Abstract
Interactions between intestinal microbiota and the central nervous system profoundly influence brain structure and function. Over the past 15 years, intense research efforts have uncovered the significant association between gut microbial dysbiosis and neurologic, neurodegenerative, and psychiatric disorders; however, our understanding of the effect of gut microbiota on quantitative neuroimaging measures of brain microstructure and function remains limited. Many current gut microbiome studies specifically focus on discovering correlations between specific microbes and neurologic disease states that, while important, leave critical mechanistic questions unanswered. To address this significant gap in knowledge, quantitative structural and functional brain imaging has emerged as a vital bridge and as the next step in understanding how the gut microbiome influences the brain. In this review, we examine the current state-of-the art, raise awareness of this important topic, and aim to highlight immense new opportunities – in both research and clinical imaging – for the imaging community in this emerging field of study. Our review also highlights the potential for preclinical imaging of germ-free and gnotobiotic models to significantly advance our understanding of the causal mechanisms by which the gut microbiome alters neural microstructure and function.
Keywords: gut microbiome, gut-brain axis, magnetic resonance imaging, fMRI, diffusion tensor imaging
INTRODUCTION
Over the past two decades, surging interest in gut microbiome (GM) research has uncovered the GM’s expansive role in human health and disease [1, 2]. As with nearly all major organ systems, the central nervous system (CNS) is no less susceptible to changes in the GM. Facilitated by metabolic, endocrine, and immune actions, the gut-brain axis (GBA) is now understood to represent a robust bidirectional relationship between the GM and the CNS, in which secreted bacterial products like short chain fatty acids, bioactive molecules, or neuroactive gut bacterial metabolites influence neuronal structure and function [3] and link the emotional and cognitive centers of the brain with peripheral systems. Emerging evidence has highlighted the GM’s ability to shape complex social and emotional behaviors in both mice and humans [3–5] and has brought to light the role of the GM in neurologic and neuropsychiatric health throughout the lifespan.
Research into the GBA has revealed that perturbations of the GM are associated with a wide variety of CNS diseases (Figure 1). These include psychiatric illnesses such as schizophrenia, anxiety, depression, and obesity [6–8]; neurologic disorders like autism spectrum disorder (ASD), Parkinson’s disease, and Alzheimer’s disease [9–11]; and neurocognitive dysfunction in intestinal diseases like irritable bowel syndrome (IBS) [12]. Spurred by technologic, computational, and methodological advances over the past decade, a growing number of GM studies have begun to leverage metagenomic approaches to analyze GMs and have uncovered distinct associations between neurologic disease states and specific strains of bacteria. While early metagenomic studies were typically restricted to preclinical models [13–15], the vast majority of reports have turned their attention to human clinical samples in an attempt to identify bacterial species that may be directly associated with specific disease states [16–20].
Figure 1:
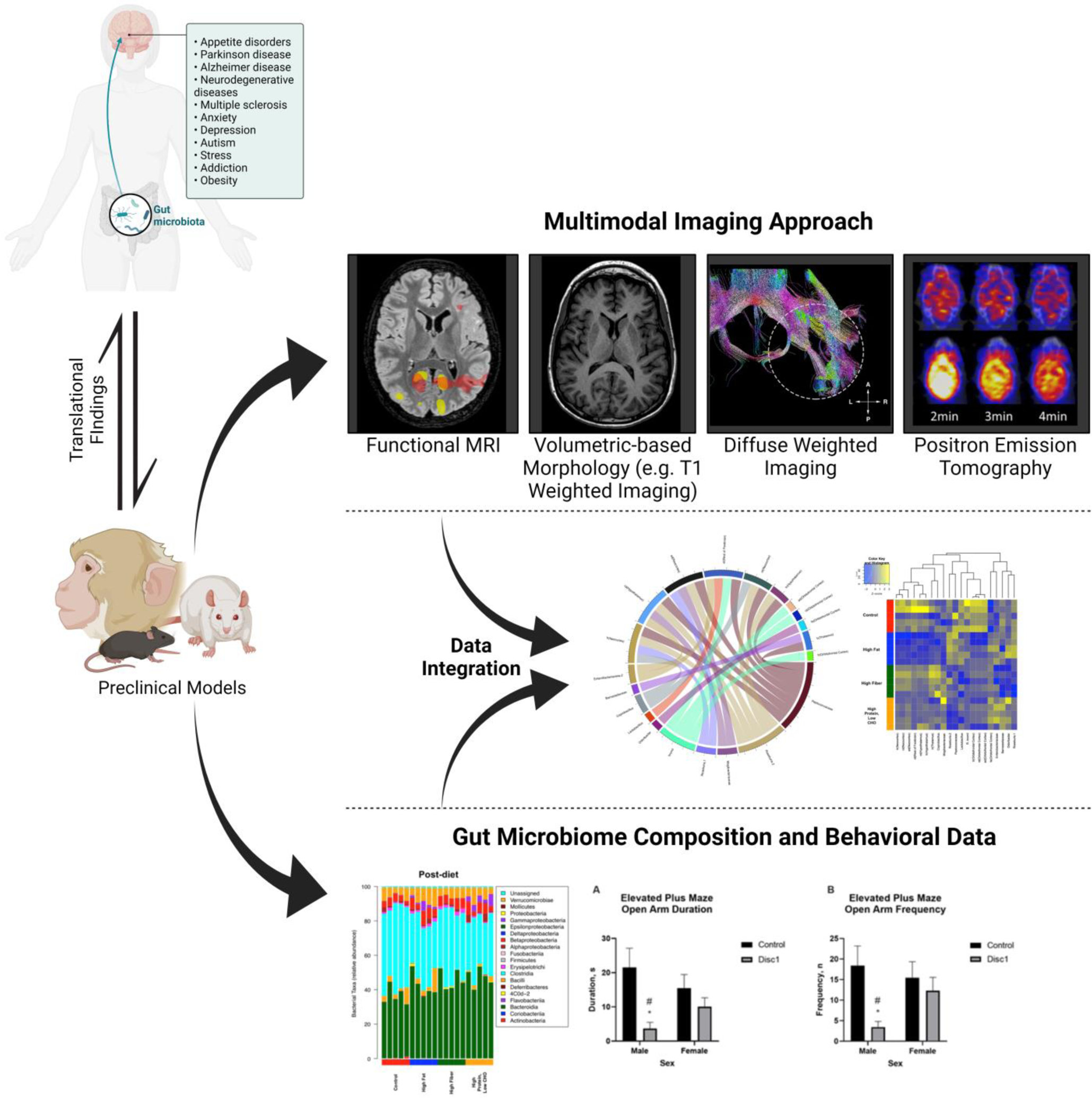
Multimodal imaging approach and gut microbiome composition and behavioral data
While more of these GM-disease associations are readily being discovered [21–24], studies investigating the causal role of the GM in disease remain limited. The few pioneering studies include reports such as Erny et al [25] who demonstrate that germ-free (GF) mice and antibiotic treatment of specific-pathogen free mice had malformed microglia, disrupted circulating microglial maturation factors, and changes in genes associated with microglial maturation and work from Sharon and colleagues [26], who showed that fecal matter transplantation of patients with autism spectrum disorder (ASD) into mice promotes altered ASD-like behavior in mice with concomitant changes in neuronal excitability. Causal and mechanistically oriented studies such as these are critical to advancing the field and, though preclinical in nature, collectively represent the first steps towards linking the GM, gut microbial dysbiosis, and specific bacterial genera and species to neurologic and neuropsychiatric illness.
As these first vital steps towards understanding the causal relationship between the GM and neurologic health begin to take place, neuroimaging tools, such as magnetic resonance imaging (MRI), rapidly emerges as an essential bridge towards clinically translating our emerging understanding of the GBA to the clinic. Neuroimaging techniques can provide unique insights into GBA-associated psychiatric conditions and neurobiological disorders [27–30]. Quantitative neuroimaging data can also be extracted and mined to detect patterns in functional or structural changes associated with specific disease states [31]. As new causal and mechanistic links between the GM and CNS are made, associated neuroimaging biomarkers will also be critical for translating these insights into improved clinical diagnostics and subsequent therapeutic monitoring. In the era of multi-omics and big data, the combination of radiologic outputs and microbial metagenomic data heralds untapped potential for both the development and subsequent clinical application of novel GM-neuroimaging biomarkers (Figure 1) [32]. In this review, we highlight and discuss the current technologies, roles, and applications of neuroimaging in studies of the GBA and then explore new directions, opportunities, and the clinical translational potential of neuroimaging in studies of the GBA.
Neuroimaging the Gut-Brain-Axis
Functional Neuroimaging
Functional MRI (fMRI) is an imaging technique that measures brain activity by detecting changes associated with blood flow under the hypothesis that cerebral blood flow levels and neuronal activation are tightly coupled [33]. Thus, using blood-oxygen-level-dependent (BOLD) imaging, the hemodynamic activity of specific brain regions can be assessed by detection of relative levels of oxyhemoglobin and deoxyhemoglobin based on their magnetic susceptibility. Alterations in neural activity can be evoked either by asking or training a subject to perform a task (task-based) or spontaneously when the subject is at rest (resting state) [34–37]. In both instances, resting state (rsfMRI) and task-based fMRI studies have uncovered significant alterations in brain function and connectivity due to alterations in the gut microbiome. In one example, GM perturbations were correlated with differences in functional connectivity (FC) in a study of 22 patients with amnestic mild cognitive impairment and found that differences in GM composition was negatively associated with activity of the cerebellar vermis [38]. Similarly, Labus et al [39] conducted a study of 65 patients with irritable bowel syndrome (IBS) and 21 healthy patients and found associations between GM variations, gastrointestinal (GI) sensorimotor function, and FC. rsfMRI has also been used to detect effects on the brain following the consumption of probiotics and after treatment with antibiotics. These novel studies represent an emerging strategy to assess the effect of specific gut microbiota on neural function and structure. In one such study Tillisch, et al [5] studied the effects of consuming fermented milk products and probiotics in 12 healthy women and discovered altered activity within a periaqueductal, gray-seeded network following treatment. In another study, 45 healthy volunteers consuming probiotics had differences in FC compared to control subjects in the default mode network (DMN), salience network, visual network, and middle and superior frontal gyrus network [40]. In addition to consumption studies, systems-based investigations have also facilitated the discovery of further complex associations between the gut microbiome and functional connectivity in the brain. rsfMRI scans of 39 1-year-old infants were analyzed and correlated to both stool microbiome analysis and cognitive testing at 1 and 2 years of age and found that GM diversity and cognitive outcomes at 2 years were associated with increased FC between the supplementary motor area and the inferior parietal lobule [41]. Another study of 99 female patients with IBS found higher connectivity in the left DMN to the left basal ganglia and from the right somatosensory network to the right basal ganglia to develop a brain-gut interactome map [42]. As described by the previous studies, the use of rsfMRI for GBA research provides an explanation for how the GM may be affecting resting state neural activity.
Complementing resting state approaches, task-based fMRI can provide novel insights into the functional organization and influence of gut microbiota on brain regions that are activated during specific cognitive demands or actions. For example, in the aforementioned Tillisch et al [5] consumption study, a standardized emotional attention task demonstrated decreased BOLD signal in the primary viscerosensory and somatosensory cortices when compared to a control subjects. Another study by Tillisch et al [43] administered an emotion induction task to 40 healthy woman and found an increased negative affect and decreased hippocampal BOLD response to negative images in women with greater abundances of Prevotella. In another study, 44 probiotic-treated IBS patients were given a task presenting fearful and happy faces, resulting in reduced engagement of the amygdala and frontal and temporal cortices as well as heightened engagement of the occipital regions in response to fear stimuli relative to control subjects; however, no compositional differences in the GM were noted [44]. A similar study by Bagga et. al [45] of 45 healthy women treated with probiotics resulted in a subtle change in GM composition and improved behavioral measures of positive affect and memory performance. Additionally, differences in the activity of cingulum, precuneus, inferior parietal lobule, thalamus, and parahippocampal gyrus was observed during an emotional decision-making task and an emotional recognition memory task [45]. These latter studies demonstrate the utility of task-based fMRI by establishing a foundation for a mechanism as to how GM alterations may be influencing responsive neural activity and its associated behavior.
Structural Neuroimaging: Voxel-based Morphometry
Voxel-based morphometry (VBM) is a computational technique of MRI data that can be used to characterize neuroanatomical variations in brain tissue on a voxel-by-voxel basis [46]. Typically using T1-weighted volumetric imaging, VBM performs statistical tests across all voxels in the image to identify brain volume differences between groups. These measurements contribute to our understanding of neural organization and in combination with GM data, can describe GM-induced volumetric changes. For example, in a study of 89 1-year-olds who were clustered by specific GM abundance, T1 weighted-imaging (T1WI) showed GM-specific grey matter volume differences in the right superior occipital gyrus and after one-year, additional GM-specific volume differences in the left and right caudate nucleus [47]. Similarly, in another study of 29 IBS patients clustered by GM composition, GM-dependent brain volumes were identified with increases in brain volume in sensory regions and decreased volumes in insular and prefrontal cortices, as well as varied cortical thickness and surface area in the insula, right globus pallidus, and motor cortex [48].
With the establishment of strong associations between GM composition and brain structure and function, new questions arise regarding the biological mechanisms mediating these observed changes. However, to characterize the complexity of GM-induced structural changes, extensive experimental control is required. Preclinical animal models provide a degree of experimental control not currently accessible in human studies. This control enables higher resolution and confidence when determining the effects of the GM on brain structure. For example, Lu et al [49] scanned specific pathogen-free (SPF) and germ-free (GF) mice and used T1WI to show regional expansion of the olfactory bulbs and prefrontal cortex in GF mice. Alongside behavioral correlates associated with these brain regions, they demonstrated that the presence of commensal bacteria is necessary for normal neural morphologic development. Another preclinical study which performed T1WI and T2WI neuroimaging of rhesus macaques discovered that nursing-associated increases in Proteobacteria was correlated with decreases in total brain volume, specifically of cortical grey matter volume in both hemispheres [50]. Unfortunately, methodological variations in VBM such as spatial transformations or smoothing procedures can affect results in a manner that may be indistinguishable from variation caused by biological differences [51]. Notably, VBM techniques lack the resolution necessary to identify microstructural changes that may be occurring both at a gastrointestinal or CNS level. Though limited in quantity, these studies use volume-based morphometric imaging techniques to detect gross structural changes occurring in the brain and thus the adaptation of these MR techniques to GBA research provides much needed insight into GM-induced structural variations of grey matter.
Structural Neuroimaging: Quantitative Diffusion Weighted Imaging
The development of VBM and other morphometric analyses unlocked the ability to quantitatively characterize neuroanatomy and, more importantly, to quantify changes occurring in the brain in the disease state. A conspicuous shortcoming of morphometric approaches, such as VBM, is the non-specificity of the gross structural changes observed. To garner greater insight and specificity, new techniques such as diffusion weighted imaging (DWI) were developed [52]. DWI techniques such as diffusion tensor imaging (DTI) measure the rate and pattern of water diffusion in biological tissues. As water diffusion in tissue is influenced by the biophysical organization of the local tissue environment, differences in water diffusivity allow for inferences to be made of tissue microstructure [53]. The first and most extensively used model to relate the microstructural environment of the brain to water diffusion is the diffusion tensor model, which provides measures of fractional anisotropy (FA) and mean diffusivity (MD) that have been interpreted as microstructural indices of white matter structural integrity [52, 54]. Notably, several of the studies previously discussed have called for DTI as a necessary next step in describing the overall mechanism underlying functional abnormalities [47, 55] while others have incorporated DTI into their multimodal approaches. For example, in the Lu et al study [49], DTI was used to observe increased FA in the fimbria, anterior commissure, corpus callosum, optic tract, internal capsule, and the periventricular white matter in SPF mice at earlier developmental stages. The study from Bagga et al [45] used DTI as a complement to their functional analysis to demonstrate no significant differences in FA or mean diffusivity (MD) when compared to baseline before treatment.
These studies use DTI as a complementary modality. However, there are other studies using DTI as their primary imaging technique. A recent study of the GBA used human-to-mouse fecal matter transplant to colonize GF mice with the GM of patients with Attention-Deficit Hyperactivity Disorder (ADHD). DTI analysis revealed decreased FA in right and left hippocampus, and in the right internal capsule and right optic tract, while identifying increased MD in the right hippocampus and fornix, and increased radial diffusivity (RD) in corpus callosum and bilateral hippocampus and right internal capsule when comparing ADHD to control subject gut transplanted animals [56]. An earlier study of 20 patients with obesity revealed that men with obesity had decreased GM diversity and those with a relative increased abundance of Actinobacteria were associated with increased with FA in the amygdala and thalamus [57]. More recently, our group has reported diet-dependent FA increases in the left frontal neocortex as well as identified extensive areas of diet-dependent decreases in RD, axial diffusivity, and MD within rats [58]. Importantly, we were able to identify specific bacterial populations that can be linked to diffusion tensor measurements in ROIs. Although DTI is proving to be an extremely useful modality, its use is currently confined to the brain and has yet to provide the high enough resolution for the spinal cord and even less for peripheral nervous tissue. Additionally, care must be taken when analyzing DTI data (i.e. preprocessing assumptions, poor tractographic interpretation, overprocessing [59]). Still DTI provides tremendous insight into imaging endophenotypes across a range of neurologic and psychiatric diseases and, with high sensitivity, can further our understanding of the modulatory influence that the GM has on neural microstructure.
Metabolic Neuroimaging: Positron Emission Tomography
Positron emission tomography (PET) is a neuroimaging modality that implements the use of radiotracers to visualize and quantify physiologic function and disease. Recently, Giron et al [60] described the potential utility of PET in GBA research, listing various radiotracers being used to label bacteria and bacterial metabolites in addition to common metabolic radiotracers such as [18F]-fluorodeoxyglucose (FDG), a radiolabeled glucose analog. While the aforementioned imaging modalities provide structural information, PET provides cellular-level information which cannot be ascertained from MRI, establishing PET’s role in diagnostic and prognostic assessment.
While others have used PET for peripheral imaging in relation to the GM [61], the current application of PET for neuroimaging the GBA is extremely sparse. One such study used PET to investigate the amyloid pathology in 89 patients with varied cognitive performance. Using [18F]-florbetpir, a radiotracer binding amyloid plaques, they found correlations between specific GM metabolites and regions (frontal, anterior cingulate, precuneus cortex) found to have increased amyloid load as measured via [18F]-florbetpir PET [62]. Another PET study used a mouse model to characterize the bacterial composition of high fat diet dams and correlated this to memory and exploratory behavior dysfunction in the offspring while additionally demonstrating that intranasal insulin administration lowered cerebral glucose metabolism in the thalamus, hypothalamus, hippocampus, amygdala, globus pallidum, striatum, frontal, medial, sensory, and temporal cortex, and cerebellum of the control animals, measured via 18F-FDG PET [63].
The number of studies using PET for neuroimaging the GBA still remains small. While this may be due to the field’s infancy and current lack of neuroimaging incorporation, the utility of PET is evident. Unfortunately, the use of radioactive tools presents a concern for specific patient populations like pregnant individuals and pediatric populations. Additionally, PET’s inability to account for anatomical or structural data results in information that cannot be spatially correlated to specific neural regions, highlighting another limitation of PET-only studies.
CONCLUSION
Neuroimaging of the gut brain axis will be pivotal for expanding our knowledge of the temporal and spatial impact of the gut microbiota on brain structure and function, as well as for realizing translational innovations in the clinic and at the bedside. While early preclinical studies established the fundamental role of the GM in neurologic and neuropsychiatric health, current studies are focused on determining what specific GM populations are associated with disease, particularly in humans [3, 64]. Although these GM compositional associations do not provide a complete mechanistic framework to determine how the GM impacts the brain, they do set the stage for future more hypothesis-driven investigations to better understand the precise role and manner by which gut microbiota contribute to neurologic health throughout the lifespan.
The studies discussed in this review highlight the utility of neuroimaging to assess the functional, structural, and metabolic neural alterations associated with GM variations. It should be noted that the discussed imaging modalities do not encompass the entirety of techniques currently utilized to describe the actions of the GBA. Other studies use macromolecule proton fraction mapping [49], magnetic resonance spectroscopy [65–67] and R2* MRI [68] to investigate these differences. Yet, to understand the causal relationships within these GM-induced neural changes, greater experimental control is necessary to account for the variability and undiscovered factors of the GM. While many GBA neuroimaging studies have focused on humans with relatively small sample sizes (Table 1), preclinical models offer an accessible means to extend our understanding of the mechanisms by which the GM impacts the brain with rigorous experimental controls and with higher-powered imaging studies. Germ-free and gnotobiotic animal models are being used to examine the role of GM populations in health and disease [15, 25, 26, 56, 69–71]. Adapting neuroimaging techniques to these models can provide longitudinal insights in a non-invasive manner. And, while limited, some have begun to use neuroimaging to reveal the functional and structural neuromodulatory effects of specific non-pathologic or disease-associated GM populations in these preclinical models [49]. In conjunction with behavioral and GM compositional correlates [7, 8, 49, 72–75], neuroimaging modalities can serve as an exciting complementary tool to reveal the underlying causal mechanisms by which the gut microbiome impacts the CNS in both health and disease.
TABLE 1:
Summary of neuroimaging studies of the gut-brain axis
| Species | Imaging Modality | Methodology Altering GM | Outcome | Reference |
|---|---|---|---|---|
| Human | rsfMRI | None – prospective study | GM composition was negatively associated with activity of the cerebellar vermis | [38] |
| Human | rsfMRI | None – prospective study | GM composition was associated with GI sensorimotor function and FC | [39] |
| Human | rsfMRI task-based fMRI | Consumption study | Consumption of fermented milk products resulted in altered activity within a periaqueductal, gray-seeded network; decreased BOLD in primary viscerosensory and somatosensory cortices | [5] |
| Human | rsfMRI | Consumption study | Consumption of probiotics resulted in FC differences in the DMN, salience network, visual network, and middle and superior frontal gyrus network | [40] |
| Human | rsfMRI | None – prospective study | GM composition and cognitive outcomes in 2-year-olds was associated with FC between the supplementary motor area and the inferior parietal lobule | [41] |
| Human | rsfMRI | None – prospective study | Patients with IBS were associated with higher FC in the left DMN to left basal ganglia and from the right somatosensory network to the right basal ganglia | [42] |
| Human | Task-based fMRI | None – prospective study | Prevotella abundance was associated with decreased hippocampal BOLD response to negative images | [43] |
| Human | Task-based fMRI | Consumption study | Probiotic treatment of IBS patients shown emotional faces showed a reduced engagement of the amygdala and frontal and temporal cortices, and heightened engagement of the occipital regions | [44] |
| Human | Task-based fMRI, DTI | Consumption study | Probiotic consumption resulted in a subtle GM composition change and in response to tasks, differences in the activity of the cingulum, precuneus, inferior parietal lobule, thalamus, and parahippocampal gyrus were observed; no differences in FA or MD were observed | [45] |
| Human | Structural brain imaging | None – prospective study | GM-dependent grey matter volume differences in the right superior occipital gyrus were noted and after a year, GM-specific differences were observed in the left and right caudate nucleus | [47] |
| Human | Structural brain imaging | None – prospective study | GM-specific groups of patients with IBS showed increased volumes in sensory regions and decreased volumes in insula, right globus pallidus, and motor cortex | [48] |
| Human | DTI | None – prospective study | Decreased GM diversity in patients with obesity and an abundance of Actinobacteria was positively correlated with FA in the amygdala and thalamus | [57] |
| Human | 18F-Florbetapir Amyloid PET | None – prospective study | Increased amyloid uptake in the frontal, anterior cingulate and precuneus cortex was associated with an increase in bacterial byproducts in plasma | [62] |
| Mouse | Structural brain imaging, DTI | Germ-free mice | GF mice showed regional expansion of the olfactory bulbs and prefrontal cortex; FA was increased in the fimbria, anterior commissure, corpus callosum, optic tract, internal capsule, and the periventricular white matter | [49] |
| Mouse | DTI | Fecal matter transplant to germ-free mice | Fecal matter transplant from patients with ADHD to GF mice showed decreased FA in the right and left hippocampus and in the right internal capsule and right optic tract, and increased MD in the right hippocampus and fornix | [56] |
| Mouse | 18F-FDG PET-CT | High fat diet consumption | Maternal high-fat diet consumption resulted in brain insulin resistance and reduction of memory and exploratory behavior in offspring with an age-dependent change in bacterial composition | [63] |
| Rat | DTI | None – prospective study | Diet-dependent FA increases were observed in the left frontal neocortex | [58] |
| Rhesus macaques | Structural brain imaging | Transition to solid food in developing macaques | Nursing-associated increases in Proteobacteria was negatively correlated with total brain volume, specifically cortical grey matter of both hemispheres | [50] |
Key points:
Alterations to the gut microbiome can significantly influence brain structure and function in health and disease.
Quantitative neuroimaging can help elucidate the effect of gut microbiota on the brain and with future translational advances, neuroimaging will be critical for both diagnostic assessment and therapeutic monitoring.
Abbreviations:
- GM
gut microbiome
- CNS
central nervous system
- GBA
gut-brain axis
- ASD
autism spectrum disorder
- IBS
irritable bowel syndrome
- GF
germ-free
- FC
functional connectivity
REFERENCES
- 1.Gevers D, Kugathasan S, Denson LA, et al. (2014) The treatment-naive microbiome in new-onset Crohn’s disease. Cell host & microbe 15:382–392. 10.1016/j.chom.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murga-Garrido SM, Hong Q, Cross T-WL, et al. (2021) Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 9:117. 10.1186/s40168-021-01061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryan JF, O’Riordan KJ, Cowan CSM, et al. (2019) The Microbiota-Gut-Brain Axis. Physiological Reviews 99:1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 4.Hsiao EY, McBride SW, Hsien S, et al. (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–1463. 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tillisch K, Labus J, Kilpatrick L, et al. (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144:1394–1401.e14014. 10.1053/j.gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait C, Sayuk GS (2021) The Brain-Gut-Microbiotal Axis: A framework for understanding functional GI illness and their therapeutic interventions. European Journal of Internal Medicine 84:1–9. 10.1016/j.ejim.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 7.Bear T, Dalziel J, Coad J, et al. (2021) The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms 9:723. 10.3390/microorganisms9040723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballini A, Scacco S, Boccellino M, et al. (2020) Microbiota and Obesity: Where Are We Now? Biology 9:415. 10.3390/biology9120415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James DM, Davidson EA, Yanes J, et al. (2021) The Gut-Brain-Microbiome Axis and Its Link to Autism: Emerging Insights and the Potential of Zebrafish Models. Frontiers in cell and developmental biology 9:662916. 10.3389/fcell.2021.662916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elfil M, Kamel S, Kandil M, et al. (2020) Implications of the Gut Microbiome in Parkinson’s Disease. Movement Disorders 35:921–933. 10.1002/mds.28004 [DOI] [PubMed] [Google Scholar]
- 11.He Y, Li B, Sun D, Chen S (2020) Gut Microbiota: Implications in Alzheimer’s Disease. Journal of clinical medicine 9:2042. 10.3390/jcm9072042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barandouzi ZA, Lee J, Maas K, et al. (2021) Altered Gut Microbiota in Irritable Bowel Syndrome and Its Association with Food Components. Journal of personalized medicine 11:35. 10.3390/jpm11010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ley RE, Bäckhed F, Turnbaugh P, et al. (2005) Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America 102:11070 LP – 11075. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garner CD, Antonopoulos DA, Wagner B, et al. (2009) Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infection and immunity 77:2691–2702. 10.1128/IAI.01570-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen L, Ley RE, Volchkov PY, et al. (2008) Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455:1109–1113. 10.1038/nature07336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Zhang X, Yu Z, et al. (2018) Altered gut microbiota profile in patients with generalized anxiety disorder. Journal of Psychiatric Research 104:130–136. 10.1016/j.jpsychires.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Ling Z, Zhang Y, et al. (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity 48:186–194. 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 18.Turna J, Grosman Kaplan K, Anglin R, Van Ameringen M (2016) “WHAT’S BUGGING THE GUT IN OCD?” A REVIEW OF THE GUT MICROBIOME IN OBSESSIVE–COMPULSIVE DISORDER. Depression and Anxiety 33:171–178. 10.1002/da.22454 [DOI] [PubMed] [Google Scholar]
- 19.Tilg H, Kaser A (2011) Gut microbiome, obesity, and metabolic dysfunction. The Journal of clinical investigation 121:2126–2132. 10.1172/JCI58109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloemendaal M, Szopinska-Tokov J, Belzer C, et al. (2021) Probiotics-induced changes in gut microbial composition and its effects on cognitive performance after stress: exploratory analyses. Translational psychiatry 11:300. 10.1038/s41398-021-01404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn IS, Yoon J, Diamante G, et al. (2021) Disparate Metabolomic Responses to Fructose Consumption between Different Mouse Strains and the Role of Gut Microbiota. Metabolites 11:342. 10.3390/metabo11060342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson AL, Xia K, Azcarate-Peril MA, et al. (2021) Infant gut microbiome composition is associated with non-social fear behavior in a pilot study. Nature Communications 12:3294. 10.1038/s41467-021-23281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng T, Hilal MG, Wang Y, et al. (2021) Differences in Gut Microbiome Composition and Antibiotic Resistance Gene Distribution between Chinese and Pakistani University Students from a Common Peer Group. Microorganisms 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pace RM, Williams JE, Robertson B, et al. (2021) Variation in Human Milk Composition Is Related to Differences in Milk and Infant Fecal Microbial Communities. Microorganisms 9:1153. 10.3390/microorganisms9061153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erny D, Hrabě de Angelis AL, Jaitin D, et al. (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience 18:965–977. 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharon G, Cruz NJ, Kang D-W, et al. (2019) Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 177:1600–1618.e17. 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T, Chen Z, Gong Q (2021) White Matter-Based Structural Brain Network of Major Depression BT - Major Depressive Disorder: Rethinking and Understanding Recent Discoveries. In: Kim Y-K (ed). Springer Singapore, Singapore, pp 35–55 [DOI] [PubMed] [Google Scholar]
- 28.Oliva A, Torre S, Taranto P, et al. (2021) Neural correlates of emotional processing in panic disorder: A mini review of functional magnetic resonance imaging studies. Journal of Affective Disorders 282:906–914. 10.1016/j.jad.2020.12.085 [DOI] [PubMed] [Google Scholar]
- 29.Gearhardt AN, Yokum S, Orr PT, et al. (2011) Neural Correlates of Food Addiction. Archives of General Psychiatry 68:808–816. 10.1001/archgenpsychiatry.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012) Schizophrenia, neuroimaging and connectomics. NeuroImage 62:2296–2314. 10.1016/j.neuroimage.2011.12.090 [DOI] [PubMed] [Google Scholar]
- 31.Gillies RJ, Kinahan PE, Hricak H (2015) Radiomics: Images Are More than Pictures, They Are Data. Radiology 278:563–577. 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Santis S, Moratal D, Canals S (2019) Radiomicrobiomics: Advancing Along the Gut-brain Axis Through Big Data Analysis. Neuroscience 403:145–149. 10.1016/j.neuroscience.2017.11.055 [DOI] [PubMed] [Google Scholar]
- 33.Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America 87:9868–9872. 10.1073/pnas.87.24.9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashworth E, Brooks SJ, Schiöth HB (2021) Neural activation of anxiety and depression in children and young people: A systematic meta-analysis of fMRI studies. Psychiatry Research: Neuroimaging 311:111272. 10.1016/j.pscychresns.2021.111272 [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Fox PT, Miller KL, et al. (2009) Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences 106:13040 LP – 13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schurz M, Radua J, Aichhorn M, et al. (2014) Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews 42:9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 37.Lee MH, Smyser CD, Shimony JS (2013) Resting-state fMRI: a review of methods and clinical applications. AJNR American journal of neuroradiology 34:1866–1872. 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P, Jia X-Z, Chen Y, et al. (2021) Gut microbiota interacts with intrinsic brain activity of patients with amnestic mild cognitive impairment. CNS neuroscience & therapeutics 27:163–173. 10.1111/cns.13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labus JS, Osadchiy V, Hsiao EY, et al. (2019) Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome 7:45. 10.1186/s40168-019-0656-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagga D, Aigner CS, Reichert JL, et al. (2019) Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. European journal of nutrition 58:1821–1827. 10.1007/s00394-018-1732-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao W, Salzwedel AP, Carlson AL, et al. (2019) Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology 236:1641–1651. 10.1007/s00213-018-5161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osadchiy V, Mayer EA, Gao K, et al. (2020) Analysis of brain networks and fecal metabolites reveals brain-gut alterations in premenopausal females with irritable bowel syndrome. Translational psychiatry 10:367. 10.1038/s41398-020-01071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillisch K, Mayer EA, Gupta A, et al. (2017) Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosomatic medicine 79:905–913. 10.1097/PSY.0000000000000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. (2017) Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 153:448–459.e8. 10.1053/j.gastro.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 45.Bagga D, Reichert JL, Koschutnig K, et al. (2018) Probiotics drive gut microbiome triggering emotional brain signatures. Gut microbes 9:486–496. 10.1080/19490976.2018.1460015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mechelli A, Price CJ, Ashburner KJF and J (2005) Voxel-Based Morphometry of the Human Brain: Methods and Applications. Current Medical Imaging 1:105–113 [Google Scholar]
- 47.Carlson AL, Xia K, Azcarate-Peril MA, et al. (2018) Infant Gut Microbiome Associated With Cognitive Development. Biological psychiatry 83:148–159. 10.1016/j.biopsych.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labus JS, Hollister EB, Jacobs J, et al. (2017) Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 5:49. 10.1186/s40168-017-0260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Synowiec S, Lu L, et al. (2018) Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PloS one 13:e0201829–e0201829. 10.1371/journal.pone.0201829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rendina DN, Lubach GR, Lyte M, et al. (2021) Proteobacteria abundance during nursing predicts physical growth and brain volume at one year of age in young rhesus monkeys. The FASEB Journal 35:e21682. 10.1096/fj.202002162R [DOI] [PubMed] [Google Scholar]
- 51.Henley SMD, Ridgway GR, Scahill RI, et al. (2010) Pitfalls in the use of voxel-based morphometry as a biomarker: examples from huntington disease. AJNR American journal of neuroradiology 31:711–719. 10.3174/ajnr.A1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophysical journal 66:259–267. 10.1016/S0006-3495(94)80775-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panagiotaki E, Schneider T, Siow B, et al. (2012) Compartment models of the diffusion MR signal in brain white matter: A taxonomy and comparison. NeuroImage 59:2241–2254. 10.1016/j.neuroimage.2011.09.081 [DOI] [PubMed] [Google Scholar]
- 54.Basser PJ, Pierpaoli C (1996) Microstructural and Physiological Features of Tissues Elucidated by Quantitative-Diffusion-Tensor MRI. Journal of Magnetic Resonance, Series B 111:209–219. 10.1006/jmrb.1996.0086 [DOI] [PubMed] [Google Scholar]
- 55.Wang YF, Zheng LJ, Liu Y, et al. (2019) The gut microbiota-inflammation-brain axis in end-stage renal disease: perspectives from default mode network. Theranostics 9:8171–8181. 10.7150/thno.35387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tengeler AC, Dam SA, Wiesmann M, et al. (2020) Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 8:44. 10.1186/s40168-020-00816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-Real J-M, Serino M, Blasco G, et al. (2015) Gut Microbiota Interacts With Brain Microstructure and Function. The Journal of Clinical Endocrinology & Metabolism 100:4505–4513. 10.1210/jc.2015-3076 [DOI] [PubMed] [Google Scholar]
- 58.Ong IM, Gonzalez JG, McIlwain SJ, et al. (2018) Gut microbiome populations are associated with structure-specific changes in white matter architecture. Translational Psychiatry 8:6. 10.1038/s41398-017-0022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soares J, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Frontiers in Neuroscience 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giron MC, Mazzi U (2021) Molecular imaging of microbiota-gut-brain axis: searching for the right targeted probe for the right target and disease. Nuclear Medicine and Biology 92:72–77. 10.1016/j.nucmedbio.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 61.Boursi B, Werner TJ, Gholami S, et al. (2018) Functional imaging of the interaction between gut microbiota and the human host: A proof-of-concept clinical study evaluating novel use for 18F-FDG PET-CT. PloS one 13:e0192747–e0192747. 10.1371/journal.pone.0192747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marizzoni M, Cattaneo A, Mirabelli P, et al. (2020) Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. Journal of Alzheimer’s Disease 78:683–697. 10.3233/JAD-200306 [DOI] [PubMed] [Google Scholar]
- 63.Sanguinetti E, Guzzardi MA, Tripodi M, Panetta D, Selma-Royo M, Zega A, Telleschi M, Collado MC, & Iozzo P (2019). Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Scientific reports, 9(1), 12609. 10.1038/s41598-019-48090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez JE, Kahana DD, Ghuman S, et al. (2021) Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Frontiers in endocrinology 12:667066. 10.3389/fendo.2021.667066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janik R, Thomason LAM, Stanisz AM, et al. (2016) Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. NeuroImage 125:988–995. 10.1016/j.neuroimage.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 66.He Y, Kosciolek T, Tang J, et al. (2018) Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. European Psychiatry 53:37–45. 10.1016/j.eurpsy.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 67.Simpson T, Deleuil S, Echeverria N, et al. (2019) The Australian Research Council Longevity Intervention (ARCLI) study protocol (ANZCTR12611000487910) addendum: neuroimaging and gut microbiota protocol. Nutrition journal 18:1. 10.1186/s12937-018-0428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blasco G, Moreno-Navarrete JM, Rivero M, et al. (2017) The Gut Metagenome Changes in Parallel to Waist Circumference, Brain Iron Deposition, and Cognitive Function. The Journal of Clinical Endocrinology & Metabolism 102:2962–2973. 10.1210/jc.2017-00133 [DOI] [PubMed] [Google Scholar]
- 69.Luczynski P, Whelan SO, O’Sullivan C, et al. (2016) Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. European Journal of Neuroscience 44:2654–2666. 10.1111/ejn.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farzi A, Fröhlich EE, Holzer P (2018) Gut Microbiota and the Neuroendocrine System. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 15:5–22. 10.1007/s13311-017-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moughnyeh MM, Brawner KM, Kennedy BA, et al. (2021) Stress and the Gut-Brain Axis: Implications for Cancer, Inflammation and Sepsis. Journal of Surgical Research 266:336–344. 10.1016/j.jss.2021.02.055 [DOI] [PubMed] [Google Scholar]
- 72.So D, Whelan K, Rossi M, et al. (2018) Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. The American Journal of Clinical Nutrition 107:965–983. 10.1093/ajcn/nqy041 [DOI] [PubMed] [Google Scholar]
- 73.Chevalier G, Siopi E, Guenin-Macé L, et al. (2020) Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nature Communications 11:6363. 10.1038/s41467-020-19931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MahmoudianDehkordi S, Arnold M, Nho K, et al. (2019) Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 15:76–92. 10.1016/j.jalz.2018.07.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li B, He Y, Ma J, et al. (2019) Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimer’s & Dementia 15:1357–1366. 10.1016/j.jalz.2019.07.002 [DOI] [PubMed] [Google Scholar]


