Abstract
BACKGROUND:
Postoperative delirium is frequent in older adults and associated with postoperative neurocognitive disorder (PND). Studies evaluating perioperative medication use and delirium have generally evaluated medications in aggregate and been poorly controlled; the association between perioperative medication use and PND remains unclear. We sought to evaluate the association between medication use and postoperative delirium and PND in older adults undergoing major elective surgery.
METHODS:
This is a secondary analysis of a prospective cohort study of adults ≥70 years without dementia undergoing major elective surgery. Patients were interviewed preoperatively to determine home medication use. Postoperatively, daily hospital use of 7 different medication classes listed in guidelines as risk factors for delirium were collected; administration prior to delirium was verified. While hospitalized, patients were assessed daily for delirium using the Confusion Assessment Method and a validated chart review method. Cognition was evaluated preoperatively and one month after surgery using a neurocognitive battery. The association between prehospital medication use and postoperative delirium was assessed using a generalized linear model with a log link function, controlling for age, gender, type of surgery, Charlson comorbidity index, and baseline cognition. The association between daily postoperative medication use (when class exposure ≥5%) and time-to-delirium was assessed using time-varying Cox models adjusted for age, gender, surgery type, Charlson comorbidity index, APACHE-II score, and baseline cognition. Mediation analysis was utilized to evaluate the association between medication use, delirium, and cognitive change from baseline to one month.
RESULTS:
Among 560 patients enrolled, 134(24%) developed delirium during hospitalization. The multivariable analyses revealed no significant association between prehospital benzodiazepine [Relative Risk (RR) 1.44;95% Confidence Interval (CI) 0.85,2.44], beta blocker (RR 1.38;95% CI 0.94,2.05), NSAID (RR 1.12;95% CI 0.77,1.62), opioid (RR 1.22;95% CI 0.82,1.82), or statin (RR 1.34;95% CI 0.92,1.95) exposure and delirium. Postoperative hospital benzodiazepine use [adjusted Hazard Ratio (aHR) 3.23;95% CI 2.10,4.99] was associated with greater delirium. Neither postoperative hospital antipsychotic (aHR 1.48;95% CI 0.74,2.94) nor opioid (aHR 0.82;95% CI 0.62,1.11) use prior to delirium were associated with delirium. Antipsychotic use (either pre- or post-surgery) was associated with a 0.34 point (standard error 0.16) decrease in the GCP at 1 month through its effect on delirium (p=0.03), despite no total effect being observed.
CONCLUSIONS:
Administration of benzodiazepines to older adults hospitalized after major surgery is associated with increased postoperative delirium. As association between in-hospital, postoperative medication use and cognition at 1 month, independent of delirium, was not detected.
Postoperative delirium, characterized by an acute onset of fluctuating attention and mental status alteration from baseline in the 7 days after surgery, occurs in up to 50% of older adults undergoing major surgery.1,2 It is associated with a longer hospital stay, a greater likelihood for transfer to a nursing facility at discharge, and cognitive decline that can persist for years and result in dementia.1,3,4 The risk for delirium in this setting depends on a number of different baseline, surgical, and hospital factors. A clear understanding of modifiable postoperative delirium risk factors is critical when formulating delirium reduction efforts. While current practice guidelines5,6 make broad recommendations to avoid medications known to be associated with a high risk for delirium in older adults undergoing major surgery,7 the individual medications that should be avoided remains unclear and the perioperative period(s) when use should be eschewed remains unknown.
Among 29 published studies evaluating preoperative factors associated with postoperative delirium, only 4 explicitly sought to evaluate the risk of preoperative medication class use.8 Among 19 published presurgical risk prediction models for postoperative delirium, only 5 included medications.8–12 Only four studies,9–12 three published more than 25 years ago,9–11 have investigated the influence of postsurgical medication use on postoperative delirium. In these studies, only postoperative benzodiazepine was consistently associated with delirium.9–12 However, these studies failed to differentiate medications newly-initiated after surgery from those continued from home and in some cases failed to distinguish whether the medication was administered prior to delirium occurrence.9,11,12 Although postoperative delirium is strongly associated with postoperative neurocognitive disorder (PND), the interplay among medications, delirium, and cognitive decline had not been rigorously investigated. For example, while benzodiazepines have been associated with cognitive decline, the impact of postoperative delirium on this cognitive trajectory has yet to be explored.13 To better understand the important potential role of pre- and post-surgical medication use on postoperative delirium and PND in older adults undergoing major surgery we sought to investigate three associations: 1) Presurgical medication use and postoperative delirium; 2) Inpatient, postsurgical medication use and postoperative delirium; and 3) the delirium-independent relationship between inpatient, postsurgical medication use and cognition 1 month after surgery.
METHODS
Study Design and Population
Our study population was derived from the Successful Aging after Elective Surgery (SAGES) study, a prospective cohort study of community-dwelling older adults undergoing major elective surgery. The study design and methods have been described previously in detail.14,15 Briefly, participants were ≥70 years old, English-speaking, able to communicate verbally, scheduled to undergo elective surgery at one of two Harvard-affiliated academic medical centers with an anticipated length of stay of at least 3 days, and were available for in-person follow-up interviews. Eligible surgical procedures included total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and colectomy.
Patients with evidence of dementia, delirium or hospitalization within 3 months preceding the surgical visit, a terminal condition, a history of schizophrenia or psychosis, or a history of alcohol abuse or withdrawal were excluded. Dementia screening occurred at initial medical record screening, telephone recruitment, and baseline enrollment interview. Patients with any one of the following were excluded: diagnosis of dementia or who had documented dementia in the medical record, affirmation during telephone recruitment of a diagnosis of dementia or Alzheimer’s disease from a physician, patients who failed a capacity assessment of their understanding of the informed consent, patients with a score of <69 or its education-adjusted equivalent on the Modified Mini-Mental (3MS) test during the baseline interview.16
The study was approved by the Institutional Review Boards at Beth Israel Deaconess Medical Center and Brigham and Women’s Hospital, and Hebrew Senior Life, the study coordinating center, all located in Boston, MA. Written informed consent was obtained from all subjects prior to their participation.
Exposure
Trained research assistants conducted a 90-minute baseline interview in participants’ homes about 2 weeks prior to the planned surgery to obtain consent, collect baseline data and current medications.14,15 Pre-hospital medications were collected and confirmed using two approaches. First, study interviewers recorded all medication names with direct visual inspection of labels of medication vials/bottles in the patient’s home. Second, all study medications were confirmed by review of admission medication listings abstracted from the medical record. The names of all pre-hospital medication were entered alphanumerically in the study database. After hospital discharge, a trained research physician reviewed the hospital medical record to determine the daily administration (both scheduled or as needed) and dosing route for 7 a priori-determined classes of medications [i.e., anticholinergics, antipsychotics, benzodiazepines, beta adrenergic blockers, non-steroidal anti-inflammatory drugs (NSAIDS), opioids, and statins] for up to seven days from the immediate postoperative period to hospital discharge. These medication classes have previously been reported in practice guidelines (e.g., the AGS Beers Criteria) or published reports to be associated with delirium in hospitalized patients.7–10 Data on medication dose or daily frequency was not collected.
Medications were coded by class using the American Hospital Formulary System (AHFS) classifications.17 Additionally, total anticholinergic activity was quantified with each medication being assigned a value from 0 to 3, based on the strength of the agent’s anticholinergic activity according to the Anticholinergic Cognitive Burden scale (ACB) and the Anticholinergic Drug Score (ADS).17 If a class of medications had a prevalence < 5% in either the pre-hospital or postsurgical period then the medication class was excluded from this time period.
Outcomes
A trained research assistant evaluated each patient daily during the day shift for delirium from the immediate postoperative period to hospital discharge using the Confusion Assessment Method (CAM).18 The CAM algorithm is the most widely used instrument worldwide and has a high sensitivity (91–97%), specificity (85–94%), and interrater reliability (κ=0.92).18 The presence of delirium was augmented using a validated chart review method, which facilitated a more complete 24-hour overview and improved sensitivity for delirium detection.19
The General Cognitive Performance (GCP) composite (scaled, 0–100), a weighted composite variable for the assessment of global cognitive function, was created specifically for the study. The GCP combines the results of multiple neuropsychological tests and has been scaled to have a mean in the general population of 50 with a standard deviation of 10. It has been demonstrated to be highly sensitive to longitudinal cognitive change with minimal floor and ceiling effects.20 In SAGES, the GCP scores were adjusted with a correction factor derived from a comparable sample of non-surgical patients to account for learning effects related to multiple retesting.21
One month after discharge, home based interviews were conducted by a group of trained research assistants who were blinded to in-hospital delirium status. Postoperative cognition at 1 month was chosen (vs. later time periods) to ensure and to decrease the potential for unmeasured confounding due to interim outpatient medication use. Earlier time periods were not available in the data set.
Covariates
The baseline, pre-hospital interview collected information from the patient on age, gender, race and ethnicity, marital status, living situation, and education. Baseline cognition as measured by the GCP was also collected during this interview. Baseline Charlson comorbidity index22, surgical type, and postoperative Acute Physiology and Chronic Health Evaluation (APACHE) II score23 were determined from a review of the medical record. Surgery type (categorized as orthopedic, vascular, or gastrointestinal) was also determined during this review. Pain scores were obtained from patients daily using the Brief Pain Inventory.15 These covariates were used for confounding adjustment in the statistical analyses as detailed below.
Statistical Analysis
Three separate analytic structures were used to answer our three objectives: one for prehospital medications, one for postoperative medications administered during hospitalization, and one assessing cognition accounting for both of the previous time periods.
Objective 1: Pre-hospital medications
Pre-hospital medications were evaluated as independent variables in a generalized linear model with a log link function (log-binomial regression), yielding relative risks for postoperative delirium. Univariable analysis was conducted for each of the six medication classes meeting the 5% prevalence breakpoint (i.e., anticholinergic medications, benzodiazepines, beta blockers, NSAIDS, opioids, and statins) to estimate unadjusted associations. Multivariable analysis was then conducted by including all 6 medication classes in a single model and controlling for six well-established postoperative delirium risk factors: age, gender, baseline GCP, Charlson comorbidity index, APACHE-II score, and surgery type.1–4, 9, 24, 25 For anticholinergic activity the number of medications with anticholinergic activity according to the ACB included was used in the primary model. To test the robustness of our findings for pre-hospital anticholinergic medication use, we created separate models using the number of medications with anticholinergic activity according to the ADS and total number of medications prior to hospitalization.
Objective 2: Postoperative medications
During hospitalization we created Cox models to evaluate time-to-postoperative delirium given medication use often changes daily. The survival analysis framework functioned as an accelerated failure model, with our results representing the acceleration of the incidence of delirium by the medications under study. A single multivariable model was created including the three medication classes that met the in-hospital 5% prevalence criterion (i.e., antipsychotics, benzodiazepines, and opioids). The model controlled for the time-varying nature of medication class exposure and the same six delirium risk factors used in the pre-hospital medication analysis. We created a second model that also controlled for worst pain during hospitalization. Given clinical decisions to administer opioids via the injectable (vs. oral) route during hospitalization may vary and the injectable route is more likely to used immediately after surgery, we conducted sensitivity analyses, stratifying opioid classes as to whether they were more likely to be administered via the intravenous (versus oral) route. We also conducted a sensitivity analysis controlling for prehospital use of benzodiazepines and of opioids; this analysis was not conducted for antipsychotics given the small prevalence of use in the pre-hospital period.
Objective 3: Mediation analysis
Mediation analysis was conducted using the Causalmed procedure26, a method by which generalized linear models are used to describe relationships between outcome (in our study, cognition using the GCP), treatment (i.e., medication class), mediator (i.e., post-operative delirium), and potential confounding (i.e., age, gender, Charlson comorbidity index, baseline cognition, surgery type, and postoperative APACHE-II score) variables.1–4, 9, 26 Mediation analysis was used to evaluate which portion of the drug-induced cognitive decline at 1 month is mediated by delirium. In order to make this assessment, we assumed no residual confounding of the exposure-mediator, exposure-outcome, or mediator-outcome relationships after adjusting for the included covariates.27 We tested the assumption of no interaction between the exposure and the mediator to ensure no interaction variable needed to be included.27 All medication exposure was confirmed to precede delirium development for individuals who were delirium positive before being entered into the model. Postoperative cognition at 1 month was chosen (vs later time periods) to ensure biologic plausibility and to decrease the potential for unmeasured confounding due to interim outpatient medication use. Earlier time periods were not available in the dataset.
The analytic procedure allowed for binary treatment and mediator variables along with a continuous outcome. The outcome (GCP) was modeled using a normal distribution with an identity link function (linear regression) while the mediator (delirium) was modeled using a binomial distribution and a logit link (logistic regression).28 Mediation analysis yields total, direct, and indirect effects for each treatment of interest. The direct effect is the effect of the medication on cognition not mediated by delirium, assessed in a model controlling for other baseline covariates and not controlling for delirium. The indirect effect represents the effect of the medication on cognition that is mediated by the effects the medication has on delirium. This is modeled in two parts, assessing the effect of medications on delirium and the effect of delirium on cognition; each part of the model controls for other baseline confounders. The total effect represents the sum of the direct and indirect effects, modeled as the effect of medications on cognition in a model controlling for baseline confounders and for delirium. The direct and indirect effects were decomposed from the total effect as described by Valeri et al.29
All results are the reported effect size with a corresponding p-value. P-values less than 0.05 were considered significant. All analyses were conducted using SAS/STAT software version 9.4 for Windows (SAS, Cary, NC). Given the 560 participants available from the SAGES cohort, 134 of whom developed delirium, and assuming a significance level (α) of 0.05, our a priori estimate yielded 88% power to detect a relative risk of 1.5 given a 24% event rate.
RESULTS
Of the 566 SAGES patients enrolled between June 18, 2010, and August 8, 2013, six were subsequently excluded for possible dementia leaving a final study cohort of 560 patients. The 560 patients, on average, were 76.0 ± 5.0 years old and had an admission APACHE-II score of 11.9 ± 2.9 (Table 1). More than half (58%) were women and most (81%) underwent an orthopedic surgical procedure. Among the 134 (24%) who developed postoperative delirium during hospitalization, 100 (82%) did so by the second postoperative day (Appendix 1). Patients who developed delirium (versus those who did not) had a lower baseline general cognitive performance (54.7 ± 6.5 vs. 58.5 ± 7.3). Patients who developed delirium experienced higher maximal (8 [4–9] vs. 7[4–8) and average pain scores (5[3–7] vs. 4[2–6]) compared to those who never developed delirium.
Table 1.
Baseline Characteristics and Patient Demographics
| Total cohort (n=560) | Delirium (n=134) | No delirium (n=426) | |
|---|---|---|---|
| Age, mean years ± SD | 76.6 ± 5.0 | 77.4 ± 4.8 | 76.4 ± 5.0 |
| Female sex, n (%) | 326 (58%) | 81 (61%) | 245 (58%) |
| Nonwhite race, n (%) | 42 (8%) | 13 (9.7%) | 29 (7%) |
| Gastrointestinal, n (%) | 71 (13%) | 18 (13.4%) | 53 (12%) |
| Married, n (%) | 332 (59%) | 79 (59.0%) | 253 (59%) |
| Lives alone, n (%) | 167 (30%) | 39 (29.1%) | 128 (30%) |
| Education, mean years ± SD | 15.0 ± 3.0 | 14.7 ± 3.0 | 15.1 ± 2.9 |
| Charlson comorbidity index, median score [IQR] | 1 [0–2] | 1 [0–2] | 1 [0–2] |
| Postoperative APACHE II, mean score (SD) | 11.9 ± 2.9 | 13.3 ± 3.5 | 11.4 ± 2.6 |
| General cognitive performance, mean ± SD | 57.6 ± 7.3 | 54.7 ± 6.5 | 58.5 ± 7.3 |
| Worst pain during hospitalization, median [IQR] | 7 [4–8] | 8 [4–9] | 7 [4–8] |
| Average pain during hospitalization, median [IQR] | 4 [2–6] | 5 [3–7] | 4 [2–6] |
Abbreviation: APACHE II, Acute Physiology and Chronic Health Evaluation II; IQR, interquartile range; SD, standard deviation
Preoperative Medication Use and Postoperative Delirium
Patients taking a preoperative beta blocker were significantly more likely to experience postoperative delirium (Table 2). Preoperative antipsychotic use was excluded as prevalence was only 0.5%. A significant association between preoperative use of any of the medication classes and postoperative delirium, after controlling for age, gender, comorbidity, baseline cognition and postsurgical severity of illness, was not detected (Table 2). Results were similar when we used the ADS (vs. the ACB) as a measure of anticholinergic burden or included the total number of preoperative medications administered.
Table 2.
Association between Prehospital Medication Use and Postoperative Delirium
| Delirium (n=134) |
No delirium (n=426) |
Unadjusted relative risk (95% CI) |
Adjusted relative riskc (95% CI) |
Adjusted relative riskd (95% CI) |
Adjusted relative riske (95% CI) |
|
|---|---|---|---|---|---|---|
| Benzodiazepines | 17 (12.7%) | 43 (10.1%) | 1.21 (0.73 to 2.01) | 1.44 (0.85 to 2.44) | 1.41 (0.82 to 2.43) | 1.40 (0.84 to 2.44) |
| Beta blockers | 70 (52.2%) | 159 (37.3%) | 1.58 (1.13 to 2.22) | 1.38 (0.94 to 2.05) | 1.41 (0.99 to 2.02) | 1.41 (0.98 to 2.02) |
| NSAIDs | 86 (64.2%) | 253 (59.4%) | 1.17 (0.82 to 1.66) | 1.12 (0.77 to 1.62) | 1.12 (0.77 to 1.62) | 1.11 (0.76 to 1.62) |
| Opioids | 33 (24.6%) | 84 (19.7%) | 1.24 (0.84 to 1.83) | 1.22 (0.82 to 1.82) | 1.20 (0.78 to 1.84) | 1.21 (0.79 to 1.85) |
| Statins | 88 (65.7%) | 235 (55.2%) | 1.40 (0.98 to 2.01) | 1.34 (0.92 to 1.95) | 1.34 (0.92 to 1.95) | 1.33 (0.91 to 1.95) |
| Number of anticholinergic medicationsa | 1.4 ± 1.2 | 1.2 ± 1.1 | 1.12 (0.96 to 1.29) | 1.03 (0.88 to 1.20) | -- | -- |
| Number of anticholinergic medicationsb | 1.6 ± 1.3 | 1.3 ± 1.2 | 1.14 (0.99 to 1.30) | -- | 1.03 (0.87 to 1.22) | -- |
| Total number of medications | 8.6 ± 3.9 | 7.8 ± 3.7 | 1.04 (0.99 to 1.09) | -- | -- | 1.01 (0.96 to 1.06) |
According to the Anticholinergic Cognitive Burden scale
According to the Anticholinergic Drug Score
Model adjusted for age, gender, surgery type, Charlson comorbidity index, and cognition at baseline
Model adjusted for number of anticholinergic medications according to the Anticholinergic Drug Score, age, gender, surgery type, Charlson comorbidity index, and cognition at baseline
Model adjusted for total number of medications, age, gender, surgery type, Charlson comorbidity index, and cognition at baseline
Abbreviation: CI, confidence interval; NSAIDs, Non-steroidal anti-inflammatory drugs; Statins, HMG-CoA reductase inhibitors.
Inpatient Postoperative Medication Use and Postoperative Delirium
Among the 7 postoperative medication classes where data was collected, only antipsychotics, benzodiazepines and opioids met the a priori prevalence threshold of 5%. Daily in-hospital, postoperative medication exposure for benzodiazepines, antipsychotics and opioids, stratified by postoperative delirium occurrence, is presented in the Figure. Patients who developed delirium had a greater exposure to antipsychotics, benzodiazepines, and opioids as a class. The unadjusted and adjusted models each revealed postoperative benzodiazepine use to be significantly associated with greater postoperative delirium (aHR 3.23; 95% CI 2.10 to 4.99; Table 3). Neither the association between antipsychotic use (aHR 1.48; 95% CI 0.74 to 2.94) nor opioid use (aHR 0.82; 95% CI 0.62 to 1.11) and delirium occurrence was statistically significant.
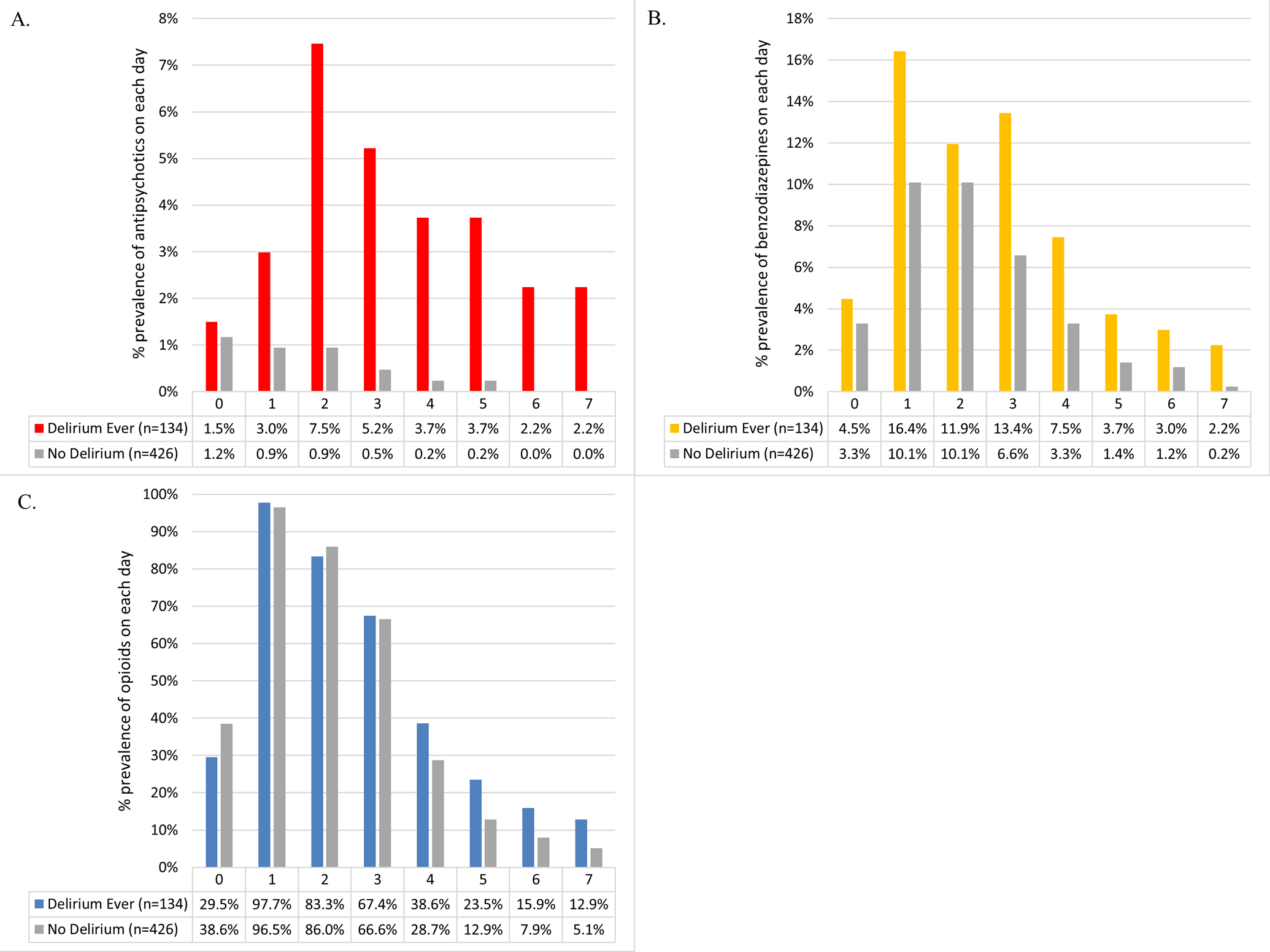
Daily in-hospital antipsychotic, benzodiazepine, and opioid use stratified by delirium status. Medication use within each group is presented as number of patients in the group exposed to that medication divided by total number of patients in that group. Panel A shows the use of antipsychotics. Panel B shows the use of benzodiazepines. Panel C shows the use of opioids.
Table 3.
Survival model showing accelerated delirium incidence for medication classes administered in hospital
| Unadjusted hazard ratio (95% CI) |
Model 1 Adjusted hazard ratioa (95% CI) |
Model 2 Adjusted hazard ratiob (95% CI) |
|
|---|---|---|---|
| Antipsychotics | 2.96 (0.96 to 9.09) | 1.48 (0.74 to 2.94) | 1.48 (0.75 to 2.93) |
| Benzodiazepines | 2.31 (1.33 to 3.99) | 3.23 (2.10 to 4.99) | 3.03 (1.97 to 4.67) |
| Any opioid | 1.01 (0.64 to 1.60) | 0.82 (0.61 to 1.10) | 0.82 (0.62 to 1.11) |
Models adjusted for age, gender, surgery type, Charlson comorbidity index, APACHE-II score, and cognition at baseline.
Models adjusted for age, gender, surgery type, Charlson comorbidity index, APACHE-II score, cognition at baseline, and worst pain.
The opioid sensitivity analysis revealed postoperative, in-hospital administration of an injectable opioid was associated with an estimated 69% higher risk of delirium (aHR 1.69; 95% CI 1.32 to 2.17) whereas oral opioid exposure was associated with a roughly 25% decrease in this risk (aHR 0.73; 95% CI 0.58 to 0.92; Appendix 2). Within this analysis (and all other sensitivity analyses evaluating injectable and oral opioids separately), antipsychotics were associated with an increase in the risk of delirium (aHR 2.46; 95% CI 1.53 to 3.95). Both fentanyl (aHR 3.73; 95% CI 1.37 to 10.2) and hydromorphone (aHR 1.54; 95% CI 1.02 to 2.31) were independently associated with an increase in postoperative delirium (Appendix 3). Model estimates did not change when the duration of observation was limited to include only delirium occurring in either the first three or first four postoperative days. Likewise, estimates did not change when the worst observed pain score during hospitalization was considered. Sensitivity analysis controlling for pre-hospital use of these medication classes did not alter the point estimates or confidence intervals reported (Appendix 4). Post-hoc models evaluating the impact of not controlling for severity of illness (Appendix 5) and controlling for hospital site (Appendix 6) did not affect the associations between medication use and delirium we report.
Inpatient Postsurgical Medication Use and Post-discharge Cognition
Post-discharge cognitive assessments were completed on 548 patients at 1 month. Compared to baseline (57.6 ± 7.3), cognition was lower at 1 month (56.9 ± 7.9; p<0.001). Patients who developed postsurgical delirium (as compared to those that did not) had lower general cognitive performance at 1 month (53.0 ± 7.0 vs. 58.1 ± 7.8, p<0.001). Mediation analysis failed to detect an association between the use of any of the eight medication classes included in the analysis and cognition at 1 month (Table 4). Antipsychotics had a statistically significant effect on cognition at 1 month (−0.34, standard error 0.16; p=0.03) but this was solely related to their effect on delirium.
Table 4.
Mediation analysis predicting cognition at 1 month after hospital discharge
| Number of Patients Taking Medication | Total Effect of Medication on Cognitiona | Direct Effect of Medication on Cognitionb | Indirect Effect of Medication on Cognitionc | ||||
|---|---|---|---|---|---|---|---|
| Slope (SE) | P-value | Slope (SE) | p-value | Slope (SE) | P-value | ||
| Antipsychotics | 32 (5.7%) | −0.15 (0.61) | .81 | 0.19 (0.61) | 0.75 | −0.34 (0.16) | .03 |
| Benzodiazepines | 127 (22.7%) | −0.01 (0.34) | .98 | 0.11 (0.34) | 0.74 | −0.12 (0.07) | .09 |
| Beta blockers | 229 (40.9%) | 0.03 (0.29) | .91 | 0.13 (0.29) | 0.66 | −0.10 (0.05) | .07 |
| NSAIDs | 339 (60.5%) | 0.12 (0.29) | .67 | 0.15 (0.29) | 0.59 | −0.03 (0.05) | .49 |
| Statins | 323 (57.7%) | −0.16 (0.29) | .58 | −0.07 (0.28) | 0.80 | −0.08 (0.05) | .09 |
| Hydromorphone | 402 (71.8%) | −0.07 (0.31) | .82 | −0.01 (0.31) | 0.97 | −0.06 (0.05) | .23 |
| Morphine | 127 (22.7%) | 0.26 (0.33) | .43 | 0.34 (0.33) | 0.30 | −0.08 (0.05) | .20 |
| Oxycodone | 416 (74.3%) | 0.37 (0.32) | .25 | 0.31 (0.32) | 0.33 | 0.07 (0.05) | .23 |
| Tramadol | 72 (12.9%) | −0.28 (0.41) | .50 | −0.16 (0.41) | 0.69 | −0.11 (0.08) | .14 |
Medication categories reflect use prior to hospitalization, during hospitalization, and at hospital discharge. All models are adjusted for age, gender, surgery type, Charlson comorbidity index, APACHE-II score, and cognition at baseline.
The total effect represents the combined direct and indirect effect of the medication on cognition at 1 month.
The direct effect represents the effect of the medication on cognition that is not mediated by delirium.
The indirect effect represents the effect of the medication on cognition that is mediated by the effects the medication has on delirium.
Abbreviations: APACHE, Acute Physiologic and Chronic Health Evaluation; NSAIDs = Non-steroidal anti-inflammatory drugs; SE, standard error; Statins = HMG-CoA reductase inhibitors
DISCUSSION
This study, utilizing a large clinical cohort of older adults undergoing major surgery, found postsurgical, inpatient, benzodiazepine use is significantly associated with greater delirium. Our analysis is innovative because it incorporated time-varying methods to analyze time from postsurgical medication exposure to delirium occurrence and considered potential confounding factors for delirium like acute pain. Unlike other studies evaluating pre-hospital medication use and postoperative delirium,24, 30–32 we were not able to find a significant association between any class of medication administered prior to hospitalization and postoperative delirium. This may be due to inadequate power for some of the classes. Compared to other postoperative delirium risk studies,9–12 we failed to find a significant association between antipsychotic or opioid use and postoperative delirium.
The study results build on strong evidence from other studies reporting perioperative benzodiazepine use is an important risk for postoperative delirium.10,32 The lack of association we detected between presurgical benzodiazepine use and postoperative delirium may be due to the low number of patients taking a benzodiazepine prior to surgery. In older adult populations where chronic use of benzodiazepines is higher, pre-surgical use of benzodiazepines should be further investigated.
Our study is the first published study to explore the relationship between postsurgical (in hospital) medication use, postoperative delirium and post-hospital cognition. Among the medication classes evaluated, we did not find an association between postoperative medication use and cognition at 1 month that was independent of delirium. While a small, but significant, association was found between in-hospital antipsychotic use and 1-month cognition, this association was entirely driven by the large effect size of antipsychotics on delirium.
The failure of our study to find an association between either preoperative beta blocker or statin use and delirium run counter to the results of other perioperative delirium risk factor studies.30, 31 These studies included a different population of surgical patients (i.e., vascular/cardiac surgery) who may have different postoperative delirium risk factors and did not control for established pre-surgical predictors of delirium such as baseline cognition. Among three other studies evaluating preoperative opioid use -postoperative delirium risk,24, 25, 33 two found an association24, 25 and one did not.33 The two studies finding an association did not control for key delirium risk factors like cognition and pain.24, 25 The more rigorous third study, where postoperative factors including pain were considered, reported results similar to ours.33 Although one prior analysis of preoperative anticholinergic exposure reported a positive association with delirium,34 this study was conducted in a different surgical population (i.e., cardiac) and also did not adjust for baseline cognition.
Antipsychotics, when rigorously evaluated for the prevention of postoperative delirium, have been found to be ineffective and potentially harmful.35 Our failure to detect an association between antipsychotic use and delirium, given the magnitude of the point estimate, suggests our analysis was underpowered to detect this association due to low postoperative antipsychotic use. However, sensitivity analyses evaluating injectable and oral opioids separately revealed a significant association between in hospital antipsychotic use and delirium.
Our analysis found no association between overall postsurgical opioid use and delirium. With the exception of meperidine, an analgesic almost never currently used in surgical practice, our results are consistent with prior literature.10, 12, 36 The increased delirium we found with injectable (vs oral) opioid use was an unexpected finding. While we did control for factors that might influence injectable (vs. oral) opioid use, including level of pain, patient acuity, and the post-operative day,37 this finding should be considered exploratory and hopefully will help justify future prospective research. Although non-opioid analgesic approaches have been shown to reduce opioid exposure and improve pain control, only one randomized study has evaluated the use of a non-opioid analgesic (acetaminophen) on postoperative delirium.38
Our results have important clinical applications. Providers managing post-operative older adults should minimize benzodiazepine use by utilizing nonpharmacologic methods to reduce agitation (e.g., out of bed mobility) or improve sleep (e.g. earplugs and/or eye masks at night). When these nonpharmacologic interventions are bundled together, delirium is reduced.39 Recent postoperative delirium practice guidelines strongly recommend against benzodiazepine or antipsychotic use in older adults undergoing major surgery.5,6 Although the relationship between opioids and delirium after surgery needs further research, the potential benefits of using ERAS protocols promoting the use of nonopioid interventions to manage pain, should be considered.5,6
Our paper has several important strengths. Delirium was evaluated using a well-validated, highly reproducible method designed to maximize sensitivity and specificity. Use of time-varying analysis ensured all medication exposure preceded delirium occurrence and allowed for estimation of time-to-delirium after medication initiation. We controlled for five a-priori selected variables in each primary model known to confound the association between medication use and postoperative delirium. Additionally, our study is one of the first utilize mediation analysis techniques to try to explain a causal association between postoperative hospital medication use and post-hospital cognition as mediated by delirium. While no clinically significant association was found, our analysis shows that such investigations are possible and future research should be explored to look for casual links between postoperative delirium risks and post-operative cognitive changes.
Traditional methods allow the mediation effect to be estimated as the product of the exposure-mediator and mediator-outcome relationships, both of which are required to be significant before mediation can be claimed.40 However, modern mediation methods estimate both of these relationships in parallel and assess direct and indirect effects as degradations of the total effect.29 It is possible through this method that direct and indirect mediation effects can be in opposing directions, leading to a total effect that is not significant despite significant mediating effects. We believe this is the case in our analysis, where we found a single significant indirect effect despite no significant total effects.
Our study has potential limitations. The associations we report in our observational study do not infer causality. Since the 2010 to 2013 enrollment period, advances in surgery and post-operative care may affect the medication-postoperative delirium results we report. These include changes in anesthetic practices, wider adoption of enhanced recovery after surgery programs, and implementation of nonpharmacologic delirium reduction strategies.39 Our analytic power was constrained by medication prevalence. While medication data was collected in a rigorous manner, pre-hospital medication adherence was not evaluated. Although medication exposure and the risk for delirium has been found to be dose-dependent in other settings, medication dose data was not collected. While we accounted for established baseline and postoperative factors known to affect postoperative delirium occurrence, other factors not included in our analyses may have affected the results we report.
Postoperative care, including the use of delirium-reducing efforts, was standardized across the two study centers, yet data on the use of these interventions was not collected and they may have been different. However, a post-hoc analysis controlling for the hospital where the individual was admitted did not show any differences in the estimates we report. Additionally, despite the operative factors like choice of anesthesia and cerebral perfusion adequacy known to influence delirium occurrence, most pre-hospital postoperative delirium risk factor studies do not account for this period. The prescriber’s rationale for each postoperative medication was unknown given the retrospective nature of the study. By postoperative day 30, PND may have resolved. While reverse causation bias is possible in our mediation analysis, the maintenance of risk factor temporality greatly reduces this risk. In addition, mediation analysis could be subject to confounding from unmeasured variables not captured in the cohort.
In conclusion, inpatient benzodiazepine administration remains an important risk factor for postoperative delirium in older adults. Clinicians should avoid prescribing benzodiazepines in the post-operative setting in this patient population and follow current guidelines regarding antipsychotic and opioid use.5–7 Future research should evaluate the dose-related effects of neuroactive medications on postoperative delirium and post-operative neurocognitive disorder among older adults undergoing major surgery. Studies should focus on both patients who require postoperative hospitalization as well as those who are discharged home on the same day after surgery.
Supplementary Material
KEY POINTS.
Question: Is there an association between medication use and postoperative delirium and post-hospital cognition among older adults undergoing elective surgical procedures?
Findings: While benzodiazepine use during hospitalization is associated with increased postoperative delirium, an association between in-hospital, postoperative medication use and cognition at 1 month, independent of delirium, was not detected.
Meaning: Avoidance of benzodiazepines in the postoperative period will reduce delirium and slow subsequent cognitive decline; further research is necessary to clarify the risks of antipsychotic and opioid use on delirium and their relationship with posthospital cognition in the post-surgical population.
Funding:
Supported by the National Institute on Aging of the National Institutes of Health under award numbers P01AG031720 (SKI), R24AG054259 (SKI), K24AG035075 (ERM), and F31AG066460 (MSD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
GLOSSARY
- ACB
Anticholinergic Cognitive Burden scale
- ADS
Anticholinergic Drug Score
- AGS
American Geriatrics Society
- AHFS
American Hospital Formulary System
- aHR
adjusted Hazard Ratio
- APACHE
Acute Physiology and Chronic Health Evaluation
- CAM
Confusion Assessment Method
- GCP
General Cognitive Performance
- IRB
Institutional Review Board
- NSAID
nonsteroidal anti-inflammatory drugs
- PND
post-operative neurocognitive disorder
- SAGES
Successful Aging after Elective Surgery
Footnotes
DISCLOSURES
Name: Matthew S. Duprey, PharmD, PhD
Contributions: This author helped with conception of the study, analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: John W. Devlin, PharmD
Contributions: This author helped with conception of the study, analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: John L. Griffith, PhD
Contributions: This author helped with study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: Thomas G. Travison, PhD
Contributions: This author helped with conception of the study, data collection, study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: Becky A. Briesacher, PhD
Contributions: This author helped with study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: Richard Jones, PhD
Contributions: This author helped with conception of the study, data collection, study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: Jane S. Saczynski, PhD
Contributions: This author helped with study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Name: Eva M. Schmitt, PhD
Contributions: This author helped with conception of the study, data collection, study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Yun Gou, MA
Contributions: This author helped with data collection, study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Edward R. Marcantonio, MD, SM
Contributions: This author helped with conception of the study, data collection, study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Sharon K. Inouye, MD, MPH
Contributions: This author helped with conception of the study, data collection, study analysis, interpretation, and manuscript drafting, revising and submission.
Conflict of Interest: None
Reprints will not be available from the authors.
References:
- 1.Rudolph JL, Marcantonio ER. Review articles: Postoperative delirium: Acute change with long-term implications. Anesth Analg. 2011;112(5):1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesth Analg. 2018;127(5):1189–1195. [DOI] [PubMed] [Google Scholar]
- 3.Devore EE, Fong TG, Marcantonio ER, et al. Prediction of long-term cognitive decline following postoperative delirium in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(12):1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults. J Am Geriatr Soc. 2015;63(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes CG, Boncyk CS, Culley DJ, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth Analg. 2020;130(6):1572–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 8.Kassie GM, Nguyen TA, Kalisch Ellett LM, Pratt NL, Roughead EE. Preoperative medication use and postoperative delirium: A systematic review. BMC Geriatr. 2017;17(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 10.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522. [PubMed] [Google Scholar]
- 11.Tune L, Carr S, Cooper T, Klug B, Golinger RC. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5(2):208–210. [DOI] [PubMed] [Google Scholar]
- 12.Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: A systematic review. Anesth Analg. 2006;102(4):1255–1266. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhou XH, Meranus DH, Wang L, Kukull WA. Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis Assoc Disord. 2016;30(2):113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt EM, Saczynski JS, Kosar CM, et al. The Successful Aging after elective Surgery (SAGES) study: Cohort description and data quality procedures. J Am Geriatr Soc. 2015;63(12):2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: The Successful Aging after elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818 e811–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng EL, Chui HC. The modified mini-mental state (3ms) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 17.Duprey MS, Devlin JW, Briesacher BA, Travison TG, Griffith JL, Inouye SK. Approaches to optimize medication data analysis in clinical cohort studies. J Am Geriatr Soc. 2020;68(12):2921–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 19.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: Chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62(3):518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RN, Rudolph JL, Inouye SK, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol. 2010;32(10):1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasunilashorn SM, Fong TG, Albuquerque A, et al. Delirium severity post-surgery and its relationship with long-term cognitive decline in a cohort of patients without dementia. J Alzheimers Dis. 2018;61(1):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache ii: A severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 24.Litaker D, Locala J, Franco K, Bronson DL, Tannous Z. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001;23(2):84–89. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Bin Abd Razak HR, Yeo SJ. Incidence of postoperative delirium in patients undergoing total knee arthroplasty-an asian perspective. Ann Transl Med. 2017;5(16):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderWeele TJ. A unification of mediation and interaction: A 4-way decomposition. Epidemiology. 2014;25(5):749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascha EJ, Dalton JE, Kurz A, Saager L. Statistical grand rounds: Understanding the mechanism: Mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117(4):980–994. [DOI] [PubMed] [Google Scholar]
- 28.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with sas and spss macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katznelson R, Djaiani G, Mitsakakis N, et al. Delirium following vascular surgery: Increased incidence with preoperative beta-blocker administration. Can J Anaesth. 2009;56(11):793–801. [DOI] [PubMed] [Google Scholar]
- 31.Katznelson R, Djaiani GN, Borger MA, et al. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology. 2009;110(1):67–73. [DOI] [PubMed] [Google Scholar]
- 32.Murakawa K, Kitamura Y, Watanabe S, Hongo S, Shinomiya K, Sendo T. Clinical risk factors associated with postoperative delirium and evaluation of delirium management and assessment team in lung and esophageal cancer patients. J Pharm Health Care Sci. 2015;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrends M, DePalma G, Sands L, Leung J. Association between intraoperative blood transfusions and early postoperative delirium in older adults. J Am Geriatr Soc. 2013;61(3):365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tully PJ, Baker RA, Winefield HR, Turnbull DA. Depression, anxiety disorders and type d personality as risk factors for delirium after cardiac surgery. Aust N Z J Psychiatry. 2010;44(11):1005–1011. [DOI] [PubMed] [Google Scholar]
- 35.Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: A systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC. The comparative risk of delirium with different opioids: A systematic review. Drugs Aging. 2017;34(6):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soffin EM, Waldman SA, Stack RJ, Liguori GA. An evidence-based approach to the prescription opioid epidemic in orthopedic surgery. Anesth Analg. 2017;125(5):1704–1713. [DOI] [PubMed] [Google Scholar]
- 38.Subramaniam B, Shankar P, Shaefi S, et al. : Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: A meta-analysis. JAMA Intern Med. 2015;175(4):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


