Abstract
Objective(s):
Calcium dobesilate (CaD) has anti-oxidant, anti-inflammatory, and anti-apoptotic effects. In this study, the protective effects of CaD against hepatorenal damage induced by carbon tetrachloride (CCl4) in mice were evaluated.
Materials and Methods:
Thirty male mice were randomly divided into five groups: Control, CaD 100 mg/kg, CCl4, CCl4+CaD 50 mg/kg, and CCl4+CaD 100 mg/kg. CaD (50 and 100 mg/kg) was administered orally once a day for 4 weeks. The liver and kidney indices (serum creatinine, blood urine nitrogen, alanine aminotransferase, and aspartate aminotransferase levels) were determined. Also, liver and kidney tissue oxidant/anti-oxidant markers (glutathione peroxidase, malondialdehyde, total anti-oxidant capacity, and superoxide dismutase) were measured. Cleaved caspase-3, Bax, cytochrome-c, and Bcl-2 protein levels were measured by immunoblotting method in the liver and kidney tissues. The liver and kidney histopathological changes were evaluated by the Hematoxylin and Eosin (H&E) staining method.
Results:
CCl4 induced significant oxidative stress and apoptosis in kidney and liver tissues that was concomitant with histopathological abnormalities in these organs in the CCl4 group versus the control (P<0.05). However, CaD (100 mg/kg) could significantly improve the histopathological change in the liver and kidney tissues of CCl4+CaD 100 mg/kg mice versus the CCl4 group (P<0.05). In addition, CaD (100 mg/kg) attenuated the pro and anti-apoptotic markers in the liver and kidney tissues of CCl4+CaD 100 mg/kg mice versus the CCl4 group (P<0.05).
Conclusion:
CaD (100 mg/kg) has a protective effect against hepatorenal injury induced by CCl4 at least via its anti-apoptotic and anti-oxidant properties.
Key Words: Apoptosis, Calcium dobesilate, Carbon tetrachloride, Mice, Oxidative stress
Introduction
Carbon tetrachloride (CCl4), an industrial solvent, has been used in animal models to explore renal injury in rats (1, 2). It was indicated that the kidney is not the only objective organ of CCl4 toxicity, it causes damage in other organs, such as the liver (3), brain (4, 5), testis (6, 7), lungs (8), and blood (9) by generation of free radicals. During the metabolism of CCl4, trichloromethyl (CCL3) free radicals cause lipid peroxidation (10, 11) and trichloromethyl peroxyl radical (CCl3O2) causes renal injuries (12). Damage by CCl4 includes altering the endogenous anti-oxidants in tissues which are manifested by histopathological lesions (13). Furthermore, liver and kidney triglycerides, cholesterol, and free fatty acids increase in the CCl4 administration animal (14). Anti-oxidants are essential substances that are capable of guarding the body against free radical-induced oxidative stress damage (15, 16). Calcium 2, 5-dihydroxybenzenesulfonate (calcium dobesilate; CaD) is a vascular protective drug that is used for treatment of diabetic retinopathy and chronic venous insufficiency (17, 18). CaD, as an angioprotective drug, can inhibit platelet activity and reduce blood viscosity and also capillary permeability (19). In addition, recent studies have demonstrated that CaD exerts protective effects against diabetic nephropathy (10). Additionally, CaD has multiple mechanisms of action that include anti-oxidant properties which reduce lipid pre-oxidation caused by free oxygen radicals and anti-inflammatory properties that decrease the release of inflammatory cytokines, such as platelet-activating factor (20-23). Moreover, it has been shown that CaD corrects capillary dysfunctions, decreases free oxygen radicals, increases nitric oxide synthase, and prevents desquamation in endothelium cells (24, 25). Considering the anti-inflammatory and anti-oxidant properties of CaD, we evaluated the protective effect of calcium dobesilate administration against hepatorenal damage induced by CCl4 in male mice.
Materials and Methods
Animal
In this experimental study, 30 male BALB/c mice (30±5 g) were acquired from Rafsanjan University of Medical Sciences Animal House, Rafsanjan, Iran. The male BALB/c mice were kept in a room with 12 hr light/dark cycle, a humidity of 45%, and a temperature of 20–23 °C with access to food and water ad libitum. The BALB/c mice were maintained in the colony room for one week before starting the experiment. The ethics committee of Rafsanjan University of Medical Sciences approved the study (IR.RUMS.REC.1399.055). Also, this study was done in agreement with the Guidelines for Animal Care and Use (National Institutes of Health Publication No. 85-23) revised in 2010.
Drugs
CCl4 (10 mmol/kg) (Sigma Chemical Co., St. Louis, MO, USA) was freshly dissolved in 50% olive oil (1:1) (26) for intraperitoneal (IP) administration. CaD was procured from Sigma-Aldrich Company, Germany. CaD (50 and 100 mg/kg) (27) was freshly prepared each day during the experiment, and it was dissolved in saline solution for oral administration.
Experimental groups and experimental protocol
Animals were randomly divided into 5 groups (six mice/group) as follows:
Group 1 (Control): This group received olive oil (as a vehicle of CCl4) by IP injection on the 1st day, then an hour later, animals were treated with saline by oral gavage daily for 4 weeks (28).
Group 2 (CaD): This group received olive oil by IP injection on the 1st day, then an hour later, animals were treated with CaD (100 mg/kg) (27) by oral gavage daily for 4 weeks (28).
Group 3 (CCl4): This group received CCl4 (10 mmol/kg) (26) in 50% olive oil (1:1) by IP injection on the 1st day, then an hour later, animals were treated with saline (oral gavage) daily for 4 weeks (28).
Group 4 (CCl4 + CaD 50 mg/kg): This group received CCl4 (10 mmol/kg) by IP injection on the 1st day, then an hour later, animals were treated with CaD (50 mg/kg) (27) by oral gavage daily for 4 weeks (28).
Group 5 (CCl4 + CaD 100 mg/kg): This group received CCl4 (10 mmol/kg) by IP injection on the 1st day, then an hour later, animals were treated with CaD (100 mg/kg) (27) by oral gavage daily for 4 weeks (28).
The mice received a single dose of olive oil or CCl4 (10 mmol/kg) on the 1st day. Then an hour later, each animal was given the same volumes of saline or CaD (50 or 100 mg/kg) during the experimental period. 24 hr after the last treatment, mice were anesthetized using diethyl ether. The paw pinch reflex as an indicator of anesthetic depth was checked in all animals just before blood sampling. Serum samples were prepared via blood centrifugation (6000 rpm for 15 min) at room temperature. After blood sampling, mice were sacrificed under deep anesthesia. The liver and kidney tissues were removed immediately and divided into two parts. One part was kept in formalin (10%) for histological evaluations by Hematoxylin and Eosin (H&E) staining. The other parts of liver and kidney tissues were homogenized in ice-cold lysis buffer solution (phosphate buffer solution; PBS) enriched with protease inhibitor cocktail (Sigma, USA). The homogenized samples were centrifuged for 20 min at 6000 rpm (at 4 °C); then the supernatant was harvested and kept at -80 °C until molecular evaluation of oxidative stress and apoptosis biochemical parameters (29-31).
Biochemical parameters measurement
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels were measured using the relevant kits (Pars Azmoon, Co., Tehran, Iran) by an automatic autoanalyzer (HECTIC, Japan). Serum creatinine (Cr) and blood urine nitrogen (BUN) levels were determined via quantitative commercial kits (Pars Azmoon, Co., Tehran, Iran) (32, 33).
Hepatorenal oxidative stress measurement
To evaluate oxidative stress status in the liver and kidney tissues, the glutathione peroxidase (GPx) and superoxide dismutase (SOD) enzyme activity levels, malondialdehyde (MDA) concentration, and total anti-oxidant capacity (TAC) status were measured via available commercial assay kits (ZellBio, Germany) according to the manufacturer’s instructions using an automatic microplate reader (BioTek, USA) (16, 34).
Hepatorenal apoptosis measurement
The immunoblotting method was done to evaluate cleaved caspase-3, cytochrome-c, Bax, and Bcl-2 proteins expression levels as apoptosis biomarkers in the liver and kidney tissues. In brief, each protein sample was isolated via 12.5% polyacrylamide gel electrophoresis and subsequently transferred to polyvinylidene difluoride (PVDF) membrane by electric current. The PVDF membranes were incubated (overnight at the temperature of 4 °C and pH 7.4) in blocking buffer (20 mM Tris–HCl, 150 mM NaCl, 0.1% Tween 20 with 5% nonfat milk). After 3 times (5 min each at room temperature) washing the membrane with washing buffer (20 mM Tris–HCl, 150 mM NaCl, 0.1% Tween 20), each PVDF membrane was incubated with rabbit polyclonal anti-Bax, anti-caspase-3, anti-cytochrome-c, and anti-Bcl-2 antibodies (1: 1000, Abcam, USA) for 3 hr at room temperature. Subsequently, the blots were washed with washing buffer and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000, Abcam, USA) at room temperature for 1 hr. Then each blot was detected via an enhanced chemiluminescence method. Band densitometry analysis was done using the ImageJ software. Beta (β)-actin immunoblotting (1:5000, Abcam, USA) was applied as a loading control (16, 30).
Histopathological analysis
The liver and kidney sections were stained using the routine H&E method (magnification 100×). Each stained slide was observed via a light microscope (Nikon Labophot, Japan) in a blind manner to score kidney and liver tissue damage (KTDS and LTDS) for histopathological analysis. Each kidney section was measured for inflammatory cell infiltration, glomerular atrophy, and tubular necrosis. The kidney tubulointerstitial injury was evaluated semi-quantitatively according to previous studies (16, 30). Also, each liver section was measured for congestion and pyknosis. KTDS and LTDS were graded from 0 to 3 by a pathologist blinded to our study (0-0.5= normal, 1= mild, 2= moderate, and 3= severe) (32, 35, 36).
Data analysis
Data were analyzed using the GraphPad Prism software package (ver. 6.01, GraphPad Software, USA). The normality of the results was analyzed using the Kolmogorov-Smirnov test. The results are expressed as mean ± SD. For parametric data, one-way ANOVA followed by Tukey’s test was done to evaluate the significance level between the groups. For non-parametric data, the Kruskal-Wallis test was performed. P<0.05 was considered a significant level.
Results
Effect of calcium dobesilate on biochemical parameters
The results showed that serum Cr, BUN, AST, and ALT levels were increased in the CCl4-treated mice compared with the control group (P<0.01) (Table 1).
Table 1.
Effect of calcium dobesilate on functional indicators of the kidney and liver in mice with CCl4 administration. Values are expressed as mean ± SD n= 6 per group. ٭٭P˂0.01 versus the control group. #P˂0.05 and ##P˂0.01 versus CCL4 group
CCl4: Carbon tetrachloride, CaD: Calcium dobesilate, BUN: Blood urea nitrogen, Cr: Creatinine, AST: Aspartate aminotransferase, ALT: Alanine transaminase
CaD (50 and 100 mg/kg) did significantly affect BUN as compared with the CCl4 administrated group. In addition, CaD (100 mg/kg) reduced the Cr level in the CCl4+CaD 100 mg/kg group compared with CCl4-treated mice (P<0.05). Furthermore, CaD (100 mg/kg) could significantly reduce both AST (P<0.05) and ALT (P<0.01) levels in the CCl4+CaD 100 mg/kg group compared with the CCl4 administrated mice (Table 1). However, CaD (50 mg/kg) had no significant effect on AST (P=0.996) and ALT (P=0.989) levels in the CCl4+CaD 50 mg/kg group versus the CCl4 group (Table 1).
Effect of calcium dobesilate on oxidative stress parameters
Free radical injury after CCl4 administration was evaluated using lipid peroxidation in the kidney and liver tissues, which was assessed by MDA concentration. According to Table 2, CCl4 significantly increased the MDA concentration in kidney and liver tissues in mice in comparison with the control group (P<0.05). Administration of CaD (100 mg/kg) significantly decreased the MDA concentration in the kidney (P<0.05) and liver (P<0.01) tissues in the CCl4 + CaD 100 mg/kg group versus CCl4 mice (Table 2). Moreover, CaD (50 mg/kg) could decrease the MDA concentration significantly in the liver tissue in CCl4 + CaD 50 mg/kg group when compared with the CCl4-group (P<0.05, Table 2).
Table 2.
Effect of calcium dobesilate on oxidative stress parameters of kidney and liver tissues in mice with CCl4 administration. Values are expressed as mean ± SD. n= 6 per group. ٭P˂0.05 and ٭٭P˂0.01 versus the control group. #P˂0.05 and ##P˂0.01 versus the CCl4 group
CCl4: Carbon tetrachloride, CaD: Calcium dobesilate, MDA: Malondialdehyde, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, TAC: Total antioxidant capacity
According to the results, CCl4 significantly decreased SOD and GPx activity (as two important anti-oxidant enzymes) in the kidney and liver tissues in the CCl4-group compared with the control mice (P<0.01, Table 2). As shown in Table 2, administration of CaD (50 and 100 mg/kg) could significantly increase the SOD activity level in the kidney and liver tissues in CCl4 + CaD 50 mg/kg and CCl4 + CaD 100 mg/kg mice versus CCl4 mice (P<0.05). Furthermore, CaD (50 and 100 mg/kg) significantly elevated the GPx activity in the liver tissue in CCl4 + CaD 50 mg/kg and CCl4 + CaD 100 mg/kg mice when compared with CCl4 mice (P<0.05, Table 2). Also, administration of CaD (100 mg/kg) significantly increased the GPx activity (P<0.05) in the kidney of the CaD treated mice compared with the CCl4-group. However, no significant difference was seen in the kidney GPx activity level in CCl4 + CaD 50 mice versus the CCl4 group (Table 2).
According to Table 2, CCl4 significantly reduced the TAC level in kidney (P<0.01) and liver (P<0.05) tissues in mice rather than in controls. Also, CaD (100 mg/kg) could significantly increase the TAC level in kidney and liver tissues in the CCl4 + CaD 100 mg/kg group compared with the CCl4-group (P<0.05, Table 2).
Effect of calcium dobesilate on kidney and liver apoptosis
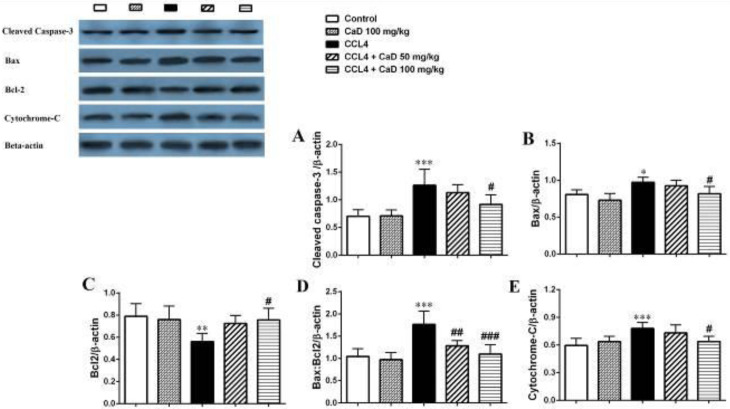
Our result also showed that CCl4 significantly increased the cleaved caspase-3, Bax, and cytochrome-c proteins expression levels in the kidney and liver tissues in the CCl4 administered mice compared with the control group (P<0.05, Figures 1 and 2). However, CaD (100 mg/kg) could decrease the cleaved caspase-3, Bax, and cytochrome-c proteins expression levels in the kidney and liver tissues in the CCl4 + CaD 100 mg/kg treated group versus the CCl4 group (P<0.05, Figures 1 and 2).
Figure 1.
Western blot analysis of the cleaved caspase-3 (A), Bax (B), Bcl-2 (C), Bax/Bcl-2 ratio (D), and cytochrome-c (E) proteins expression in the kidney tissue of mice with CCl4 administration. Values are expressed as mean ± SD. n= 6 per group
*P˂0.05, ٭٭P˂0.01, and ٭٭٭P˂0.001 versus the control group. #P˂0.05, ##P˂0.01, and ###P˂0.001 versus CCl4 group. CCl4: Carbon tetrachloride, CaD: Calcium dobesilate
Figure 2.
Western blot analysis of the cleaved caspase-3 (A), Bax (B), Bcl-2 (C), Bax/Bcl-2 ratio (D), and cytochrome-c (E) proteins expression in the liver tissue of the mice with CCL4 administration. Values are expressed as mean ± SD. n= 6 per group
*P˂0.05, ٭٭P˂0.01, and ٭٭٭P˂0.001 versus the control group. #P˂0.05, ##P˂0.01, and ###P˂0.001 versus CCl4 group. CCl4: Carbon tetrachloride, CaD: Calcium dobesilate
Moreover, as shown in Figures 1C and 2C, CCl4 significantly decreased the Bcl-2 protein expression level in the kidney and liver tissues in the CCl4 administered group rather than the control (P<0.05). Also, CaD at doses of 100 mg/kg increased the Bcl-2 protein expression level in the kidney and liver tissues in CCl4 + CaD 100 mg/kg treated mice compared with CCl4 administered mice (P<0.05, Figures 1C and 2C).
As shown in Figures 1D and 2D, CaD at the dose of 100 mg/kg significantly decreased the Bax:Bcl-2 ratio in the kidney tissue in the CCl4 + CaD 100 mg/kg group compared with CCl4 administered mice (P<0.001). Also, CaD at doses of 50 and 100 mg/kg significantly decreased the Bax:Bcl-2 ratio in the liver tissue (P<0.01 and P<0.001, respectively) in CaD-treated groups (CCl4 + CaD 50 mg/kg and CCl4 + CaD 100 mg/kg) compared with CCl4 administered mice.
Effect of calcium dobesilate on kidney and liver tissue damage
The H&E staining results showed that there was no pathologic condition in the kidney and liver tissues of the control group (Figures 3A and 4A). Furthermore, the histopathologic results showed that CCl4 increases kidney and liver damage, so that there were mild and severe kidney and liver damages in CCl4 administered mice when compared with the control group (P<0.001, Figures 3B and 4B), and CaD (100 mg/kg) could attenuate the kidney and liver tissue damage caused by CCl4 (Figures 3A and 4A). Therefore, KTDS and LTDS were significantly decreased in the CCl4 + CaD 100 mg/kg treated mice when compared with the CCl4 administered group (P<0.05 and P<0.01, respectively, Figures 3B and 4B).
Figure 3.
Hematoxylin and Eosin-stained sections (magnification 100×) in the kidney tissue of mice with CCl4 administration. Scores 1–3 were considered as leukocyte infiltration, glomerular atrophy, and tubular necrosis in the kidney tissue sections. However, scores 0 to 0.5 were considered normal tissue. B: Kidney tissue damage score (KTDS) in CCl4-induced hepatorenal damage mice, four weeks after administration of saline/CaD. Values are expressed as mean ± SD. n= 6 per group. Arrow: glomerular atrophy/tubular necrosis; Circle: leukocyte infiltration
٭٭٭P˂0.001 versus the control group. #P˂0.05 versus CCl4 group. CCl4: Carbon tetrachloride, CaD: Calcium dobesilate
Figure 4.
Hematoxylin and Eosin-stained sections (magnification 100×) in the liver tissue of mice with CCl4 administration. 1: Scores 1–3 were considered as congestion, fat deposit, and pyknotic cells in the liver tissue sections. However, scores 0 to 0.5 were considered normal tissue. 2: liver tissue damage score (LTDS) in CCl4 induced hepatorenal damage mice, four weeks after administration of saline/CaD. Values are expressed as mean ± SD. n= 6 per group. ٭٭٭P˂0.001 versus the control group. Arrow: pyknosis/fat deposit; Circle: congestion. ##P˂0.01 versus CCl4 group
CCl4: Carbon tetrachloride, CaD: Calcium dobesilate
Discussion
In the present study, we examined the protective effects of CaD (50 and 100 mg/kg) against hepatorenal damage induced by CCl4 in mice. Our result showed that CCl4 increases BUN, Cr, ALT, and AST levels. In line with our study, it has been shown that CCl4 treatment causes significant hepatorenal injuries and increases BUN, Cr, ALT, and AST levels (37, 38). Our results also showed that CCl4 increases the MDA levels and decreases GPx, SOD activities, and TAC in the liver and kidney tissues. In accordance with our results, it has been reported that CCl4 decreases SOD, GPx, and catalase activity in the kidney and also induces oxidative stress in the liver and kidney tissues (37, 38). Moreover, our results showed that administration of CaD at the dose of 100 mg/kg can attenuate the hepatorenal injuries induced by CCl4 via reducing BUN, Cr, AST, and ALT levels (Table 1). Also, our results showed that CaD (100 mg/kg) can ameliorate oxidative stress via decreasing MDA concentration and increasing the GPx, SOD activities, and TAC in the liver and kidney tissues of CCl4 administrated mice (Table 2). In this regard, previous reports revealed that CaD has anti-oxidant properties (39); Seker et al. found that CaD has protective effects against intestinal ischemia-reperfusion injury via increasing the TAC indicator (22). In another study, it was revealed that CaD reduces the retinal injury induced by ischemia/reperfusion by increasing the content of GPx (40). Moreover, it has been shown that CaD is a potent free radical scavenger and reduces the level of MDA (as a marker of lipid peroxidation) (41). Besides, over-generation of MDA could result in pathological changes in the kidneys and liver (30, 42). Our results also showed that CCl4 can induce apoptosis via increasing cleaved caspase-3, Bax, and cytochrome-c proteins expression levels, and lowering Bcl-2 protein expression level in the kidney and liver (Figures 1 and 2). However, CaD (100 mg/kg) could attenuate apoptosis in the liver and kidney tissues via at least decreasing the cleaved caspase-3, Bax, and cytochrome-c proteins expression levels and increasing the Bcl-2 protein expression level. In line with our study, Zhang et al. reported that CaD has an important role in inhibiting apoptosis factors in the kidney and liver tissues (43). On the other hand, it is specified that oxidative stress triggers apoptosis (44), which results in liver and kidney degeneration (45). In our study, it is possible that CaD (100 mg/kg) also has attenuated the hepatorenal apoptosis induced by CCl4 via reducing oxidative stress along with ameliorating the pro- and anti-apoptotic markers. Furthermore, our histopathologic results showed that CCl4 increases kidney and liver damage, and CaD (100 mg/kg) could attenuate KTDS and LTDS induced by CCl4 (Figures 3 and 4). In the present study, renal tubular necrosis, glomerular atrophy, renal leukocyte infiltration, hepatic congestion, sinusoid dilatation, and pyknosis of hepatocytes, can be regarded as proof of hepatorenal damage in addition to increased abovementioned biochemical parameters in CCl4 administered mice. Moreover, the liver and kidney histological results are in accord with the abovementioned changes in the biochemical and oxidative factors. In favor of our results, it has previously been shown that CCl4 induces oxidative damage and increases histopathological change in the liver and kidney tissue (46). In this regard, it has been reported that CaD can reduce the renal histopathological change induced by gentamicin and ameliorate nephrotoxicity (47).
Conclusion
Our study showed that CaD at the dose of 100 mg/kg can decrease BUN and Cr levels in the CCl4 administrated mice. Also, CaD (100 mg/kg) can attenuate the oxidative stress in the kidney and liver tissue of the CCl4 administrated mice via its anti-oxidant effect. Moreover, CaD (100 mg/kg) could ameliorate the apoptotic indicators and histopathological changes in the liver and kidney tissues of the CCl4 administered mice. Overall, the findings of the present study showed that CaD (100 mg/kg) possibly has a protective effect against CCl4 hepatorenal toxicity. However, additional studies must be performed to improve understanding of the mechanisms and degree of this protective effect.
Authors’ Contributions
JH Conceived and designed the experiments; EH, AK, and JH Performed the experiments; AK, MR, MA, and JH Analyzed the data; MA, MR, and JH Contributed reagents/materials/analysis tools; and AK, EH, and JH Wrote the article.; All authors agree to be accountable for all aspects of the work, ensuring integrity and accuracy. The authors declare that all data were generated in-house and that no paper mill was used.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgment
This study was supported by Rafsanjan University of Medical Sciences, Rafsanjan, Iran (Grant # 98274).
References
- 1.Khan MR, Rizvi W, Khan GN, Khan RA, Shaheen S. Carbon tetrachloride-induced nephrotoxicity in rats: protective role of Digera muricata. J Ethnopharmacol. 2009;122:91–99. doi: 10.1016/j.jep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Khan RA, Khan MR, Sahreen S, Bokhari J. Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem Toxicol. 2010;48:2469–2476. doi: 10.1016/j.fct.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)-induced oxidative damage in rats. Chem Biol Interact. 2008;171:283–293. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Guzik-Kornacka A, Sliwa A, Plucinska G, Lukasiuk K. Status epilepticus evokes prolonged increase in the expression of CCl3 and CCl4 mRNA and protein in the rat brain. Acta Neurobiol Exp (Wars) 2011;71:193–207. doi: 10.55782/ane-2011-1840. [DOI] [PubMed] [Google Scholar]
- 5.Anand KV, Anandhi R, Pakkiyaraj M, Geraldine P. Protective effect of chrysin on carbon tetrachloride (CCl4)-induced tissue injury in male Wistar rats. Toxicol Ind Health. 2011;27:923–933. doi: 10.1177/0748233711399324. [DOI] [PubMed] [Google Scholar]
- 6.Fadhel ZA, Amran S. Effects of black tea extract on carbon tetrachloride-induced lipid peroxidation in liver, kidneys, and testes of rats. Phytother Res. 2002;16 Suppl 1:S28–32. doi: 10.1002/ptr.793. [DOI] [PubMed] [Google Scholar]
- 7.Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, Hegde A, et al. Effect of Phyllanthus niruri Linn treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol. 2008;46:514–520. [PubMed] [Google Scholar]
- 8.Mizuguchi S, Takemura S, Minamiyama Y, Kodai S, Tsukioka T, Inoue K, et al. S-allyl cysteine attenuated CCl4-induced oxidative stress and pulmonary fibrosis in rats. Biofactors. 2006;26:81–92. doi: 10.1002/biof.5520260108. [DOI] [PubMed] [Google Scholar]
- 9.Soliman AM, Fahmy SR. Protective and curative effects of the 15 KD isolated protein from the Peganum harmala L seeds against carbon tetrachloride induced oxidative stress in brain tests and erythrocytes of rats. Eur Rev Med Pharmacol Sci. 2011;15:888–899. [PubMed] [Google Scholar]
- 10.Janbaz KH, Saeed SA, Gilani AH. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557–563. doi: 10.1016/s0367-326x(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Kader MS, Abulhamd AT, Hamad AM, Alanazi AH, Ali R, Alqasoumi SI. Evaluation of the hepatoprotective effect of combination between hinokiflavone and Glycyrrhizin against CCl(4) induced toxicity in rats. Saudi Pharm J. 2018;26:496–503. doi: 10.1016/j.jsps.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruprah M, Mant TG, Flanagan RJ. Acute carbon tetrachloride poisoning in 19 patients: implications for diagnosis and treatment. Lancet. 1985;1:1027–1029. doi: 10.1016/s0140-6736(85)91624-1. [DOI] [PubMed] [Google Scholar]
- 13.Alshammari GM, Balakrishnan A, Chinnasamy T. 2-Hydroxy-4-methoxy benzoic acid attenuates the carbon tetra chloride-induced hepatotoxicity and its lipid abnormalities in rats via anti-inflammatory and anti-oxidant mechanism. Inflamm Res. 2017;66:753–763. doi: 10.1007/s00011-017-1054-2. [DOI] [PubMed] [Google Scholar]
- 14.Marimuthu S, Adluri RS, Rajagopalan R, Menon VP. Protective role of ferulic acid on carbon tetrachloride-induced hyperlipidemia and histological alterations in experimental rats. J Basic Clin Physiol Pharmacol. 2013;24:59–66. doi: 10.1515/jbcpp-2012-0053. [DOI] [PubMed] [Google Scholar]
- 15.Kaeidi A, Maleki M, Shamsizadeh A, Fatemi I, Hakimizadeh E, Hassanshahi J. The therapeutic approaches of renal recovery after relief of the unilateral ureteral obstruction: A comprehensive review. Iran J Basic Med Sci. 2020;23:1367–1373. doi: 10.22038/ijbms.2020.41984.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaeidi A, Taghipour Z, Allahtavakoli M, Fatemi I, Hakimizadeh E, Hassanshahi J. Ameliorating effect of troxerutin in unilateral ureteral obstruction induced renal oxidative stress, inflammation, and apoptosis in male rats. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:879–888. doi: 10.1007/s00210-019-01801-4. [DOI] [PubMed] [Google Scholar]
- 17.Berthet P, Farine JC, Barras JP. Calcium dobesilate: pharmacological profile related to its use in diabetic retinopathy. Int J Clin Pract. 1999;53:631–636. [PubMed] [Google Scholar]
- 18.Tejerina T, Ruiz E. Calcium dobesilate: pharmacology and future approaches. Gen Pharmacol. 1998;31:357–360. doi: 10.1016/s0306-3623(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Li S, Sun D. Calcium dobesilate and micro-vascular diseases. Life Sci. 2019;221:348–353. doi: 10.1016/j.lfs.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Cihan Ozbek I, Arslan C, Cantürk E, Süzer O. The effects of calcium dobesilate on the mechanical function of rat hearts. J Cardiovasc Surg (Torino) 2009;50:395–401. [PubMed] [Google Scholar]
- 21.Bozkurt AK, Konukoğlu D, Ustündağ N, Yüceyar L, Mayda AS. Calcium dobesilate ameliorates lung injury following lower limb ischemia/reperfusion. Drugs Exp Clin Res. 2002;28:127–132. [PubMed] [Google Scholar]
- 22.Seker A, Bardakci O, Eryilmaz S, Kocarslan S, Incebiyik A, Yucel Y, et al. Does calcium dobesilate protect against intestinal ischemia-reperfusion injury induced in rats? Eur Rev Med Pharmacol Sci. 2016;20:2168–2173. [PubMed] [Google Scholar]
- 23.Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, et al. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59:2637–2645. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz E, Lorente R, Tejerina T. Effects of calcium dobesilate on the synthesis of endothelium-dependent relaxing factors in rabbit isolated aorta. Br J Pharmacol. 1997;121:711–716. doi: 10.1038/sj.bjp.0701184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suschek C, Kolb H, Kolb-Bachofen V. Dobesilate enhances endothelial nitric oxide synthase-activity in macro- and microvascular endothelial cells. Br J Pharmacol. 1997;122:1502–1508. doi: 10.1038/sj.bjp.0701512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermenean A, Ardelean A, Stan M, Herman H, Mihali C-V, Costache M, et al. Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chem Biol Interact. 2013;205:138–147. doi: 10.1016/j.cbi.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Unal Y, Tuncal S, Kosmaz K, Kucuk B, Kismet K, Cavusoglu T, et al. The effect of calcium dobesilate on liver damage in experimental obstructive jaundice. J Invest Surg. 2019;32:238–244. doi: 10.1080/08941939.2018.1451936. [DOI] [PubMed] [Google Scholar]
- 28.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 29.Bass JJ, Wilkinson DJ, Rankin D, Phillips BE, Szewczyk NJ, Smith K, et al. An overview of technical considerations for Western blotting applications to physiological research. Scand J Med Sci Sports. 2017;27:4–25. doi: 10.1111/sms.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaeidi A, Sahamsizadeh A, Allahtavakoli M, Fatemi I, Rahmani M, Hakimizadeh E, et al. The effect of oleuropein on unilateral ureteral obstruction induced-kidney injury in rats: the role of oxidative stress, inflammation and apoptosis. Mol Biol Rep. 2020;47:1371–1379. doi: 10.1007/s11033-019-05237-0. [DOI] [PubMed] [Google Scholar]
- 31.Taghipour Z, Kaviani E, Kaeidi A, Shamsizadeh A, Hassanshahi J, Fatemi I. Atorvastatin attenuates D-galactose-induced hepatorenal toxicity in mice: an experimental study with histopathological evaluations. Physiology and Pharmacology. 2019;23:36–43. [Google Scholar]
- 32.Hakimizadeh E, Hassanshahi J, Kaeidi A, Nematollahi MH, Taghipour Z, Rahmani M, et al. Ceftriaxone improves hepatorenal damages in mice subjected to D-galactose-induced aging. Life Sciences. 2020;258:118119. doi: 10.1016/j.lfs.2020.118119. [DOI] [PubMed] [Google Scholar]
- 33.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Renal failure. 2012;34:1046–1051. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 34.Amini N, Sarkaki A, Dianat M, Mard SA, Ahangarpour A, Badavi M. Protective effects of naringin and trimetazidine on remote effect of acute renal injury on oxidative stress and myocardial injury through Nrf-2 regulation. Pharmacological Reports. 2019;71:1059–1066. doi: 10.1016/j.pharep.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Khosravi M-S, Samimiat A, Mazaheri B, Ashrafi F, Talebi A, Nematbakhsh M. Hydration with Mannitol and Dextrose May Promote Cisplatin-Induced Nephrotoxicity: Test of Five Protocols of Hydration during Cisplatin Therapy in Rat Models. J Toxicol. 2021:2021. doi: 10.1155/2021/5547341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moslemi F, Talebi A, Nematbakhsh M. Protective effect of zinc supplementation on renal ischemia/reperfusion injury in rat: gender-related difference. Int J Prev Med. 2019:10. doi: 10.4103/ijpvm.IJPVM_279_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohseni R, Karimi J, Tavilani H, Khodadadi I, Hashemnia M. Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-β signaling pathways in carbon tetrachloride (CCl(4))-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol. 2019;41:163–171. doi: 10.1080/08923973.2019.1566926. [DOI] [PubMed] [Google Scholar]
- 38.Venkatanarayana G, Sudhakara G, Sivajyothi P, Indira P. Protective effects of curcumin and vitamin E on carbon tetrachloride-induced nephrotoxicity in rats. Excli J. 2012;11:641–650. [PMC free article] [PubMed] [Google Scholar]
- 39.Garay RP, Hannaert P, Chiavaroli C. Calcium dobesilate in the treatment of diabetic retinopathy. Treat Endocrinol. 2005;4:221–232. doi: 10.2165/00024677-200504040-00003. [DOI] [PubMed] [Google Scholar]
- 40.Szabo ME, Haines D, Garay E, Chiavaroli C, Farine JC, Hannaert P, et al. Anti-oxidant properties of calcium dobesilate in ischemic/reperfused diabetic rat retina. Eur J Pharmacol. 2001;428:277–286. doi: 10.1016/s0014-2999(01)01196-7. [DOI] [PubMed] [Google Scholar]
- 41.Unal Y, Tuncal S, Kosmaz K, Kucuk B, Kismet K, Cavusoglu T, et al. The Effect of Calcium Dobesilate on Liver Damage in Experimental Obstructive Jaundice. J Invest Surg. 2019;32:238–244. doi: 10.1080/08941939.2018.1451936. [DOI] [PubMed] [Google Scholar]
- 42.Mehrzadi S, Fatemi I, Esmaeilizadeh M, Ghaznavi H, Kalantar H, Goudarzi M. Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed Pharmacother. 2018;97:233–239. doi: 10.1016/j.biopha.2017.10.113. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Liu W, Wu S, Jin J, Li W, Wang N. Calcium dobesilate for diabetic retinopathy: a systematic review and meta-analysis. Science China Life Sci. 2015;58:101–107. doi: 10.1007/s11427-014-4792-1. [DOI] [PubMed] [Google Scholar]
- 44.Hassanshahi J, Mirzahosseini-Pourranjbar A, Hajializadeh Z, Kaeidi A. Anticancer and cytotoxic effects of troxerutin on HeLa cell line: an in-vitro model of cervical cancer. Molecular Biology Reports. 2020;47:6135–6142. doi: 10.1007/s11033-020-05694-y. [DOI] [PubMed] [Google Scholar]
- 45.Hagen TM. Oxidative stress, redox imbalance, and the aging process. Anti-oxid Redox Signal. 2003;5:503–506. doi: 10.1089/152308603770310149. [DOI] [PubMed] [Google Scholar]
- 46.Okolo KO, Siminialayi IM, Orisakwe OE. Carbon tetrachloride induced hepatorenal toxicity in rats: possible protective effects of wild Pleurotus tuber-regium. Clinical Phytoscience. 2017;3:1–7. [Google Scholar]
- 47.Jafarey M, Changizi Ashtiyani S, Najafi H. Calcium dobesilate for prevention of gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2014;8:46–52. [PubMed] [Google Scholar]