
Among the 26 women veterans with probable PTSD who completed the 3-month follow-up assessment, 16 (61.5%) reported a clinically significant reduction in PTSD symptoms, 16 (61.5%) demonstrated remission of PTSD symptoms, and 15 (57.7%) demonstrated both a clinically significant reduction in and remission of PTSD symptoms at 3-month follow-up. This change was accounted for by significant improvements in intrusive symptoms, avoidance symptoms, negative changes in cognition and mood, and changes in arousal and reactivity at post-treatment. These significant improvements in intrusive symptoms and changes in arousal and reactivity were maintained at 3-month follow-up.
Discussion
We found that women veterans with and without probable PTSD who underwent CBT-I showed improvements in insomnia, sleep quality, and diary sleep efficiency and TST from baseline to post-treatment and 3-month follow-up. This builds on previous studies, showing that CBT-I improves sleep among patients with comorbid psychiatric conditions, including PTSD (DeViva et al., 2018; Talbot et al., 2014). Our findings show that women veterans with comorbid insomnia and PTSD experience sleep improvements with CBT-I, and while PTSD is sometimes viewed as a potential barrier to achieving benefits from insomnia treatment, in our study, women veterans with probable PTSD experienced significantly greater improvements in diary sleep efficiency at post-treatment and TST at 3-month follow-up compared to women veterans without probable PTSD. Neither women with nor without probable PTSD demonstrated significant improvement in objective sleep efficiency. The discrepancy between subjective and objective sleep efficiency measures has been repeatedly observed across a variety of populations (Campanini et al., 2017; Short, Gradisar, Lack, Wright, & Carskadon, 2012). The finding that women veterans experienced benefits in terms of increased TST is different from many other studies and meta-analytic reviews of CBT-I that do not show increased TST (Wu et al., 2015). The large change in TST in the women with PTSD was also remarkable; the average increase of over one hour is clinically significant and should be replicated in future research.
While improvements in sleep measures were expected, findings also demonstrated the positive impact of CBT-I on mental health symptoms and QoL. Women veterans with probable PTSD reported significant reductions in nightmares per week at post-treatment and 3-month follow-up relative to women veterans without probable PTSD, although nightmares per week was low in women without PTSD at baseline. Reduced nightmares per week may be related to increased total sleep time, as previous studies demonstrate that nightmares are more frequent when patients are sleep deprived (Creamer, Brock, Matsangas, Motamedi, & Mysliwiec, 2018; Tamanna, Parker, Lyons, & Ullah, 2014). We also found significant improvements in anxiety and depression symptoms, which builds on previous studies showing CBT-I improves mood symptoms in people without PTSD (Cunningham & Shapiro, 2018). Women veterans, regardless of probable PTSD status, reported improvements in mental health QoL at post-treatment and 3-month follow-up. These findings are consistent with research demonstrating the benefits of CBT-I on health-related QoL (Van Houdenhove, Buyse, Gabriëls, & Van den Bergh, 2011). It is unclear why improvement in physical health QoL was not observed. Consistent with prior research comparing individuals with high and low levels of PTSD symptoms (Pacella, Hruska, & Delahanty, 2013), we found that women veterans in the probable PTSD group reported poorer physical health QoL compared to those without probable PTSD at baseline; however, we did not observe changes in physical health QoL with CBT-I.
Another aim of the proposed study was to examine the impact of CBT-I on PTSD symptoms. We found that most women veterans with probable PTSD reported clinically significant reductions in PTSD symptoms (≥10 point-reduction on the PCL-5) at post-treatment and 3-month follow-up, suggesting meaningful clinical change. All PTSD symptoms clusters demonstrated significant improvements at post-treatment, but only improvements in intrusive symptoms and changes in arousal and reactivity were maintained at 3-month follow-up. Scheduled worry time (cognitive therapy strategy) and relaxation strategies are utilized in CBT-I (Pigeon, 2010) and may contribute directly to improvements in intrusive thoughts and hyperarousal. Additionally, intrusive symptoms include nightmares, which (as noted above) decreased significantly in women veterans with probable PTSD. The absence of a significant effect on avoidance symptoms at 3-month follow-up is not entirely unexpected. With the exception of behavioral experiments, CBT-I did not directly target avoidance symptoms. Patients with PTSD may delay falling asleep at night in an effort to avoid nightmares (Aurora et al., 2010). Strategies in CBT-I may be used to limit this tendency; however, the current CBT-I protocol did not specifically target nightmares and there are no strategies in CBT-I that specifically target other PTSD avoidance symptoms. The absence of a significant effect on cognition and mood at 3-month follow-up was unexpected, given the large literature demonstrating the effects of CBT-I on depression symptoms (Cunningham & Shapiro, 2018) and our current finding that PHQ-9 scores decreased at post-treatment and 3-month follow-up.
That being said, it is important to note that CBT-I targets problematic thoughts related to sleep, not problematic thoughts related to trauma, nor does CBT-I include techniques to reduce avoidance such as exposure or behavioral activation. While adaptive responses to sleep-related thoughts may generalize to cognitions more broadly, these techniques may not necessarily impact trauma-specific cognitions. Nevertheless, CBT-I reduced depression and PTSD hyperarousal symptoms. There is clear overlap between these disorders (e.g., concentration difficulties, irritability). Future studies should examine the specific symptom changes that may account for the positive impact of CBT-I in patients with comorbid psychiatric conditions.
Strengths and Limitations
To our knowledge, this was the first study to examine the impact of CBT-I in a sample of exclusively women veterans. This study included an established 5-session CBT-I treatment protocol and a comprehensive assessment battery of well-established measures and methods. While this study possessed multiple strengths, several limitations should be noted. This was a secondary analysis of a larger behavioral sleep intervention study and current findings may not generalize beyond women veterans. Medical record review revealed that 17 participants with probable PTSD had mental health encounters in the year prior to the study. The following year, 10 participants had fewer and 7 had more mental health encounters, suggesting findings are not simply the result of concurrent treatment and/or motivation to engage in treatment. Nevertheless, future studies should examine the impact of concurrent/sequenced treatment engagement on CBT-I outcomes. Information regarding traumatic events and PTSD symptoms was limited to the LEC-5 and PCL-5, which are not sufficient to confirm a diagnosis of PTSD. Thus, we have limited our group description to "probable PTSD." It is possible that the PCL-5 measured general distress in the current study and greater mental health symptom reductions may be partially attributable to range restriction. Regardless, findings demonstrate patients with high distress benefit from CBT-I at least as much as patients with lower distress.
The current study was exploratory in nature and, as such, analyses did not include alpha adjustments for multiple comparisons; therefore, it is possible that chance might explain one particular finding, but chance alone is unlikely to explain the overall pattern of results showing significant benefits across a range of outcomes. Additionally, PCL-5 cutoff scores were examined to demonstrate that changes in PTSD symptoms were clinically important. Future studies should incorporate comprehensive PTSD assessment and explore the temporal relationships among changes in insomnia and other symptoms and the mechanisms of change that may account for PTSD symptom improvement following CBT-I.
Implications for Clinical Practice
Current findings suggest that probable PTSD should not discourage providers from initiating CBT-I among women veterans with insomnia disorder. Presenting evidence-based treatment options for both PTSD and insomnia and supporting patient preferences is consistent with a shared decision-making approach (Elwyn et al., 2012). Previous research has demonstrated that when veterans are presented with descriptions of evidence-based treatments for PTSD and insomnia, veterans who screen positive for insomnia and PTSD report greater willingness to seek insomnia versus PTSD treatment (Gutner, Pedersen, & Drummond, 2018). There is also a growing body of literature highlighting the importance of trauma-informed care (Machtinger, Cuca, Khanna, Rose, & Kimberg, 2015). While trauma-focused treatments specifically target PTSD symptoms, trauma-informed care involves tailoring existing interventions to meet the unique needs of patients who have experienced trauma (Kelly, Boyd, Valente, & Czekanski, 2014). Current findings suggest avoidance symptoms and trauma-related cognitions may not be adequately addressed by CBT-I. Future research should develop and test trauma-informed enhancements to CBT-I to better meet the needs of patients with comorbid PTSD.
Supplementary Material
Funding Statement:
This project was funded by VA/HSR&D IIR-HX002300 (PI: Martin), NIH/NHLBI K24HL143055 (PI: Martin). Dr. Martin's effort was also funded by a VA HSR&D Senior Research Career Scientist Award (Project # RCS 20-191) and NIH/NHLBI K24 HL143055. This project was also supported by VA/HSR&D CSHIIP LIP 65-74 (PI: Carlson). Dr. Carlson and Dr. Kelly were supported by the VA Office of Academic Affiliations through the Advanced Fellowship Programs in HSR&D and Women’s Health and the Advanced Fellowship Program in Geriatrics. Support was also provided by the VA Greater Los Angeles Geriatric Research, Education and Clinical Center. Dr. Yano’s effort is funded by a VA HSR&D Senior Research Career Scientist Award (Project # RCS 05-195).
Footnotes
Disclosure: The authors have no conflicts to disclose. The views expressed in this study are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
References
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, … Taylor DJ (2015). The SBSM guide to actigraphy monitoring: Clinical and research applications. Behavioral Sleep Medicine, 13(sup1), S4–S38. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. [Google Scholar]
- Aurora R, Zak R, Auerbach S, Casey K, Chowduri S, Krippot A, … Bista S (2010). Best practice guide for the treatment of nightmare disorder in adults. Journal of Clinical Sleep Medicine, 6(4), 389–401. [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. [DOI] [PubMed] [Google Scholar]
- Beard C, Hsu K, Rifkin L, Busch A, & Björgvinsson T (2016). Validation of the PHQ-9 in a psychiatric sample. Journal of Affective Disorders, 193, 267–273. [DOI] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, & Keane TM (2016). Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychological Assessment, 28(11), 1379. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Campanini MZ, Lopez-Garcia E, Rodríguez-Artalejo F, González AD, Andrade SM, & Mesas AE (2017). Agreement between sleep diary and actigraphy in a highly educated Brazilian population. Sleep Medicine, 35, 27–34. [DOI] [PubMed] [Google Scholar]
- Carlson GC, Kelly MR, Grinberg AM, Mitchell MN, McGowan S, Culver NC, Kay M, Alessi CA, Washington DL, Yano EM, Martin JL (2020). Insomnia precipitating events among Women Veterans: The impact of traumatic and nontraumatic events on sleep and mental health symptoms. Behavioral Sleep Medicine, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep, 35(2), 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 1551–159. [DOI] [PubMed] [Google Scholar]
- Creamer JL, Brock MS, Matsangas P, Motamedi V, & Mysliwiec V (2018). Nightmares in United States military personnel with sleep disturbances. Journal of Clinical Sleep Medicine, 14(3), 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JE, & Shapiro CM (2018). Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. Journal of Psychosomatic Research, 106, 1–12. [DOI] [PubMed] [Google Scholar]
- DeViva JC, McCarthy E, Bieu RK, Santoro GM, Rinaldi A, Gehrman P, & Kulas J (2018). Group cognitive-behavioral therapy for insomnia delivered to veterans with posttraumatic stress disorder receiving residential treatment is associated with improvements in sleep independent of changes in posttraumatic stress disorder. Traumatology, 24(4), 293–300. [Google Scholar]
- Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, … Martin JL (2020). Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine systematic review, meta-analysis and GRADE assessment. Journal of Clinical Sleep Medicine, 0(0), jcsm. 8988. doi:doi: 10.5664/jcsm.8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, & Quack D (2010). Emotion regulation difficulties in trauma survivors: The role of trauma type and PTSD symptom severity. Behavior Therapy, 41(4), 587–598. [DOI] [PubMed] [Google Scholar]
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, … Rollnick S (2012). Shared decision making: A model for clinical practice. Journal of General Internal Medicine, 27(10), 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellis LA, & Gehrman PR (2011). Cognitive behavioral treatment for insomnia in veterans with long-standing posttraumatic stress disorder: A pilot study. Journal of Aggression, Maltreatment & Trauma, 20(8), 904–916. [Google Scholar]
- Gutner CA, Pedersen ER, & Drummond SP (2018). Going direct to the consumer: Examining treatment preferences for veterans with insomnia, PTSD, and depression. Psychiatry Research, 263, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Jouldjian S, Washington DL, Alessi CA, & Martin JL (2013). Insomnia and symptoms of post-traumatic stress disorder among women veterans. Behavioral Sleep Medicine, 11(4), 258–274. [DOI] [PubMed] [Google Scholar]
- Kelly U, Boyd MA, Valente SM, & Czekanski E (2014). Trauma-informed care: Keeping mental health settings safe for veterans. Issues in Mental Health Nursing, 35(6), 413–419. [DOI] [PubMed] [Google Scholar]
- Krakow B, Hollifield M, Schrader R, Koss M, Tandberg D, Lauriello J, … Edmond T (2000). A controlled study of imagery rehearsal for chronic nightmares in sexual assault survivors with PTSD: A preliminary report. Journal of Traumatic Stress, 13(4), 589–609. [DOI] [PubMed] [Google Scholar]
- Krakow B, Schrader R, Tandberg D, Hollifield M, Koss MP, Yau CL, & Cheng DT (2002). Nightmare frequency in sexual assault survivors with PTSD. Journal of Anxiety Disorders, 16(2), 175–190. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, & Löwe B (2010). The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. General Hospital Psychiatry, 32(4), 345–359. [DOI] [PubMed] [Google Scholar]
- Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, & Herzberg PY (2008). Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical Care, 266–274. [DOI] [PubMed] [Google Scholar]
- Machtinger EL, Cuca YP, Khanna N, Rose CD, & Kimberg LS (2015). From treatment to healing: The promise of trauma-informed primary care. Women's Health Issues, 25(3), 193–197. [DOI] [PubMed] [Google Scholar]
- Martin JL, Schweizer CA, Hughes JM, Fung CH, Dzierzewski JM, Washington DL, … Josephson KR (2017). Estimated prevalence of insomnia among women veterans: Results of a postal survey. Women's Health Issues, 27(3), 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Brownlow JA, & Gehrman PR (2020). Sleep in PTSD: Treatment approaches and outcomes. Current Opinion in Psychology, 34, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysliwiec V, Martin JL, Ulmer CS, Chowdhuri S, Brock MS, Spevak C, & Sall J (2020). The Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea: Synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Annals of Internal Medicine, 172(5), 325–336. [DOI] [PubMed] [Google Scholar]
- National Center for Postraumatic Stress Disorder (n. d.). PTSD Checklist for DSM-5 (PCL-5). Retrieved from https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsdchecklist.asp
- Pacella ML, Hruska B, & Delahanty DL (2013). The physical health consequences of PTSD and PTSD symptoms: A meta-analytic review. Journal of Anxiety Disorders, 27(1), 33–46. [DOI] [PubMed] [Google Scholar]
- Pigeon WR (2010). Treatment of adult insomnia with cognitive–behavioral therapy. Journal of Clinical Psychology, 66(11), 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, & Denberg TD (2016). Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 165(2), 125–133. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, & Moher D (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine, 152(11), 726–732. [DOI] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright H, & Carskadon MA (2012). The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Medicine, 13(4), 378–384. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Suris A, & Lind L (2008). Military sexual trauma: A review of prevalence and associated health consequences in veterans. Trauma, Violence, & Abuse, 9(4), 250–269. [DOI] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, … Ruoff L (2014). Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: A randomized controlled trial. Sleep, 37(2), 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanna S, Parker JD, Lyons J, & Ullah M (2014). The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). Journal of Clinical Sleep Medicine, 10(6), 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2008). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992 [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, & Ritchie K (2005). Sleep and depression. The Journal of Clinical Psychiatry, 66(10), 1254–1269. [DOI] [PubMed] [Google Scholar]
- Van Houdenhove L, Buyse B, Gabriëls L, & Van den Bergh O (2011). Treating primary insomnia: Clinical effectiveness and predictors of outcomes on sleep, daytime function and health-related quality of Life. Journal of Clinical Psychology in Medical Settings, 18(3), 312–321. [DOI] [PubMed] [Google Scholar]
- Wagley JN, Rybarczyk B, Nay WT, Danish S, & Lund HG (2013). Effectiveness of abbreviated CBT for insomnia in psychiatric outpatients: Sleep and depression outcomes. Journal of Clinical Psychology, 69(10), 1043–1055. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr, Kosinski M, & Keller SD (1996). A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care, 220–233. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013a). The Life Events Checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD. Retrieved from www.ptsd.va.gov [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP (2013b). PTSD Checklist for DSM-5 (PCL-5). Retrieved from www.ptsd.va.gov. [Google Scholar]
- Wu JQ, Appleman ER, Salazar RD, & Ong JC (2015). Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: A meta-analysis. JAMA Internal Medicine, 175(9), 1461–1472. [DOI] [PubMed] [Google Scholar]
- Zayfert C, & DeViva JC (2004). Residual insomnia following cognitive behavioral therapy for PTSD. Journal of Traumatic Stress: Official Publication of The International Society for Traumatic Stress Studies, 17(1), 69–73. [DOI] [PubMed] [Google Scholar]
- Zinzow HM, Grubaugh AL, Monnier J, Suffoletta-Maierle S, & Frueh BC (2007). Trauma among female veterans: A critical review. Trauma, Violence, & Abuse, 8(4), 384–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


