Abstract
Background:
Integrins avβ6 and avβ8 are expressed by keratinocytes and transactivate latent TGFβ. In a murine model, integrin mediated activation of TGFβ has been shown to be critical in maintaining skin homeostasis, specifically playing roles in epidermal retention of Langerhans cells and resident memory cells T cells (Trm).
Objective:
We examine expression of Integrins β6 and β8 in human skin, inflammatory skin disease, benign nevi, and melanoma and hypothesize that integrin expression is dysregulated in disease.
Methods:
Using immunohistochemistry, we stained tissue from normal human skin (n=8), psoriasis (n=6), atopic dermatitis (n=6), lichen planus (n=5), benign nevi (n=24), and melanoma (n=25) with anti-integrin β6 and anti-integrin β8 to survey expression pattern. We also performed a retrospective chart review in the melanoma cohort to examine if integrin β6 and β8 expression was associated with increased Breslow depth and worse prognostic staging.
Results:
Here, we show that human keratinocytes express integrins β6 and β8, similar to murine keratinocytes. We also found that inflammatory skin conditions have increased Integrin β6, but not Integrin β8 expression. Furthermore, we identified that melanomas have greatly increased expression of integrin β8 compared to nevi. Additionally, high expression of integrin β8 was correlated with greater Breslow depth at diagnosis and with worse prognostic staging.
Conclusion:
These findings demonstrate that like murine keratinocytes, human keratinocytes express integrin β6 and β8 under steady state conditions. Moreover, altered integrin expression may participate in the development or maintenance of cutaneous inflammation as well as tumor immune evasion.
Keywords: TGFb, melanoma
Introduction
The integrin protein family consists of heterodimeric molecules that are composed of an alpha subunit and a beta subunit. They play major roles in mediating cell-cell adhesions, activating intracellular signaling pathways, and have been shown to be important in a wide range of biological processes including development, immune responses, cell migration, and cell proliferation [1]. There are 18 alpha subunits and 8 beta subunits, which results in 24 distinct types of integrin heterodimers, each with their own specific ligands and tissue expression profiles. Integrins β6 and β8 solely pair with Integrin alpha v. As heterodimers, Integrin avβ6 and Integrin avβ8 selectively bind extracellular ligands that express the Arg-Gly-Asp (RGD) peptide motif giving them the unique ability to bind the latency-associated peptides of transforming growth factor beta (TGFβ) and release biologically active TGFβ from its latency complex [2–4].
In murine skin, Integrin avβ6 and avβ8 expressed by keratinocytes are the primary mediators of TGFβ activation in the epidermis [5]. We have previously reported that avβ8 is expressed primarily by follicular keratinocytes while avβ6 is expressed by interfollicular keratinocytes [5, 6]. Additionally, TGFβ activation by avβ6 and avβ8 expressed by keratinocytes is required to maintain epidermal residence of CD8+ resident memory T cells (Trm) and Langerhans cells (LC) during steady-state [5]. In Itgb6−/− mice, LC located in the interfollicular epidermis failed to maintain epidermal residence while in K14ΔItgb8 mice, LC were absent from the follicular epidermis. In Itgb6−/− Itgb8ΔKC mice in the epidermis, LC were absent. Similarly, after cutaneous vaccinia virus infection, T cells were able to enter the epidermis of Itgb6−/− Itgb8ΔKC mice but failed to differentiate into Trm and establish epidermal residency [5]. In the context of inflammation, murine keratinocytes alter expression of Integrins avβ6 and avβ8. In response to In vitro and in vivo stimuli such as recombinant IL-1β or TNF-α, keratinocytes increase expression of avβ6 but not avβ8 resulting in increased activation of latent TGFβ [6]. In contrast, UVB irradiation reduced keratinocyte expression of both avβ6 and avβ8 resulting in reduced activation of latent TGFβ [5, 6]. Despite the clear importance of keratinocyte expression of integrins avβ6 and avβ8 in mice, their expression pattern in human skin is poorly described.
Integrin avβ6’s and avβ8’s role in carcinogenesis is now beginning to be explored. Various cancers, including colon, pancreatic, breast, ovarian, endometrial, oral squamous cell, and brain metastases, have increased expression of Integrin avβ6 [7–9]. Similarly, Itgb8 mRNA expression has been reported in head and neck squamous cell carcinoma, non-small cell lung cancer, and gynecological cancers [10]. Increased expression of the TGFβ-activating integrins has been associated with increased tumor invasion in an in vitro model, and studies of integrin avβ6 expression in different carcinomas suggest that high expression is associated with decreased survival and is an unfavorable prognostic factor [8, 11].
Integrin avβ6 and avβ8 may be contributing to carcinogenesis in several ways through mediating TGFβ signaling. It is well known that TGFβ promotes the epithelial-to-mesenchymal transition and has modulatory effects on immune cells [12]. Increased expression of integrin avβ6 and TGFβ signaling have been associated with increased invasion and metastasis in oral squamous cell carcinoma, which suggests that TGFβ activating integrins may play a role in promoting invasion [13, 14]. Moreover, TGFβ activation by integrin avβ8 expressed on tumor cells has been shown to suppress macrophage production of chemokines and decrease expression of genes associated with anti-tumor immunity [11, 15]. Recently, tumor expressed Integrin avβ8 has also recently been shown to promote the differentiation and enrichment of immunosuppressive T-regulatory cells dependent on activation of TGFβ [10]. Thus integrin-mediated TGFβ activation likely plays a key role in the tumor progression and potentially immunoevasion.
Here, we examined integrin β6 and β8 expression in normal human skin, human skin affected by inflammatory diseases, and benign nevi and melanomas. We found that similar to murine skin, normal human keratinocytes expressed both integrin β6 and β8, albeit at low levels. Expression of integrin β6 was increased in three distinct inflammatory conditions, but expression of integrin β8 remained largely unchanged. Moreover, we found that melanomas express higher levels of integrin β8 and Integrin β6 compared to nevi. However, only integrin β8 expression was associated with increased Breslow depth and worse prognostic stage.
Materials and Methods
Mice
C57BL/6 (WT) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Itgβ6−/− and Itgβ8ΔKC mice were previously described [5]. Experiments used female age-matched mice between 6–12 weeks of age. All mice were maintained under specific-pathogen-free conditions and all animal experiments were approved by University of Pittsburgh Institutional Animal Care and Use Committee.
Reagents
Antibodies used for flow cytometry and immunofluorescence of murine tissues were all previously described [6]. Antibodies used for human immunohistochemistry: Anti-integrin β6 (polyclonal) was purchased from ThermoFisher (Rockford, IL). Anti-integrin β8 (polyclonal) was purchased from Abcam (Cambridge, MA). BOND polymer refine red detection was purchased from Leica Microsystems (Buffalo Grove, IL).
Immunofluorescence and Imaging (Mice)
Mouse whole skin was collected and mounted in OCT medium and 8 uM tissue cross sections were prepared and stained as previously described [6].
Flow Cytometry
Epidermal single-cell suspensions were prepared and stained for flow cytometry as previously described [6].
Patient Samples
This study was approved by our institution’s Institutional Review Board. Free text searches for re-excision specimens (for normal human skin), psoriasis, atopic dermatitis, lichen planus, benign melanocytic nevi, and melanoma were performed and slides retrieved and diagnoses confirmed by a trained dermatopathologist (JH) and blank slides were cut and de-identified prior to immunohistochemical staining. After cases were stained and scored, cases were re-identified for retrospective chart review. All cases were collected retrospectively prior to this study and available from the University of Pittsburgh Department of Dermatopathology tissue bank.
Immunohistochemistry and Scoring
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue. Deparaffinization, antigen retrieval, immunostaining for integrin β6 and Integrin β8, and chromogen detection were performed by a fully automated Leica Bond III (Leica Biosystems, Buffalo Grove, IL). Slides were semi-quantitatively scored for intensity and/or percent of cells staining positive by a trained dermatopathologist (JH). Breslow depths were measured digitally using NDP. view software on whole slide images (Hamamatsu Photonics, Japan).
Statistics
Statistical analyses were performed with Prism 8 software (Graphpad). A Fisher’s exact test was used to compare the semi-quantitative scoring distributions between normal skin and inflammatory skin conditions, and nevi and melanoma samples. A two-tailed unpaired Student’s t-test was used for comparison of means of two groups. P < 0.05 was considered significant.
Results
Integrin β6 and β8 are both expressed in steady state murine and human skin
In murine epidermis, keratinocytes (KC) can be divided into three subsets based on their location relative to the hair follicle. Interfollicular keratinocytes (IFE) are the keratinocytes residing between hair follicles, isthmus keratinocytes (IM) compose the follicle isthmus, and the bulge keratinocytes (B) compose the follicle bulge. Isthmus and bulge keratinocytes can be differentiated by the expression of epithelial cell adhesion molecule (EpCAM), which is specific to IM KCs [16]. In order to corroborate our prior RTqPCR analysis of sorted keratinocytes [5], we confirmed that mouse keratinocytes express both Integrin avβ6 and avβ8 in a regional specific pattern using immunofluorescence microscopy (IF). IF of skin from wild-type mice demonstrated Integrin avβ6 expression in IFE KCs and bulge KCs, but not IM KCs, where Integrin avβ8 was predominantly expressed by IM and bulge KCs (Figure 1a). Skin harvested from Itgβ6−/− and Itgβ6−/− Itgbβ8ΔKC mice were included as specificity controls (Figure 1a). Analysis of epidermal single cell suspensions revealed an expression pattern similar to that observed with immunofluorescence microscopy (Figure 1b, Supplementary Figure 1a, b). Thus, consistent with our earlier report based on mRNA expression of Itgb6 and Itgb8, mice expression of avβ6 is predominantly expressed by IFE KC while avβ8 is expressed primarily by IM KC and both integrins are highly expressed by bulge KC [5].
Figure 1. Murine and human keratinocytes both express Integrin β6 and Integrin β8.
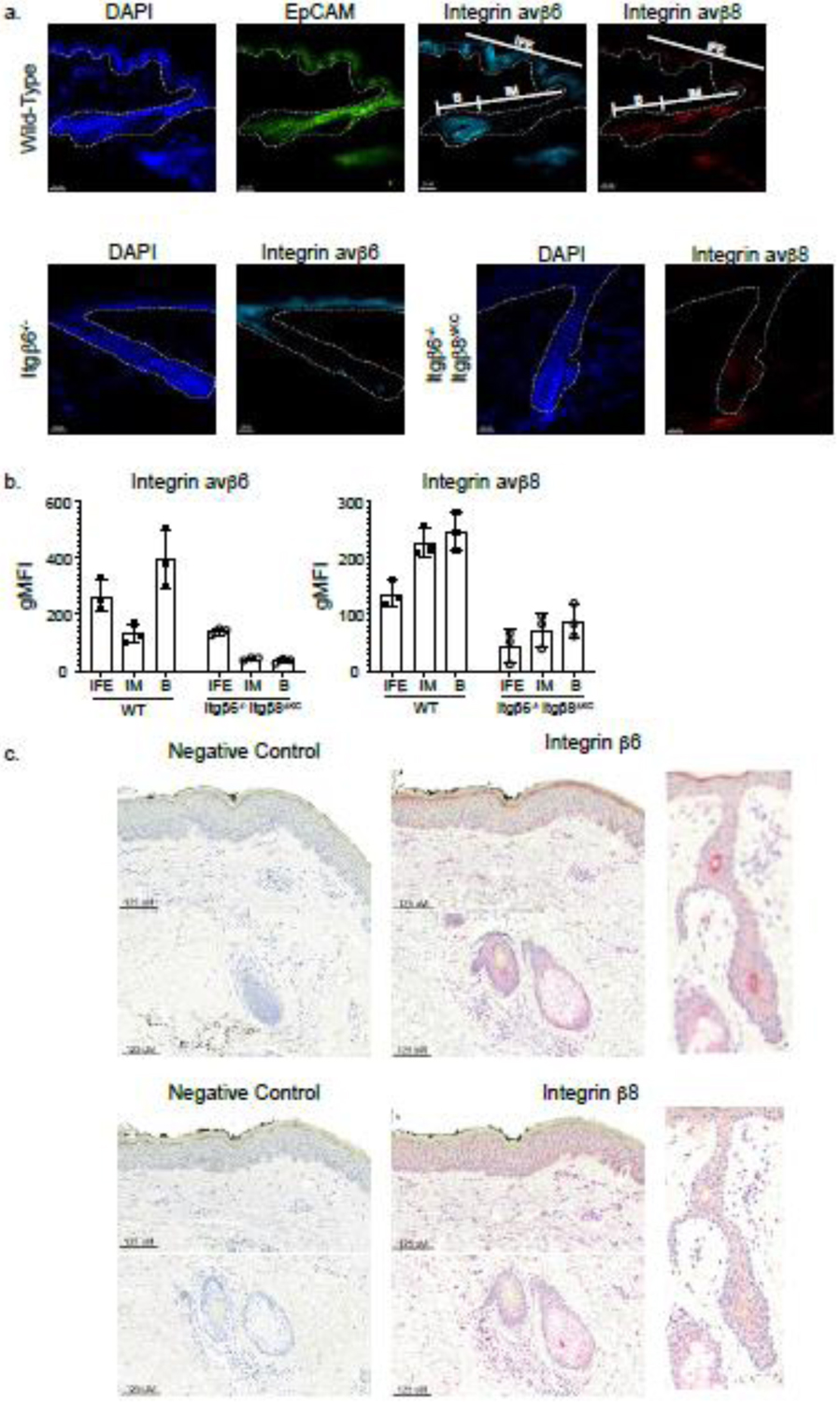
(a) Representative immunofluorescence images of steady-state flank skin transverse sections from wild-type mice or Integrin β6−/− and Integrin β6−/−Integrin β8ΔKC mice are stained for Integrin β6 (teal) and Integrin β8 (red). EpCAM, a marker for isthmus keratinocytes, is stained in green. Regions of interfollicular keratinocytes (IFE), isthmus keratinocytes (IM), and hair bulb (B) are labeled. The white dotted line demarcates the dermal-epidermal junction. (b) Integrin expression by KCs was confirmed by flow cytometry, with IFE KC gated as CD45.2−, CD207−, MHCII−, CD34−, EpCAM−, Sca1+, IM KC gated as CD45.2−, CD207−, MHCII−, CD34−, EpCAM+, Sca1−, and bulge (B) KC gated as CD45.2−, CD207−, MHCII−, CD34+, EpCAM−, Sca1−. (C) Representative immunohistochemistry of steady state human skin and hair follicle (serial sections taken from shoulder) stained with Integrin β6 or Integrin β8 (red). Integrin β6 was scored as 2+ intensity for KCs in the stratum granulosum, 0 intensity for KCs in all other epidermal layers, and 1+ intensity in the hair follicle. Integrin β8 was scored as 2+ intensity for all KCs in the epidermis and hair follicle. Negative control includes the secondary antibody and chromogen only.
We next examined integrin β6 and β8 expression in human keratinocytes. Using immunohistochemistry, we stained normal human skin (n=8) for Integrin β6 and β8. We found that Integrin β6 demonstrated a membranous staining pattern and was expressed by IFE keratinocytes in the stratum granulosum and by keratinocytes in the hair follicle (Figure 1c). Integrin β8 demonstrated a perinuclear staining pattern and was also expressed broadly and diffusely by keratinocytes in all layers of the epidermis including the hair follicle. Integrin β8 expression was also detected by dermal cells, which could represent fibroblasts based on their spindle morphology (Figure 1c). Thus, similar to murine keratinocytes, steady state human keratinocytes also express integrin β6 and β8. Unlike mouse skin, we observed expression of both integrins by keratinocytes throughout the epidermis and hair follicle and expression of Integrin β8 by a population of dermal cells.
Expression of integrin β6 but not Integrin β8 increases in inflammatory skin disease
We have shown in mice increased KC expression of Integrin β6 but not Integrin β8 in response to infection and pro-inflammatory cytokines TNFα and IL-1β [6]. We sought to determine whether this was recapitulated in human skin. Skin biopsies of psoriasis (n=6), atopic dermatitis (n=6) and lichen planus (n=5) were examined by immunohistochemistry for integrin β6 and β8 expression. Cases were semi-quantitatively scored based on intensity of staining, on a scale from 0 to 3+, with 0 signifying negative staining and 3+ signifying the highest intensity staining.
Examination of integrin β6 expression showed clear differences between all inflammatory conditions and normal skin (Figure 2a). Psoriasis, like normal skin, demonstrated 2+ intensity of integrin β6 staining in the stratum granulosum. However, psoriasis cases showed diffusely increased integrin β6 expression throughout the epidermis, in contrast to normal skin, where integrin β6 staining is confined to the stratum granulosum (Fisher’s exact test p = 0.003) (Figure 2a, b). Integrin β6 expression in atopic dermatitis followed a similar pattern to psoriasis, with significantly increased 1+ intensity staining in the stratum spinosum in all six cases (Fisher’s exact test p = 0.003) (Figure 2a, b). Cases of lichen planus showed a distinct expression pattern for integrin β6. In addition to showing a pattern in the stratum spinosum similar to that observed in psoriasis and atopic dermatitis samples, KC in the stratum granulosum showed 1+ staining, compared to 2+ in normal KCs (Fisher’s exact test p = 0.008) and most strikingly, KCs in the stratum basale showed 1+ and 2+ intensity staining localized in close proximity to the lichenoid inflammatory infiltrate, compared to negative staining in normal skin (Fisher’s exact test p = 0.0098) (Figure 2a, b).
Figure 2. Inflammatory skin disorders increase expression of Integrin β6, but not Integrin β8.
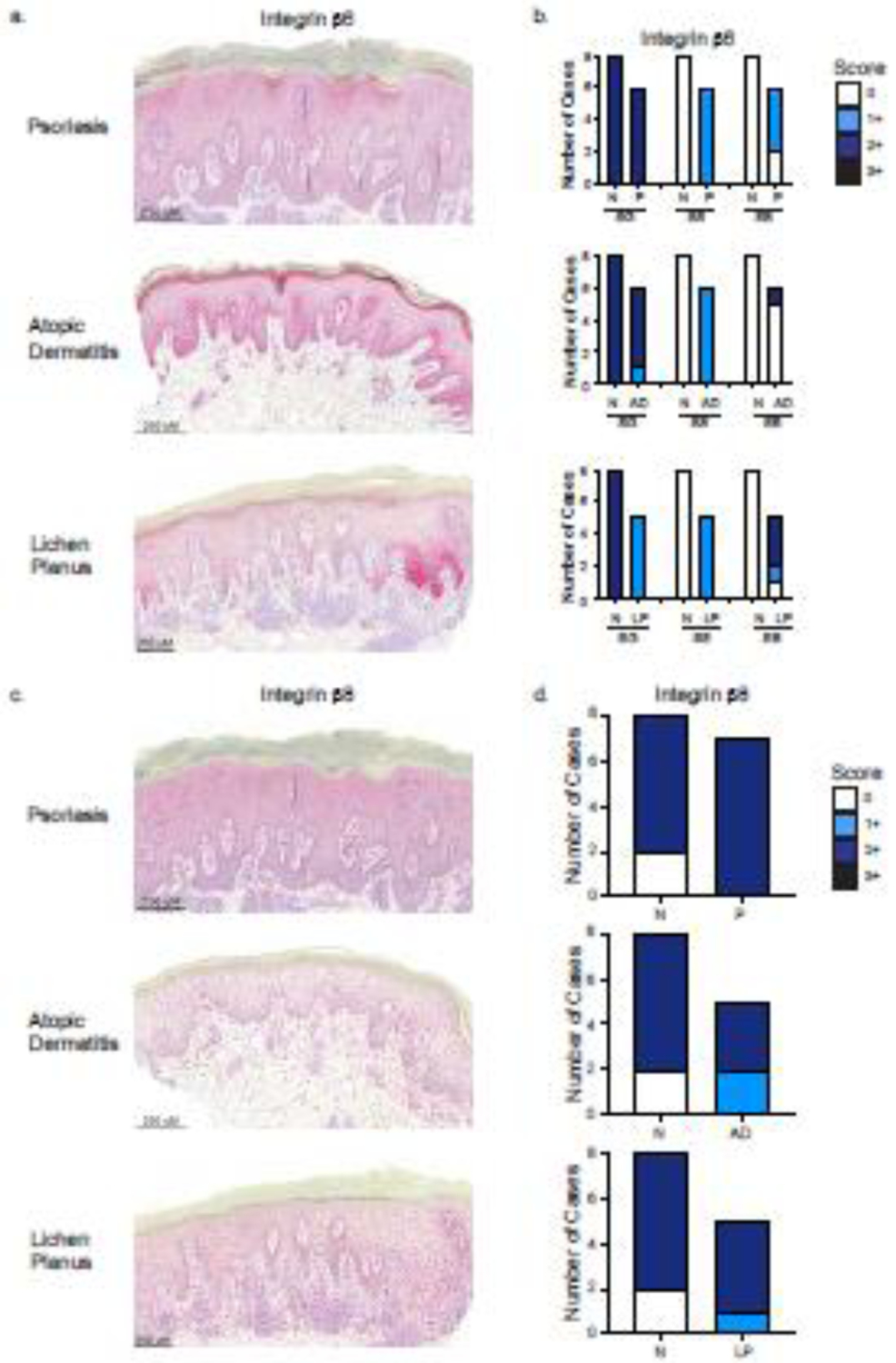
(a) Representative immunohistochemistry images of skin from psoriasis (P), atopic dermatitis (AD), and lichen planus (LP) patients stained with Integrin β6. For β6 expression, image of psoriasis shows 2+ intensity for KCs in stratum granulosum (SG), 1+ intensity for stratum spinosum (SS) and stratum basale (SB). AD shows 2+ intensity for KCs in the SG, 1+ intensity for the SS, and 2+ intensity in SB. LP shows 1+ intensity for KCs in SG, 1+ intensity for KCs in SS, and 2+ intensity in SB. (b) Comparison of semi-quantitative intensity scoring of Integrin β6 expression in P, AD, and LP to normal skin (N) by epidermal layer. (c) For Integrin β8 expression, image of psoriasis shows 2+ intensity in all KCs, atopic dermatitis shows 1+ intensity in all KCs, and lichen planus shows 1+ intensity in all KCs. (d) Comparison of semi-quantitative intensity scoring of Integrin β8 expression in psoriasis (P), atopic dermatitis (AD), and lichen planus (LP) to normal skin (N). Scores range from 0 (negative staining) to 3+ (highest intensity staining).
Integrin β8 was expressed diffusely throughout the epidermis in all cases of psoriasis, atopic dermatitis, and lichen planus (Figure 2c, d). Histological scoring also showed that integrin β8 expression did not differ between any of the inflammatory conditions and normal skin (Figure 2d). Taken together, these cases show that inflammatory skin conditions are associated with increased integrin β6, but not β8, expression. These findings are similar to the patterns of expression in observed in inflamed murine skin suggesting that our findings in mice maybe translatable to cutaneous inflammation in humans.
Melanomas have increased Integrin β8 expression compared to nevi
In addition to inflammatory skin diseases, integrin β6 and β8 expression has not been extensively examined in skin cancers. Because integrin β6 and β8 are important in TGFβ activation in the skin, the expression of these integrins in cancer may contribute to a tumor microenvironment enriched in active TGFβ. We chose to focus on assessing integrin β6 and β8 expression in melanoma with a direct comparison to benign nevi. We used immunohistochemistry and stained for integrin β6 and β8 expression in melanoma (n=25) and nevi (n=24) (Figure 3a). All melanoma cases had an associated lymphocytic infiltrate. Cases were scored based on intensity of the stain (0 to 3+, with 3+ representing the highest intensity) and on percentage of melanocytes or melanoma cells that stained positive (0, 0–25%, 25–50%, >50%). We found that all melanocytic nevi lacked expression of Integrin β6 (Figure 3b). Similar to nevi, fifteen melanoma cases did not express integrin β6. However, nine melanoma cases demonstrated 1+ intensity, two melanoma cases demonstrated 2+ intensity, and one case demonstrated 3+ intensity, for integrin β6 staining (Figure 3b). Further, out of the ten melanoma cases that did express integrin β6, nine cases showed positive staining in 0–25% of melanoma cells and only one case showed positive staining in 25–50% of melanoma cells. No cases showed positive Integrin β6 staining in over 50% of melanoma cells (Figure 3b). A Fisher’s exact test showed that melanomas were more likely to express increased intensity (1+ or greater) of integrin β6 staining compared to nevi and were significantly more likely to have more integrin β6+ melanoma cells (Fisher’s exact test p = 0.006).
Figure 3. Melanomas primarily increase expression of Integrin β8 compared to nevi.
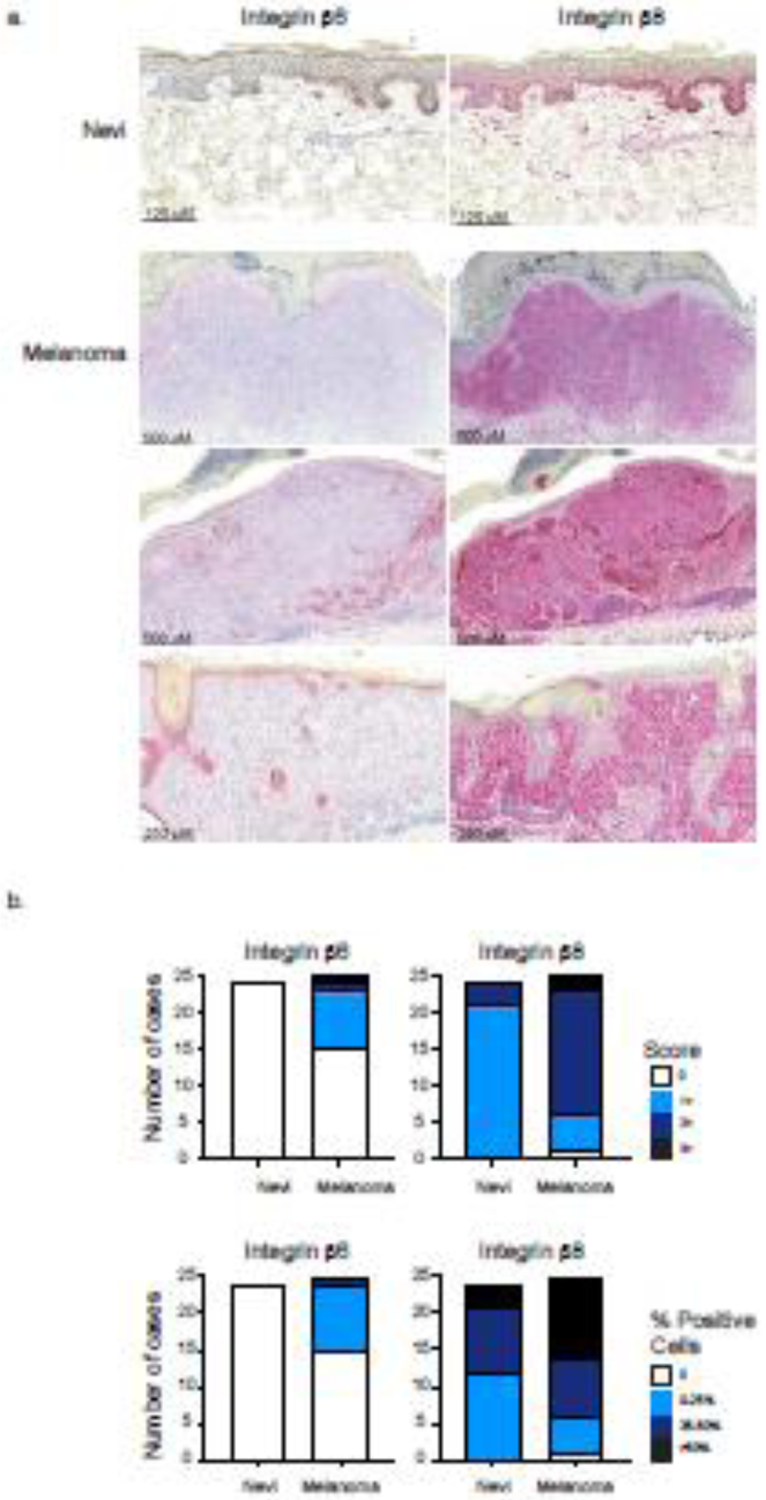
(a) Representative immunohistochemistry images of nevi and melanoma stained with Integrin β6 or Integrin β8 (red). Pictured nevi demonstrates 0 intensity scoring and 0% positive staining melanocytes for Integrin β6 expression and demonstrates 1+ intensity scoring and >50% positive staining melanocytes for Integrin β8 expression. Pictured melanomas from top to bottom demonstrate 0, 2+ (0–25% Itgβ6+ melanoma cells staining positive), and 0 intensity scoring for Integrin β6 and 2+, 3+, and 2+ intensity scoring for Integrin β8. All pictured melanomas show >50% Integrin β8+ melanoma cells. (b) Comparison of scored intensities of Integrin β6 and Integrin β8 expression in melanoma and nevi. Cases were scored as previously described in addition to scoring the percentage of positive staining melanocytes or melanoma cells.
In contrast, we found that 21 nevi demonstrated 1+ intensity and 3 nevi demonstrated 2+ intensity for integrin β8 staining (Figure 3b). Compared to nevi, only five melanomas scored 1+ intensity, with 19 melanomas scoring 2+ or 3+ intensity. Melanomas were more likely to express significantly increased intensity (2+ or greater) of integrin β8 staining compared to nevi (Fisher’s exact test p < 0.0001). In terms of frequency of positive staining cells, 12 nevi showed positive staining for integrin β8 in 0–25% of melanocytes, 9 nevi cases showed positive staining for integrin β8 in 25–50% of melanocytes, and 3 nevi cases showed positive staining for integrin β8 in over 50% of melanocytes (Figure 3b). Analysis also revealed a higher percentage of integrin β8 positive melanoma cells compared to melanocytes found in nevi. 11 melanoma cases showed positive integrin β8 staining in over 50% of melanoma cells, 8 cases showed positive integrin β8 staining in 25–50% of melanoma cells, and 5 cases showed positive integrin β8 staining in 0–25% of melanoma cells (Figure 3b). A Fisher’s exact test showed that melanomas were more likely to have greater amounts of integrin β8 expressing cells (50% or greater) compared to nevi (Fisher’s exact test p < 0.0255). Positive integrin β8 expression was also seen in the inflammatory infiltrate in all melanoma cases.
Taken together, these results show that melanoma cells have higher expression of integrin β8 compared to benign nevi. Although melanomas showed a statistically significant increase in integrin β6 expression, the overall percentage of melanoma cells expressing integrin β6 was considerably lower in comparison to the number of integrin β8 expressing cells.
Retrospective review of melanoma cases indicates that high Integrin β8 expression is associated with increased Breslow depth and higher risk melanoma
We examined the association of Breslow depth and integrin β6 and β8 expression. Because Breslow depth has significant prognostic value in melanoma, we hypothesized that increased integrin β6 and β8 expression positively correlated with increased Breslow depths. We found that increasing percentage of integrin β6+ melanoma cells was not significantly associated with greater Breslow depths (Figure 4a). However, melanomas with >50% Integrin β8+ cells had significantly greater Breslow depths compared to melanomas with 25–50% and 0–25% Integrin β8+ cells, respectively (p<0.05, p<0.01). These findings suggest that more invasive melanomas express higher amounts of Integrin β8, but not Integrin β6.
Figure 4. Increased Integrin β8 expression is positively correlated with Breslow depth and associated with high-risk melanomas.
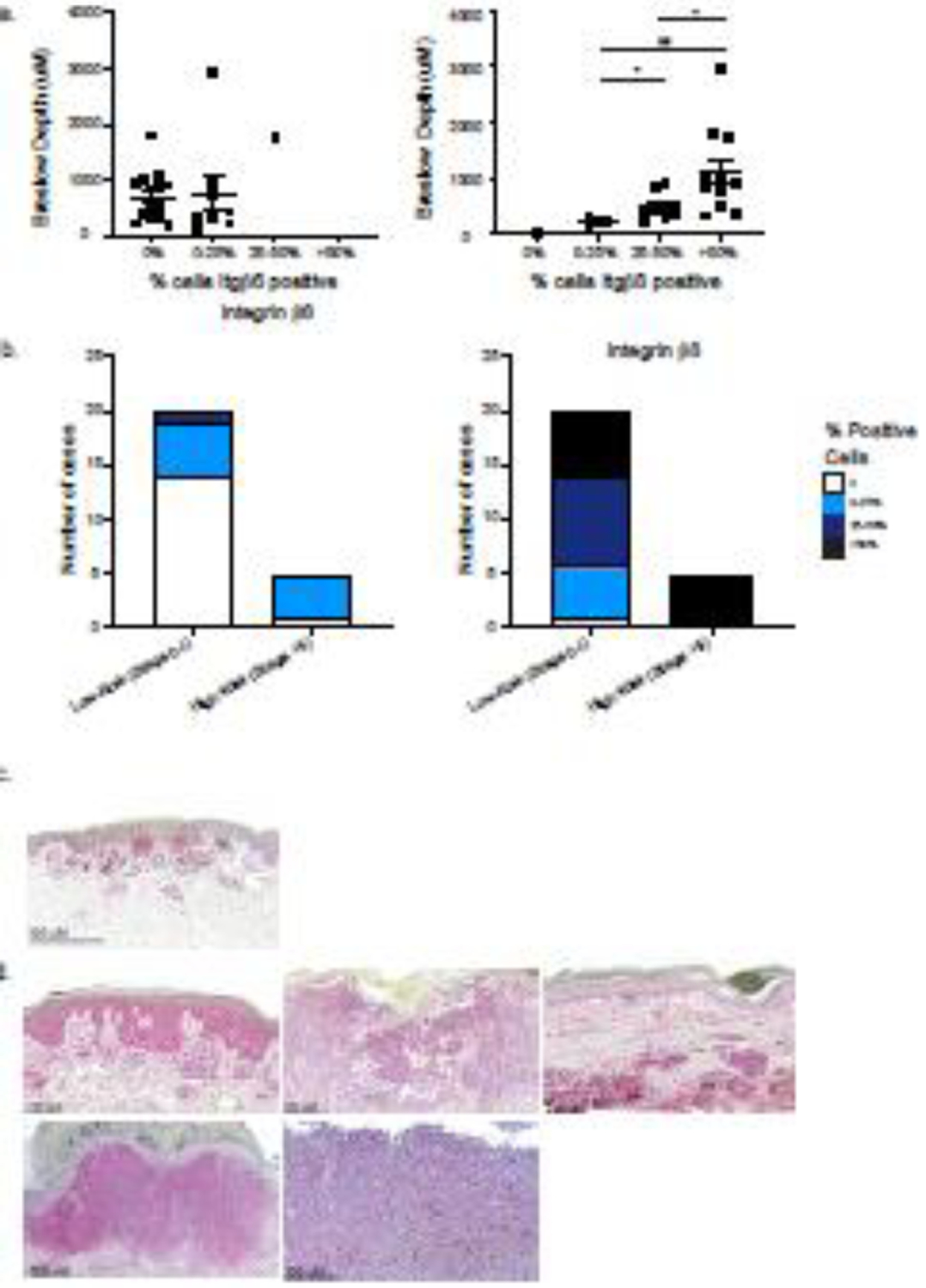
(a) Association of percent of Integrin β6+ or Integrin β8+ melanoma cells and Breslow depth (um). Each symbol represents data from an individual patient. *p<0.05, **p<0.01. Breslow depth was not measured in cases of melanoma in situ (n=2) (b) Melanoma staging information was collected from the corresponding pathology reports. Melanomas were stratified into low risk (pathologic prognostic stage 0 and I) and into high risk (pathologic prognostic stage II and greater) groups, and percentage of Integrin β6 and β8 positive cells were compared between the two groups. (c) Representative image of a low-risk melanoma (Stage IA). (d) Images of all high-risk (Stage II or greater) melanomas.
After observing that melanomas with increased Breslow depths were positively associated with integrin β8 expression, we performed a retrospective chart review to determine whether melanomas with high Integrin β8 expression (>50% melanoma cells staining positive for Integrin β8) were associated with poorer staging. In our study, patients were diagnosed with melanomas ranging from Stage 0 to IV, according to AJCC 8 melanoma pathologic TNM prognostic stage groups [17]. All melanomas were treated surgically with wide local excision or Mohs micrographic surgery and had negative margins after excision. Mean age (sd) at diagnosis was 61.73 (14.3) years. 52% of patients were male and 48% of patients were female. Patient demographics are displayed in Table 1.
Table 1.
Summary of patient demographics from melanoma cases stained for integrin β6 and integrin β8.
| Sex | |
| • Male | 0.52 (13/25) |
| • Female | 0.48 (12/25) |
| Average age (y) at diagnosis | 61.73 |
| • Male | 63.39 |
| • Female | 59.94 |
| Pathologic Prognostic Staging | |
| • 0 | 0.08 (2/25) |
| • IA | 0.52 (13/25) |
| • IB | 0.2 (5/25) |
| • IIA | 0.08 (2/25) |
| • IIB | 0.04 (1/25) |
| • IIIC | 0.04 (1/25) |
| • IV | 0.04 (1/25) |
| Melanoma Subtype | |
| • Superficial spreading | 0.68 (17/25) |
| • Lentigo Maligna | 0.12 (3/25) |
| • Nodular | 0.12 (3/25) |
| • Nevoid | 0.04 (1/25) |
| • Cutaneous metastasis | 0.04 (1/25) |
| Site | |
| • Head/Neck | 0.2 (5/25) |
| • Trunk | 0.32 (8/25) |
| • Upper Extremity | 0.4 (10/25) |
| • Lower Extremity | 0.08 (2/25) |
| Locoregional recurrence | |
| • Yes | 0 (0/25) |
| • No | 0.96 (2425) |
| • Dysplastic nevi | 0.04 (1/25) |
| Metastasis | |
| • Yes | 0.04 (1/25), lung |
| • No | 0.96 (24/25) |
| Disease Specific Death | |
| • Yes | 0.5 (1/2) |
| • No | 0.5 (1/2) |
Patient demographics including sex, average age at diagnosis, pathologic prognostic staging, melanoma subtype, melanoma site, locoregional recurrence, metastasis, and disease specific death were obtained through systematic chart review.
Low risk melanomas had a pathologic TNM prognostic stage of Ib or less (Figure 4c). High risk melanomas were considered to be melanomas with a pathologic TNM prognostic stage of II or greater (Figure 4d). The cases of high-risk melanoma included nodular subtype (3/5), a cutaneous metastasis from an unknown primary (1/5), and a superficial spreading subtype (1/5). The skin sites included scalp (2/5), trunk (1/5), lower extremity (1/5), and upper extremity (1/5) (Table 1). We found that high risk melanomas were significantly associated with increased integrin β8 expression (Fisher’s exact test p = 0.0087) (Figure 4b, 4d). Notably, all cases of nodular melanoma in this cohort expressed high integrin β8 expression. In contrast, high risk melanomas were not significantly associated with increased integrin β6 expression (Fisher’s exact test p = 0.1206) (Figure 4b). Overall, these results suggest that expression of integrin β8, but not integrin β6, is positively associated with high risk and more invasive melanomas and may be a potential biomarker for more aggressive disease.
Discussion
Here, we have shown that steady state human keratinocytes in the epidermis express integrin β6 and β8 at low levels. Integrin β6 expression is mainly found in the stratum granulosum, whereas integrin β8 expression is found in all epidermal layers. Both integrin β6 and β8 are expressed by KCs in the hair follicle. In inflammatory skin diseases, including psoriasis, atopic dermatitis, and lichen planus, we demonstrated that KCs increase integrin β6 expression while integrin β8 expression remains largely unchanged. We also found that nevi and most melanomas do not express integrin β6. Melanocytic nevi expressed integrin β8 at low levels in a minority of cells. In contrast, melanoma cells expressed high levels of integrin β8 in a majority of cells. There was a strong positive correlation between the percentage of melanoma cells expressing integrin β8 and Breslow depth and worse prognostic stage. Based on these results, we conclude that expression of TGFβ activating integrins in steady-state and inflammatory contexts appears similar in murine and human skin. Moreover, the high expression of β8 by melanoma cells compared with melanocytic nevi and the correlation between β8 expression and Breslow depth and prognostic stage may be of diagnostic value.
These data complement our recent findings, where we showed that inflammatory cytokines TNF-α and IL-1β increase integrin avβ6, but not integrin avβ8 mRNA and protein expression in mouse keratinocytes [6]. They also suggest that integrin β8 expression is largely unaffected by inflammatory stimuli. Interestingly, cases of lichen planus showed a distinct pattern of increased integrin β6 expression in localized areas of the stratum basale in close proximity to the inflammatory infiltrate. However, the significance of integrin β6 upregulation in these conditions is not yet clear. One possibility is that inflammatory mediators released by infiltrating leukocytes directly cause an increase in integrin β6 expression by KCs. Another possibility is that increased integrin β6 expression by KCs in inflammatory conditions promotes increased re-epithelialization and contributes to the hyperkeratosis and acanthotic features commonly observed in these conditions. It has previously been shown that avβ6 mediated activation of TGFβ is essential for re-epithelialization of wounded human skin, both in vivo and in vitro [18]. Additionally, epidermal keratinocytes upregulate integrin avβ6 during wound healing, specifically late-stage where there is presence of granulation tissue and epithelial coverage [19]. Thus, it is possible that the chronic inflammation found in psoriasis, atopic dermatitis, and lichen planus causes upregulation of integrin β6 and subsequent uncontrolled epidermal epithelialization. Investigation into the role of integrin avβ6 in inflammatory skin pathologies represents an area of future study.
High expression of avβ6 has been well reported for numbers types of cancers [20–23]. In contrast, expression of avβ8 has been less well studied in only two reports of avβ8 protein expression [9, 15]. We now provide data from a set of 25 cases of cutaneous melanoma and show that 96% express integrin β8. Moreover, expression of high levels of β8 is strongly associated with melanoma compared with nevi. This raises the possibility that expression of β8 could assist in distinguishing between these two entities. Interestingly, we noted an association between the degree of β8 expression and Breslow depth and prognostic staging. Thus, expression of β8 could be used to aid both the diagnosis and the prognosis of melanoma. Currently, our analysis has been limited to a relatively small cohort for which long-term outcomes were not available. Follow up studies with a larger cohort of patient samples coupled with a molecular analysis of lesions and outcomes data would be required to validate the utility of β8 expression in the diagnosis and management of melanoma.
Integrin avβ8’s role in cancer is just beginning to be explored with a focus towards its effects on immune cells. Recent work has shown that tumor cells express avβ8 that activates TGFβ sourced from immune cells which promotes the differentiation and enrichment of regulatory T-cells, leading to increased tumor growth [10]. Furthermore, our group has shown that antigen specific and bystander CD8+ Trm compete for active TGFβ and that high levels of TGFβ can promote the accumulation of bystander Trm in mice [24]. We speculate that cancers expressing high levels of integrin avβ8 have the ability to create a TGFβ rich environment, thus promoting the accumulation of non-tumor antigen specific T-cells and preventing effective anti-tumor immunity. We anticipate that modulating integrin-mediated TGFβ activation for the treatment of both inflammation and neoplasia will yield important therapeutic approaches for multiple human diseases.
Supplementary Material
Supplementary Figure 1. Keratinocyte (KC) subset gating strategy and validation of anti-integrin avβ6 and avβ8 staining. (a) Gating strategy used to identify different KC subsets (IFE, IM, and Bulge) from epidermal single cell suspension derived from the flank skin of WT and Integrin β6−/−Integrin β8ΔKC mice. Epidermal single cell suspensions were prepared and analyzed as described in Methods. (b) IFE, IM, and Bulge KCs from epidermal single cell suspensions from flank skin of WT and Integrin β6−/−Integrin β8ΔKC mice stained with anti-integrin avβ6 and anti-integrin avβ8.
Highlights:
Expression of β6 and β8 in human skin recapitulates expression in murine skin.
Skin from inflammatory conditions show increased integrin β6 expression.
Compared to benign nevi, melanomas show increased integrin β8 expression.
Increased integrin β8 expression may be a prognostic factor in melanoma.
Acknowledgments
We thank the members of the Kaplan laboratory and members throughout the departments of Dermatology and Immunology for their helpful discussion. This work was supported by NIH grant 5R01AR060744 (D.H.K, B.A.N.) and the Melanoma Research Foundation (B.A.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: The authors have no conflict of interest to declare.
CRediT authorship contribution statementB.A.N and D.H.K. designed and interpreted the study. J.H. selected study specimens, performed histological scoring and provided conceptual assistance. J.S.D.D. performed experiments. S.N. kindly provided anti-avβ8(C6D4) antibody; B.A.N. and D.H.K wrote the manuscript and all authors edited it.
References
- 1.Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell, 2002. 110(6): p. 673–87. [DOI] [PubMed] [Google Scholar]
- 2.Travis MA and Sheppard D, TGF-β activation and function in immunity. Annu Rev Immunol, 2014. 32: p. 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aluwihare P, et al. , Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci, 2009. 122(Pt 2): p. 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, et al. , Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol, 2007. 176(6): p. 787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed J, et al. , Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nature Immunology, 2016. 17(4): p. 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De La Cruz Diaz JS, et al. , TNF-α and IL-1β Do Not Induce Langerhans Cell Migration by Inhibiting TGFβ Activation. JID Innovations, 2021. 1(3): p. 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht JL, et al. , Overexpression of the alphavbeta6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol, 2008. 16(6): p. 543–7. [DOI] [PubMed] [Google Scholar]
- 8.Niu J and Li Z, The roles of integrin αvβ6 in cancer. Cancer Letters, 2017. 403: p. 128–137. [DOI] [PubMed] [Google Scholar]
- 9.Vogetseder A, et al. , αv-Integrin isoform expression in primary human tumors and brain metastases. Int J Cancer, 2013. 133(10): p. 2362–71. [DOI] [PubMed] [Google Scholar]
- 10.Seed RI, et al. , A tumor-specific mechanism of T(reg) enrichment mediated by the integrin αvβ8. Sci Immunol, 2021. 6(57). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashido Y, et al. , Overexpression of integrin αv facilitates proliferation and invasion of oral squamous cell carcinoma cells via MEK/ERK signaling pathway that is activated by interaction of integrin αvβ8 with type Ⅰ collagen. Int J Oncol, 2014. 45(5): p. 1875–82. [DOI] [PubMed] [Google Scholar]
- 12.Khan Z and Marshall JF, The role of integrins in TGFβ activation in the tumour stroma. Cell Tissue Res, 2016. 365(3): p. 657–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, et al. , Epigenetic regulation of integrin β6 transcription induced by TGF-β1 in human oral squamous cell carcinoma cells. J Cell Biochem, 2018. 119(5): p. 4193–4204. [DOI] [PubMed] [Google Scholar]
- 14.Li YY, Zhou CX, and Gao Y, Interaction between oral squamous cell carcinoma cells and fibroblasts through TGF-β1 mediated by podoplanin. Exp Cell Res, 2018. 369(1): p. 43–53. [DOI] [PubMed] [Google Scholar]
- 15.Takasaka N, et al. , Integrin αvβ8-expressing tumor cells evade host immunity by regulating TGF-β activation in immune cells. JCI Insight, 2018. 3(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagao K, et al. , Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nature immunology, 2012. 13(8): p. 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershenwald JE, et al. , Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin, 2017. 67(6): p. 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duperret EK, et al. , The integrin αv-TGFβ signaling axis is necessary for epidermal proliferation during cutaneous wound healing. Cell Cycle, 2016. 15(15): p. 2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haapasalmi K, et al. , Keratinocytes in Human Wounds Express αvβ6 Integrin. Journal of Investigative Dermatology, 1996. 106(1): p. 42–48. [DOI] [PubMed] [Google Scholar]
- 20.Zhang ZY, et al. , Integrin alphanvbeta6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol (R Coll Radiol), 2008. 20(1): p. 61–6. [DOI] [PubMed] [Google Scholar]
- 21.Desai K, et al. , High expression of integrin β6 in association with the Rho-Rac pathway identifies a poor prognostic subgroup within HER2 amplified breast cancers. Cancer Med, 2016. 5(8): p. 2000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazelbag S, et al. , Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol, 2007. 212(3): p. 316–24. [DOI] [PubMed] [Google Scholar]
- 23.Marsh D, et al. , alpha vbeta 6 Integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res, 2008. 68(9): p. 3295–303. [DOI] [PubMed] [Google Scholar]
- 24.Hirai T, et al. , Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche. Immunity, 2021. 54(1): p. 84–98.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Keratinocyte (KC) subset gating strategy and validation of anti-integrin avβ6 and avβ8 staining. (a) Gating strategy used to identify different KC subsets (IFE, IM, and Bulge) from epidermal single cell suspension derived from the flank skin of WT and Integrin β6−/−Integrin β8ΔKC mice. Epidermal single cell suspensions were prepared and analyzed as described in Methods. (b) IFE, IM, and Bulge KCs from epidermal single cell suspensions from flank skin of WT and Integrin β6−/−Integrin β8ΔKC mice stained with anti-integrin avβ6 and anti-integrin avβ8.


