Abstract
Ferumoxytol (FMX) is an iron oxide nanoparticle that is FDA approved for the treatment of iron deficiency anemia. FMX contains an Fe3O4 core. Currently, the redox chemistry of Fe3O4 nanoparticles remains relatively unexplored. FMX has recently gained interest as an anti-cancer agent. Ionizing radiation (IR) is a treatment modality employed to treat several types of cancer. Utilizing electron paramagnetic resonance (EPR) spectroscopy, we found that the products produced from the radiolysis of water can oxidize the Fe3O4 core of FMX. Because of the limited diffusion of the HO2• and HO• produced, these highly oxidizing species have little direct effect on FMX oxidation. We have determined that H2O2 is the primary oxidant of FMX. In the presence of labile Fe2+, we found that reducing species generated from the radiolysis of H2O are able to reduce the Fe3+ sites of the Fe3O4 core. Importantly, we also have shown that IR stimulates the release of ferric iron from FMX. Because of its release of iron, FMX may serve as an adjuvant to enhance radiotherapy.
Keywords: ferumoxytol (FMX), magnetite, redox chemistry, Fricke dosimetry, iron oxide radiolysis, EPR spectroscopy
INTRODUCTION
Ferumoxytol (Feraheme®, FMX) is an FDA-approved therapeutic for the treatment of iron-deficiency anemia [1, 2]. FMX is a 30 nm, neutral charged superparamagnetic iron oxide nanoparticle (SPION) with an Fe3O4 core (formally, 2Fe3+,1Fe2+ oxide) encapsulated within a carboxylated polymer coating [3]. In addition to iron deficiency anemia, FMX has also been utilized as a magnetic resonance imaging (MRI) contrast agent in the imaging of glioma tumors in patients that are unable to receive gadolinium [4–6]. Recently, FMX has shown promise as an anti-cancer agent [7]. It has been shown to enhance leukemia cell killing in cells with low ferroportin expression. These observations suggest the hypothesis that FMX-induced cell death may potentially be an iron- and reactive oxygen species (ROS)-dependent process. Thus, the Fe3O4 core of FMX may be redox-active, thereby providing the iron needed for detrimental oxidations.
Ionizing radiation (IR) is a common cancer therapeutic used as a treatment modality in a variety of cancer types. Recent literature suggests that SPIONs may function as radiosensitizers, by increasing DNA damage via enhanced production of ROS [8]. IR readily leads to the oxidation of Fe2+ to Fe3+; because iron can enhance cellular free radical oxidation reactions [9, 10], approaches that increase redox active iron in cancer cells may increase radiosensitization.
The oxidation of ferrous iron following the radiolysis of H2O (Fricke dosimetry), is a widely accepted dosimetric technique that has been utilized since 1927 [11]. Fricke dosimetry allows for IR dose estimation to water (Dw) by evaluating changes in optical density (OD) associated with the oxidation of ferrous (Fe2+) to ferric (Fe3+) iron [12, 13]. For a given Dw, the radiolytic yield of Fe3+ (G (Fe3+)) can be approximated by measuring changes in OD (alias absorbance) (equation [1]) [12, 14]):
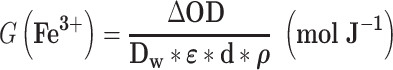 |
(1) |
where (G (Fe3+) is defined as the number of Fe3+ ions produced per 100 eV of energy deposition, ε is the molar extinction coefficient (here the extinction coefficient would be that of Fe3+ at 303 nm minus the extinction coefficient of Fe2+ at 303 nm, 2174 M−1 cm−1 [11]), d is the absorption pathlength and ρ is the density of the solution (taken as 1.00 g cm−3).
A major focus of this project was to develop a reliable method to detect FMX and determine its concentration in water-based solutions. We hypothesized that electron paramagnetic resonance (EPR) spectroscopy would be an ideal approach because there are two low-spin (S = 1/2) Fe3+ in each Fe3O4 with minimal contribution from the low-spin Fe2+ (S = 0) allowing for oxidation state specificity. In addition, EPR would provide a useful tool to evaluate levels of FMX in complex environments such as cell culture media, blood, or tissue. Our goal is to apply these principles to understand the radiation chemistry of Fe3O4 nanoparticles to evaluate any chemical changes upon exposure to IR.
MATERIALS AND METHODS
Chemical preparations
FMX (Feraheme®) was diluted to the appropriate concentration in 18 MΩ H2O. For pH dependency experiments, 50 μM FMX in 18 MΩ H2O was titrated to the appropriate pH with either 1 M HCl or 1 M NaOH. For mechanistic experiments, FMX was diluted to 50 μM in 18 MΩ H2O supplemented with either 50 mM Na-pyruvate, D-mannitol, or ferrous ammonium sulfate (FAS). Na-pyruvate (ThermoFisher Scientific; 11 360 070), D-mannitol, and FAS were diluted to 50 mM from a 100 mM stock. Samples were irradiated with a 60Co source.
EPR spectroscopic evaluation of FMX
FMX concentrations were determined by measuring the peak-to-peak signal intensity of the EPR spectra of the low-spin Fe3O4 complex at g = 2 relative to a standard curve. The following scan parameters were used: center field = 3508.97 G, sweep rate = 2000 G/42 s, time constant = 327.68 ms, frequency = 9.85 GHz, power attenuation = 18 dB, modulation frequency = 100 kHz, modulation amplitude = 0.7 G, with spectra being generated from a signal average of 2 scans. The Fe3+ concentration of FMX was calculated based on a 2:1 stoichiometry of 2Fe3+:Fe2+ contained within a magnetite crystal and a FMX molecular weight of 731 kDa [15]. Samples were examined by EPR within 10 min of irradiation.
Detection of Fe release
Detection of Fe-release from FMX was accomplished by diluting the appropriate FMX samples into a ferrozine buffer (5 mM ferrozine diluted in double-distilled H2O) ± 5 mM ascorbate. The formation of the Fe2+-ferrozine complex, absorption at 562 nm (ε562 = 27.9 mM−1 cm−1) [16], was evaluated using a Beckman DU800 UV–Vis spectrometer. Ferrozine buffer containing 5 mM ascorbate was used to reduce all the chelatable iron to Fe2+ ([Fe]total). The amount of Fe3+ released was calculated as the difference between [Fe]total (ferrozine +5 mM ascorbate) and [Fe2+] (ferrozine alone) (equation [2]):
 |
(2) |
RESULTS AND DISCUSSION
FMX is readily detected using EPR spectroscopy
The goal of this project was to develop a reliable method to detect FMX and determine its concentration in water-based solutions. We hypothesized that EPR spectroscopy would be an ideal approach because there are two low-spin (S = 1/2) Fe3+ in each Fe3O4 with minimal contribution from the low-spin Fe2+ (S = 0) allowing for oxidation state specificity. In addition, EPR would provide a useful tool to evaluate levels of FMX in complex environments such as cell culture medium, blood and tissue. Using EPR, we detected an Fe3O4 concentration-dependent-signal at g ≈ 2 (detected at ≈ 3500 G) with a second absorption at g ≈ 2.3 (detected at ≈3100 G) (Fig. 1A) [17]. This suggests detection of the low-spin (S = 1/2) Fe3+ contained within the octahedral sublattice (g = 2) along with the tetrahedral lattice (g‖ = 2, g⊥ = 2.3) of the magnetite structure (Fig. 1B) [18]. We quantified the peak-to-peak intensity of the signal and verified that it had a direct linear dependence on concentration (Fig. 1C). Only Fe3+ would contribute to this signal because of the low-spin nature of the crystal structure; low-spin Fe2+ (S = 0) is EPR silent. Because Fe3O4 has a 2:1 Fe3+:Fe2+ stoichiometry, we could approximate the Fe3+ content and its linear proportionality with the g ≈ 2 peak-to-peak signal intensity (Fig. 1D). The peak at g ≈ 2 has contributions from both the tetrahedral lattice and octahedral sublattice, thus acting as a more robust marker of the total Fe3+ content [18]. Therefore, the EPR spectroscopic method should provide an accurate measure of the Fe3+ content in FMX.
Fig. 1.
FMX is readily detected with EPR spectroscopy in room temperature aqueous solution. (A) Increasing concentrations of FMX in 18 MΩ H2O, pH ≈ 7 were examined using an X-band Bruker EMX spectrometer. (B) Fe3O4 structure with low-spin, octahedral Fe2+and Fe3+ and a central, tetrahedral Fe3+. (C) FMX concentration-dependence was determined by measuring the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G). (D) [Fe3+] concentration dependence was performed by approximating the FMX Fe3+ content and comparing to the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G).
FMX undergoes IR-induced oxidation
Traditional Fricke dosimetry is performed by detecting changes in OD following the oxidation of Fe2+ to Fe3+ by the products of the radiolysis of water [13]. However, because the EPR signal intensity at g ≈ 2 is linearly proportional to both FMX and the content of Fe3+ in FMX, EPR signal intensity can also be used. We hypothesized that FMX can undergo Fricke-type chemical reactions leading to the oxidation of its Fe2+ sites and these changes could be detected by EPR. The approximate radiolytic yield (G(Fe3+)) would be given by equation (3):
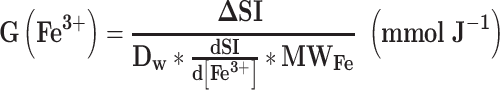 |
(3) |
where ΔSI is the change in EPR signal intensity at g ≈ 2, Dw is the dose of IR to water, dSI/d[Fe3+] is the change in signal intensity per μM Fe3+ = 77.8 A.U. per μM Fe3+ (Fig. 1C), and MWFe is the molecular weight of Fe (55.84 g mol−1). (Note: 77.8 A.U. per μM Fe3+ is specific to the physical setup and instrument settings of these specific experiments.)
To determine if Fe3O4 could undergo ionization following the radiolysis of water, 50 μM FMX in double-distilled H2O was irradiated with increasing doses (Dw). We observed an increase in EPR signal intensity with increasing doses of IR (Fig. 2A). This suggests that IR may lead to the oxidation of the Fe2+ sites within the cluster; the increase in EPR signal intensity is consistent with equation (3) for doses <10 Gy. Thus, FMX oxidation is also consistent with traditional Fricke dosimetric measures of FeSO4 at clinically relevant doses that show a linear proportionality between OD changes and Dw [13].
Fig. 2.

FMX undergoes IR-induced oxidation. (A) 50 nM FMX in 18 MΩ H2O was irradiated at increasing doses (0–10 Gy) using a 60Co source at 0.6 Gy min−1. EPR spectroscopic evaluation of the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G) was done within 10 min of sample irradiation. (B) Temporal dependence of IR-induced oxidation of FMX. 50 nM FMX in 18 MΩ H2O was irradiated with 10 Gy (0.6 Gy min−1) using a 60Co source. EPR spectroscopic evaluation of the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G) was done within 10 min of sample irradiation and then every 20 min up to 100 min following irradiation.
To determine if there was any long-term temporal dependence associated with FMX oxidation following radiation, G(Fe3+) was calculated at multiple time points (Fig. 2B). We found that following the initial radiolytic oxidation of FMX, there was a steady decline in radiolytic yield overtime. After 60 min, G(Fe3+) becomes negative and then remains stable for up to 100 min. This suggests that the initial oxidation event stimulates the decomposition of FMX that continues until all the oxidized surface charges have been removed, leaving behind a slightly smaller Fe3O4 core.
Next, we determined if the oxidation of Fe3O4 was dependent on the dose-rate of the IR. We found that FMX oxidation reaches a maximum at 0.6 Gy min−1 (Fig. 3). This is consistent with a dose rate-dependent suppression of G(Fe3+) at a dose rates <100 Gy s−1 with a monoenergetic beam [19]. O’Leary et al. proposed that this effect is the result of recombination of free radicals following the radiolysis of water at high dose rates. Our data support this notion, but it may be further compounded due to the diffusion limitations of the crystal core, as Fe3O4 oxidation is limited by the rate of diffusion of O2 into the core [20]. Thus, lower IR dose rates likely enhance Fe3O4 oxidation by providing a steady flow of oxidation reactions over a longer period of time, thereby increasing the probability of diffusion of O2 into the Fe3O4 core and lowering the probability of recombination events.
Fig. 3.

FMX oxidation is dose rate dependent. 50 nM FMX in 18 MΩ H2O was irradiated at increasing dose rates (10 Gy) using a 60Co and altering the source to sample distance. EPR spectroscopic evaluation of the peak-to-peak signal intensity at g ≈ 2 (3500 G) was done within 10 min of sample irradiation.
FMX undergoes non-traditional Fe radiochemistry
We then determined the main drivers of FMX oxidation by the radiolysis of water. Classically, the radiolytic yield of Fe3+ in the Fricke system upon the radiolysis of water is described by (equation [4]) [21]:
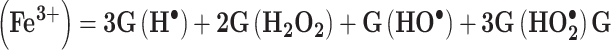 |
(4) |
The radiolysis of H2O, under our experimental system results in many different reactive species, including e−aq, H•, HO•, O2•- and its conjugate acid HO2•, as well as H2O2 and other products in small yields.
The deposition of energy into water can result in homolytic bond cleavage, yielding H• and HO•:
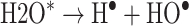 |
(5) |
Or this energy can ionize water:
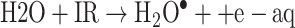 |
(6) |
then:
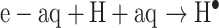 |
(7) |
and:
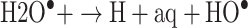 |
(8) |
Both e−aq and H• wiil rapidly react with O2 to form superoxide or its conjugate acid, the hydroperoxyl radical:
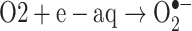 |
(9) |
 |
(10) |
HO2•can then efficiently oxidize Fe2+ (equation [11]):
 |
(11) |
Because the fraction of HO2• present of the O2•-/HO2• dyad is pH-dependent, we examined whether the oxidation of FMX is also pH-dependent. We found that at low pH there is a decrease in FMX signal intensity indicative of a decrease in Fe3+ (Fig. 4A). This is consistent with the low-temperature reduction of Fe3O4 under acidic conditions (< 500°C) [22, 23]. We found that maximal FMX oxidation occurred following 10 Gy IR at pH = 5; oxidation was reduced at both lower and higher pH ranges (Fig. 4B). This suggests that HO2• may not play a critical role in FMX radiochemistry because the HO2• population increases under more acidic conditions (pKa = 4.8) and typically functions as an oxidizing species to increase G(Fe3+) (equation [4]) [24, 25].
Fig. 4.

The effects of the radiolysis of water on FMX is pH dependent. (A) 50 nM FMX in 18 MΩ H2O with variable pH (titrated with either 1 M HCl or 1 M NaOH) and then was irradiated with 10 Gy using a 60Co source at 0.6 Gy min−1. (B) EPR spectroscopic evaluation of the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G) was done within 10 min of sample irradiation to determine the radiolytic yield, G(Fe3+). Results of triplicate measures ± SD.
Next, we determined whether H2O2 or HO• has a greater effect on the oxidation of FMX by IR. Ionization of H2O by radiation leads to H2O2 via the hydroperoxyl radical (equation [11]). The generation of H2O2 can oxidize two Fe2+ ions via Fenton chemistry. This first oxidation occurs via Fenton chemistry directly (equation [12]):
 |
(12) |
The second oxidation occurs indirectly, i.e. by the HO• produced from Fenton chemistry (equation [13]):
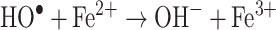 |
(13) |
The same oxidation of Fe2+ by HO• may occur directly from IR as HO• is one of the oxidants produced by the radiolysis of H2O (equations [5] and [8]). To examine this possibility, FMX was placed in H2O supplemented with either 50 mM pyruvate, to act as an Fe-independent H2O2 scavenger, or 50 mM mannitol, to scavenge HO• [26–28]. That the addition of pyruvate lowered the apparent value of G(Fe3+) to essentially 0, following 10 Gy IR; that is, there is no change in signal intensity when comparing irradiated and unirradiated samples, ΔSI of equation (3) is essentially 0. Mannitol decreased G(Fe3+) by approximately 30%, from 31 to 22 mmol J−1 (Fig. 5). Because HO• is highly reactive, the decrease in radiolytic yield provided by mannitol is likely the result of its reaction with HO•, thereby preventing site-specific reactions within the Fe3O4 core.
Fig. 5.

Oxidation of FMX is primarily dependent on H2O2. Control: Irradiation of 50 nM FMX in18 MΩ H2O with 10 Gy using a 60Co source at 0.6 Gy min−1 yields G(Fe3+) = 31 mol J−1 (using equation [3]). When this solution of FMX contains with 50 mM Na-pyruvate the apparent G(Fe3+) is essentially 0. 50 mM D-mannitol decreased the apparent G(Fe3+) by about 30% of control. EPR spectroscopic evaluation of the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G) was done within 10 min of sample irradiation. Results of triplicate measures ± SD.
Lastly, we aimed to evaluate the impact of reducing species (e.g. e−aq and O2●-) produced following the radiolysis of H2O in our system. These species should be considered for the redox chemistry associated with mixed iron oxides such as Fe3O4 because reductants may affect the Fe3+ sites. While the radiolysis of Fe typically considers the oxidation of Fe2+ (equation [4]), the reduction of Fe3+ may be relevant chemically given the 2:1 Fe3+:Fe2+ stoichiometry of Fe3O4. To evaluate the impact of these reducing species produced, we irradiated FMX with 10 Gy in H2O containing 50 mM Fe2+ (FAS). Thus, we can leverage the rapid oxidation of Fe2+ to absorb HO●, H2O2, H● and HO2● allowing reduction chemistry to occur. We found that the addition of 50 mM Fe2+ to the H2O resulted in a 134% decrease in radiolytic yield from 43 mol J−1 to −15 mol J−1 (Fig. 6). The generation of a negative G(Fe3+) by the addition of labile Fe2+ is indicative of a site-specific reduction of the Fe3+ sites by reducing species produced from the radiolysis of H2O such as e−aq and O2●-. These results are unsurprising as the Fe3+-OOH core of ferritin has been shown to be labilized by IR using a pulsed-radiolysis approach that was attributed to the e−aq produced from the radiolysis of H2O [29]. Additionally, this may illustrate the potential for site-specific reactions with oxygen inside the crystal core by H● further enabling Fe reduction chemistry to occur. Therefore, in the presence of labile or freely chelatable Fe2+ (as is seen in living systems) the reduction of the Fe3+ sites of FMX by radiolytically produced species such as e−aq and O2●- may become increasingly relevant.
Fig. 6.

FMX can be reduced by IR in the presence of labile Fe2+. Control: Irradiation of 50 nM FMX in18 MΩ H2O with 10 Gy using a 60Co source at 0.6 Gy min−1 yields G(Fe3+) = 43 mol J−1 (using equation [3]). When this solution of FMX contains with 50 mM Fe2+ (FAS) the apparent G(Fe3+) is reduced to −15 mmol J−. EPR spectroscopic evaluation of the peak-to-peak signal intensity at g ≈ 2 (≈ 3500 G) was done within 10 min of sample irradiation. Results of triplicate measures ± SD.
A key question remains: Does IR enhance Fe release from the Fe3O4 core into the supporting solvent? To address this, solutions containing 50 μM FMX in distilled water were irradiated with 10 Gy IR and then analyses for iron were employed as presented in Methods. Following IR there was a significant increase in total Fe released from FMX of 218.6 ± 54.2 nM (14.7 ± 5.4%; P < 0.05) (Fig. 7). As we previously detected the oxidation of the Fe2+ sites contained with FMX, we hypothesized that these atoms would be those likely released. Consistent with this hypothesis, we found that there was a 301.1 ± 45.1 nM (31.6 ± 19.5%) increase (P < 0.05) in Fe3+ released from the core. This result was accompanied by a 82.4 ± 10.6 nM (22.8 ± 1.4%) decrease (P < 0.05) in Fe2+ being released. These findings suggest that IR enhances the release of Fe from FMX by oxidizing the Fe2+ sites. Taken together, these data suggest that redox reactions associated with the radiolysis of H2O can enhance the release of Fe from the FMX core.
Fig. 7.

IR liberates Fe3+ from the FMX core. 50 nM FMX in 18 MΩ H2O was irradiated with 10 Gy using a 60Co source at 0.6 Gy min−1. Free Fe2+ was evaluated by diluting samples in 5 mM ferrozine buffer and evaluating the absorbance at 562 nm (ε562 = 27.9 mM−1 cm−1). Total Fe was done by diluting samples in 5 mM ferrozine with 5 mM ascorbate. Free Fe3+ was calculated as the difference between [Fe]total and [Fe2+]. Results of triplicate measures ± SD. *P < 0.05 using a paired, two-tailed Student’s T-test.
CONCLUSION
In this study, we have made the following observations regarding FMX radiochemistry:
EPR spectroscopy is a useful tool for evaluating FMX concentrations and Fe3O4 redox chemistry;
IR can lead to the oxidation of FMX;
FMX undergoes non-traditional Fe-radiochemistry as H2O2 appears to be the primary oxidant due to its ability to diffuse into the crystal core;
HO2• and HO• likely only contribute site-specific oxidations because their chemistries are diffusion-rate limited;
In the presence of labile Fe2+ (as seen in living systems), radiolytically produced species such as e−aq and O2●- can reduce the Fe3+ sites of FMX and should not be ignored;
IR can stimulate the release of Fe3+ from the FMX core.
While significant pre-clinical work remains, these data suggest the possibility of FMX as a clinically relevant, redox active Fe reserve to enhance radiotherapy.
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not represent the views of the National Institutes of Health. The ESR Facility at The University of Iowa as well as the Department of Radiation Oncology provided invaluable support.
Contributor Information
Michael S Petronek, Department of Radiation Oncology, Free Radical and Radiation Biology, The University of Iowa, Iowa City, IA 52242-1181, USA.
Douglas R Spitz, Department of Radiation Oncology, Free Radical and Radiation Biology, The University of Iowa, Iowa City, IA 52242-1181, USA.
Garry R Buettner, Department of Radiation Oncology, Free Radical and Radiation Biology, The University of Iowa, Iowa City, IA 52242-1181, USA.
Bryan G Allen, Department of Radiation Oncology, Free Radical and Radiation Biology, The University of Iowa, Iowa City, IA 52242-1181, USA.
FUNDING
This work was funded by NIH grants T32 CA078586, P01 CA217797, P01 CA244091, R01 CA169046, R21 CA256301 and the Gateway for Cancer Research grant G-7-1500. Core facilities were supported in part by the Carver College of Medicine and the Holden Comprehensive Cancer Center, NIH P30 CA086862.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1. Rosner MH, Auerbach M. Ferumoxytol for the treatment of iron deficiency. Expert Rev Hematol 2011;4:399–406. [DOI] [PubMed] [Google Scholar]
- 2. Auerbach M, Chertow GM, Rosner M. Ferumoxytol for the treatment of iron deficiency anemia. Expert Rev Hematol 2018;11:829–34. [DOI] [PubMed] [Google Scholar]
- 3. Bullivant JP, Zhao S, Willenberg BJ et al. Materials characterization of Feraheme/ferumoxytol and preliminary evaluation of its potential for magnetic fluid hyperthermia. Int J Mol Sci 2013;14:17501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neuwelt EA, Várallyay CG, Manninger S et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery 2007;60:601–11. [DOI] [PubMed] [Google Scholar]
- 5. Gahramanov S, Muldoon LL, Varallyay CG et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology 2013;266:842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dadfar SM, Roemhild K, Drude NI et al. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev 2019;1:302–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trujillo-Alonso V, Pratt EC, Zong H et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol 2019;14:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fathy M, Fahmy H, Saad O et al. Silica-coated iron oxide nanoparticles as a novel nano-radiosensitizer for electron therapy. Life Sci 2019;234:116756. [DOI] [PubMed] [Google Scholar]
- 9. Buettner G, Anne JB. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res 1996;145:532–41. [PubMed] [Google Scholar]
- 10. Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: an electron paramagnetic resonance spin trapping study. Free Radic Biol Med 1999;26:1447–56. [DOI] [PubMed] [Google Scholar]
- 11. Fricke H, Morse S. The chemical action of roentgen rays on dilute ferrosulphate solutions as a measure of dose. Am J Roentgenol Radium Therapy Nucl Med 1927;18:430–2. [Google Scholar]
- 12. deAlmeida CE, Ochoa R, Lima MC de et al. A feasibility study of Fricke dosimetry as an absorbed dose to water standard for 192Ir HDR sources. PLoS One 2014;9:e115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klassen NV, Shortt KR, Seuntjens J et al. Fricke dosimetry: the difference betweenG(Fe3+) for60Co gamma-rays and high-energy x-rays. Phys Med Biol 1999;44:1609–24. [DOI] [PubMed] [Google Scholar]
- 14. Schreiner LJ. Review of Fricke gel dosimeters. J Phys Conf Ser 2004;1:9–21. [Google Scholar]
- 15. Balakrishnan VS, Rao M, Kausz AT et al. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Investig 2009;39:489–96. [DOI] [PubMed] [Google Scholar]
- 16. Stookey L. Ferrozine-a new spectrophotometric reagent for iron. Anal Chem 1970;42:779–81. [Google Scholar]
- 17. Tian S, Jiang J, Zang S et al. Determination of IgG by electron spin resonance spectroscopy using Fe3O4 nanoparticles as probe. Microchem J 2018;141:444–50. [Google Scholar]
- 18. Chokkareddy R, Kumar BN, Kabane B et al. Bio-Sensing Performance of Magnetite Nanocomposite for Biomedical Applications. In: Sabela and Chaudhery Mustansar H. (eds.). Nanomaterials: Biomedical, Environmental, and Engineering Applications. Scrivener Publishing LLC, Hoboken, NJ, 2018, 165–196. [Google Scholar]
- 19. O’Leary M, Boscolo D, Breslin N et al. Observation of dose-rate dependence in a Fricke dosimeter irradiated at low dose rates with monoenergetic X-rays. Sci Rep 2018;8:4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Z, Chanéac C, Berger G et al. Mechanism and kinetics of magnetite oxidation under hydrothermal conditions. RSC Adv 2019;9:33633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meesat R, Sanguanmith S, Meesungnoen J et al. Utilization of the ferrous sulfate (Fricke) dosimeter for evaluating the Radioprotective potential of Cystamine: experiment and Monte Carlo simulation. Radiat Res 2012;177:813–26. [DOI] [PubMed] [Google Scholar]
- 22. Teplov OA. Kinetics of the low-temperature hydrogen reduction of magnetite concentrates. Russian Metallurgy (Metally) 2012;2012:8–21. [Google Scholar]
- 23. Hansel CM, Benner SG, Fendorf S. Competing Fe(II)-induced mineralization pathways of Ferrihydrite. Environ Sci Technol 2005;39:7147–53. [DOI] [PubMed] [Google Scholar]
- 24. Kozmér Z, Arany E, Alapi T et al. Determination of the rate constant of hydroperoxyl radical reaction with phenol. Radiat Phys Chem 2014;102:135–8. [Google Scholar]
- 25. Kwon BG. An advanced kinetic method for HO2∙/O2-∙ determination by using terephthalate in the aqueous solution. Environ Eng Res 2012;17:205. [Google Scholar]
- 26. Goldstein S, Czapski G. Mannitol as an OH· scavenger in aqueous solutions and in biological systems. Int J Radiat Biol Relat Stud Phys Chem Med 1984;46:725–9. [DOI] [PubMed] [Google Scholar]
- 27. Desesso JM, Scialli AR, Goeringer GC. D-mannitol, a specific hydroxyl free radical scavenger, reduces the developmental toxicity of hydroxyurea in rabbits. Teratology 1994;49:248–59. [DOI] [PubMed] [Google Scholar]
- 28. Guarino VA, Oldham WM, Loscalzo J et al. Reaction rate of pyruvate and hydrogen peroxide: assessing antioxidant capacity of pyruvate under biological conditions. Sci Rep 2019;9:19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolszczak M, Gajda J. Iron release from ferritin induced by light and ionizing radiation. Res Chem Intermed 2010;36(5):549–63. [Google Scholar]



