Abstract
Purpose:
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis Syndrome (TENS) are severe and potentially lethal adverse drug reactions characterized by acute inflammation of the skin, mucous membranes, and ocular surface that typically occurs within weeks of a culprit drug ingestion.
The purpose of this study is to report a retrospective trend analysis of SJS spectrum diagnoses and associated culprit drugs in patients admitted to the Loyola University Medical Center (LUMC) Burn Unit, the major referral center in the Chicagoland region for patients with SJS disease spectrum.
Methods:
The electronic medical records (EMR) of 163 patients with a diagnosis of SJS/TENS admitted to the LUMC Burn Unit from 2000 to 2019 were reviewed. Clinical data in addition to the well-established algorithm of drug causality for epidermal necrolysis (ALDEN) allowed us to identify the single most probable culprit drug in 131 cases.
Results:
From 2000 to 2019, the most common spectrum classification was TENS (48.1%), followed by SJS (33.6%) and SJS-TEN Overlap Syndrome (18.3%). Anticonvulsants were found to be the most probable culprit class in 30% of cases followed by Trimethoprim-Sulfamethoxazole in 19% of cases. Beta-lactams were the most probable culprit class in 11% of cases while NSAIDs and allopurinol were each the most probable culprit class/drug in 8.4% of cases.
Conclusions:
This is one of the largest single center series of SJS/TENS cases in the United States. Further study into culprit drug distribution by region as well as continuous monitoring of trends is crucial in order to advise prescribing practices.
Keywords: Adverse drug reaction, Culprit drug, Offending agent, SJS, TENS
1. Introduction
1.1. Definition
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis Syndrome (TENS) are severe and potentially lethal immunologically mediated adverse drug reactions (IM-ADR) characterized by the inflammation of the skin and mucous membranes associated with keratinocyte apoptosis and epidermal necrosis. Secondary complications include sepsis, blindness, respiratory, and genitourinary scarring and dysfunction [1].
Medications trigger SJS/TENS in greater than 80% of adults, and the reaction typically occurs within the first few weeks after the administration of an inciting agent (culprit drug) [2]. SJS and TENS are thought to be the same disease across a spectrum of severity defined by the percentage of total body surface area (TBSA) involved with SJS (<10%), SJS-TEN Overlap Syndrome (10–30%) and TENS (>30%) [1]. Unlike SJS, a TENS diagnosis does require the presence of mucosal lesions [3].
Differential diagnoses for SJS/TENS include erythema multiforme (EM), erythematous drug eruptions, acute generalized exanthematous pustulosis (AGEP), generalized bullous fixed drug eruptions, phototoxic eruptions as well as others. While no exact SJS/TENS diagnostic criteria are accepted universally, the diagnosis is typically made based on a history of recent drug exposure, characteristic features of the rash, involvement of the mucosal membranes (oral, ocular, and genital), and with skin biopsy [4]. For patients with suspected SJS/TENS spectrum disease, a punch biopsy of >4 mm is typically taken from an area of affected skin. Alternatively, a deep shave biopsy can also be performed. The classic histological features of SJS/TENS spectrum disease are initially apoptotic keratinocytes scattered in the basal layer of the epidermis. Additionally, a T lymphocyte predominant mononuclear inflammatory infiltrate in the papillary dermis may be seen in perivascular regions. Later in the disease subepidermal bullae may be seen in addition to full-thickness epidermal necrosis [4,5]. In comparison, the classic histological features of EM are basal cell vacuolar degeneration in addition to scattered necrotic keratinocytes and lymphocyte exocytosis findings [6].
1.2. Epidemiology
Between 2004–2013 the FDA found a yearly incidence of 1460 cases or 5 cases per 1,000,000.
Based on large studies performed in Germany and France, the estimated incidence of SJS/TENS is 1–2 per 1,000,000 per year [1]. Men and women are affected equally. Prior studies have identified several classes of culprit agents implicated in SJS/TENS reactions including antibiotics (sulfonamides), allopurinol, NSAIDs, anticonvulsants (carbamazepine) and other less common culprit agents such as anti-osteoporotic agents and BRAF/MEK inhibitors used to treat BRAF-mutated metastatic melanomas [7–17].
A culprit drug was identified in 75% of cases [1]. When treated with supportive care, patients have a median hospital stay of 18.5 days at a cost of $44,695 USD per patient. Firm data on the best treatment is elusive, and most patients are treated based on institution experience as well as expert opinion [18].
Although it is a rare disease, SJS/TENS represents a substantial health burden with a mean total cost of $128 million annually in the US [19]. There are still many research gaps across the multiple disciplines (e.g., dermatology, immunology, clinical pharmacology, ophthalmology, gynecology) to optimally manage and prevent SJS/TENS. This will demand a collective effort to advance and translate science into prediction, prevention, early diagnosis and more targeted and effective treatments to improve short and long-term patient outcomes.
1.3. Pathophysiology
Drug-associated SJS/TENS is an antigen-driven, HLA-restricted inflammatory process whose effector cells include CD8+ T cells, NK and NK T cells. Soluble factors, including TNF-α, IL-15, and the cytolytic peptide granulysin produced by cytotoxic lymphocytes may mediate systemic inflammation and keratinocyte cell death. Granulysin found in high concentrations in blister fluid and plasma levels may also be associated with the severity of acute disease and mortality [20–22].
1.4. Immunogenetics
Over the last 15 years, there have been significant advancements in our understanding of the immunopathogenesis, and genetic risk factors for SJS/TENS. These advancements have fueled preventive efforts leading to successful pre-prescription screening programs in some countries [23–27]. A genetic predisposition to SJS/TENS is attributed to specific HLA types including HLA-B 15:02, HLA-B 58:01, HLA-A 31:01, and HLA-A −2:06 as well as certain ethnic populations that are likely to carry these alleles [28]. Further, some studies suggest that certain HLA types may be culprit drug-specific [29–35]. HLA-B 58:01 is associated with SJS/TENS following allopurinol administration in Han Chinese, Caucasian, and Japanese populations [33–35]. However, the HLA-B 15:02 allele is also strongly associated with an increased risk of developing SJS/TENS in Han Chinese and Thai populations following carbamazepine administration [29,30]. Still, HLA-B*15:02 is carried in fewer than 1% of those with a European, Hispanic, or African ancestry, and only 60% of allopurinol SJS/TENS is associated with HLA-B*58:01 in European populations [36]. This has led not only to successful HLA-B*15:02 screening programs in Taiwan, Singapore, and other parts of Southeast Asia that have led to near elimination of carbamazepine associated SJS/TENS, it also furthered our understanding of the immunopathogenesis of SJS/TENS. In the US, HLA-B 15:02 screening of patients of Han Chinese descent is recommended by the FDA [37].
Further discovery is needed to identify genetic factors associated with risk of SJS/TENS for drugs used and populations represented in North America. The immunopathogenesis of SJS/TENS is distinct. Despite this progress, there remains troubling clinical and research gaps. For example, the lack of an evidence-based approach to guide therapeutic interventions above aggressive supportive care in acute SJS/TENS, the lack of identified genetic predictors for most drugs that cause SJS/TENS, the lack of available predictive biomarkers for early diagnosis, and the lack of understanding why such a small proportion (2–6%) of those carrying an HLA risk allele will develop SJS/TENS still require investigation [38].
1.5. Management
SJS/TENS treatment practices vary widely but primarily consist of prompt withdrawal of the causative agent and supportive care [39]. The toxic epidermal necrolysis-specific severity of illness score (SCORTEN) is a score commonly used as a predictor of mortality in patients with SJS/TENS that involves parameters evaluated in the first 24 h of the patient’s admission including age, heart rate, cancer/hematologic malignancy, TBSA involvement, blood urea nitrogen level, serum glucose level, and serum bicarbonate level [40].
In 2015, a North American SJS/TENS practice survey of 30 burn center directors and 17 academic dermatology centers highlighted that (despite lack of evidence) 80% of sites cited IVIG as their first choice of therapy [1,41]. Early intensive supportive care remains the only consensus or evidence-based therapeutic options for SJS/TENS. Non-targeted immune-modulating therapies such as intravenous immunoglobulin (IVIG) and non-pulsed dosing of systemic steroids have shown no benefit over aggressive supportive care alone. A meta-analysis of observational studies for IVIG, corticosteroids, and cyclosporine showed a potential study level benefit of corticosteroids that disappeared when patient level data was analyzed (0.54 [95%CI, 0.29–1.01]) [42,43]. IVIG was not beneficial [42]. The only published randomized double-blind placebo-controlled trial examined 5 days of thalidomide which was stopped early due to an increase in mortality in the treatment arm [42]. Smaller studies, a retrospective review, a phase II non-randomized trial, and in data analyzed from the RegiSCAR cohort support the efficacy of cyclosporine in halting the progression of SJS/TENS and lowering mortality without treatment limiting adverse effects [42]. Multiple small non-blinded studies suggest rapid re-epithelialization and a lack of mortality with etanercept, while one randomized control trial showed a 50% reduction in mortality and 3 days faster healing compared to steroids corresponds with a >50% reduction in serum and blister fluid granulysin and TNF-α [44].
1.6. Complications
Although with aggressive supportive care mortality should be less than 20%, in adults >65 years of age with TENS, mortalities as high as 70% have been reported [45]. The mortality associated with TENS in the setting of aggressive supportive care at experience centers is approximately 20%, however in the elderly and immunocompromised populations this can exceed 50%. The short-term morbidity associated with SJS/TENS including sepsis, respiratory complications, gastrointestinal and genital tract obstruction and eye disease is well recognized. However long-term morbidity is also considerable and includes vision loss, blindness, reproductive/genitourinary complications, chronic respiratory disease, psychiatric illness, disfigured painful skin, severely constricted therapeutic choices and shortened life-span. The global clinical and financial burden of SJS/TENS is considerable, due to prolonged hospital stays, mortality of up to 50% in the elderly and considerable long-term multi-system physical and mental health morbidity that is still poorly understood qualitatively and quantitatively [1].
1.7. Culprit drug identification
Loyola University Medical Center (LUMC) serves as a major SJS/TENS referral center in the Chicagoland area. LUMC falls within the top 10–25% of burn centers for overall SJS/TENS admissions and the vast majority of patients with the final diagnosis of SJS/TENS spectrum disease are biopsy-proven. Patients with suspected acute SJS/TENS are often transported to regional referral centers from the local hospitals they present to. Thus, there is often a lack of detailed clinical information available on such patients for the receiving physicians to document when assessing the patient on presentation. Therefore, it is often the case that a specific inciting agent is not identified on presentation. Additionally, there are a significant number of patients who have undergone the initiation of numerous medications of which multiple have been previously documented as potential causative agents for SJS/TENS. Therefore, it is crucial to have a standardized protocol that could be employed to identify the possible inciting agents for a given patient and then using a standard algorithm identify the most probable culprit agent among those possible agents.
One such protocol is the Algorithm of Drug Causality for Epidermal Necrolysis (ALDEN) which was developed by Sassolas et al. and described in a 2010 paper [46]. ALDEN was designed to act as a validated algorithm that could not only be used clinically to determine the most probable culprit drug in an individual case but also for large-scale pharmacovigilance drug monitoring studies. ALDEN was applied to the cases from the EuroSCAR case-control analysis study, which included cases from 1997 to 2001, and strongly correlated with the results of the EuroSCAR study. While clinical experience and judgement must always play a center role in determining drug causality in the acute setting, ALDEN can be used as a reference tool for identifying the most probable culprit drug [46].
The algorithm considers six key parameters that can be retrospectively applied to the medical record for each SJS/TENS case (Table 1). This algorithm considers six main criteria to determine the most probable culprit drug when more than one possible culprit drug is suspected. These criteria include: (1) delay from initial drug component intake to onset of reaction (Index Day); (2) drug present in the body on index day; (3) prechallenge/rechallenge; (4) dechallenge; (5) Type of drug (notoriety); and (6) other cause. See further discussion in Section 2.
Table 1 –
ALDEN algorithm for the assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis.
| Criterion | Rule | Interpretation | Point value |
|---|---|---|---|
| 1. Delay from initial drug component intake to onset of reaction (Index Day) [−3 to 3] | 5–28 Days or If previous rxn to same drug: 1–4 days | Suggestive | +3 |
| 29–56 Days | Compatible | +2 | |
| 1–4 Days or If previous rxn to same drug: 5–56 Days | Likely | +1 | |
| >56 Days | Unlikely | −1 | |
| Started on or after the index day | Excluded | −3 | |
| 2. Drug present in the body on index day [−3 to 0] | Drug continued up to index day or stopped at a time point less than 5× the elimination half-life before the index day | Definite | 0 |
| Drug stopped at a time point prior to the index day by more than five times the elimination half-life but liver to kidney function alterations or suspected drug interactions are present | Doubtful | −1 | |
| Drug stopped at a time point prior to the index day by more than five times the elimination half-life without liver or kidney function alterations or suspected drug interactions. | Excluded | −3 | |
| 3. Prechallenge/rechallenge [−2 to 4] | SJS/TEN after use of the same drug | Positive specific for disease and drug | +4 |
| SJS/TEN after use of similar drug or other reaction with same drug | Positive specific for disease or drug | +2 | |
| Other reaction after use of similar drug | Positive unspecific | +1 | |
| No known previous exposure to this drug | Not done/unknown | 0 | |
| Exposure to this drug without any reaction (before or after reaction) | Negative | −2 | |
| 4. Dechallenge [−2 or 0] | Drug stopped (or unknown) | Neutral | 0 |
| Drug continued without harm | Negative | −2 | |
| 5. Type of drug (notoriety) [−1 to 3] | Drug of the high-risk list according to previous case-control studies | Strongly associated | +3 |
| Drug with definite but lower risk according to previous case-control studies | Associated | +2 | |
| Several previous reports, ambiguous epidemiology results (drug “under surveillance”) | Suspected | +1 | |
| All other drugs including newly released ones | Unknown | 0 | |
| No evidence of association from previous epidemiology study with sufficient number of exposed controls | Not Suspected | −1 | |
| Intermediate score: total of all previous criteria [−11 to 10] | |||
| 6. Other cause | Rank all drugs from highest to lowest intermediate score–if at least one has an intermediate score >3, subtract 1 point from the score of each of the other drugs taken by the patient (another cause more likely). | Possible | −1 |
| Final score: [−12 to 10] | |||
Table adapted from: Sassolas, B., et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 88, 60–68 (2010).
1.8. Purpose
This purpose of this study was four-fold. Our first goal was to retrospectively investigate the distribution of disease classification on the SJS/TENS spectrum (SJS vs TENS vs SJS-TEN Overlap Syndrome) at LUMC presentation. The second was to identify the most common culprit drugs in SJS/TENS patients presenting to LUMC and to isolate trends in culprit drugs over time. Our third objective was to apply the ALDEN algorithm as a means to delineate the most probable culprit drug from other possible culprit drugs in patients receiving multiple agents. Finally, we wanted to established the ALDEN criteria as a standardized method of determining the most probable culprit drug in patients at presentation as it is well-documented association between time to offending agent discontinuation and a decrease in mortality [8,47–49]. By elucidating trends in SJS/TENS culprit drugs, we hope to add to the fund of knowledge to advise providers on the current most likely culprit drugs.
2. Methods
2.1. Inclusion and exclusion criteria
This study included patients with the diagnosis of SJS/TENS who were admitted to the LUMC Burn Unit between the years of 2000 and 2019. Patients were excluded from the study if they had a clinical assessment that was not indicative of SJS/TENS spectrum disease, a negative biopsy result, or a clinical history of autoimmune bullous disease or any other diagnosis that might mimic SJS/TENS. Additionally, patients were also excluded from the study if they were not hospitalized following their presentation with SJS/TENS, were discharged within 1 day of presentation, if they did not end up receiving a final diagnosis of SJS/TENS, or if their diagnosis of SJS/TENS was changed after hospitalization. Finally, patients were excluded if their medical record did not contain adequate information to apply the ALDEN algorithm—most of which consisted of patients who were seen prior to 2000 or between 2000–2006 who did not continue to be followed at LUMC— discussed further in Section 3.1.
2.2. Data collection
The EPIC electronic medical record (EMR) system was implemented at LUMC in 2007. Thus, for this study information was gathered from the paper charts of patients who presented to between the years of January 2000 to December 2006 and who did not continue to be followed at our institution. We reviewed the EMRs of patients who were seen between January 2000–2006 who continued to follow at LUMC, and thus had their paper records scanned into their EMR in 2007, as well as patients presenting to LUMC after 2007. The following clinical data were reviewed: date of admission, SJS/TENS subtype diagnosis, biopsy confirmation, possible/probable inciting agents, and hospital mortality. Hard copy data was kept in a locked cabinet in a locked office. Electronic data was kept on a Loyola secure server. All data was collected and stored in REDCAP.
2.3. Culprit drug identification
We used the well-established ALDEN algorithm to identify the most probable culprit drug in cases where multiple agents were involved (Table 1).
-
Delay from initial drug to index day:
It has been previously reported that most SJS/TENS reactions typically occur within a few weeks following the initial administration of a culprit drug. Thus, the first criteria the assessed by ALDEN is the number of days from the initiation of a medication to onset of the reaction which is described as the index day.
-
Drug present in the body on index day:
The likelihood of the drug being present in the body on index day is assessed by considering the elimination half-life of each specific agent run through the algorithm. The scoring takes into account whether a drug was continued up until index day/discontinued less than 5 elimination half lives of the specific drug. Additionally, the possibility of drug interactions as well as liver and kidney function are taken into account in the scoring.
-
Prechallenge/rechallenge:
The prechallenge/rechallenge parameter accounts for whether a patient had a past reactions to the same drug or a similar agent. Similar agent was defined by two substances were within the same chemical subgroup based on the World Health Organization’s (WHO’s) anatomic therapeutic chemical (ATC) codes. If the reaction was a SJS/TENS reaction vs another reaction plays a role in scoring for this aspect. Additionally, if a patient previously took this culprit drug without any reaction the likelihood that this drug is the most probable culprit decreases.
-
Dechallenge:
The likelihood of a specific substance being the probable culprit is decreased if the drug was continued throughout and beyond the SJS/TENS reaction.
-
Type of drug (notoriety):
Drug notoriety was established by Sassolas et al. using the data collected from the 1995 RegiSCAR study by Roujeau et al. to estimate the relative risks of various agents and to place them in categories ranging from “Strongly Associated Drugs” to “Not Suspected Drugs [50].” More details on specific categories can be found in Table 1.
-
Other cause:
The “other cause” parameter is used to account for other causes of SJS/TENS spectrum disease such as infection by Mycoplasma pneumoniae which remains a contested point among researchers [51–53]. This parameter is only calculated after the five previous categories have been addressed for each drug the patient had been taking.
2.4. Biostatistics
There were no planned (i.e., powered a-priori) null hypothesis tests reported in this review. Instead, we report summary frequencies and statistics to describe a sample of 131 patients at one institution who are affected by SJS, TENS, or SJS-TEN Overlap Syndrome. These summaries include patients’ age, sex, race, decade of admission and culprit drug which are provided for the total sample as well as stratified by patients’ diagnosis and decade of admission. We used SAS version 9.4 (Cary, NC) for these summaries.
3. Results
3.1. Selection process
Between the years of 2000 and 2019, 163 patients presented to the LUMC Burn Unit with an initial diagnosis of SJS/TENS spectrum disease. Of these 163 cases, a specific SJS/TENS spectrum subtype was not identified in the record and %TBSA was not provided in 32/163 (19.6%). Between the years of 2000 and 2019, a total of 131 patients diagnosed with SJS/TENS spectrum disease in which a single most probable culprit drug could be identified were admitted to the LUMC Burn Unit. It is important to note that 111/131 (84.7%) of these cases were biopsy confirmed. It was unknown whether a biopsy was performed in the remainder of the cases (n = 20) (Fig. 1).
Fig. 1 –
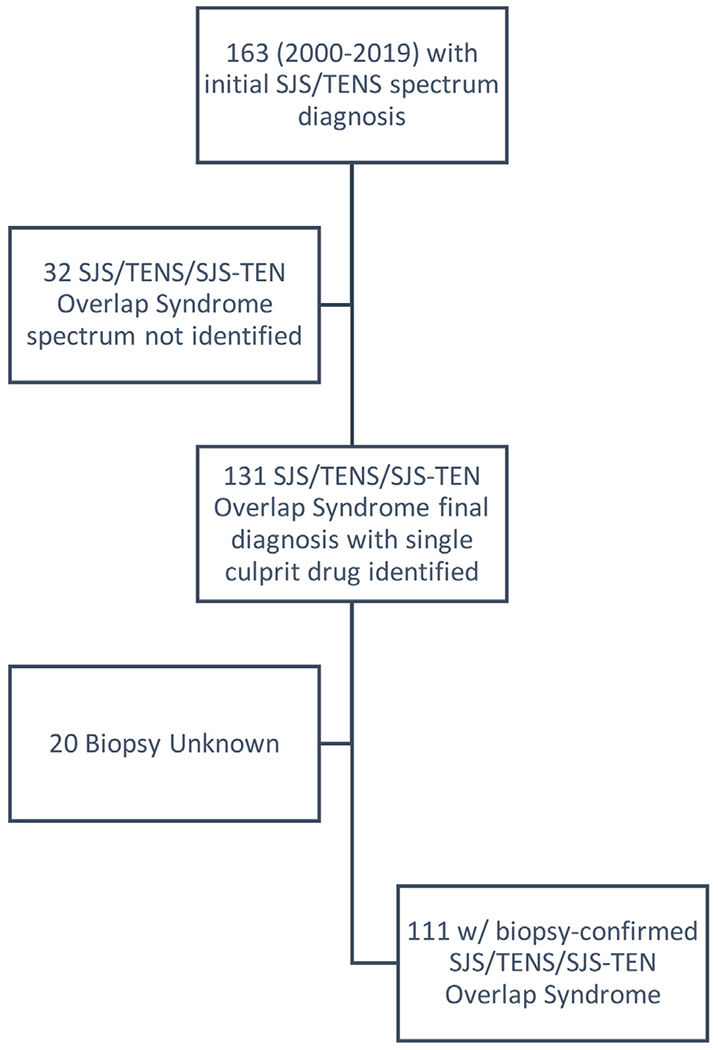
Retrospective case selection process.
3.2. Demographics
Overall, the patients had a mean age of 44.9 years (SD = 23.0) and the majority 78/131 (60%) were female. The majority of patients identified as white 65/131 (50%) followed by Black/African American 40/131 (31%), Asian 14/131 (11%), and other 12/131 (9.2%). Of the total 131 patients admitted with a final diagnosis of SJS/TENS between 2000 and 2019, 45 (34%) cases were admitted in 2003–2009 and 86 (66%) cases in 2010–2019. This equates to a 4.5 patients/year for the 2000–2009 time period and 8.6 patients/year for the 2010–2019 time period. However, this difference in admitted patients/year is likely due to the fact that a majority of the patients excluded from our initial study group (n = 170) were from the earlier years (Table 2).
Table 2 –
Demographics.
| SJS (n = 44) | TENS (n = 63) | OVERLAP (n = 24) | Total (n = 131) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 45.74 | 23.02 | 43.96 | 24.85 | 46.00 | 17.85 | 44.93 | 22.96 | ||
| Count | % | Count | % | Count | % | Count | % | ||
| Sex | Female | 28 | 64 | 33 | 52 | 17 | 71 | 78 | 60 |
| Male | 16 | 36 | 30 | 48 | 7 | 29 | 53 | 40 | |
| Race | White | 15 | 34 | 35 | 56 | 15 | 63 | 65 | 50 |
| Black | 15 | 34 | 18 | 29 | 7 | 29 | 40 | 31 | |
| Asian | 7 | 16 | 5 | 7.9 | 2 | 8.3 | 14 | 11 | |
| Other | 7 | 16 | 5 | 7.9 | 0 | 0.0 | 12 | 9.2 | |
| Admission Decade | 2003–2009 | 5 | 11 | 29 | 46 | 11 | 46 | 45 | 34 |
| 2010–2019 | 39 | 89 | 34 | 54 | 13 | 54 | 86 | 66 | |
Demographic data including age, sex, race, and admission date. Race: there were no cases of white Hispanics or American Indians among our sample population.
3.3. SJS/TENS spectrum diagnosis
From 2000 to 2019, the most common spectrum classification was TENS (63/131; 48.1%), followed by SJS (44/131; 33.6 %) and SJS-TEN Overlap Syndrome (24/131; 18.3%). The distribution of SJS/TENS diagnoses from 2000 to 2009 (n = 45) was 29/45 (64.4%) TENS, 11/45 (24.4%) SJS-TEN Overlap Syndrome, 5/45 (11.1%) SJS. The distribution from 2010 to 2019 (n = 86) was 39/86 (45.3%) SJS, 34/86 (39.5%) TENS, and 13/86 (15.1%) SJS-TEN Overlap Syndrome (Fig. 2).
Fig. 2 –
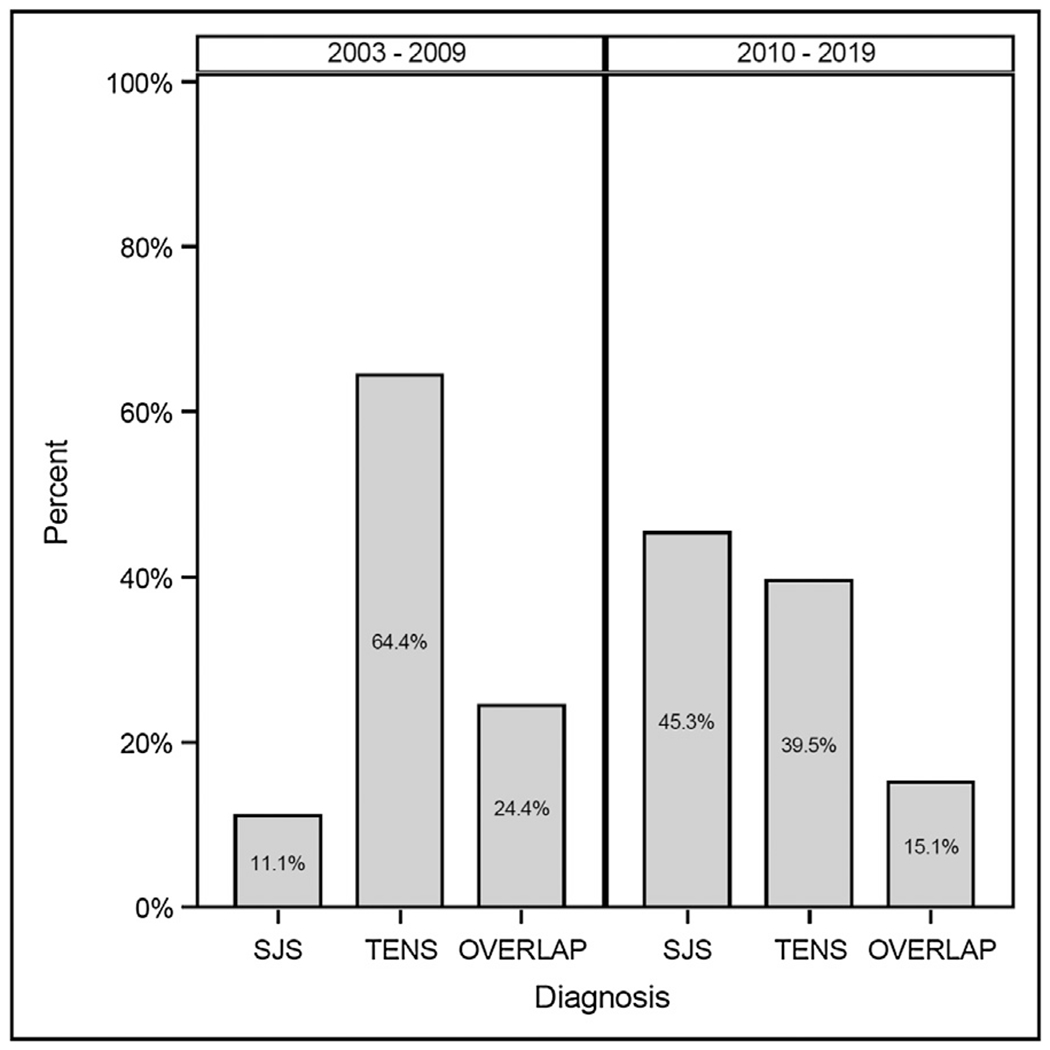
Spectrum diagnosis by Admit Date.
SJS: Stevens-Johnson Syndrome, TENS: Toxic Epidermal Necrolysis Syndrome, OVERLAP: SJS-TEN Overlap Syndrome.
3.4. Culprit drugs
Using medical record data and the ALDEN algorithm, a single most probable culprit drug was able to be identified in the 131 patients with a diagnosis of SJS/TENS spectrum disease. From 2000 to 2019, anticonvulsants were found to be the most probable culprit class in 39/131 (30%) cases followed by Trimethoprim-Sulfamethoxazole [TMP-SMX] in 25/131 (19%) of cases. Beta-lactams were the most probable culprit class in 15/131 (11%) of cases while NSAIDs and allopurinol were each the most probable culprit class/drug in 11/131 (8.4% of cases). Fluoroquinolones and other antibiotics were each the probable class in 10/131 (7.7%) cases and immunosuppressants were the most probable class in 2/131 (1.5%) cases. The “other” category, which included 1 case of suspected M. pneumoniae infection, was the most probable drug class/cause in 8/131 (6.1%) of cases (Table 3).
Table 3 –
Drug class by decade and diagnosis.
| Decade: 2003–2009 | SJS (n = 5) | TENS (n = 29) | SJS-TEN Overlap Syndrome (n = 11) | Total (N = 45) |
|---|---|---|---|---|
| Allopurinol | 1 (20%) | 1 (3.5%) | 0 | 2 (4.4%) |
| Anticonvulsant | 1 (20%) | 8 (28%) | 4 (36%) | 13 (29%) |
| Beta-lactam | 0 | 5 (17%) | 1 (9.1%) | 6 (13%) |
| Fluoroquinolone | 1 (20%) | 2 (6.9%) | 0 | 3 (13%) |
| Immunosuppressant | 0 | 0 | 0 | 0 |
| NSAID | 0 | 3 (10%) | 2 (18%) | 5 (11%) |
| Other | 0 | 2 (6.9%) | 1 (9.1%) | 3 (6.7%) |
| Other antibiotics | 1 (20%) | 3 (10%) | 1 (9.1%) | 5 (11%) |
| TMP-SMX | 1 (20%) | 5 (17%) | 2 (18%) | 8 (18%) |
|
| ||||
| Decade: 2010–2019 | SJS (n = 39) | TENS (n = 34) | SJS-TEN Overlap Syndrome (n = 13) | Total (N = 86) |
|
| ||||
| Allopurinol | 4 (10%) | 4 (12%) | 1 (7.7%) | 9 (10%) |
| Anticonvulsant | 13 (33%) | 10 (29%) | 3 (23%) | 26 (30%) |
| Beta-lactam | 1 (2.6%) | 5 (15%) | 3 (23%) | 9 (10%) |
| Fluoroquinolone | 6 (15%) | 0 | 1 (7.7%) | 7 (8.1%) |
| Immunosuppressant | 0 | 2 (5.9%) | 0 | 2 (2.3%) |
| NSAID | 1 (2.6%) | 4 (12%) | 1 (7.7%) | 6 (7.0%) |
| Other | 5 (13%) | 0 | 0 | 5 (5.8%) |
| Other antibiotics | 3 (7.7%) | 1 (2.9%) | 1 (7.7%) | 5 (5.8%) |
| TMP-SMX | 6 (15%) | 8 (24%) | 3 (23%) | 17 (20%) |
|
| ||||
| Overall | SJS (n = 44) | TENS (n = 63) | SJS-TEN Overlap Syndrome (n = 24) | Total (N = 131) |
|
| ||||
| Allopurinol | 5 (11%) | 5 (7.9%) | 1 (4.2%) | 11 (8.4%) |
| Anticonvulsant | 14 (32%) | 18 (29%) | 7 (29%) | 39 (30%) |
| Beta-lactam | 1 (2.3%) | 10 (16%) | 4 (17%) | 15 (11%) |
| Fluoroquinolone | 7 (16%) | 2 (3.2%) | 1 (4.2%) | 10 (7.7%) |
| Immunosuppressant | 0 | 2 (3.2%) | 0 | 2 (1.5%) |
| NSAID | 1 (2.3%) | 7 (11%) | 3 (13%) | 11 (8.4%) |
| Other | 5 (11%) | 2 (3.2%) | 1 (4.2%) | 8 (6.1%) |
| Other antibiotics | 4 (9.1%) | 4 (6.4%) | 2 (8.3%) | 10 (7.6%) |
| TMP-SMX | 7 (16%) | 13 (21%) | 5 (21%) | 25 (19%) |
Anticonvulsants: lamotrigine, phenytoin, carbamazepine, valproic acid, levitiracem, gabapentin; TMP-SMX: Trimethoprim-Sulfamethoxazole; Beta-lactams: ceftriaxone, ampicillin, piperacillin-tazobactam, penicillin G, amoxicillin, amoxicillin-clavulanate, imipenem; NSAIDs: ibuprofen, naproxen; Fluoroquinolones: ciprofloxacin, oxacillin, levofloxacin, moxifloxacin; other antibiotics: azithromycin, erythromycin, vancomycin, minocycline, rifampin, clindamycin; Other: acetaminophen, propylthiouracil, oseltamivir, loratadine, butalbital, amlodipine, possible mycoplasma infection; Immunosuppressants: rituximab, methotrexate.
Of the cases from 2000 to 2009 (n = 45), anticonvulsants were most probable in 13/45 (29%) cases and Trimethoprim-Sulfamethoxazole was most probable in 8/45 (18%) cases. Beta-lactams were the most probable in 6/45 (13%) cases. NSAIDs and other antibiotics were each most probable in 5/45 (11%) cases. Fluoroquinolones were most probable in 3/45 (13%) cases and allopurinol was most probable in 2/45 (4.4%) cases. Agents from the other category were most probable for 3/45 (6.7%) of cases (Table 3).
In cases from 2010 to 2019 (n = 86), anticonvulsants were most probable in 26/86 (30%) cases and Trimethoprim-Sulfamethoxazole was most probable in 17/86 (20%) cases. Allopurinol and Beta-lactams were each most probable in 9/86 (10%) cases and Fluoroquinolones were most probable in 7/86 (8.1%) of cases while NSAIDs were most probable in 6/86 (7.0%) of cases. Other antibiotics were most probable in 5/86 (5.8%) of cases and immunosuppressants were most probable in 2/86 (2.3%) of cases. The other category, which included 1 case of suspected M. pneumoniae, was most probable in 5/86 (5.8%) cases (Table 3, Fig. 3).
Fig. 3 –
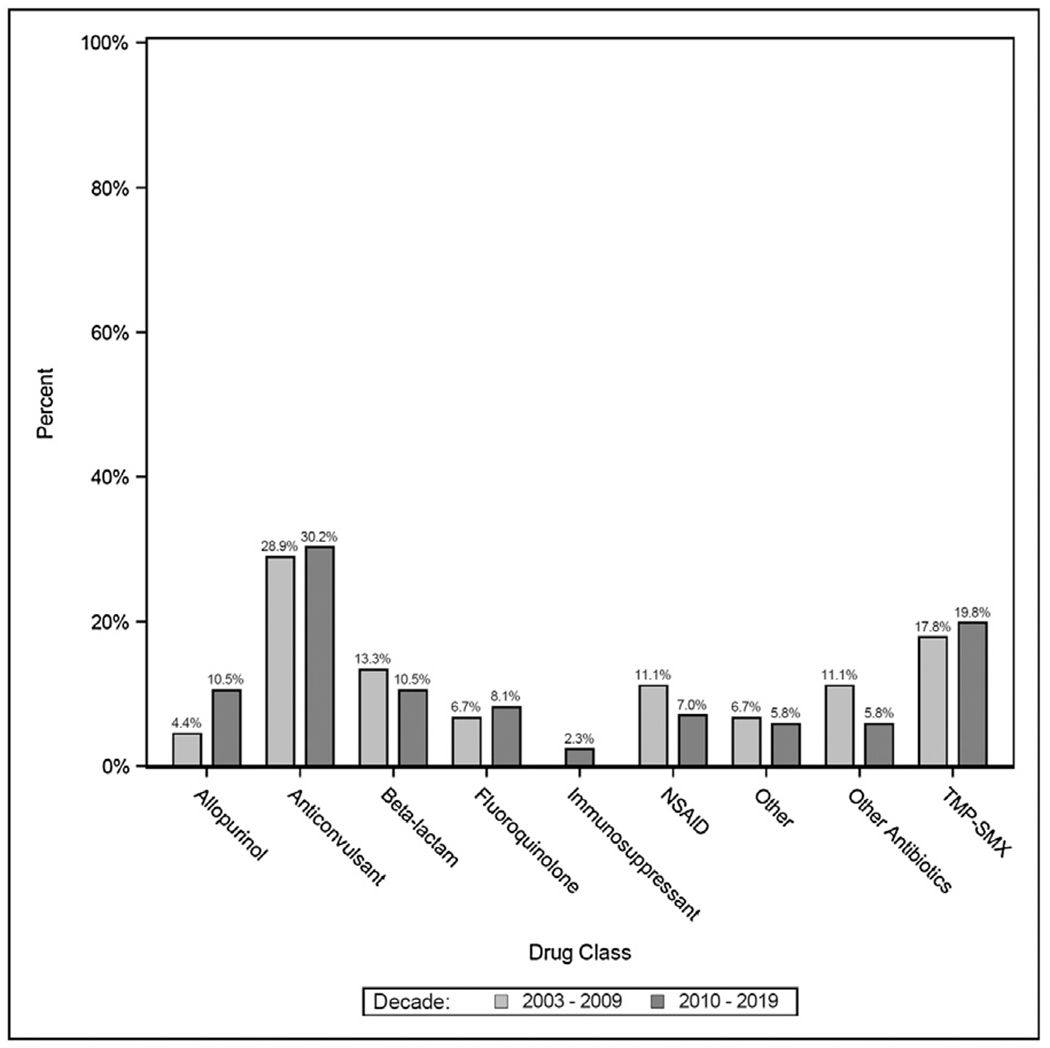
Culprit drug class by Admission Decade.
Anticonvulsants: lamotrigine, phenytoin, carbamazepine, valproic acid, levitiracem, gabapentin; TMP-SMX: Trimethoprim-Sulfamethoxazole; Beta-lactams: ceftriaxone, ampicillin, piperacillin-tazobactam, penicillin G, amoxicillin, amoxicillin-clavulanate, imipenem; NSAIDs: ibuprofen, naproxen; Fluoroquinolones: ciprofloxacin, oxacillin, levofloxacin, moxifloxacin; other antibiotics: azithromycin, erythromycin, vancomycin, minocycline, rifampin, clindamycin; Other: acetaminophen, propylthiouracil, oseltamivir, loratadine, butalbital, amlodipine, possible mycoplasma infection; Immunosuppressants: rituximab, methotrexate.
3.5. Culprit drugs by spectrum diagnosis
Among cases from 2000 to 2019 in which anticonvulsants were found to be the most probable culprit agent (n = 39), 18/39 (46%) cases presented as TENS, 14/39 (36%) presented as SJS, and 7/39 (18%) presented as SJS-TEN Overlap Syndrome. In the cases in which Trimethoprim-Sulfamethoxazole was the most probable agent (n = 25), 13/25 (52%) presented as TENS, 7/25 (28%) presented as SJS, and 5/25 (21%) presented as SJS-TEN Overlap Syndrome. In the cases in which Beta-lactams were the most probable agents (n = 15), 10/15 (67%) presented as TENS, 4/15 (27%) presented as SJS-TEN Overlap Syndrome, and 1/12 (6.7%) presented as SJS. In the cases in which NSAIDs were the most probable agent (n = 11), 7/11 (64%) cases presented as TENS, 3/11 (27%) presented as SJS-TEN Overlap Syndrome, and 1/11 (9.1%) presented as SJS. In the cases in which allopurinol was the most probable agent (n = 11), 5/11 (45%) cases presented as TENS, 5/11 (45%) presented as SJS, and 1/11 (9.1%) presented as SJS-TEN Overlap Syndrome. In the cases in which Fluoroquinolones were the most probable agent (n = 10), 7/10 (70%) cases presented as SJS, 2/10 (20%) presented as TENS, and 1/10 (10%) presented as SJS-TEN Overlap Syndrome. In cases in which other antibiotics were the most probable agents (n = 10), 4/10 (40%) were TENS, 4/10 (40%) were SJS, and 2/10 (20%) were SJS-TEN Overlap Syndrome. In cases where immunosuppressants were the most probable agents (n = 2), 2/2 (100%) were TENS. For cases where the most probable culprit agent was included in the other category (n = 8), 5/8 (63%) were SJS, 2/8 (25%) were TENS, and 1/8 (13%) was SJS-TEN Overlap Syndrome (Table 3).
4. Discussion
4.1. SJS/TENS spectrum diagnosis
From 2000 to 2019, there has been an increase in the proportion of patients with SJS from 11.1% between 2000–2009 to 45.3% between 2010–2019. Additionally, the incidence of TENS has decreased over the same time period from 64.4% in 2000–2009 to 39.5% in 2010–2019. TENS was the most common presentation among our study population from 2000 to 2019 responsible for 48.1% (63/131) of cases. This is in contrast to Micheletti et al. where SJS-TEN Overlap Syndrome was the most common presentation accounting for 45.5% (158/337) of cases. SJS was the second most common presentation in both our study, responsible for 33.9% (42/124) of cases, and the study by Micheletti et al. where it was responsible for 31.7% (110/337) of cases [14]. At LUMC, we suspect that some factors that may have influenced this shift are changes in referral patterns stemming from changes in LUMC burn unit leadership as well as dynamic changes in the major health systems and referral patterns in the Chicagoland area over time.
4.2. Culprit drugs
In patients admitted to LUMC from 2000 to 2019 with a SJS/TENS spectrum diagnosis in which a single probable culprit drug was able to be identified (n = 131), anticonvulsants (n = 39), Trimethoprim-Sulfamethoxazole (n = 25) and Beta-lactams (n = 15) were the most common inciting agent classes/agents. Allopurinol and NSAIDs were each responsible for 11 cases, and were the 4th most common inciting agent classes/agents in our study population.
Our results align with previous culprit studies where anticonvulsants were found to be the most common class of culprit drug. One of the most commonly cited culprit studies was the EuroSCAR study which collected data culprit drug information from 379 patients in various European countries between 1997 and 2001. The EuroSCAR study found that anticonvulsants, specifically carbamazepine, were the most common culprit drugs among their patients [10]. More recently, a Canadian study that collected data from 64 patients between 2000 and 2015 as well as a Korean study that collected data from 187 patients in 2011 both found that anticonvulsants were the most common class of culprit drugs among their patients. The Korean study also found carbamazepine to be the most common agent out of the anticonvulsants while the Canadian study found phenytoin to be the most common culprit of the anticonvulsant agents [8,12]. A Study in Japan including 87 patients between 2000–2013 also found anticonvulsants, specifically carbamazepine, to be the most common culprit drug class along their patient population [11]. However, unlike the studies discussed above, lamotrigine was found to be the most common anticonvulsant in our study –responsible for 21/39 cases followed by phenytoin responsible for 8/39 cases while carbamazepine was responsible for only 4/39 cases.
Further, a 2019 study by Park et al. examined the specific agents involved in antiepileptic-induced drug reactions between 2010–2015 in 56 patients with SJS and 16 patients with TENS/SJS-TEN Overlap Syndrome. They found that carbamazepine was responsible for 28/56 cases (50%) of SJS in their patient population followed by phenytoin in 9/56 (16.1%) cases and valproic acid in 7/56 (12.5%) of cases. They also found that carbamazepine was the most common inciting agent in their patients with TENS/SJS-TEN Overlap Syndrome (n = 16) and was responsible for 7/16 (43.8%) of cases followed by lamotrigine which was responsible for 4/16 (25%) of cases and valproic acid responsible for 2/16 (12.5%) of cases [54].
Conversely, a study performed on 377 patients in the United States between 2000 and 2015 found that antibiotic agents were the most common class of culprit drug with Trimethoprim specifically designated as the culprit drug in 26.3% of cases they examined [14]. This is in contrast to our study where Trimethoprim-Sulfamethoxazole was the second most common culprit agent –responsible for 25/131 (19%) of cases. It is important to note that the Micheletti study was performed between the same years as our study.
Additionally, a study performed on 928 patients in China from 2000 to 2015 found that antibiotics were the most common class of culprit drugs among their patient population—although no data about specific agents was reported [9]. A smaller study performed on 41 patients in Brazil from 2007 to 2017 also found antibiotics to be the most common class of culprit drugs with Beta-lactams as the most common agents [7]. Finally, a study performed on 189 patients in Malaysia between 2006–2015 found that antibiotics were the most common class of culprit drug, specifically Beta-lactams however the most common specific agent among their entire study population was allopurinol [13]. This is in contrast to our data where Trimethoprim-Sulfamethoxazole was our most common single inciting agent (25 cases) followed by lamotrigine (21 cases) and then allopurinol (11 cases).
The proportion of our patients with allopurinol-induced reactions (11/131; 8.4%) was very similar to the proportion of patients with allopurinol-induced reactions in the Michelletti et al. study (8.6%). Compared to Micheletti et al. study we did however have a larger proportion of patients with NSAID-induced reactions 8.4% (11/131) compared to 5.3%, and Fluoroquinolone-induced reactions, 7.6% (10/131) compared to 3.6%. Our proportion of anticonvulsant-induced studies was higher compared to Micheletti et al., 30% (39/131) vs 23.7%, while out proportion of Trimethoprim-Sulfamethoxazole induced reactions was less compared to their study, 19% (25/131) vs 26.3%.
The “other antibiotics” category included 3 cases of macrolide-induced reactions (azithromycin (1/3 reactions) and erythromycin (2/3 reactions)). A review paper published in 2020 analyzed 25 publications with a total of 27 patients who were suspected to have macrolide-induced SJS/TENS reactions. Of those cases, azithromycin was responsible for 11/27, clarithromycin was responsible for 7/27 cases, and erythromycin was responsible for 5/27 cases. While macrolides were only found to be responsible for a minority of our reported drug reactions (3/131), they are some of the most commonly prescribed antibiotics in practice and thus it is important to understand that they can be inducers of SJS/TENS [55].
4.3. Culprit drugs by year
In our study population, the incidence of anticonvulsant-induced has remained relatively stable from 2000 to 2019 being responsible for 29% (13/45) of cases from 2000 to 2009 and 30% (26/86) of cases from 2010 to 2019. Additionally, the incidences of Fluoroquinolone-induced reactions, 6.7% (3/45) of cases from 2000 to 2009 and 8.1% (7/86) of cases from 2010 to 2019, have also remained stable (Table 3).
From 2000 to 2009 there were no cases in which immunosuppressants were found to be the most probable culprit agents while from 2010 to 2019 there were 2 cases. Additionally, the incidence of allopurinol-induced reactions increased from 4.4% (2/45) in 2000–2009 to 10% (9/86) in 2010–2019. In contrast, the incidence of NSAID-induced reactions has decreased from 11% in 2000–2009 to 7.0% in 2010–2019 (Table 3).
4.4. Culprit drugs and spectrum diagnosis
From 2000 to 2019, the most common class of inciting agents in patients with TENS spectrum presentations was anti-convulsants (29%; 18/63) followed by Trimethoprim-Sulfamethoxazole (21%; 13/63) and Beta-lactams (16%; 10/63). In patients with SJS spectrum presentations, anticonvulsants were also the most common class of inciting agent (32%; 14/44) followed by both Trimethoprim-Sulfamethoxazole (16%; 7/44) and Fluoroquinolones (16%; 7/44). Among patients with SJS-TEN Overlap Syndrome presentation, anticonvulsants (29%; 7/24) and Trimethoprim-Sulfamethoxazole (21%; 5/24) were the most common classes of inciting agents (Table 3, Fig. 4).
Fig. 4 –
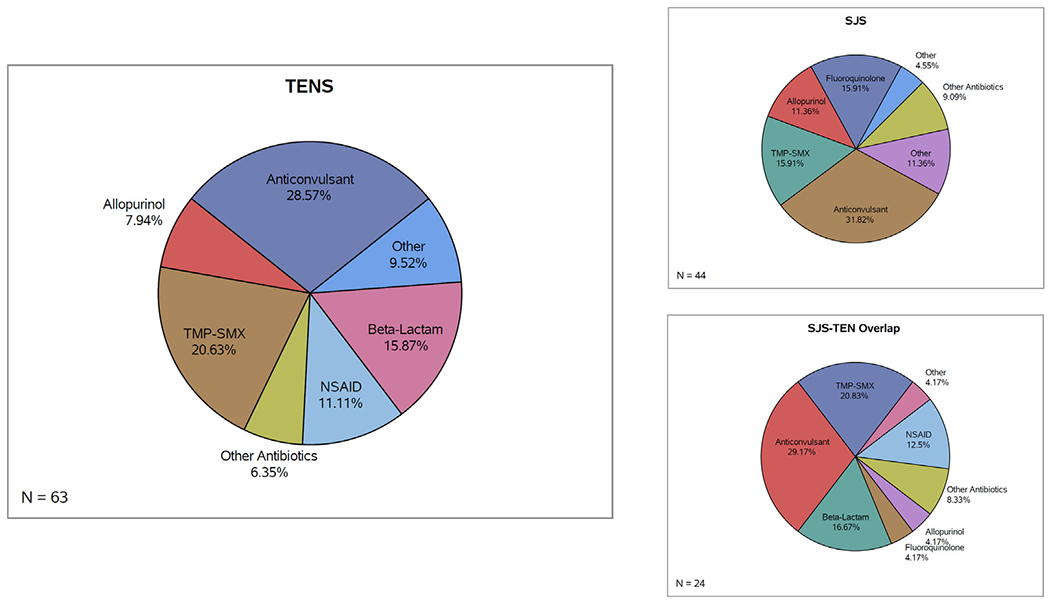
Drug class among SJS, TENS, and SJS-TEN Overlap Syndrome patients.
Anticonvulsants: lamotrigine, phenytoin, carbamazepine, valproic acid, levitiracem, gabapentin; TMP-SMX: Trimethoprim-Sulfamethoxazole; Beta-lactam: ceftriaxone, ampicillin, piperacillin-tazobactam, penicillin G, amoxicillin, amoxicillin-clavulanate, imipenem; NSAIDs: ibuprofen, naproxen; Fluoroquinolones: ciprofloxacin, oxacillin, levofloxacin, moxifloxacin; other antibiotics: azithromycin, erythromycin, vancomycin, minocycline, rifampin, clindamycin; Other: acetaminophen, propylthiouracil, oseltamivir, loratadine, butalbital, amlodipine, possible mycoplasma infection; Immunosuppressants: rituximab, methotrexate.
Both of the cases involving immunosuppressants as the most probable inciting agent led to TENS presentation of the disease. Interestingly, a large proportion of NSAID-induced reactions (64%; 7/11) were TENS spectrum presentations while a large portion of Fluoroquinolone-induced reactions (70%; 7/10) were SJS spectrum presentations (Table 3).
4.5. Limitations
Limitations of this study include biases that typically accompany retrospective studies including bias in diagnosis and selection of cases as well as the non-standardized determination of causality by treating providers. Additionally, it was difficult to account for all relevant confounders that could affect a patient’s reaction to a drug. There is also a general heterogeneity of the patient population, supportive care, as well as pharmacotherapeutic decisions implemented by providers. There also may have been errors in data entry either during initial input into the EMR system or during the collection phase of our study.
Available information on %TBSA and other relevant clinical data from cases that fell within the 2002–2005-time interval was limited. Thus, SJS spectrum classifications as well as a single probable inciting agent could not be accurately determined for many of these cases. We hope that by tracking inciting agents in future cases we will be able to determine the most probable inciting agent using ALDEN in the context of clinical presentation—with more accurate and accessible information available through EMR.
4.6. Future directions
As previously discussed, due to the nature of patients being transferred emergently from other hospitals with suspected SJS, it can be hard to retrospectively obtain exact information pertaining to the patient’s current medications and when medications were started. Additionally, it can be difficult to know whether the patient was on the drug before and whether or not they experienced an allergic reaction. A great deal of this information would likely be more accurately obtained if patients were asked ALDEN relevant questions during their SJS admission. We plan to make this a standard in the LUMC Burn Unit.
Further, a 2019 case report by Kim et al. lymphocyte activate testing (LAT) reported that lymphocyte activation tests (LATs) may be a useful method of culprit drug identification in patients who have experienced SJS/TENS culprit drug reactions. The benefits of this in vitro methodology include the high specificity of 97.8% and the avoidance of having to re-challenge the drug in the patient however the low sensitivity of 16.75% is a downside of the method [56]. Additionally, with the widespread advancement in genetic techniques, the widespread HLA genotyping of individuals who have suffered SJS/TENS reactions may help to gain a more solid understanding of the different HLA genotypes among these patient and help solidify any associations between specific HLA genotype and a specific culprit drug [56].
The EuroSCAR study collected data on culprit drugs among patients from 1997 to 2001. The ALDEN algorithm was validated using this data and the drug notoriety table for the ALDEN algorithm was obtained using this data set. If there are significant trends in most common culprit drugs across time, the drug notoriety database used for ALDEN may need to be updated and constructed using data from more recent years. Additionally, the drug notoriety database was constructed using cases from Europe (EuroSCAR). However, literature suggests that the most common culprit drugs vary greatly by region. Thus, if ALDEN is to be used in the future as a uniform algorithm for determining a most probable culprit drug in patient cases, we believe that the drug notoriety scale may need to be region-specific.
5. Conclusion
This is one of the largest single center series of SJS/TENS/SJS-TEN Overlap Syndrome cases in the US. Between 2000–2019 there has been an increased proportion of patients presenting with SJS as well as a decreased proportion of patients presenting with TENS. In Loyola/Chicagoland patients admitted to LUMC, anticonvulsants, Trimethoprim-Sulfamethoxazole, and Beta-lactams continue to be the most common culprit agents. Allopurinol, NSAIDs, and Fluoroquinolones have also been shown to be common inciting agents. The incidence of allopurinol as a culprit agent has increased from 2000–2009 to 2010–2019. For the more severe TENS cases, the anti-convulsant lamotrigine (9 out of the 18 anticonvulsant cases for TENS) was the most common inciting agent.
Our data supports trends presented in the EuroSCAR (1997–2001) and RegiSCAR (2003–2012) studies. The ALDEN algorithm provides an important and validated method for determining the probable culprit drug in SJS/TENS spectrum reactions. Trends in culprit drugs can shift over time based on changes in prescribing practices. Different inciting agents may lead to more or less severe presentations of SJS/TENS spectrum reactions. Therefore, these trends need to be monitored in order to advise providers on which agents present the greatest risk to patients.
Acknowledgements
The authors would like to thank the Loyola Clinical Research Office, Illinois Society for the Prevention of Blindness, and the Perritt Charitable Foundation for their support of this research project.
Funding
This work was supported by the Illinois Society for the Prevention of Blindness; and The Richard A. Perritt Charitable Foundation.
Footnotes
Declarations of interest
None.
REFERENCES
- [1].White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract 2018;6(January–February (1)):38–69, doi: 10.1016/j.jaip.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Oakley AM, Krishnamurthy K. Stevens Johnson Syndrome. StatPearls Treasure Island (FL): StatPearls Publishing; 2020. Copyright © 2020, StatPearls Publishing LLC. [PubMed] [Google Scholar]
- [3].Ueta M. Genetic predisposition to Stevens-Johnson Syndrome with severe ocular surface complications. Cornea 2015;34(November (Suppl 11)):S158–65, doi: 10.1097/ico.0000000000000605. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 201369(August (2)), doi: 10.1016/j.jaad.2013.05.002 187.e1–16; quiz 203–204. [DOI] [PubMed] [Google Scholar]
- [5].Rzany B, Hering O, Mockenhaupt M, Schröder W, Goerttler E, Ring J, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 1996;135(July (1)):6–11. [PubMed] [Google Scholar]
- [6].Howland WW, Golitz LE, Weston WL, Huff JC. Erythema multiforme: clinical, histopathologic, and immunologic study. J Am Acad Dermatol 1984;10(March (3)):438–46, doi: 10.1016/s0190-9622(84)80090-0. [DOI] [PubMed] [Google Scholar]
- [7].Medeiros MP, Carvalho CHC, Santi CG, Avancini J. Stevens-Johnson syndrome and toxic epidermal necrolysis - retrospective review of cases in a high complexity hospital in Brazil. Int J Dermatol 2020;59(February (2)):191–6, doi: 10.1111/ijd.14544. [DOI] [PubMed] [Google Scholar]
- [8].Miliszewski MA, Kirchhof MG, Sikora S, Papp A, Dutz JP. Stevens-Johnson Syndrome and toxic epidermal necrolysis: an analysis of triggers and implications for improving prevention. Am J Med 2016;129(November (11)):1221–5, doi: 10.1016/j.amjmed.2016.03.022. [DOI] [PubMed] [Google Scholar]
- [9].Deng Q, Fang X, Zeng Q, Lu J, Jing C, Huang J. Severe cutaneous adverse drug reactions of Chinese inpatients: a meta-analysis. An Bras Dermatol 2017;92(May–June (3)):345–9, doi: 10.1590/abd1806-4841.20175171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128(January (1)):35–44, doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- [11].Yamane Y, Matsukura S, Watanabe Y, Yamaguchi Y, Nakamura K, Kambara T, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients—treatment and outcome. Allergol Int 2016;65(January (1)):74–81, doi: 10.1016/j.alit.2015.09.001. [DOI] [PubMed] [Google Scholar]
- [12].Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Searching for the culprit drugs for Stevens-Johnson Syndrome and toxic epidermal necrolysis from a nationwide claim database in Korea. J Allergy Clin Immunol Pract 20208(February (2)), doi: 10.1016/j.jaip.2019.09.032. 690–695.e2 [DOI] [PubMed] [Google Scholar]
- [13].Loo CH, Tan WC, Khor YH, Chan LC. A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia. Med J Malaysia 2018;73(April (2)):73–7. [PubMed] [Google Scholar]
- [14].Micheletti RG, Chiesa-Fuxench Z, Noe MH, Stephen S, Aleshin M, Agarwal A, et al. Stevens-Johnson Syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol 2018;138(11):2315–21, doi: 10.1016/j.jid.2018.04.027. [DOI] [PubMed] [Google Scholar]
- [15].Chen CB, Chen YE, Chu MT, Wang CW, Chung-Yee Hui R, Lu CW, et al. The risk of anti-osteoporotic agent-induced severe cutaneous adverse drug reactions and their association with HLA. J Eur Acad Dermatol Venereol 2021;35(September):712–20, doi: 10.1111/jdv.16924. [DOI] [PubMed] [Google Scholar]
- [16].Torres-Navarro I, de Unamuno-Bustos B, Botella-Estrada R. Systematic review of BRAF/MEK inhibitors-induced Severe Cutaneous Adverse Reactions (SCARs). J Eur Acad Dermatol Venereol 2020(August), doi: 10.1111/jdv.16894. [DOI] [PubMed] [Google Scholar]
- [17].Lin CC, Chen CB, Wang CW, Hung SI, Chung WH. Stevens-Johnson syndrome and toxic epidermal necrolysis: risk factors, causality assessment and potential prevention strategies. Expert Rev Clin Immunol 2020;16(April (4)):373–87, doi: 10.1080/1744666x.2020.1740591. [DOI] [PubMed] [Google Scholar]
- [18].Phillips EJ. In: Bouchard C, editor. NATIENS synopsis. [Google Scholar]
- [19].Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol 2016;136(July (7)):1387–97, doi: 10.1016/j.jid.2016.03.023. [DOI] [PubMed] [Google Scholar]
- [20].Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14(December (12)):1343–50, doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- [21].Chung WH, Chang WC, Stocker SL, Juo CG, Graham GG, Lee MH, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis 2015;74(December (12)):2157–64, doi: 10.1136/annrheumdis-2014-205577. [DOI] [PubMed] [Google Scholar]
- [22].Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson Syndrome/toxic epidermal necrolysis. J Invest Dermatol 2017;137(May (5)):1065–73, doi: 10.1016/j.jid.2016.11.034. [DOI] [PubMed] [Google Scholar]
- [23].Chang WC, Abe R, Anderson P, Anderson W, Ardern-Jones MR, Beachkofsky TM, et al. SJS/TEN 2019: from science to translation. J Dermatol Sci 2020;98(April (1)):2–12, doi: 10.1016/j.jdermsci.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008;358(February (6)):568–79, doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- [25].Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011;364(March (12)):1126–33, doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- [26].Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med 2013;369(October (17)):1620–8, doi: 10.1056/NEJMoa1213096. [DOI] [PubMed] [Google Scholar]
- [27].Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 2015;351(September):h4848, doi: 10.1136/bmj.h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ueta M Stevens-Johnson syndrome/toxic epidermal necrolysis with severe ocular complications. Expert Rev Clin Immunol 2020;16(March (3)):285–91, doi: 10.1080/1744666X.2020.1729128. [DOI] [PubMed] [Google Scholar]
- [29].Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2004;428(April (6982)):486, doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- [30].Locharernkul C, Loplumlert J, Limotai C, Korkij W, Desudchit T, Tongkobpetch S, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia 2008;49(December (12)):2087–91, doi: 10.1111/j.1528-1167.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- [31].Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet 2011;20(March (5)):1034–41, doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- [32].McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med 2011;364(March (12)):1134–43, doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;102(March (11)):4134–9, doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008;18(February (2)):99–107, doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- [35].Tohkin M, Kaniwa N, Saito Y, Sugiyama E, Kurose K, Nishikawa J, et al. A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenomics J 2013;13(February (1)):60–9, doi: 10.1038/tpj.2011.41. [DOI] [PubMed] [Google Scholar]
- [36].Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H, et al. A marker for Stevens-Johnson syndrome … : ethnicity matters. Pharmacogenomics J 2006;6(July–August (4)):265–8, doi: 10.1038/sj.tpj.6500356. [DOI] [PubMed] [Google Scholar]
- [37].Fang H, Xu X, Kaur K, Dedek M, Zhu GD, Riley BJ, et al. A screening test for. Front Pharmacol 2019;10:149, doi: 10.3389/fphar.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol 2015;136(August (2))219–34, doi: 10.1016/j.jaci.2015.05.050 quiz 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shanbhag SS, Chodosh J, Fathy C, Goverman J, Mitchell C, Saeed HN. Multidisciplinary care in Stevens-Johnson syndrome. Ther Adv Chronic Dis 2020;11:2040622319894469, doi: 10.1177/2040622319894469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zavala S, O’Mahony M, Joyce C, Baldea AJ. How does SCORTEN score? J Burn Care Res 2018;39(June (4)):555–61, doi: 10.1093/jbcr/irx016. [DOI] [PubMed] [Google Scholar]
- [41].Dodiuk-Gad RP, Olteanu C, Jeschke MG, Cartotto R, Fish J, Shear NH. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol 201573(November (5)), doi: 10.1016/j.jaad.2015.08.008 876–877 e2. [DOI] [PubMed] [Google Scholar]
- [42].Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic immunomodulating therapies for Stevens-Johnson Syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol 2017;153(June (6)):514–22, doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998;352(November (9140)):1586–9, doi: 10.1016/S0140-6736(98)02197-7. [DOI] [PubMed] [Google Scholar]
- [44].Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest 2018;128(March (3)):985–96, doi: 10.1172/JCI93349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Paulmann M, Mockenhaupt M. Severe drug hypersensitivity reactions: clinical pattern, diagnosis, etiology and therapeutic options. Curr Pharm Des 2016;22(45):6852–61, doi: 10.2174/1381612822666160928125152. [DOI] [PubMed] [Google Scholar]
- [46].Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 2010;88(July (1)):60–8, doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- [47].Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000;136(March (3)):323–7. [DOI] [PubMed] [Google Scholar]
- [48].Downey A, Jackson C, Harun N, Cooper A. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol 2012;66(June (6)):995–1003, doi: 10.1016/j.jaad.2011.09.029. [DOI] [PubMed] [Google Scholar]
- [49].Mockenhaupt M. Bullous drug reactions. Acta Derm Venereol 2020;100(February (5)):adv00057, doi: 10.2340/00015555-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995;333(December (24)):1600–7, doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- [51].Vujic I, Shroff A, Grzelka M, Posch C, Monshi B, Sanlorenzo M, et al. Mycoplasma pneumoniae-associated mucositis—case report and systematic review of literature. J Eur Acad Dermatol Venereol 2015;29(March (3)):595–8, doi: 10.1111/jdv.12392. [DOI] [PubMed] [Google Scholar]
- [52].Meyer Sauteur PM, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis—part of the Stevens-Johnson syndrome spectrum. J Dtsch Dermatol Ges 2012;10(October (10)):740–6, doi: 10.1111/j.1610-0387.2012.07951.x. [DOI] [PubMed] [Google Scholar]
- [53].Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72(February (2)):239–45, doi: 10.1016/j.jaad.2014.06.026. [DOI] [PubMed] [Google Scholar]
- [54].Park CS, Kang DY, Kang MG, Kim S, Ye YM, Kim SH, et al. Severe cutaneous adverse reactions to antiepileptic drugs: a nationwide registry-based study in Korea. Allergy Asthma Immunol Res 2019;11(September (5)):709–22, doi: 10.4168/aair.2019.11.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pejčić AV. Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of macrolide antibiotics: a review of published cases. Int J Dermatol 2020(August), doi: 10.1111/ijd.15144. [DOI] [PubMed] [Google Scholar]
- [56].Kim EY, Kim MY, Park CS, Choi JH, Ghim JL, Kim HS, et al. Antiepileptic drug-induced severe cutaneous adverse reactions and HLA alleles: a report of five cases with lymphocyte activation test. Transl Clin Pharmacol 2019;27(June (2)):64–8, doi: 10.12793/tcp.2019.27.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]


