Abstract
Manganese (Mn), iron (Fe), copper (Cu), and zinc (Zn) are essential nutrients which aid in the proper functioning of cells, but high concentrations of these metals can be toxic to various organs. Little is known about the endogenous concentrations of these metals in the cochlea, the auditory portion of the inner ear which is extremely small and difficult to access. To fill this gap, a trace quantitative digestion and inductively coupled plasma mass spectrometry method was developed to determine the concentrations of these metals in the stria vascularis, organ of Corti, and spiral ganglion, three critically important parts of the cochlea (≤ 1.5 mg); these values were compared to those in specific brain regions (≤ 20 mg) of rats. Rats were sacrificed and the cochlea and brain regions were carefully isolated, digested, and analyzed to determine baseline concentrations of Mn, Fe, Cu, and Zn. In the cochlea, Mn, Fe, Cu, and Zn concentrations ranged from 3.2–6, 73–300, non-detect, and 13 –200 μg/g respectively. In the brain, Mn, Fe, Cu, and Zn concentrations ranged from 1.3–2.72, 21–120, 5.0–10.6, and 33–47 μg/g respectively. Significant differences (p < 0.05) were observed between the tissue types within the cochlea, and between the cochlea and brain. This validated method provides the first quantitative assessment of these metals in the three key subdivisions of the cochlea compared to the levels in the brain; Mn, Fe, and Zn levels were considerably higher in the cochlea than brain.
Keywords: ICP-MS, cochlea, trace analysis, auditory pathway, iron, manganese, zinc, copper
Introduction
Essential transition metals, including iron (Fe), zinc (Zn), copper (Cu), cobalt (Co) and manganese (Mn), play a critical role in normal functioning of all cells because they act as required cofactors for a variety of enzymes in addition to their participation in maintaining basic mitochondrial behavior and integrity (Kuo et al., 2001, Nose et al., 2006, Roth et al., 2013). They catalyze a diverse array of redox reactions that are critical for cellular respiration, chemical detoxification, metabolism, and neurotransmitter synthesis (Erikson et al., 2002, Sharp, 2003, Jenkitkasemwong et al., 2012). These metals are required nutrients, but when exposure greatly exceeds homeostatic nutritional requirements, they can become neurotoxicants (Aschner, 1997, Erikson et al., 2002, Reaney et al., 2006, Mizuno and Kawahara, 2013, Liu et al., 2014). Overexposure to other heavy metals, such as lead (Pb), strontium, rubidium, and cadmium (Cd), which have no known biological function, can also stimulate cell toxicity, presumably by interacting and interfering with metal binding components of the cell (Bartley and Reber, 1961, Fieve and Meltzer, 1974, Alexander and Meltzer, 1975, Murata et al., 1993). Heavy metal neurotoxicity normally progresses as a continuum of symptoms and consequences, which depend on the properties of the metal, the dose, the duration of exposure and genetic factors.
All organs in the body can potentially be adversely affected by heavy metals, yet considerable specificity exists with regard to their interaction with distinct cellular sites within any given tissue. In terms of metal toxicity, one organ which has received relatively minor attention is the cochlea even though studies have clearly identified a number of heavy metals associated with hearing impairment, including Cd (Ozcaglar et al., 2001, Agirdir et al., 2002, Choi et al., 2012), Pb (Choi et al., 2012), arsenic (Jacobsen, 1998); Co (Ikeda et al., 2010, Pelclova et al., 2012, Apostoli et al., 2013) and Mn (Ding et al., 2011b, Choi et al., 2012). Several of these metals have been shown to accumulate in the auditory portion of the inner ear, and based on their known toxicological properties, have the potential to selectively influence cell viability within the individual cellular compartments within the cochlea, thus potentially affecting hearing. Heavy metal exposure studies from our laboratory (Ding et al., 2011b, Ding et al., 2014, Liu et al., 2014) using cochlear organotypic cultures have demonstrated preferential toxicity to specific cellular components within the cochlea. Toxicity is most commonly associated with the production of reactive oxygen species which leads to apoptosis; though other cell death pathways such as autophagy and necrosis may be involved (Oberto et al., 1996, Zou et al., 2001, Hirata, 2002, Zhang et al., 2011, Unuma et al., 2013, Zhang et al., 2013, Wang et al., 2014). The rate limiting steps controlling metal toxicity are not fully understood but likely involve uptake of the metals into the different cells within the cochlea. Previous studies from our lab have identified different isoforms of the major transporter for many of these metals; divalent metal transporter I (DMT1), Zip8, Zip14 and transferrin receptor, in 3 day old and mature cochlea (Ding et al., 2014). These four proteins were selectively expressed in different cell populations within the cochlea and their cellular and subcellular distribution changed with development. Thus, the cochlea, like other organs differentially expresses specific proteins required for the uptake of several potentially toxic metals.
Although treatment of rats with heavy metals can lead to their accumulation in the cochlea, the normal levels of these metals within the different regions of the cochlea that contain the neural, sensory cells and supporting cells is largely unknown. Since abnormal levels of essential metals can potentially have deleterious effects on the cochlea, it is important to determine the normal concentration and distribution of these metals within the various regions in order to better understand their normal and potential impact on function. Accordingly, in this paper, the levels of four essential metals, Mn, Fe, Cu, and Zn, were assessed in the modiolus (M) where the spiral ganglion neurons (SGN) reside, the basilar membrane (BM) containing the sensory hair cells, and the stria vascularis (SV) which contains supports cells responsible for the import and export of key ions and molecules into the cochlea. To put the data from the cochlea into perspective, we also measured the concentration of Mn, Fe, Cu and Zn in two regions of the brain within the auditory pathway, the inferior colliculus (IC) and cochlear nucleus (CN), as well as the hippocampus (HP), striatum (S) and globus pallidus (GP) in the central nervous system (CNS).
Experimental procedures
Animals
Female SASCO® Sprague Dawley Rats ranging in age from 3–4 months were used for these experiments. Experiments were performed according to the rules and regulations of the Institutional Animal Care and Use Committee of the State University of New York at Buffalo and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue Harvesting
Rats were anesthetized with ketamine (40 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.), then decapitated with a small guillotine. The bulla was quickly removed under a dissection microscope and placed in phosphate-buffered saline. The bony walls of the cochlea were opened. The organ of Corti containing the hair cells, the spiral ligament including the SV, and the soft tissue in the cochlear M including the SGN, were carefully dissected out. Brains were removed from the skull and sliced by using 1 mm intervals coronal rat brain slicer matrices (Plastics One Inc.) on ice. Regions of interest including the S, IC,GP, CN, and HP were identified using Paxinos & Watson rat brain atlas, and separated using a biopsy punch (Glowinski and Iversen, 1966). Each component of the cochlea and brain was placed in a separate metal-free polypropylene centrifuge tube. Individual tubes contained tissues from four pooled animals.
Solid Sample Digestion
Rat tissue and animal food samples were digested using trace metal-grade nitric acid (Aristar Ultra) and hydrogen peroxide (J. T. Baker) following a method adapted from USEPA 3050B (1996) for use at the micro scale. The samples were air dried at room temperature under filter paper and weighed to the nearest tenth of a microgram using a microbalance (Mettler Toledo). Polytetrafluoroethylene coated tweezers and metal-free polypropylene centrifuge tubes and caps were used to prevent metal contamination. Samples were refluxed with concentrated trace metal nitric acid for 2–3 h in metal-free 1.5 ml polypropylene centrifuge tubes in a hot block. Samples were refluxed for another 1–3 h after the addition of hydrogen peroxide. Method reagent blanks were prepared using the same procedure, and contained only the chemical reagents. An internal standard of 103Rh (Sigma Aldrich) was added (50 μl of 1 ng/μl in 2% nitric acid) to the samples prior to injection. The internal standard was used for volume and instrument drift correction. Samples were diluted to 5 ml with Nanopure® water, and then filtered with 0.45-μm polypropylene membrane syringe filters (VWR International).
Water Samples
Samples of rat drinking water were obtained and adjusted to 2% nitric acid concentration using trace metal-grade nitric acid. Concentrated acid was used to reduce dilution effects. The internal standard was added prior to injection (50 μl of 1 ng/μl 103Rh in 2% nitric acid) to account for volume differences and instrument drift.
Elemental Analysis
Samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) on an X-Series 2 instrument (Thermo Scientific) using a 1 ml sample loop. Manganese, Fe, Cu, and Zn, were quantified using isotope standards for 55Mn, 57Fe, 65Cu, and 64Zn respectively. All samples were normalized to the 103Rh signal. Iron was analyzed using collision cell technology to reduce polyatomic interferences. Operating conditions of the ICP-MS are presented in Table 1. Quantification was performed with external calibration curves (n ≥ 3) in 2% nitric acid at concentrations from 0.1 to 250 ng/ml. A standard metal mix (82026–108, BDH Aristar® Plus, VWR International) was used to quantitate all metals.
Table 1.
Operating Conditions of the Inductively Coupled Plasma Mass Spectrometer
| Parameter | Setting |
|---|---|
|
| |
| Forward power | 1403.92 W |
| Cooling gas flow (argon) | 13.02 L/min |
| Auxiliary gas flow (argon) | 0.70 L/min |
| Nebulizer gas flow (argon) | 0.98 L/min |
| Pole bias [(Mn, Cu, Zn) / Fe] | −5.80 V / −17.00 V |
| Hexapole bias [(Mn, Cu, Zn) / Fe] | −4.00 V / −20.00 V |
| Scanning mode/s | Survey and peak jumping |
| Collision cell gas (H2/He) (Fe analysis only) | 2.00 |
Statistical Analysis
Outlying data points were removed using a Q-test at the 95% confidence level. Statistical analysis was performed using Origin Pro 9.0. One-way analysis of variance (ANOVA) was performed using a Tukey post-hoc test with significance at p < 0.05.
Analytical Figures of Merit
Method limits of detection (mLOD) and quantification (mLOQ) were determined for each element and tissue type including homogenized brain (HB) as summarized in Table 2. Like tissue digests were pooled and spiked at 4 levels (0, 2.5, 10 and 50 ng/ml); each level was injected 7 times to produce a calibration curve. Correction for the endogenous concentrations was performed using multipoint standard addition. The mLOD and mLOQ were determined by dividing 3 or 10 times the standard deviation of the matrix blank by the slope of the calibration curve respectively. Reported values represent recovery-corrected concentrations present prior to sample preparation. The HB limits were applied to the CN and HP as no tissue specific limits were obtained. Extraction recoveries in homogenized brain were evaluated at three spiking levels: 10, 20 and 100 μg/g with n = 3 samples per level. As analyte free matrix could not be obtained, spiking values were in addition to the endogenous concentrations. Recoveries were corrected by background subtracting the endogenous levels. Values are reported as averages and standard errors for the recovery levels used. Mn and Cu values are reported for all three recovery levels, Fe values are reported at the 20 and 100 μg/g levels, and Zn recoveries are reported only for the 100 μg/g level. Recovery values are 108 ± 1%, 110 ± 10%, 112 ± 5%, and 120 ± 20% for Mn, Fe, Zn, and Cu respectively.
Table 2.
Method Limits of Detection and Quantification for Mn, Fe, Cu, and Zn
| Limit (μg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| BM | M | SV | S | GP | IC | HB | ||
| Mn | mLOD | 11.5 | 59.6 | 13.6 | 49.4 | 65.5 | 67.2 | 26.7 |
| mLOQ | 38.3 | 199 | 45.3 | 165 | 218 | 224 | 89.0 | |
| Fe | mLOD | 1.15×103 | 7.51×103 | 9.07×102 | 5.41×103 | 2.11×103 | 2.95×103 | 1.93×103 |
| mLOQ | 3.83×103 | 2.50×104 | 3.02×103 | 1.80×104 | 7.02×103 | 9.69×103 | 6.43×103 | |
| Cu | mLOD | 28.0 | 101 | 32.0 | 292 | 195 | 684 | 298 |
| mLOQ | 93.4 | 337 | 107 | 974 | 649 | 2.28×103 | 992 | |
| Zn | mLOD | 1.06×103 | 4.77×103 | 1.34×103 | 2.17×103 | 1.99×103 | 3.10×103 | 1.51×103 |
| mLOQ | 3.52×103 | 1.59×104 | 4.46×103 | 7.24×103 | 6.63×103 | 1.03×104 | 5.03×103 | |
The HB limits were applied to the CN and HP tissues.
Results
Analysis of Metals in Food and Drinking Water
The concentrations of Mn, Fe, Zn and Cu present in rat drinking water and standard laboratory rodent chow are shown in Table 3. As expected, all four metals were present in rat chow and drinking water at varying concentrations.
Table 3.
Concentrations of Mn, Fe, Cu and Zn in food
| Food (μg/g) | Water (ng/ml) | |
|---|---|---|
|
| ||
| Mn | 91 ± 3 | 0.226 ± 0.003 |
| Fe | 200 ± 40 | 135 ± 3 |
| Cu | 18 ± 4 | 18.90 ± 0.05 |
| Zn | 47 ± 2 | 9.84 ± 0.04 |
Mean (±SEM) concentrations of Mn, Fe, Cu and Zn in food (n=9) and water (n=6). Methods are described in Experimental Procedures.
Manganese in the Cochlea and CNS
Mn concentrations were determined for three regions of the cochlea, the M, BM, and SV, and five regions of the brain. As illustrated in Fig. 1, Mn was detected in all of the tissues examined, though the highest concentrations were found in the cochlear samples. In the cochlea, tissue concentrations ranged from about 3.0 to 5.8 μg/g tissue dry weight with highest levels expressed in the BM, followed by the M and then the SV. Statistical analysis revealed that levels of Mn within these areas of the cochlea were significantly different from each other. The data further indicates that Mn concentrations in brain were statistically lower than the cochlea, ranging from approximately 1.2 to 2.8 μg/g tissue dry weight, with the highest levels present in the IC.
Fig. 1.
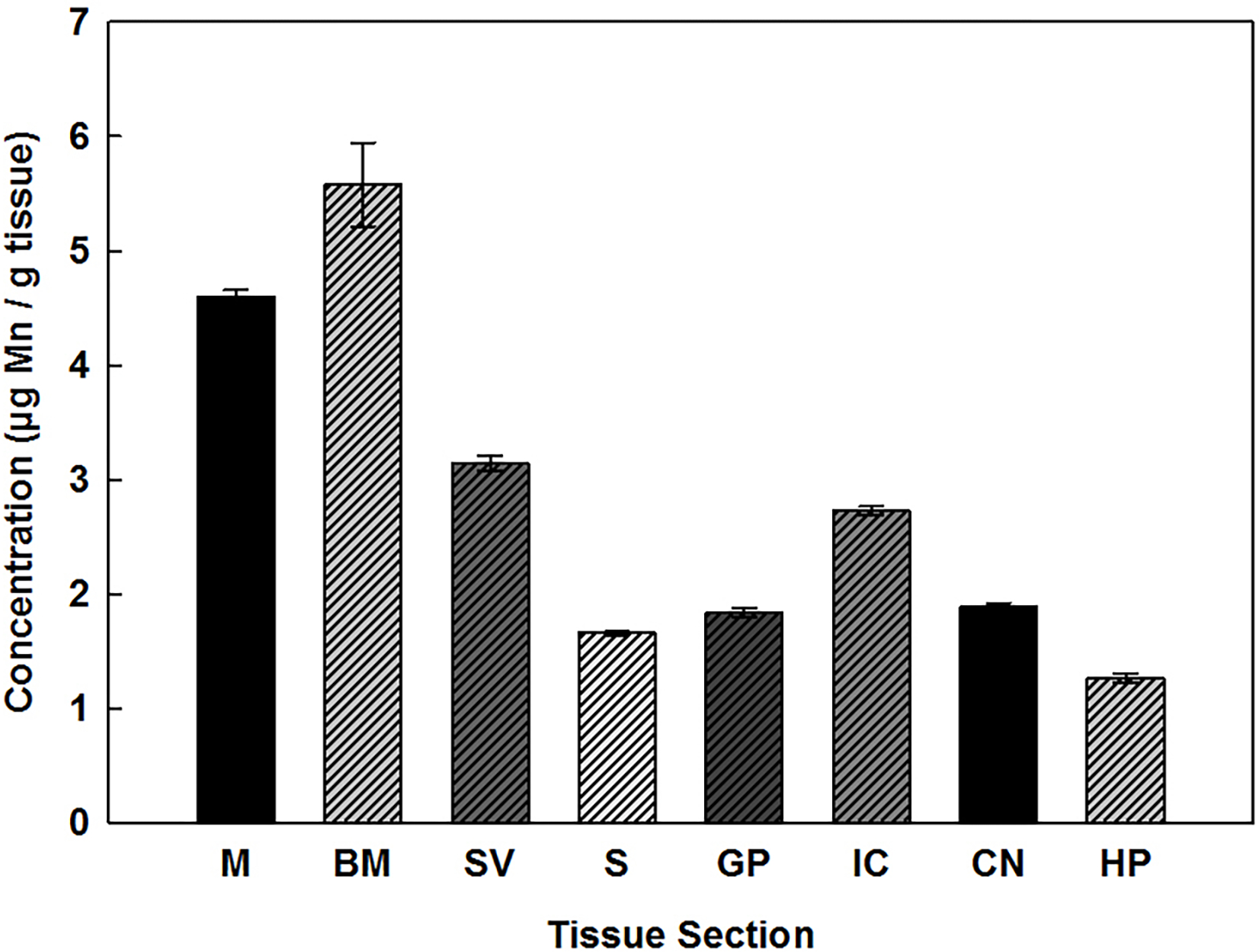
Mn concentrations (μg/g) present in the cochlea: M, BM, SV, and brain: S, GP, IC, CN, and HP of rats. Error bars represent standard error (n = 9). g of tissue are expressed as dry weight.
Iron in Cochlea and CNS
All regions of the cochlea and brain contained detectable levels of Fe as shown in Fig. 2. In the cochlea, tissue concentrations of Fe ranged from about 70 to 300 μg/g tissue dry weight with the highest levels expressed in the SV, followed by the BM and then the M. However, only the SV and M were statistically different from one another. In brain, Fe concentrations ranged from approximately 20 to 120 μg/g tissue dry weight with highest levels expressed in the IC and lowest in the CN, yet statistical comparison indicated that none of the brain areas were significantly different from each other.
Fig. 2.
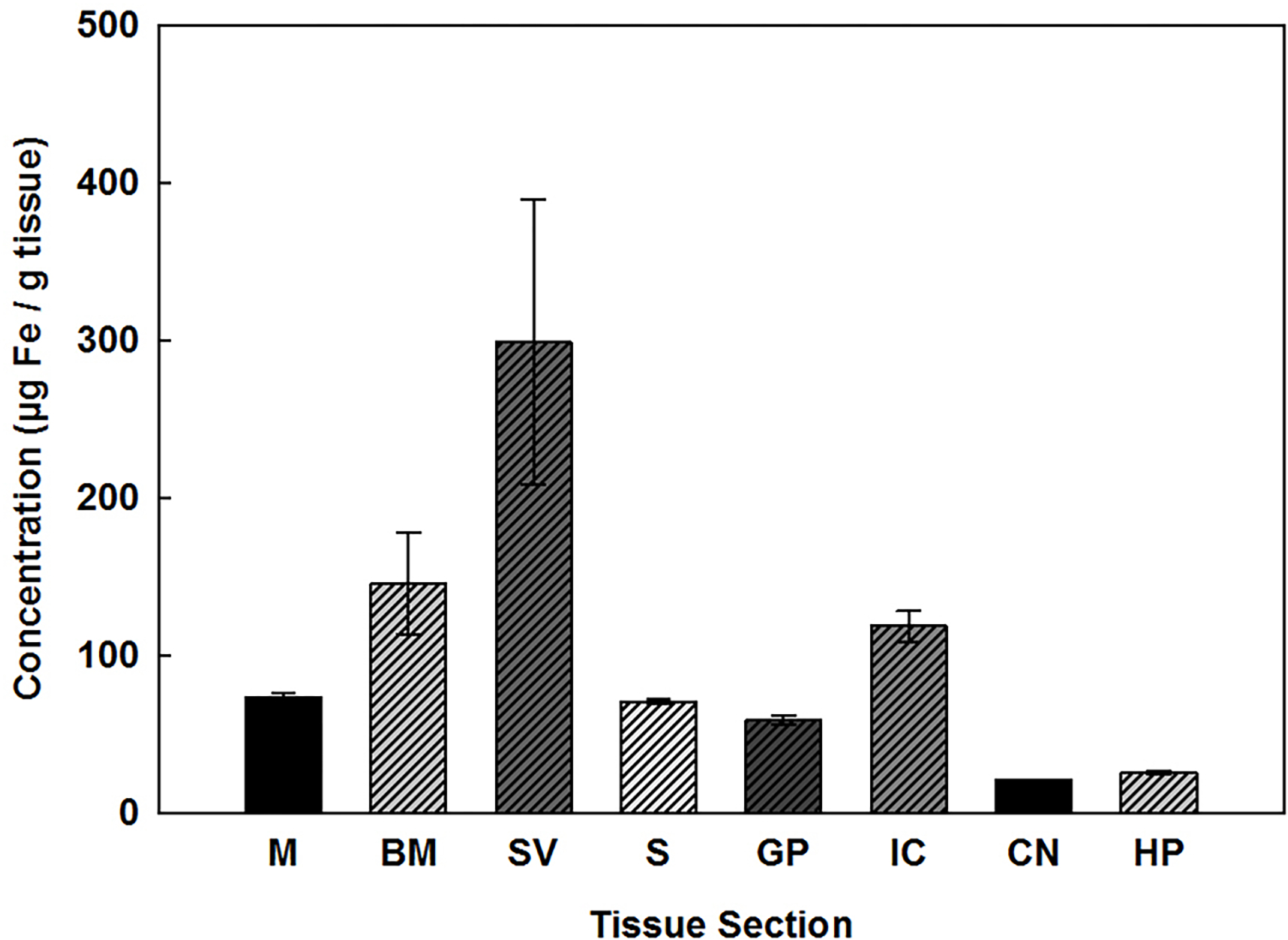
Fe concentrations (μg/g) present in the cochlea: M, BM, SV, and brain: S, GP, IC, CN, and HP of rats. Error bars represent standard error (n ≥ 4). g of tissue are expressed as dry weight. The cochlear nucleus is represented by a pooled sample, and therefore has no calculated error.
Zinc in Cochlea and CNS
Zn concentrations measured in the cochlea and brain are presented in Fig. 3. Within the cochlea, highest levels were observed in the SV which contained approximately 210 μg/g of tissue. The SV was statistically different from both the M and BM which contained essentially identical concentrations of Zn at around 140 μg/g of tissue. Like Mn, all of the cochlear sections contain statistically higher levels of Zn than any of the brain areas examined. The levels of Zn within brain were similar ranging between 30 and 45 μg/g of tissue.
Fig. 3.
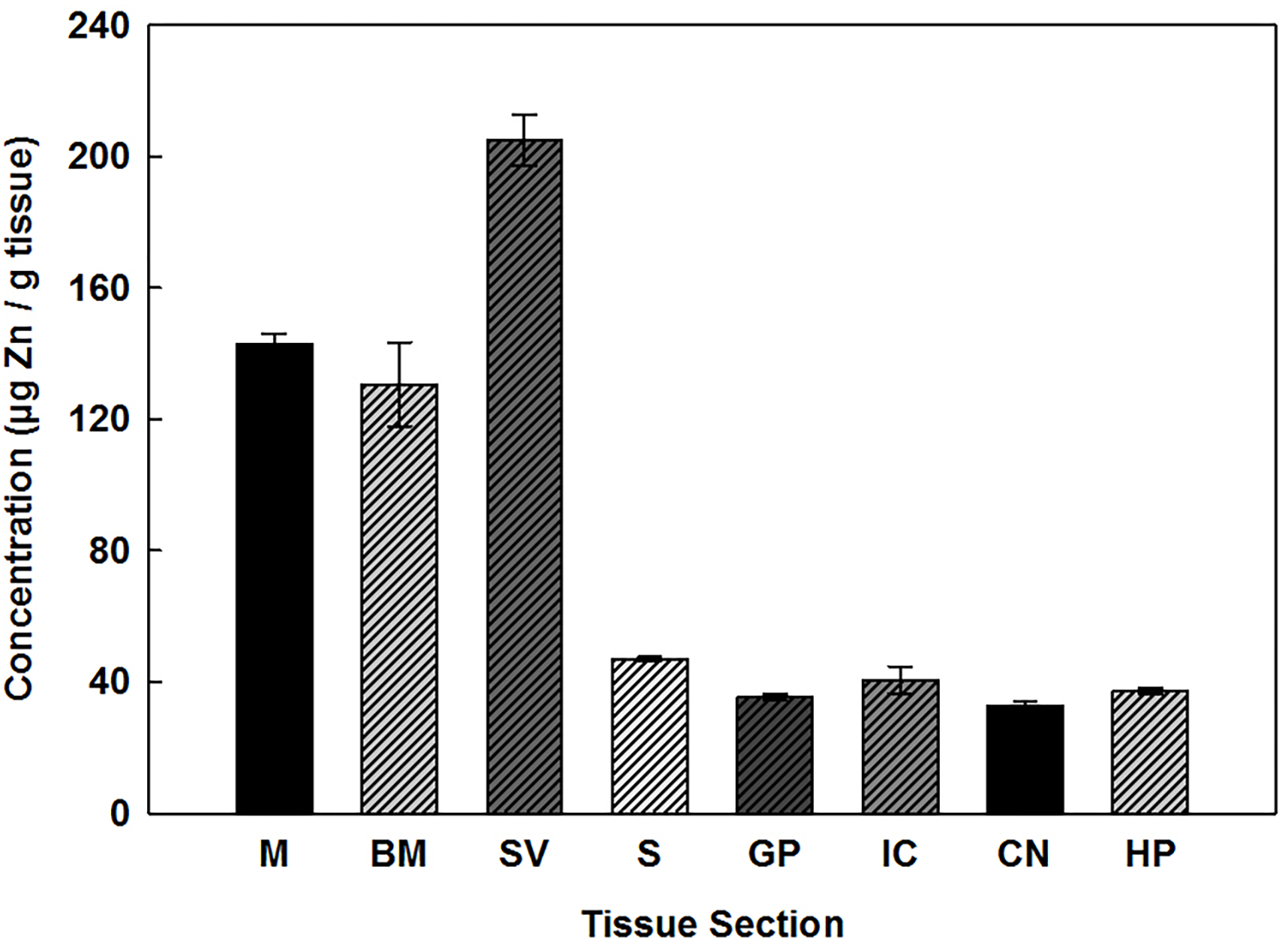
Cu concentrations (μg/g) present in the cochlea: M, BM, SV, and brain: S, GP, IC, CN, and HP of rats. Error bars represent standard error (n ≥ 6). g of tissue are expressed as dry weight. The absence of a data bar indicates that values are less than the limits of detection.
Copper in the Cochlea and CNS
Cu was detected in all of the brain specimens examined and values are displayed as μg/g tissue dry weight in Fig. 4. Within the brain, the highest levels of Cu were observed in the IC which contained approximately 11 μg/g of tissue followed by the S which contained about 9 μg/g of tissue. Levels of Cu in the other brain areas ranged between 5 and 6 μg/g of tissue. Statistical analysis of the brain tissue revealed that with the exception of GP, when compared to CN and HP, all other areas were statistically different from each other. As opposed to the trend for the other three metals examined, the levels of Cu present in all three compartments of the cochlear tissues of the rat were below detectable limits.
Fig. 4.
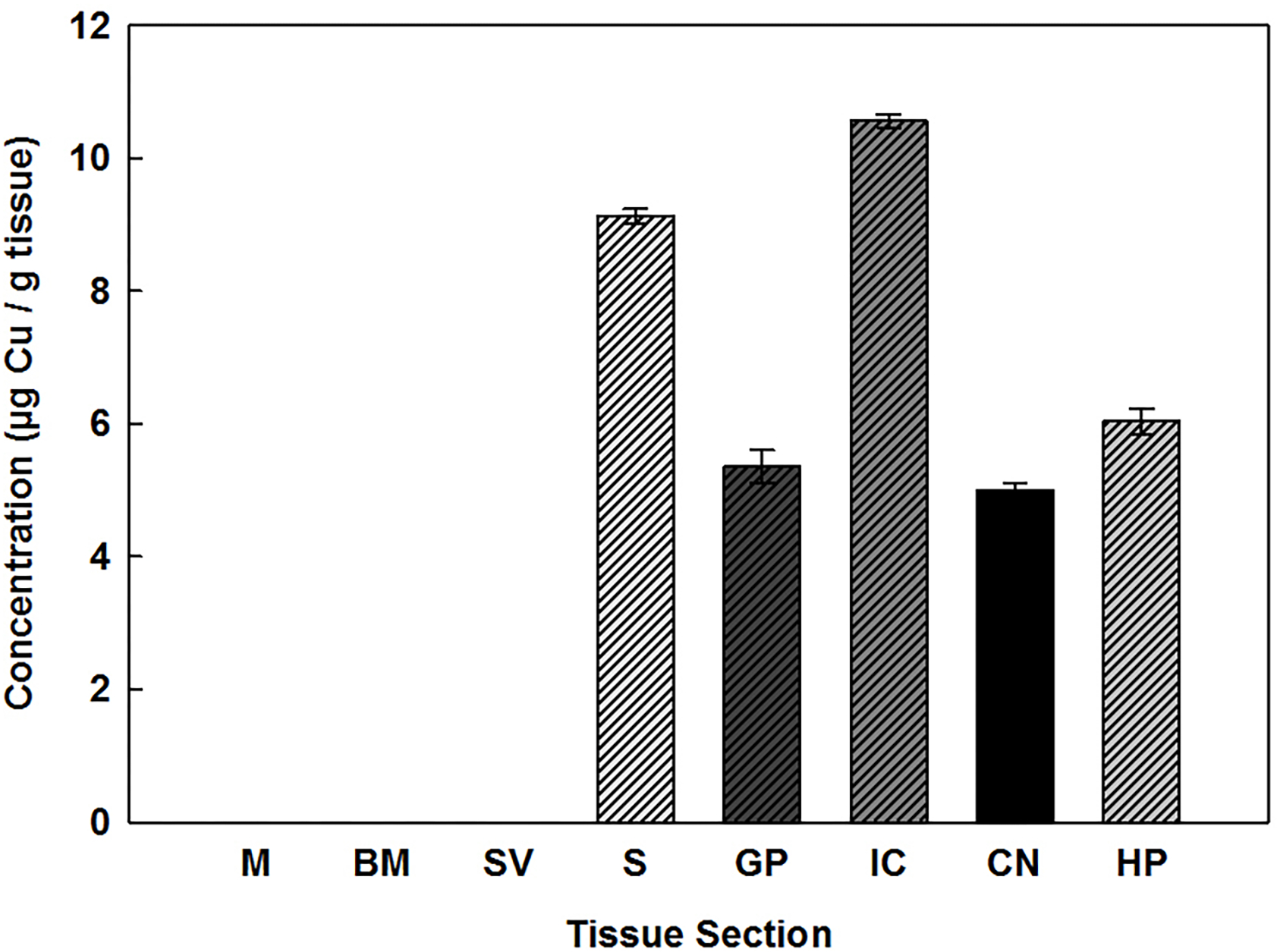
Zn concentrations (μg/g) present in the cochlea: M, BM, SV, and brain: S, GP, IC, CN, and HP of rats. Error bars represent standard error (n = 9). Grams of tissue are expressed as dry weight.
Discussion
The cochlea has been reported to accumulate a variety of essential and nonessential metals including Mn, Pb, Co and Cd. Accumulation is regulated, at least in part, by their affinity for the different transport systems which are ultimately responsible for uptake across the blood-cochlea barrier and subsequent transport into the cells within the cochlea. The unique and selective properties of the different metal transporters discreetly controls endogenous levels of the essential metals within the cochlea, thereby, influencing normal cellular processes as well as potentially provoking neurotoxic responses leading to hearing impairment in conditions of excess metal exposures. The overall net accumulation of each metal is, thus, contingent on the discerning and opposing capacities of the import and export transport systems within each part of the cochlea. Since unequal compartmentalization of metals in the cochlea can lead to distinctive hearing deficits, it is important to evaluate endogenous levels of these metals in normal animals in order to understand or explain the origins of metal-induced damage to different anatomical structures in the cochlea.
In this paper, endogenous levels of four essential transition metals, Fe, Mn, Cu and Zn, were examined. All four metals share, at least in part, common transport proteins for cellular uptake including, DMT1, ZIP8, and ZIP 14 (Garrick et al., 2006, Jenkitkasemwong et al., 2012), though a number of other proteins are responsible for the uptake of Cu including Crt1 (Kuo et al., 2001) which is important in the ototoxic actions of the anti-cancer drug cisplatin (Sharp, 2003, Ding et al., 2011a). Export proteins include ferroportin for Zn, Fe and Mn, and ATP7A and B for Cu (Gupta, 2014, Kaler, 2014). SLC30A10 is a selective exporter for Mn and Zn, and mutations in this gene lead to Mn toxicity characterized by dystonic movements similar to those observed in Parkinson’s disease (Quadri et al., 2012, Stamelou et al., 2012). It should be noted that the measured intracellular levels of these metals are also significantly influenced by binding proteins within the cell involved in their sequestration.
Prior studies in our laboratory have reported the selective expression of several metal transporters in different cell populations of the cochlea (Ding et al., 2014) including DMT1 and the two ZIP proteins, 8 and 14. In addition, our studies demonstrated that the Cu transporter, Ctr1 and the Cu export pumps, ATP7A and ATP7B, are selectively localized within the organ of Corti, which contains the hair cells, the vascular epithelium of the SV, and the SGN (Ding et al., 2011a). In general, the pattern of existing transporters represents redundant and overlapping systems for maintaining homeostatic levels of essential metals.
Cochlear Compartments
The metal concentrations in the cochlea were measured in three functional subdivisions, the M, BM, and SV. The M, located within the central core of the cochlea, is mainly populated by SGN myelinated by Schwann cells. The BM is the sensory hair cells which transduce sound vibrations into neural activity. The sensory cells rest on the BM and are surrounded by a diverse array of supporting cells. The SV and spiral ligament form the lateral wall of scala media. The marginal, intermediate and basal cells of the SV, which contains numerous small blood vessels, play an important role in fluid and ion transport into scala media and production of endolymph which contains a high concentration of potassium and low concentration of sodium. Quantitative analysis of metal content in the individual cochlear components was particularly challenging as tissue sample sizes were extremely small (≤ 1.5 mg dry weight). Nevertheless, the micro-scale extraction procedure and ICP-MS method developed, validated, and described in this paper enabled us to accurately assess endogenous levels of all four metals with the exception of Cu. In general, our results show a consistent trend for the levels of these metals within individual tissue compartments with Fe ≥ Zn >> Mn. Cu concentrations were below detectable levels in all specimens of the cochlea examined, yet the levels of Cu in food and water were well within our measurement range. The reasons for this are not known, and are puzzling given that cuprous ions are transported by DMT1, which was previously identified in the cochlea (Ding et al., 2014). Cu is also a component of ceruloplasmin and hephaestin which are required for the export and oxidation of Fe from cells and uptake across the endothelium of the blood-cochlea barrier. Despite our inability to detect Cu in the samples, the apparent low abundance of Cu in the cochlea must be sufficient to account for the accumulation of Fe in the inner ear (McCarthy and Kosman, 2014). Our findings are also surprising, given that the Cu transporter, Ctr1, and Cu export pumps ATP7A and ATP7B, have previously been identified in the organ of Corti, epithelium of the SV, and SGN (Ding et al., 2011a). Thus, all the machinery is present within the cochlea for Cu utilization despite our inability to detect them with our methods. With regard to the relative concentrations of the other three essential metals within the individual components of the cochlea, there was no clear evidence of selective localization of Fe, Zn or Mn. Although the differences in the concentrations were in some cases statistically significant, the differences were relatively small.
Brain Regions
One of the most significant findings in this study was the observation that, with the exception of Cu, the levels of Fe, Mn, and Zn were considerably higher in the cochlea compared with any of the brain regions examined, specifically the IC, CN, GP, S, and HP. The IC and CN were selected as they are part of the auditory pathway in the brainstem and midbrain (Rose et al., 1966, Young et al., 1992). Additionally, the IC is one of the more metabolically active parts of the brain (Sokoloff, 1981) in which Mn is known to accumulate in rats (Takeda et al., 1999), and due to its high metabolic rate, the IC is an important region to monitor for metal concentration changes. The GP and S are components of the basal ganglia, and were chosen due to their involvement in motor dysfunction related to metal toxicity. The GP, a Mn rich region of the brain, readily accumulates additional Mn which leads to neuron death (Bird et al., 1967, Larsen et al., 1979, Larsen et al., 1981, Aschner, 1997, Roth et al., 2013).
The trend for the overall distribution of the metal levels in all the brain samples measured in this study follows the order Fe ≥ Zn >> Cu > Mn. Previously reported values of these metals in rat brain (Table 4) follow a similar trend and our measurements fall within the concentration range previously reported (Donaldson et al., 1973, Fjerdingstad et al., 1974a, 1977, Seth et al., 1977, Chan et al., 1983, Han et al., 1999, Reaney et al., 2006).
Table 4.
Range of Metal Concentrations in Rat Brain Compared to Previous Literature
| Element | Previous Studies μg/g | Present Study μg/g | ||||
|---|---|---|---|---|---|---|
| Min. | Max. | Min. | Max. | |||
|
| ||||||
| Mn | 0.887 | - | 5.878 | 1.31 | - | 2.72 |
| Fe | 71 | - | 149 | 21 | - | 120 |
| Cu | 8.61 | - | 54.85 | 5.0 | - | 10.6 |
| Zn | 31.9 | - | 400 | 33 | - | 47 |
Concentrations are expressed in μg/g tissue dry weight. Values from (Donaldson et al., 1973, Fjerdingstad et al., 1974b, Fjerdingstad et al., 1977, Seth et al., 1977, Chan et al., 1983, Han et al., 1999, Reaney et al., 2006)
In general, the levels of Zn within the brain tissues studied do not follow the same pattern as Mn and Fe, suggesting that Zn may transported via different transporters than Mn and Fe. It has been suggested, however, that Zn is transported by DMT1 though it has a considerably lower affinity for the transporter than either Fe or Mn (Garrick et al., 2006). Interestingly, the relative pattern for Cu measured within our brain tissue sections are similar to those of Fe suggesting that similar transport mechanisms may be involved in their accumulation. This is supported by the observation that Fe deficiency, which causes an increase in DMT1, results in an increase in both Zn and Mn concentrations in brain (Erikson et al., 2004). Additionally, Fe is known to be transported by Crt1, which may account for the similarities in uptake patterns (Nose et al., 2006). However, the non-detectable Cu concentrations in the ear tissues suggest that the distribution of transporters present in the brain tissues is distinct from that in the cochlea.
Conclusions
A sensitive analytical method for the trace quantification of metals in tissue samples, less than 20 mg was successfully developed and validated. Endogenous concentrations of Fe, Zn, Mn and Cu were measured in three separate compartments of the cochlea for the first time and compared to those in several parts of the central auditory system plus other sections of the brain. Importantly, our results indicate that Fe, Mn, and Zn levels in the cochlea are greater than those detected in the other regions of rat brain specimens examined. Surprisingly, Cu levels within the cochlea were below the detection limit (28.0 – 101 μg/g) and therefore far below those in the brain.
Acknowledgements
Research supported by National Institute for Occupational Safety and Health (NIOSH) award #R010HH010311–01. Stacia Wegst-Uhrich acknowledges her IGERT fellowship support from the National Science Foundation (NSF), grant #DGE-0654305, titled “Ecosystem Restoration through Interdisciplinary Exchange” Traineeship Program. We acknowledge the NSF Major Research Instrumentation Program CHE0959565 for the ICP-MS. We are grateful to the Colón lab for the use of the microbalance.
Footnotes
Conflict of Interest
The authors state that they have no conflicts of interest that affect the objectivity of this publication.
Contributor Information
Stacia R. Wegst-Uhrich, Department of Chemistry, University at Buffalo, NY 14260
Elizabeth J. Mullin, Department of Chemistry, University at Buffalo, NY 14260
Dalian Ding, Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital, Central South University, University at Buffalo, NY 14214; Changsha, Hunan 410008, China.
Senthilvelan Manohar, Center for Hearing and Deafness, University at Buffalo, NY 14214.
Richard Salvi, Center for Hearing and Deafness, University at Buffalo, NY 14214.
Diana S. Aga, Department of Chemistry, University at Buffalo, NY 14260
Jerome A. Roth, 11 Cary Hall, Department of Pharmacology and Toxicology, University at Buffalo, NY 14214.
References
- Acid Digestion of Sediments, Sludges, and Soils In: Method 3050B Washington D.C.: United States Environmental Protection Agency; (1996). [Google Scholar]
- Agirdir B, Bilgen I, Dinc O, Ozçağlar H, Fişenk F, Turhan M, Oner G (2002) Effect of zinc ion on cadmium-induced auditory changes. Biol Trace Elem Res 88:153–163. [DOI] [PubMed] [Google Scholar]
- Alexander GJ, Meltzer HL (1975) Onset of audiogenic seizures in rodents after intake of near-toxic doses of rubidium chloride. The Journal of pharmacology and experimental therapeutics 194:480–487. [PubMed] [Google Scholar]
- Apostoli P, Catalani S, Zaghini A, Mariotti A, Poliani PL, Vielmi V, Semeraro F, Duse S, Porzionato A, Macchi V, Padovani A, Rizzetti MC, De Caro R (2013) High doses of cobalt induce optic and auditory neuropathy. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 65:719–727. [DOI] [PubMed] [Google Scholar]
- Aschner M (1997) Manganese neurotoxicity and oxidative damage. In: Metals and oxidative damage in neurological disorders (Connor JR, ed), pp 77–93 New York: Plenum. [Google Scholar]
- Bartley JC, Reber EF (1961) Toxic effects of stable strontium in young pigs. The Journal of nutrition 75:21–28. [DOI] [PubMed] [Google Scholar]
- Bird ED, Grant LG, Ellis WH (1967) Measurement of the Effect of Phenothiazine on the Manganese Concentration in the Basal Ganglia of Subhuman Primates by Activation Analysis. In: Nuclear Activation Techniques in the Life Sciences, vol. 44, pp 491–499 Amsterdam. [Google Scholar]
- Chan AW, Minski MJ, Lai JC (1983) An application of neutron activation analysis to small biological samples: simultaneous determination of thirty elements in rat brain regions. J Neurosci Methods 7:317–328. [DOI] [PubMed] [Google Scholar]
- Choi Y-H, Hu H, Mukherjee B, Miller J, Park SK (2012) Environmental Cadmium and Lead Exposures and Hearing Loss in U.S. Adults: The National Health and Nutrition Examination Survey, 1999 to 2004. Environmental Health Perspectives 120:1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM, Salvi RJ (2011a) Cisplatin ototoxicity in rat cochlear organotypic cultures. Hearing research 282:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Roth J, Salvi R (2011b) Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology 32:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Salvi R, Roth JA (2014) Cellular localization and developmental changes of Zip8, Zip14 and transferrin receptor 1 in the inner ear of rats. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 27:731–744. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Pierre TS, Minnich JL, Barbeau A (1973) Determination of Na+, K+, Mg2+, Cu2+, Zn2+, and Mn2+ in Rat-Brain Regions. Canadian Journal of Biochemistry 51:87–92. [DOI] [PubMed] [Google Scholar]
- Erikson KA, Shihabi ZK, Aschner JL, Aschner M (2002) Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res 87:143–156. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M (2004) Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J Nutr Biochem 15:335–341. [DOI] [PubMed] [Google Scholar]
- Fieve RR, Meltzer HL (1974) Proceedings: Rubidium salts--toxic effects in humans and clinical effects as an antidepressant drug. Psychopharmacology bulletin 10:38–50. [PubMed] [Google Scholar]
- Fjerdingstad E, Danscher G, Fjerdingstad EJ (1974a) Zinc content in hippocampus and whole brain of normal rats. Brain Res 79:338–342. [DOI] [PubMed] [Google Scholar]
- Fjerdingstad E, Danscher G, Fjerdingstad EJ (1977) Changes in Zinc and Lead Content of Rat Hippocampus and Whole Brain Following Intravital Dithizone Treatment as Determined by Flameless Atomic-Absorption Spectrophotometry. Brain Research 130:369–373. [DOI] [PubMed] [Google Scholar]
- Fjerdingstad EJ, Danscher G, Fjerdingstad E (1974b) Hippocampus: selective concentration of lead in the normal rat brain. Brain research 80:350–354. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knopfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM (2006) DMT1: which metals does it transport? Biological research 39:79–85. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL (1966) Regional Studies of Catecholamines in the Rat Brain-I. Journal of Neurochemistry 13:655–669. [DOI] [PubMed] [Google Scholar]
- Gupta S (2014) Cell therapy to remove excess copper in Wilson’s disease. Annals of the New York Academy of Sciences 1315:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Li W, Jamil U, Dargan K, Orefice M, Kemp FW, Bogden JD (1999) Effects of weight loss and exercise on the distribution of lead and essential trace elements in rats with prior lead exposure. Environ Health Perspect 107:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y (2002) Manganese-induced apoptosis in PC12 cells. Neurotoxicology and teratology 24:639–653. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Takahashi K, Kabata T, Sakagoshi D, Tomita K, Yamada M (2010) Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle & nerve 42:140–143. [DOI] [PubMed] [Google Scholar]
- Jacobsen P (1998) Chemically-Induced Hearing Disorders. In: Encyclopedia of Occupational Health and Safety, vol. 1 (Stellman JM, ed) Geneva: International Labour Office. [Google Scholar]
- Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD (2012) Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 25:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG (2014) Translational research investigations on ATP7A: an important human copper ATPase. Annals of the New York Academy of Sciences 1314:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proceedings of the National Academy of Sciences of the United States of America 98:6836–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen NA, Pakkenberg H, Damsgaard E, Heydorn K (1979) Topographical distribution of arsenic, manganese, and selenium in the normal human brain. J Neurol Sci 42:407–416. [DOI] [PubMed] [Google Scholar]
- Larsen NA, Pakkenberg H, Damsgaard E, Heydorn K, Wold S (1981) Distribution of Arsenic, Manganese, and Selenium in the Human-Brain in Chronic Renal-Insufficiency, Parkinsons-Disease, and Amyotrophic Lateral Sclerosis. Journal of the Neurological Sciences 51:437–446. [DOI] [PubMed] [Google Scholar]
- Liu H, Ding D, Sun H, Jiang H, Wu X, Roth JA, Salvi R (2014) Cadmium-induced ototoxicity in rat cochlear organotypic cultures. Neurotoxicity research 26:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RC, Kosman DJ (2014) Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PloS one 9:e89003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno D, Kawahara M (2013) The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia. International journal of molecular sciences 14:22067–22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Araki S, Yokoyama K, Uchida E, Fujimura Y (1993) Assessment of central, peripheral, and autonomic nervous system functions in lead workers: neuroelectrophysiological studies. Environmental research 61:323–336. [DOI] [PubMed] [Google Scholar]
- Nose Y, Kim BE, Thiele DJ (2006) Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4:235–244. [DOI] [PubMed] [Google Scholar]
- Oberto A, Marks N, Evans HL, Guidotti A (1996) Lead (Pb+2) promotes apoptosis in newborn rat cerebellar neurons: pathological implications. The Journal of pharmacology and experimental therapeutics 279:435–442. [DOI] [PubMed] [Google Scholar]
- Ozcaglar HU, Agirdir B, Dinc O, Turhan M, Kilincarslan S, Oner G (2001) Effects of cadmium on the hearing system. Acta Oto-Laryngologica 121:393–397. [DOI] [PubMed] [Google Scholar]
- Pelclova D, Sklensky M, Janicek P, Lach K (2012) Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clinical toxicology 50:262–265. [DOI] [PubMed] [Google Scholar]
- Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. American journal of human genetics 90:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR (2006) Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicological sciences : an official journal of the Society of Toxicology 93:114–124. [DOI] [PubMed] [Google Scholar]
- Rose JE, Gross NB, Geisler CD, Hind JE (1966) Some neural mechanisms in the inferior colliculus of the cat which may be relevant to localization of a sound source. Journal of Neurophysiology 29:288–314. [DOI] [PubMed] [Google Scholar]
- Roth J, Ponzoni S, Aschner M (2013) Manganese homeostasis and transport. Metal ions in life sciences 12:169–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth PK, Husain R, Mushtaq M, Chandra SV (1977) Effect of manganese on neonatal rat: manganese concentration and enzymatic alterations in brain. Acta Pharmacol Toxicol (Copenh) 40:553–560. [PubMed] [Google Scholar]
- Sharp PA (2003) Ctr1 and its role in body copper homeostasis. The international journal of biochemistry & cell biology 35:288–291. [DOI] [PubMed] [Google Scholar]
- Sokoloff L (1981) Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab 1:7–36. [DOI] [PubMed] [Google Scholar]
- Stamelou M, Tuschl K, Chong WK, Burroughs AK, Mills PB, Bhatia KP, Clayton PT (2012) Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Movement disorders : official journal of the Movement Disorder Society 27:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Ishiwatari S, Okada S (1999) Manganese uptake into rat brain during development and aging. Journal of Neuroscience Research 56:93–98. [DOI] [PubMed] [Google Scholar]
- Unuma K, Aki T, Matsuda S, Funakoshi T, Yoshida K, Uemura K (2013) Inducer of heme oxygenase-1 cobalt protoporphyrin accelerates autophagy and suppresses oxidative damages during lipopolysaccharide treatment in rat liver. Hepatology research : the official journal of the Japan Society of Hepatology 43:91–96. [DOI] [PubMed] [Google Scholar]
- Wang L, Ding D, Salvi R, Roth JA (2014) Nicotinamide adenine dinucleotide prevents neuroaxonal degeneration induced by manganese in cochlear organotypic cultures. Neurotoxicology 40:65–74. [DOI] [PubMed] [Google Scholar]
- Young ED, Spirou GA, Rice JJ, Voigt HF, Rees A (1992) Neural Organization and Responses to Complex Stimuli in the Dorsal Cochlear Nucleus [and Discussion]. Philosophical Transactions: Biological Sciences 336:407–413. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cao R, Cai T, Aschner M, Zhao F, Yao T, Chen Y, Cao Z, Luo W, Chen J (2013) The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotoxicity research 24:478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen X, Yang Y, Zhou X, Liu J, Ding F (2011) Neuroprotection against cobalt chloride-induced cell apoptosis of primary cultured cortical neurons by salidroside. Molecular and cellular biochemistry 354:161–170. [DOI] [PubMed] [Google Scholar]
- Zou W, Yan M, Xu W, Huo H, Sun L, Zheng Z, Liu X (2001) Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J Neurosci Res 64:646–653. [DOI] [PubMed] [Google Scholar]


