Abstract
The purpose of this study was to outline the course and profile of adverse events specific to boron neutron capture therapy (BNCT) for head and neck cancer. This was a sub-analysis of the phase II JHN002 trial. Patients received 400 mg/kg borofalan(10B), followed by neutron irradiation. The course of adverse events after BNCT was documented in the JHN002 Look Up study. Patients were grouped into face/front (FF), face/lateral (FL) and neck (N) beam groups according to the point of skin incidence of the epithermal neutron beam axis, and the profile of adverse events dependent on beam incidence position was examined. The courses of adverse events in eight recurrent squamous cell carcinoma (R-SCC) and 13 recurrent or locally advanced non-SCC cases were analyzed. Median interval to complete recovery was 23 days (interquartile range (IQR), 14–48 days) for oral mucositis, 40 days (IQR, 24–56 days) for dermatitis, 58 days (IQR, 53–80 days) for dysgeusia and 156 days (IQR, 82–163 days) for alopecia. In the FF beam group, parotitis (P = 0.007) was less frequent, while oral mucositis (P = 0.032), fatigue (P = 0.002), conjunctivitis (P = 0.001), epistaxis (P = 0.001) and abdominal discomfort (P = 0.029) tended to be more frequent than in the FL and N beam groups. Courses and irradiation site-specific profiles of adverse events in BNCT for head and neck cancer were identified. This profile may be useful for considering interventions to prevent exacerbation of treatment-related adverse events on BNCT.
Keywords: borofalan(10B), adverse events, phase II study, head and neck cancer, boron neutron capture therapy (BNCT)
INTRODUCTION
The JHN002 study, a phase II trial of boron neutron capture therapy (BNCT), demonstrated the utility of BNCT as a cell-selective heavy ion beam treatment for unresectable head and neck cancer after chemoradiotherapy with a lack of available local treatment options, depending on the tumor cell accumulation of borofalan(10B) [1]. In the study, eight recurrent squamous cell carcinoma (R-SCC) and 13 recurrent or locally advanced non-squamous cell carcinoma (R/LA-nSCC) patients who underwent accelerator-based BNCT presented a 71% response rate, including complete response of 50% and partial response of 25% in R-SCC patients and CR of 8% and PR of 62% in R/LA-nSCC patients, and a locoregional progression-free survival of 11.5 months for R-SCC patients. In Japan, the treatment is currently provided in a few hospitals under the health insurance system that covers all citizens. Unlike photon and particle therapies, the epithermal neutrons used for BNCT do not travel straight through the body of the patient, but rather diffuse into the surroundings. Neutrons injected into tissues are therefore widely transmitted off-axis from the beam rather than straight, and 10B(n,α)7Li reactions occur in normal tissues with boron accumulation [2]. This can lead to the development of unexpected adverse events in normal tissues where boron accumulation is more likely to occur. Predicting the onset of adverse events thus depends on tumor location and neutron beam incidence position in BNCT and is more complicated than in other radiotherapies [3–7].
In the JHN002 study of recurrent or locally advanced head and neck cancer, the frequency and variety of adverse events were observed in a boron accumulation-dependent manner, and occurrence of adverse events depending on drug accumulation is not applicable to any radiotherapy. However, the propensity of adverse events to develop depending on the incident beam has not been analyzed in detail [1]. On the other hand, all 21 subjects with adverse events required treatment. This suggests that an understanding of the developmental profile of adverse events may be useful for predicting the need for hospitalization after BNCT and in administering treatment to prevent the onset or exacerbation of adverse events.
The present study therefore aimed to outline the course and profile of the development of adverse events specific to BNCT for head and neck cancer.
METHODS
Study design and participants
The study design and data of the JHN002 trial have been reported previously [1]. The trial began in July 2016 and ended in November 2019. A total of 21 patients diagnosed with recurrent squamous cell carcinoma, or recurrent or locally advanced non-squamous carcinoma of the head and neck underwent BNCT using a cyclotron-based epithermal neutron generator and 10B. The median age of eligible patients was 62 years (range, 32–78 years), and all eight patients with R-SCC had previously received radiotherapy with a median dose of 65.5 Gy (range, 59.4–76.0 Gy), with six showing local recurrence and two showing major regional lymph node metastases. The histology of R/LA-nSCC included five adenoid cystic carcinomas, three mucoepidermoid carcinomas, two acinic cell carcinomas, two salivary ductal carcinomas and one malignant melanoma. The study was approved by the review boards of the institution and all subjects provided written informed consent.
Treatment procedure
Preplan was performed on diagnostic computed tomography (CT) to determine the rough beam conditions and specific patient treatment positions. Based on the preplan, the actual plan was performed and the final beam conditions and treatment positions were determined. Planning was performed using SERA which is a treatment planning system for BNCT [8], and tumor and mucosa of the oral cavity, pharynx and larynx were delineated, along with other organs at risk (OAR) contours. A maximum dose of 12 Gy-equivalents (Gy-Eq) was prescribed for the mucosa, and tumor dose was passively determined with a median minimum tumor dose of 31.1 Gy-Eq (interquartile range, 26.1–34.3 Gy-Eq). Data sets of the tissue composition used to calculate the dose were from the International Commission on Radiation Units and Measurements (ICRU) report No. 46 [9], and the relative biological effectiveness of irradiated beam components from the neutron generator were determined as 1 for gamma rays, 2.4 for fast neutrons and 2.9 for thermal neutrons. Compound biological effectiveness factors for boron derived from 10B were 4.9 for mucosa, 4.0 for tumor, 2.5 for skin and 1.34 for other normal tissues. Tumor boron concentrations were calculated as a constant of 3.5 as a ratio to the whole-blood boron concentration [1, 10–12].
Assessment of adverse event profiles
Information on adverse events was collected according to the JHN002 study (clinical trial registration No. JapicCTI-194 640) and JHN002 Look Up study (clinical trial registration No. UMIN000040501) protocols. Safety was assessed every month for up to 90 days and every 3 months for up to 2 years after 90 days in JHN002.
Follow-up was continued until discontinuation due to patient death, withdrawal of consent, tumor progression, or the decision of the investigator due to difficulties in study follow-up or in consideration of further treatment. Severities of adverse events and laboratory abnormalities were graded using the Common Terminology Criteria for Adverse Events version 4.03 from the National Cancer Institute. Treatment for adverse events was investigated from medical records.
Grouping of subjects by neutron beam incidence position
To classify the profile of adverse events based on the point and direction of neutron beam incidence, subjects were classified into three groups according to beam conditions. Subjects with the incident beam coming from the caudal side rather than the inferior margin of the mandible were classified into the neck (N) beam group, while subjects with the incident beam coming from the cranial side were further divided into two groups: a face/front (FF) beam group, in which the beam was delivered anterior to the center of the zygomatic arch; and a face/lateral (FL) beam group, in which the beam was delivered posteriorly. The frequency and grading of adverse events in these groups were analyzed.
Statistical analysis
Qualitative data are reported as proportions, and quantitative data are reported as median and range or interquartile range. The association between position of epithermal neutron beam incidence and probability of adverse events was examined using the Kruskal-Wallis test. All statistical analyses were performed using Prism 8 for macOS software version 8.4.3 (GraphPad Software, Inc., San Diego, CA).
RESULTS
The duration of follow-up for adverse events was 8.9 months (interquartile range, 4.3-24.4 months). The reason for the shortened observation period was that tumors remained on the tumor assessment at 3 months or the recurrence following 90 days after BNCT was recognized, and observation was interrupted to provide additional treatment [1]. The grading of the adverse events investigated in this study were listed in Table 1 based on the previous report [1]. Courses of adverse events in eight R-SCCs and 13 R/LA-nSCCs were analyzed, with nausea (17; 81%), vomiting (10; 48%), loss of appetite (14; 67%), parotitis (14; 67%), submandibular adenitis (7; 33%), conjunctivitis (7; 33%) and fatigue (9; 43%) categorized as the group with symptoms that recovered in ≤30 days. Nausea occurred at 2 days (range, 1–2 days) in 17 patients and disappeared at a median of 8 days (range, 3–64 days). These patients were treated with metoclopramide (59%), dexamethasone (53%) and granisetron (41%). Parotitis was also observed at a median of 1 day (range, 1–2 days) and recovered at a median of 8 days (range, 5–100 days). Pain associated with the parotitis led to the administration of acetaminophen (64%), non-steroidal anti-inflammatory drugs (NSAIDs) (50%) and opioids (29%) in several patients. Concomitant hyperamylasemia occurred the day after BNCT in all cases, and recovered at a median of 8 days (range, 5–16 days) with no treatment. Improvement in loss of appetite was distributed within 8–64 days. Mosapride and enteral nutrition were used in 43% and 29% of these patients, respectively, until improvement. For conjunctivitis, fluorometholone eye drops (86%), hyaluronic acid eye drops (14%) and levofloxacin eye drops (14%) were prescribed (Fig. 1 and Table 2). All patients were managed with critical pass and discharged within 7 days after BNCT. There were no patients who need postponement of discharge.
Table 1.
Grading of adverse events analyzed in this study [1]
| Acute toxicity (n = 21) | Late toxicity (n = 12) | ||||
|---|---|---|---|---|---|
| Treatment-Related Adverse Event | Grade 1 or 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | Grade 1 or 2 n (%) | Grade 3 n (%) |
| Conjunctivitis | 7 (33) | 0 | 0 | 2 (17) | 0 |
| Parotitis | 14 (67) | 0 | 0 | 1 (8) | 0 |
| Submandibular glanditis | 7 (33) | 0 | 0 | 0 | 0 |
| Nausea | 17 (81) | 0 | 0 | 0 | 0 |
| Vomiting | 10 (48) | 0 | 0 | 0 | 0 |
| Loss of appetite | 14 (67) | 0 | 0 | 0 | 0 |
| Fatigue | 9 (43) | 0 | 0 | 0 | 0 |
| Hyperamylasemia | 2 (10) | 1 (5) | 15 (71) | 0 | 0 |
| Hyperprolactinemia | 12 (57) | 0 | 0 | 0 | 0 |
| Dry mouth | 9 (43) | 0 | 0 | 4 (33) | 0 |
| Dysgeusia | 15 (71) | 0 | 0 | 4 (33) | - |
| Oral mucositis | 12 (57) | 1 (5) | 0 | 2 (17) | 0 |
| Alopecia | 20 (95) | 0 | 0 | 12 (100) | - |
| Dermatitis | 8 (38) | 1 (5) | 0 | 1 (8) | 0 |
Fig. 1.
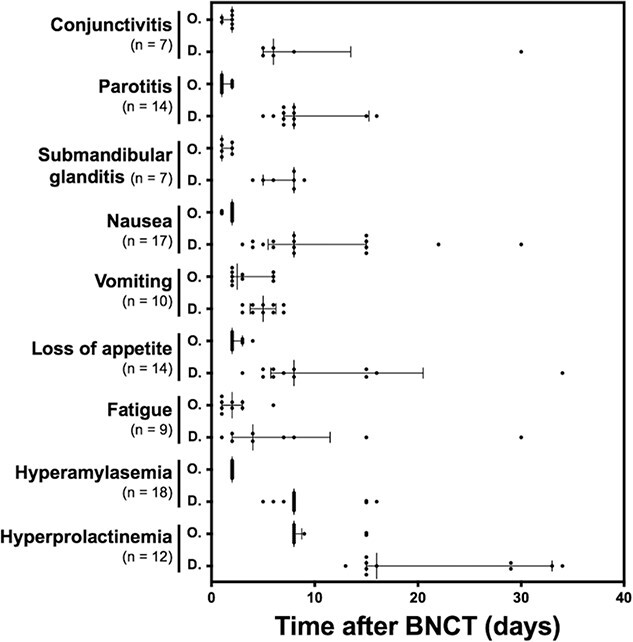
Course of adverse events requiring ≤1 month from onset to recovery. Each dot represents observed onset and disappearance of the adverse event. Each bar depicts median and interquartile range of the timing for the onset and disappearance of the adverse event. Abbreviations: O, onset; D, disappearance.
Table 2.
Treatment for adverse events after BNCT in JHN002.
| Adverse events | Agents prescribed after BNCT | n (%) |
|---|---|---|
| Conjunctivitis | Fluorometholone eye drops | 6 (86) |
| Hyaluronic acid eye drops | 1 (14) | |
| Levofloxacin eye drops | 1 (14) | |
| Parotitis | Acetaminophen | 9 (64) |
| NSAIDs | 7 (50) | |
| Opioids | 4 (29) | |
| Nausea/Vomiting | Metoclopramide | 10 (59) |
| Dexamethasone | 9 (53) | |
| Granisetron | 7 (41) | |
| Domperidone | 2 (12) | |
| Diphenhydramine | 1 (6) | |
| Prochlorperazine | 1 (6) | |
| Loss of appetite | Mosapride | 6 (43) |
| enteral nutrition | 4 (29) | |
| Oral mucositis | Gargle | 8 (62) |
| Dexamethasone oral ointment | 7 (54) | |
| Acetaminophen | 6 (46) | |
| NSAIDs | 6 (46) | |
| Opioids | 1 (8) | |
| Dermatitis | Betamethasone ointment | 3 (33) |
| Dimethyl isopropylazulene ointment | 1 (11) | |
| Gentamicin ointment | 1 (11) |
Abbreviations: BNCT, boron neutron capture therapy; NSAIDs, non-steroidal anti-inflammatory drugs
Symptoms prolonged for >30 days after treatment included alopecia (20; 95%), dysgeusia (15; 71%), dry mouth (9; 43%) and dermatitis (9; 43%). Of the seven patients followed-up for adverse effects for >6 months, alopecia disappeared completely in five patients including one patient with prior radiotherapy of total 70 Gy. Although the remaining two patients, both with prior radiotherapy of total 60 Gy, were followed-up for 266 days and 741 days, no disappearance of alopecia was observed during these periods. In all cases, alopecia was not accompanied by erythema of the scalp or other signs of inflammation. Median time from onset required to completely recover from each symptom was 23 days (range, 14–48 days) for oral mucositis, 156 days (range, 82–163 days) for alopecia, 58 days (range, 53–80 days) for dysgeusia, 37 days (range, 14–83 days) for dry mouth and 40 days (range, 24–56 days) for dermatitis (Fig. 2). Oral mucositis was treated with gargle (62%), dexamethasone oral ointment (54%), acetaminophen (46%), NSAIDs (46%) and opioids (8%). Dermatitis was managed with betamethasone ointment (33%), dimethyl isopropylazulene ointment (11%) and gentamicin ointment (11%) (Table 2). The patients who developed an intracranial infection needed 2-months hospitalized treatment with surgical drainage and antibiotics.
Fig. 2.
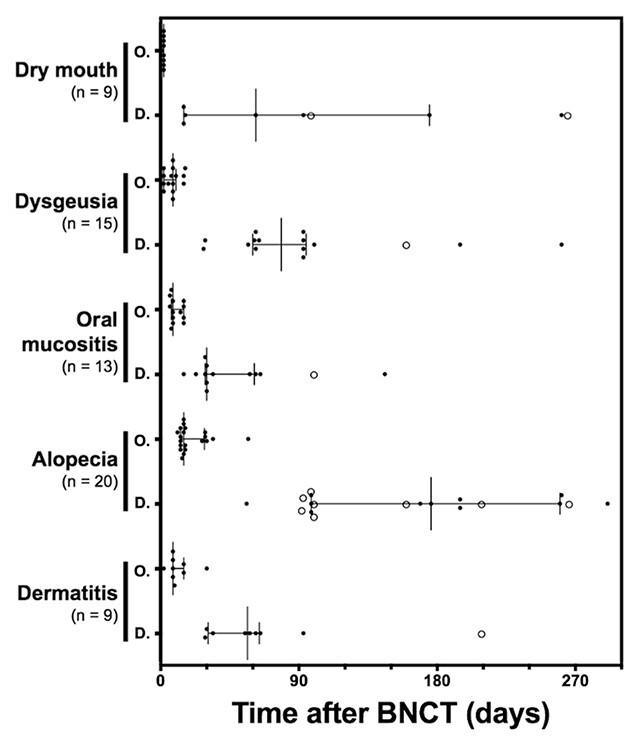
Course of adverse events requiring >1 month from onset to recovery. Each bar depicts median and interquartile range of the timing for the onset and disappearance of the adverse event. Each dot represents observed onset and disappearance of the adverse event. On the other hand, each circle represents the end of observation without disappearance due to being censored in the study. Abbreviations: O, onset; D, disappearance.
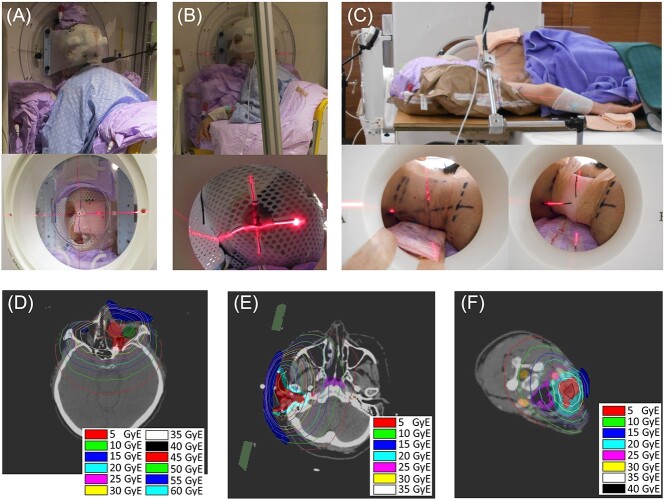
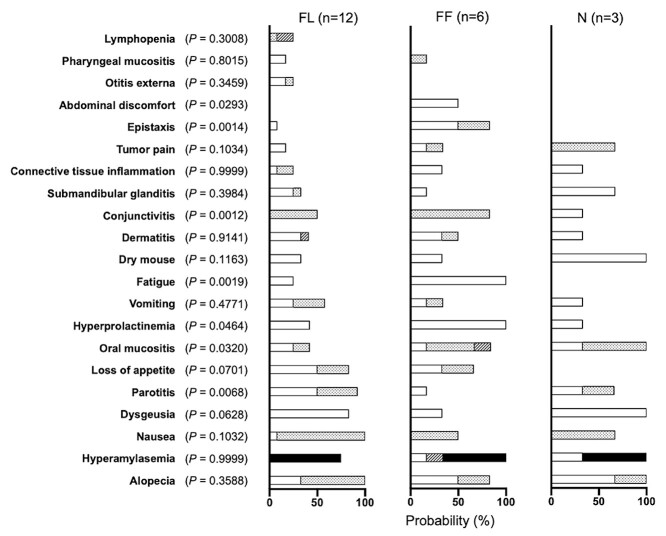
In groupings based on positions of neutron beam incidence, tumor locations included the parotid gland in 10 patients, and the external auditory canal in two patients in the FL beam group, the maxillary sinuses in four patients, the nasal cavity in one patient, and the mandibular gingiva in one patient in the FF beam group, and level II lymph nodes in two patients and level III lymph nodes in one patient in the N beam group (Table 3). The patient position and the tumor dose distribution for a representative case in each group were shown in Fig. 3. In the FF beam group, parotitis (P = 0.007) was less frequent, while the incidences of oral mucositis (P = 0.032), hyperprolactinemia (P = 0.046), fatigue (P = 0.002), conjunctivitis (P = 0.001), epistaxis (P = 0.001) and abdominal discomfort (P = 0.029) tended to be higher than in the FL and NL beam groups (Fig. 3).
Table 3.
Classification by skin incidence point of the epithermal neutron beam axis
| Parameters | FL (n = 12) |
FF (n = 6) |
N (n = 3) |
|---|---|---|---|
| Median age, years (range) | 64 (32–78) | 66 (40–76) | 53 (37–54) |
| Sex, n (%) Male Female |
5 (42) 7 (58) |
3 (50) 3 (50) |
2 (67) 1 (33) |
| KPS, n (%) 100% 90% 80% |
7 (58) 5 (42) 0 |
2 (33) 3 (50) 1 (17) |
3 (100) 0 0 |
| Tumor location, n | Parotid gland, 10 EAC, 2 |
Maxillary sinus, 4 Nasal cavity, 1 Mandibular gingiva, 1 |
Level IIa LN, 2 Level III LN, 1 |
| Prior radiotherapy | 2 (17) | 4 (67) | 2 (67) |
| Median cumulative dose, Gy (range) | 0 (0–76) | 59.7 (0–70) | 65 (0–66) |
| Prior chemotherapy | 2 (17) | 3 (50) | 2 (67) |
| Pathology SCC nSCC |
2 (17) 10 (83) |
4 (67) 2 (33) |
2 (67) 1 (33) |
| Beam parameters phi (degrees) theta (degrees) |
77.9 (74.5–87.3) 8.4 (−4.2–15.0) |
79.2 (76.4–91.0) 66.1 (36.5–87.5) |
81.2 (74.5–90.0) −1.6 (−2.0–0) |
Angle phi was the polar angle from the reference beam location at the top of the head to the current beam location, with the superior axis used as the reference direction. Angle theta was the azimuthal angle from the side of the body to the current beam location. Positive values of theta rotated the beam towards the front of the body, and negative values rotated the beam towards the back of the body [1].
Abbreviations: KPS, Karnofsky performance status; SCC, squamous cell carcinoma; nSCC, non-squamous cell carcinoma; FL, face/lateral; FF, face/front; N, neck; EAC, external auditory canal; LN, lymph node.
Fig. 3.
Patient setup and tumor dose distributions for representative cases. A, B and C showed pictures of the patient setup during treatment for representative cases of FF, FL and N groups, respectively. The patients were set up in the position determined during the preliminary patient set up and treatment planning, aligned with the center of the beam axis (laser intersection) as in the treatment plan. Tumor dose distributions for each patient according to the treatment plan are depicted in D, E and F for each patient in the picture A, B and C, respectively. The red contours in D, E and F represent the GTV, and the surrounding light red contours in D and the light blue contours in E and F represent the CTV. Note that each tumor dose distribution applies only to the evaluation of GTV and CTV dose. The blue contours represent water-equivalent boluses to increase the dose of body surface. Abbreviations: FL, face/lateral; FF, face/front; N, neck; GTV, gross tumor volume; CTV, clinical targe volume.
DISCUSSION
This is the first report to summarize the profile of adverse events after accelerator-based BNCT. Although nuclear reactors had previously been used as neutron sources for BNCT, the timing of the onset of adverse events and the course of recovery had never been reported in detail and was only made known to the clinicians involved. Furthermore, based on the excellent principle that the efficacy and toxicity of BNCT depend on the selective accumulation of boron in tumor cells, BNCT has been falsely perceived in Japan as a treatment with very few side effects for patients. This report provides an opportunity for clinicians to better understand the adverse event profile. Parotitis and submandibular glanditis are not common adverse events in photon therapy, and are thought to be symptoms and processes based on organ-specific accumulation of boron drug. Alopecia observed in JHN002 was not accompanied by scalp inflammation, and was considered to be a characteristic finding of BNCT. Regarding the onset and recovery of adverse events, as for adverse events common to photon therapy, the time to recovery from adverse events occurring with BNCT seems to be equal to or more prolonged than that required for recovery with photon therapy. However, it should be taken into account that eight of the patients in this analysis had a history of prior radiotherapy with 59.4–76 Gy.
Each nuclear reactor shows specific energy distributions depending on its power [13]. The neutron source used in the JHN002 study was based on the 9Be(p,n)9B reaction of beryllium with protons accelerated by an accelerator, and the energy spectrum has a maximum at 40 keV, which allows thermal neutrons to reach deeper-seated tumor compared to reactor neutron sources [14, 15]. The frequency of adverse events may vary based on the different conditions of each neutron source. In addition, the frequency of adverse events may vary depending on the way the patient is set up, due to differences in the area of irradiation. Several clinical trials have been reported on BNCT using nuclear reactors [3–7, 16–23]. For safety, Kankaanranta et al. reported that BNCT using the FiR 1250-kW Triga Mark II nuclear research reactor as a neutron source resulted in the incidence of Grade 3 or higher adverse events with oral and pharyngeal mucositis (53%), pain (53%), fatigue (33%), dysphagia (17%) and tumor pain (17%) as acute adverse events. They also reported the development of pneumonia (35%), radiation osteonecrosis (20%), dry mouth (20%), dysphagia (19%), cataract (19%) and dry eye (19%) as late adverse events [3]. On the other hand, Wang et al. reported grade 3 or higher adverse events with mucositis (29%), hemorrhage (12), infection (12%), dysphagia (6%), tumor pain (6%), pneumonia (6%) and laryngeal edema (6%) as acute adverse events in a BNCT using the National Tsing-Hua University 2 MW TRIGA CONV research reactor as a neutron source [6]. Most adverse events from the accelerator BNCT in the JHN002 study were of grade 2 or lower, and the frequency of grade 3 or higher adverse events tended to be low [1]. This may emphasize the validity of beam conditions and patient setup, and differences in the data distribution bias for tumor location and original general condition of the patient before treatment. As shown in Table 4, BNCT for head and neck cancer using the reactors was performed as two-fraction BNCT. It is suggested that the therapeutic toxicity of JHN002 using single fraction is lower than those of these reports using two fractions. The way of prescribing dose is also different in each report. Regarding the mucous membrane, one of the dose-prescribing organs commonly used in all reports, its shape is complex, but there is no definition of the delineation for the mucosa, and no uniformity was made in each group, which may have affected the difference in the incidence of adverse events. Delineation of the mucosa in the JHN002 study was defined as the high-intensity region on MRI T2-weighted image and the enhanced region on gadolinium-enhanced T1-weighted image, as described in Hirose et al. [1] It is conceivable that this strict definition could have prevented overdose of the mucosa. And if the mucosal overdose was effectively prevented, it may have led to a reduction in the overdose of the other organs in the whole, which may have contributed to a reduction in the incidence of other adverse events. On the other hand, as for the difference in the neutron sources used, the thermal neutron distribution of the accelerator BNCT used is seemed to be deeper than that of other reactors, and thus has the risk of enhancing adverse events. Nevertheless, the low frequency of adverse events in the JHN002 study suggests that this factor was less significant than the other factors. In addition, as shown in Figure 4, there were not many adverse events in which the tumor location had a significant influence on the incidence of adverse events, suggesting that the difference in distribution of tumor locations between studies did not have a significant effect on the frequency of adverse events.
Table 4.
Comparison of grade ≥ 3 adverse events between JHN002 and other BNCT studies
| Author | n | Neutron source/Procedures of BNCT | Occurrence of Grade ≥ 3 Acute AEs |
|---|---|---|---|
| Hirose et al. (JHN002) [1] | 21 | 30-MeV Accelerator with Beryllium target Single BNCT using 10B-BPA, administered with mucosa Dmax of 12 Gy-Eq. |
For acute AEs, hyperamylasemia, 76% (Gr4, 71%); oral mucositis, 5%; dermatitis, 5%; lymphopenia, 10%; Intracranial infection, 5%. For late AEs, intracranial infection, 8%; osteonecrosis of jaw: Gr3, 8% |
| Kankaanranta et al. [7] | 30 | FiR 1250-kW Triga Mark II nuclear research reactor Two-fractional BNCT using 10B-BPA with 3-5 week interval, administered with mucosa physical dose of 6 Gy or less and other dose restriction for normal tissue dose |
For acute AEs, oral mucositis, 53%; fatigue, 33%; oral pain, 53%; pharyngeal mucositis, 17%; dysphagia, 17%; trismus, 10%; xerostomia, 3%; nausea, 10%; tumor pain, 17%; myositis, 3%; pneumonia, 10%; osteoradionecrosis, 3%; ulceration, 3%; diplopia, 3%; soft-tissue necrosis, 3%; septicemia, 3%; oliguria, 3%. For late AEs, fatigue, 18%; xerostomia, 20%; dysphagia, 19%; cataract, 19%; pneumonia, 35%; dry eyes, 19%; osteoradionecrosis, 20%; vomiting, 6%; periodontal disease, 6%; ulceration, 6%; soft-tissue necrosis, 6%; septicemia, 12%; diplopia, 6%. |
| Wang et al. [6] | 17 | Tsing-Hua Univ. 2-MW Triga Conv research reactor Two-fractional BNCT using 10B-BPA with median 28-day interval, administered with tumor D80 of 20–25 Gy-Eq and other restrictions. |
For acute AEs, mucositis, 29%; dysphagia, 6%; tumor pain, 6%; hemorrhage, 12% (Gr4, 6%); Infection (soft tissue), 12%; pneumonia, 6%; edema (H&N), 6%; edema (laryngeal), 6% (Gr4). For late AEs, fatigue, 6%; cranial neuropathy, 12%; soft tissue necrosis, 6%; pain, 6%; keratitis, 6%; infection (soft tissue), 6%. |
Abbreviations: BNCT, boron neutron capture therapy; Univ., University; 10B-BPA, 10B-boronophenylalanine; D max, maximal dose to the indicated volume; D80, dose to 80% of the indicated volume; AE, adverse event; Gr, grade; H&N, head and neck.
Fig. 4.
Relationship between beam incidence position and propensity for adverse events. Frequency of adverse events in the FL, FF and N groups. White, dotted, striped and black bars represent the probability of Grade 1, 2, 3 and 4 adverse events, respectively. Abbreviations: FL, face/lateral; FF, face/front; N, neck.
Mucositis occurs in most patients with head and neck cancer treated using chemoradiotherapy. Bonner et al. reported that grade 3 mucositis occurred in 56% of patients in a phase III trial of radiotherapy plus cetuximab [24]. Care for prevention of adverse event exacerbations is thus considered important. Although mucositis exacerbated to grade 3 or higher in only one case in the present study, maintaining the cleanliness of the oral environment by regularly scheduled gargling immediately after BNCT and early application of steroid ointment through careful observation may avoid exacerbation of stomatitis, and further investigations for merits of these intervention are required. The usefulness of cooling with an ice ball to reduce blood flow and metabolism of the oral mucosa during boron drug administration and gargling with inhibitors of amino acid transporters acting on 10B-BPA uptake with the aim of reducing drug accumulation in the oral mucosa, may also become the key interventions. Similarly, as a prophylaxis for parotitis and submandibular glanditis, the possibility of excretion of 10B-BPA from glandular tissues before neutron irradiation by promoting salivary drainage through oral administration of lemon juice or vitamin C may also be considered.
In chemoradiotherapy, gastrointestinal symptoms such as loss of appetite, nausea and vomiting developed depending on the chemotherapy used in combination with radiotherapy, and nausea, vomiting and anorexia were observed in the RTOG1016 study in 19.1%, 12.1% and 22.4% of received patients, respectively, in the CDDP plus IMRT groups, as well as 8.1%, 4.1% and 15.5% in the cetuximab plus IMRT group, respectively [25]. Compared to those results, the incidence of gastrointestinal symptoms in JHN002 was much higher. Nausea and vomiting are reported as the most distressing toxicities to patients, and some guidelines recommend strict antiemetic therapy for difficult-to-control delayed emesis [26]. In the JHN002 study, 80% of patients developed nausea and 48% experienced vomiting; the cause of BNCT-induced nausea and vomiting is still poorly understood and was treated with common dopamine receptor antagonists, corticosteroid and 5-HT3 receptor antagonist and H1 receptor antagonist. In JG002, a phase II trial of accelerator BNCT for recurrent malignant glioma, the incidence of nausea and vomiting was not high, at 25.9% and 14.8%, respectively. This suggests that nausea and vomiting are not drug-induced adverse events, but some kind of irradiation site-specific effect of the head and neck tissues, rather than a direct effect on the central nervous system. From this point of view, it is possible that the nausea same as which occurs in photon therapy may be strongly induced based on the tissue-specific accumulation of boron drug. Alternatively, the possibility is also not denied that an unknown different mechanism is working on the incidence. In fact, although one of the objectives of this study was to evaluate whether there was an irradiation site-specific mode of onset of nausea and vomiting, unfortunately, the irradiation site specificity could not be clarified. The small number of cases evaluated may have been a limitation of this study, and further investigation is necessary in the future. Considering the frequency of emesis in BNCT, the nausea and vomiting caused by BNCT are very appropriately classified as an intermediate risk in the chemotherapy emetic risk category, and standard guideline-based prophylactic antiemetic therapy should be provided. Based on the study results, prophylactic antiemetic treatment should be started from the onset of emesis; more specifically, one or two days after treatment. However, even after the JHN002 trial, it is still unclear which drugs are effective in treatment for nausea and vomiting, and the first step should be to find effective antiemetic agents for BNCT-induced nausea and vomiting.
In this analysis, as a profile of irradiation site-specific adverse events, it was considered that irradiation from the front of face would induce epistaxis and conjunctivitis as a result of a wider distribution of thermal neutrons from the incident point and higher doses given to the nasal mucosa and eyes. On the other hand, with regard to the oral cavity dose, the anterior beam delivers the dose to a wide area from the palate to the buccal mucosa, but the incidence of stomatitis may have been increased due to a previous history of irradiation in 67% of the cases. The incidence of parotitis and alopecia remained high in the lateral neck irradiation, which was same as in the lateral face irradiation, despite the fact that the irradiation was delivered from an inferior side than the mandible. This was thought to be due to the large lateral diffusion of neutrons in the tissue without a direct track, and the large contribution of the 10B(n,α)7Li reaction in the parotid gland and scalp hair roots with high boron accumulation or high CBE or both. Therefore, even if the glandular tissue or scalp is farther from the center of the irradiation axis, the occurrence of parotitis and alopecia based on the neutron distribution characteristics of BNCT is an event that should be fully explained to the patient before treatment. In the cases irradiated from the lateral of the face, including patients with tumors localized in the parotid gland and external auditory canal, nausea was observed in all cases, and vomiting was most frequently observed compared to the other groups. However, we did not find significant differences in any of the adverse events from the other groups. The number of patients in this analysis was small, and the statistical power to find significant differences may have been weak. Significant results may also have been biased and should be interpreted with caution. In order to find accurate correlations between irradiated site and the frequency of adverse events, further accumulation and continuous study of cases with high-quality observations of post-treatment follow-up are required.
Limitations in this study included the small number of patients, different histories of prior radiotherapy and chemotherapy, and the fact that the data were not subjective evaluations, such as patient-reported outcomes. With the widespread use of this treatment, the relationship between position of beam incidence and risk of adverse events will be elucidated even more clearly through studies of large patient populations. In addition, important adverse events that should be controlled need to be identified based on patient subjective assessment and to develop prophylactic treatments for these adverse events.
In conclusion, the present study provides a profile of the adverse events from accelerator-based BNCT for head and neck cancer. This profile may be useful for interventions to prevent the development or exacerbation of treatment-related adverse events on BNCT.
ACKNOWLEDGMENT
The authors are grateful to the patients, families and caregivers and all investigators involved in this study.
Contributor Information
Katsumi Hirose, Southern Tohoku BNCT Research Center, 7-10 Yatsuyamada, Koriyama, 963-8052, Japan; Department of Radiation Oncology, Hirosaki University Graduate School of Medicine, 5 Zaifu-cho, Hirosaki, 036-8562, Japan.
Mariko Sato, Southern Tohoku BNCT Research Center, 7-10 Yatsuyamada, Koriyama, 963-8052, Japan; Department of Radiation Oncology, Hirosaki University Graduate School of Medicine, 5 Zaifu-cho, Hirosaki, 036-8562, Japan.
Takahiro Kato, Southern Tohoku BNCT Research Center, 7-10 Yatsuyamada, Koriyama, 963-8052, Japan; Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan; School of Health Sciences, Fukushima Medical University, 10-6 Sakaemachi, Fukushima, 960-8516, Japan.
Kanako Takayama, Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan.
Motohisa Suzuki, Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan.
Hisashi Yamaguchi, Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan.
Ichiro Seto, Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan.
Yasuhiro Kikuchi, Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan.
Masao Murakami, Department of Radiation Oncology, Southern Tohoku Proton Therapy Center, 7-172 Yatsuyamada, Koriyama, 963-8052, Japan.
Yoshihiro Takai, Southern Tohoku BNCT Research Center, 7-10 Yatsuyamada, Koriyama, 963-8052, Japan.
CONFLICT OF INTEREST
The author declare they have no conflicts of interest.
FUNDING
This study was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (Grant Number JP20H03631).
CLINICAL TRIAL REGISTRATION NUMBER
JapicCTI-194 640; UMIN000040501.
References
- 1. Hirose K, Konno A, Hiratsuka J et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): an open-label phase II trial. Radiother Oncol 2021;155:182–7. [DOI] [PubMed] [Google Scholar]
- 2. Sakurai Y, Kobayashi T. The medical-irradiation characteristics for neutron capture therapy at the heavy water neutron irradiation Facility of Kyoto University Research Reactor. Med Phys 2002;29:2328–37. [DOI] [PubMed] [Google Scholar]
- 3. Kankaanranta L, Seppala T, Koivunoro H et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys 2012;82:e67–75. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki M, Kato I, Aihara T et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J Radiat Res 2014;55:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haapaniemi A, Kankaanranta L, Saat R et al. Boron neutron capture therapy in the treatment of recurrent laryngeal cancer. Int J Radiat Oncol Biol Phys 2016;95:404–10. [DOI] [PubMed] [Google Scholar]
- 6. Wang LW, Chen YW, Ho CY et al. Fractionated boron neutron capture therapy in locally recurrent head and neck cancer: a prospective phase I/II trial. Int J Radiat Oncol Biol Phys 2016;95:396–403. [DOI] [PubMed] [Google Scholar]
- 7. Kankaanranta L, Seppala T, Koivunoro H et al. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: a phase I study. Int J Radiat Oncol Biol Phys 2011;80:369–76. [DOI] [PubMed] [Google Scholar]
- 8. Nigg, DW, Wemple, CA, Wessol, DE et al. SERA -- An advanced treatment planning system for neutron therapy and BNCT. Transactions of the American Nuclear Society; Journal Volume: 80; Conference: 1999 annual meeting of the American Nuclear Society (ANS), Boston, MA (United States), 6–10 Jun 1999. [Google Scholar]
- 9. White DR, Griffith IJ, Wilson IJ. Report 46. Journal of the International Commission on Radiation Units and Measurements 1992;24. [Google Scholar]
- 10. Hiratsuka J, Fukuda H, Kobayashi T et al. The relative biological effectiveness of 10B-neutron capture therapy for early skin reaction in the hamster. Radiat Res 1991;128:186–91. [PubMed] [Google Scholar]
- 11. Coderre JA, Makar MS, Micca PL et al. Derivations of relative biological effectiveness for the high-let radiations produced during boron neutron capture irradiations of the 9L rat gliosarcoma in vitro and in vivo. Int J Radiat Oncol Biol Phys 1993;27:1121–9. [DOI] [PubMed] [Google Scholar]
- 12. Coderre JA, Morris GM, Micca PL et al. Comparative assessment of single-dose and fractionated boron neutron capture therapy. Radiat Res 1995;144:310–7. [PubMed] [Google Scholar]
- 13. Provenzano L, Koivunoro H, Postuma I et al. The essential role of radiobiological figures of merit for the assessment and comparison of beam performances in boron neutron capture therapy. Phys Med 2019;67:9–19. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka H, Sakurai Y, Suzuki M et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl Instrum Methods Phys Res, Sect B 2009;267:1970–7. [Google Scholar]
- 15. Tanaka H, Sakurai Y, Suzuki M et al. Improvement of dose distribution in phantom by using epithermal neutron source based on the be(p,n) reaction using a 30 MeV proton cyclotron accelerator. Appl Radiat Isot 2009;67:S258–61. [DOI] [PubMed] [Google Scholar]
- 16. Aihara T, Morita N, Kamitani N et al. BNCT for advanced or recurrent head and neck cancer. Appl Radiat Isot 2014;88:12–5. [DOI] [PubMed] [Google Scholar]
- 17. Joensuu H, Kankaanranta L, Seppala T et al. Boron neutron capture therapy of brain tumors: clinical trials at the Finnish facility using boronophenylalanine. J Neuro-Oncol 2003;62:123–34. [DOI] [PubMed] [Google Scholar]
- 18. Kawabata S, Miyatake S, Kajimoto Y et al. The early successful treatment of glioblastoma patients with modified boron neutron capture therapy. Report of two cases. J Neuro-Oncol 2003;65:159–65. [DOI] [PubMed] [Google Scholar]
- 19. Miyatake S, Kawabata S, Kajimoto Y et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg 2005;103:1000–9. [DOI] [PubMed] [Google Scholar]
- 20. Kageji T, Nagahiro S, Matsuzaki K et al. Boron neutron capture therapy using mixed epithermal and thermal neutron beams in patients with malignant glioma-correlation between radiation dose and radiation injury and clinical outcome. Int J Radiat Oncol Biol Phys 2006;65:1446–55. [DOI] [PubMed] [Google Scholar]
- 21. Kawabata S, Miyatake S, Kuroiwa T et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 2009;50:51–60. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto T, Nakai K, Kageji T et al. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol 2009;91:80–4. [DOI] [PubMed] [Google Scholar]
- 23. Kawabata S, Hiramatsu R, Kuroiwa T et al. Boron neutron capture therapy for recurrent high-grade meningiomas. J Neurosurg 2013;119:837–44. [DOI] [PubMed] [Google Scholar]
- 24. Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8. [DOI] [PubMed] [Google Scholar]
- 25. Gillison ML, Trotti AM, Harris J et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berger MJ, Ettinger DS, Aston J et al. NCCN guidelines insights: Antiemesis, version 2.2017. J Natl Compr Cancer Netw 2017;15:883–93. [DOI] [PubMed] [Google Scholar]