Summary
Background:
Poor persistence to oral HIV pre-exposure prophylaxis (PrEP) diminishes clinical and public health benefits. This study synthesizes evidence regarding discontinuation, adherence, and re-initiation of PrEP among geographically diverse PrEP users.
Methods:
We conducted a systematic review and meta-analysis evaluating studies published in PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (inception to December 18, 2020). We included studies that presented data on PrEP discontinuation, defined as investigator-reported loss-to-follow-up or participant self-reported PrEP stoppage. Data were extracted and assessed for risk of bias. We used a random-effects meta-analysis to pool estimates and I-square and tau-squared to evaluate heterogeneity. This study was registered with PROSPERO (CRD42020155675) and followed PRISMA guidelines.
Findings:
The meta-analysis included 59 longitudinal studies (n=43,917). Forty-one percent of participants discontinued PrEP within six months (95% CI: 18.8–63.5%), with the highest rates in observational studies. Studies in Sub-Saharan Africa pooled a higher rate of discontinuation (47.5% [95% CI: 29.4–66.4%]) versus other regions (P<0.001). Studies with adherence interventions pooled a lower discontinuation rate than those without (24.7% vs. 36.7%, P=0.015). Studies of gay or bisexual men who have sex with men or transgender women (GBMSM/TGW) reported higher HIV incidence among PrEP users (>0.5/100 person-years) and marginally higher discontinuation (32.6% vs. 23.8%, P=0.096). GBMSM/TGW offered daily or non-daily dosing options had lower discontinuation rates than daily alone (21.6% vs. 31.5%, P<0.001). The pooled rate of suboptimal adherence within six months was 37.7% (95% CI: 8.4–66.9%). Among persons who discontinued PrEP, 47.3% (95% CI: 31.5%−63.2%) re-initiated PrEP within a year of PrEP initiation.
Interpretation:
Seventy percent of PrEP users stopped or had suboptimal adherence within six months of initiation. Among those who discontinued, fifty percent restarted PrEP later. Strategies to encourage re-initiating PrEP for new or persistent risk should be a focus of future PrEP implementation strategies.
Funding:
NIH and NSFC
Keywords: HIV, Pre-exposure Prophylaxis, discontinuation, adherence, re-initiation
Introduction
Oral pre-exposure prophylaxis (PrEP) for HIV, using tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) in combination with emtricitabine, has shown high effectiveness in preventing HIV infection when the drug is used with adequate adherence.1,2,3–5 In 2016, the World Health Organization (WHO) recommended that people at substantial risk of HIV infection be offered PrEP as part of comprehensive HIV prevention.6 By the end of 2020, 928,750 individuals were receiving PrEP in 76 countries and regions,7,8 and 54 countries have included PrEP as part of national HIV prevention strategies alongside clinical guidelines for PrEP implementation.9–14
However, high rates of premature PrEP discontinuation have hindered this efficacious prevention strategy. The discontinuation rate within one year of PrEP initiation varies enormously across populations, ranging from 2% to 80% among gay, bisexual and other men who have sex with men and transgender women (GBMSM/TGW)15–18 serodiscordant couples, 19–21 female sex workers (FSW),22,23 and people who inject drugs (PWID).24 More importantly, HIV seroconversion frequently occurs shortly after the discontinuation of PrEP,25–27, and HIV incidence rebounds from 0.0–0.1/100 person-years (100PY) among PrEP users to 2.1–3.6/100PY among those who discontinued PrEP.18,28
Data summarizing PrEP discontinuation are limited. Gaps in our current knowledge regarding stopping and restarting PrEP may compromise maximizing the benefit of this prevention strategy.29 When defined as the proportion of enrolled or initiated study participants who returned for a follow-up visit, 63% of persons continued on PrEP at six months and 71% at 12 months.30 However, this definition included persons retained in a study but who had stopped using PrEP.31–34 Given increasingly recognized dynamic patterns of PrEP use, it is critical to appreciate the full scope of PrEP use, including the correlates of continuation, reasons for discontinuation, and rates of re-initiation among those who discontinued. A comprehensive review of current evidence on oral PrEP adherence, discontinuation, and re-initiation, is urgently needed. While other forms of PrEP (e.g., long-acting injectable PrEP, topical microbicide, etc.) are now approved, and still other forms (e.g., long-acting implants, sub-dermal patches, etc.) under development, the evidence synthesized from studies of oral PrEP will inform and improve the implementation of PrEP in the future.
This study synthesizes the estimated rates of oral PrEP discontinuation, suboptimal adherence among those who continued using PrEP, and re-initiation among those who discontinued, across key populations from diverse geographic locations. We summarize the potential reasons and correlates related to PrEP discontinuation.
Methods
Search strategy and selection criteria
We conducted this systematic review and meta-analysis according to PRISMA guidelines (Figure 1).35 Our protocol was registered with PROSPERO (CRD42020155675). We searched three electronic databases (Medline via PubMed, EMBASE, and Cochrane Central Register of Controlled Trials) for studies reporting on adherence and persistence of oral PrEP, using the following terms: “oral pre-exposure prophylaxis”, “stop”, “discontinuation”, “drop out”, “retention” or “adherence” (see Supplementary 2, page 10 for full search terms). We also searched trial registries from ClinicalTrials.gov and the abstracts from the International AIDS Conference and the annual Conference on Retroviruses and Opportunistic Infections meeting (PROVIDE DATES SEARCHEE) to include published literature as well as ongoing PrEP studies. We searched for studies published in English from database inception to December 18, 2020. Only longitudinal studies (randomized controlled trials and longitudinal observational studies) were included. Two investigators (CL and ZH) independently screened the titles and abstracts, and discrepancies were resolved by a third investigator (JZ). Full texts for the screened studies were read to extract data on the proportions of PrEP users who discontinued PrEP. In the event of multiple publications from one study population, we included the publication with the largest sample size that also had key PrEP discontinuation and/or adherence outcomes. Among the included discontinuation studies, we investigated the proportion of participants with suboptimal adherence among those who continued PrEP and re-initiation among those who discontinued.
Figure 1.
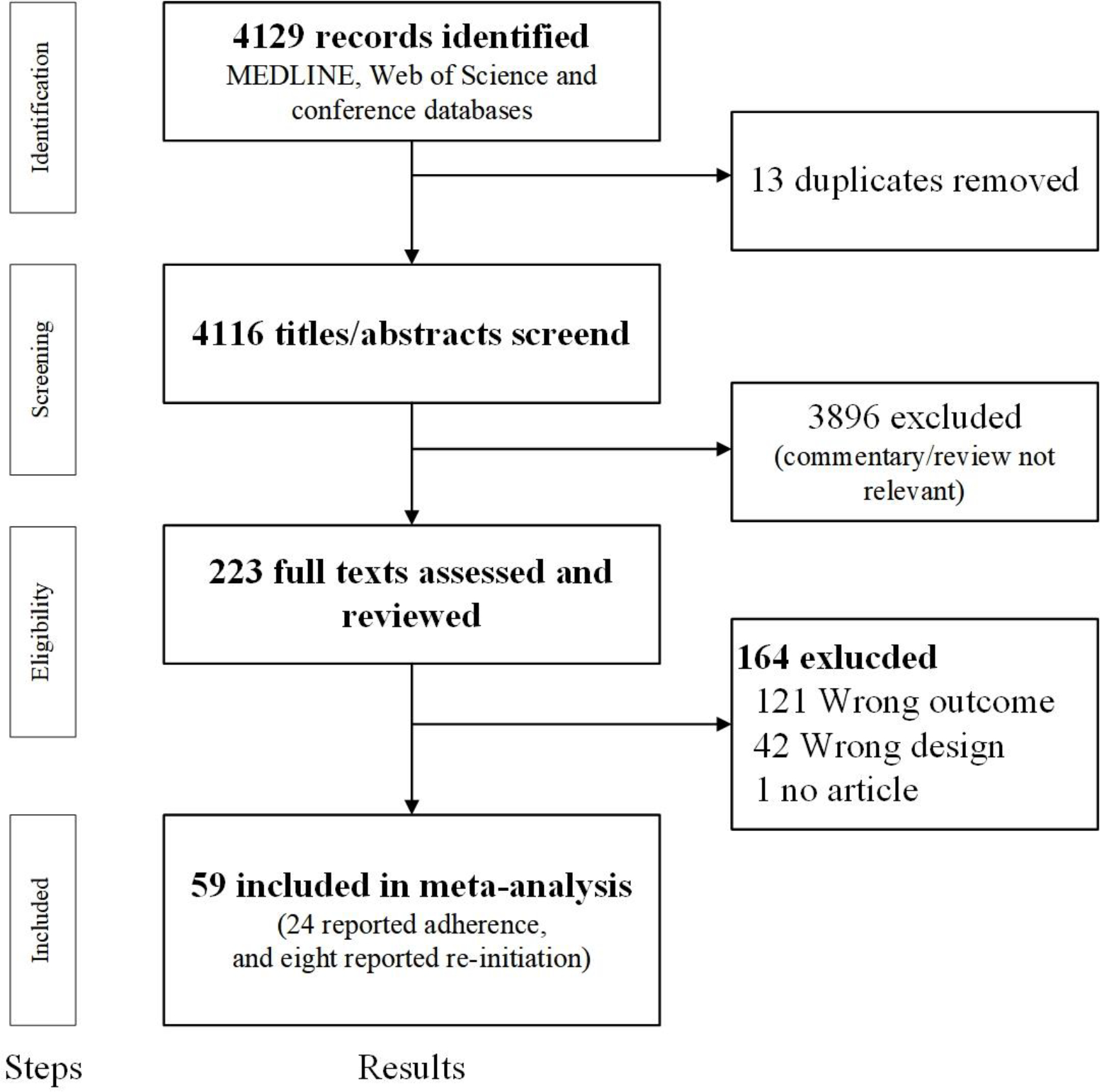
Study selection
Definitions and measurements
Discontinuation was defined as participants self-reporting stopping PrEP, or study team/medical staff-reporting having stopped PrEP refills, or participants having been lost-to-follow-up (LTFU) without reaching a predefined study endpoint and with no evidence of transferring care. The definition of LTFU included participants who did not return for scheduled follow-up visits, who could not be reached by study team/medical staff, or those without TDF/FTC possession calculated by retrospective chart review and pharmacy refill data despite assumed ongoing HIV risk. This definition excluded participants who have evidence of transferring care but does include participants who discontinued but then re-initiated PrEP (Supplementary 5, page 15–20).
We defined suboptimal adherence as having taken fewer doses than required to achieve a protective drug level, according to reported HIV risk (i.e., penile-anal exposure or penile-vaginal exposure). Although daily PrEP dosing is recommended for any individual who has a substantial risk of HIV infection, non-daily PrEP dosing may be appropriate for MSM and TGW with only penile-anal exposure to HIV and less frequent intercourse. The adherence measurements included self-report, pill counting, pharmacy/study refill records or tenofovir-diphosphate (TFV-DP) concentration testing in blood. If there was more than one adherence measurement, we prioritized TFV-DP over pill count and over self-report. We used different thresholds of adherence for daily versus non-daily dosing. For daily dosing, adherence was defined as at least four doses per week (self-report), an intracellular concentration TFV-dp of 16 fmol per million peripheral blood mononuclear cells (PBMCs),36 concentrations of TFV-dp of at least 700–719 fmol/punch in dry blood spots samples37, TFV levels of 0.023ng/mg in hair sample 38, TFV-dp levels of 1000 ng/mL in urine sample39, TFV reported being taken for more than 90% of the days in follow-up40, or a medication possession ratio >80% (the number of tablets dispensed at the prior visit divided by days).25 For non-daily dosing for MSM/TGW, pill counts were adjusted to account for missing pills from the expected “2–1-1” regimen41. Suboptimal adherence was further classified if TFV was not detected in plasma, indicating a gap of drug intake greater than one week 42. There are no clearly defined protective TFV-DP level thresholds for PrEP adherence among cisgender women, though observational data suggesting tenofovir concentrations of 35–40 ng/ml in plasma indicates daily dosing for cisgender women, which is the recommended dosing frequency for this group. 43,44 Furthermore, the PrEP drug level threshold during pregnancy and post-partum periods are highly variable, complicating the use of drug levels to determine PrEP adherence during these periods45, with differences in median steady-state TFV-DP in pregnancy (965 fmol/punch, IQR: 691–1166) and in post-partum (1406 fmol/punch, IQR: 1053–1859, P = 0.006). We did not distinguish if cis-gender women were pregnant or immediately post-partum.
We categorized the study population into GBMSM/TGW, cisgender girls and women, heterosexual men and women, serodiscordant couples, FSW, and PWID. Given the limited number of transgender women (TGW) in each study and the fact that the data among TGW was not distinguished from the data of GBMSM in most studies, we included them in GBMSM/TGW subgroup. Mean, or median lengths were used to categorize follow-ups according to how they were reported in the primary data source. We used the exact number of discontinuation cases from the reported follow-up visit if this information was unavailable. Study designs were categorized as “randomized controlled trials” (RCT) focusing on the efficacy of PrEP, “demonstration projects” focusing on the effectiveness of PrEP, and “real-world implementation” focusing on the routine clinical dissemination of PrEP. We further categorized studies by their geographical regions of enrollment according to UNAIDS’ definition (North America, South America, Sub-Saharan Africa, Middle East, and North Africa, Europe, Asia and Pacific, and the Caribbean) 46. If the study was conducted in more than one region, we categorized the country with the largest/larger sample size. “Youth” was defined as persons aged between 15 and 24 years.47 “High HIV incidence” was defined as greater than 0.5/100 person-years. PrEP regimens were classified as daily and non-daily dosing. Daily dosing was one pill of oral PrEP every 24 hours. The approved non-daily dosing was the “2–1-1” dosing for GBMSM/TGW (also known as on-demand, event-driven, or sex-driven), which is two PrEP pills at least two to 24 hours before sex, one pill 24 hours after the first dose and another pill 48 hours after the first dose.
Data extraction
Two investigators (CL and ZH) independently extracted data from the final list of selected studies, including study characteristics (e.g., study year, design, region, regimen, mean and median follow-up time, etc.), patient characteristics (e.g., age, population, gender at birth), and the number of persons categorized as discontinued, suboptimal adherence and re-initiation.
Data analysis
We pooled independent study estimates and calculated the 95% confidence interval (CI) using random-effects models due to the high heterogeneity of included studies. Heterogeneity across assessments was assessed using the I2, tau-squared, and visual inspection for overlapping of 95% CI. We considered the level of heterogeneity significant if I2>75%. We also evaluated whether study estimates varied by the study population, study design, country regions, PrEP regimen provided in the study, and the follow-up period. Leave-one-out sensitivity analyses were conducted to explore how sensitive associations were between study characteristics and PrEP discontinuation.
We further assessed the quality of evidence by study characteristics based on the Quality Assessment Tool for Quantitative Studies, including selection bias, study design, confounders, blinding, data collection methods, withdrawals, and drop-outs. 48 We also assessed publication bias using funnel plots for asymmetry. All analyses were conducted using Comprehensive Meta-Analysis software (Version 3.3.070).
Role of the funding source
Funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 4,129 records were identified, 4,116 de-duplicated records were screened for relevance using the titles and abstracts, resulting in assessing 223 full-text articles. Fifty-nine articles that captured PrEP discontinuation were included, of which 24 reported adherence among those who continued, and eight reported re-initiation among those who discontinued (Figure 1).
Fifty-five observational studies and four RCTs were included, providing estimates for 43,917 individuals from 20 countries/regions (Supplementary 7, page 22). Thirty-two (54.2%) studies were conducted in North America, 13 (22.0%) in Africa, six (11.5%) in Asia and Pacific, six (11.5%) in Europe, and two (3.8%) in South America. Nearly half of participants were GBMSM/TGW (46.6%, 20,461/43,917, 39 studies). Cisgender girls and women, heterosexual men and women, and HIV negative partners in serodiscordant relationships accounted for 9.9% (4,390/43,917, four studies), 8.9% (3,903/43,917, three studies), and 8.8% (3,875/43,917, four studies), respectively (Table 1). There was no evidence of publication bias for the proportion of PrEP users who discontinued. (Supplement 8, page 23)
Table 1.
Meta-Analysis for the Discontinuation among PrEP Users
| Subgroups | Number of studies | Number of participants | Pooled estimate on rate of discontinuation (95% CI) | Heterogeneity (I2, %) | Tau square | P Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | 59 | 43,917 | ||||
| Study follow-up length* | ||||||
| 6 months and less | 16 | 7,321 | 41.0 (18.8–63.5) | 99.0 | 2.0 | 0.607 |
| 7–12 months | 31 | 28,541 | 35.6 (28.9–42.2) | 98.9 | 0.5 | |
| 13 months and more | 12 | 8,055 | 34.8 (13.9–55.6) | 99.5 | 2.2 | |
| Study Design | ||||||
| RCT | 4 | 3,748 | 16.3 (8.0–30.5) | 98.6 | 0.7 | 0.017 |
| Observational (Demonstration) | 22 | 17,590 | 34.0 (22.7–47.5) | 99.5 | 1.8 | |
| Observational (real world implementation) | 33 | 22,579 | 39.5 (34.4–44.9) | 98.1 | 0.4 | |
| Region | ||||||
| North America | 32 | 20,068 | 37.8 (32.9–43.0) | 97.7 | 0.3 | P<0.001 |
| Sub-Saharan | 13 | 12,889 | 47.5 (29.4–66.4) | 99.6 | 2.0 | |
| Asia and Pacific | 6 | 7,118 | 33.4 (19.5–50.9) | 99.4 | 0.8 | |
| Europe | 6 | 2,167 | 17.4 (13.0–22.9) | 85.6 | 0.1 | |
| South America | 2 | 1,675 | 8.9 (2.4–28.4) | 98.3 | 1.0 | |
| Definition of discontinuation | ||||||
| Study team reported stopping refills or LTFU | 30 | 25,723 | 39.5 (32.3–47.3) | 99.0 | 0.7 | 0.427 |
| Participants self-reported stopping PrEP | 14 | 7,044 | 29.4 (15.1–49.3) | 99.4 | 2.6 | |
| Both | 15 | 11,150 | 33.1 (24.7–42.8) | 98.9 | 0.6 | |
| Age (Median or Mean) of study subjects | ||||||
| Mainly Adults (age≥25) | 49 | 38,689 | 36.3 (30.5–42.5) | 99.2 | 0.8 | 0.592 |
| Mainly Youth (age≤24) | 10 | 5,228 | 31.0 (16.6–50.4) | 99.1 | 1.7 | |
| HIV incidence of the study | ||||||
| Higher than 0.5/100PY | 15 | 13,290 | 31.6 (21.7–43.4) | 99.3 | 1.0 | 0.081 |
| Lower than 0.5/100PY | 24 | 16,424 | 30.6 (22.0–40.9) | 99.3 | 1.2 | |
| Did not provide | 20 | 14,203 | 44.8 (35.4–54.6) | 98.5 | 0.8 | |
| Provided interventions for adherence other than standardized follow-up service | ||||||
| Yes | 6 | 1,234 | 24.7 (18.2–32.5) | 87.0 | 0.2 | 0.015 |
| No | 53 | 42,683 | 36.7 (31.0–42.9) | 99.2 | 0.9 | |
| GBMSM/TGW | 39 | 20,461 | ||||
| Follow-up length* | ||||||
| 6 months and less | 10 | 2,277 | 31.5 (19.2–47.0) | 97.4 | 1.1 | 0.864 |
| 7–12 months | 19 | 13,835 | 30.4 (23.1–38.7) | 98.9 | 0.6 | |
| 13 months and more | 10 | 4,349 | 26.7 (15.9–41.1) | 98.5 | 1.1 | |
| Study Design | ||||||
| RCT | 2 | 519 | 14.9 (8.6–24.5) | 74.9 | 0.2 | P=0.001 |
| Observational (Demonstration) | 12 | 9,440 | 21.0 (13.6–31.0) | 98.9 | 0.8 | |
| Observational (real world implementation) | 25 | 10,502 | 36.3 (29.5–43.8) | 97.9 | 0.6 | |
| Region | ||||||
| North America | 26 | 10,299 | 36.0 (29.7–42.8) | 97.5 | 0.5 | P<0.001 |
| Sub-Saharan | 0 | 0 | -- | -- | -- | |
| Asia and Pacific | 5 | 6,320 | 28.3 (15.5–45.9) | 99.3 | 0.7 | |
| Europe | 6 | 2,167 | 17.4 (13.0–22.9) | 85.6 | 0.1 | |
| South America | 2 | 1,675 | 8.9 (2.4–28.4) | 98.3 | 1.0 | |
| Definition of discontinuation | ||||||
| Study team reported stopping refills or LTFU | 16 | 7,976 | 32.1 (24.0–41.4) | 98.2 | 0.7 | 0.794 |
| Participants self-reported stopping PrEP | 10 | 3,118 | 26.5 (14.9–42.5) | 98.0 | 1.3 | |
| Both | 13 | 9,367 | 29.3 (20.3–40.3) | 98.9 | 0.8 | |
| Regimen of PrEP provided in the study | ||||||
| Daily | 33 | 16,792 | 31.5 (25.4–38.3) | 98.5 | 0.7 | P=0.001 |
| Non-daily | 1 | 361 | 17.5 (13.9–21.7) | 0.0 | 0.0 | |
| Both | 5 | 3,308 | 21.6 (7.9–46.8) | 99.3 | 1.7 | |
| Age (Median or Mean) of subjects | ||||||
| Mainly Adults (age≥25) | 33 | 19,699 | 30.3 (24.2–37.2) | 98.8 | 0.8 | 0.436 |
| Mainly Youths (age≤24) | 6 | 762 | 26.1 (18.9–35.0) | 83.1 | 0.2 | |
| HIV incidence of the study | ||||||
| Higher than 0.5/100PY | 10 | 8,644 | 32.6 (20.9–47.0) | 99.2 | 0.9 | 0.096 |
| Lower than 0.5/100PY | 17 | 9,365 | 23.8 (17.5–31.5) | 98.1 | 0.6 | |
| Did not provide | 12 | 2,452 | 36.8 (27.5–47.0) | 95.5 | 0.5 | |
| Provided interventions for adherence other than standardized follow-up service | ||||||
| Yes | 6 | 1,234 | 24.7 (18.2–32.5) | 87.0 | 0.2 | 0.235 |
| No | 33 | 19,227 | 30.6 (24.5–37.6) | 98.8 | 0.8 | |
| Cisgender girls and women | 4 | 4,390 | ||||
| Follow-up length* | ||||||
| 6 months and less | 3 | 2,380 | 43.3(27.5–60.6) | 95.6 | 0.3 | P<0.001 |
| 7–12 months | 1 | 2,010 | 10.1 (8.9–11.5) | 00.0 | 0.0 | |
| 13 months and more | 0 | 0 | -- | |||
| Heterosexual men and women | 3 | 3,903 | 72.4 (12.4–98.0) | 99.8 | 6.6 | |
| Sero-discordant couples | 4 | 3,875 | 42.0 (10.9–81.1) | 99.7 | 3.2 | |
| Clinical and pharmacy records without population specified | 5 | 9,748 | 45.7 (34.0–57.9) | 98.6 | 0.3 | |
| FSW | 3 | 742 | 50.7 (25.7–75.4) | 98.0 | 1.0 | -- |
| PWID | 1 | 798 | 62.0 (58.6–65.3) | 00.0 | 0.0 | -- |
Quality of studies and Risk of Bias
The included studies had relatively poor quality in terms of study design, with a moderate risk of bias (Supplementary 4, page 13–14). Discontinuation was commonly self-reported or defined by LTFU. Adherence estimates relied only on self-report in five of 24 studies. Among the 55 observational studies, 22 were demonstration projects, and 33 were real-world setting implementation experiences. Most studies used convenient samples, which might be subject to selection bias. The measurements of discontinuation and adherence were inconsistent across studies, which might have introduced heterogeneity. The sensitivity analysis result is stable (Supplementary 6, page 21–22). No publication bias was observed by the symmetry of the funnel plot (Supplementary 8, page 23).
PrEP discontinuation
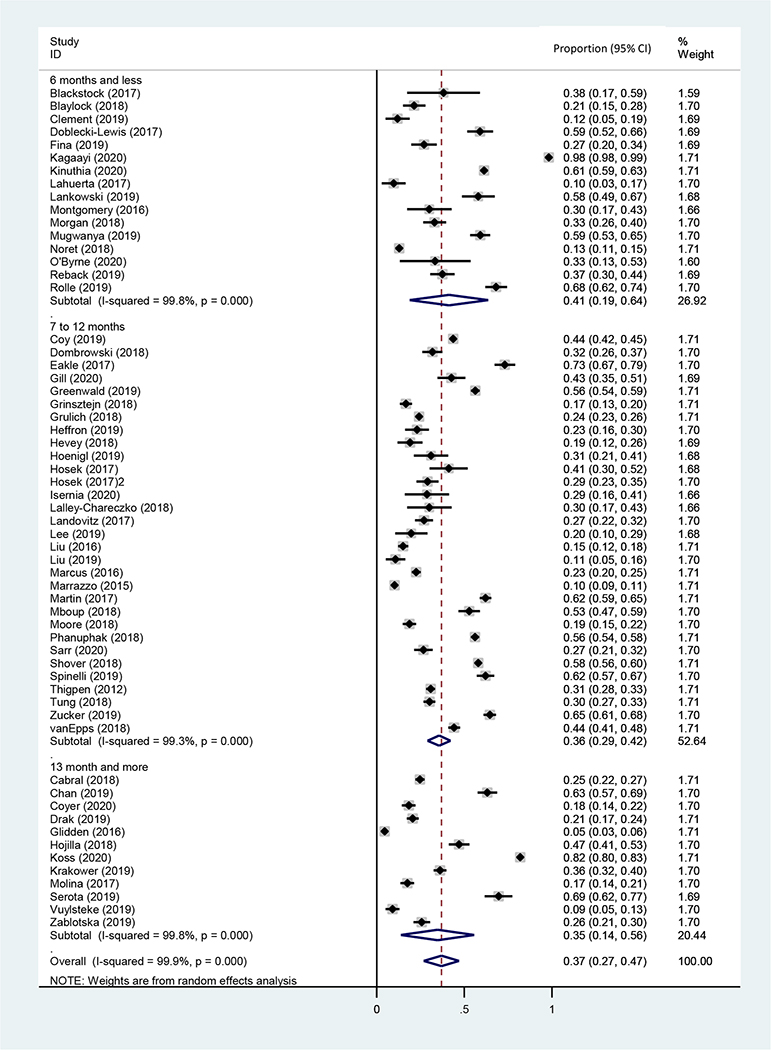
The pooled proportion of PrEP discontinuation within six months after PrEP initiation was 41.0% (95% CI: 18.8–63.5%, N=7,321, 16 studies, I2=99.0%) (Table 1 and Figure 2A). There was a significant difference of pooled PrEP discontinuation rates comparing study designs: 16.3% (95% CI: 8.0%−30.5%, N=3,748, four studies, I2=98.6%, P=0.021) from RCTs, 34.0% (95% CI: 22.7%−47.5%, N=17,590, 22 studies, I2=99.5%) from demonstration projects, and 39.5% (95% CI:34.4%−44.9%, N=22,579, 33 studies, I2=98.1%) from real world implementation (P=0.017). There was a significant regional variation in PrEP discontinuation: 37.8% (95% CI: 32.9–43.0%, N=20,068, 32 studies, I2=97.7%) in North America, 47.5% (95% CI: 29.4–66.4%, N=12,889, 13 studies, I2=99.6%) in Sub-Saharan Africa, 33.4% (95% CI: 19.5–50.9%, N=7,118, six studies, I2=99.4%) in Asia and Pacific, 17.4% (95% CI: 13.0–22.9%, N=2,167, six studies, I2=85.6%) in Europe, and 8.9% (95% CI: 2.4–28.4%, N=1,675, two studies, I2=98.3%) in South America (P<0.001). Studies that included adherence interventions besides standard follow-up services reported a significant lower rate of discontinuation than those studies that did not including adherence interventions (24.7% vs. 36.7%, six studies vs. 53 studies, P=0.015). Multiple definitions of discontinuation were deployed across studies.
Figure 2A.
Forest plots for the rate of discontinuation by time period
Regarding study populations, the pooled rate of PrEP discontinuation in the six months following PrEP initiation was 43.3% (95% CI: 27.5–60.6%, N=2,380, three studies, I2=95.6%) among cisgender girls and women and 31.5% ( 95% CI 19.2–47.0%, N=2,277, 10 studies, I2=97.4%) among GBMSM/TGW. Among all the studies conducted among GBMSM/TGW (n=39), the PrEP discontinuation was significantly higher in observational compared to RCTs (Demonstration: 21.0%, real-world implementation: 36.3%, RCT: 14.9%, P=0.001). North America-based studies pooled a significantly higher rate of discontinuation than other regionsd. In terms of dosing frequency, six studies offered a non-daily regimen of PrEP for GBMSM/TGW, among which two studies were demonstration studies42,49 and four were real-world implementation.28,50–52 The rate of PrEP discontinuation was significantly higher in daily-PrEP only studies (31.5%, 95% CI: 25.4–38.3%, N=16,792, 33 studies, I2=98.5%) compared to studies offering non-daily options (17.5%, 95% CI: 13.9–21.7%, N=361, one studies, I2=0%) or studies offering both strategies (21.6%, 95% CI: 7.9–46.8%, N=3,308, five studies, I2=99.3%, P=0.001). We observed a marginally higher rate of PrEP discontinuation rat in studies with an HIV incidence compared to studies with low incidence (32.6% vs. 23.8%, 10 studies vs. 17 studies, P=0.096).
Suboptimal adherence to PrEP of those who continued PrEP
Table 2 summarizes the pooled rates of what is presumed to be suboptimal PrEP adherence based on the definition above. Twenty-four studies reported PrEP adherence among participants who continued PrEP. The pooled rate of suboptimal adherence within six months was 37.7% (95% CI: 8.4–66.9%, N=330, four studies, I2=95.9%) (Figure 2B). Studies of participants with a median age≤ 24 had a significantly higher rate of suboptimal adherence than those with a median age >24 (62.5% vs. 33.7%, five studies vs. 19 studies, P=0.004). The rates also varied significantly across different measurements of adherence: 18.4% (95% CI: 9.8%−32.0%, N=5,891, three studies, I2=98.9%) via pill counting or refill records, 43.7% (95% CI: 15.6%−76.5%, N=1,629, five studies, I2=99.2%) via self-reporting, and 43.1% (95% CI: 33.6%−53.1%, N=2,663, 16 studies, I2=95.3%, P=0.017) via drug concentrations.
Table 2.
Meta-analysis for the suboptimal adherence among PrEP Users who continued
| Subgroups | Number of studies | Number of participants | Pooled estimate of suboptimal adherence (95% CI) | Heterogeneity (I2, %) | Tau square | P Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | 24 | 10,183 | ||||
| Study follow-up length* | ||||||
| 6 months and less | 4 | 330 | 37.7 (8.4–66.9) | 95.9 | 1.8 | 0.932 |
| 7–12 months | 17 | 9,170 | 41.6 (32.1–51.1) | 98.6 | 0.9 | |
| 13 months and more | 3 | 683 | 43.1 (17.5–68.7) | 97.6 | 0.9 | |
| Study Design | ||||||
| RCT | 4 | 1,697 | 38.1 (19.0–61.9) | 97.4 | 0.9 | 0.158 |
| Observational (Demonstration) | 12 | 6,330 | 49.0 (37.4–60.8) | 98.1 | 0.7 | |
| Observational (real world implementation) | 8 | 2,156 | 25.7 (11.6–47.5) | 98.2 | 1.8 | |
| Region | ||||||
| North America | 14 | 3,443 | 34.2 (22.0–49.1) | 98.0 | 1.3 | 0.175 |
| Sub-Saharan | 6 | 1,756 | 51.7 (27.3–75.2) | 98.3 | 1.6 | |
| Asia and Pacific | 2 | 4,273 | 53.2 (14.5–88.4) | 99.7 | 1.9 | |
| Europe | 1 | 336 | 28.6 (24.0–33.6) | 00.0 | 0.0 | |
| South America | 1 | 375 | 26.1 (21.9–30.8) | 00.0 | 0.0 | |
| Age (Median or Mean) of study subjects | ||||||
| Mainly Adults (age≥25) | 19 | 9,687 | 33.7 (25.1–43.6) | 98.4 | 0.8 | 0.004 |
| Mainly Youths (age≤24) | 5 | 486 | 62.5 (45.6–76.7) | 90.6 | 0.5 | |
| Measurement of adherence | ||||||
| Pill Counts and refills records | 3 | 5,891 | 18.4 (9.8–32.0) | 98.9 | 0.4 | 0.017 |
| Self-report | 5 | 1,629 | 43.7 (15.6–76.5) | 99.2 | 0.6 | |
| Drug concentrations | 16 | 2,663 | 43.1 (33.6–53.1) | 95.3 | 2.7 | |
| HIV incidence of the study | ||||||
| Higher than 0.5/100PY | 6 | 1,704 | 51.1 (23.2–78.3) | 98.6 | 2.3 | 0.653 |
| Lower than 0.5/100PY | 12 | 7,299 | 35.5 (25.4–47.0) | 98.4 | 0.7 | |
| Did not provide | 6 | 1,180 | 35.9 (14.3–65.4) | 98.3 | 2.3 | |
| Provided intervention for adherence | ||||||
| Yes | 5 | 729 | 36.1 (17.1–60.7) | 96.6 | 1.3 | 0.753 |
| No | 19 | 9,454 | 40.2 (30.3–51.0) | 98.5 | 0.9 | |
| GBMSM | 16 | 6,882 | -- | |||
| Follow-up length* | ||||||
| 6 months and less | 3 | 264 | 27.5 (5.0–73.3) | 96.9 | 2.9 | 0.764 |
| 7–12 months | 11 | 6,051 | 34.3 (25.6–44.2) | 96.8 | 0.5 | |
| 13 months and more | 2 | 567 | 47.4 (15.5–81.7) | 98.7 | 1.3 | |
| Study Design | ||||||
| RCT | 2 | 441 | 31.3 (9.2–67.2) | 97.7 | 1.1 | 0.555 |
| Observational (Demonstration) | 7 | 5,257 | 41.8 (32.2–52.0) | 96.3 | 0.3 | |
| Observational (real world implementation) | 7 | 1,184 | 28.3 (11.2–55.1) | 97.9 | 2.2 | |
| Regimen of PrEP provided in the study | ||||||
| daily | 15 | 6,546 | 35.8 (26.9–45.7) | 97.2 | 0.6 | 0.172 |
| Non-daily | 1 | 336 | 28.6 (24.0–33.6) | 00.0 | 0.0 | |
| Both | 0 | 0 | -- | -- | ||
| Region | ||||||
| North America | 13 | 2,471 | 36.8 (24.1–51.6) | 97.4 | 1.2 | 0.280 |
| Sub-Saharan | 0 | 0 | -- | -- | -- | |
| Asia and Pacific | 1 | 3,700 | 30.1 (28.7–31.6) | 00.0 | 0.0 | |
| Europe | 1 | 336 | 28.6 (24.0–33.6) | 00.0 | 0.0 | |
| South America | 1 | 375 | 26.1 (21.9–30.8) | 00.0 | 0.0 | |
| Age (Median or Mean) of subjects | ||||||
| Mainly Adults (age≥25) | 12 | 6,433 | 29.1 (21.8–37.7) | 96.6 | 0.4 | 0.005 |
| Mainly Youths (age≤24) | 4 | 449 | 57.0 (39.3–73.1) | 91.4 | 0.5 | |
| Measurement of suboptimal adherence | ||||||
| Self-report | 4 | 1,056 | 35.6 (9.6–74.1) | 98.9 | 2.8 | 0.508 |
| Pill Counts | 1 | 3,700 | 30.1 (28.7–31.6) | 00.0 | 0.0 | |
| Drug concentrations | 11 | 2,126 | 35.9 (26.3–46.8) | 95.1 | 0.5 | |
| HIV incidence of the study | ||||||
| Higher than 0.5/100PY | 3 | 298 | 55.5 (29.6–78.7) | 89.8 | 0.8 | 0.193 |
| Lower than 0.5/100PY | 8 | 5,470 | 30.9 (26.3–35.9) | 87.6 | 0.1 | |
| Did not provide | 5 | 1,114 | 32.3 (10.5–66.0) | 98.6 | 2.5 | |
| Provided intervention for adherence | ||||||
| Yes | 5 | 729 | 36.1 (17.1–60.7) | 96.6 | 1.3 | 0.945 |
| No | 11 | 6,153 | 35.2 (25.8–45.9) | 97.4 | 0.5 | |
| Cisgender girl and women | 1 | 66 | 56.1 (44.0–67.5) | 00.0 | 0.0 | |
| Heterosexual men and women (follow-up length ranged from 1 months to more than 12 months) | 2 | 1,256 | 49.1 (3.7–96.1) | 98.1 | 5.3 | -- |
| Sero-discordant couples (follow-up length was 1–6 months) | 1 | 116 | 33.6 (25.6–42.7) | 00.0 | 0.0 | |
| Clinical and pharmacy records without population specified (follow-up length was 7–12 months) | 1 | 972 | 12.1 (10.2–14.3) | 00.0 | 0.0 | |
| FSW (follow-up length was 7–12 months) | 2 | 318 | 62.4 (51.5–72.7) | 76.3 | 0.1 | |
| PWID (follow-up length was 7–12 months) | 2 | 573 | 75.0 (71.3–78.4) | 00.0 | 0.0 | |
Figure 2B.
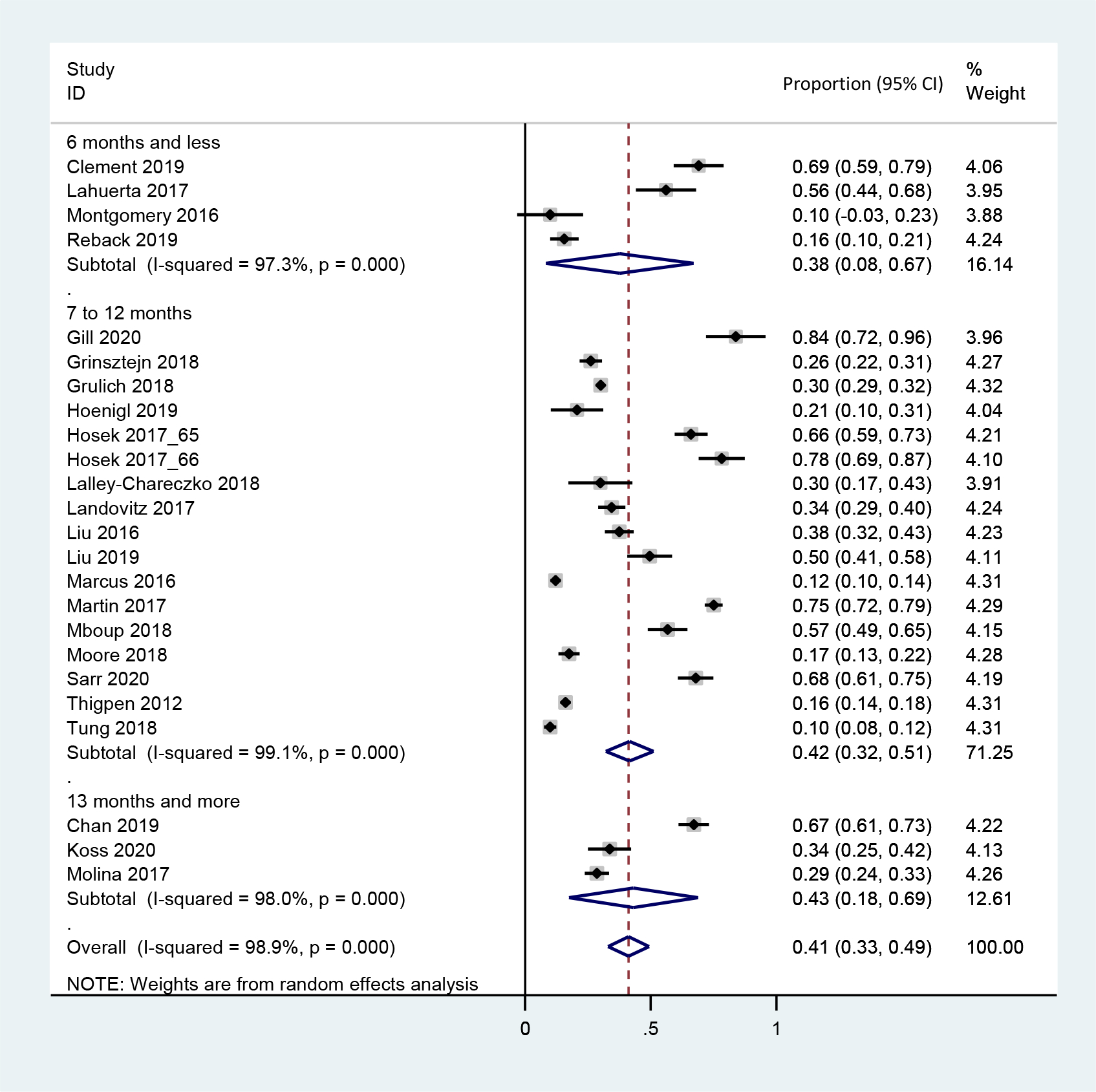
Forest plot for the rate of suboptimal adherence by time period
Re-initiation of PrEP among those who discontinued PrEP
Eight studies collected data on PrEP re-initiation among those who discontinued PrEP. Pooled re-initiation rate was 47.3% (95% CI: 31.5–63.2%, N=2,658, I2=96.9%) (Figure 2C). We did not observe any statistically significant difference of re-initiation rates according to study design (demonstration 28.4% vs. real-world implementation 42.3%, four studies vs. four studies, P=0.449) or by HIV incidence rates (higher incidence 54.9% vs. lower incidence 30.1%, two studies vs. five studies, P=0.193), with statistical testing limited due to small sample size (Table 3).
Figure 2C.
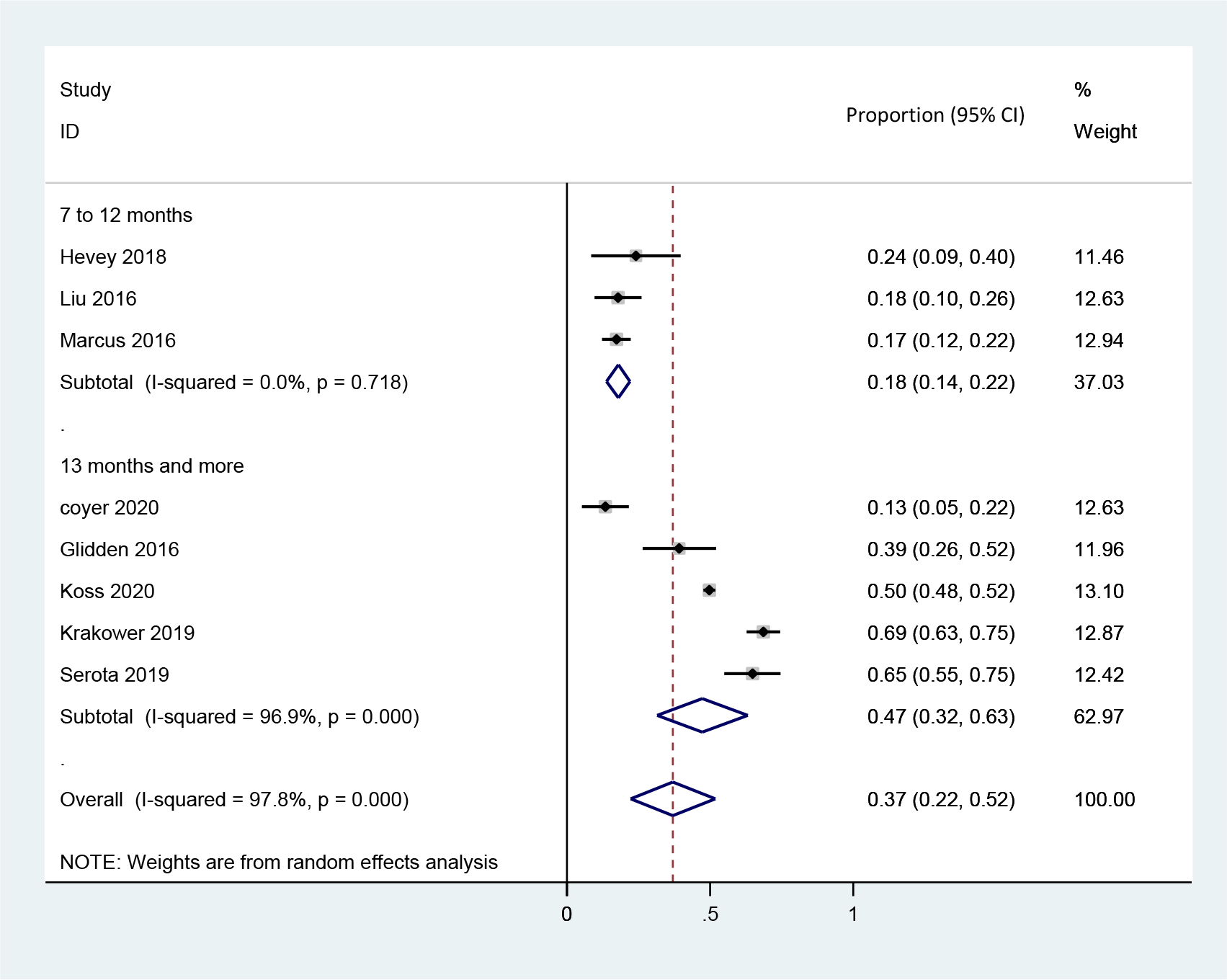
Forest plots for the rate of re-initiation by time period
Table 3.
Meta-analysis for the re-initiation among PrEP Users who discontinued
| Subgroups | Number of studies | Number of participants | Pooled estimate of suboptimal adherence (95% CI) | Heterogeneity (I2, %) | Tau square | P Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | 8 | 2,990 | ||||
| Study follow-up length* | ||||||
| Six months and less | 0 | 0 | -- | -- | P<0.001 | |
| 7–12 months | 3 | 332 | 18.0 (13.8–22.1) | 94.1 | 0.4 | |
| 13 months and more | 5 | 2,658 | 47.3 (31.5–63.2) | 96.9 | 0.0 | |
| Study Design | ||||||
| RCT | 0 | 0 | -- | -- | 0.449 | |
| Observational (Demonstration) | 4 | 2,412 | 28.4 (13.7–49.8) | 94.5 | 0.8 | |
| Observational (real world implementation) | 4 | 578 | 42.3 (16.7–72.8) | 97.6 | 1.7 | |
| Region | ||||||
| North America | 5 | 662 | 36.5 (15.3–64.7) | 97.3 | 1.7 | P<0.001 |
| Sub-Saharan | 1 | 2,205 | 49.7 (47.6–51.8) | 00.0 | 0.0 | |
| Asia and Pacific | 0 | 0 | -- | -- | -- | |
| Europe | 1 | 67 | 13.4 (7.1–23.8) | 00.0 | 0.0 | |
| South America | 1 | 56 | 39.3 (27.5–52.5) | 00.0 | 0.0 | |
| Age (Median or Mean) of study subjects | ||||||
| Mainly Adults (age≥25) | 7 | 2,961 | 36.9 (23.6–52.5) | 96.5 | 0.7 | 0.263 |
| Mainly Youths (age≤24) | 1 | 29 | 24.1 (12.0–42.7) | 00.0 | 0.0 | |
| HIV incidence of the study | ||||||
| Higher than 0.5/100PY | 2 | 295 | 54.9 (27.0–80.0) | 93.6 | 0.7 | 0.193 |
| Lower than 0.5/100PY | 5 | 2,666 | 30.1 (15.1–50.9) | 96.9 | 1.0 | |
| Did not provide | 1 | 29 | 24.1 (12.0–42.7) | 00.0 | 0.0 | |
| Provided intervention for adherence | ||||||
| Yes | 0 | 0 | -- | -- | -- | 1.000 |
| No | 8 | 2,990 | 35.4 (23.1–49.9) | 96.1 | 0.7 | |
Reasons and correlates for PrEP discontinuation
We included and reviewed 30 studies reporting reasons for and correlates of PrEP discontinuation. We categorized these reasons into three levels: individual, interpersonal, and structural. The most common reasons for stopping PrEP was due to low perceived risk of HIV infection (21 studies), experiencing side-effects (25 studies), concerns for the long-term side-effects of PrEP (five studies), challenges with medication adherence or pill burden (seven studies), choosing prevention methods other than PrEP (three studies), and relocation (seven studies). Interpersonal reasons included lack of family support (one study), while structural reasons were primarily related to cost or lack of health insurance (ten studies), and inaccessibility to care (nine studies). (Supplementary 12, page 30) We synthesized correlates of discontinuation from 16 longitudinal studies. The most-reported factors positively associated with discontinuation were individual-level factors, such as younger age (six studies), being female (one study), and being transgender (three studies). (Supplementary 10, page 23).
Discussion
This review updates and synthesizes the rates and correlates of PrEP discontinuation in global literature, extending previous work by quantifying adherence among those who continued PrEP and describing re-initiation among persons who discontinued PrEP. Our meta-analysis revealed four out of ten participants discontinued PrEP within six months after initiation. Among the remainder who continued PrEP, more than one-third were using it with a frequency that was not expected to provide adequate adherence to prevent HIV acquisition after accounting for differences in the regimen. Taken together, this suggests that less than one-third of PrEP initiators used PrEP properly within six months following initiation. Among those who discontinued PrEP, about half restarted it within the year after the first initiation, further demonstrating the fluidity and dynamic patterns of oral PrEP use.
In this review, we attempted to classify PrEP discontinuation in two ways: by study team-reporting and by participants’ self-reporting. There was no statistically significant difference in PrEP discontinuation rates between study team-reporting LTFU or stopped refills and self-reported stoppage. Our discontinuation definition excluded cases with evidence of transferring care, and five studies reported data of transferring care among LTFU52–56. The LTFU designation is likely an overestimate, as rates were influenced by the study follow-up frequency and study design. This was especially true among retrospective chart reviews that relied heavily on refill records and clinician notes to calculate drug possession and possible discontinuation dates.
Although LTFU may be considered as termination of PrEP access (and thus a reliable estimate of discontinuation), participants may be LTFU from the research study but continue to access PrEP via other channels, particularly as PrEP becomes more available globally. This shift in non-study access to PrEP enforces the importance of documenting or verifying care transfers to better estimate retention on PrEP and clinical outcomes after recorded PrEP discontinuation.
There is substantial variation in defining PrEP discontinuation among the studies included in this review and the literature. The complexity of discontinuation rests in the fundamental difference between ART and PrEP. The former requires lifelong use for effectiveness, and the latter is only needed during the period of substantial risk for HIV acquisition. Unfortunately, the capacity of study investigators, and indeed participants to some extent, to accurately assess and report a need for PrEP based on objective HIV risk continues to complicate our ability to define and distinguish clinically meaningful premature PrEP discontinuation from that which may be an appropriate stoppage. One strategy is to examine the reasons for participants discontinuing PrEP and describe correlates of discontinuation. Exploring these discontinuation reasons can help distinguish appropriate discontinuation and inform possible strategy to improve persistence among those at ongoing elevated risk of HIV infection.
We observed a marginal correlation between higher discontinuation rates and HIV incidence among GBMSM/TGW, further supporting the effectiveness of PrEP in reducing HIV acquisition. Some examples of the study design helped dictate and distinguish appropriate discontinuation events. For example, in the Partners Demonstration Project57, PrEP was a “bridge” for HIV prevention among serodiscordant couples until the partner living with HIV had achieved viral suppression. In Kinuthia et al., PrEP was co-dispensed with HIV self-test kits for secondary distribution to male partners21. Some participants discontinued PrEP after confirming their partner’s HIV-negative status via the HIV self-tests. Hence, our pooled rates of PrEP discontinuation included both inappropriate and appropriate discontinuations, highlighting the importance of differentiating these categories to achieve prevention-effective PrEP use in future studies58, instead of calls for near-perfect adherence regardless of risk exposure.
There was also high heterogeneity in PrEP adherence assessments. This review prioritized adherence data measured by drug concentration when several measurements were used in a single study. However, tenofovir concentration was only measured in two-thirds of studies. Given that we observed higher suboptimal adherence rates when adherence was measured by TFV-dp concentrations compared to pill counting or refill record review, our estimates are likely an underestimate. Interestingly, the difference between suboptimal adherence when assessed using patient self-report (43.7%) versus TFV concentration (43.1%) was not substantial. Considering the already resource-strained healthcare systems in which PrEP is being provided, patients’ self-reported PrEP use history may be a more convenient and affordable approximation to PrEP adherence. Digital health PrEP adherence tools may further reduce reporting bias when recalling pill-taking history and are a compelling advance in PrEP monitoring and clinical care59,60.
We observed highly variable discontinuation rates comparing different key populations. Within six months of PrEP initiation, more than 40% of cisgender girls and women discontinued PrEP. This subgroup was drawn from the general population in HIV high burden settings, including adolescent girls and young women (25 and under) from studies conducted in sub-Saharan Africa. Importantly, regional differences in discontinuation also noted the highest rates in sub-Saharan Africa. Data was limited regarding PrEP continuation during pregnancy and the post-partum periods as only two studies allowed enrollment of pregnant or breastfeeding participants. Barriers to PrEP persistence are likely to vary by gender, age, pregnancy status, and cultural context. Strategies to improve engagement and PrEP persistence for cisgender girls and women are urgently needed, as are studies that specifically evaluate PrEP use during the high-HIV acquisition risk period of pregnancy and the immediate post-partum period.
Our results suggest that one-third of GBMSM/TGW discontinued PrEP within six months of initiation. Studies that offered daily and non-daily regimen options reported a significantly lower discontinuation rate than studies that only offered daily PrEP. This correlation suggests that providing choices of PrEP regimen and dosing frequency can help GBMSM/TGW better cope with fluctuations in risk – utilizing PrEP “as needed” rather than a complete cessation of PrEP. Providing options in PrEP dosing frequency for GBMSM/TGW is likely superior to limiting to daily dosing11,42,49, but there is no data to support this strategy for cisgender women or PWID.
While in real-world settings, the full potential of oral PrEP for HIV prevention has been undermined by poor persistence, the recent promising results from the HIV Prevention and Trials Network (HPTN) 083 and 084 efficacy trials of long-acting injectable PrEP61,62 are a compelling alternative to oral formulations, particularly for people who struggle with adherence. In contrast to once daily or event-driven PrEP regimens, long-acting (LA PrEP) reduces dosing frequency to once every two months. However, the successful implementation of LA PrEP will depend highly on the capacity of local HIV care systems and effective public messaging. Unlike oral regimens that may be distributed for up to 3-month at a time, injectable PrEP could require more frequent clinic visits and higher costs. LA PrEP provides the critical addition of new choices for biomedical HIV prevention but is unlikely to replace oral PrEP entirely, and the impact of LA PrEP on premature discontinuation will necessarily be the study of future research.
Our study has several implications. From a policy perspective, the high rate of discontinuation within the first 6 months of PrEP suggests additional attention is needed on providing comprehensive HIV prevention in the era of PrEP, strengthening counseling provided before initiation of PrEP and while on PrEP. Although many studies suggest increased incidence after stopping PrEP, suggesting inappropriate discontinuation, identifying strategies to help counsel patients appropriately on when it may be ok to “pause PrEP” and ensuring they have adequate resources to reengage in PrEP care is critical. This will require more objective assessment of HIV infection risk and prediction tools for discontinuation tailored to different key populations. Our findings also emphasize the importance of designing interventions that encourage PrEP re-initiation, messaging that may be different from those of persons considering starting PrEP for the first time. Cost-effectiveness analyses and mathematical models should include the rate of discontinuation as an essential indicator to assess the economic and epidemiological impact of PrEP implementation.63,64
Our study has several limitations. First, there was substantial heterogeneity across studies. The low quality of included studies and the inconsistent outcome measurement likely introduced additional heterogeneity in our pooled results. While PrEP research evolved rapidly from RCT to demonstration to real-world implementation, we observed the expected finding of poorer retention outside of controlled study settings. Additional contributors to observed heterogeneity were due to our inclusion of various key populations, PrEP regimens, and diverse geographic settings – all of which increased heterogenicity but are critical given the desire to evaluate our research outcome on a global scale. Second, most studies did not provide evidence on transferring care among people who were LTFU but may continue or re-initiate PrEP outside of the study, resulting in an overestimation of discontinuation. Third, most of the included studies were conducted in North America, and the target populations were mainly GBMSM and individuals aged 25 and above. Our sub-analyses may lead to a bias that underestimates the PrEP persistence, including suboptimal adherence, globally. Fourth, some studies did not report disaggregated data for cisgender men and women. In these studies, we used four pills/week as a level of adherence for cisgender women, and no tenofovir was detected for MSM using the “2–1-1” regimen, which systematically underestimates that which is expected to result in a protective drug level. Fifth, we did not include grey literature or literature in languages other than English. However, several studies suggest exclusion of non-English studies does not impact systematic reviews.65,66 Finally, there are very minimum data that represent/reflect PrEP among PWID, and additional research among PWID is needed.
Conclusion
Seventy percent of PrEP users either stopped or had suboptimal PrEP adherence within six months of initiation. Among those who discontinued, nearly half restarted PrEP one year after the first initiation. Strategies to encourage re-initiating PrEP for new or persistent risk should be a focus of future PrEP implementation and are critical considerations even in the era of long-acting PrEP.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials for studies presenting data on discontinuation of oral PrEP from inception to December 18, 2020. One previous meta-analysis of PrEP continuation included studies published up to 2018, and results suggested a PrEP retention rate of 63% at six months follow-up. However, the definition of continuation had clinically significant gaps as it did not account for persons retained in studies but who had stopped PrEP, nor those who transferred care; furthermore, since 2018, critical new evidence surrounding PrEP persistence has emerged. An updated, comprehensive summary of the evidence surrounding rates of PrEP discontinuation, suboptimal adherence, and re-initiation among geographically diverse populations at elevated risk of HIV (key populations) is critical.
Added value of this study
We conducted a global systematic review and meta-analysis to update and extend previous work by quantifying the rates of PrEP discontinuation, suboptimal adherence among those who continued PrEP, and re-initiation among those who stopped PrEP during the observed period of follow-up. The findings confirm prior studies, demonstrating that PrEP discontinuation in the first six months following initiation is common worldwide. By accounting for suboptimal adherence (i.e., adherence that would not be expected to result in HIV-protective drug levels), we estimate that less than 30% of PrEP initiators received the HIV protective benefit of PrEP within six months of initiation. Demonstration projects (so-called “real-world settings”), studies conducted in Sub-Saharan Africa, and studies that did not include an adherence intervention had higher rates of PrEP discontinuation.
Implications of all the available evidence
Our analysis suggests PrEP discontinuation was common within six months of PrEP initiation in a wide range of geographic locations and HIV risk-populations. Poor PrEP persistence, with premature discontinuation, suboptimal adherence, and infrequent restarts despite persistent or recurrent risk, fundamentally undermines efforts to maximize the prevention potential of PrEP. Efforts to prevent premature discontinuation and support re-initiation of PrEP for new or persistent risk need to be strengthened and should be a focus of future PrEP implementation strategies.
Acknowledgments
This work was supported by the Mega-Projects of national science research (13th Five-Year Plan [2017ZX10201101–002-007]), the Mega-Projects of national science research (12th Five-Year Plan [2012ZX10001006–001-010]), National Natural Science Foundation of China (81903371, 81872674), the Mega-Projects of national science research (2018ZX10101001–001-003), the National Key Research and Development Program of China (2017YFE0103800), the National Institutes of Health (NIAID K24AI143471, R34MH109359, and R34MH119963), Guangdong Medical Science and Technology Research Fund (A2020509), the National Social Science Fund of China (No. 19CSH018), and UNC CFAR P30 AI050410. Sarah E Rutstein was supported by T32AI007001.
Footnotes
Declaration of interests
We declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jing Zhang, The NHC Key Laboratory of AIDS Immunology and National Clinical Research Center for Laboratory Medicine of the First Affiliated Hospital of China Medical University; Key Laboratory of AIDS Immunology, Chinese Academy of Medical Sciences; Key Laboratory of AIDS Immunology of Liaoning Province; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases.
Chunyan Li, Department of Health Behavior of University of North Carolina.
Junjie Xu, The NHC Key Laboratory of AIDS Immunology and National Clinical Research Center for Laboratory Medicine of the First Affiliated Hospital of China Medical University; Key Laboratory of AIDS Immunology, Chinese Academy of Medical Sciences; Key Laboratory of AIDS Immunology of Liaoning Province; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases.
Zhili Hu, The NHC Key Laboratory of AIDS Immunology and National Clinical Research Center for Laboratory Medicine of the First Affiliated Hospital of China Medical University; Key Laboratory of AIDS Immunology, Chinese Academy of Medical Sciences; Key Laboratory of AIDS Immunology of Liaoning Province; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases.
Sarah E Rutstein, Department of Medicine of University of North Carolina.
Joseph D. Tucker, Department of Medicine of University of North Carolina University of North Carolina Project-China; Faculty of Infectious and Tropical Diseases of London School of Hygiene and Tropical Medicine.
Jason J Ong, Faculty of Infectious and Tropical Diseases of London School of Hygiene and Tropical Medicine; Monash University.
Yongjun Jiang, The NHC Key Laboratory of AIDS Immunology and National Clinical Research Center for Laboratory Medicine of the First Affiliated Hospital of China Medical University; Key Laboratory of AIDS Immunology, Chinese Academy of Medical Sciences; Key Laboratory of AIDS Immunology of Liaoning Province; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases.
Wenqing Geng, The NHC Key Laboratory of AIDS Immunology and National Clinical Research Center for Laboratory Medicine of the First Affiliated Hospital of China Medical University; Key Laboratory of AIDS Immunology, Chinese Academy of Medical Sciences; Key Laboratory of AIDS Immunology of Liaoning Province; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases.
Sarah T Wright, Health Sciences Library of University of North Carolina.
Myron S. Cohen, Department of Medicine of University of North Carolina
Hong Shang, The NHC Key Laboratory of AIDS Immunology and National Clinical Research Center for Laboratory Medicine of the First Affiliated Hospital of China Medical University; Key Laboratory of AIDS Immunology, Chinese Academy of Medical Sciences; Key Laboratory of AIDS Immunology of Liaoning Province; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases.
Weiming Tang, Department of Medicine of University of North Carolina; University of North Carolina Project-China; Dermatology Hospital of Southern Medical University.
Data Sharing Statement
All data will be made available upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Pre-exposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine 2010; 363(27): 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine 2012; 4(151): 151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (London, England) 2016; 387(10013): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JM, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. The New England journal of medicine 2015; 373(23): 2237–46. [DOI] [PubMed] [Google Scholar]
- 5.Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020; 396(10246): 239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinelli MA, Laborde N, Kinley P, et al. Missed opportunities to prevent HIV infections among pre-exposure prophylaxis users: a population-based mixed methods study, San Francisco, United States. J Int AIDS Soc 2020; 23(4): e25472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nearly a million have started taking PrEP worldwide-only a third of UNAIDS’s 2020 target. AIDSMAP. Available at https://www.aidsmap.com/news/feb-2021/nearly-million-have-started-takingprep-worldwide-only-third-unaids-2020-target. Accessed on May 17, 2021..
- 8.380,000 people on PrEP globally, mostly in the USA and Africa. Available at http://www.aidsmap.com/news/oct-2018/380000-people-prep-globally-mostly-usa-and-africaupdated. Accessed on September 14, 2019. 2018.
- 9.CDC. HIV Among Gay and Bisexual Men. Available from: https://www.cdc.gov/hiv/group/msm/index.html. Accessed on November 26, 2021. 2020.
- 10.Gill K, Johnson L, Dietrich J, et al. Acceptability, safety, and patterns of use of oral tenofovir disoproxil fumarate and emtricitabine for HIV pre-exposure prophylaxis in South African adolescents: an open-label single-arm phase 2 trial. The Lancet Child & adolescent health 2020; 4(12): 875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyer L, van den Elshout MAM, Achterbergh RCA, et al. Understanding pre-exposure prophylaxis (PrEP) regimen use: Switching and discontinuing daily and event-driven PrEP among men who have sex with men. EClinicalMedicine 2020; 29–30: 100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available at https://covid19.who.int/. Accessed 18th Feburary 2021.. 2021. [Google Scholar]
- 13.Our World in Data. Statistics and Research. Coronavirus (COVID-19) Vaccinations. Available at https://ourworldindata.org/covid-vaccinations. Accessed 18 Feburary 2021.. 2021. [Google Scholar]
- 14.TheBody. The HIV/AIDS resources. COVID-19 Vaccination for People Living With HIV: What Do We Know So Far? Availabe at https://www.thebody.com/article/covid-19-vaccine-people-living-with-hiv. Accessed 18 Feburary 2021.. 2020.
- 15.Grulich AE, Guy R, Amin J, et al. Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. The lancet HIV 2018; 5(11): e629–e37. [DOI] [PubMed] [Google Scholar]
- 16.Whitfield THF, Parsons JT, Rendina HJ. Rates of Pre-exposure Prophylaxis Use and Discontinuation Among a Large U.S. National Sample of Sexual Minority Men and Adolescents. Archives of sexual behavior 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phanuphak N, Sungsing T, Jantarapakde J, et al. Princess PrEP program: the first key population-led model to deliver pre-exposure prophylaxis to key populations by key populations in Thailand. Sex Health 2018; 15(6): 542–55. [DOI] [PubMed] [Google Scholar]
- 18.Shover CL, Shoptaw S, Javanbakht M, et al. Mind the gaps: prescription coverage and HIV incidence among patients receiving pre-exposure prophylaxis from a large federally qualified health center in Los Angeles, California : Mind the Gaps: Cobertura de recetas e incidencia de VIH entre pacientes recibiendo profilaxis pre-exposicion de un centro de salud grande y federalmente calificado en Los Angeles, CA. AIDS and behavior 2019; 23(10): 2730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabral A, J MB, Ngure K, et al. Intimate Partner Violence and Self-Reported Pre-exposure Prophylaxis Interruptions Among HIV-Negative Partners in HIV Serodiscordant Couples in Kenya and Uganda. J Acquir Immune Defic Syndr 2018; 77(2): 154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral pre-exposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine 2012; 367(5): 423–34. [DOI] [PubMed] [Google Scholar]
- 21.Kinuthia J, Pintye J, Abuna F, et al. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: results from an implementation programme. The lancet HIV 2020; 7(1): e38–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eakle R, Gomez GB, Naicker N, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project. PLoS medicine 2017; 14(11): e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mboup A, Behanzin L, Guedou FA, et al. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc 2018; 21(11): e25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin M, Vanichseni S, Suntharasamai P, et al. Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. The lancet HIV 2017; 4(2): e59–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus JL, Hurley LB, Hare CB, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J Acquir Immune Defic Syndr 2016; 73(5): 540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV pre-exposure prophylaxis during primary care. J Int AIDS Soc 2019; 22(2): e25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kai Jonas NY. HIV Prevention After Discontinuing Pre-Exposure Prophylaxis: Conclusions From a Case Study. Frontiers in public health 2018; 6(137). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald ZR, Maheu-Giroux M, Szabo J, et al. Cohort profile: l’Actuel Pre-Exposure Prophylaxis (PrEP) Cohort study in Montreal, Canada. BMJ Open 2019; 9(6): e028768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutstein SE, Smith DK, Dalal S, Baggaley RC, Cohen MS. Initiation, discontinuation, and restarting HIV pre-exposure prophylaxis: ongoing implementation strategies. Lancet HIV 2020; 7(10): e721–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stankevitz K, Grant H, Lloyd J, et al. Oral pre-exposure prophylaxis continuation, measurement and reporting. Aids 2020; 34(12): 1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaylock JM, Hakre S, Decker CF, et al. HIV PrEP in the Military: Experience at a Tertiary Care Military Medical Center. Military medicine 2018; 183(suppl_1): 445–9. [DOI] [PubMed] [Google Scholar]
- 32.Marcus JL, Hurley LB, Hare CB, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. Jaids-Journal of Acquired Immune Deficiency Syndromes 2016; 73(5): 540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doblecki-Lewis S, Liu A, Feaster D, et al. Healthcare Access and PrEP Continuation in San Francisco and Miami After the US PrEP Demo Project. J Acquir Immune Defic Syndr 2017; 74(5): 531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan E, Ryan DT, Newcomb ME, Mustanski B. High Rate of Discontinuation May Diminish PrEP Coverage Among Young Men Who Have Sex with Men. AIDS and behavior 2018; 22(11): 3645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PRISMA 2020 checklist. Available at http://www.prismastatement.org/PRISMAStatement/Checklist.aspx. Accessed on May 25, 2021..
- 36.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Science translational medicine 2012; 4(151): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo-Mancilla JR, Zheng J-H, Rower JE, et al. Tenofovir, Emtricitabine, and Tenofovir Diphosphate in Dried Blood Spots for Determining Recent and Cumulative Drug Exposure. Aids Research and Human Retroviruses 2013; 29(2): 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koss CA, Charlebois ED, Ayieko J, et al. Uptake, engagement, and adherence to pre-exposure prophylaxis offered after population HIV testing in rural Kenya and Uganda: 72-week interim analysis of observational data from the SEARCH study. The lancet HIV 2020; 7(4): E249–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for Pre-exposure Prophylaxis to Prevent HIV-1 Acquisition in Young Adult Men Who Have Sex With Men and Transgender Women of Color Using a Urine Adherence Assay. J Acquir Immune Defic Syndr 2018; 79(2): 173–8. [DOI] [PubMed] [Google Scholar]
- 40.van Epps P, Maier M, Lund B, et al. Medication Adherence in a Nationwide Cohort of Veterans Initiating Pre-exposure Prophylaxis (PrEP) to Prevent HIV Infection. J Acquir Immune Defic Syndr 2018; 77(3): 272–8. [DOI] [PubMed] [Google Scholar]
- 41.Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. The lancet HIV 2019. [DOI] [PubMed] [Google Scholar]
- 42.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. The lancet HIV 2017; 4(9): e402–e10. [DOI] [PubMed] [Google Scholar]
- 43.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66(3): 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cottrell ML, Yang KH, Prince HM, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis 2016; 214(1): 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stranix-Chibanda L, Anderson PL, Kacanek D, et al. Tenofovir diphosphate concentrations in dried blood spots from pregnant and post-partum adolescent and young women receiving daily observed pre-exposure prophylaxis in sub-Saharan Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.UNAIDS. Global AIDS update. Seizing the Moment. Tackling entrenched inequalities to end epidemics. Available at https://aids2020.unaids.org/chapter/foreword/seizing-the-moment/.Accessed on September 28, 2021.. 2020. [Google Scholar]
- 47.Youth. United Nations. Available at https://www.un.org/en/global-issues/youth. Accessed on March 23, 2021..
- 48.Quality Assessment Tool for Quantitative Studies. Available at https://www.nccmt.ca/registry/resource/pdf/14.pdf. Accessed May 31, 2020.
- 49.Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. J Int AIDS Soc 2019; 22(10): e25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fina L, Phillips AL, Jones AT, et al. Early experience of implementing a national HIV pre-exposure prophylaxis service in Wales, United Kingdom 2017. Sex Health 2019; 16(1): 56–62. [DOI] [PubMed] [Google Scholar]
- 51.Isernia V, Phung B, Lepretre AM, et al. Pre-exposure HIV prophylaxis (PrEP) among transgender women: 3 years of follow-up in a university hospital in Paris. Sex Transm Infect 2020. [DOI] [PubMed] [Google Scholar]
- 52.Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. Aids 2018; 32(15): 2161–9. [DOI] [PubMed] [Google Scholar]
- 53.Dombrowski JC, Golden MR, Barbee LA, Khosropour CM. Patient Disengagement From an HIV Preexposure Prophylaxis Program in a Sexually Transmitted Disease Clinic. Sex Transm Dis 2018; 45(9): e62–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health 2018; 15(6): 556–61. [DOI] [PubMed] [Google Scholar]
- 55.Drak D, Barratt H, Templeton DJ, O’Connor CC, Gracey DM. Renal function and risk factors for renal disease for patients receiving HIV pre-exposure prophylaxis at an inner metropolitan health service. PLoS One 2019; 14(1): e0210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS and behavior 2018; 22(4): 1096–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heffron R, Ngure K, Odoyo J, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa. Gates open research 2017; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haberer JE, Bangsberg DR, Baeten JM, et al. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. Aids 2015; 29(11): 1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chai PR, Goodman G, Bustamante M, et al. Design and Delivery of Real-Time Adherence Data to Men Who Have Sex with Men Using Antiretroviral Pre-exposure Prophylaxis via an Ingestible Electronic Sensor. AIDS and behavior 2021; 25(6): 1661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smiley SL, Milburn NG, Nyhan K, Taggart T. A Systematic Review of Recent Methodological Approaches for Using Ecological Momentary Assessment to Examine Outcomes in US Based HIV Research. Current HIV/AIDS reports 2020; 17(4): 333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. The New England journal of medicine 2021; 385(7): 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delany-Moretlwe S HJ, Bock P, et al. Long acting injectable cabotegravir is safe and effective in preventing HIV infection in cisgender women: interim results from HPTN 084. HIV R4P virtual conference; online; Jan 27, 2021 (abstract number: LB1479). 2021. [Google Scholar]
- 63.Cambiano V, Miners A, Dunn D, et al. Cost-effectiveness of pre-exposure prophylaxis for HIV prevention in men who have sex with men in the UK: a modelling study and health economic evaluation. Lancet Infect Dis 2018; 18(1): 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayer KH, Chan PA, Patel RR, Flash CA, Krakower DS. Evolving Models and Ongoing Challenges for HIV Preexposure Prophylaxis Implementation in the United States. Jaids 2018; 77(2): 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 2012; 28(2): 138–44. [DOI] [PubMed] [Google Scholar]
- 66.Nussbaumer-Streit B, Klerings I, Dobrescu AI, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol 2020; 118: 42–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.