Abstract
This study is an international multi-institutional retrospective study comparing the clinical outcomes between intracavitary brachytherapy (ICBT) and the hybrid of intracavitary and interstitial brachytherapy (HBT) for locally advanced cervical cancer patients treated with definitive radiation therapy. Locally advanced cervical cancer, the initial size of which is larger than 4 cm and treated by concurrent chemoradiotherapy and image-guided adaptive brachytherapy, were eligible for this retrospective study. Patients who received HBT at least once were included in the HBT group, and patients who received only ICBT were included in the ICBT group. Anonymized data from 469 patients from 13 institutions in Japan, one from Korea and one from Thailand, were analyzed. Two hundred eighty and 189 patients were included in the ICBT group and the HBT group, respectively. Patients in the HBT group had more advanced stage, non-Scc histopathology, a higher rate of uterine body involvement, larger tumor at diagnosis, larger tumor before brachytherapy and a lower tumor reduction ratio. With a median follow-up of 51.3 months (2.1–139.9 months), 4-y local control (LC), progression-free survival (PFS) and overall survival (OS) for the entire patient population were 88.2%, 64.2% and 83%, respectively. The HBT group received a higher HR-CTV D90 than that of the ICBT group (68.8 Gy vs 65.6 Gy, P = 0.001). In multivariate analysis, the non-Scc histological subtype, HR-CTV D95 ≤ 60 Gy, reduction ratio ≤ 29% and total treatment time (TTT) ≥ 9 weeks were identified as the independent adverse prognostic factors for LC. Regarding LC, no difference was found between ICBT and HBT (4-y LC 89.3% vs 86.8%, P = 0.314). After adjustment for confounding factors by propensity score matching, no advantage of applying HBT was demonstrated regarding LC, PFS, or OS. Despite the fact that HBT patients had more adverse clinical factors than ICBT patients, HBT delivered a higher dose to HR-CTV and resulted in comparable LC.
Keywords: uterine cervical cancer, hybrid of intracavitary and interstitial brachytherapy (HBT), combined intracavitary/interstitial brachytherapy, image-guided adaptive brachytherapy
INTRODUCTION
The definitive radiotherapy for cervical cancer consists of pelvic external beam radiation therapy (EBRT) covering the primary tumor and regional drainage lymphatic area followed by intracavitary brachytherapy (ICBT) [1, 2]. ICBT involves inserting two ovoids or ring applicators into the vagina, and a tandem applicator is inserted into the uterine canal, and a high dose is delivered directly to the primary tumor located in the uterine cervix. After magnetic resonance imaging (MRI) or computed tomography (CT) images with the brachytherapy applicators in place became available for brachytherapy dose calculation, studies on the relationship between delivered dose and local control (LC) were published, and it was discovered that a favorable clinical outcome is expected when the tumor responds well to the preceding EBRT and the tumor is confined within the proximity of brachytherapy applicators. On the other hand, if a residual tumor still exists that is far from the brachytherapy applicators after EBRT, the possibility of developing local recurrences is high [3]. To address this issue, the concept of 3D image-guided adaptive brachytherapy (3D-IGABT) was advocated in Europe [1, 4] and the United States [5, 6], and favorable clinical outcomes were reported [3, 7, 8]. A French group conducted a non-randomized prospective clinical trial comparing 2D ICBT (2D-ICBT) and 3D-IGABT, demonstrating that 3D-IGABT was superior in terms of toxicity and efficacy [9]. As a result, 3D-IGABT is considered the standard brachytherapy technique in definitive radiation therapy for locally advanced cervical cancer.
It is widely accepted that multi-catheter interstitial brachytherapy (ISBT) can effectively treat large tumors [10, 11]. However, only a few institutions can perform ISBT because it requires expertise and human resources, patients must be in bed overnight with the needles in place for ISBT multiple sessions until all brachytherapy treatment is completed. Following that, the hybrid of intracavitary/interstitial brachytherapy (HBT) was introduced, with promising clinical results [12–14]. Along with intracavitary applicators, a few extra interstitial needles are inserted into the involved parametrium during HBT. Because this method is much easier to perform than multi-catheter ISBT, it is anticipated that many institutions will begin to perform HBT. Yoshida et al. [15] conducted a simulation analysis and found that tumors smaller than 4 × 3 × 3 cm can be treated with ICBT, while tumors larger than 5 × 4 × 4 cm can be treated by multi-catheter ISBT, and tumors in between can be treated by HBT. However, currently, there exist no guidelines indicating which tumor should be treated with which types of brachytherapy.
There has been no prospective clinical trial comparing ICBT and HBT to investigate the superiority of HBT over conventional ICBT since the concept of HBT in the field of gynecological brachytherapy was introduced in the field of gynecological brachytherapy. As a result, it is unclear whether a more invasive procedure such as HBT is truly beneficial for patients with locally advanced uterine cervical cancer when compared to conventional ICBT.
Our study group originally intended to conduct a randomized clinical trial that directly compared clinical outcomes between ICBT and HBT. However, because the superiority of HBT over ICBT is evident due to the dose coverage superiority of HBT over ICBT, approximately 30% of participants thought it was unethical to perform such a clinical trial. As a result, a multi-institutional retrospective study comparing clinical outcomes of patients treated with ICBT and HBT was planned to determine whether or not there is a clinical improvement after the introduction of HBT. If clinical equipoise between ICBT and HBT is observed, it is ethically permissible to conduct a phase III clinical trial comparing clinical results of ICBT and HBT. If this is not the case, a validating single-arm clinical trial should be performed. The aim of this multi-institutional retrospective study is to compare the clinical outcomes of ICBT and HBT for locally advanced uterine cervical cancer patients treated with definitive chemoradiotherapy.
MATERIALS AND METHODS
Inclusion criteria for this study were as follows: previously untreated uterine cervical cancer patients who were 20 years old or older and were treated with definitive chemoradiotherapy between 2000 and 2016 with a follow-up period longer than 2 years were included in this retrospective study — patients who died within 2 years after treatment were also included. Before treatment, the initial maximum tumor size should be greater than 4 cm as measured by MRI. MRI should also be used to determine tumor size prior to brachytherapy. These two MRIs taken prior to chemoradiotherapy and brachytherapy were used to calculate the tumor reduction ratio. Brachytherapy dose calculation should be based either on CT or MRI (3D-IGABT). The following were the study’s exclusion criteria: (i) patients who had induction chemotherapy, hysterectomy, or history of pelvic irradiation, (ii) patients who had treatment for invasive cancer other than uterine cervical cancer within the previous 5 years, (iii) histopathology other than squamous cell carcinoma (Scc), adenocarcinoma, or adenosquamous cell carcinoma, (iv) patients with extra-pelvic disease extension except for 1–3 para-aortic lymph node(s) (PALN) less than 2 cm in size; the patients with 4 PALNs and/or larger than 2 cm PALN, and (v) patients who received only palliative doses less than 40 Gy or brachytherapy less than twice.
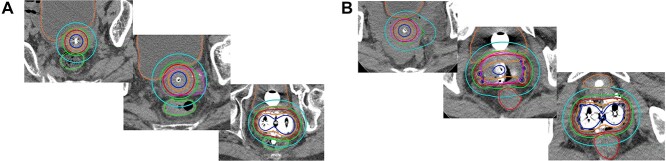
The HBT group included patients who had received HBT at least once. For example, a patient who received HBT twice and experienced favorable tumor shrinkage and subsequently received ICBT twice was included in the HBT group. Figure 1 depicts an example of a comparison of dose distributions in T3b patients treated with ICBT and HBT.
Fig. 1.
Shows an example of a comparison of dose distributions in T3b patients treated with ICBT (A) and HBT (B). The solid red line represents 100% isodose line (6 Gy), the dark blue line, 200% isodose line (12 Gy), the orange line, 150% (9 Gy), the green line, 80% (4.8 Gy), the sky blue line, 50% (3 Gy) and the pink line represents the HR-CTV, respectively. In Fig. 1A, even though the dwell time of the left ovoid was set longer than the dwell time of the right ovoid in order to cover as much of the left side parametrial extension as possible, the 100% isodose line could not adequately cover a large portion of the left parametrial extension. On the other hand, in Fig. 1B, since interstitial needles were inserted to cover bilateral parametrial extension, HR-CTV was well covered by the 100% isodose line.
The following clinical data were collected: patient’s age, the International Federation of Gynecology and Obstetrics (FIGO) stage (2008), histological subtypes, the presence of uterine body involvement, pyometra, parametrial invasion, or hydronephrosis, largest tumor size at diagnosis and before brachytherapy both assessed by MRI (cm), total treatment time (TTT) (week), dose of whole pelvis (WP) irradiation (Gy), dose of pelvic irradiation with a central shield (CS), dose of lymph node boost, systemic chemotherapy regimen, acute toxicities and late toxicities assessed by the Common Terminology Criteria for Adverse Events v4.0, and brachytherapy dosimetric parameters (high-risk clinical target volume [HR-CTV]; D90, D95, rectum D2cc and bladder D2cc). While the majority of institutions participating in this study used 3D conformal radiation therapy with CS in the latter part of pelvic irradiation, some institutions use intensity modulated radiation therapy (IMRT). In IMRT, CTV included gross tumor volume, the uterine body, uterine cervix, parametrium and upper part of vagina. A planning target volume was created, adding adequate margins to CTV to compensate for organ motion, and no CS-like IMRT plan was applied. Therefore, the CTV dose was used as the central pelvic dose in this study. In this study, late adverse events of greater than grade 1 were counted as events. Because the dose contribution from CS to the primary site is difficult to assess [16], dose contribution only from WP was used in calculating the total dose of EBRT and brachytherapy and expressed in the form of the equivalent dose in 2 Gy fractions (EQD2) according to the linear-quadratic model [17].
The primary endpoint was the LC rate. The progression of the primary site was considered a local failure, and the LC rate was calculated from the start of the definitive radiation therapy to the date of local failure confirmation. Primary sites include the uterine cervix, uterine body, parametrium, or vagina in patients with initial vaginal invasion. Secondary endpoints included overall survival (OS), progression-free survival (PFS), and the late complication rate for the rectum, the bladder and the vagina. The Kaplan–Meier method was used to calculate survival curves. Univariate and multivariate analysis were used to investigate factors that influenced the recurrence or development of complications. Statistical significance was defined as a P-value of <0.05. Multivariate analysis using the Cox regression analysis was performed on factors with a P-value of <0.05. The hazard ratios were estimated using Cox proportional-hazards models. Baseline characteristics that were imbalanced between the two groups and had an influence on prognosis were used to perform 1:1 propensity score matching. Statistical analyses were conducted using IBM SPSS Statistics version 25.0 (SPSS, Inc., Chicago, IL) and EZR (XXX Medical Center, YYY University) [18], that is a freely available modified version of R (The R Foundation for Statistical Computing, Vienna, Austria) commander designed to add statistical functions frequently used in biostatistics.
RESULTS
Anonymized data sets from 498 patients were collected from 13 institutions in Japan, including those involved in the Working Group of the Gynecological Tumor Committee of the Japanese Radiation Oncology Study Group (JROSG), one from Korea and one from Thailand. Only one institution, which provided 10 patients’ data for this study, calculates brachytherapy doses based on MRI. The exclusion criteria resulted in the exclusion of 29 patients, leaving 469 patients in the analysis (Fig. 2). Table 1 shows patient characteristics. Two hundred eighty and 189 patients were included in the ICBT group and the HBT group, respectively. The ICBT group had a longer follow-up period than that of the HBT group, with statistical significance (60 months vs 45.5 months, P < 0.001). Patients in the HBT group had a more advanced stage, non-Scc histopathology, a higher rate of uterine body involvement, a larger tumor at diagnosis, a larger tumor before brachytherapy, and a lower tumor reduction ratio, implying that patients in the HBT group had poorer prognostic factors than patients in the ICBT group.
Fig. 2.
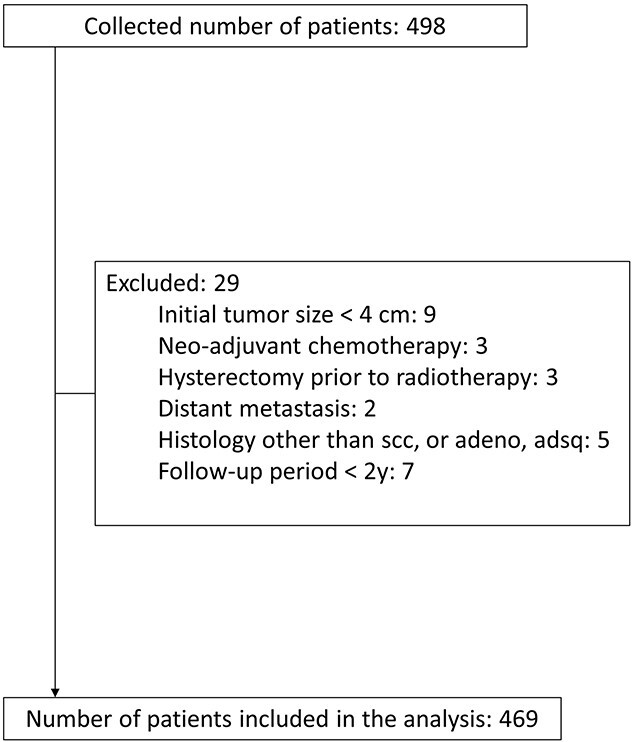
Shows the CONSORT flow diagram of the study.
Table 1.
Patient characteristics
| ICBT (n = 280) | HBT (n = 189) | P-value | ||
|---|---|---|---|---|
| Age (median), years | 55 (26-86) | 58 (26-81) | 0.237 | |
| Follow-up period (median), months | 55.3 (2.1-139.9) | 44.6 (3.0-105.3) | <0.001* | |
| FIGO stage (2008) | ||||
| IB2-II | 189 (67.5%) | 75 (39.7%) | <0.001* | |
| III-IVA | 91 (32.5%) | 114 (60.3%) | ||
| Histological subtypes | ||||
| Squamous cell carcinoma | 268 (95.7%) | 170 (89.9%) | 0.005* | |
| Adenocarcinoma | 7 (2.5%) | 17 (9%) | ||
| Adenosquamous carcinoma | 5 (1.8%) | 2 (1.1%) | ||
| Uterine body invasion | ||||
| Yes | 90 (32.1%) | 92 (48.7%) | <0.001* | |
| No | 190 (67.9%) | 96 (50.8%) | ||
| N/A | 0 (0%) | 1 (0.5%) | ||
| Pyometra | 0.140 | |||
| Yes | 77 (27.5%) | 148 (78.3%) | ||
| No | 203 (72.5%) | 41 (21.7%) | ||
| Parametrium invasion | 0.013* | |||
| Yes | 232 (82.9%) | 171 (90.5%) | ||
| No | 48 (17.1%) | 18 (9.5%) | ||
| Hydronephrosis | <0.001* | |||
| Yes | 38 (13.6%) | 58 (30.7%) | ||
| No | 242 (86.4%) | 131 (69.3%) | ||
| Pelvic LN metastasis | ||||
| Yes | 147 (52.5%) | 106 (56.1%) | 0.445 | |
| No | 133 (47.5%) | 83 (43.9%) | ||
| Tumor size at diagnosis (median, cm) | 5.4 (4.0-12.0) | 5.7 (4.1-14.5) | 0.001* | |
| Tumor size before brachytherapy (median, cm) | 3.8 (0.0-6.6) | 4.3 (2.0-10.3) | <0.001* | |
| Reduction ratio (%) | 31 (0-100) | 25 (0-71) | <0.001* | |
| Total treatment time (median, weeks) | 7 (5-11) | 7 (5-14) | 0.600 | |
FIGO: the International Federation of Gynecology and Obstetrics
LN: lymph node
ICBT: intracavitary brachytherapy
HBT: hybrid brachytherapy
*Statistical significance was defined as a P-value of <0.05.
Table 2 shows the treatment details. When compared to the HBT group, the patients in the ICBT group used more often WP alone as EBRT and fewer brachytherapy fractions compared to the HBT group, suggesting that large tumors in the ICBT group were treated primarily with EBRT and less brachytherapy was used, presumably because ICBT cannot adequately cover the entire tumor. The mean lymph node boost irradiation dose was significantly higher in the ICBT group. Cisplatin was the most commonly used systemic chemotherapy agent in both groups. HR-CTV D90, D95 and rectum D2cc in the HBT group received significantly higher doses than in the ICBT group, while bladder D2cc showed no statistical difference.
Table 2.
Treatment details
| ICBT (n = 280) | HBT (n = 189) | P-value | ||
|---|---|---|---|---|
| EBRT strategy | ||||
| 3D-CRT, WP + CS | 263 (93.9%) | 173 (91.5%) | 0.003* | |
| 3D-CRT, WP alone | 17 (6.1%) | 9 (4.8%) | ||
| IMRT | 0 (0%) | 7 (3.7%) | ||
| Central Pelvic EBRT dose, (median, Gy) | 30.6 (20.0-54.0) | 30.0 (26.0-54.0) | 0.602 | |
| LN boost (median, Gy) | 0 (0-10) | 6 (0-22.8) | 0.001* | |
| Systemic chemotherapy agents | ||||
| CDDP | 227 (81%) | 152 (80.5%) | <0.001* | |
| CDDP +5-FU | 0 (0%) | 1 (0.5%) | ||
| CDDP + S-1 | 1 (0.4%) | 0 (0%) | ||
| NDP | 4 (1.4%) | 7 (3.7%) | ||
| CBDCA | 0 (0%) | 1 (0.5%) | ||
| TP | 29 (10.4%) | 3 (1.6%) | ||
| Unknown | 19 (6.8%) | 25 (13.2%) | ||
| No. of BT fractions | ||||
| 2 fractions | 12 (4.3%) | 1 (0.5%) | <0.001* | |
| 3 fractions | 52 (18.6%) | 18 (9.5%) | ||
| 4 fractions | 211 (75.3%) | 152 (80.5%) | ||
| 5 fractions | 5 (1.8%) | 14 (7.4%) | ||
| 6 fractions | 0 (0%) | 4 (2.1%) | ||
| BT, EQD2 (a/b = 10, Gy) | ||||
| HR-CTV D90 (median) | 65.6 (41.3-102.0) | 68.8 (49.4-97.3) | 0.001* | |
| HR-CTV D95 (median) | 60.6 (37.7-95.8) | 65.0 (41.6-91.9) | <0.001* | |
| BT, EQD2 (a/b = 3, Gy) | ||||
| Rectum D2cc (median) | 51.4 (33.6-87.2) | 58.4 (35.1-91.5) | <0.001* | |
| Bladder D2cc (median) | 65.7 (36.5-113.4) | 68.4 (40.7-108.8) | 0.178 | |
EBRT: external beam radiation therapy
3D-CRT: 3-dimensional conformal radiation therapy
WP: whole pelvis
CS: central shield
IMRT: intensity modulated radiation therapy
LN: lymph node
CDDP: cisplatin
5-FU: 5-fluorouracil
S-1: an oral fluoropyrimidine
NDP: nedaplatin
CBDCA: carboplatin
TP: paclitaxel and carboplatin
BT: brachytherapy
EQD2: equivalent doses delivered in 2 Gy fractions
HR-CTV: high-risk clinical target volume
*Statistical significance was defined as a P-value of <0.05.
Efficacy
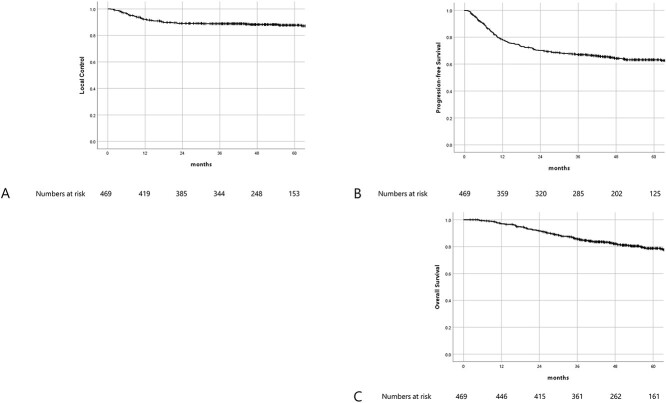
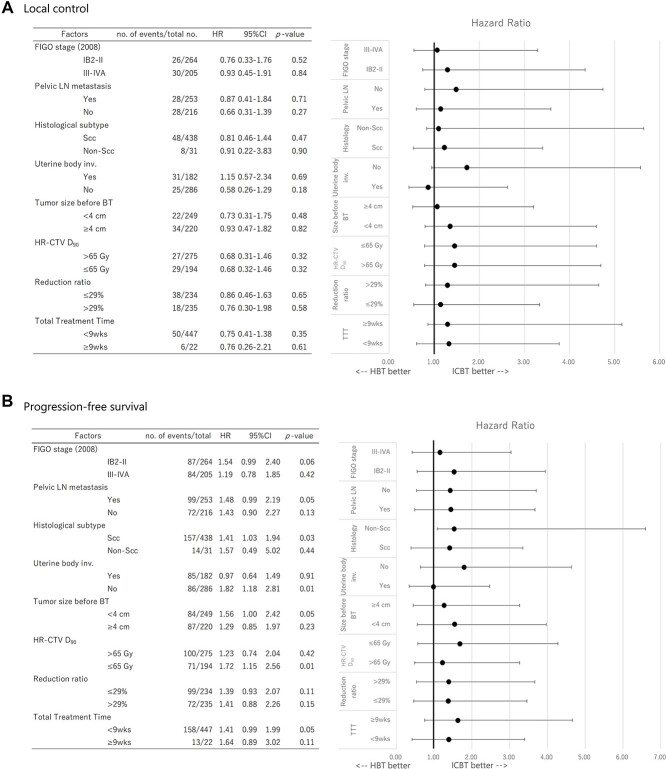
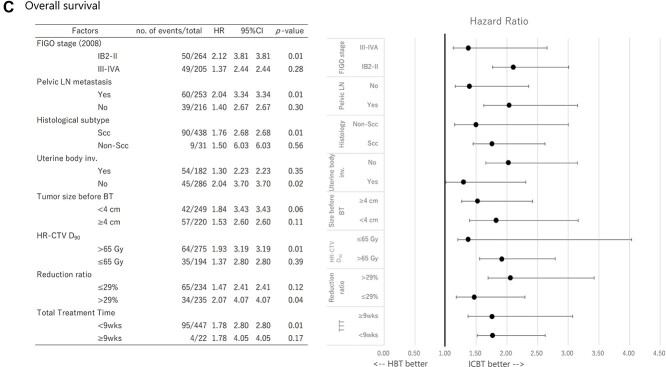
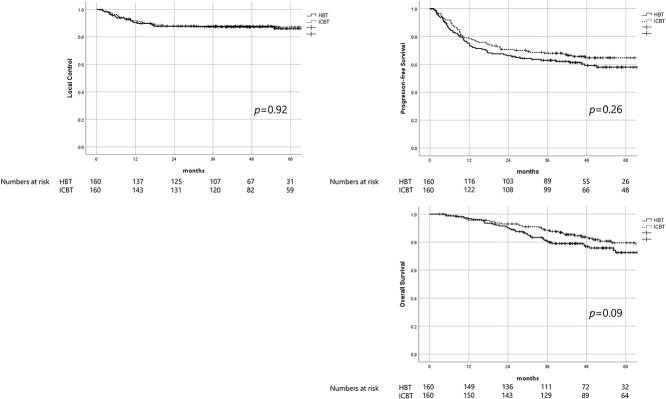
With a median follow-up of 51.3 months (2.1–139.9 months), 4-y LC, PFS and OS for the entire patient population were 88.2%, 64.2% and 83%, respectively (Fig. 3). Clinicopathological factors that influenced LC, PFS or OS are summarized in Table 3. In univariate analysis, histological subtype, uterine body invasion, tumor size before brachytherapy ≥4 cm, HR-CTV D95 ≤ 60 Gy, reduction ratio ≤ 29% and TTT ≥ 9 weeks were found to be negatively associated with LC. In multivariate analysis, non-Scc histological subtype, HR-CTV D95 ≤ 60 Gy, reduction ratio ≤ 29% and TTT ≥ 9 weeks were identified as the independent negative prognostic factors for LC. In univariate analysis, uterine body invasion, tumor size at diagnosis ≥7 cm, reduction ratio ≤ 29% and TTT ≥ 9 weeks were found to be negatively associated with PFS. In multivariate analysis, uterine body invasion, reduction ratio ≤ 29% and TTT ≥ 9 weeks were identified as the independent negative prognostic factors for PFS. In univariate analysis, uterine body invasion, tumor size before brachytherapy ≥4 cm, reduction ratio ≤ 29% and HBT were found to be negatively associated with OS. In multivariate analysis, uterine body invasion, reduction ratio ≤ 29% and HBT were identified as the independent negative prognostic factors for OS.
Fig. 3.
Shows Kaplan–Meier survival curves. Fig. 3A–C shows LC, PFS and OS, respectively.
Table 3.
Hazard ratios for OS, LC and PFS in cervical cancer
| Factors | Pts | OS | multivariate analysis | LC | multivariate analysis | PFS | multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-y OS (%) | P-value | HR (95%CI) | P-value | 4-y LC (%) | P-value | HR (95%CI) | P-value | 4-y PFS (%) | P-value | HR (95%CI) | P-value | |||
| FIGO stage (2008) | ||||||||||||||
| IB2-II | 264 (56.3%) | 84.8 | 0.085 | 90.4 | 0.094 | 67.3 | 0.058 | |||||||
| III-IVA | 205 (43.7%) | 78.3 | 85.3 | 60.0 | ||||||||||
| Pelvic LN metastasis | ||||||||||||||
| Yes | 253 (53.9%) | 80.6 | 0.125 | 88.9 | 0.571 | 62.4 | 0.111 | |||||||
| No | 216 (46.1%) | 83.8 | 87.4 | 66.3 | ||||||||||
| Histological subtype | ||||||||||||||
| Scc | 438 (93.4%) | 82.8 | 0.056 | 89.2 | 0.007* | 3.55 (1.66-7.60) | 0.001* | 65.1 | 0.119 | |||||
| Non-Scc | 31 (6.6%) | 72.1 | 73.6 | 50.9 | ||||||||||
| Uterine body inv. | ||||||||||||||
| Yes | 182 (38.8%) | 75.2 | 0.002* | 0.53 (0.35-0.79) | 0.002* | 82.5 | 0.024* | 52.7 | 0.001* | 0.57 (0.42-0.76) | <0.001* | |||
| No | 286 (61%) | 86.4 | 91.8 | 71.4 | ||||||||||
| N/A | 1 (0.2%) | |||||||||||||
| Pyometra | ||||||||||||||
| Yes | 118 (25.2%) | 80.9 | 0.719 | 69.7 | 0.566 | 57.4 | 0.309 | |||||||
| No | 351 (74.8%) | 82.4 | 74.1 | 66.4 | ||||||||||
| Parametrium inv. | ||||||||||||||
| Yes | 66 (14.1%) | 82.2 | 0.070 | 71.5 | 0.209 | 63.1 | 0.252 | |||||||
| No | 403 (85.9%) | 87.4 | 81.2 | 70.7 | ||||||||||
| Hydronephrosis | ||||||||||||||
| Yes | 96 (20.5%) | 84.3 | 0.654 | 74.4 | 0.663 | 61.2 | 0.554 | |||||||
| No | 373 (79.5%) | 81.5 | 72.6 | 64.9 | ||||||||||
| Tumor size at diagnosis | ||||||||||||||
| <7 cm | 386 (82.3%) | 83.5 | 0.095 | 89.5 | 0.096 | 66.3 | 0.029* | |||||||
| ≥7 cm | 83 (17.7%) | 75.5 | 82.7 | 54.5 | ||||||||||
| Tumor size before BT | ||||||||||||||
| <4 cm | 249 (53.1%) | 86.8 | 0.014* | 91.3 | 0.021* | 67.0 | 0.129 | |||||||
| ≥4 cm | 220 (46.9%) | 76.7 | 84.7 | 61.0 | ||||||||||
| HR-CTV D90 | ||||||||||||||
| HR-CTV D90 > 65Gy | 275 (58.6%) | 81.4 | 0.229 | 90.5 | 0.080 | 64.5 | 0.707 | |||||||
| HR-CTV D90 ≤ 65Gy | 194 (41.4%) | 83.1 | 85.0 | 63.8 | ||||||||||
| HR-CTV D95 | ||||||||||||||
| HR-CTV D95 > 60Gy | 269 (57.4%) | 80.9 | 0.099 | 90.3 | 0.020* | 2.14 (1.25-3.64) | 0.005* | 63.9 | 0.076 | |||||
| HR-CTV D95 ≤ 60Gy | 181 (38.6%) | 82.1 | 83.7 | 62.0 | ||||||||||
| Unknown | 19 (4%) | |||||||||||||
| Reduction ratio | ||||||||||||||
| ≤29% | 234 (49.9%) | 74.5 | <0.001* | 2.02 (1.33-3.07) | 0.001* | 84.0 | 0.004* | 2.49 (1.41-4.38) | 0.002* | 58.2 | 0.010* | 1.49 (1.1-2.0) | 0.010* | |
| >29% | 235 (50.1%) | 89.6 | 92.4 | 70.2 | ||||||||||
| Total Treatment Time | ||||||||||||||
| <9wks | 447 (95.3%) | 82.1 | 0.943 | 88.9 | 0.021* | 0.33 (0.17-0.93) | 0.033* | 65.2 | 0.021* | 0.56 (0.32-0.98) | 0.042* | |||
| ≥9wks | 22 (4.7%) | 81.0 | 73.5 | 43.8 | ||||||||||
| Type of BT | ||||||||||||||
| ICBT | 280 (59.7%) | 85.3 | 0.004* | 1.51 (1.00-2.27) | 0.049* | 89.3 | 0.314 | 68.4 | 0.014* | |||||
| HBT | 189 (40.3%) | 77.0 | 86.8 | 57.8 | ||||||||||
FIGO: the International Federation of Gynecology and Obstetrics
LN: lymph node
BT: brachytherapy
HR-CTV: high-risk clinical target volume
ICBT: intracavitary brachytherapy
HBT: hybrid brachytherapy
OS: overall survival
LC: local control
PFS: progression-free survival
*Statistical significance was defined as a P-value of <0.05.
The forest plots were drawn based on the results of Cox regression analysis regarding LC, PFS and OS between ICBT and HBT (Fig. 4). Results derived from the analysis showed no subgroup of patients benefited from HBT regarding LC, PFS and OS. To perform a statistical adjustment for pre-existing imbalance of baseline characteristics between the two groups, 1:1 propensity score matching was performed based on (confounding factors included were: stage, histology, uterine body invasion, tumor size before brachytherapy and reduction ratio). As a result, 320 patients (ICBT group: 160 patients, HBT group: 160 patients) were extracted. The matched-pair patients’ characteristics are summarized in Table 4. As shown in Table 4, no statistical difference was found between the two groups except the follow-up period. After adjustment of potential confounding factors between the two groups with propensity score matching, survival curves comparison was performed with the log-rank test regarding LC, PFS and OS (Fig. 5), however, no statistically significant difference was found between the two groups.
Fig. 4.
The forest plots show the results of Cox regression analysis regarding LC (4A), PFS (4B) and OS (4C) for ICBT and HBT.
Table 4.
Patient characteristics (patched-pair)
| ICBT (n = 160) | HBT (n = 160) | P-value | ||
|---|---|---|---|---|
| Age (median), years | 57 (28-86) | 58 (26-81) | 0.942 | |
| Follow-up period (median), months | 50.8 (5.2-138.4) | 45.5 (3.0-105.3) | <0.001* | |
| FIGO stage (2008) | ||||
| IB2-II | 73 (45.6%) | 72 (45%) | 1 | |
| III-IVA | 87 (54.4%) | 88 (55%) | ||
| Histological subtypes | ||||
| Squamous cell carcinoma | 149 (93.1%) | 150 (93.8%) | 0.226 | |
| Adenocarcinoma | 6 (3.8%) | 9 (5.6%) | ||
| Adenosquamous carcinoma | 5 (3.1%) | 1 (0.6%) | ||
| Uterine body invasion | ||||
| Yes | 59 (36.9%) | 77 (48.1%) | 0.054 | |
| No | 101 (63.1%) | 83 (51.9%) | ||
| Pyometra | ||||
| Yes | 45 (28.1%) | 35 (21.9%) | 0.198 | |
| No | 115 (71.9%) | 125 (78.1%) | ||
| Parametrium invasion | ||||
| Yes | 148 (92.5%) | 143 (89.4%) | 0.223 | |
| No | 12 (7.5%) | 17 (10.6%) | ||
| Hydronephrosis | ||||
| Yes | 33 (20.6%) | 40 (25%) | 0.221 | |
| No | 127 (79.4%) | 120 (75%) | ||
| Pelvic LN metastasis | ||||
| Yes | 89 (55.6%) | 88 (55%) | 1 | |
| No | 71 (44.4%) | 72 (45%) | ||
| Tumor size at diagnosis (median, cm) | 5.9 (4.0-12) | 5.7 (4.1-11) | 0.835 | |
| Tumor size before brachytherapy (median, cm) | 4.1 (1.6-6.6) | 4.1 (2.0-9.0) | 0.087 | |
| Reduction ratio (%) | 29 (0-74) | 28 (0-71) | 0.084 | |
| Total treatment time (median, weeks) | 7 (5-11) | 7 (5-14) | 0.914 | |
FIGO: the International Federation of Gynecology and Obstetrics
LN: lymph node
ICBT: intracavitary brachytherapy
HBT: hybrid brachytherapy
*Statistical significance was defined as a P-value of <0.05.
Fig. 5.
Shows the Kaplan–Meier LC, PFS and OS curves stratified by ICBT and HBT after adjustment of potential confounding factors between the two groups with propensity score matching.
Toxicities
A total of 29 patients (6.2%) experienced ≥ G3 late radiation-related toxicities. Table 5 summarizes the relationship between rectum or bladder D2cc and late gastrointestinal (GI), genitourinary (GU) and vaginal toxicities. Unfortunately, the vaginal dose was not collected in this study. Instead, rectal dose was used as a surrogate marker for vaginal toxicities since it was thought that rectal dose may surrogate the vaginal dose because the ICRU recto-vaginal reference point is located on the anterior wall of the rectum [19]. While rectum D2cc was associated with late GI and vaginal toxicities, bladder D2cc was associated with late GU toxicities. Table 6 summarizes the relationship between the type of brachytherapy and late GI, GU and vaginal toxicities. While HBT was associated with increased late vaginal and ≥ G1 late GI toxicities, there was no difference in late ≥ G2 or ≥ G3, GI and GU toxicities.
Table 5.
Relationship between rectal and bladder dose and late GI, vaginal and GU toxicity
| Late GI toxicity ≥ G1 | Late GI toxicity ≥ G2 | Late GI toxicity ≥ G3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 130) | No (n = 339) | P-value | Yes (n = 48) | No (n = 421) | P-value | Yes (n = 17) | No (n = 452) | P-value | |
| Mean total rectum D2cc (EQD2, α/β = 3, Gy) | 58.8 | 53.2 | <0.001* | 59.6 | 54.2 | 0.001* | 61.9 | 54.5 | 0.007* |
| Late Vaginal toxicity ≥ G1 | Late Vaginal toxicity ≥ G2 | Late Vaginal toxicity ≥ G3 | |||||||
| Mean total rectum D2cc (EQD2, α/β = 3, Gy) | Yes (n = 53) | No (n = 416) | P-value | Yes (n = 15) | No (n = 454) | P-value | Yes (n = 8) | No (n = 461) | P-value |
| 59.6 | 54.2 | <0.001* | 59.3 | 54.7 | 0.111 | 59.1 | 54.7 | 0.266 | |
| Late GU toxicity ≥ G1 | Late GU toxicity ≥ G2 | Late GU toxicity ≥ G3 | |||||||
| Mean total bladder D2cc (EQD2, α/β = 3, Gy) | Yes (n = 77) | No (n = 392) | P-value | Yes (n = 36) | No (n = 433) | P-value | Yes (n = 9) | No (n = 460) | P-value |
| 71.2 | 64.1 | <0.001* | 73.4 | 64.6 | 0.001* | 72.3 | 65.1 | 0.155 | |
EQD2: equivalent doses delivered in 2 Gy fractions
GI toxicity: gastrointestinal toxicity
GU toxicity: genitourinary toxicity
*Statistical significance was defined as a P-value of <0.05.
Table 6.
. Relationship between type of brachytherapy and late GI, vaginal and GU toxicity
| Type of Brachytherapy | Late GI toxicity ≥ G1 | P-value | Late GI toxicity ≥ G2 | P-value | Late GI toxicity ≥ G3 | P-value |
|---|---|---|---|---|---|---|
| ICBT (280) | 67 (23.9%) | 0.026* | 28 (10%) | 0.838 | 10 (3.6%) | 0.940 |
| HBT (189) | 63 (33.3%) | 20 (10.6%) | 7 (3.7%) | |||
| Late Vaginal toxicity ≥ G1 | Late Vaginal toxicity ≥ G2 | Late Vaginal toxicity ≥ G3 | ||||
| ICBT (280) | 18 (6.4%) | <0.001* | 5 (1.8%) | 0.034* | 4 (1.4%) | 0.412 |
| HBT (189) | 35 (18.5%) | 10 (5.3%) | 4 (2.1%) | |||
| Late GU toxicity ≥ G1 | Late GU toxicity ≥ G2 | Late GU toxicity ≥ G3 | ||||
| ICBT (280) | 45 (16.1%) | 0.805 | 24 (8.6%) | 0.375 | 4 (1.4%) | 0.271 |
| HBT (189) | 32 (16.9%) | 12 (6.3%) | 5 (2.6%) |
ICBT: intracavitary brachytherapy
HBT: hybrid brachytherapy
GI toxicity: gastrointestinal toxicity
GU toxicity: genitourinary toxicity
*Statistical significance was defined as a P-value of <0.05.
DISCUSSION
In this multi-national multi-institutional retrospective study involving Asian countries comparing the clinical outcomes of ICBT and HBT for locally advanced uterine cervical cancer, equivalent LC was observed in ICBT and HBT, despite patients treated with HBT having several adverse prognostic factors such as advanced stage, non-Scc, larger tumor size at diagnosis/before brachytherapy and a worse reduction ratio with statistical significance. To the best of our knowledge, this is the largest retrospective study comparing clinical outcomes between ICBT and HBT for Asian uterine cervical cancer patients.
In terms of LC, HBT could not demonstrate superiority to ICBT. Moreover, as shown in Fig. 4, the forest plots showed no subgroup of patients benefited from HBT regarding either LC, PFS, or OS. On the other hand, HBT was associated with a lower OS in this study, despite the fact that the mean lymph node boost dose in the HBT group was significantly higher (Table 1). It was presumably due to negative prognostic factors such as larger tumor size, advanced stage, poor reduction and uterine body invasion in patients included in the HBT group. To adjust such base-line imbalances between the two groups, propensity score matching analysis was performed using these prognostic factors; however, there was no statistical difference between HBT and ICBT in terms of LC, PFS and OS (Fig. 5). One possible explanation for this result is that the criteria for adapting HBT differed from institution to institution, and the indication of HBT could also have been changed during long the study period between 2000 and 2016 in the same institution, so it is possible that some patients who could have been cured even with ICBT were actually treated with HBT. Another possible reason why the advantage of HBT could not be clearly shown in this study was that the quality of HBT was not assessed and if any additional interstitial needles were used, the patient was categorized in the HBT group. As a result, it could be that patients treated by HBT may not always have been treated by brachytherapy with desirable dose distribution. Another possible reason could be that MRI, which is a gold standard image-guide modality of 3D-IGABT with superior tissue resolution to CT, was not used in most institutions included in this study and resulted in inadequate dose distribution, even though favorable clinical outcomes using CT-based 3D-IGABT with careful contouring, paying attention to the weakness of CT findings and taking account of recent MRI findings, have been reported. Although it is unclear which types of patients require HBT, some researchers attempted to answer this clinical question: Yoshida et al. [15] showed that HR-CTV size between 4 × 3 × 3 cm and 5 × 4 × 4 cm could be better treated by HBT, and Gonzalez et al. [20] found that tumor volume ≥ 35 cc or significant tumor asymmetry would benefit more from HBT. Despite the fact that the HBT group had more patients with advanced stage, uterine body invasion and parametrium invasion (Table 1) and rectum D2cc was higher in HBT patients, bladder D2cc was comparable between the two groups, suggesting that HBT is better than ICBT at delivering a higher dose to the target volume while sparing organs at risk (OAR). Taken together, despite the fact that HBT patients had more adverse clinical factors, HBT delivered a higher dose to HR-CTV and resulted in comparable LC. Thus, although the benefit of HBT was not clearly demonstrated after adjustment of imbalanced backgrounds with the propensity score matching analysis in this study, possibly because of uncollected imbalanced confounding factors that would influence clinical outcomes due to the nature of the retrospective study, actually HBT yielded comparable LC to ICBT despite the fact that the HBT group had more advanced clinical features than the ICBT group, additionally, other reports also have also shown favorable clinical results with HBT [12–14], HBT may be a promising and effective treatment method for locally advanced uterine cervical cancer.
In this study, the non-Scc histological subtype was associated with worse LC, which was consistent with previous reports [21, 22]. Furthermore, a tumor reduction ratio ≤ 29% was found to be a strong independent prognostic factor for LC, PFS and OS. Based on data from the international study on MRI-guided brachytherapy in locally advanced cervical cancer (EMBRACE) study, Jastaniyah et al. [23] clearly classified tumor response into six groups, and Mayr et al. [24] demonstrated in a retrospective analysis involving 115 patients that residual volumes at 40-50 Gy assessed by MRI were an independent prognostic factor for LC and OS. Minkoff et al. [22] also found that GTV at first brachytherapy >7.5 cc was associated with poorer 2-y LC, PFS and OS. These findings are consistent with our finding that tumor reduction ratio is an independent prognostic factor for uterine cervical cancer definitive radiotherapy.
While HR-CTV D90 is a well-recognized parameter that predicts LC [2, 5, 21, 23, 25], HR-CTV D95 was found to be a better predictor of LC in this study. D90 ignores 10% of the target volume, whereas D95 ignores only 5%, and this difference could be translated into a significant difference when applied to the large tumors. And the absolute volume of this ignored volume would be larger when the tumor is larger. Therefore, it could be said that when the ignored percentage is smaller, such influence will become smaller, especially for larger tumors. Okazaki et al. [26] reported that, in addition to HR-CTV D90, HR-CTV D98 was associated with tumor control. Therefore, HR-CTV DX greater than D90 may thus be a better surrogate dose-volume parameter for LC. In this study, HR-CTV D95 > 60 Gy was found to be associated with better LC. Although 60 Gy is much lower than the doses written in the guidelines from the Gynaecological Groupe Europeen de Curietherapie-European Scoiety for Radiotherapy & Oncology (GEC-ESTRO) [1] or American Brachytherapy Society (ABS) [7], the dose contribution from CS was not considered in the calculation due to the complexity [17], and the dose contribution to HR-CTV D90 from CS with 4 cm width being 13–35% depending on the size of the tumor [27], so the actual dose delivered to the target would have been higher than 60 Gy. CS has the advantage of being able to easily reduce the rectal and bladder doses. However, this technique has a significant disadvantage in that the actual delivered dose may be difficult to assess unless complicated image registration is not performed. If our society continues to use CS, attempting to obtain the actual delivered dose should be assessed precisely in some practical and simple ways.
In this study, uterine body invasion was found to be an unfavorable prognostic factor, which was consistent with the previous report [14]. The current emphasis on incorporating interstitial needles is primarily on covering lateral tumor spread. However, when there is severe asymmetric uterine body invasion that cannot be adequately covered by tandem, it may be encouraged that interstitial needles be inserted to supplement the uterine body tumor asymmetry. When LC was compared between ICBT and HBT for 182 patients only with uterine body invasion, no statistically significant difference was found (4-y LC 81% vs 84.3%, P = 0.691), indicating that the current cohort of patients’ intent for using HBT was not primarily to cover the uterine body invasion. Interstitial needle insertion guided by a combination of transrectal and transabdominal ultrasonography would be an appropriate technique to navigate needle position for deeply situated tumors such as uterine body invasion in real-time fashion [28].
TTT is an established prognostic factor for uterine cervical cancer [29, 30]. In addition to these previous reports, TTT ≥ 9 weeks was found to be an independent negative prognostic factor in this study. However, because HBT requires more expertise and labor than conventional ICBT, it is conceivable that longer TTT is required when referring patients to a distant institution capable of HBT. As a result, such a logistical issue should be addressed, with at least one institution in a large medical area offering HBT.
Although mean rectum D2cc was higher in HBT than ICBT (Table 2), no statistical difference was found concerning late ≥ G2 toxicities between groups (Table 6). If higher HR-CTV doses were due to simply allowing a higher dose, both the rectal and bladder dose should have been increased. In reality, this was not the case (Table 2). Thus, with HBT, higher HR-CTV D90 and D95 doses (Table 2) were delivered with equivalent late GI toxicities, which is a significant advantage for using HBT for locally advanced cervical cancer. Nonetheless, as previously reported [31, 32], rectum D2cc and bladder D2cc were associated with late rectal and urogenital toxicities (Table 5). According to the findings of this study, non-Scc histology or a reduction rate ≤ 29% were associated with worse LC. For such unfavorable tumors, dose escalation is required to improve clinical outcomes. Therefore, when escalating target doses to ensure LC, doses to the surrounding normal OARs should be kept as low as possible. As shown in the treatment of prostate cancer [33], gel spacers may be useful in lowering OAR doses in the management of gynecologic malignancies [32, 34–37]. Rectum D2cc was also associated with late vaginal toxicities, lending credence to the ICRU rectovaginal reference point, which is located at the intersection of the tandem and vaginal source positions and 5 mm dorsal of the posterior vaginal wall [19].
Although there was no statistical difference ≥ G3 vaginal toxicities between the two groups, the HBT group had more ≤ G2 vaginal toxicities (Table 6). Because no data on vaginal doses or interstitial needle pathways were collected in the current study, no confirmatory description can be made. However, higher vaginal doses or needle insertion through the vaginal wall could have contributed to the higher incidence of G2 vaginal toxicities in the HBT group.
The majority of the patients in this study were treated with CS to protect OARs such as the rectum and the bladder, which is obviously not a standard treatment strategy in the United States or most European countries [1, 6]. Therefore, even if 13–35% of a CS dose was actually delivered to the target volume as mentioned before, the median HR-CTV D90 in this study was lower than the recommended HR-CTV D90 of 85 Gy EQD2 by the GEC-ESTRO. Recently, results of EMBRACE-I, a large multi-institutional prospective observational study involving ≥1300 patients, have been reported [38], in which as much as ≥90% of 5-year LC was consistently achieved regardless of T stage. On the other hand, up to 14.6% of patients experienced grade 3–5 late toxicities, rising to 18.4% when focusing on Stage III-IVA, which is unacceptable to our society, and this high late toxicity rate is the reason why our society’s guideline recommendation still does not adopt 85 Gy EQD2 as a prescription goal. Because the majority of uterine cervical cancer patients are related to the human papillomavirus (HPV) and similar to the HPV-related oropharyngeal cancer [39, 40], de-intensification for uterine cervical cancer could be considered as demonstrated by a subgroup analysis of the current patient cohort [41]. Even if IMRT is used for EBRT, a 1–2 cm margin is required to compensate for internal uterine motion, resulting in exposure to the surrounding bowel. Although it is understandable that some patients with radio-resistance require a higher dose of >85 Gy EQD2, it is unsafe to administer a high dose to all patients regardless of tumor response due to the high rate of late radiation-related toxicities. In this context, CS may be a viable option for effectively and easily reducing OAR doses, resulting in a lower rate of late severe toxicities, as shown in this retrospective study, while maintaining reasonable LC.
This study has a number of limitations. Because this was a retrospective study, the treatment protocols across the participating institutions differed, including EBRT dose, technique, contouring guidelines, imaging protocol, number of brachytherapy fractions, usage of inhomogeneity correction in brachytherapy dose calculation, or systemic chemotherapy. Furthermore, the definition of an indication for applying HBT varied between institutions; therefore, tumors that could also be treated with ICBT were potentially treated with HBT. There was selection bias in deciding whether to use ICBT or HBT, and as shown in Table 1, more advanced patients were more likely to be treated with HBT. Contouring uncertainty in IGABT, which potentially plays a significant role in dose-volume histogram parameters such as D90 and D95, was not addressed in the study. Important information such as HR-CTV volume prior to treatment and at the time of brachytherapy was missing. Despite these limitations listed above, along with recent studies supporting the efficacy of HBT [12–14], the current study, with an adequate number of patients and a long follow-up period, supports the efficacy of HBT in treating locally advanced uterine cervical cancer. However, since the follow-up period in the HBT group was shorter than that of the ICBT group (Table 1), it has to be said that the results derived from this study are inconclusive.
CONCLUSION
Despite the fact that HBT patients had more adverse clinical factors than ICBT patients, HBT delivered a higher dose to HR-CTV and resulted in comparable LC, even though clear superiority of HBT over ICBT could not be shown after adjusting for potential confounding clinical factors with propensity score matching. HBT may be a promising treatment method for patients with locally advanced uterine cervical cancer.
ACKNOWLEDEGMENT
The authors are grateful for all doctors who were involved in data collection for this retrospective study. The authors would also like to express their heartful gratitude to Ryunosuke Machida, a biostatistician, for providing us with valuable and insightful advice on the statistical analyses of this article. Part of the patients’ data was provided from institutions involved in the Working Group of the Gynecological Tumor Committee of the Japanese Radiation Oncology Study Group (JROSG).
CONFLICT OF INTEREST
Dr. Itami reports personal fees from HekaBio, grants and personal fees from Itochu, grants from Elekta, personal fees from AlphaTAU, personal fees from ViewRay, personal fees from Palette Science, outside the submitted work.
Dr. Igaki reports personal fees from HekaBio, personal fees from AstraZeneca, personal fees from Itochu, outside the submitted work.
This study receives no financial support from any company, so there are no conflicts of interests to declare.
FUNDING
This study was partially supported by The Japan Agency for Medical Research and Development (AMED, 19ck0106305h0003) and the National Cancer Center Research and Development Fund (26-A-18 and 26-A-28).
ETHICAL STATEMENT
All researchers involved in this study acted and performed the research according to the Declaration of Helsinki and Ethical guidelines for medical and health research involving human subjects. The research started after receiving approval from the local institutional ethical review board (the approval number was 2018-245). Because of the retrospective and observational nature of this study, the requirement for written informed consent was waived on the condition that a document declaring an opt-out policy by which any potential patient and/or relatives could refuse to be included in this study was uploaded to the National Cancer Center Hospital Web page. Under the philosophy of individual respect for personality, information that can identify a person was anonymized and the collected data was securely stored and handled in accordance with the regulations.
Contributor Information
Naoya Murakami, Department of Radiation Oncology, National Cancer Center Hospital, Tokyo 104-0045, Japan.
Ken Ando, Department of Radiation Oncology, Gunma Prefectural Cancer Center, Gunma 373-8550, Japan; Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan.
Masumi Murata, Department of Radiation Oncology, Gunma Prefectural Cancer Center, Gunma 373-8550, Japan.
Kazutoshi Murata, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan; QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Tatsuya Ohno, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan.
Tomomi Aoshika, Department of Radiation Oncology, Saitama Medical University International Medical Center, Saitama 350-1298, Japan.
Shingo Kato, Department of Radiation Oncology, Saitama Medical University International Medical Center, Saitama 350-1298, Japan.
Noriyuki Okonogi, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Anneyuko I Saito, Department of Radiation Oncology, Juntendo University Faculty of Medicine, Tokyo 113-8431, Japan.
Joo-Young Kim, Department of Radiation Oncology, National Cancer Center, Goyang 410-769, Korea.
Yasuo Yoshioka, Radiation Oncology Department, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan.
Shuhei Sekii, Department of Radiation Oncology, Hyogo Cancer Center, Hyogo 673-8558, Japan; Department of Radiation Therapy, Kita-Harima Medical Center, Hyogo 675-1392, Japan.
Kayoko Tsujino, Department of Radiation Oncology, Hyogo Cancer Center, Hyogo 673-8558, Japan.
Chairat Lowanichkiattikul, Division of Radiation Oncology, Department of Radiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 73170, Thailand.
Poompis Pattaranutaporn, Division of Radiation Oncology, Department of Radiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 73170, Thailand.
Yuko Kaneyasu, Department of Radiation Oncology, National Hospital Organization Fukuyama Medical Center, Hiroshima, Japan.
Tomio Nakagawa, Department of Radiation Oncology, National Hospital Organization Fukuyama Medical Center, Hiroshima, Japan.
Miho Watanabe, Department of Radiology, Chiba University Hospital, Chiba 260-8677, Japan.
Takashi Uno, Department of Radiology, Chiba University Hospital, Chiba 260-8677, Japan.
Rei Umezawa, Department of Radiation Oncology, Tohoku University Graduate School of Medicine, Miyagi 980-8574, Japan.
Keiichi Jingu, Department of Radiation Oncology, Tohoku University Graduate School of Medicine, Miyagi 980-8574, Japan.
Ayae Kanemoto, Department of Radiation Oncology, Niigata Cancer Center Hospital, Niigata 951-8566, Japan.
Masaru Wakatsuki, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan; Department of Radiology, Jichi Medical University Hospital, Tochigi 329-0498, Japan.
Katsuyuki Shirai, Department of Radiology, Jichi Medical University Hospital, Tochigi 329-0498, Japan.
Hiroshi Igaki, Department of Radiation Oncology, National Cancer Center Hospital, Tokyo 104-0045, Japan.
Jun Itami, Department of Radiation Oncology, National Cancer Center Hospital, Tokyo 104-0045, Japan.
References
- 1. Potter R, Haie-Meder C, Van Limbergen E et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 2. Potter R, Tanderup K, Kirisits C et al. The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol 2018;9:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murakami N, Kasamatsu T, Wakita A et al. CT based three dimensional dose-volume evaluations for high-dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer 2014;14:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haie-Meder C, Potter R, Van Limbergen E et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45. [DOI] [PubMed] [Google Scholar]
- 5. Nag S, Cardenes H, Chang S et al. Proposed guidelines for image-based intracavitary brachytherapy for cervical carcinoma: report from Image-Guided Brachytherapy Working Group. Int J Radiat Oncol Biol Phys 2004;60:1160–72. [DOI] [PubMed] [Google Scholar]
- 6. Viswanathan AN, Beriwal S, De Los Santos JF et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy 2012;11:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Potter R, Dimopoulos J, Georg P et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83:148–55. [DOI] [PubMed] [Google Scholar]
- 8. Sturdza A, Potter R, Fokdal LU et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016;120:428–33. [DOI] [PubMed] [Google Scholar]
- 9. Charra-Brunaud C, Harter V, Delannes M et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: results of the French STIC prospective study. Radiother Oncol 2012;103:305–13. [DOI] [PubMed] [Google Scholar]
- 10. Murakami N, Kobayashi K, Kato T et al. The role of interstitial brachytherapy in the management of primary radiation therapy for uterine cervical cancer. J Contemp Brachytherapy 2016;8:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Syed AM, Puthawala AA, Abdelaziz NN et al. Long-term results of low-dose-rate interstitial-intracavitary brachytherapy in the treatment of carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2002;54:67–78. [DOI] [PubMed] [Google Scholar]
- 12. Dimopoulos JC, Kirisits C, Petric P et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys 2006;66:83–90. [DOI] [PubMed] [Google Scholar]
- 13. Murakami N, Kobayashi K, Shima S et al. A hybrid technique of intracavitary and interstitial brachytherapy for locally advanced cervical cancer: initial outcomes of a single-institute experience. BMC Cancer 2019;19:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fokdal L, Sturdza A, Mazeron R et al. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol 2016;120:434–40. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida K, Yamazaki H, Kotsuma T et al. Simulation analysis of optimized brachytherapy for uterine cervical cancer: can we select the best brachytherapy modality depending on tumor size? Brachytherapy 2016;15:57–64. [DOI] [PubMed] [Google Scholar]
- 16. Tamaki T, Ohno T, Noda SE et al. Filling the gap in central shielding: three-dimensional analysis of the EQD2 dose in radiotherapy for cervical cancer with the central shielding technique. J Radiat Res 2015;56:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol 1985;58:515–28. [DOI] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirchheiner K, Nout RA, Lindegaard JC et al. Dose-effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol 2016;118:160–6. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez Y, Giap F, Klages P et al. Predicting which patients may benefit from the hybrid intracavitary+interstitial needle (IC/IS) applicator for advanced cervical cancer: A dosimetric comparison and toxicity benefit analysis. Brachytherapy 2021;20:136–45. [DOI] [PubMed] [Google Scholar]
- 21. Horne ZD, Karukonda P, Kalash R et al. Single-Institution Experience in 3D MRI-Based Brachytherapy for Cervical Cancer for 239 Women: Can Dose Overcome Poor Response? Int J Radiat Oncol Biol Phys 2019;104:157–64. [DOI] [PubMed] [Google Scholar]
- 22. Minkoff D, Gill BS, Kang J et al. Cervical cancer outcome prediction to high-dose rate brachytherapy using quantitative magnetic resonance imaging analysis of tumor response to external beam radiotherapy. Radiother Oncol 2015;115:78–83. [DOI] [PubMed] [Google Scholar]
- 23. Jastaniyah N, Yoshida K, Tanderup K et al. A volumetric analysis of GTVD and CTVHR as defined by the GEC ESTRO recommendations in FIGO stage IIB and IIIB cervical cancer patients treated with IGABT in a prospective multicentric trial (EMBRACE). Radiother Oncol 2016;120:404–11. [DOI] [PubMed] [Google Scholar]
- 24. Mayr NA, Wang JZ, Lo SS et al. Translating response during therapy into ultimate treatment outcome: a personalized 4-dimensional MRI tumor volumetric regression approach in cervical cancer. Int J Radiat Oncol Biol Phys 2010;76:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jurgenliemk-Schulz IM, Tersteeg RJ, Roesink JM et al. MRI-guided treatment-planning optimisation in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol 2009;93:322–30. [DOI] [PubMed] [Google Scholar]
- 26. Okazaki S, Murata K, Noda SE et al. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J Radiat Res 2019;60:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamaki T, Noda SE, Ohno T et al. Dose-volume histogram analysis of composite EQD2 dose distributions using the central shielding technique in cervical cancer radiotherapy. Brachytherapy 2016;15:598–606. [DOI] [PubMed] [Google Scholar]
- 28. Shimizu Y, Murakami N, Chiba T et al. High-dose-rate interstitial brachytherapy for deeply situated gynecologic tumors guided by combination of transrectal and transabdominal ultrasonography: a technical note. Front Oncol 2022;11:808721. 10.3389/fonc.2021.808721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanciano RM, Pajak TF, Martz K et al. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns-of-care study. Int J Radiat Oncol Biol Phys 1993;25:391–7. [DOI] [PubMed] [Google Scholar]
- 30. Perez CA, Grigsby PW, Castro-Vita H et al. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys 1995;32:1275–88. [DOI] [PubMed] [Google Scholar]
- 31. Mazeron R, Fokdal LU, Kirchheiner K et al. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiother Oncol 2016;120:412–9. [DOI] [PubMed] [Google Scholar]
- 32. Murakami N, Nakamura S, Kashihara T et al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy 2020;19:154–61. [DOI] [PubMed] [Google Scholar]
- 33. Hamstra DA, Mariados N, Sylvester J et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys 2017;97:976–85. [DOI] [PubMed] [Google Scholar]
- 34. Iijima K, Murakami N, Nakamura S et al. Configuration analysis of the injection position and shape of the gel spacer in gynecologic brachytherapy. Brachytherapy 2021;20:95–103. [DOI] [PubMed] [Google Scholar]
- 35. Kashihara T, Murakami N, Tselis N et al. Hyaluronate gel injection for rectum dose reduction in gynecologic high-dose-rate brachytherapy: initial Japanese experience. J Radiat Res 2019;60:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viswanathan AN, Damato AL, Nguyen PL. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol 2013;31:e446–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Damato AL, Kassick M, Viswanathan AN. Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound. Brachytherapy 2017;16:949–55. [DOI] [PubMed] [Google Scholar]
- 38. Potter R, Tanderup K, Schmid MP et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol 2021;22:538–47. [DOI] [PubMed] [Google Scholar]
- 39. Ang KK, Harris J, Wheeler R et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen AM, Felix C, Wang PC et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol 2017;18:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murakami N, Ando K, Murata M et al. Why not de-intensification for uterine cervical cancer? Gynecol Oncol 2021;163:105–9. [DOI] [PubMed] [Google Scholar]