ABSTRACT
It has recently emerged that microglia, the tissue-resident macrophages of the central nervous system, play significant non-innate immune roles to support the development, maintenance, homeostasis and repair of the brain. Apart from being highly specialized brain phagocytes, microglia modulate the development and functions of neurons and glial cells through both direct and indirect interactions. Thus, recognizing the elements that influence the homeostasis and heterogeneity of microglia in normal brain development is crucial to understanding the mechanisms that lead to early disease pathogenesis of neurodevelopmental disorders. In this Review, we discuss recent studies that have elucidated the physiological development of microglia and summarize our knowledge of their non-innate immune functions in brain development and tissue repair.
KEY WORDS: Microglia, Brain development, Heterogeneity, Neurons, Synapse, Oligodendrocytes
Summary: This Review summarizes the crucial role of microglia – brain-specific, yolk sac-derived macrophages – in neural development, highlighting temporal and spatial heterogeneity in their interactions with neuronal and non-neuronal cells to support brain development and regeneration.
Introduction
Microglia are highly motile, surveillant brain-resident macrophages that constitute a significant proportion of neuroglia in the central nervous system (CNS) (Lawson et al., 1990; Nimmerjahn et al., 2005). Microglia that form during CNS development go on to self-renew throughout the lifetime of the healthy animal, thereby maintaining the tissue-resident population (Ajami et al., 2007; Prinz et al., 2014; Tay et al., 2017b). Although microglia are largely known for their immune response-driven phagocytosis of pathogens and debris, as well as changes in inflammatory phenotypes in neurodegenerative diseases, they are also involved in many non-immune aspects of CNS development, homeostasis and repair throughout life (Tay et al., 2017a). Without a doubt, microglia are the de facto workhorses of the CNS immune system.
Here, we discuss recent findings and highlight reviews that examine this topic in greater detail. Notably, microglia do not form a monolithic population within the CNS, despite originating from common sources in early brain development. Aside from their dual developmental origin, the complex microenvironment where microglia reside shapes the phenotype and function of the cells (Bennett and Bennett, 2020). As a crucial regulator of the brain microenvironment, evidence of molecular and state-specific functional heterogeneity of microglia throughout the CNS is unsurprising. However, we are only at the beginning of understanding microglial heterogeneity. In addition, there have been an increasing number of examples that demonstrate how microglial interactions with other brain cell types modulate CNS development and repair (Greenhalgh et al., 2020; Werneburg et al., 2017). More strikingly, recent evidence from human mutations and preclinical models has revealed mild (e.g. dysregulation of microglial state) to devastating (e.g. pediatric-onset leukoencephalopathy) consequences in brains that lack healthy microglia (Oosterhof et al., 2019; Spittau et al., 2020). To elucidate the mechanisms that lead to early disease pathogenesis of neurodevelopmental disorders, we find it timely to expound the factors that impact microglial specification, heterogeneity and functional diversity in physiological CNS development in this Review. In particular, we examine the influence microglia exert on other brain cell types for normal brain development and regeneration.
Specification of microglia
Developmental origins of microglia
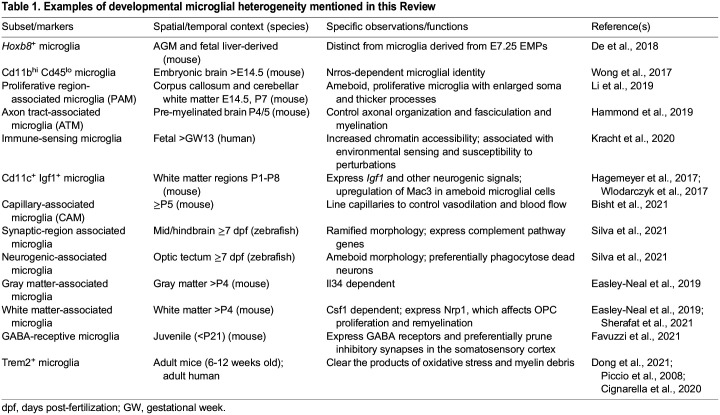
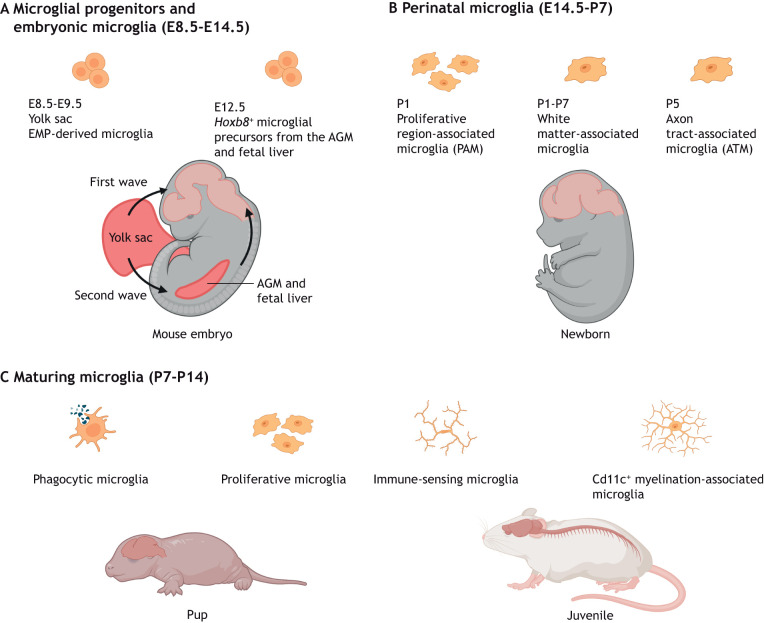
In contrast to neuroepithelium-derived glia, such as astrocytes and oligodendrocytes, microglia originate from early erythromyeloid progenitors (EMPs) derived from yolk sac hematopoiesis at embryonic day (E) 7.25-7.5 (Alliot et al., 1999; Ginhoux et al., 2010; reviewed by Becker and Becker, 2022). Embryonic microglia infiltrate the developing murine neuroepithelium by E9.5, around the period that primary brain vesicles are formed and prior to the appearance of the first neurons at E11.0 (Chen et al., 2017; Ginhoux et al., 2010). In addition, a subpopulation of Hoxb8+ microglia (Table 1) has been suggested to arise from a second wave of yolk sac hematopoiesis that gives rise to microglial precursors, which expand their population in the aorta-gonad-mesonephros (AGM) and fetal liver. Fate mapping has revealed that these Hoxb8+ AGM and fetal liver microglial precursors then colonize the embryonic CNS from E12.5 (De et al., 2018; Fehrenbach et al., 2018) (Fig. 1A). Although the origin of this second source of Hoxb8+ microglia has only been shown in one study, genetically preventing hematopoiesis after E11 reduces the number of microglia in sampled mouse brains at E16.5, E18.5, postnatal day (P) 0 and P1, thus supporting the notion that a subpopulation of microglia is generated independently of early EMPs (Fehrenbach et al., 2018).
Table 1.
Examples of developmental microglial heterogeneity mentioned in this Review
Fig. 1.
Microglial specification, heterogeneity and functional diversity across stages of CNS development and maturation. (A) During early CNS development, the brain is infiltrated by erythromyeloid progenitors (EMPs) from the yolk sac between E8.5 and E9.5 from the first wave of hematopoiesis. A second wave of yolk sac hematopoiesis generates Hoxb8+ microglial precursors that migrate to the CNS from the aorta-gonad-mesonephros (AGM) and fetal liver from E12.5. (B,C) Proliferative microglial cells are a common feature during the perinatal (E14.5-P7) and early maturation (P7-P14) stages. (B) Specific proliferative microglial clusters, such as the white matter-associated microglia and axon tract-associated microglia, are observed within the first week after birth. (C) Microglial maturation and expansion of the population continue within various brain compartments. Phagocytic microglia, immune-sensing microglia and Cd11c+ myelination-associated microglia can be found in fetal human and juvenile murine brains.
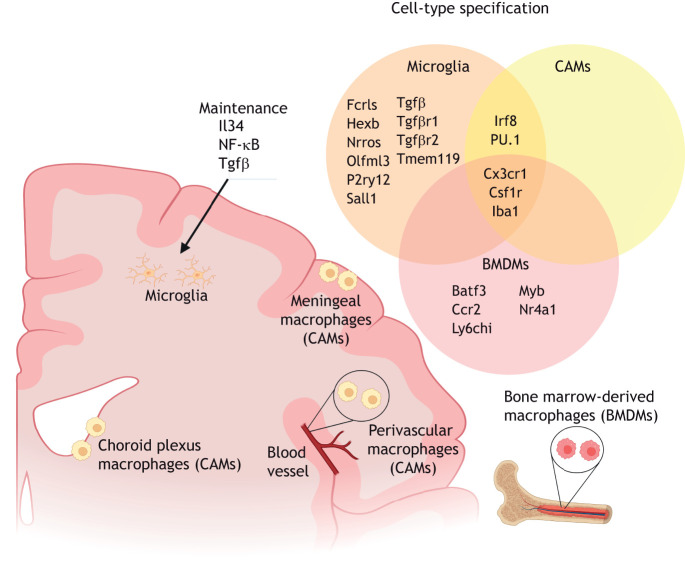
The transcription factor PU.1 (also known as Spi1) is required for commitment to the myeloid lineage in EMPs (Back et al., 2004), and interferon (Ifn) regulatory factor 8 (Irf8) is a master regulator of microglial identity (Van Hove et al., 2019) (Fig. 2). Together, Irf8 and PU.1 function as heterodimers during the early specification of microglial precursors between E9 and E10.5, and are required to determine the molecular and morphological phenotype of microglia (Beers et al., 2006; Kierdorf et al., 2013; Minten et al., 2012; Schulz et al., 2012). Between E10.5 and E14.5 transforming growth factor beta (Tgfβ) signaling drives EMPs to acquire a microglial molecular identity, by inducing the upregulation of spalt-like transcription factor 1 (Sall1), which encodes a zinc-finger transcription factor that defines the identity and roles of microglia (Buttgereit et al., 2016) (Fig. 2).
Fig. 2.
Microglial identity. Microglia reside in the brain parenchyma and can be distinguished by their expression of the combination of Fcrls, Hexb, Nrros, Olfml3, P2ry12, Sall1, Tgfβ, Tgfβ receptors 1 and 2, and Tmem119. Microglial identity is maintained by signaling through Il34, NF-κB and Tgfβ. In the CNS border compartments, choroid plexus, meningeal and perivascular macrophages, otherwise known as CNS-associated macrophages (CAMs) or border-associated macrophages (BAMs), are distinct from microglia but share the expression of the transcription factors Irf8 and PU.1 in their lineage specification. Bone marrow-derived macrophages (BMDMs), which are not typically localized in the brain, share the common expression of Csf1r and Cx3cr1 with microglia and CAMs, but selectively express Batf3, Ccr2, Ly6chi, Myb and Nr4a1.
Ontogenically similar to microglia, the CNS-associated meningeal, choroid plexus and perivascular macrophages (CAMs) are located at CNS interfaces and are also largely derived from early yolk sac EMPs to form tissue-resident macrophages in their respective compartments (Goldmann et al., 2016) (Fig. 2). CAMs are morphologically distinct from homeostatic ramified microglia, but, like microglia, also express PU.1 and Irf8 (Goldmann et al., 2016). Microglia are distinguished from CAMs by the early expression of hexosaminidase B (Hexb) and Sall1, suggesting that high expression of these two proteins is specific to the microglial population (Buttgereit et al., 2016; Kim et al., 2021; Masuda et al., 2020). Furthermore, although the development of microglia is dependent on Tgfβ signaling, fate mapping of CAMs [also referred to as border-associated macrophages (BAMs) as a result of their localization in the border compartments of the CNS] has revealed the early segregation of CAMs into a Tgfβ-independent lineage (Utz et al., 2020) (Fig. 2).
Microglia and CAMs do not express Myb, Batf3 and Nr4a1, which are instead associated with hematopoietic stem cell (HSC), bone marrow-derived macrophages (BMDMs) (Goldmann et al., 2016). A subpopulation of Ly6chi Ccr2+ BMDMs are able to replace microglia to a certain extent under specific conditions (Priller et al., 2001; Prinz et al., 2014) (Fig. 2). Studies have demonstrated the replacement of microglia by BMDMs as an artifact of irradiation (Mildner et al., 2007), or through freeing of the brain parenchymal microglial niche by genetic manipulation to cause partial microglial depletion (Cronk et al., 2018; Lund et al., 2018). BMDMs acquire distinct microglia-like phenotypes once engrafted into the brain microenvironment, including a regular, tiled distribution, ramified morphology and the expression of some microglia signature genes, such as Fc receptor-like S scavenger receptor (Fcrls) and Tgfβ receptor genes. However, BMDMs do not fully recapitulate the microglial identity (Bennett et al., 2018; Cronk et al., 2018; Lund et al., 2018; Shemer et al., 2018); long-term integrated brain BMDMs display a distinct transcriptome, epigenetic regulation and immune responses compared with endogenous microglia (Shemer et al., 2018). Nevertheless, these observations emphasize the therapeutic potential of BMDMs in modulating the pathogenesis and disease burden of neurodevelopmental disorders.
Maturation of microglia
As for all macrophages, the normal proliferation, maturation and survival of microglia is dependent on colony-stimulating factor 1 (Csf1) receptor (Csf1r) signaling, which acts through both the Csf1 and interleukin (Il) 34 ligands expressed by neural cells to signal to microglia in a brain region-dependent manner (Elmore et al., 2014; Bohlen et al., 2017; Erblich et al., 2011; Greter et al., 2012; Nandi et al., 2012; Wang et al., 2012). Similarly, deficiency in the Csf1r adaptor protein DNAX activation protein of 12 kDa (DAP12; also known as Tyrobp) in mice leads to an eventual reduction in microglial cell numbers due to defects in Csf1r-dependent proliferation and survival (Otero et al., 2009). The complete lack of Nrros, a myeloid-expressed protein that regulates reactive oxygen species (ROS) production by controlling NADPH oxidase 2 (Nox2; also known as Cybb) stability, also results in the loss of bona fide embryonic microglia marked by Cd11b (Itgam)hi Cd45 (Ptprc)lo (Table 1) by E14.5 (Wong et al., 2017) (Fig. 2). In the Nrros knockout mouse, perivascular macrophage-like cells have been observed to take the place of microglia in the CNS at P2 (Wong et al., 2017). Furthermore, runt-related transcription factor 1 (Runx1) appears to be required for the proliferation and maturation of ameboid microglia during the first 2 weeks of postnatal forebrain development by promoting the ramified microglial morphology found in homeostatic adult brain (Zusso et al., 2012).
In summary, subtle differences in the fate specification program of microglia likely distinguish them from their close CAM myeloid relatives. However, in addition to intrinsic fate-determining factors, microglial identity is subject to external influences (Box 1).
Box 1. External factors that influence microglial identity and activity.
Tissue microenvironments drive chromatin modifications and enhancer selections that impact the transcriptional regulation of local microglial phenotypes (Gosselin et al., 2014; Lavin et al., 2014). Relocating human and mouse microglia from the brain to tissue culture leads to such broad epigenetic remodeling that even Tgfβ addition cannot fully reverse (Bohlen et al., 2017; Gosselin et al., 2017). Conversely, transplantation of CNS naïve HSC-derived myeloid cells into Csf1r knockout brains devoid of microglia induces the donor cells to partially adopt a microglial identity (Bennett et al., 2018).
Although there are reports of sexual dimorphism in adult microglia and sex differences in microglia behavior (Guneykaya et al., 2018; Hanamsagar et al., 2017; Nelson et al., 2017; Schwarz et al., 2012), it is currently unclear whether genetic sex differences or sex hormones play a role in microglial specification. It is important to investigate the impact of sex on microglia to understand the sex biases that underlie several neurodevelopmental and neurodegenerative disorders (Hanamsagar and Bilbo, 2016; Villa et al., 2019).
Extrinsic cues may be important to the regulation of microglial homeostasis. Several aspects of gut-brain crosstalk influence microglial state and functions: prenatal mice raised under germ-free conditions display dysregulated microglial development and maturation in a sex- and brain region-dependent manner (Thion et al., 2018). Tgfα release by microglia is activated by gut-derived metabolites, such as tryptophan, through the aryl hydrocarbon receptor expressed by microglia (Rothhammer et al., 2018). Finally, maternal intake of omega-3 fatty acids is essential for hippocampal development in offspring, a process that is mediated by microglial phagocytosis (Madore et al., 2020). Other environmental factors that could indirectly interact with brain microglia to alter their homeostatic functions are discussed elsewhere (Tay et al., 2018).
Microglia identification and heterogeneity
Concerted efforts have enabled the recent identification of additional microglial cell markers and transcriptomic signatures to study precisely their origins, specification and functions in the brain. To unveil these factors, it is important to target microglia specifically within the brain environment, while distinguishing them from other brain cell types and close myeloid relatives, such as circulating monocytes and BMDMs. The fractalkine receptor or CX3C chemokine receptor 1 (Cx3cr1) GFP reporter mouse has served as an invaluable tool in the visualization of brain microglia for over two decades (Jung et al., 2000). The subsequent availability of the inducible Cre-driver under the control of the Cx3cr1 promoter has permitted temporal labeling of microglia and microglia-specific gene targeting (Goldmann et al., 2013; Yona et al., 2013). However, because Cx3cr1 is also expressed in CAMs (Fig. 2), clear identification of microglia requires additional information, such as location, morphology and the expression of other markers (Goldmann et al., 2016; Jung et al., 2000). The identification of genes enriched in microglia has led to the recent expansion of resources for targeting rodent and human microglia in development and disease (Butovsky et al., 2014). Genetic tools based on markers, such as Sall1 and Hexb, and others, now distinguish microglia from monocytes and BMDMs, as well as CAMs (Bennett et al., 2016; Buttgereit et al., 2016; Kaiser and Feng, 2019; Masuda et al., 2020; McKinsey et al., 2020). One caveat, however, is that – with the exception of Hexb – these microglial markers are downregulated in some disease conditions, such as demyelination (Masuda et al., 2020). Here, we provide a brief perspective on the regulation of microglia homeostasis and activity, as well as their heterogeneity in space and time.
Regulation of microglial homeostasis
Several of the factors that promote microglial identity are also required for the survival, self-renewal and homeostatic state of microglia. Steady-state microglia are typically considered to be the mature, ramified and dynamic cells that actively survey their local microenvironments without overt increase in inflammatory response (Tay et al., 2017a). Reactive microglia (which appear ameboid and upregulate proinflammatory proteins and antigen-presenting markers), primed microglia (which produce an exaggerated inflammatory response to a second stimulus) and senescent microglia (which have enlarged soma and thick processes, and accumulate cellular debris) are generally considered dysfunctional and associated with pathology and aging. Notably, the proliferative, phagocytic or ameboid microglia that appear with spatial and temporal specificity during normal CNS development are integral to the diverse functions performed by microglia and should not be confused with undesirable microglial states that lead to neurotoxicity (Tay et al., 2017a). Stable Tgfβ signaling maintains the unique homeostatic microglial transcriptomic signature comprising Sall1, Hexb, transmembrane protein 119 (Tmem119), purinergic receptor P2RY12 (P2ry12), Tgfbr1, Fcrls and olfactomedin-like 3 (Olfml3) (Fig. 2), as well as immune reactivity (Butovsky et al., 2014; Zöller et al., 2018). In mice that lack expression of Tgfβ1 in the CNS, no microglia that express the microglial markers ionized calcium-binding adapter molecule 1 (Iba1) and P2ry12 are observed in the brains of P20 and older animals (Butovsky et al., 2014). Epigenetic factors, such as the class I histone deacetylases Hdac1 and Hdac2, are required for microglial survival during development, but are functionally redundant in homeostatic adult microglia (Datta et al., 2018). Microglial homeostasis and function are also post-transcriptionally regulated by microRNAs (miRNAs) in an age-dependent manner (Varol et al., 2017). For instance, knocking out Dicer (Dicer1), a protein that processes miRNAs, in Cx3cr1+ yolk sac EMPs leads to a global increase in DNA damage in P0 newborn microglia, but microglial depletion of Dicer in adults does not have the same outcome. Instead, loss of Dicer in adult microglia drives hyper-reactivity and results in hippocampal neuronal impairment (Varol et al., 2017). Maintaining microglial self-renewal and normal tiling pattern in the CNS requires Csf1r signaling, largely through Il34 (Greter et al., 2012). Regeneration of microglial homeostasis and cell density after transient chemical ablation by a Csf1r inhibitor in the adult mouse brain requires a stepwise maturation process reminiscent of CNS development (Zhan et al., 2019). However, the requirement for the activation of NF-κB signaling, and the upregulation of the transcription factor Mafb, may be specific to the regulation of adult microglial homeostasis and is independent of the developmental and perinatal maturation programs (Matcovitch-Natan et al., 2016; Zhan et al., 2019).
Microglia-specific inactivation of Sall1 causes the adoption of an inflammatory phenotype in steady-state microglia that leads to altered brain development, disrupted tissue homeostasis and impaired neurogenesis (Buttgereit et al., 2016). miR-124 reportedly modulates mouse and zebrafish microglial phagocytosis and motility as the cells switch from the ameboid to the ramified phenotype (Ponomarev et al., 2011; Svahn et al., 2016). Furthermore, a study of juvenile rodent microglia has reported that the ramified surveillant phenotype of motile microglia is regulated by the two-pore domain potassium channel Thik-1 (Kcnk13) (Madry et al., 2018). Aside from cell-intrinsic aspects that mediate microglial homeostasis, we next examine the non-cell-autonomous signals that modulate the steady state phenotype. Signaling between neurons and microglia via Cx3cr1 is important for maintaining microglia in their non-reactive state. Notably, Cx3cr1 is also necessary for regulating microglial behavior, including their timely recruitment into the CNS, cell density in the early postnatal stage and phagocytic activity that impact their role in physiological synaptic pruning and synaptic plasticity (Hoshiko et al., 2012; Paolicelli et al., 2011; Rogers et al., 2011; Ueno et al., 2013; Zhan et al., 2014). Furthermore, the dysregulation of microglial activity does not necessarily involve a switch to an inflammatory state. In fact, elevated mammalian target of rapamycin (mTOR) signaling in microglia activates a reactive phenotype, independently of inflammatory responses, and is correlated with early-onset seizures or death in juvenile mice (Zhao et al., 2018).
In summary, several endogenous and external elements (Box 1) act independently or in concert to regulate microglial homeostasis and function during CNS development and maturation.
Microglial heterogeneity in space and time
In the adult CNS, morphological diversity of microglia across brain compartments has been described for more than three decades (Lawson et al., 1990) and non-uniform microglial expression of various myeloid and inflammatory markers across the CNS has also been summarized in a recent review (Tan et al., 2020). One of the earliest studies that revealed microglial diversity was a genome-wide analysis of microglia from discrete brain regions across the adult lifespan of the mouse, in which microglia reportedly possessed distinct regionally variable transcriptional identities, immunophenotypes and metabolic requirements (Grabert et al., 2016).
Subsequent studies have also distinguished between gray and white matter-associated microglia. For instance, white matter microglia upregulate ubiquitin-specific protease 18 to maintain homeostasis by negatively regulating basal interferon signaling (Goldmann et al., 2015). Furthermore, white matter-associated microglia are activated in an age-dependent manner, in contrast to gray matter microglia, possibly in relation to the former's role in clearing myelin debris (Safaiyan et al., 2021) (Table 1) (Fig. 1B). More recently, studies using function-blocking antibodies have revealed the differential dependence on Il34 and Csf1 for maintaining microglia in gray matter and white matter, respectively, in adult mice (Easley-Neal et al., 2019). Temporally, Il34 appears to play a lesser role in maintaining the microglial population compared with Csf1 during prenatal development and the region-specific dependency on each Csf1r ligand is only apparent after P4 (Easley-Neal et al., 2019). Follow-up studies on these findings using inducible genetic targeting approaches would be valuable to verify these results because of the relatively unknown penetrance and efficacy of the blocking antibodies in brain tissue in developmental and postnatal stages. A similar example is the transient appearance of a proliferative and reactive Cd11c (Itgax)+ Mac3 (Lamp2)+ Igf1+ (insulin-like growth factor 1) microglial subset associated with white matter but absent in gray matter (Hagemeyer et al., 2017; Wlodarczyk et al., 2017) (Fig. 1B).
The broad accessibility of single-cell RNA sequencing (scRNA-seq), high-dimensional mass and fluorescence cytometry, and spatial transcriptomics, has delivered unparalleled new information on the heterogeneity of brain microglia in various spatial and temporal contexts of health or disease (Böttcher et al., 2019; Kubick et al., 2020; Mrdjen et al., 2018). Multi-dimensional clustering of single-cell transcriptomes has typically revealed greater temporal heterogeneity (in terms of more clusters) among embryonic and early postnatal microglia than in adult microglia of healthy mice (Hammond et al., 2019; Li et al., 2019; Masuda et al., 2019; Matcovitch-Natan et al., 2016). For instance, unsupervised clustering of microglia from the mouse brain at E16.5, 3 weeks of age and 16 weeks of age gave rise to six, three and one cluster(s), respectively (Masuda et al., 2019). The increasing microglial homogeneity from the third postnatal week is also reflected in the stabilization of microglial turnover and cell density from P42, which remain constant in the healthy adult brain (Nikodemova et al., 2015). A subpopulation of ameboid proliferative region-associated microglia (PAM) (Table 1) have been identified at E14.5 and P7, particularly in the developing murine corpus callosum and cerebellar white matter at one week after birth (Li et al., 2019) (Fig. 1B,C). Here, PAM possess a specific transcriptome that resembles that of aging- and neurodegenerative disease-related microglia, including the secretion of the chemokine ligands Ccl3 and Ccl4. Morphologically, PAM have been shown to possess enlarged soma and thicker processes, and largely express the surface protein Clec7a (Li et al., 2019). Similarly, a subset of ameboid axon tract-associated microglia (ATM) (Table 1) in the P4/P5 pre-myelinated mouse brain has been described. The functions of these ATM have been identified as the control of axonal organization and fasciculation, as well as myelination and homeostasis of oligodendrocytes (Hammond et al., 2019). This early postnatal ATM population has not been found at any other time points and they have been shown to upregulate the expression of osteopontin (also known as Spp1), Igf1, neuromedin B, immune response-associated galectin genes, lysosomal-associated membrane protein 1 (Lamp1) and Cd68 (Hammond et al., 2019) (Fig. 1B).
scRNA-seq analyses of aborted fetuses have also enabled the transcriptomic-based tracing of human microglia at different developmental stages and have identified the early acquisition of immune-sensing properties by fetal microglia (Bian et al., 2020; Fan et al., 2018; Kracht et al., 2020) (Table 1) (Fig. 1C).
Taken together, it is likely that the studies mentioned have identified consistent populations of microglia during CNS maturation that share similarities in gene regulation with microglial subsets that arise during normal aging, adult white matter microglial reconstitution or neurodegeneration (Benmamar-Badel et al., 2020; Hohsfield et al., 2021) (Fig. 1). Although there is now some evidence that the adult pool of microglia is derived from two temporally distinct sources (De et al., 2018; Fehrenbach et al., 2018; Ginhoux et al., 2010), past studies have not taken this into consideration. Thus, it is currently unclear whether the second source of microglia is specified by the same intrinsic factors to a similar extent during CNS development. It is reasonable to believe that a different response to the transcription factors PU.1 and Irf8, and Tgfβ and Csf1r signaling pathways could impact microglial heterogeneity during development. Furthermore, the microglial heterogeneity that has been reported so far is independent of sex differences (Hammond et al., 2019) (Box 1). Considering the diverse needs of the CNS during development, spatial and temporal microglial heterogeneity is likely tied to the various developmental processes that microglia help to regulate, including neurogenesis, axonogenesis and myelination, as we discuss below (Stratoulias et al., 2019).
Function of microglia
Guided by their spatial and temporal heterogeneity, microglia are crucial architects of the developing brain. Microglia promote brain development and homeostasis through complex interactions with various neuronal and non-neuronal cell types (Figs 3 and 4). Below, we consider these unique interactions between microglia and other cell types that underlie normal brain development and CNS tissue regeneration.
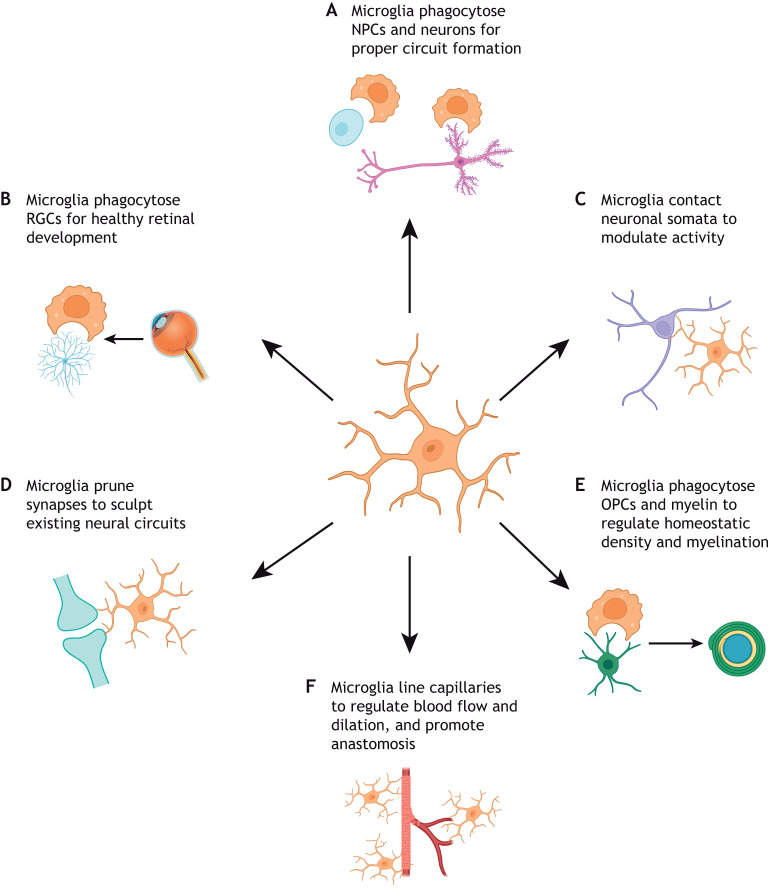
Fig. 3.
Microglia physically interact with neural cell types to promote healthy brain development. Microglia are capable of modulating brain development through direct contact with various cell types of the CNS. (A) Elimination of NPCs and neurons via phagocytosis is crucial for the formation and function of neuronal circuitry. (B) In particular, microglial phagocytosis of retinal ganglion cells (RGCs) to regulate proper eye development has been well documented. (C) Microglia can also modulate neuronal activity by directly interacting with neuronal somata. (D) Microglia physically interact with neuronal dendrites and routinely prune synapses to direct brain and eye development. (E) Microglia phagocytose oligodendrocyte precursor cells (OPCs) and myelin to regulate homeostatic density and myelination. (F) Microglia bridge endothelial tip cells to promote fusion and vascularization, and capillary-associated microglia are present throughout postnatal life, lining brain capillaries to control blood flow and dilation.
Fig. 4.
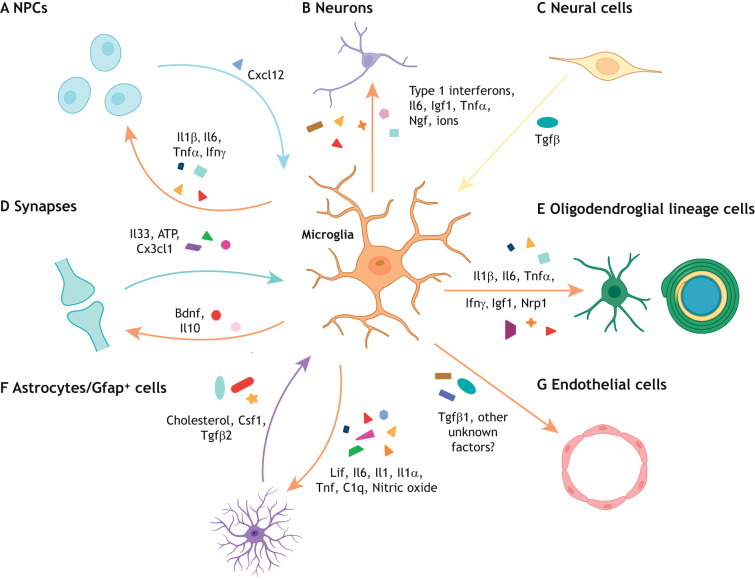
Microglia secrete cytokines to regulate CNS development. Microglia regulate brain development through the secretion of cytokines that have distinct, complex effects on different neural cell types depending on their cellular, spatial and temporal context. (A) Reactive microglia can release inflammatory cytokines (Il1β, Il6, Tnfα and Ifnγ) that promote neurogenesis in specific brain regions. However, the absence of these cytokines (Il6, type 1 interferons) promotes proper neuronal maturation in other brain regions. Reciprocally, neural precursor cells (NPCs) can recruit microglia to specific brain regions through Cxcl12 secretion. (B) Microglial-derived factors (type 1 interferons, Il6, Igf1, Tnfα, Ngf and superoxide ions) can either support or impair neuronal survival to ensure proper circuit formation in a context-specific manner. (C) CNS-wide disruption of Tgfβ signaling induces dysmature microglia, which impact both neuronal and oligodendroglial cell populations. (D) At the synapse, microglial Il10 and Bdnf regulate synaptic formation and maturation, whereas neuronal Il33, ATP and fractalkine signaling can modulate microglia maturation and function. (E) Inflammatory cytokines (Il1β, Il6, Tnfα and Ifnγ) as well as other factors expressed by microglial subsets (Igf1 and Nrp1) can regulate OPC proliferation, oligodendrogenesis, and myelination in different spatial contexts. (F) Microglia-to-astrocyte crosstalk is crucial to astrocyte development (Lif, Il6, Il1, nitric oxide) and reactivity (Tnf, C1q, Il1α) in both health and disease contexts. Similarly, astrocyte-derived cholesterol, Csf1 and Tgfβ2 are essential for microglial survival. (G) Finally, Tgfβ1 is expressed by microglia to regulate vascularization, but there are likely other microglial-derived factors that promote angiogenesis that remain to be elucidated.
Neural precursor cells and neurogenesis
Neural precursor cells (NPCs) crucially give rise to both neurons and a variety of glial cell populations (Martínez-Cerdeño and Noctor, 2018). Early work documenting the presence of microglia during normal development, and subsequent cell death in both mice and birds, first hinted at the importance of microglia in shaping the newly formed CNS (Antony et al., 2011; Lang and Bishop, 1993; Marín-Teva et al., 1999). Recent research has further identified key interactions between microglia and NPCs, which have profound effects on neurogenesis and overall brain development in vertebrates. Microglia regulate developmental neurogenesis in multiple proliferative brain regions via phagocytosis (Fig. 3A). For instance, microglia in the subgranular zone of the murine dentate gyrus phagocytose apoptotic neuroblasts in the early postnatal period to regulate their incorporation into hippocampal circuits (Sierra et al., 2010). Dysregulation of Sall1 abrogates neuroblast differentiation in the murine dentate gyrus via disruption of microglial gene expression, morphology and reactivity (Buttgereit et al., 2016). During embryonic development, microglia also colonize the subventricular zone (SVZ) and inner SVZ of macaque and rat brains to phagocytose NPCs and regulate NPC population density (Cunningham et al., 2013). Notably, the phagocytic capacity of microglia is heavily context dependent; during zebrafish neurodevelopment, a phenotypically distinct microglial subpopulation, called neurogenic-associated microglia (Table 1), localizes to the neurogenic regions of the optic tectum and primarily phagocytoses dead neurons. In contrast, microglia located in the zebrafish midbrain and hindbrain, called synaptic-region associated microglia (Table 1), express genes associated with phagocytosis of synaptic material and complement pathway activation (Silva et al., 2021). Taken together, these findings emphasize the multi-faceted ways microglia can regulate neurogenesis via phagocytosis.
Although the mechanism(s) that drive microglial selection of NPC phagocytosis remain unclear, an increasing body of work has elucidated the role of microglial secreted factors in regulating NPCs and neurogenesis to support brain development. When cultured alone, murine NPCs lose the capacity to produce committed neuroblasts, but co-culture with either microglia or microglial-conditioned media rescues this ability. These findings suggest that microglial-secreted factors are sufficient to promote SVZ neurogenesis (Walton et al., 2006). Indeed, microglial reactivity and the subsequent secretion of inflammatory cytokines [e.g. Il1β, Il6, tumor necrosis factor α (Tnfα) and Ifnγ], in early postnatal development promotes both neurogenesis and oligodendrogenesis in the rat SVZ (Shigemoto-Mogami et al., 2014) (Fig. 4A). Recent work has suggested that the crosstalk between microglia and NPCs may also be bidirectional; basal progenitor cells in the murine ventricular zone and SVZ actively recruit microglia through the expression of Cxcl12 during cortical development. In turn, disruption of essential microglial Csf1r signaling reduces the population of basal progenitor cells, implicating microglia in proper progenitor cell maintenance (Arnò et al., 2014). Furthermore, embryonic murine microglia are recruited by Cxcl12 to both the SVZ and meninges from the cortical plate. The absence of microglia-secreted factors (e.g. Il6 and type 1 interferons) in the cortical plate is instrumental in the proper maturation of post-migratory cortical plate neurons (Hattori et al., 2020). These studies highlight both the phagocytic (Fig. 3A) and secretory (Fig. 4A) mechanisms by which microglia mediate NPC development and neurogenesis. Future studies linking bidirectional signaling mechanisms, secretory or otherwise, to phagocytosis will clarify the complex crosstalk between NPCs and microglia during neurodevelopment.
Neurons
Microglia are central regulators of the survival and activity of newborn neurons after embryonic development, both promoting and inhibiting cell death when appropriate. To highlight this, numerous studies have investigated the role of microglia during pre- and postnatal eye development (Anderson et al., 2019; Jobling et al., 2018; Lang and Bishop, 1993; Marín-Teva et al., 1999). During murine prenatal development, retinal microglia primarily associate with newborn retinal ganglion cells (RGCs) and regulate neuronal elimination via complement-dependent phagocytosis (Anderson et al., 2019) (Fig. 3B). During postnatal murine eye development, microglial fractalkine signaling modulates microglial-cone contacts, as well as proper cone photoreceptor cilium gene expression and maturation. Genetic ablation of Cx3cr1 signaling induces retinal dysfunction and the loss of cone photoreceptors (Jobling et al., 2018), demonstrating the crucial role of microglia in cell survival. Recent studies have also demonstrated the importance of microglial cytokines for neuronal survival. Previously, microglial-derived Tnfα (Sedel et al., 2004), nerve growth factor (Ngf) (Frade and Barde, 1998) and superoxide ions (Marín-Teva et al., 2004) have been identified as salient mediators of neuronal survival during mouse development (Fig. 4B). More recent work has found that microglia postnatally accumulate near subcerebral and callosal projection axons and that microglial-derived Igf1 is crucial in preventing the apoptosis of layer V cortical neurons (Ueno et al., 2013). Moreover, disruption of microglial Tgfβ signaling (achieved by αVβ8 integrin deletion in the CNS) induces the formation of dysmature microglia, which impair oligodendrocyte maturation and promote the loss of GABAergic interneurons, ultimately leading to severe motor dysfunction and death (Arnold et al., 2019) (Fig. 4C). In addition to secretory mechanisms, microglial processes can directly contact neuronal somata to modulate neuronal function through purinergic junctions in both adult mice and humans (Cserép et al., 2020) (Fig. 3C). Collectively, these findings indicate that microglia are vital to sustain numerous neuronal populations, yet, crucially, microglia are also imperative to the establishment and refinement of the neuron–neuron communication that forms the foundation of neural signaling.
Synaptogenesis, synaptic activity and pruning
Microglia are also involved in the formation of functional neural circuits by modulating synaptogenesis, synapse maturation and synaptic activity. A number of early studies have demonstrated that the loss of functional microglia can alter both excitatory and inhibitory synaptic activity, as well as long-term potentiation (Béchade et al., 2013; Bessis et al., 2007; Roumier et al., 2004). More recent work has elucidated novel mechanisms underlying microglial-mediated alterations to synaptic activity in different cellular, spatial and temporal contexts. For example, microglial loss in prenatal mice can disrupt the proper laminar positioning of a subset of neocortical Lhx6+ inhibitory interneurons, which is required for circuit function (Squarzoni et al., 2014). Alternatively, microglia can physically interact with the dendrites of pyramidal neurons within the somatosensory cortex to initiate actin accumulation and augment calcium ion transients, allowing for filopodia and spine formation in mice. Pharmacological blockade of microglia decreases spine density and excitatory synapse formation (Miyamoto et al., 2016). In contrast, microglial depletion in postnatal development increases excitatory and inhibitory synapse number in the somatosensory cortex by disrupting the preferential pruning of inhibitory parvalbumin interneuron synapses by GABA-receptive microglia (Favuzzi et al., 2021) (Table 1). Notably, microglial depletion in utero increases inhibitory parvalbumin interneuron synaptic connections with layer IV neurons in the juvenile (P20) barrel cortex, but decreases them in adults (P60) (Thion et al., 2019). Adding further to this complexity, microglial depletion in adult mice increases synapse number, as well as the activity, of excitatory neurons and inhibitory parvalbumin interneurons in the visual cortex (Liu et al., 2021) (Fig. 3D). These seemingly contradictory findings saliently highlight the necessity of studying the temporal and spatial heterogeneity of microglia during development.
As with neurogenesis, microglia can secrete cytokines as a mechanism to aid developmental synaptogenesis and synaptic maturation (Fig. 4D). In vitro, microglial-secreted Il10 increases the number of hippocampal dendritic spines, excitatory synapses and inhibitory synapses of rat hippocampal neurons (Lim et al., 2013). In vivo, microglial brain-derived neurotrophic factor (Bdnf) signaling in juvenile mice promotes motor learning-related synapse formation and structural plasticity, and ultimately supports glutamatergic synaptic activity and cognitive function (Parkhurst et al., 2013). Postnatal microglial recruitment to thalamocortical synapse clusters in the murine somatosensory cortex is mediated by fractalkine signaling and genetic ablation of this signaling delays the maturation of postsynaptic glutamate receptors at these synapses (Hoshiko et al., 2012). Intriguingly, during human brain development, integrated scRNA-seq has revealed a human cytokine-associated microglia subtype, and xenotransplantation of human prenatal microglia in cerebral organoids increases the synchronization and frequency of neural activity, suggesting a role for microglia in circuit formation and accelerated synaptic maturation (Popova et al., 2021).
Reciprocal crosstalk between microglia and neurons is another key component regulating synaptic activity. Activated hippocampal neurons in adolescent mice secrete Il33, which induces experience-dependent microglial spine remodeling and engulfment of the extracellular matrix, which in turn augments excitatory postsynaptic current frequency (Nguyen et al., 2020). In adult mice, striatal microglia migrate to the synapses of activated thalamocortical projection neurons that secrete ATP. Microglia then convert this ATP to adenosine, which limits neuronal synchrony and firing frequency to prevent seizure (Badimon et al., 2020). However, when hippocampal microglia release ATP, it can be amplified by P2y1r-expressing astrocytes, leading to increased excitatory postsynaptic currents (Pascual et al., 2012). Using several transgenic mouse models to disrupt microglial homeostasis during CNS development, another study has demonstrated that microglia are important for the negative regulation of neuronal activity in an ATP-dependent manner to prevent overactivation and seizures (Badimon et al., 2020).
In addition to building functional circuits, microglia are also essential for synaptic refinement, in which established synapses are either eliminated or reinforced and maintained (Schafer et al., 2013). Surveilling microglia phagocytose live synaptic material in the hippocampi of young mice and microglial loss leads to an increase in dendritic spines and the development of immature synapses (Paolicelli et al., 2011). Microglial loss and impaired synaptic pruning during neurodevelopment has also been associated with autism spectrum disorder; Cx3cr1 knockout mice exhibit a reduced number of excitatory synapses per axon, which is associated with deficits in long-range hippocampal connectivity, decreased social interactions and increased repetitive behavior (Zhan et al., 2014). These works suggest a neuroprotective role for synaptic pruning. Indeed, reactive microglia in adult mice physically disrupt inhibitory presynaptic terminals at cortical neuron somata (Fig. 3C) and the subsequent increase in neuronal activity prevents neuronal apoptosis post-injury (Chen et al., 2014).
Synaptic pruning in the postnatal retinogeniculate system depends on both intact microglial complement signaling and neuronal signaling. In mice, the C1q protein initiates classical complement signaling and is expressed by RGCs in the dorsal lateral geniculate nucleus (dLGN) in response to astrocyte exposure. Complement pathway activation ultimately leads to the deposition of the C3 complement proteins, which mark cells for phagocytosis, and thus promotes dLGN synapse refinement and proper segregation of RGC inputs from each eye (Stevens et al., 2007). Microglia in the dLGN preferentially prune less-active RGC presynaptic terminals and loss of microglial complement signaling induces increased synaptic density and impaired eye segregation (Schafer et al., 2012), thereby demonstrating that synaptic pruning is essential for proper eye development. However, in a murine multiple sclerosis model, microglia engulf synapses in the dLGN during early-stage disease. Inhibition of synaptic C3 prevents pruning and rescues vision loss independently of demyelination and C1q (Werneburg et al., 2020), suggesting that alterations to synaptic pruning may underlie the neurodegeneration associated with multiple sclerosis. In summary, microglia control synaptogenesis and synapse refinement in both health and disease through numerous mechanisms depending on their unique cellular, spatial and temporal contexts.
Beyond the synapse: axonogenesis, myelinogenesis and oligodendrocyte precursor cells
In addition to the synapse, microglia are known to influence the generation and pruning of neuronal axons and the myelin that ensheathes them. Studies in Drosophila initially provided evidence that glial cells can alter axons. These glia engulf the fragmented axons of mushroom body γ neurons in a microglial-like manner to facilitate proper larval development (Watts et al., 2004). In the prenatal murine forebrain, microglia regulate the outgrowth of dopaminergic axons (Squarzoni et al., 2014). In the context of optic nerve injury, RGC axonal regeneration requires Clec7a (also known as dectin-1), which is expressed only by myeloid cells (including microglia) in the murine retina (Baldwin et al., 2015), thus suggesting that microglia are instrumental for proper axonogenesis. However, in embryonic human brains, reactive microglia are present in the pons, olivary bodies, hippocampus, optical tract and anterior limb of the internal capsule, but are absent from most regions with growing axons, indicating that reactive microglia may limit axonogenesis (Cho et al., 2013). These works shed light on both pro- and anti-axonogenic roles for microglia during neurodevelopment and further work is necessary to fully elucidate the context-specific nuances in microglial regulation of axonogenesis.
Microglia are also crucial regulators of myelinogenesis, which is the process of myelin formation around bare axons. Myelin is a lipid-rich sheath produced by oligodendrocytes that enwraps neuronal axons to insulate and promote the saltatory conduction of action potentials. As such, the differentiation of oligodendrocyte precursor cells (OPCs) into mature oligodendrocytes and properly formed myelin is crucial for healthy brain development. Genetic or pharmacological ablation of microglia soon after birth in mice leads to decreased numbers of OPCs and oligodendrocytes and a transient decrease in white matter-specific myelinated axons (Hagemeyer et al., 2017) (Fig. 3E). Aberrant perinatal activation of inflammatory signaling in microglia is associated with white matter dysregulation of myelination; however, blocking the microglial intracellular protein complex (known as the inflammasome) in mouse brain explants can reverse the developmental hypomyelination (Holloway et al., 2021). Furthermore, murine microglial ablation in adults results in a rapid decrease in OPCs of the corpus callosum (Hagemeyer et al., 2017). The type 1 integral membrane protein neuropilin 1 (Nrp1), expressed specifically by white matter-associated microglia, facilitates OPC proliferation during development and remyelination after acute demyelination via PDGF-AA signaling (Sherafat et al., 2021), thus underscoring the potent role of microglia in oligodendroglial lineage cell maintenance throughout life. Microglia migrating from the ventricular zone can phagocytose viable OPCs in the murine corpus callosum, a process that is reduced with knockdown of fractalkine signaling; this reduced OPC phagocytosis resulting from fractalkine signaling knockdown is subsequently associated with an increase in adulthood of mature oligodendrocytes that produce thinner myelin, thereby suggesting that microglia contribute to myelin and myelin-forming cell homeostasis (Nemes-Baran et al., 2020). Indeed, in zebrafish, microglia phagocytose excess myelin in the optic tectum to ensure proper developmental myelination in a neuronal activity-dependent manner (Hughes and Appel, 2020). Furthermore, young human patients who carry homozygous mutations for the CSF1R completely lack the myelinated nerve bundles that connect both hemispheres of the brain (Oosterhof et al., 2019).
Another mechanism by which microglia regulate developmental myelination is through cytokine release (Fig. 4E). Suppression of microglial reactivity with minocycline in early life decreases secretion of inflammatory cytokines and oligodendrogenesis in the SVZ (Shigemoto-Mogami et al., 2014). Intriguingly, though a Cd11c+ subset of microglia has been shown to poorly produce proinflammatory cytokines during experimental autoimmune encephalitis in mice (Wlodarczyk et al., 2014), a Cd11c+ postnatal microglial population (Table 1) can express Igf1 and other neurogenic signals implicated in developmental myelination (Wlodarczyk et al., 2017) (Fig. 4E). Finally, as in neurogenesis, CNS-specific deletion of murine αVβ8 integrin causes dysmature microglia, leading to deficits in oligodendrocyte maturation (Arnold et al., 2019). Taken together, these works emphasize pivotal neuron-glia and glial-glial interactions that support healthy CNS development.
Astrocytes and astrogenesis
Prenatal and postnatal interactions between microglia and astrocytes also underscore the essential role of microglia for glia-glia interactions pertaining to development. Experiments culturing NPCs from Spi1−/− mice, which lack microglia, have reduced staining for glial fibrillary acidic protein-positive (Gfap+) cells relative to mice with intact microglia, thus suggesting that microglia may be involved in astrogenesis (Antony et al., 2011). Further work has specified how secreted factors may regulate astrogenesis and astrocyte development (Fig. 4F). Recombinant, proinflammatory cytokine Il1 increases Gfap+ cell number in embryonic mixed-glial cerebral cortex cultures (Giulian et al., 1988). Likewise, culturing embryonic rat SVZ cells in microglial conditioned media, which specifically contains Il6 and leukemia inhibitory factor (Lif), also increases the percentage of Gfap+ cells (Nakanishi et al., 2007). Nitric oxide has also been implicated in the differentiation of hippocampal radial glia into mature astrocytes. Nitric oxide synthase 2 (Nos2) is highly upregulated by inflammatory microglia and a key producer of nitric oxide; genetic ablation of Nos2 shows no evidence of disrupting the generation of glutamine synthetase-positive astrocytes, but does reduce astrocyte differentiation in mice as measured by reduced Gfap expression (Béchade et al., 2011). Thus, microglial secreted factors are a primary mechanism by which microglia regulate putative astrocyte development.
Finally, recent work has drawn attention to the reciprocal nature of crosstalk between microglia and astrocytes that supports cellular survival. Microglial survival ex vivo requires a number of astrocyte-derived factors including Csf1, Il34, Tgfβ2 and cholesterol (Bohlen et al., 2017). Microglial-astrocyte communication has functional implications in a neurodegenerative context as well; neuroinflammatory microglia secrete Il1α, Tnf and C1q, which induce A1 astrocyte reactivity (Fig. 4F). In mice, A1 astrocytes fail to promote multiple regenerative mechanisms ranging from neuronal process outgrowth and synaptogenesis to phagocytosis, leading to neuronal and oligodendrocyte death (Liddelow et al., 2017). As such, microglial-astrocyte crosstalk represents yet another axis for microglial maintenance of brain homeostasis.
Angiogenesis
Microglia have been implicated in multiple aspects of functional blood vessel generation during CNS development. Previous imaging work initially identified microglia in close physical proximity to newly formed blood vessels in several animal models and humans (Arnold and Betsholtz, 2013; Cuadros et al., 1992; Dalmau et al., 1997; Monier et al., 2007). In the mammalian hindbrain, brain macrophages physically bridge endothelial tip cells to promote vessel fusion/anastomosis, with genetic ablation of CNS macrophages impairing hindbrain vascularization (Fantin et al., 2010). Recent work has identified a distinct microglial subpopulation in mice (Table 1), deemed capillary-associated microglia, which line brain capillaries and regulate vasodilation and blood flow (Fig. 3F). These capillary-associated microglia can be detected as early as P5 and maintain their density from P15 through to 12 months of age (Bisht et al., 2021). A number of studies have also investigated microglial control of retinal vascularization. In the rat retina, clodronate liposome depletion of microglia, but not systemic macrophages, early in postnatal development reduces blood vessel growth and density (Checchin et al., 2006). In the murine retina, as with the hindbrain (Fantin et al., 2010), microglia are physically present at tip cells and promote anastomosis (Rymo et al., 2011). However, in murine aortic ring cultures, microglia can additionally promote vessel sprouting via secreted factors (Rymo et al., 2011). Intriguingly, murine microglia must migrate through extracellular matrix of varying stiffness, which in turn regulates their bipolarization and expression of Tgfβ1 and ultimately determines normal retinal vascularization (Dudiki et al., 2020) (Fig. 4G). In summary, microglial regulation of developmental angiogenesis is multimodal, providing many avenues for further mechanistic research on neurodevelopmental interactions between microglia and endothelial cells.
Conclusion
The works summarized here highlight the dynamic nature of microglial heterogeneity and crucial microglial interactions with neuronal and non-neuronal cell types that regulate proper CNS development. Understanding how these developmental, transcriptionally distinct microglial subsets determine microglial function and reactivity remains a crucial question in the field. As such, dissecting the functional roles of diverse microglial populations in health will yield indispensable insights into what microglial-mediated processes may go awry in disease (Box 2). With this knowledge, we will be one step closer to determining how microglia can be effectively targeted for therapeutic benefit in different cellular, spatial and temporal contexts.
Box 2. Therapeutic potential of targeting microglia to regenerate CNS tissue.
As microglia are central architects in generating a healthy CNS during development, ongoing research seeks to leverage this regulatory capacity for tissue regeneration in the context of multiple sclerosis. Microglia can promote demyelination via Csf1r signaling, and inhibiting this signaling can alleviate demyelination, oligodendrocyte loss and reactive astrogliosis (measured by Gfap staining) after cuprizone treatment (Marzan et al., 2021). In the injured optic nerve, both reactive microglia and OPCs impair de novo myelination of newly regenerated axons. A combination approach blocking OPC Gpr17 signaling and depleting microglia via Csf1r inhibition promotes robust remyelination (Wang et al., 2020), suggesting that eliminating microglia may be advantageous. However, the caveat of lack of specificity associated with Csf1r inhibition remains (Dai et al., 2002; Lei et al., 2020). Thus, more research is necessary to understand the long-term benefits and consequences of persistent myeloid depletion.
Intriguingly, recent work has elucidated beneficial roles of microglia in promoting remyelination. In response to neuronal potassium release, microglia preferentially interact with nodes of Ranvier to promote remyelination (Ronzano et al., 2021). Additionally, stimulation of sterol synthesis in microglia can promote inflammation resolution and oligodendrocyte differentiation at demyelinating lesions (Berghoff et al., 2021). Finally, Trem2+ microglia (Table 1) can be found in adult humans (Piccio et al., 2008) and mice; in mice they have been shown to clear the products of oxidative stress induced by multiple sclerosis, which otherwise can kill neurons and oligodendrocytes and cause neurodegeneration (Dong et al., 2021). An alternative therapeutic route may be to reprogram, rather than eliminate, pathologic microglia; Trem2 agonism promotes the clearance of myelin debris after cuprizone administration and allows for an increase in OPC number, OPC differentiation and remyelination (Cignarella et al., 2020). Together, these works suggest that there may be significant clinical utility in targeting pathologic microglia, but underscore the need to refine the specificity of these approaches with respect to cellular, spatial and temporal dynamics to promote myelin regeneration successfully.
Acknowledgements
This Review is in memory of C. Mehl and M. Spelleken. All figures were created with BioRender.com.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
L.C.M. was supported by the National Cancer Institute (PHS grant number CA09302), U.S. Department of Health and Human Services. E.M.G. was supported by the U.S. Department of Defense (W81XWH-21-1-0846); the National Multiple Sclerosis Society (PP-1907-34759); the Weintz Family COVID-19 Research Fund; the Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University 2021 Innovator Grant Program; and a NARSAD Young Investigator Grant (#2997) from the Brain and Behavior Research Foundation. T.L.T. was supported by the Klaus Tschira Stiftung Boost Fund (KT-10); a Freiburg Institute for Advanced Studies, Albert-Ludwigs-Universität Freiburg Junior Fellowship; the Albert-Ludwigs-Universität Freiburg Research Innovation Fund; Wissenschaftlichen Gesellschaft Freiburg (Helmut Holzer Prize); the German Research Foundation (Deutsche Forschungsgemeinschaft; EXC 1086); and the Ministry of Economics, Science and Arts of Baden-Württemberg (Sustainability Program for Projects of the Excellence Initiative II). Deposited in PMC for release after 12 months.
References
- Ajami, B., Bennett, J. L., Krieger, C., Tetzlaff, W. and Rossi, F. M. V. (2007). Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538-1543. 10.1038/nn2014 [DOI] [PubMed] [Google Scholar]
- Alliot, F., Godin, I. and Pessac, B. (1999). Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev. Brain Res. 117, 145-152. 10.1016/S0165-3806(99)00113-3 [DOI] [PubMed] [Google Scholar]
- Anderson, S. R., Zhang, J., Steele, M. R., Romero, C. O., Kautzman, A. G., Schafer, D. P. and Vetter, M. L. (2019). Complement targets newborn retinal ganglion cells for phagocytic elimination by microglia. J. Neurosci 39, 2025-2040. 10.1523/JNEUROSCI.1854-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony, J. M., Paquin, A., Nutt, S. L., Kaplan, D. R. and Miller, F. D. (2011). Endogenous microglia regulate development of embryonic cortical precursor cells. J. Neurosci. Res. 89, 286-298. 10.1002/jnr.22533 [DOI] [PubMed] [Google Scholar]
- Arnò, B., Grassivaro, F., Rossi, C., Bergamaschi, A., Castiglioni, V., Furlan, R., Greter, M., Favaro, R., Comi, G., Becher, B.et al. (2014). Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat. Commun. 5, 5611. 10.1038/ncomms6611 [DOI] [PubMed] [Google Scholar]
- Arnold, T. and Betsholtz, C. (2013). The importance of microglia in the development of the vasculature in the central nervous system. Vasc. Cell 5, 4. 10.1186/2045-824X-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, T. D., Lizama, C. O., Cautivo, K. M., Santander, N., Lin, L., Qiu, H., Huang, E. J., Liu, C., Mukouyama, Y., Reichardt, L. F.et al. (2019). Impaired αVβ8 and TGFβ signaling lead to microglial dysmaturation and neuromotor dysfunction. J. Exp. Med 216, 900-915. 10.1084/jem.20181290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back, J., Dierich, A., Bronn, C., Kastner, P. and Chan, S. (2004). PU.1 determines the self-renewal capacity of erythroid progenitor cells. Blood 103, 3615-3623. 10.1182/blood-2003-11-4089 [DOI] [PubMed] [Google Scholar]
- Badimon, A., Strasburger, H. J., Ayata, P., Chen, X., Nair, A., Ikegami, A., Hwang, P., Chan, A. T., Graves, S. M., Uweru, J. O.et al. (2020). Negative feedback control of neuronal activity by microglia. Nature 586, 417-423. 10.1038/s41586-020-2777-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin, K. T., Carbajal, K. S., Segal, B. M. and Giger, R. J. (2015). Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc. Natl. Acad. Sci. USA 112, 2581-2586. 10.1073/pnas.1423221112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béchade, C., Pascual, O., Triller, A. and Bessis, A. (2011). Nitric oxide regulates astrocyte maturation in the hippocampus: Involvement of NOS2. Mol. Cell. Neurosci. 46, 762-769. 10.1016/j.mcn.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Béchade, C., Cantaut-Belarif, Y. and Bessis, A. (2013). Microglial control of neuronal activity. Front. Cell. Neurosci 7, 32. 10.3389/fncel.2013.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, T. and Becker, C. G. (2022). Regenerative neurogenesis: the integration of developmental, physiological and immune signals. Development 149, dev199907. 10.1242/dev.199907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers, D. R., Henkel, J. S., Xiao, Q., Zhao, W., Wang, J., Yen, A. A., Siklos, L., McKercher, S. R. and Appel, S. H. (2006). Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 103, 16021-16026. 10.1073/pnas.0607423103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmamar-Badel, A., Owens, T. and Wlodarczyk, A. (2020). Protective microglial subset in development, aging, and disease: lessons from transcriptomic studies. Front. Immunol. 11, 430. 10.3389/fimmu.2020.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M. L. and Bennett, F. C. (2020). The influence of environment and origin on brain resident macrophages and implications for therapy. Nat. Neurosci. 23, 157-166. 10.1038/s41593-019-0545-6 [DOI] [PubMed] [Google Scholar]
- Bennett, M. L., Bennett, F. C., Liddelow, S. A., Ajami, B., Zamanian, J. L., Fernhoff, N. B., Mulinyawe, S. B., Bohlen, C. J., Adil, A., Tucker, A.et al. (2016). New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 113, E1738-E1746. 10.1073/pnas.1525528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, F. C., Bennett, M. L., Yaqoob, F., Mulinyawe, S. B., Grant, G. A., Hayden Gephart, M., Plowey, E. D. and Barres, B. A. (2018). A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98, 1170-1183. 10.1016/j.neuron.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff, S. A., Spieth, L., Sun, T., Hosang, L., Schlaphoff, L., Depp, C., Düking, T., Winchenbach, J., Neuber, J., Ewers, D.et al. (2021). Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci, 24, 47-60. 10.1038/s41593-020-00757-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis, A., Béchade, C., Bernard, D. and Roumier, A. (2007). Microglial control of neuronal death and synaptic properties. Glia 55, 233-238. 10.1002/glia.20459 [DOI] [PubMed] [Google Scholar]
- Bian, Z., Gong, Y., Huang, T., Lee, C. Z. W., Bian, L., Bai, Z., Shi, H., Zeng, Y., Liu, C., He, J.et al. (2020). Deciphering human macrophage development at single-cell resolution. Nature 582, 571-576. 10.1038/s41586-020-2316-7 [DOI] [PubMed] [Google Scholar]
- Bisht, K., Okojie, K. A., Sharma, K., Lentferink, D. H., Sun, Y.-Y., Chen, H.-R., Uweru, J. O., Amancherla, S., Calcuttawala, Z., Campos-Salazar, A. B.et al. (2021). Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 12, 5289. 10.1038/s41467-021-25590-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen, C. J., Bennett, F. C., Tucker, A. F., Collins, H. Y., Mulinyawe, S. B. and Barres, B. A. (2017). Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759-773. 10.1016/j.neuron.2017.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher, C., Schlickeiser, S., Sneeboer, M. A. M., Kunkel, D., Knop, A., Paza, E., Fidzinski, P., Kraus, L., Snijders, G. J. L., Kahn, R. S.et al. (2019). Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 22, 78-90. 10.1038/s41593-018-0290-2 [DOI] [PubMed] [Google Scholar]
- Butovsky, O., Jedrychowski, M. P., Moore, C. S., Cialic, R., Lanser, A. J., Gabriely, G., Koeglsperger, T., Dake, B., Wu, P. M., Doykan, C. E.et al. (2014). Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131-143. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit, A., Lelios, I., Yu, X., Vrohlings, M., Krakoski, N. R., Gautier, E. L., Nishinakamura, R., Becher, B. and Greter, M. (2016). Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 17, 1397-1406. 10.1038/ni.3585 [DOI] [PubMed] [Google Scholar]
- Checchin, D., Sennlaub, F., Levavasseur, E., Leduc, M. and Chemtob, S. (2006). Potential role of microglia in retinal blood vessel formation. Invest. Ophthalmol. Vis. Sci. 47, 3595-3602. 10.1167/iovs.05-1522 [DOI] [PubMed] [Google Scholar]
- Chen, Z., Jalabi, W., Hu, W., Park, H.-J., Gale, J. T., Kidd, G. J., Bernatowicz, R., Gossman, Z. C., Chen, J. T., Dutta, R.et al. (2014). Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 5, 4486. 10.1038/ncomms5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, V. S., Morrison, J. P., Southwell, M. F., Foley, J. F., Bolon, B. and Elmore, S. A. (2017). Histology atlas of the developing prenatal and postnatal mouse central nervous system, with emphasis on prenatal days E7.5 to E18.5. Toxicol. Pathol. 45, 705-744. 10.1177/0192623317728134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, K. H., Cheong, J. S., Kim, J. H., Abe, H., Murakami, G. and Cho, B. H. (2013). Site-Specific distribution of CD68-positive microglial cells in the brains of human midterm fetuses: a topographical relationship with growing axons. BioMed Res. Int. 2013, e762303. 10.1155/2013/762303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarella, F., Filipello, F., Bollman, B., Cantoni, C., Locca, A., Mikesell, R., Manis, M., Ibrahim, A., Deng, L., Benitez, B. A.et al. (2020). TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. (Berl.) 140, 513-534. 10.1007/s00401-020-02193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk, J. C., Filiano, A. J., Louveau, A., Marin, I., Marsh, R., Ji, E., Goldman, D. H., Smirnov, I., Geraci, N., Acton, S.et al. (2018). Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 215, 1627-1647. 10.1084/jem.20180247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserép, C., Pósfai, B., Lénárt, N., Fekete, R., László, Z. I., Lele, Z., Orsolits, B., Molnár, G., Heindl, S., Schwarcz, A. D.et al. (2020). Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 367, 528-537. 10.1126/science.aax6752 [DOI] [PubMed] [Google Scholar]
- Cuadros, M. A., Moujahid, A., Martin-Partido, G. and Navascués, J. (1992). Microglia in the mature and developing quail brain as revealed by a monoclonal antibody recognizing hemopoietic cells. Neurosci. Lett. 148, 11-14. 10.1016/0304-3940(92)90792-6 [DOI] [PubMed] [Google Scholar]
- Cunningham, C. L., Martínez-Cerdeño, V. and Noctor, S. C. (2013). Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. .33, 4216-4233. 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, X.-M., Ryan, G. R., Hapel, A. J., Dominguez, M. G., Russell, R. G., Kapp, S., Sylvestre, V. and Stanley, E. R. (2002). Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111-120. 10.1182/blood.V99.1.111 [DOI] [PubMed] [Google Scholar]
- Dalmau, I., Finsen, B., Tønder, N., Zimmer, J., González, B. and Castellano, B. (1997). Development of microglia in the prenatal rat hippocampus. J. Comp. Neurol. 377, 70-84. [DOI] [PubMed] [Google Scholar]
- Datta, M., Staszewski, O., Raschi, E., Frosch, M., Hagemeyer, N., Tay, T. L., Blank, T., Kreutzfeldt, M., Merkler, D., Ziegler-Waldkirch, S.et al. (2018). Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 48, 514-529. 10.1016/j.immuni.2018.02.016 [DOI] [PubMed] [Google Scholar]
- De, S., Van Deren, D., Peden, E., Hockin, M., Boulet, A., Titen, S. and Capecchi, M. R. (2018). Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development 145, dev152306. 10.1242/dev.152306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y., D'Mello, C., Pinsky, W., Lozinski, B. M., Kaushik, D. K., Ghorbani, S., Moezzi, D., Brown, D., Melo, F. C., Zandee, S.et al. (2021). Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 24, 489-503. 10.1038/s41593-021-00801-z [DOI] [PubMed] [Google Scholar]
- Dudiki, T., Meller, J., Mahajan, G., Liu, H., Zhevlakova, I., Stefl, S., Witherow, C., Podrez, E., Kothapalli, C. R. and Byzova, T. V. (2020). Microglia control vascular architecture via a TGFβ1 dependent paracrine mechanism linked to tissue mechanics. Nat. Commun. 11, 986. 10.1038/s41467-020-14787-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley-Neal, C., Foreman, O., Sharma, N., Zarrin, A. A. and Weimer, R. M. (2019). CSF1R Ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions. Front. Immunol 10, 2199. 10.3389/fimmu.2019.02199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore, M. R. P., Najafi, A. R., Koike, M. A., Dagher, N. N., Spangenberg, E. E., Rice, R. A., Kitazawa, M., Matusow, B., Nguyen, H., West, B. L.et al. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380-397. 10.1016/j.neuron.2014.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich, B., Zhu, L., Etgen, A. M., Dobrenis, K. and Pollard, J. W. (2011). Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE 6, e26317. 10.1371/journal.pone.0026317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, X., Dong, J., Zhong, S., Wei, Y., Wu, Q., Yan, L., Yong, J., Sun, L., Wang, X., Zhao, Y.et al. (2018). Spatial transcriptomic survey of human embryonic cerebral cortex by single-cell RNA-seq analysis. Cell Res. 28, 730-745. 10.1038/s41422-018-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin, A., Vieira, J. M., Gestri, G., Denti, L., Schwarz, Q., Prykhozhij, S., Peri, F., Wilson, S. W. and Ruhrberg, C. (2010). Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829-840. 10.1182/blood-2009-12-257832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi, E., Huang, S., Saldi, G. A., Binan, L., Ibrahim, L. A., Fernández-Otero, M., Cao, Y., Zeine, A., Sefah, A., Zheng, K.et al. (2021). GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184, 4048-4063. 10.1016/j.cell.2021.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach, M. K., Tjwa, M., Bechmann, I. and Krueger, M. (2018). Decreased microglial numbers in Vav1-Cre+:dicer knock-out mice suggest a second source of microglia beyond yolk sac macrophages. Ann. Anat. Anat. Anz 218, 190-198. 10.1016/j.aanat.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Frade, J. M. and Barde, Y.-A. (1998). Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron 20, 35-41. 10.1016/S0896-6273(00)80432-8 [DOI] [PubMed] [Google Scholar]
- Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., Mehler, M. F., Conway, S. J., Ng, L. G., Stanley, E. R.et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841-845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian, D., Young, D. G., Woodward, J., Brown, D. C. and Lachman, L. B. (1988). Interleukin-1 is an astroglial growth factor in the developing brain. J. Neurosci. 8, 709-714. 10.1523/JNEUROSCI.08-02-00709.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann, T., Wieghofer, P., Müller, P. F., Wolf, Y., Varol, D., Yona, S., Brendecke, S. M., Kierdorf, K., Staszewski, O., Datta, M.et al. (2013). A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci 16, 1618-1626. 10.1038/nn.3531 [DOI] [PubMed] [Google Scholar]
- Goldmann, T., Zeller, N., Raasch, J., Kierdorf, K., Frenzel, K., Ketscher, L., Basters, A., Staszewski, O., Brendecke, S. M., Spiess, A.et al. (2015). USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34, 1612-1629. 10.15252/embj.201490791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann, T., Wieghofer, P., Jordão, M. J. C., Prutek, F., Hagemeyer, N., Frenzel, K., Amann, L., Staszewski, O., Kierdorf, K., Krueger, M.et al. (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797-805. 10.1038/ni.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin, D., Link, V. M., Romanoski, C. E., Fonseca, G. J., Eichenfield, D. Z., Spann, N. J., Stender, J. D., Chun, H. B., Garner, H., Geissmann, F.et al. (2014). Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327-1340. 10.1016/j.cell.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin, D., Skola, D., Coufal, N. G., Holtman, I. R., Schlachetzki, J. C. M., Sajti, E., Jaeger, B. N., O'Connor, C., Fitzpatrick, C., Pasillas, M. P.et al. (2017). An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222. 10.1126/science.aal3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert, K., Michoel, T., Karavolos, M. H., Clohisey, S., Baillie, J. K., Stevens, M. P., Freeman, T. C., Summers, K. M. and McColl, B. W. (2016). Microglial brain region−dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504-516. 10.1038/nn.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh, A. D., David, S. and Bennett, F. C. (2020). Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 21, 139-152. 10.1038/s41583-020-0263-9 [DOI] [PubMed] [Google Scholar]
- Greter, M., Lelios, I., Pelczar, P., Hoeffel, G., Price, J., Leboeuf, M., Kündig, T. M., Frei, K., Ginhoux, F., Merad, M.et al. (2012). Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050-1060. 10.1016/j.immuni.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya, D., Ivanov, A., Hernandez, D. P., Haage, V., Wojtas, B., Meyer, N., Maricos, M., Jordan, P., Buonfiglioli, A., Gielniewski, B.et al. (2018). Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 24, 2773-2783. 10.1016/j.celrep.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Hagemeyer, N., Hanft, K.-M., Akriditou, M.-A., Unger, N., Park, E. S., Stanley, E. R., Staszewski, O., Dimou, L. and Prinz, M. (2017). Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. (Berl) 134, 441-458. 10.1007/s00401-017-1747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. R., Dufort, C., Dissing-Olesen, L., Giera, S., Young, A., Wysoker, A., Walker, A. J., Gergits, F., Segel, M., Nemesh, J.et al. (2019). Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253-271. 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar, R. and Bilbo, S. D. (2016). Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 160, 127-133. 10.1016/j.jsbmb.2015.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar, R., Alter, M. D., Block, C. S., Sullivan, H., Bolton, J. L. and Bilbo, S. D. (2017). Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65, 1504-1520. 10.1002/glia.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori, Y., Naito, Y., Tsugawa, Y., Nonaka, S., Wake, H., Nagasawa, T., Kawaguchi, A. and Miyata, T. (2020). Transient microglial absence assists postmigratory cortical neurons in proper differentiation. Nat. Commun. 11, 1631. 10.1038/s41467-020-15409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohsfield, L. A., Najafi, A. R., Ghorbanian, Y., Soni, N., Crapser, J., Figueroa Velez, D. X., Jiang, S., Royer, S. E., Kim, S. J., Henningfield, C. M.et al. (2021). Subventricular zone/white matter microglia reconstitute the empty adult microglial niche in a dynamic wave. eLife 10, e66738. 10.7554/eLife.66738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, R. K., Ireland, G., Sullivan, G., Becher, J., Smith, C., Boardman, J. P., Gressens, P. and Miron, V. E. (2021). Microglial inflammasome activation drives developmental white matter injury. Glia 69, 1268-1280. 10.1002/glia.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko, M., Arnoux, I., Avignone, E., Yamamoto, N. and Audinat, E. (2012). Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J. Neurosci. 32, 15106-15111. 10.1523/JNEUROSCI.1167-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, A. N. and Appel, B. (2020). Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055-1066. 10.1038/s41593-020-0654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling, A. I., Waugh, M., Vessey, K. A., Phipps, J. A., Trogrlic, L., Greferath, U., Mills, S. A., Tan, Z. L., Ward, M. M. and Fletcher, E. L. (2018). The role of the microglial Cx3cr1 pathway in the postnatal maturation of retinal photoreceptors. J. Neurosci. 38, 4708-4723. 10.1523/JNEUROSCI.2368-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, S., Aliberti, J., Graemmel, P., Sunshine, M. J., Kreutzberg, G. W., Sher, A. and Littman, D. R. (2000). Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106-4114. 10.1128/MCB.20.11.4106-4114.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, T. and Feng, G. (2019). Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6, ENEURO.0448-18.2019. 10.1523/ENEURO.0448-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf, K., Erny, D., Goldmann, T., Sander, V., Schulz, C., Perdiguero, E. G., Wieghofer, P., Heinrich, A., Riemke, P., Hölscher, C.et al. (2013). Microglia emerge from erythromyeloid precursors via Pu.1– and Irf8-dependent pathways. Nat. Neurosci. 16, 273-280. 10.1038/nn.3318 [DOI] [PubMed] [Google Scholar]
- Kim, J.-S., Kolesnikov, M., Peled-Hajaj, S., Scheyltjens, I., Xia, Y., Trzebanski, S., Haimon, Z., Shemer, A., Lubart, A., Van Hove, H.et al. (2021). A binary Cre transgenic approach dissects microglia and CNS border-associated macrophages. Immunity 54, 176-190. 10.1016/j.immuni.2020.11.007 [DOI] [PubMed] [Google Scholar]
- Kracht, L., Borggrewe, M., Eskandar, S., Brouwer, N., Chuva de Sousa Lopes, S. M., Laman, J. D., Scherjon, S. A., Prins, J. R., Kooistra, S. M. and Eggen, B. J. L. (2020). Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science 369, 530-537. 10.1126/science.aba5906 [DOI] [PubMed] [Google Scholar]
- Kubick, N., Henckell Flournoy, P. C., Klimovich, P., Manda, G. and Mickael, M. (2020). What has single–cell RNA sequencing revealed about microglial neuroimmunology? Immun. Inflamm. Dis. 8, 825-839. 10.1002/iid3.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, R. A. and Bishop, J. M. (1993). Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74, 453-462. 10.1016/0092-8674(93)80047-I [DOI] [PubMed] [Google Scholar]
- Lavin, Y., Winter, D., Blecher-Gonen, R., David, E., Keren-Shaul, H., Merad, M., Jung, S. and Amit, I. (2014). Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312-1326. 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, L. J., Perry, V. H., Dri, P. and Gordon, S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151-170. 10.1016/0306-4522(90)90229-W [DOI] [PubMed] [Google Scholar]
- Lei, F., Cui, N., Zhou, C., Chodosh, J., Vavvas, D. G. and Paschalis, E. I. (2020). CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc. Natl. Acad. Sci. USA 117, 23336-23338. 10.1073/pnas.1922788117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Cheng, Z., Zhou, L., Darmanis, S., Neff, N. F., Okamoto, J., Gulati, G., Bennett, M. L., Sun, L. O., Clarke, L. E.et al. (2019). Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207-223. 10.1016/j.neuron.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., Bennett, M. L., Münch, A. E., Chung, W.-S., Peterson, T. C.et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481-487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S.-H., Park, E., You, B., Jung, Y., Park, A.-R., Park, S. G. and Lee, J.-R. (2013). Neuronal synapse formation induced by microglia and interleukin 10. PLoS ONE 8, e81218. 10.1371/journal.pone.0081218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.-J., Spangenberg, E. E., Tang, B., Holmes, T. C., Green, K. N. and Xu, X. (2021). Microglia elimination increases neural circuit connectivity and activity in adult mouse cortex. J. Neurosci. 41, 1274-1287. 10.1523/JNEUROSCI.2140-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, H., Pieber, M., Parsa, R., Han, J., Grommisch, D., Ewing, E., Kular, L., Needhamsen, M., Espinosa, A., Nilsson, E.et al. (2018). Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 9, 4845. 10.1038/s41467-018-07295-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore, C., Leyrolle, Q., Morel, L., Rossitto, M., Greenhalgh, A. D., Delpech, J. C., Martinat, M., Bosch-Bouju, C., Bourel, J., Rani, B.et al. (2020). Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 11, 6133. 10.1038/s41467-020-19861-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry, C., Kyrargyri, V., Arancibia-Cárcamo, I. L., Jolivet, R., Kohsaka, S., Bryan, R. M. and Attwell, D. (2018). Microglial ramification, surveillance, and interleukin-1β release are regulated by the two-pore domain K+ channel THIK-1. Neuron 97, 299-312.e6. 10.1016/j.neuron.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Teva, J. L., Cuadros, M. A., Calvente, R., Almendros, A. and Navascués, J. (1999). Naturally occurring cell death and migration of microglial precursors in the quail retina during normal development. J. Comp. Neurol 412, 255-275. [DOI] [PubMed] [Google Scholar]
- Marín-Teva, J. L., Dusart, I., Colin, C., Gervais, A., van Rooijen, N. and Mallat, M. (2004). Microglia promote the death of developing purkinje cells. Neuron 41, 535-547. 10.1016/S0896-6273(04)00069-8 [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño, V. and Noctor, S. C. (2018). Neural progenitor cell terminology. Front. Neuroanat 12, 104. 10.3389/fnana.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzan, D. E., Brügger-Verdon, V., West, B. L., Liddelow, S., Samanta, J. and Salzer, J. L. (2021). Activated microglia drive demyelination via CSF1R signaling. Glia 69, 1583-1604. 10.1002/glia.23980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T., Sankowski, R., Staszewski, O., Böttcher, C., Amann, L., Sagar, Scheiwe, C., Nessler, S., Kunz, P., van Loo, G.et al. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388-392. 10.1038/s41586-019-0924-x [DOI] [PubMed] [Google Scholar]
- Masuda, T., Amann, L., Sankowski, R., Staszewski, O., Lenz, M., d'Errico, P., Snaidero, N., Costa Jordão, M. J., Böttcher, C., Kierdorf, K.et al. (2020). Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol. 21, 802-815. 10.1038/s41590-020-0707-4 [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan, O., Winter, D. R., Giladi, A., Vargas Aguilar, S., Spinrad, A., Sarrazin, S., Ben-Yehuda, H., David, E., Zelada González, F., Perrin, P.et al. (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670. 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- McKinsey, G. L., Lizama, C. O., Keown-Lang, A. E., Niu, A., Santander, N., Larpthaveesarp, A., Chee, E., Gonzalez, F. F. and Arnold, T. D. (2020). A new genetic strategy for targeting microglia in development and disease. eLife 9, e54590. 10.7554/eLife.54590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner, A., Schmidt, H., Nitsche, M., Merkler, D., Hanisch, U.-K., Mack, M., Heikenwalder, M., Brück, W., Priller, J. and Prinz, M. (2007). Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544-1553. 10.1038/nn2015 [DOI] [PubMed] [Google Scholar]
- Minten, C., Terry, R., Deffrasnes, C., King, N. J. C. and Campbell, I. L. (2012). IFN Regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS. PLoS ONE 7, e49851. 10.1371/journal.pone.0049851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, A., Wake, H., Ishikawa, A. W., Eto, K., Shibata, K., Murakoshi, H., Koizumi, S., Moorhouse, A. J., Yoshimura, Y. and Nabekura, J. (2016). Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun 7, 12540. 10.1038/ncomms12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier, A., Adle-Biassette, H., Delezoide, A.-L., Evrard, P., Gressens, P. and Verney, C. (2007). Entry and Distribution of Microglial Cells in Human Embryonic and Fetal Cerebral Cortex. J. Neuropathol. Exp. Neurol 66, 372-382. 10.1097/nen.0b013e3180517b46 [DOI] [PubMed] [Google Scholar]
- Mrdjen, D., Pavlovic, A., Hartmann, F. J., Schreiner, B., Utz, S. G., Leung, B. P., Lelios, I., Heppner, F. L., Kipnis, J., Merkler, D.et al. (2018). High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380-395.e6. 10.1016/j.immuni.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Nakanishi, M., Niidome, T., Matsuda, S., Akaike, A., Kihara, T. and Sugimoto, H. (2007). Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur. J. Neurosci. 25, 649-658. 10.1111/j.1460-9568.2007.05309.x [DOI] [PubMed] [Google Scholar]
- Nandi, S., Gokhan, S., Dai, X.-M., Wei, S., Enikolopov, G., Lin, H., Mehler, M. F. and Stanley, E. R. (2012). The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 367, 100-113. 10.1016/j.ydbio.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, L. H., Warden, S. and Lenz, K. M. (2017). Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain. Behav. Immun. 64, 11-22. 10.1016/j.bbi.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes-Baran, A. D., White, D. R. and DeSilva, T. M. (2020). Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 32, 108047. 10.1016/j.celrep.2020.108047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, P. T., Dorman, L. C., Pan, S., Vainchtein, I. D., Han, R. T., Nakao-Inoue, H., Taloma, S. E., Barron, J. J., Molofsky, A. B., Kheirbek, M. A.et al. (2020). Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182, 388-403.e15. 10.1016/j.cell.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova, M., Kimyon, R. S., De, I., Small, A. L., Collier, L. S. and Watters, J. J. (2015). Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J. Neuroimmunol. 278, 280-288. 10.1016/j.jneuroim.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn, A., Kirchhoff, F. and Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314-1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Oosterhof, N., Chang, I. J., Karimiani, E. G., Kuil, L. E., Jensen, D. M., Daza, R., Young, E., Astle, L., van der Linde, H. C., Shivaram, G. M.et al. (2019). Homozygous mutations in CSF1R cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am. J. Hum. Genet. 104, 936-947. 10.1016/j.ajhg.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, K., Turnbull, I. R., Poliani, P. L., Vermi, W., Cerutti, E., Aoshi, T., Tassi, I., Takai, T., Stanley, S. L., Miller, M.et al. (2009). Macrophage colony stimulating factor induces macrophage proliferation and survival through a pathway involving DAP12 and β-catenin. Nat. Immunol. 10, 734-743. 10.1038/ni.1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., Giustetto, M., Ferreira, T. A., Guiducci, E., Dumas, L.et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456-1458. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Parkhurst, C. N., Yang, G., Ninan, I., Savas, J. N., Yates, J. R., Lafaille, J. J., Hempstead, B. L., Littman, D. R. and Gan, W.-B. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596-1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual, O., Achour, S. B., Rostaing, P., Triller, A. and Bessis, A. (2012). Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 109, E197-E205. 10.1073/pnas.1111098109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio, L., Buonsanti, C., Cella, M., Tassi, I., Schmidt, R. E., Fenoglio, C., Rinker, J., 2nd, Naismith, R. T., Panina-Bordignon, P., Passini, N.et al. (2008). Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 131, 3081-3091. 10.1093/brain/awn217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev, E. D., Veremeyko, T., Barteneva, N., Krichevsky, A. M. and Weiner, H. L. (2011). MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 17, 64-70. 10.1038/nm.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova, G., Soliman, S. S., Kim, C. N., Keefe, M. G., Hennick, K. M., Jain, S., Li, T., Tejera, D., Shin, D., Chhun, B. B.et al. (2021). Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell 28, 2153-2166.e6. 10.1016/j.stem.2021.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller, J., Flügel, A., Wehner, T., Boentert, M., Haas, C. A., Prinz, M., Fernández-Klett, F., Prass, K., Bechmann, I., de Boer, B. A.et al. (2001). Targeting gene-modified hematopoietic cells to the central nervous system: Use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 7, 1356-1361. 10.1038/nm1201-1356 [DOI] [PubMed] [Google Scholar]
- Prinz, M., Tay, T. L., Wolf, Y. and Jung, S. (2014). Microglia: unique and common features with other tissue macrophages. Acta Neuropathol. (Berl.) 128, 319-331. 10.1007/s00401-014-1267-1 [DOI] [PubMed] [Google Scholar]
- Rogers, J. T., Morganti, J. M., Bachstetter, A. D., Hudson, C. E., Peters, M. M., Grimmig, B. A., Weeber, E. J., Bickford, P. C. and Gemma, C. (2011). CX3CR1 Deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 31, 16241-16250. 10.1523/JNEUROSCI.3667-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzano, R., Roux, T., Thetiot, M., Aigrot, M. S., Richard, L., Lejeune, F. X., Mazuir, E., Vallat, J. M., Lubetzki, C. and Desmazières, A. (2021). Microglia-neuron interaction at nodes of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nat. Commun. 12, 5219. 10.1038/s41467-021-25486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer, V., Borucki, D. M., Tjon, E. C., Takenaka, M. C., Chao, C.-C., Ardura-Fabregat, A., de Lima, K. A., Gutiérrez-Vázquez, C., Hewson, P., Staszewski, O.et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724-728. 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier, A., Béchade, C., Poncer, J.-C., Smalla, K.-H., Tomasello, E., Vivier, E., Gundelfinger, E. D., Triller, A. and Bessis, A. (2004). Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J. Neurosci. 24, 11421-11428. 10.1523/JNEUROSCI.2251-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo, S. F., Gerhardt, H., Sand, F. W., Lang, R., Uv, A. and Betsholtz, C. (2011). A Two-Way Communication between Microglial Cells and Angiogenic Sprouts Regulates Angiogenesis in Aortic Ring Cultures. PLoS ONE 6, e15846. 10.1371/journal.pone.0015846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan, S., Besson-Girard, S., Kaya, T., Cantuti-Castelvetri, L., Liu, L., Ji, H., Schifferer, M., Gouna, G., Usifo, F., Kannaiyan, N.et al. (2021). White matter aging drives microglial diversity. Neuron 109, 1100-1117.e10. 10.1016/j.neuron.2021.01.027 [DOI] [PubMed] [Google Scholar]
- Schafer, D. P., Lehrman, E. K., Kautzman, A. G., Koyama, R., Mardinly, A. R., Yamasaki, R., Ransohoff, R. M., Greenberg, M. E., Barres, B. A. and Stevens, B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691-705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. P., Lehrman, E. K. and Stevens, B. (2013). The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 61, 24-36. 10.1002/glia.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, C., Perdiguero, E. G., Chorro, L., Szabo-Rogers, H., Cagnard, N., Kierdorf, K., Prinz, M., Wu, B., Jacobsen, S. E. W., Pollard, J. W.et al. (2012). A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86-90. 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- Schwarz, J. M., Sholar, P. W. and Bilbo, S. D. (2012). Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 120, 948-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel, F., Béchade, C., Vyas, S. and Triller, A. (2004). Macrophage-derived tumor necrosis factor α, an early developmental signal for motoneuron death. J. Neurosci. 24, 2236-2246. 10.1523/JNEUROSCI.4464-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer, A., Grozovski, J., Tay, T. L., Tao, J., Volaski, A., Süß, P., Ardura-Fabregat, A., Gross-Vered, M., Kim, J.-S., David, E.et al. (2018). Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat. Commun. 9, 5206. 10.1038/s41467-018-07548-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherafat, A., Pfeiffer, F., Reiss, A. M., Wood, W. M. and Nishiyama, A. (2021). Microglial neuropilin-1 promotes oligodendrocyte expansion during development and remyelination by trans-activating platelet-derived growth factor receptor. Nat. Commun. 12, 2265. 10.1038/s41467-021-22532-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami, Y., Hoshikawa, K., Goldman, J. E., Sekino, Y. and Sato, K. (2014). Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 34, 2231-2243. 10.1523/JNEUROSCI.1619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, A., Encinas, J. M., Deudero, J. J. P., Chancey, J. H., Enikolopov, G., Overstreet-Wadiche, L. S., Tsirka, S. E. and Maletic-Savatic, M. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483-495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, N. J., Dorman, L. C., Vainchtein, I. D., Horneck, N. C. and Molofsky, A. V. (2021). In situ and transcriptomic identification of microglia in synapse-rich regions of the developing zebrafish brain. Nat. Commun. 12, 5916. 10.1038/s41467-021-26206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittau, B., Dokalis, N. and Prinz, M. (2020). The role of TGFβ signaling in microglia maturation and activation. Trends Immunol. 41, 836-848. 10.1016/j.it.2020.07.003 [DOI] [PubMed] [Google Scholar]
- Squarzoni, P., Oller, G., Hoeffel, G., Pont-Lezica, L., Rostaing, P., Low, D., Bessis, A., Ginhoux, F. and Garel, S. (2014). Microglia modulate wiring of the embryonic forebrain. Cell Rep. 8, 1271-1279. 10.1016/j.celrep.2014.07.042 [DOI] [PubMed] [Google Scholar]
- Stevens, B., Allen, N. J., Vazquez, L. E., Howell, G. R., Christopherson, K. S., Nouri, N., Micheva, K. D., Mehalow, A. K., Huberman, A. D., Stafford, B.et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164-1178. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Stratoulias, V., Venero, J. L., Tremblay, M. and Joseph, B. (2019). Microglial subtypes: diversity within the microglial community. EMBO J. 38, e101997. 10.15252/embj.2019101997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svahn, A. J., Giacomotto, J., Graeber, M. B., Rinkwitz, S. and Becker, T. S. (2016). miR-124 Contributes to the functional maturity of microglia. Dev. Neurobiol. 76, 507-518. 10.1002/dneu.22328 [DOI] [PubMed] [Google Scholar]
- Tan, Y.-L., Yuan, Y. and Tian, L. (2020). Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 25, 351-367. 10.1038/s41380-019-0609-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay, T. L., Savage, J. C., Hui, C. W., Bisht, K. and Tremblay, M.-È. (2017a). Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol. 595, 1929-1945. 10.1113/JP272134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay, T. L., Mai, D., Dautzenberg, J., Fernández-Klett, F., Lin, G., Sagar, D., Drougard, M., Stempfl, A., Ardura-Fabregat, T. and et al., A. (2017b). A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 20, 793-803. 10.1038/nn.4547 [DOI] [PubMed] [Google Scholar]
- Tay, T. L., Béchade, C., D'Andrea, I., St-Pierre, M.-K., Henry, M. S., Roumier, A. and Tremblay, M.-E. (2018). Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front. Mol. Neurosci. 10, 421. 10.3389/fnmol.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion, M. S., Low, D., Silvin, A., Chen, J., Grisel, P., Schulte-Schrepping, J., Blecher, R., Ulas, T., Squarzoni, P., Hoeffel, G.et al. (2018). Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500-516. 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion, M. S., Mosser, C.-A., Férézou, I., Grisel, P., Baptista, S., Low, D., Ginhoux, F., Garel, S. and Audinat, E. (2019). Biphasic impact of prenatal inflammation and macrophage depletion on the wiring of neocortical inhibitory circuits. Cell Rep. 28, 1119-1126.e4. 10.1016/j.celrep.2019.06.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, M., Fujita, Y., Tanaka, T., Nakamura, Y., Kikuta, J., Ishii, M. and Yamashita, T. (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543-551. 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- Utz, S. G., See, P., Mildenberger, W., Thion, M. S., Silvin, A., Lutz, M., Ingelfinger, F., Rayan, N. A., Lelios, I., Buttgereit, A.et al. (2020). Early fate defines microglia and non-parenchymal brain macrophage development. Cell 181, 557-573.e18. 10.1016/j.cell.2020.03.021 [DOI] [PubMed] [Google Scholar]
- Van Hove, H., Martens, L., Scheyltjens, I., De Vlaminck, K., Pombo Antunes, A. R., De Prijck, S., Vandamme, N., De Schepper, S., Van Isterdael, G., Scott, C. L.et al. (2019). A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021-1035. 10.1038/s41593-019-0393-4 [DOI] [PubMed] [Google Scholar]
- Varol, D., Mildner, A., Blank, T., Shemer, A., Barashi, N., Yona, S., David, E., Boura-Halfon, S., Segal-Hayoun, Y., Chappell-Maor, L.et al. (2017). Dicer deficiency differentially impacts microglia of the developing and adult brain. Immunity 46, 1030-1044.e8. 10.1016/j.immuni.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Villa, A., Della Torre, S. and Maggi, A. (2019). Sexual differentiation of microglia. Front. Neuroendocrinol 52, 156-164. 10.1016/j.yfrne.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Walton, N. M., Sutter, B. M., Laywell, E. D., Levkoff, L. H., Kearns, S. M., Marshall, G. P., Scheffler, B. and Steindler, D. A. (2006). Microglia instruct subventricular zone neurogenesis. Glia 54, 815-825. 10.1002/glia.20419 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Szretter, K. J., Vermi, W., Gilfillan, S., Rossini, C., Cella, M., Barrow, A. D., Diamond, M. S. and Colonna, M. (2012). IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753-760. 10.1038/ni.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., He, X., Meng, H., Li, Y., Dmitriev, P., Tian, F., Page, J. C., Lu, Q. R. and He, Z. (2020). Robust myelination of regenerated axons induced by combined manipulations of GPR17 and microglia. Neuron 108, 876-886.e4. 10.1016/j.neuron.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, R. J., Schuldiner, O., Perrino, J., Larsen, C. and Luo, L. (2004). Glia engulf degenerating axons during developmental axon pruning. Curr. Biol. 14, 678-684. 10.1016/j.cub.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Werneburg, S., Feinberg, P. A., Johnson, K. M. and Schafer, D. P. (2017). A microglia-cytokine axis to modulate synaptic connectivity and function. Curr. Opin. Neurobiol. 47, 138-145. 10.1016/j.conb.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg, S., Jung, J., Kunjamma, R. B., Ha, S.-K., Luciano, N. J., Willis, C. M., Gao, G., Biscola, N. P., Havton, L., Crocker, A. (2020). Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167-182.e7. 10.1016/j.immuni.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk, A., Løbner, M., Cédile, O. and Owens, T. (2014). Comparison of microglia and infiltrating CD11c+ cells as antigen presenting cells for T cell proliferation and cytokine response. J. Neuroinflammation 11, 57. 10.1186/1742-2094-11-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk, A., Holtman, I. R., Krueger, M., Yogev, N., Bruttger, J., Khorooshi, R., Benmamar-Badel, A., Boer-Bergsma, J. J., Martin, N. A., Karram, K.et al. (2017). A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292-3308. 10.15252/embj.201696056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, K., Noubade, R., Manzanillo, P., Ota, N., Foreman, O., Hackney, J. A., Friedman, B. A., Pappu, R., Scearce-Levie, K. and Ouyang, W. (2017). Mice deficient in NRROS show abnormal microglial development and neurological disorders. Nat. Immunol. 18, 633-641. 10.1038/ni.3743 [DOI] [PubMed] [Google Scholar]
- Yona, S., Kim, K.-W., Wolf, Y., Mildner, A., Varol, D., Breker, M., Strauss-Ayali, D., Viukov, S., Guilliams, M., Misharin, A.et al. (2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79-91. 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, Y., Paolicelli, R. C., Sforazzini, F., Weinhard, L., Bolasco, G., Pagani, F., Vyssotski, A. L., Bifone, A., Gozzi, A., Ragozzino, D.et al. (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400-406. 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
- Zhan, L., Krabbe, G., Du, F., Jones, I., Reichert, M. C., Telpoukhovskaia, M., Kodama, L., Wang, C., Cho, S., Sayed, F.et al. (2019). Proximal recolonization by self-renewing microglia re-establishes microglial homeostasis in the adult mouse brain. PLoS Biol. 17, e3000134. 10.1371/journal.pbio.3000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Liao, Y., Morgan, S., Mathur, R., Feustel, P., Mazurkiewicz, J., Qian, J., Chang, J., Mathern, G. W., Adamo, M. A.et al. (2018). Noninflammatory changes of microglia are sufficient to cause epilepsy. Cell Rep 22, 2080-2093. 10.1016/j.celrep.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller, T., Schneider, A., Kleimeyer, C., Masuda, T., Potru, P. S., Pfeifer, D., Blank, T., Prinz, M. and Spittau, B. (2018). Silencing of TGFβ signalling in microglia results in impaired homeostasis. Nat. Commun. 9, 4011. 10.1038/s41467-018-06224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusso, M., Methot, L., Lo, R., Greenhalgh, A. D., David, S. and Stifani, S. (2012). Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. J. Neurosci. Off. J. Soc. Neurosci. 32, 11285-11298. 10.1523/JNEUROSCI.6182-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]