Abstract
Objective(s):
Antimicrobial peptide compounds (AMPs) play important roles in the immune system. They also exhibit significant anti-tumor and antibacterial properties. Most AMPs are cationic and are able to bind bacterial cell membranes through electrostatic affinity. Ib-AMP4 is a plant-derived AMP that exerts rapid bactericidal functions. In the present study, the antibacterial efficiency of the produced recombinant Ib-AMP4 in elimination of Methicillin-resistant Staphylococcus aureus (MRSA) bacterial infection, was investigated under in vitro and in vivo situations.
Materials and Methods:
The synthesized Escherichia coli codon-optimized gene sequences of the Ib-AMP4 were expressed in E. coli BL21 (DE3) pLysS. The recombinant Ib-AMP4 was purified and refolding conditions were optimized. The antibacterial efficiency of the refolded peptide against MRSA was tested under in vivo and in vitro situations for treatment of skin and systematic infection of MRSA in a mouse model.
Results:
Antibacterial assays confirmed the antibacterial function of Ib-AMP4 against MRSA. SEM results proved the destructive effects of applying Ib-AMP4 on MRSA biomembrane. Time-kill curve and growth kinetic assay illustrated rapid antibacterial activity of the produced Ib-AMP4. Moreover, Ib-AMP4 showed significant infection treatment ability in a mouse model and all infected mice receiving Ib-AMP4 protein survived and there was no trace of bacteria in their blood samples.
Conclusion:
The results confirmed the rapid antibacterial potential of the produced recombinant Ib-AMP4 to be used for efficient treatment of MRSA infection.
Key Words: Antimicrobial activity, Recombinant Ib-AMP4, Skin and systematic – infection, Staphylococcus aureus
Introduction
Antibiotic resistance is considered one of the major public health challenges. By the way, most conventional antibiotics are rendered ineffective by drug-resistant pathogenic bacteria. This phenomenon as a growing global crisis has turned into a serious human health threat (1). Therefore, research for finding alternative antibacterial drugs has been increased in recent years. Antimicrobial peptides (AMPs) are small proteins able to kill various microorganisms. The antibacterial properties of AMPs are due to their ability to disrupt cell biomembranes by electrostatic attachment to bacterial cell wall, whereas conventional antibiotics destroy cell walls by binding to specific targets. This different mechanism of function rarely causes AMPs resistance observation among bacterial cells (2, 3).
Ib-AMP4 is a plant defense antimicrobial peptide with a net charge of +6, derived from impatiens balsamina seeds. Cysteine residues at positions 6, 7, 16, and 20 of the Ib-AMP4 sequence (QWGRRCCGWGPGRRYCRRWC) make two intermolecular disulfide bonds in the arrangement Cys6–Cys16 and Cys7–Cys20 that increase its stability in the liquid substrate. The highly cationic Ib-AMP4 peptide were investigated for significant bactericidal properties against both Gram-positive and Gram-negative bacteria (4). Solid-phase chemical synthesis leading to small amounts of peptides have been produced via solid-phase chemical synthesis. Whereas, methods based on biotechnological techniques are capable of generating higher quantities of peptides efficiently. Using a bacterial host for recombinant production of AMPs has resulted in production of considerable amounts of peptides (5).
This study describes application of the recombinant fusion protein Ib-AMP4 in the treatment of infection caused by MRSA in vivo. Ib-AMP4 was studied for the activities against fungi and bacteria in vitro. Besides, it was also applied for bacterial discrimination in previous reports. Ib-AMP4 has never been applied in vivo to assess its ability against pathogens. This is the first report of in vivo study of Ib-AMP4.
Materials and Methods
Materials and chemicals
Ampicillin, Chloramphenicol, Nutrient agar, Nutrient broth, and Mueller Hinton media (MHA, MHB) were purchased from Merck (Germany), and resazurin sodium salt of BDH (INGLAD) and Ni-NTA kit from Qiagen (USA). All other chemicals used were of analytical grade.
Bacterial strains
The antibacterial activities of this peptide were assessed against Staphylococcus aureus (MRSA) (ATCC 25923). E. coli DH5α was used as the cloning host, E. coli BL21 (DE3) pLYsS was employed as the recombinant protein expression host, and pET32a was applied as the recombinant expressive vector.
Construction of the expression vector pET32a-IbAMP4
The entire gene sequence, which comprises 5’ BamHI, an Ib-AMP4 (UniProt: 024006), and Xho I 3’ was designed and codon-optimized for E. coli expression. The whole construct was synthesized by Biomatik Company (Cambridge, ON, Canada) and inserted into the plasmid pET32a (Novagene) as the expression vector. The pET32a-IbAMP4 construct was transferred
into competent E. coli DH5a bacteria. PCR and mini-preparation plasmid were applied to confirm bacterial colonies that carried recombinant constructs (6).
Production of recombinant Ib-AMP4
For expression purposes, competent E. coli BL21 (DE3) cells were prepared based on standard protocols and were transformed by the successfully extracted expression vector pET32a-IbAMP4 construct (7, 8). Selected transformant bacterial colonies grown on plates containing ampicillin were incubated with a 2 ml NB medium. Then, 300 μl of overnight culture was inoculated into 50 ml NB broth (100 μg/ml ampicillin), and incubated at 37 °C and 220 rpm. The cells’ optical density at 600 nm was measured occasionally, and at OD600 nm ~ 0.6, isopropyl thio β-D-galactosidase (IPTG) (1 mM) was added to induce protein expression. After a 4 hr incubation time, the cells were harvested by centrifugation at 5000 rpm for 20 min and the pellets were stored at – 20 °C. SDS-polyacrylamide gel electrophoresis (SDS-PAGE12%) was applied to assay the quality of the purified recombinant Ib-AMP4 protein (9).
Purification of Ib-AMP4
Denaturing conditions using 8 M urea, followed by Ni-NTA agarose resin affinity chromatography were used for purification of the recombinant proteins. Subsequently, SDS-PAGE12% and the absorbance assay at 280 and 260 nm were applied to assay the quality and quantity of the purified recombinant Ib-AMP4 protein, respectively.
Urea assay test
To ensure the absence of urea in the protein solution, a urea assay test was done with kit Berthelot (Iran) according to the kit protocol.
Refolding of Ib-AMP4
Using urea for protein purification denatures the active folding of proteins. Accordingly, the refolding process was done by applying the prepared dialysis tubing; 10K molecular weight cut-off (MWCO) (Thermo Scientific SnakeSkin. Various pH (4.5, 5, 7, 8, and 8.5) were tested to gain the most efficient dialysis conditions. The dialysis process was carried out in PBS buffer 20 mM at 4 °C for 24 hr. Then, the refolded protein was analyzed by SDS-PAGE (12%) (10). The quantity of protein was obtained with assessment of the ratio of absorbance at 280 and 260 nm then calculated from the following equation:
(1.55 × OD280) – (0.76 × OD260) = The amount of protein (mg/ml)
Concentrate of refolded Ib-AMP4
The refolded recombinant protein was concentrated by a 10 kDa Amicon centrifugal filter (Merck Millipore, Darmstadt, Germany) (11).
In vivo tests
Determination of antibacterial minimum inhibitory concentration (MIC)
The MIC test of Ib-AMP4 against MRSA (ATCC 25923) was performed based on CLSI protocol MO7-A10 (12). In advance, a standardized inoculum of bacterial cell suspension 1×106 CFU/ml was prepared. Subsequently, 50 µl of MH broth was poured into wells of the 96-well microplates from column 1:10. After that, a two-fold serial dilution of the dialyzed recombinant Ib-AMP4 was made. Column 11, involving 100 µl of bacterial inoculum, was regarded as the positive growth control, and Column12 with 100 µl of the MH was considered as the sterility control. After 24 hr incubation time, 20 µl of resazurin dyes (0.02 %(w/v)) were poured into each well and incubated for another 2 hr at 37 °C. The concentration of protein in the final blue color well was recorded as the MIC value. All experiments were repeated triplicate (13).
Time-kill
A starting inoculum of 106 CFU/ml of tested bacteria in the exponential phase was used for the time-kill assay. Ib-AMP4 with 2×MIC concentrations was employed to assay the peptide effects on cell survival. Samples were collected at 0, 10, 20, 30, 40, 50, and 60 min after incubation and plated on MH agar for colony counting. After subsequent 24 hr of incubation at 37 °C, the decline of the viable bacterial cell was determined (14).
SEM microscopy
Overnight cultures of the bacterial cell were adjusted to 1 × 106 CFU/ml cell density. Afterward,
bacterial cells were treated with IbAMP4 at the determined FIC values for 2 hr at 37 °C. Untreated controls were prepared in a BHI medium with the same cell density. The bacterial cells were centrifuged at 12,000×g for 15 min, then washed two or three times and resuspended in sterile PBS. Ten microliters of the tested bacterial suspension were spread onto a microscope slide and fixed in 2.5% glutaraldehyde. Then, samples were coated with gold. The cell morphology was analyzed by SEM (15).
In vivo Tests
Animal model
All of the in vivo experiments were confirmed appropriate by the Ethical Committee of Arak University of Medical Sciences. The Syrian mice were adult males; they weighed about 20–25 grams and were obtained from the Pasteur Institute. Animals were kept in a suitable animal room with 20–25 °C temperature, 50%–70% humidity, and a 12:12 hr light/ dark cycle and were divided into groups of 3. Syrian mice were nourished by specific rodent pellets and urban tap water. We used topical and systematic methods to infect the mice.
Culture of MRSA
The overnight culture of MRSA was grown in fresh BHI and the bacteria were then centrifuged at 10000 ×g for 10 min, washed, and resuspended in sterile phosphate-buffered saline (PBS). This process was repeated twice, and the bacterial suspension was adjusted to the ultimate density of 1.5 × 108 colony-forming units CFU/ml.
Topical method
The animals were anesthetized with 50 mg/kg ketamine and 5 mg/kg xylazine. The shaven area of the panniculus carnosus muscle was selected for a 25 mm×20 mm thickness excisional wound. The wound was not sutured or covered. The prepared cell suspension (1.5× 108 CFU of MRSA, 10 μl) was equally inoculated on the induced wounds.
Infectious wounds in the Syrian mice
For ensuring the proper infection of wounds, wound sampling was carried out 2 days after the infection, and the samples were cultured on blood agar medium.
Infection treatment
The animals were categorized into three groups of seven mice, randomly. Group 1 was treated with mupirocin 2% ointment (positive control), group 2 was treated topically with the simple ointment prepared from recombinant protein Ib-AMP4 (4×MIC) and group 3 did not receive any treatment (negative control). Each Syrian mouse was secured in a cage and fed individually and kept under controlled environmental conditions. The Syrian mice were anesthetized by ketamine and then a 2 × 2 cm region of the posterior part of the neck was shaved for skin injury.
Wound infection was performed by MRSA (1.5×108 CFU/ml). Treatment of the skin infections followed 1 hr after wound colonization. Treatment continued for 7 days, once every 12 hr. During the wound healing period, the wound areas were measured.
The percentage of wound healing was calculated as follows:
Wound healing percentage = ((A0 – Ascar)/A0) × 100 %
Where A0 and Ascar are original wound and scared areas, respectively.
Systemic infection
Intraperitoneal (IP)
In the systematic method, MRSA bacteria were injected the intraperitoneally (IP). 1 ml of bacteria was injected IP into each Syrian mouse with 2×1010 CFU/ml concentration. Then, the produced peptide (1 ml with 4×MIC concentrations) was injected IP 2 times with an interval of 12 hr for 48 hr (16).
The Syrian mice were divided into six groups of five.
Group 1: Drug control (positive control) (vancomycin): 200 μg / ml
Group 2: Control group (negative control) (mice infected with MRSA bacteria)
Group 3: Recombinant protein treatment group with a concentration of 200 μg/ ml
Group 4: Recombinant protein treatment group with a dose of 100 μg/ ml
Group 5: Recombinant protein treatment group with a dose of 50 μg/ ml
Group 6: Recombinant protein treatment group with a dose of 25 μg/ ml
Supplementary tests
Control Group
After the death of the Syrian mice in the control group, blood samples were collected to confirm the bacteria. 5 ml of blood from rat heart was added to BHI sterile liquid culture medium and then incubated overnight at 37 °C. To confirm bacteria, a pure plate method and differential culture medium were used to identify MRSA.
Protein-receiving groups
72 hr following the first protein injection, one Syrian mouse from each group was killed as a sample. Afterward, about 5 ml of blood was collected from the heart of each sample and added to a sterile BHI culture medium. It was then incubated for 18 hr. Subsequently, 10 μl of the above culture medium was cultured in a blood agar medium for confirmatory tests.
Extraction and culture of spleen
Seventy-two hr after the first protein injection, one Syrian mouse from each group was killed as a sample. Their spleens were then extracted and washed with PBS buffer. Under sterile conditions and using a laminar hood, we perfused the spleen. Ultimately, about 100 µl of the contents inside the spleen were cultured on agar.
Results
Expression of Ib-AMP4 in Escherichia coli BL21 (DE3) pLYsS
Protein production in various tested induction conditions was analyzed with SDS-PAGE. The greatest yield was achieved by applying 1.5 × NB and 0.5 mM IPTG. Overnight incubation of induced medium under 200 rpm shaking at 37 °C was the most convenient condition. The produced 22 kDa protein after 24 hr and 2 hr of the induction is indicated with corresponding arrows in Figure 1.
Figure 1.
SDS-PAGE analysis of Ib-AMP4 in E. coli BL21 (DE3) pLysS
Lane 1: Protein marker; Lane 2: pET32a- Ib-AMP4 before induction; Lanes 3: pET32a- Ib-AMP4 after 2 hr; Lane 4: pET32a-lys after 24 hr
Purification of Ib-AMP4
SDS-PAGE was used to analyze the quality of purified protein by nickel nitrilotriacetic acid-agarose (Figure 2).
Figure 2.
SDS-PAGE analysis of purification of recombinant Ib-AMP4
Lane 1: Protein marker; Lane 2,3,4,5: fusion protein retrieved by nickel affinity chromatography
Refolding of Ib-AMP4
The results of the dialysis in phosphate-buffered saline (PBS) buffers at different pHs revealed that pH 7 was the most effective for proper protein refolding. Ib-AMP4 concentration after the dialysis procedure was about 100 µg/ml which was not sufficient for further assays. The problem was solved by utilizing amplicon centrifuging filters, yielding proteins with 400 µg/ml concentration.
In vitro tests
Determination of MIC
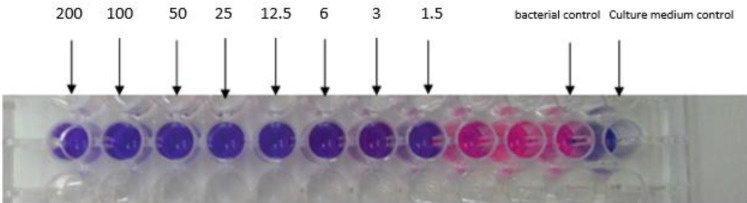
The MIC value of Ib-AMP4 against MRSA is presented in Figure 6. The MIC value of recombinant Ib-AMP4 against MRSA was recorded at 1.5 μg/ml. As shown in Figure 6, the highest concentration of protein was poured into the first well of a 96 well plate. Then the protein concentration was serially diluted by half (well numbers 1-10). Positive control was MHB (well number 12) and negative control was a culture of MRSA (well number 11). Resazurin was used for visualization of the MIC results. The blue color indicates a decrease in the number or absence of viable bacteria. While the red color represents the growth of the bacteria (Figure 3).
Figure 6.
A: In vivo wound closure. B: Table associated with wound size in the studied Syrian mice
Figure 3.
Results of the Minimum Inhibitory Concentration (MIC) test for Ib-AMP4 protein against MRSA bacteria (μg/ml). The pink color represents the growth of the organism, and the purple color represents no growth
Time-kill
Time-kill test was employed to investigate of time-dependent impacts of recombinant IbAMP4 exposure on bacterial cell viability. The result of time-kill confirmed complete cell death occurred within 50 min (Figure 4).
Figure 4.

Time-kill test results curve for Ib-AMP4 protein methicillin-resistant Staphylococcus aureus on MRSA bacteria
SEM microscopy
The SEM results illustrated the cell wall destruction induced by recombinant IbAMP4 on MRSA cells. This phenomenon led to cell wall deformation and consequently end to cell component leakage. These defects cause cell death.
In vivo tests
Topical method
Figure 6 illustrates a representative Syrian mice wound healing model from each group (control, recombinant Ib-AMP4, and mupirocin) on days four and seven after treatment. The results confirmed that treatment with recombinant Ib-AMP4 caused a faster wound closure in Syrian mice when compared with other groups.
Moreover, assessment of wound healing percentage at defined days indicated the most increased healing percentage was recorded for group B in comparison with other groups (group A= 60 %, group B= 96%, group C= 80% ) (Figure 7).
Figure 7.
Graphical representation of wound healing percentage during 7 days (A) Negative control: no treatment. (B) Treated with recombinant Ib-AMP4. (C) Drug control: Treated with mupirocin 2%
Systematic method
Infection of Syrian mice
The IP method was used to administer MRSA to infect Syrian mice.
Negative and positive control groups
The results of the present study revealed that all Syrian mice in the negative control group died 12 hr after bacterial injection. Also, the results of Syrian mice in the positive control group showed that 60% of Syrian mice receiving the drug died within 24–48 hr. The presence of MRSA in blood samples taken from dead Syrian mice was confirmed. Moreover, MRSA was just detected in bacteria taken from dead Syrian mice. Therefore, infection with MRSA had occurred as expected (Figure 8).
Figure 8.
Results of differential tests: A. positive mannitol salt agar, B. Sensitivity tonovobiocin and cefixime, C. positive coagulase, D. positive DNase
Protein group
The recombinant Ib-AMP4 protein was injected into the Syrian mice (IP). The injectable doses of protein were 200 μg / ml, 100 μg / ml, 50 μg / ml, and 25 μg / ml. Moreover, the protein injection time was immediately after the bacterial injection. The number of injections was four times with an interval of 12 hr. The findings of this study show that all Syrian mice receiving all the proteins survived. Also, no symptoms of bacteria were detected in the blood and spleen samples of Syrian mice in the peptides used groups (Figure 9).
Figure 9.
A. Cultures from spleen samples of Syrian mice receiving Ib-AMP4 protein. B. Culture from blood samples of Syrian mice receiving Ib-AMP4 protein
Figure 5.

Results of recombinant protein lesions in on methicillin-resistant Staphylococcus aureus bacteria
Discussion
The growing development of drug-resistant pathogens has enhanced the risks of antibiotic-resistant microbe spread as a serious worldwide health crisis. In order to create more efficient methods for prevention of infections, the search for alternative antibiotics has turned into a worldwide health priority (17).
Due to cationic properties, AMPs are able to bind bacterial cell walls with a negative charge. Moreover due to amphiphilic properties of AMPs, they induce holes or make destructive effects on bacterial cell membranes that can lead to cell lysis and consequently cell death (18).
Methicillin-resistant Staphylococcus aureus (MRSA) infection is caused by a specific strain of S. aureus that is resistant to some antibiotics including methicillin. MRSA is considered a major cause of hospital- and community-acquired infections. Thus, providing alternative treatment for MRSA infection has been placed at the center of researchers’ attention (19).
In the present study, the antibacterial function of the recombinant Ib-AMP4 peptide against MRSA infection has been evaluated. It has been proven that Ib-AMP4 peptide indicates a broad range of antibacterial properties and suppresses the growth of both gram-positive and gram-negative bacteria efficiently (20). In this report, the Ib-AMP4 corresponding gene sequence was successfully expressed in E. coli. The recombinant protein with high purity was obtained through nickel affinity chromatography. The results of antimicrobial activity assays confirmed that the refolding process in a neutral buffer (pH=7) led to the most active form of the peptide. The refolded recombinant Ib-AMP4 exhibited significant antimicrobial function against MRSA (ATCC 25923). Considerable antibacterial properties of Ib-AMP4 were proven by previous research (21, 22). It has been shown that the antibacterial function of Ib-AMP4 is due to its ability to attach to bacterial cell membranes and expand cell lysis by creating disorders through cell membrane integrity (23).
Time-kill results indicated a sharp reduction of MRSA cell viability within the first 50 min of exposure to the refolded recombinant Ib-AMP4. In addition, in vivo studies of Ib-AMP4 potential in the healing of MRSA infected wounds, confirmed the higher efficiency of Ib-AMP4 in comparison with mupirocin ointment.
Plus, the healing efficiency of Ib-AMP4 in systematic infection was confirmed under in vivo situations by treatment of systematic MRSA-infected Syrian mice. While MRSA-infected Syrian mice died within 12–24 hr after being injected with the bacterium, all of the infected mice treated with the recombinant Ib-AMP4 survived. Noticeably, the blood samples taken from the mice treated with the produced recombinant protein did not show any traces of MRSA bacteria in the blood agar medium. While the culture of the blood samples taken from the control Syrian mice illustrated extended growth of bacteria.
Overall, all performed antimicrobial tests demonstrated significant antimicrobial activities of the recombinant Ib-AMP4. Thus, the recombinant Ib-AMP4 peptide can be considered a reliable alternative candidate for treatment of patients with infections caused by S. aureus after subsequent needed clinical trials steps.
Conclusion
Antimicrobial assessments consistently revealed the notable efficiency of the produced recombinant Ib-AMP4 against MRSA bacteria under in vitro and in vivo situations.
Authors’ Contributions
EGR and HA Designed the experiments; SSA and SS Performed experiments and collected data; EGR and HA Discussed the results and strategy; EGR, SF, and HA Supervised, directed, and managed the study; SSA and HA Approved the final version to be published.
Conflicts of Interest
The authors declare that no conflict of interest exists.
Acknowledgment
This research was supported by financial assistance from the Molecular and Medicine Research Center of Arak University of Medical Sciences, Arak, Iran. The authors would like to thank the Deputy of Research and Technology of Arak University of Medical Sciences. The results presented in this paper were part of a student thesis.
References
- 1.Avison MB. New approaches to combating antimicrobial drug resistance. Genome Biol. 2005;6:243–246. doi: 10.1186/gb-2005-6-13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkar T, Chetia M & Chatterje S. Antimicrobial peptides and proteins: From nature’s reservoir to the laboratory and beyond. Front Chem. 2021;9:691532. doi: 10.3389/fchem.2021.691532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S, Bharath Prasad AS, Mehta CH & Nayak UY. Antimicrobial peptide polymers: No escape to ESKAPE pathogens-a review. World J Microbiol Biotechnol. 2020;36:131–144. doi: 10.1007/s11274-020-02907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flórez-Castillo JM, Ropero-Vega JL, Perullini M, & Jobbágy M. Biopolymeric pellets of polyvinyl alcohol and alginate for the encapsulation of Ib-M6 peptide and its antimicrobial activity against Escherichia coli. Heliyon. 2019;5:e01872. doi: 10.1016/j.heliyon.2019.e01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoogh Abbasian S, Abtahi H, Zolfaghari MR, Soufian S, Ghaznavi-Rad E. Cloning, expression,purification and antigenic evaluation of hyaluronidase antigenic fragments recombinant protein of streptococcus pyogenes. Afr J Biotechnol. 2012;11:2376–2380. [Google Scholar]
- 6.Mirjamali N, Soufian , S , Molaee N, Sadoogh Abbasian S, Abtahi H. Cloning and expression of the enzymatic region of Streptococcal hyaluronidase. Iran J Basic Med Sci. 2014;17:667–672. [PMC free article] [PubMed] [Google Scholar]
- 7.Abtahi H, Salmanian AH, Rafati S, Nejad GB, Hassan ZM. High level expression of recombinant ribosomal protein (L7/L12) from Brucella abortus and its reaction with infected human sera. Iran Biomed J. 2004;8:13–18. [Google Scholar]
- 8.Sadoogh Abbasian S, Ghaznavi-Rad E, Akbari N, Zolfaghari M R, pakzad I, Abtahi H. Overexpression and enzymatic assessment of antigenic fragments of hyaluronidase recombinant protein from streptococcus pyogenes. Jundishapur J Microbiol. 2015;8:e13653. doi: 10.5812/jjm.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahimirad S, Razavi SH, Abtahi H, Alizadeh H, Ghorbanpour M. Recombinant production and antimicrobial assessment of beta casein-IbAMP 4 as a novel antimicrobial polymeric protein and its synergistic effects with thymol. Int J Pept Res Ther. 2018;24:213–222. [Google Scholar]
- 10.Sadoogh Abbasian S, Soufian S, Ghaznavi-Rad E, Abtahi H. High level activity of recombinant lysostaphin after computer simulation and additive-based refolding. Int J Pept Res Ther. 2019;25:1241–1249. [Google Scholar]
- 11.Fahimirad S, Abtahi H, Razavi SH, Alizadeh H, Ghorbanpour M. Production of recombinant antimicrobial polymeric protein beta casein-E 50-52 and its antimicrobial synergistic effects assessment with yhymol. Molecules. 2017;22:822–836. doi: 10.3390/molecules22060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38:1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahimirad S, Ghaznavi-Rad E, Abtahi H, Sarlak N. Antimicrobial activity, stability and wound healing performances of chitosan nanoparticles loaded recombinant LL37 antimicrobial peptide. Int J Pept Res Ther. 2021;27:2505–2515. [Google Scholar]
- 14.Rudilla H, Fusté E, Cajal Y, Rabanal F, Vinuesa T, Viñas M. Synergistic antipseudomonal effects of synthetic peptide AMP38 and carbapenems. Molecules. 2016;21:1223–1234. doi: 10.3390/molecules21091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zengin H, Baysal AH. Antibacterial and antioxidant activity of essential oil terpenes against pathogenicand spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soković M, Glamočlija J, Marin PD, Brkić D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Silvestri C, et al. LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob Agents Chemother. 2006;50:1672–1679. doi: 10.1128/AAC.50.5.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Pushpanathan M, Gunasekaran P, Rajendhran J. Antimicrobial peptides: versatile biological properties. Int J Pept. 2013;2013:675391. doi: 10.1155/2013/675391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann NH. The potential of phages to prevent MRSA infections. Res Microbiol. 2008;159:400–405. doi: 10.1016/j.resmic.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Schäfer H, Reichling J, Wink M. Bactericidal properties of the antimicrobial peptide Ib-AMP4 from Impatiens balsamina produced as a recombinant fusion-protein in Escherichia coli. Biotechnol J. 2013;8:1213–1220. doi: 10.1002/biot.201300121. [DOI] [PubMed] [Google Scholar]
- 22.Satei P, Ghaznavi-Rad E, Fahimirad S, Abtahi H. Recombinant production of Trx-Ib-AMP4 and Trx-E50-52 antimicrobial peptides and antimicrobial synergistic assessment on the treatment of methicillin-resistant Staphylococcus aureus under in vitro and in vivo situations. Protein Expr Purif. 2021;188:105949. doi: 10.1016/j.pep.2021.105949. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Reichling J, Wink M. Antibacterial activity of the recombinant antimicrobial peptide Ib-AMP4 from Impatiens balsamina and its synergy with other antimicrobial agents against drug resistant bacteria. Pharmazie. 2013;68:628–630. [PubMed] [Google Scholar]