Abstract
Objective
COVID-19 is an infectious disease that has become pandemic with a high mortality rate. This study aims to provide new insight into the relations between SARS-CoV-2 and the Endocrine system.
Materials and Methods
In this cross-sectional study, we have hospitalized 60 patients with a positive SARA-CoV-2 PCR test. The information of complete blood count and endocrine hormones was obtained when the patients were admitted to the hospital or for a maximum of 4 days onset the hospitalization.
Results
Of 60 patients with COVID-19, forty-four (73.33%) had at least one abnormality mean item >×3. In total, 26 (43.33%), 21 (35%), 18 (30%), 13 (21.67%), 31 (51.67%), 12 (20%), 30 (50%), 25 (41.67%) patients having estradiol, follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, progesterone, testosterone, cortisol and thyroid stimulating hormone (TSH) abnormal test results, respectively. There was no change in creatinine levels. FSH has shown drastic changes in both sexes’ intensity (F: 769, P<0.0001). Although TSH had many abnormalities in women, analysis has shown no significant P value (P=0.4558). Furthermore, prolactin and testosterone mean level in men and the estradiol mean level in women have shown no significant P value (P=0.2077, P=0.1446, P=0.1351, respectively).
Conclusion
Results suggest that COVID-19 affects directly or non-directly glands and related hormones.
Keywords: COVID-19, Endocrine System, SARS-CoV-2
Introduction
Coronaviruses are a common virus within animals and humans that cause multiple system infections in both species, predominantly humans respiratory tract infections, such as severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS). Coronaviruses also cause enteric, hepatic, and neurologic diseases (1-3). Since late December 2019, a newfound virus has become prevalent in Wuhan, China, previously described as (2019-nCoV), which subsequently affected all countries worldwide (4, 5).
The worldwide health organization labeled the COVID-19 as a pandemic worldwide that has led to thousands of deaths, albeit most of the cases have mild symptoms, more severe symptoms have caused respiratory failure, septic shock, and multiple organ failure dysfunctions (6). It appears that 93.1% of the sequence identity of the spike gene of the virus is relative to the RaTG13 of Bat coronaviruses. However, the other SARSCoV and SSRS-CoVs have less than 80% manifested sequence identity (7). A homometric spike glycoprotein with S1 and S2 subunits in each spike monomer operates as cellular receptors to bind the hostage. A cascade of events occurs by binding the receptors leading to the fusion between viral membrane and cell. Cryo-EM studies of the SARS-CoV spike with ACE2 cell receptor have shown that glycan-RBD (a hexapeptide in the receptorbinding domain) enforces the separation of the S1 with ACE2 as a necessary action for membrane fusion through actuating the S2 subunit from mutable perfusion to a more post-fusion state (8-13). A required regularization of the renin-angiotensin system, named angiotensin-converting enzyme 2 (ACE2), has been known as a homolog of the metalloprotease angiotensin-converting enzyme ACE (14, 15).
The expression and presence of the ACE2 within the internal organs may assume to be the potential path of the entrance of the COVID-19 virus. Highly enriched expression and distribution of ACE2 at the surface of type 2 alveolar cells of the lung, oral mucosa, tongue, stratified and upper esophagus epithelial cells, colon and ileum absorptive enterocytes, myocardial cells, cholangiocytes, proximal tubule cells of the kidney, bladder urothelial cells, seminiferous duct cells in the testis and leydig cells have been detected (16-21). These uncoverings demonstrate that organs with high expression of ACE2 receptors are exposed to the high risk of 2019-nCoV infection (18). Moreover, hormonal disorders can amplify cytokine storms and organ failure (22).
However, the data of other organs has not been comprehensively analyzed. Hence, this study aims to report the hormonal sex, adrenal, ovary, hypothalamus, thyroid, and pituitary gland function and their parameters in patients with COVID-19 hospitalized to the Bagher-AlOlum hospital in Ahar, Iran. More potential therapies would be recognized with a better concentrate on pathogenesis and affected human physiology, which might intercept glands failure in patients with SARS-CoV-2.
Study criteria and design
We conducted a cross-sectional study of patients admitted to the Bagher-Al-Olum Hospital of Ahar. As the WHO interim guidance (23). From November 12, 2020, to December 18, 2020, 85 infected patients were identified; however, only 60 were placed in this study. All of the patients were in reproduction age. Those who had abnormal CT-Scan, and CBC and Diff test results with a signed consent form, from admission to November 12, 2020, were enrolled in the study. Also, we extracted 60 normal laboratory test results from the hospital database (HIS) to set as the control group for reaching an accurate comparative study. Women with menstrual disorders and patients using medications that may affect hormonal results were excluded from the study. This study has taken approval from Tabriz University of Medical Sciences Ethics Committee, Iran (IR.TBZMED.REC.1399.129).
Confirmation and severity of SARS-CoV-2
Hospitalization of patients was based on confirmation of positive real-time polymerase chain reaction (PCR), chest CT-Scan, and abnormal CBC and Diff tests. All patients stated no history of glands disease, and their odd results were likely due to SARS-CoV-2 disease. Nasopharyngeal and throat specimens were collected by swab from dubious patients to evaluate their E gene expression by RT-PCR. According to the Infectious Diseases Society of America/ American Thoracic Society (IDSA/ATS) guideline (24), Pneumonia was defined. Combinations of azithromycin, Hydroxychloroquine, Kaletra, Vitamin C, and Interferon 1-B were applied for treatment.
Statistical analysis
GraphPad Prism version 8.4.3 (San Diego, California, USA) was used for statistical analysis. Median for continuous variables were compared using independent group t tests when the data were normally distributed; confidence interval (CI) and Significance were set as a P<0.05 for each group; otherwise, the Mann-Whitney U test was used. Also, an ANOVA test was performed to compare the mean among several independent groups, and F statistics were reported for the ratio of changes Between Groups to Within Groups.
Results
Clinical characteristic of patients with COVID-19
Blood samples due to precise analysis of the cortisol were collected at 8 AM. Of 60 patients with COVID-19, 26 (43.33%) had abnormal estradiol test, 21 (35%) had abnormal follicle stimulating hormone (FSH) test, 18 (30%) had abnormal luteinizing hormone (LH) test, 13 (21.67%) had abnormal prolactin test, 31 (51.67%) had abnormal progesterone test, 12 (20%) had abnormal testosterone test, 30 (50%) had abnormal cortisol test, 25 (41.67%) had abnormal thyroid stimulating hormone (TSH) test, and no changing in creatinine levels was observed. Twenty (33.33%) extended intensive disease, and 40 (66.67%) had the moderate disease during confinement.
Clinical characteristics of patients
At admission, about 51 (85%) of the patients had abnormal CBC and Diff test results, 35 (58.33%) fever, cough 45 (75%), body aches 49 (81.67%), headache 49 (81.67%), insomnia 27 (45%), dysgeusia 25 (41.67%), ageusia 18 (30%) in both sexes. Almost all 27 women had menstrual disorder such, polymenorrhea 12 (40%), oligomenorrhea 4 (13.33%), metrorrhagia 7 (23.33%), menometrorrhagia 4 (13.33%), and 3 (10%) had no change in their menstrual hygiene.
Results by statistical analysis
The average age of all patients with COVID-19 was almost 41 years old, 30 (50%) were male, and 30 (50%) were female. In total, for 60 patients, laboratory results have shown that 26 (43.33%), 21 (35%), 18 (30%), 13 (21.67%), 31 (51.67%), 12 (20%), 30 (50%), 25 (41.67%) patients having estradiol, FSH, LH, prolactin, progesterone, testosterone, cortisol and TSH abnormal test results, respectively. Of 60 patients, forty-four (73.33%) had at least one abnormality mean Item >×3, of which 26 were women, and 18 were confined in the intensive care unit (ICU) (Fig .1).
Fig.1.
Flowchart of the study. FSH; Follicle stimulating hormone, LH; Luteinizing hormone, and TSH; Thyroid stimulating hormone.
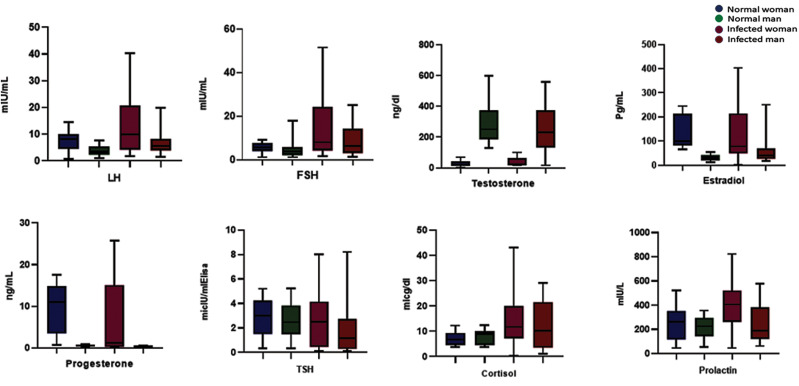
Table 1 shows the P value, minimum, maximum, and mean for all hormones in males and females. The serum level of FSH value in Infected women was >2× of normal control women (CI: 4.422-15.83, P=0.0008). Likewise, males had almost >×2 FSH mean levels than healthy control males (CI: 1.691-7.891, P=0.0030), and women had ×1-2 FSH elevated mean levels than infected men (F3,116: 9.769, P<0.0001). The average LH mean value for both infected women and men were almost ×2 normal levels (female CI: 1.575-9.993, P=0.0079, male CI: 1.376-5.076, P=0.0009), also males had almost > ×1 rose (F3,116: 12.42, P<0.0001) testosterone in both infected sex had a slight change. The mean of the infected men has shown ×1-2 decreased levels compared with normal control men (CI:-127.0-19.07, P=0.1446). Contrariwise, the infected women have shown ×1-2 increased levels of testosterone (CI: 3.653-29.73, P=0.0130). Females had >×1 changed testosterone mean level value than males (F3.116: 51.75, P<0.0001).
Table 1.
Hypothalamus-pituitary axis hormones range between normal, and infected patients
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormones | Women | Men | |||||||
| Normal | Infected | Normal range | P value | Normal | Infected | Normal range | P value | ||
|
| |||||||||
| FSH | Min | 1.2 | 1.5 | 1.2-9.0 mIU/mL | 0.0008 | 1.18 | 1.26 | 0.7-11.1 | 0.0030 |
| Max | 9 | 51.5 | 17.92 | 25 | |||||
| Median | 5.750 | 8.005 | 3.820 | 6.425 | |||||
| LH | Min | 0.8 | 1.84 | 0-14.7 mIU/mL | 0.0079 | 1 | 1.6 | 0.8-7.6 | 0.0009 |
| Max | 14.6 | 40.2 | 7.58 | 19.8 | |||||
| Median | 8.100 | 9.850 | 3.525 | 5.685 | |||||
| Testosterone | Min | 1.01 | 17.5 | ND – 73 Ng/dl | 0.013 | 130 | 16.6 | 20-49: 72 –853 | 0.1446 |
| Max | 70.1 | 100.7 | 600 | 560 | >50: 129 – 767 | ||||
| Median | 20.50 | 22.90 | 251.0 | 232.0 | |||||
| Progesterone | Min | 0.73 | 0.1 | 0.72 – 17.8 Ng/mL | 0.0299 | 0.27 | 0.80 | 0.27 – 0.90 | <0.0001 |
| Max | 17.5 | 25.8 | 0.9 | 0.57 | |||||
| Median | 11.02 | 1.175 | 0.5400 | 0.2550 | |||||
| Estradiol | Min | 65.5 | 0.28 | 72 – 246 Pg/mL | 0.1351 | 12 | 18.3 | 0-56 | 0.0124 |
| Max | 245 | 403 | 55 | 250 | |||||
| Median | 99.00 | 78.15 | 31.57 | 39.85 | |||||
| Prolactin | Min | 45 | 45 | 40 – 530 mIU/L | 0.0013 | 55 | 62 | 53 – 360 | 0.2077 |
| Max | 522 | 823 | 356 | 578 | |||||
| Median | 261.5 | 407.0 | 224.5 | 187.5 | |||||
| Cortisol | Min | 3.7 | 0.19 | 3.7- 19.4 Micg/dl | 0.0016 | 3.7 | 1 | 3.7- 19.4 | 0.0087 |
| Max | 12.25 | 43 | 12.4 | 29 | |||||
| Median | 6.735 | 11.70 | 8.90 | 10.23 | |||||
| TSH | Min | 0.33 | 0.1 | 0.32 – 5.2 micIU/mlElisa | 0.4558 | 0.32 | 0.1 | 0.32 – 5.2 | 0.0104 |
| Max | 5.2 | 8 | 5.23 | 8.2 | |||||
| Median | 3.010 | 2.500 | 2.465 | 1.160 | |||||
|
| |||||||||
FSH; Follicle stimulating hormone, LH; Luteinizing hormone, TSH; Thyroid stimulating hormone, Min; Minimum, and Max; Maximum.
Although progesterone mean levels of women dropped ×1-2 compared with the Healthy control group (CI:-7.140 to-0.3000, P=0.0299), males have experienced ×2-3 decreased levels mean value of progesterone (CI:-0.3923 to-0.2037, P<0.0001). The decreased mean levels of males were ×1-2 females (F3,116: 24.21, P<0.0001). Prolactin mean levels were elevated in both sexes, infected females experienced prolactin (PRL) levels ×1-2 Normal control group (CI: 58.03-227.9, P=0.0013). Likewise, males had ×1-2 elevated PRL mean level than the healthy control group (CI:-24.42-109.9, P=0.2077). Women had ×1-1.5 enhancement PRL mean level than men (F3,116: 9.134, P<0.0001). Furthermore, females had a minor reduction in their estradiol mean levels (CI:-55.03-6.800, P=0.1351), but Infected men experienced ×1-2 Increased mean levels (CI: 5.980-47.41, P=0.0124). Males had ×1-2 raised Estradiol mean levels than infected women (F3,116: 14.37, P<0.0001). Both sexes experienced ×1-2 ascent mean levels of cortisol (female CI: 2.423 to 9.833, P=0.0016, male CI: 1.260 to 8.303, P=0.0087). Women had >×1 increased mean level cortisol than infected men (F3,116: 6.177, P=0.0006). TSH for both sexes had a slight reduction. Female mean level TSH in women has dropped almost ×1 (CI:-1.230-0.8000, P=0.4558). Men have experienced ×1-2 dropped mean level than normal control (CI:-1.910 to-0.2200, P=0.0104). Also, men had ×1-2 decreased mean level of TSH than infected women (F3,116: 0.5781, P=0.6306), as shown in Figures 2 and 3.
Fig.2.
The hormonal changes in both sexes compared to the normal range of healthy individuals. FSH; Follicle stimulating hormone, LH; Luteinizing hormone, and TSH; Thyroid stimulating hormone.
Fig.3.
Hormone changes based on the intensity of increase and decrease. FSH; Follicle stimulating hormone, LH; Luteinizing hormone, and TSH; Thyroid stimulating hormone.
Discussion
Our study has comprehensively investigated most of the hormones, that had not been measured during Covid-19 disease. Based on our findings, most of the studied female cases had abnormal changes in their hormones compared with the studied males. The first hormone that showed increasing, and decreasing changes in both sexes was estradiol. Many reasons indicate the absence of estrogen is a consequence of dysfunction or toxicity of the hypothalamus-pituitary axis and ovary (25, 26), leading to the coronary artery and renal diseases, osteoporosis, changes in verbal memory performance and mood, and IL-6 production, which have been recognized as a critical factor in cytokine storm that occurs in Covid-19, osteoclasts, and dysgeusia (27-32). Likewise, increasing estradiol levels will affect the thyroid and enhance iodide concentration, thrombotic, ischemic stroke, and elevations of breast cancer risk (33-36). Firstly, Estradiol’s abnormal levels in patients might directly result from the damaged ovary, hypothalamus-pituitary axis, FSH, and LH abnormalities. Secondly, maybe as a derivative result of dysgeusia led by the virus itself or antiviral drugs or a combination of both. The next and more forgotten hormone in men is progesterone, which showed only decreasing changes. Progesterone is an essential hormone in men that influences spermatogenesis, sperm capacitation/acrosome reaction, and testosterone biosynthesis in the Leydig cells. Besides, the nervous system has the capacity to bio-convert progesterone into its active metabolite allopregnanolone. Other progesterone effects include blocking gonadotropin secretion, sleep improvement, and effects on tumors in the central nervous system (CNS) (meningioma, fibroma), and effects on the immune system, cardiovascular system, and kidney function, adipose tissue, behavior, and respiratory system (37, 38). As we know, in women, progesterone saves the pregnancy, regulates the period, and the ovary produces the most amount of it; also, adrenals generate a slight amount of progesterone. The abnormal amount of progesterone in both sexes is probably a result of impairment of the ovary, adrenal, or hypothalamuspituitary axis.
Both genders (male and female) had almost equal abnormalities, suggesting that adrenal and or hypothalamus-pituitary axis are most likely to be involved in the virus attack, rather than women hormons secretion. Testosterone was among those hormones with fewer fold changes. Still, there is a need to enhance the statistical community to determine whether it is an effect of SARS-CoV-2 or not since it is an essential hormone in reproductive health. Moreover, the levels of FSH, LH, TSH, and prolactin showed significant changes. The results of the current study suggests that hypothalamuspituitary axis was infected and affected by the virus, which consequently leads to cardiovascular disease, blood pressure, and Metabolic disease (39). However, a sudden increase in estrogen and progesterone can cause negative feedback of FSH and LH hormones. Also, the abnormal TSH level might be negative feedback toward T3 and T4 amount and probably reflects the thyroid gland injury. The excess of Cortisol secretion on the immune system, blood pressure, fat deposition, and symptoms and disease is well documented. Therefore, the abnormal level of cortisol and progesterone have been found in this study confirmed the hypothesis of adrenal damage. We will next determine whether these changes are the results of cytokine storm, Stress caused by fear of illness, or the amount of CRH and ACTH released by the hypothalamus and pituitary glands. There is a need to measure the CRH, ACTH, GNRH, T3, and T4 in more samples to endorse the damage of cerebral glands, thyroid, and virus access into the brain.
Conclusion
These results suggested that COVID-19 affects directly or non-directly glands and related hormones.
Acknowledgements
We want to thank Dr. Shahriyar Mamikhani, Dr. Mohammad Hakkaki Fard for all they did during COVID-19 on the quarantine section and saving lives. Although Dr. Mohammad Mirzapour, Mr. Saeid Abdi, and Ms. Helma Mohtadifar supported our study and provided us with all the equipment, we needed. This study financially supported by the Tabriz University of Medical Sciences. The authors declare no conflicts of interest.
Authors’ Contributions
N.H., L.R.; Had the idea for and designed the study. All authors had full access to all data in the study and took responsibility for the data’s integrity and accuracy. N.H., M.Sh., S.T.; Contributed to the writing of the report and analysis. H.A.; Contributed to the blood sampling of patients at the infectious ward and reporting of results. Sh.Ma.; Contributed as a supervising physician. A.Z., Sh.Me.; Had contributed to the clinical trials assessing. All authore read and approved the final manuscript.
References
- 1.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. 2020;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C, Günther S, Preiser W, Van Der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrov DS. The secret life of ACE2 as a receptor for the SARS virus. Cell. 2003;115(6):652–653. doi: 10.1016/S0092-8674(03)00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui M, Song W, Zhou H, Xu J, Chen S, Xiang Y, et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27(1):119–129. doi: 10.1038/cr.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14(8):e1007236–e1007236. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchdoerfer RN, Wang N, Pallesen J, Wrapp D, Turner HL, Cottrell CA, et al. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci Rep. 2018;8(1):15701–15701. doi: 10.1038/s41598-018-34171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y, Cao D, Zhang Y, Ma J, Qi J, Wang Q, et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092–15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Struck AW, Axmann M, Pfefferle S, Drosten C, Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012;94(3):288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 15.Prabakaran P, Xiao X, Dimitrov DS. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys Res Commun. 2004;314(1):235–241. doi: 10.1016/j.bbrc.2003.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8–8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell rna expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. Gut. 2020;69:1010–1018. [Google Scholar]
- 20.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019- nCoV infection.bioRxiv. bioRxiv; 2020. [Google Scholar]
- 21.Fan C, Lu W, Li K, Ding Y, Wang J. ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front Med (Lausanne). 2021;7:563893–563893. doi: 10.3389/fmed.2020.563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord. 2020;21(4):495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Clinical management of severe acute respiratory infection ( SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization; 2020. Available from: https://appswhoint/iris/handle/10665/331446 . (12 March 2022) [Google Scholar]
- 24.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira FR, Ferreira JR, dos Santos CM, Macêdo LE, de Oliveira RB, Rodrigues JA, et al. Estradiol reduces cumulative mercury and associated disturbances in the hypothalamus-pituitary axis of ovariectomized rats. Ecotoxicol Environ Saf. 2006;63(3):488–493. doi: 10.1016/j.ecoenv.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Utsunomiya T, Utsunomiya H, Umesaki N. Irinotecan HCl, an anticancer topoisomerase I inhibitor, frequently induces ovarian failure in premenopausal and perimenopausal women. Oncol Rep. 2008;19(5):1123–1133. [PubMed] [Google Scholar]
- 27.Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, et al. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92(1):24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- 28.Antus B, Hamar P, Kokeny G, Szollosi Z, Mucsi I, Nemes Z, et al. Estradiol is nephroprotective in the rat remnant kidney. Nephrol Dial Transplant. 2003;18(1):54–61. doi: 10.1093/ndt/18.1.54. [DOI] [PubMed] [Google Scholar]
- 29.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 30.Wolf OT, Kudielka BM, Hellhammer DH, Törber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24(7):727–741. doi: 10.1016/s0306-4530(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 31.Girasole G, Jilka RL, Passeri G, Boswell S, Boder G, Williams DC, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LoRusso PM, Piha-Paul SA, Mita M, Colevas AD, Malhi V, Colburn D, et al. Co-administration of vismodegib with rosiglitazone or combined oral contraceptive in patients with locally advanced or metastatic solid tumors: a pharmacokinetic assessment of drugdrug interaction potential. Cancer Chemother Pharmacol. 2013;71(1):193–202. doi: 10.1007/s00280-012-1996-6. [DOI] [PubMed] [Google Scholar]
- 33.Boccabella AV, Alger EA. Influence of estradiol on thyroid: serum radioiodide concentration ratios of gonadectomized and hypophysectomized rats. Endocrinology. 1964;74:680–688. doi: 10.1210/endo-74-5-680. [DOI] [PubMed] [Google Scholar]
- 34.Bagot CN, Leishman E, Onyiaodike CC, Jordan F, Gibson VB, Freeman DJ. Changes in laboratory markers of thrombotic risk early in the first trimester of pregnancy may be linked to an increase in estradiol and progesterone. Thromb Res. 2019;178:47–53. doi: 10.1016/j.thromres.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Lin JH, Jiménez MC, Manson JE, Hankinson SE, Rexrode KM. Plasma estradiol and testosterone levels and ischemic stroke in postmenopausal women. Stroke. 2020;51(4):1297–1300. doi: 10.1161/STROKEAHA.119.028588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyytinen H, Pukkala E, Ylikorkala O. Breast cancer risk in postmenopausal women using estradiol-progestogen therapy. Obstet Gynecol. 2009;113:65–73. doi: 10.1097/AOG.0b013e31818e8cd6. [DOI] [PubMed] [Google Scholar]
- 37.Oettel M, Mukhopadhyay AK. Progesterone: the forgotten hormone in men? Aging Male. 2004;7(3):236–257. doi: 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- 38.Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol. 2015;146:48–61. doi: 10.1016/j.jsbmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2013;34(1):27–46. doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]