Abstract
Objective:
To investigate the effects of the Intensive Cognitive and Communication Rehabilitation (ICCR) program for young adults with acquired brain injury (ABI) using a quasi-experimental pilot intervention study design while transitioning to remote implementation.
Method:
Twelve young adults with chronic ABI (treatment n=7; control n=5) participated in ICCR (i.e., lectures, seminars, individual CR, technology training) for six hours/day, four days/week, for one or two 12-week semesters. Outcomes included classroom metrics, individual therapy performance, including Goal Attainment Scaling (GAS), standardized cognitive-linguistic assessments, and participation and health-related quality of life (HRQOL) measures.
Results:
In the first semester (in-person and remote), treatment participants significantly improved in classroom exams; individual therapy (i.e., memory, writing, GAS); executive function and participation measures, but not QOL. In the second semester (remote), treatment participants significantly improved in classroom exams; essay writing; individual therapy (i.e., writing, GAS); and memory assessment, but not in participation or QOL. Treatment participants enrolled in consecutive semesters significantly improved in classroom exams, individual therapy (i.e., memory), participation and QOL, but not on standardized cognitive assessments. Controls demonstrated no significant group-level gains.
Conclusion:
These preliminary results highlight the benefit of intensive, integrated, and contextualized CR for this population and show promise for its remote delivery.
Keywords: cognitive rehabilitation, education, traumatic brain injury, stroke, language, executive functioning
Acquired Brain Injury and the Young Adult Population
Acquired brain injury (ABI) is a leading cause of disability in adults [1,2]. Stroke and TBI are prevailing etiologies of ABI [3] with approximately 795,000 individuals experiencing stroke [4] and nearly three million individuals sustaining a TBI in the US annually [5]. Young adults are a prominently affected age group to sustain TBI in the United States [3,5,6] and the incidence rate of young stroke has been increasing over the past several decades [7,8].
Significance of ABI in Young Adults
Young adulthood is a time of great development [9]. Sustaining an ABI during this time can disrupt participation in key growth experiences, such as attending college and establishing a career. This disruption occurs because cognitive processes that are integral to academic success, such as working memory, language, and attention [10–12], are often impaired after ABI [13,14]. This population often struggles to successfully transition to post-secondary education and a subsequent career. Missing out on these academic and social milestones can ultimately limit their independence from their families and society at large (e.g., financial, living [15,16]).
Cognitive Rehabilitation for ABI
Cognitive Rehabilitation (CR) is the most common method of intervention for cognitive impairment after ABI [17]. CR includes several different approaches, such as restorative (i.e., re-establish previously learned behaviors [18]), compensatory (i.e., establish new patterns of behavior [18]), comprehensive (i.e., address cognitive, interpersonal, and functional skills in individual and group therapy contexts on a daily basis [19]), and contextualized (i.e., target cognition in personally-relevant contexts with personally-meaningful stimuli [20]).
CR approaches are inherently embedded with principles of experience-dependent neuroplasticity (e.g., “use it or lose it”, “repetition”, “intensity”, “salience”, “age”, “transference [21,22]), which provide guidelines for promoting learning in the healthy and damaged brain after injury and are thus naturally applicable to rehabilitation programs for ABI. Each approach has strengths, yet their limitations may diminish the benefit of using a single approach in isolation. Treatment outcomes may be maximized by developing a multimodal, integrated CR program that simultaneously implements the most advantageous features of individual CR approaches.
CR Needs for Young Adults with ABI
The current continuum of post-injury care (i.e., acute, inpatient, outpatient, community) for most young adults with ABI is often disjointed and can result in incomplete recovery [23,24]. Available services for this population, including intensive comprehensive aphasia programs [25]; TBI model systems of care [26]; and coaching programs [27], may be insufficiently intensive, repetitive, salient, or contextualized to support young adults to transition to postsecondary education.
Young adults with ABI often struggle in postsecondary educational settings. They report difficulty on academic tasks (e.g., textbook readings, lack of repetition of material; [28,29]) and achieving overall success due to their cognitive impairments [30–34]. Students with ABI who have returned to college also endorse hardships associated with disability services (e.g., unaware of available accommodations [31], managing inadequately trained staff [35]). Moreover, they are often responsible for seeking out and establishing formal accommodations on their own, which can be challenging to accomplish in the presence of language and other cognitive impairment after ABI [36].
In sum, there is an obvious need for more specialized CR services for young adults with ABI pursuing college. Intervention for this population should integrate CR approaches that are grounded in principles of experience-dependent neuroplasticity within a variety of functional contexts such as classroom activities, exam preparation and performance [37], and essay writing [38,39], to encourage successful recruitment of these domains in postsecondary educational activities. This pilot intervention study provides one example of how integrated treatment can target cognitive and communication impairments in college-age individuals with ABI, varying in severity and recovery phase, to support their future academic success.
Intensive Cognitive and Communication Rehabilitation (ICCR) Program
The Intensive Cognitive and Communication Rehabilitation Program (ICCR) is a specialized CR program for young adults with ABI pursuing college [40]. The scientific premise of this program is that intensive, comprehensive, and functional rehabilitation that is delivered in the classroom setting improves functional skills like taking exams and memorizing lecture content material but also improves the underlying cognitive-linguistic domains sub-serving such skills. Thus, ICCR targets cognition through the following components: 1) group CR via college coursework; 2) individual CR via impairment- and functionally- based therapy activities; and 3) technology-facilitated CR via application- and computer-based exercises. ICCR participants attend the program six hours/day, four days/week for a 12-week long college semester. In an initial efficacy study for the ICCR program [40], treatment participants showed significant gains in standardized measures, individual treatment, and in classroom and life participation; further, nine of fifteen possible participants have enrolled in college. These outcomes suggest the promise and use of a program like ICCR.
Although the previous study provided promising results, there are several questions regarding the efficacy and mechanism of this novel treatment that remain unanswered. Therefore, in this study, we assessed the following: 1) quantitative gains in classroom and individual therapy performance; 2) interactions between treatment components and their corresponding outcomes (e.g., whether memory strategies acquired in individual therapy influenced classroom exam performance and supported gains on a standardized assessment measuring memory); and 3) whether alternative treatment delivery methods would be feasible (e.g., teletherapy), given increasing availability and interest in telerehabilitation. The following research questions were explored in the context of two semesters of the program when the program transitioned from in-person delivery to teletherapy using a quasi-experimental pilot intervention study design:
1. Do treatment participants demonstrate significant gains on classroom performance metrics within an integrated therapy context (i.e., targets cognitive domains important for academic success, such as memory, executive function, and writing)?
It was anticipated that treatment participants would demonstrate significant gains on pre- and post-semester exams covering academic content; there were numerous built-in opportunities to encourage encoding and retrieval of the newly-acquired information (e.g., multiple repetitions of the material within different activities, such as note taking, group lecture review, and practice quiz questions), agreeing with the principle of “repetition” [21]. Further, it was expected that they would show significant improvements in essay writing due to the integrated nature of the intervention (e.g., writing tasks in the classroom and individual therapy).
2. Do treatment participants demonstrate significant gains on individual therapy goals targeting cognitive domains important for academic success (i.e., memory, executive function, and writing)?
It was expected that treatment participants would demonstrate significant gains on cognitive domains targeted in individual therapy for two reasons. First, skills targeted were important for academic success and inherently relevant to participants’ goals and environment (i.e., aligning with the principle of salience; [21]). Second, twice weekly therapy activities followed evidence-based CR guidelines, and thus were expected to result in gains in the areas of memory, language (i.e., writing; [41]), and executive function [19].
3. Do treatment participants (versus control participants) demonstrate significant gains on standardized assessments measuring cognitive domains targeted in the integrated classroom and individual therapy contexts (i.e., memory, writing, and executive function)?
Treatment participants were expected to show significant gains on standardized assessments measuring performance on domains targeted in the classroom and individual therapy (i.e., memory, executive function, writing). This was anticipated since the central hypothesis of this work was that intensive, comprehensive, and functional, rehabilitation that is delivered in the classroom setting improves functional skills like taking exams and memorizing lecture content material but also improves the underlying cognitive-linguistic domains sub-serving such skills. Such results will underscore the relationships between impairment and functional communication [42,43], the associations between change in cognitive-linguistic skills and functional communication ability and participation [44] and reinforce the value of comprehensive treatment after ABI that is both impairment- and function-driven [45].
4. Do treatment participants (versus control participants) demonstrate significant gains on assessments of life participation and quality of life in conjunction with cognitive and communication gains)?
It was hypothesized that treatment participants would demonstrate gains in life participation (i.e., home, neighborhood and community, school, home and community living) and health-related quality of life (i.e., anxiety, depression, positive affect, communication, cognition) in line with previous work of similarly designed intensive rehabilitation programs [25,46–49]. This outcome was hypothesized irrespective of in-person or remote treatment delivery, as both methods of service implementation were designed to promote participants’ independence.
Methods
Participants
Twelve young adults with ABI (Males = 8) participated in this study (Table 1). They ranged in age from 19 to 38 years, in months post-onset from five to 145 months, language severity from mild to moderate (WAB-R AQ range: 60.9 to 99, Table 1), total cognitive severity from mild-moderate to severe (RBANS total range: 44 to 77, Table 1), and in education from 12 to 23 years at consent. Participants were recruited through social media; local hospitals and clinics; and by word of mouth and marketing through professional organizations and affiliates.
Table 1.
Demographic Information.
| Etiology | Age | Sex | Education (years) | Months Postonset | Language WAB AQ | Cognitive Severity (RBANS Total) | |
|---|---|---|---|---|---|---|---|
| P1 | Tumor | 23.4 | M | 15 | 4.57 | 90.8 | 54 |
| P2 | Stroke | 21.7 | M | 13 | 53.88 | 95.1 | 69 |
| P3 | Stroke | 18.9 | F | 12 | 37.02 | 99 | 77 |
| P4 | Tumor | 22.3 | M | 14 | 19.97 | 97.6 | 74 |
| P5 | Encephalitis | 26 | M | 12 | 144.94 | 93.9 | 59 |
| P6 | Tumor | 26.5 | F | 13 | 99.02 | 69.7 | 47 |
| P7 | TBI | 28.6 | M | 12 | 96.06 | 93.4 | 53 |
| P8/C1 | Tumor | 20.5 | F | 13 | 22.24 | 68.5 | 54 |
| P9/C2 | TBL | 21.05 | M | 12 | 18.69 | 60.9 | 44 |
| C3 | TBL | 32.81 | M | 18 | 99.93 | 97.1 | 64 |
| C4 | Stroke | 30.94 | M | 23 | 38.47 | 72.1 | 64 |
| C5 | TBL | 38.23 | F | 16 | 61.46 | 84.2 | 53 |
Note. TBI = traumatic brain injury; M = male; F = female
In accordance with eligibility criteria for the study, all twelve participants exhibited cognitive impairments post-ABI via standardized assessments; reported a goal of enrolling in and/or returning to post-secondary education; and demonstrated hearing and vision for functional conversation and reading, respectively. All participants provided written consent in accordance with Boston University Institutional Review Board policy and procedures.
Eligible participants were offered to start treatment or defer for a semester. Seven unique participants contributed treatment data for Spring 2020, and two unique participants contributed treatment data for Summer 2020, with five participants contributing treatment data to both the Spring 2020 and Summer 2020 semesters. Five of the twelve participants deferred treatment, meaning they could engage in everyday life activities (e.g., outpatient therapy, volunteering, work) but could not take college coursework; two of these individuals transitioned to the treatment phase in Summer 2020. See Figure 1 for timelines pertaining to participants’ enrollment in the program.
Figure 1.
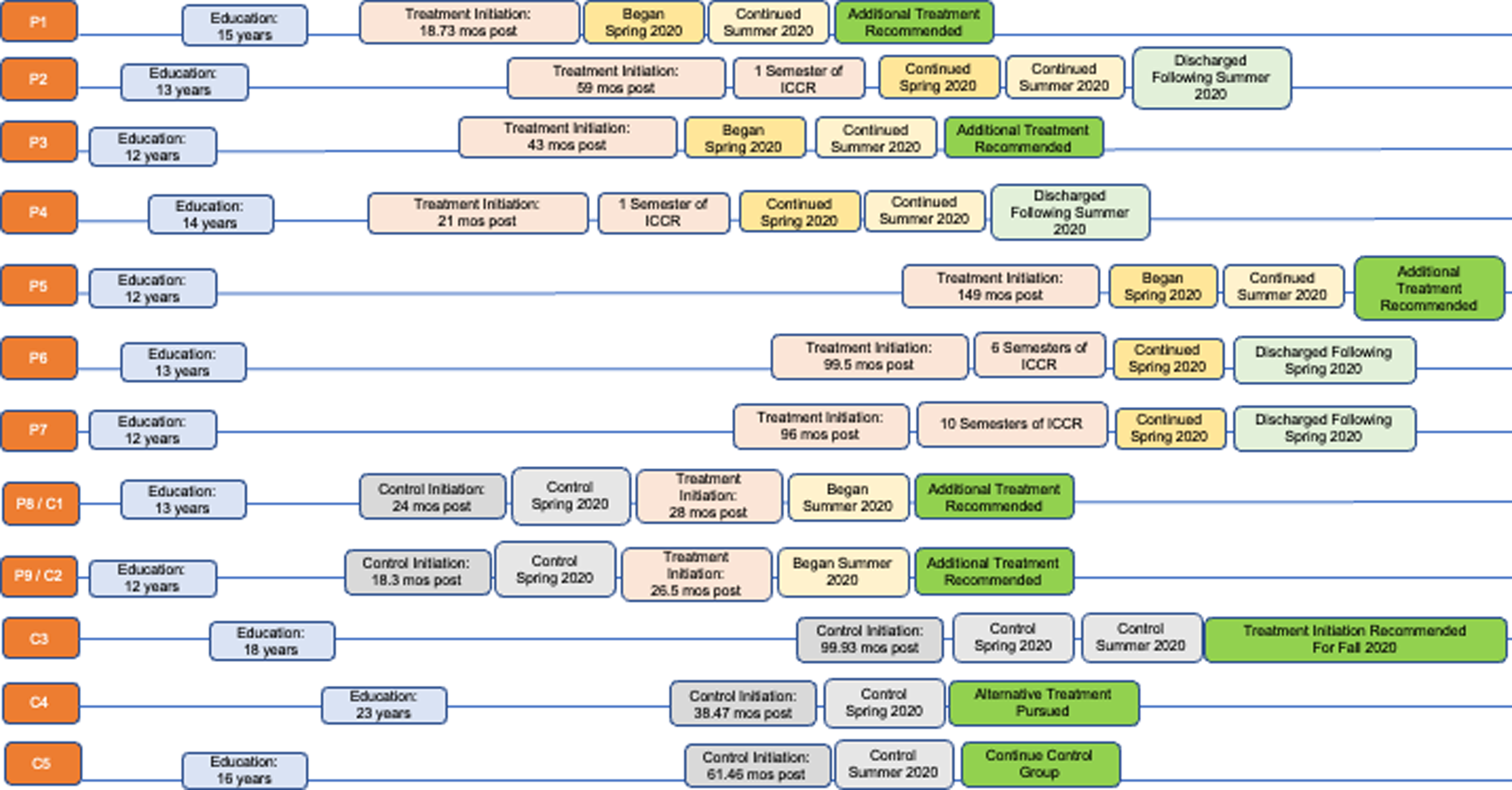
Participants and treatment timelines Note. This figure illustrates the relationship between participants, their prior level of education, their time post onset of injury at enrollment in the ICCR program, and semesters enrolled in the program, either as controls or in treatment. This figure also provides context for which participants were enrolled in the program prior to this study as well as points of discharge.
Standardized assessment performance
Treatment and control participants completed a battery of assessments before and after each 12-week semester. The post-Spring 2020 timepoint was used as pre-treatment data for the pre-Summer 2020 timepoint for individuals who participated in both semesters. Standardized assessment outcomes included subtests measuring memory (i.e., the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [51] Immediate Memory, RBANS Delayed Memory, Scales of Cognitive and Communicative Ability for Neurorehabilitation (SCCAN) [52] Memory), writing (i.e., Western Aphasia Battery-Revised (WAB) [50] Writing, SCCAN Writing), executive function (i.e., WAB Raven’s, SCCAN Problem Solving), life participation (i.e., The Child and Adolescent Scale of Participation-Youth Reported (CASP-Y; [53,54] and health-related quality of life (i.e., TBI or Neuro Quality of Life Scale TBI-QOL; [55] or Neuro-QOL [56,57] anxiety, depression, cognitive function, communication, and positive affect short-forms).
Treatment
Treatment was delivered six hours per day, four days per week within the framework of a 12-week college semester. Key components included: 1) lecture-based courses (e.g., Brain and Mental Health); 2) seminar-based courses (e.g., US History II, Writing); 3) application- and/or computer-based exercises (e.g., Constant Therapy); and 4) individual CR sessions (i.e., two, one-hour sessions weekly). One speech-language pathologist served as the primary clinician in the classroom, while another speech-language pathologist was the primary clinician in individual CR sessions.
The program has historically been delivered in-person [40], however shifted to telepractice due to the COVID-19 pandemic. For the Spring of 2020, the first seven weeks were delivered in-person (Figure 2a), and the final five weeks via telepractice (e.g., Figure 2b). The Summer 2020 semester (Figure 2c) was delivered entirely over telepractice. This shift in service delivery method provided the unique opportunity to assess the impact of the program in an alternative format. See Supplemental Section 1 and Gilmore et al. (2019) for additional details regarding the treatment.
Figure 2.
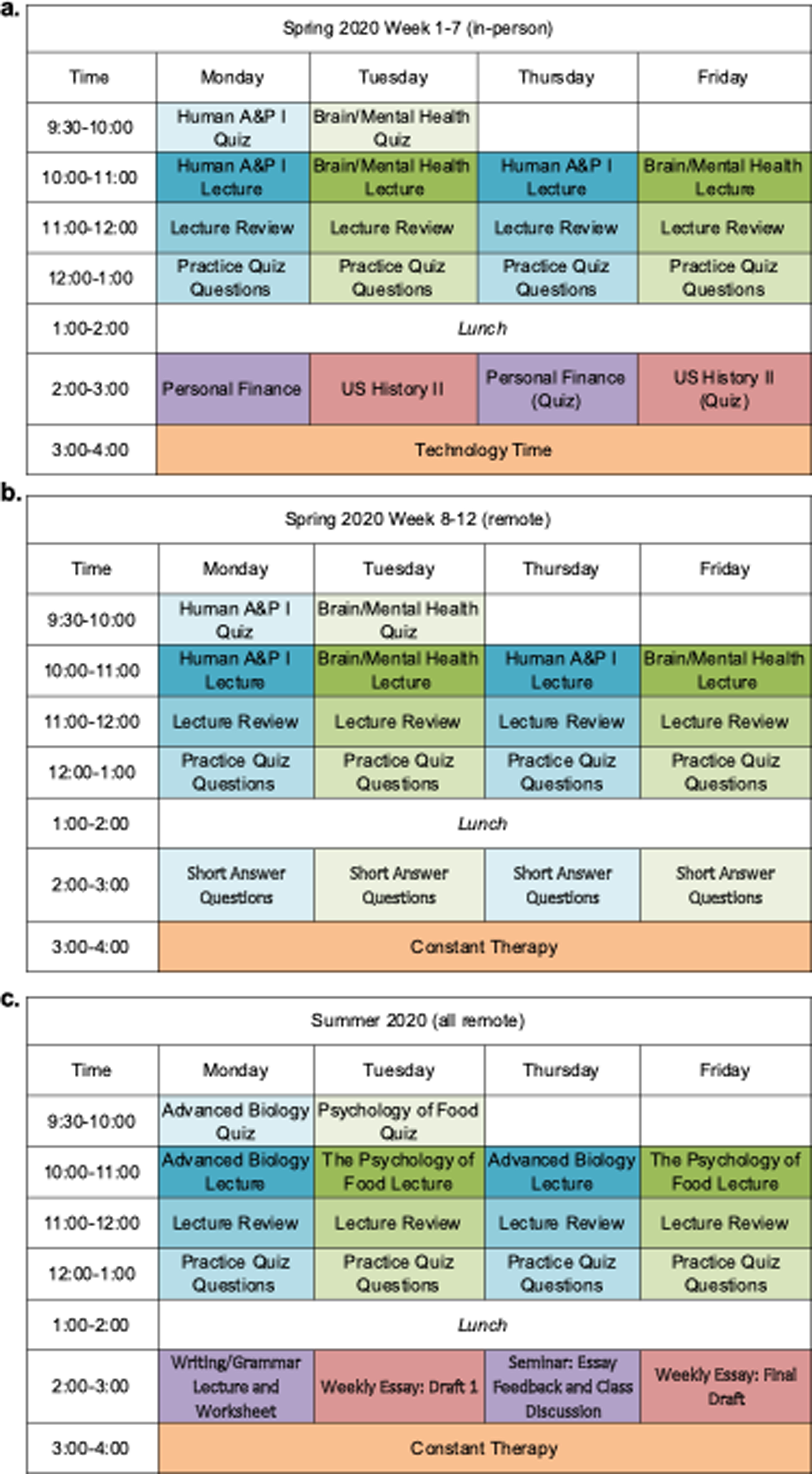
Weekly ICCR schedule by semester.
Classroom- and therapy-based performance metrics
Treatment participants’ performance in the lecture-based courses was quantitatively measured via the administration of the same 35-question cumulative exam (see Figure 3) before and after each semester. Each exam contained 30 multiple-choice questions (e.g., complete the sentence, true/false, definition, NOT questions) and five short-answer questions (e.g., “We learned about the Glycemic Index of Food in class. Please define what it is, and give an example of a high glycemic food and a low glycemic food.”). See Supplemental Section 1 for details regarding exam administration. Participants took weekly quizzes (i.e., five multiple-choice and one short-answer question) pertaining to content covered in the previous week’s lectures via the same methods as exams. These quizzes were used as a therapeutic exercise, providing students opportunities to build study habits and practice applying test-tasking strategies [37]. Performance in the seminar-based essay writing course were graded using a four-point scale (i.e., 0–3 with 3 as full credit) to assess four components (i.e., thesis, evidence, paragraph structure, and mechanics/grammar) with a total possible essay score of twelve.
Figure 3.

Sample classroom exam questions.
Treatment participants’ performance in individual therapy sessions was monitored through: 1) Memory Probes (i.e., completed each session with complexity level individualized to clients), 2) Pre- and Post-Semester Therapy Probes (i.e., tasks matching domains targeted in treatment); and 3) use of Goal Attainment Scaling (GAS)[50]) for participant-generated functional goals (e.g., “improving memory for daily events and information about other people, such as things that happened in the recent past”). See Supplemental Sections 2 and 3 for additional details.
Data Analysis
Classroom and individual therapy data were only available for treatment participants. Classroom pre- and post-exam data for each course and semester were analyzed individually using paired sample t-tests (e.g., Pre-Spring 2020 Anatomy & Physiology scores vs. Post-Spring 2020 Anatomy & Physiology scores). Overall classroom exam test-taking ability was assessed for individuals who participated in two consecutive semesters of ICCR (n = 5) by combining scores from the pre-Spring 2020 courses, combining scores from the post-Summer 2020 courses and then, comparing via paired sample t-tests. Individual therapy data were analyzed using Wilcoxon signed-rank tests to mitigate smaller sample sizes for some of the domains. Standardized assessment data were available for both treatment and control participants. Scores were compared across timepoints using paired sample t-tests. Note that control data from Spring 2020 (n = 4) were combined with the control data from Summer 2020 (n = 1) to constitute an n of 5. Further, data for the three controls who completed the health-related TBI QOLs were analyzed using Wilcoxon signed-rank tests due to the sample size. P-values were adjusted for multiple comparisons according to semester and research question using the Benjamini-Hochberg false discovery rate (FDR) of 0.05. Significant findings at the p < 0.05 level that did not survive FDR-adjustment were reported and discussed given the exploratory nature of this pilot intervention study.
Preliminary Results
Research Question 1: Classroom Performance Metrics
Spring 2020 (n = 7)
In the Brain and Mental Health Course (Figure 4), treatment participants significantly improved in their pre- to post-exam performance (Change M(SD): +10.71(6.90), t(7)=4.11, p=.006, p-adjust=.024). When exam performance was broken down by question type, participants significantly improved on multiple-choice items (Change M(SD): +6.43(5.19), t(7)=3.276, p = .017, p-adjust=.034) and short-answer items (Change M(SD): +4.29 (2.87), t(7) = 3.95, p = .008, p-adjust=.024).
Figure 4.
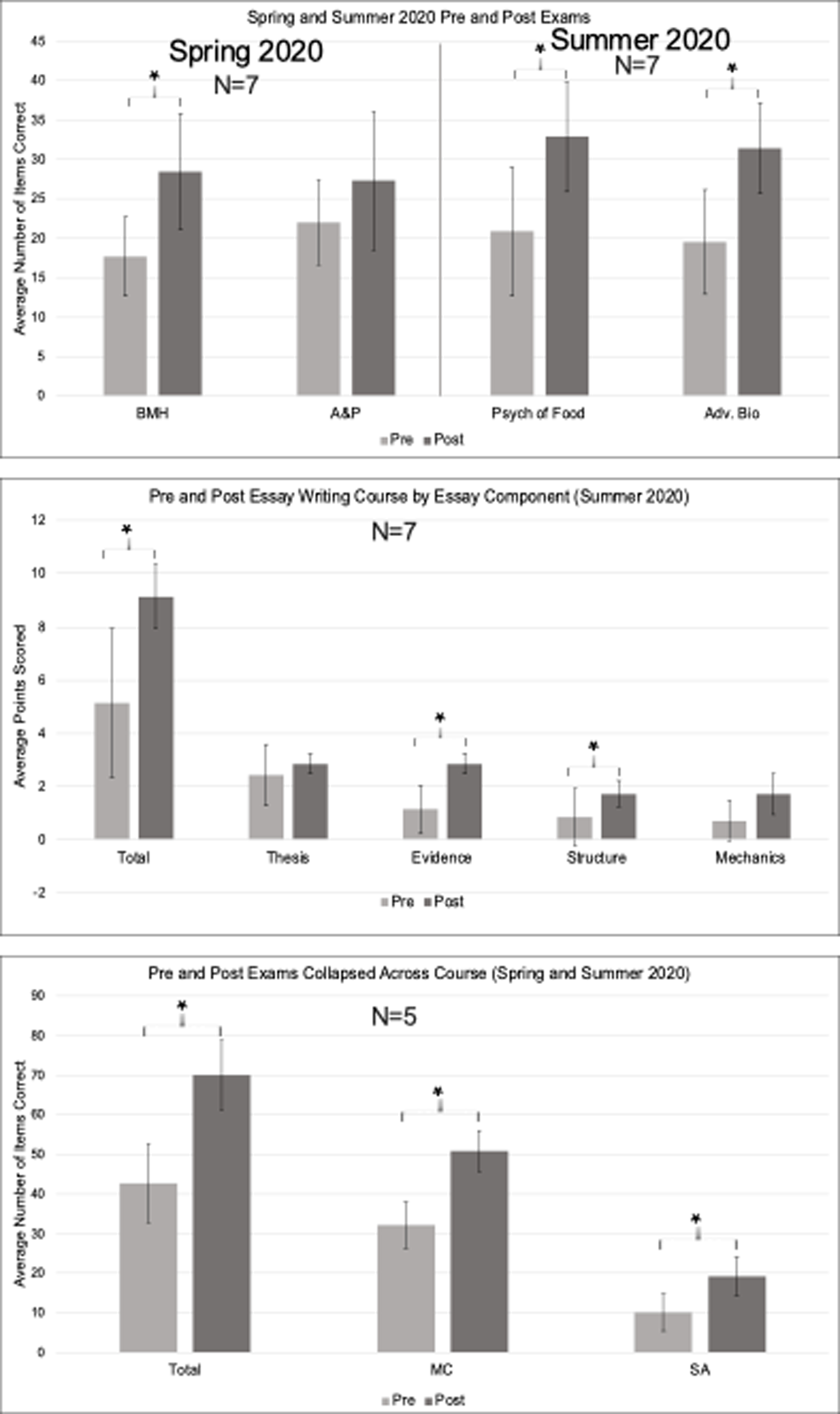
Pre- and Post-Treatment Course Content Exam Scores Note. BMH = Brain and Mental Health; A&P = Human Anatomy and Physiology; Psych of Food = Psychology of Food; Adv. Bio = Advanced Biology. MC = Multiple Choice; SA = Short Answer. * marks significance. Error bars reflect standard error.
In the Anatomy and Physiology course (Figure 4), treatment participants’ showed higher post-exam than pre-exam scores, but this change was not significant (Change M(SD): +5.28(5.82), t(7)= 2.40, p=.053, p-adjust=.0795). The null result remained when exam performance was broken down by question type: multiple-choice items (Change M(SD): +3.57(4.50), t(7) = 2.10, p = .081, p-adjust=.0972) and short-answer items (Change M(SD): +1.71 (2.43), t(7) = 1.87, p = .11, p-adjust=.11).
Summer 2020 (n = 7)
In the Psychology of Food course (Figure 4), treatment participants significantly improved in their pre- to post-exam performance (Change M(SD): +12.00(6.95), t(7) = 4.57 and p = .004, p-adjust=.0075). When exam performance was broken down by question type, participants significantly improved on multiple-choice items (Change M(SD): +9.14(5.52), t(7) = 4.38, p = .005, p-adjust=.0075) and short-answer items (Change M(SD): +2.86 (3.08), t(7) = 2.46, p = .049, p-adjust=.049).
In the Advanced Biology course (Figure 4), treatment participants significantly improved in their pre- to post-exam performance (Change M(SD): +11.86(4.63), t(7) = 6.77 , p = .001, p-adjust=.003). When exam performance was broken down by question type, participants significantly improved on multiple-choice items (Change M(SD): +8.57(3.55), t(7) =6.38, p = .001, p-adjust=.003) and short-answer items (Change M(SD): +3.29 (2.21), t(7) = 3.93, p = .008, p-adjust=.0096). See Supplemental Section 3 for additional information regarding pre- and post-exams.
Examination of the essay writing course administered in Summer 2020 showed that treatment participants demonstrated significant pre- to post-semester gains in their essay writing total performance (Figure 4, Change M(SD) 4.00(3.21), t(7) = 3.29, p = .017, p-adjust=.0425). When performance was broken down by component, participants significantly improved on the evidence (t(7) = 4.77, p = .003, p-adjust=.015), but not the thesis or mechanics/grammar aspects. Treatment participants demonstrated significant improvements in paragraph structure post-treatment, although this finding must be taken with caution as it did not withstand FDR correction (t(7) = 2.52, p = .045, p-adjust=.075).
Spring 2020 to Summer 2020 (n = 5)
Treatment participants significantly improved in their pre- to post-exam performance (Figure 4, out of 90, Change M(SD): +27.40(11.01), t(5) = 5.56, p = .005, p-adjust=.009). When exam performance was broken down by question type, participants significantly improved on multiple-choice items (out of 60, Change M(SD): +18.60(7.99), t(5) = 5.21, p = .006, p-adjust=.009) and short-answer items (out of 30, Change M(SD): +8.80 (4.09), t(5) = 4.82, p = .009, p-adjust=.009).
Research Question 2: Individual Therapy Goals
Spring 2020
Treatment participants demonstrated significant gains in memory probe performance (Figure 5, n = 7, Change M(SD) = 1.43 (1.51), z = −2.06, p = .039, p-adjust=.057) and writing post-semester probe performance (n = 5, Change M(SD) = 1.48 (0.97), z = −2.02, p = .043, p-adjust=.057), although interpretation is guarded as this analysis did not withstand FDR correction. Mean performance on the executive function post-semester probe increased, but this increase was not significant. Participants demonstrated significant gains on their functional goals as measured via GAS (n = 7, Change M(SD) = 1.14 (0.38), z = −2.53, p = .011, p-adjust=.044).
Figure 5.
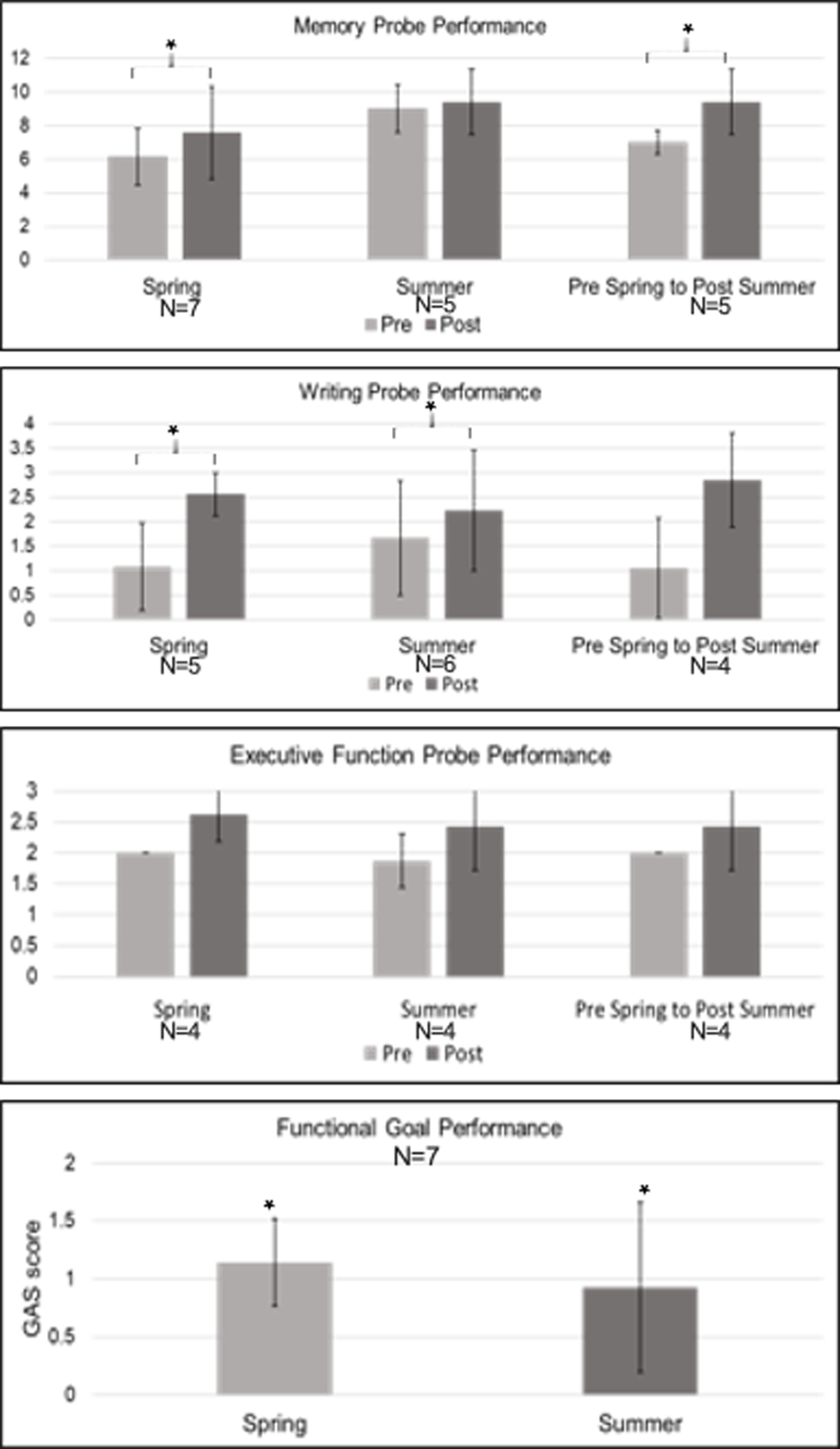
Pre- and Post-Treatment Individual Therapy Scores Note. GAS Scores are plotted as the average change score. * marks significance. Error bars reflect standard error.
Summer 2020
Treatment participants demonstrated significant gains in writing probe performance (n = 6, Change M(SD) = 0.56 (0.33), z = −2.06, p = .039, p-adjust=.078) and on their functional goals (n = 7, Change M (SD): .93 (.73), z = −2.06, p = .039, p-adjust=.078), although these findings must be taken with caution as they did not withstand FDR correction. No significant gains were shown on the memory or executive function post-semester probes.
Spring 2020 to Summer 2020
Treatment participants who participated in consecutive semesters demonstrated significant gains in memory probe performance (Figure 5, n = 5, Change M(SD) = 2.40 (2.19), z = −2.06, p = .039, p-adjust=.102), although interpretation is guarded as this finding did not withstand FDR correction. They demonstrated no significant gains on the writing or executive function post-semester probes.
Research Question 3: Standardized Assessments
Spring 2020
Treatment participants (n=7) demonstrated significant gains in executive function as measured by performance on the WAB Raven’s (Figure 6, Change M(SD): 0.05 (.004), t(7)=3.29, p = .017, p-adjust=.119, although this finding did not reach statistical significance after FDR correction. No significant gains were found on the other standardized assessment subtests related to memory, problem solving, or writing.
Figure 6.
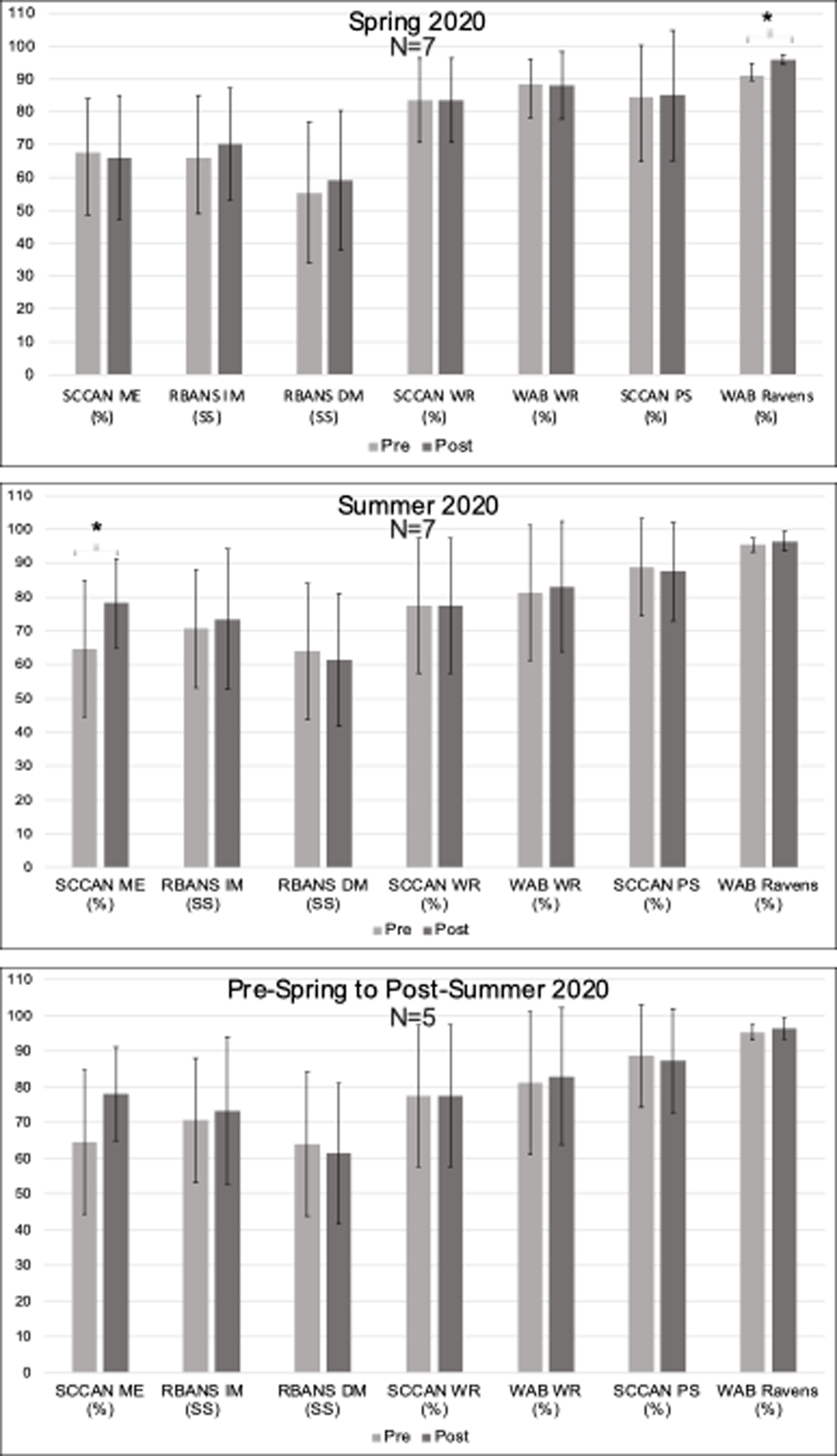
Pre- and Post-Treatment StandardizedAassessment Scores Note. SCCAN ME = Scales of Cognitive and Communication Ability for NeurorehabilitationMemory; RBANS IM and DM = Repeatable Battery for the Assessment of Neuropsychological Status Immediate and Delayed Memory; SCCAN WR = SCCAN Writing; WAB WR = Western Aphasia Battery Writing; SCCAN PS = SCCAN Problem Solving; WAB Ravens = Raven’s Coloured Progressive Matrices. * marks significance. Error bars reflect standard error.
Summer 2020
Treatment participants (Figure 6, n = 7) demonstrated significant gains in memory as measured by the SCCAN Memory (Change M(SD): 13.53 (9.04), t(7) = 3.96, p = .007, p-adjust=.049), but no significant gains were found on the other standardized assessments of memory, writing, or executive function.
Pre- Spring 2020 to Post-Summer 2020
No significant gains were demonstrated by treatment participants (n = 5) on standardized assessments measuring memory, writing, or executive function, as reflected in Figure 6.
Controls
Control participants (n=5) demonstrated no significant gains on standardized assessment measures of memory, writing, or executive functioning (Figure 7).
Figure 7.
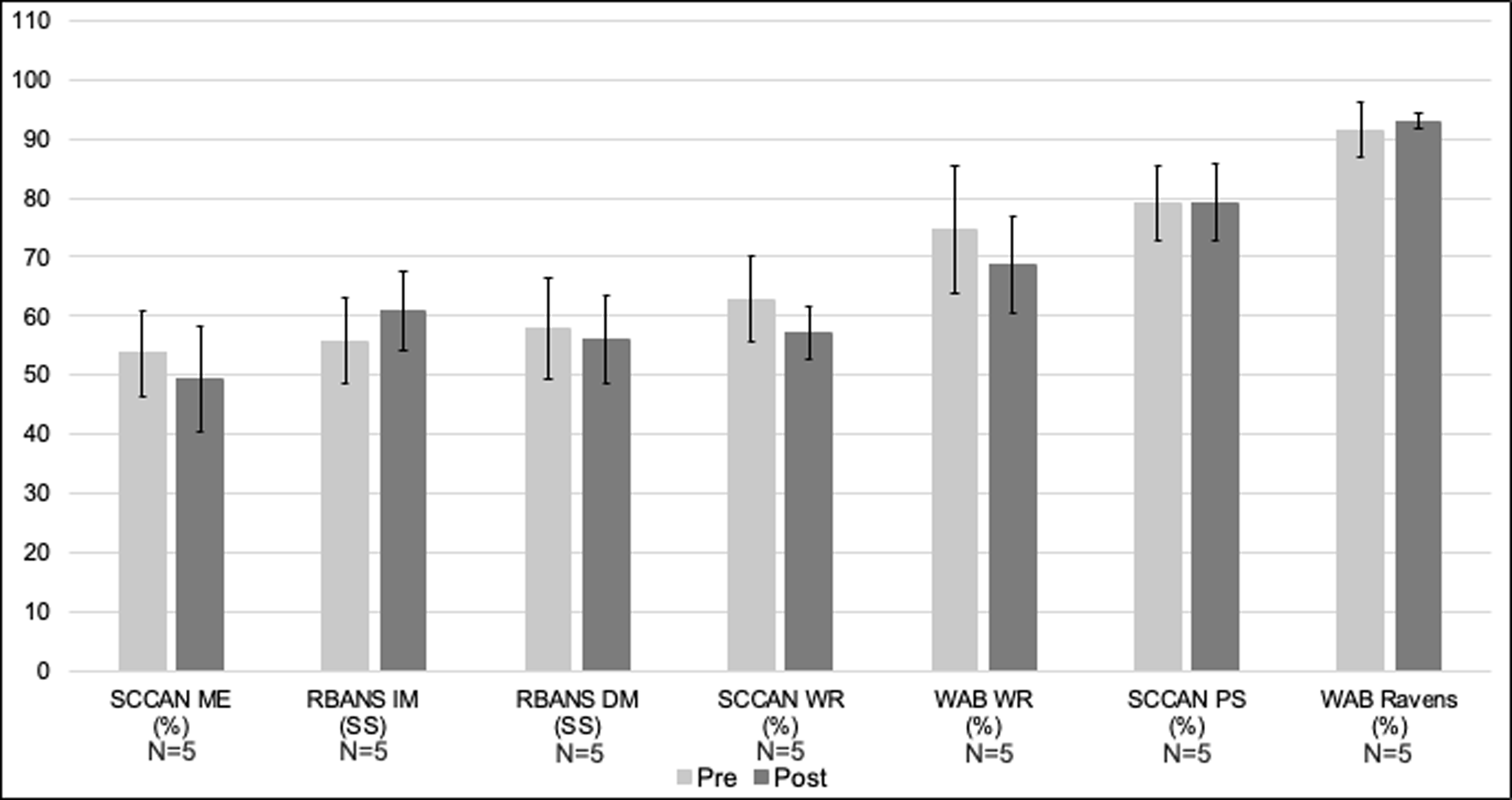
Pre- and Post-Control Phase ofcontrols’ Standardized Assessment Scores. Note. SCCAN ME = Scales of Cognitive and Communication Ability for Neurorehabilitation Memory; RBANS IM and DM= Repeatable Battery for the Assessment of Neuropsychological Status Immediate and Delayed Memory; SCCAN WR = SCCAN Writing; WAB WR = Western Aphasia Battery Writing; SCCAN PS = SCCAN Problem Solving; WAB Ravens = Raven’s Coloured Progressive Matrices. Error bars reflect standard error.
Research Question 4: Participation and Quality of Life Outcomes
Spring 2020
Treatment participants (n = 7) demonstrated significant gains on measures of Home and Community Living Activities in the Child and Adolescent Scale of Participation (CASP-Y; Figure 8, Change M(SD): 12.14 (9.48), t(7) = 3.39, p = .015, p-adjust=.075), which should be interpreted with caution as it did not withstand FDR correction. They demonstrated no significant gains on the other domains assessed. Treatment participants showed no significant gains in Neuro-QOL measures (Figure 9), and the participant who completed the TBI-QOL showed no gains outside of normal test-retest fluctuation.
Figure 8.
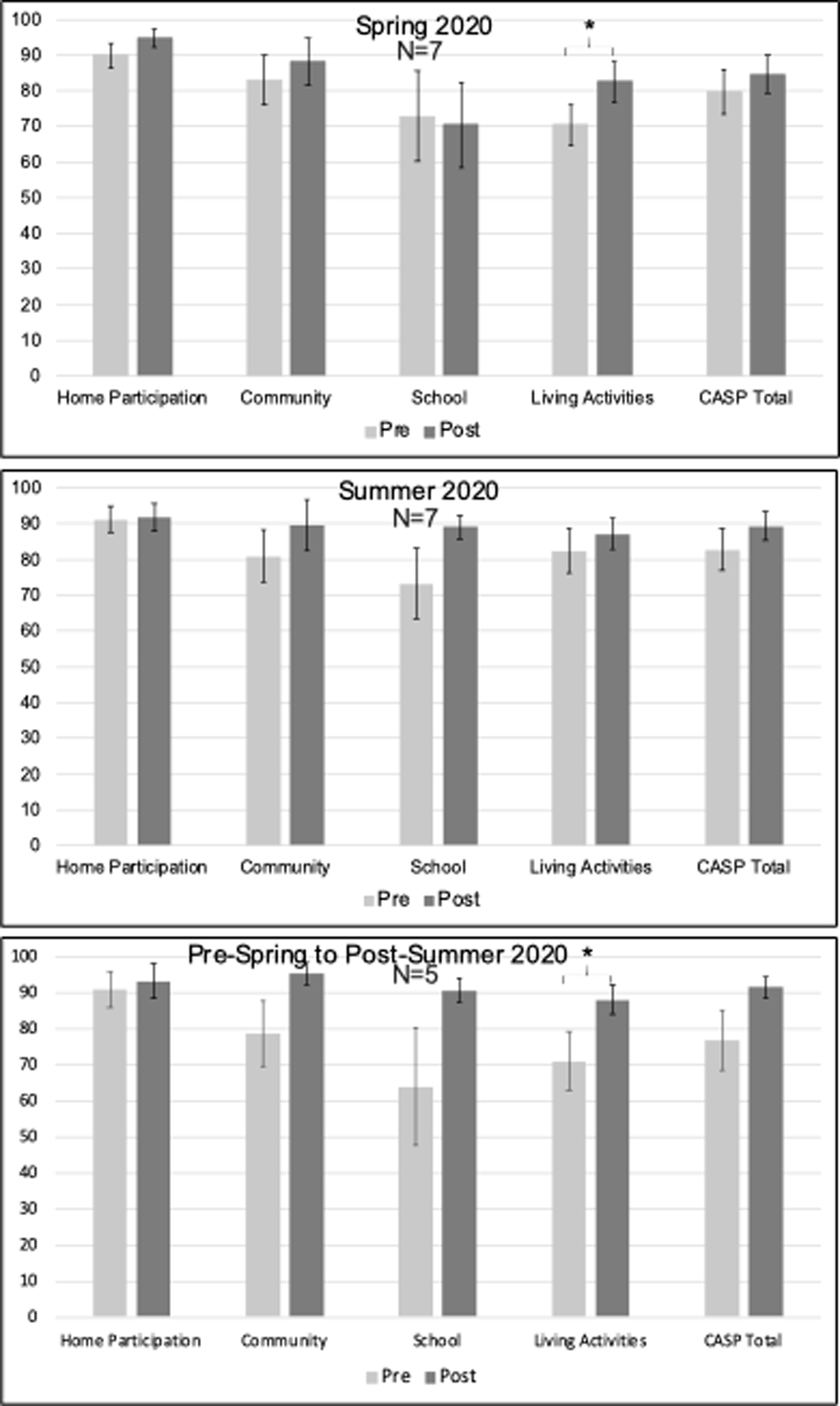
Pre- and Post-Treatment Child and Adolescent Scale of Participation – Youth Reported (CASP-Y) Scores. Note. Error bars reflect standard error.
Figure 9.
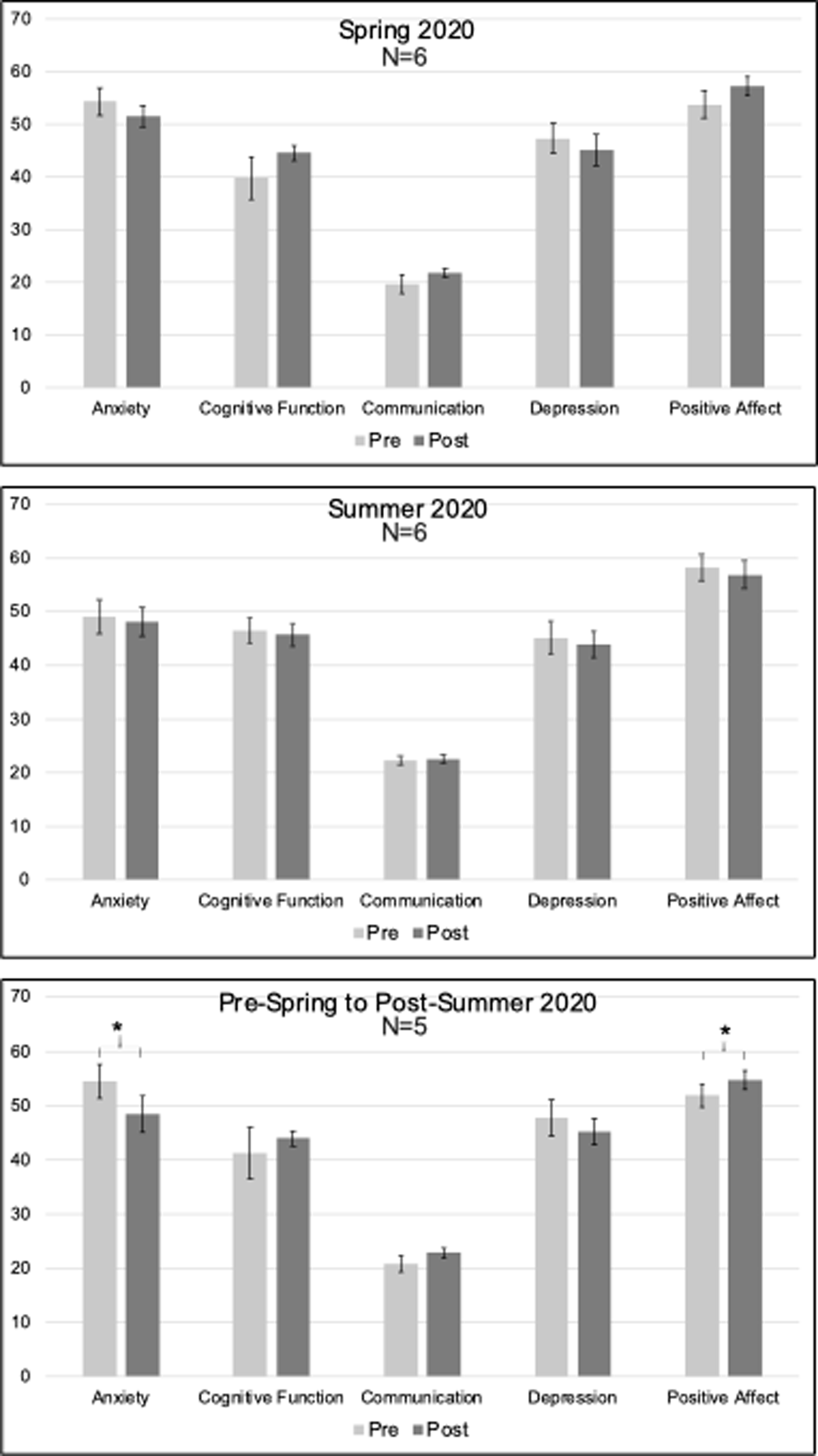
Pre- and Post-Treatment Neuro Quality-of-Life (Neuro-QOL) short-form scores Note. Error bars reflect standard error.
Summer 2020
Treatment participants (n = 7) demonstrated no significant gains on any domain within the CASP-Y, including the measure of living activities that had been significant in Spring of 2020. There were no significant gains among treatment participants on Neuro-QOL measures (n = 6) during the Summer semester (Figure 9). The single participant who completed the TBI-QOL scale again showed no gains outside of normal test-retest fluctuation.
Pre-Spring to Post-Summer 2020
While treatment participants (n = 5) demonstrated significant gains on the measure of living activities in the CASP-Y (Figure 8, Change M(SD): 17 (13.51), t(5) = 2.81, p = 0.048, p-adjust=.12), interpretation of this finding is guarded as it did not withstand FDR correction. No significant gains were found on any of the other domains measured with the CASP-Y. Treatment participants demonstrated reduced anxiety (Figure 9, Change M(SD): −6 (3.83), t(5) = −3.5, p = 0.025, p-adjust=.0925) and improved positive affect (Change M(SD): 2.8 (2.03), t(5) = 3.09, p = 0.037, p-adjust=.0925) after two consecutive semesters of treatment, although this positive result should be taken with caution as it did not withstand multiple comparison correction. No significant improvements were found in other areas of quality of life after two consecutive semesters in the intervention.
Controls
The control group (n = 5) demonstrated no significant gains on the CASP-Y domains or total score (see Figure 10). Most controls (n = 4) showed no significant changes outside of normal test-retest fluctuation on the QOLs. One control showed lower depression and higher positive affect scores on the Neuro-QOL (i.e., scores outside of one SD from the mean).
Figure 10.

Life Participation and QOL Outcome Scores for Controls Note. CASP-Y = Child and Adolescent Scale of Participation – Youth Reported. TBI-QOL = TBI Quality-of-Life. Error bars reflect standard error.
Discussion
This pilot intervention study aimed to extend findings from an initial study of the ICCR program [40] by investigating gains in classroom and individual therapy, exploring outcomes across intervention components, and determining the feasibility of implementing the program via alternative service delivery methods (e.g., teletherapy). Findings were promising as treatment participants ultimately demonstrated group-level gains withstanding FDR correction in the domains of classroom exam performance and essay writing; personal goal attainment; and memory standardized assessment performance, with controls demonstrating no such group-level gains.
The domain-level overlap in gains observed in classroom- and individual therapy-based outcomes emphasized the benefit of the integrated nature of ICCR (e.g., targeting memory in the classroom and individual therapy and seeing improvements in both). Further, participants attended ICCR six hours per day, four days per week for at least one twelve-week semester, providing opportunity for rehearsal and encoding of new information within group-based classroom activities and targeting specific impairments pertaining to memory within individual therapy. The same was true for writing; participants were writing notes and drafting essays in class, while targeting spelling, syntax, and other writing skills in individual therapy. Positive outcomes in exam performance, essay writing, and individual therapy probes drawing on similar cognitive domains underscore the value of comprehensive integrated CR that combines principles of intensity, repetition [21,22], and salience (e.g., academic context)[20]) all within one framework to meet the goals of young adults with ABI pursuing postsecondary education (e.g., requires test taking, essay writing skills [37–39]). Notably, all classroom-based performance metrics maintained significance after adjusting for Type I error, suggesting the greatest area of benefit from ICCR may be in academic performance—a valuable finding for young adults with ABI interested in pursuing college post-injury.
Gains in cognitive domains targeted in the classroom and individual therapy appeared to transfer to significant group-level gains in standardized assessments as well, albeit to a lesser extent. Still, the absence of any significant gains on standardized assessment in the control group emphasizes that the gains seen in treatment were likely attributable to the intervention and highlights the benefit of a program like ICCR for this population as opposed to standard care. This preliminary finding also suggests that intensive and targeted retraining of language and other cognitive domains within a functional context—a paradigm shift from typical rehabilitation for this population—can transfer to gains on discrete cognitive domains as measured by standardized neuropsychological assessments.
After a single semester of ICCR, treatment participants showed significant gains in life participation (i.e., CASP-Y Home and Community Living), but no significant outcomes in HRQOL scores. However, after two consecutive semesters of ICCR, treatment participants demonstrated significant improvements in both life participation and HRQOL (i.e., increased Home and Community Living Activities participation, positive affect, reduced anxiety). These preliminary results imply that a program such as ICCR can foster gains beyond the impairment level, and that there may be additional benefit from cumulative enrollment on self-perceived QOL. These broader psychosocial and functional outcomes of the intervention are noteworthy, providing a reminder of the significant impact that ABI has on activity participation and QOL [58–60], but also extending the validity of participation and QOL outcomes from other similar programs [61]. The lack of significant gains in the control group supports this intervention’s value in this population.
Finally, the shift from in-person to remote due to the COVID-19 pandemic provided an opportunity to assess the feasibility of delivering ICCR via teletherapy. Though they did not all withstand correction procedures, positive findings in the second semester of the intervention, delivered entirely remotely, suggest that teletherapy did not prevent treatment participants from making significant gains, adding to the existing evidence base for telepractice [62]. Conversely, gains in participation and QOL were observed in the Spring semester (hybrid in-person/remote) and cumulatively (from pre-Spring 2020 to post-Summer 2020), but not from Pre- to Post-Summer 2020, which was provided fully remotely, highlighting the importance of future studies exploring the extent to which ICCR benefits participation and QOL when delivered remotely. Finally, remote implementation of ICCR increased access to participants who were previously unable to relocate from their geographic location to participate in ICCR in-person, a perceived benefit of the program.
Limitations
While this study addresses an important topic in need of further study, several limitations must be acknowledged when interpreting the findings. First, the study may have been underpowered to find an effect in either the treatment or control group across domains (i.e., classroom-based, individual therapy, standardized assessments, life participation and HRQOL), and future, larger-scale efficacy studies of this intervention are imperative to replicate and extend the present study’s outcomes. These future studies should include apriori power analyses to inform study design and sample size decisions. Second, in terms of Research Question 3, only significant gains in SCCAN Memory withstood FDR correction, somewhat diminishing the extent to which this study’s findings support the hypothesis that ICCR drives gains in underlying cognitive processes as measured by standardized assessments. Future work should systematically investigate mechanisms that promote transfer between ICCR program components and their corresponding metrics and consider including additional neuropsychological assessments that may better characterize language and other cognitive impairments in young adults with ABI pursuing college. Finally, domain-level study outcomes were not consistent across treatment components (e.g., Summer 2020: classroom gains in writing, standardized assessment gains in memory). Several factors may have contributed to this inconsistency, such as variable clinical profile, severity, and distribution of symptom severity across participants, the effect of which can be modeled in future studies.
Conclusion
Overall, this study’s preliminary results provide new evidence for how ICCR’s integrated components may drive improvements across cognitive-linguistic function, life participation, and aspects of health-related QOL for young adults with ABI as evidenced by significant gains in classroom exam performance, individual therapy metrics, and standardized outcome measures, with classroom performance gains ultimately withstanding correction. These positive findings build on previous work [40] supporting the efficacy of the ICCR program for young adults with chronic ABI interested in college and emphasize the program’s holistic benefit. Because similar outcomes were observed among participants varying in etiology, recovery phase, and educational plans (educational background, treatment duration), these preliminary results lend support for the utility and effectiveness of this type of CR with a range of clinical profiles. Moreover, this study demonstrated the feasibility of delivering ICCR over teletherapy, which could potentially increase the program’s reach in the future. Overall, integrated treatment such as ICCR may be a robust option for filling the gap in the continuum of care for this population, particularly breaking down barriers of geographical location with telerehabilitation.
Taken together, this study’s strengths and limitations provide important pilot data for future large-scale studies that can investigate: 1) the level of intensity and/or proportion of services needed to maximize treatment outcomes, 2) the appropriate balance between classroom CR, individual CR, and technology-facilitated CR, and 3) the number of semesters of enrollment (dosage) in ICCR best supporting significant gains and subsequent success transitioning to and completing a college semester. These studies should explore the impact of repeated cognitive assessments and amplify statistical power to better understand the relationship between the data and results.
Supplementary Material
Acknowledgements
The authors appreciate the ICCR participants and their caregivers for their participation, current and previous members of the Aphasia Research Laboratory for their support in data collection. The office of the Dean of Sargent College of Health and Rehabilitation Sciences provided funding for the ICCR program. Natalie Gilmore was funded by NIH/NIDCD F31DC017892 (PI: Gilmore).
Footnotes
Declaration of Interest Statement
Christianna Gilbert and Anne Citorik are salaried employees at Boston University. Dr. Kiran is a scientific advisor to and cofounder of Constant Therapy Health. Constant Therapy data was collected but was not analyzed or reported in this manuscript. None of the other authors (N.G., G.M.) have any conflicts of interest to report. This study was approved, regulated, and overseen by the Boston University Institutional Review Board.
References
- [1].Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. [DOI] [PubMed] [Google Scholar]
- [2].Langlois JA, Rutland-Brown W, Wald M. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J Head Trauma Rehabil. 2006;21:375–378. [DOI] [PubMed] [Google Scholar]
- [3].Feigin VL, Barker-Collo S, Krishnamurthi R, et al. Epidemiology of ischaemic stroke and traumatic brain injury. Best Pract Res Clin Anaesthesiol. 2010;24:485–494. [DOI] [PubMed] [Google Scholar]
- [4].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Taylor CA. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ [Internet]. 2017 [cited 2018 Oct 5];66. Available from: https://www.facebook.com/CDCMMWR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rates of TBI-related Emergency Department Visits by Age Group — United States, 2001–2010 | Concussion | Traumatic Brain Injury | CDC Injury Center [Internet]. 2016. [cited 2018 Jul 7]. Available from: https://www.cdc.gov/traumaticbraininjury/data/rates_ed_byage.html.
- [7].Kissela BM, Khoury JC, Alwell K, et al. Age at stroke temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Putaala J Ischemic stroke in the young: Current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur Stroke J. 2016;1:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bonnie RJ, Stroud C, Breiner H, et al. Young Adults in the 21st Century [Internet]. 2015. [cited 2020 Aug 17]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK284782/.
- [10].Hassanbeigi A, Askari J, Nakhjavani M, et al. The relationship between study skills and academic performance of university students. Procedia - Soc Behav Sci. 2011;30:1416–1424. [Google Scholar]
- [11].Taraban R, Rynearson K, Kerr M. College Students’ Academic Performance and Self-Reports of Comprehension Strategy Use. Read Psychol. 2000;21:283–308. [Google Scholar]
- [12].Krumrei-Mancuso EJ, Newton FB, Kim E, et al. Psychosocial Factors Predicting First-Year College Student Success. J Coll Stud Dev. 2013;54:247–266. [Google Scholar]
- [13].McDonald MW, Black SE, Copland DA, et al. Cognition in stroke rehabilitation and recovery research: Consensus-based core recommendations from the second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke Off J Int Stroke Soc. 2019;1747493019873600. [DOI] [PubMed] [Google Scholar]
- [14].McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Graham JR, Pereira S, Teasell R. Aphasia and return to work in younger stroke survivors. Aphasiology. 2011;25:952–960 9p. [Google Scholar]
- [16].Smajlovic D Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag. 2015;157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cicerone KD, Dahlberg C, Kalmar K, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81:1596–1615. [DOI] [PubMed] [Google Scholar]
- [18].Institute of Medicine, editor. Cognitive Rehabilitation Therapy for Traumatic Brain Injury: Evaluating the Evidence. Washington, DC; 2011. [Google Scholar]
- [19].Cicerone KD, Goldin Y, Ganci K, et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature From 2009 Through 2014. Arch Phys Med Rehabil [Internet]. 2019 [cited 2019 Apr 4]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003999319301947. [DOI] [PubMed] [Google Scholar]
- [20].Ylvisaker M, Hanks R, Johnson-Greene D. Perspectives on rehabilitation of individuals with cognitive impairment after brain injury: rationale for reconsideration of theoretical paradigms. J Head Trauma Rehabil. 2002;17:191–209. [DOI] [PubMed] [Google Scholar]
- [21].Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. [DOI] [PubMed] [Google Scholar]
- [22].Kiran S, Thompson CK. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front Neurol [Internet]. 2019. [cited 2019 Apr 4];10. Available from: 10.3389/fneur.2019.00295/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lorenz L, Katz G. Severe Brain Injury in Massachusetts: Assessing the Continuum of Care. Issue Brief Mass Health Policy Forum. 2015;1–62. [PubMed] [Google Scholar]
- [24].Wissel J, Olver J, Sunnerhagen KS. Navigating the Poststroke Continuum of Care. J Stroke Cerebrovasc Dis. 2013;22:1–8. [DOI] [PubMed] [Google Scholar]
- [25].Rose ML, Cherney LR, Worrall LE. Intensive Comprehensive Aphasia Programs: An International Survey of Practice. Top Stroke Rehabil. 2013;20:379–387. [DOI] [PubMed] [Google Scholar]
- [26].Bushnik T Introduction: The Traumatic Brain Injury Model Systems of Care. Arch Phys Med Rehabil. 2003;84:151–152. [DOI] [PubMed] [Google Scholar]
- [27].Kennedy MRT, Krause MO. Self-Regulated Learning in a Dynamic Coaching Model for Supporting College Students With Traumatic Brain Injury: Two Case Reports. J Head Trauma Rehabil. 2011;26:212–223. [DOI] [PubMed] [Google Scholar]
- [28].Sohlberg M, Griffiths G, Fickas S. An Exploratory Study of Reading Comprehension in College Students After Acquired Brain Injury. Am J Speech Lang Pathol. 2015;24. [DOI] [PubMed] [Google Scholar]
- [29].Dinnes C, Hux K, Holmen M, et al. Writing Changes and Perceptions After Traumatic Brain Injury: “Oh, by the way, I can’t write.” AJSLP. 2018;27:1523–1538. [DOI] [PubMed] [Google Scholar]
- [30].Cahill SM, Rotter JM, Lyons KK, et al. Survivors of brain injury: the narrative experiences of being a college or university student. Can J Occup Ther Rev Can Ergother. 2014;81:93–101. [DOI] [PubMed] [Google Scholar]
- [31].Kennedy MRT, Krause MO, Turkstra LS. An electronic survey about college experiences after traumatic brain injury. NeuroRehabilitation. 2008;23:511–520. [PubMed] [Google Scholar]
- [32].Mattuzzi M, Pfenninger SE. The language-cognition-affect interface in young college student stroke survivors with aphasia. Int J Appl Linguist [Internet]. 2018. [cited 2018 Jul 19]; Available from: 10.1111/ijal.12222. [DOI] [Google Scholar]
- [33].Todis B, Glang A. Redefining Success: These preliminary results of a Qualitative Study of Postsecondary Transition Outcomes for Youth With Traumatic Brain Injury. J Head Trauma Rehabil. 2008;23:252–263. [DOI] [PubMed] [Google Scholar]
- [34].Mealings M, Douglas J, Olver J. Considering the student perspective in returning to school after TBI: a literature review. Brain Inj. 2012;26:1165–1176. [DOI] [PubMed] [Google Scholar]
- [35].Childers C, Hux K. Invisible Injuries: The Experiences of College Students with Histories of Mild Traumatic Brain Injury. J Postsecond Educ Disabil. 2016;29:389–405. [Google Scholar]
- [36].Harris JR, Depompei R. Provision of Services for Students with Traumatic Brain Injury: A Survey of Ohio Colleges. J Head Trauma Rehabil. 1997; [Google Scholar]
- [37].Sotola LK, Crede M. Regarding Class Quizzes: a Meta-analytic Synthesis of Studies on the Relationship Between Frequent Low-Stakes Testing and Class Performance. Educ Psychol Rev [Internet]. 2020. [cited 2020 Aug 27]; Available from: 10.1007/s10648-020-09563-9. [DOI] [Google Scholar]
- [38].Mongillo G, Wilder H. An examination of at-risk college freshmen’s expository literacy skills using interactive online writing activities. J Coll Read Learn. 2014;42:27–50. [Google Scholar]
- [39].Lloyd J Perceptions on the Essential Writing Skills of Entering First-Year College Students. Walden Diss Dr Stud Collect. 2018; [Google Scholar]
- [40].Gilmore N, Ross K, Kiran S. The Intensive Cognitive-Communication Rehabilitation Program for Young Adults With Acquired Brain Injury. Am J Speech Lang Pathol. 2019;28:341–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dinnes C, Hux K. A Multicomponent Writing Intervention for a College Student With Mild Brain Injury. Commun Disord Q. 2017;39:490–500. [Google Scholar]
- [42].Irwin WH, Wertz RT, Avent JR. Relationships among language impairment, functional communication, and pragmatic performance in aphasia. Aphasiology. 2010;16:823–835. [Google Scholar]
- [43].Aftonomos LB, Steele RD, Appelbaum JS, et al. Relationships between impairment-level assessments and functional-level assessments in aphasia: Findings from LCC treatment programmes. Aphasiology. 2010;15:951–964. [Google Scholar]
- [44].Meier EL, Johnson JP, Villard S, et al. Does Naming Therapy Make Ordering in a Restaurant Easier? Dynamics of Co-Occurring Change in Cognitive-Linguistic and Functional Communication Skills in Aphasia. AJSLP. 2017;26:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Galletta EE, Barrett AM. Impairment and Functional Interventions for Aphasia: Having it All. Curr Phys Med Rehabil Rep. 2014;2:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Persad C, Wozniak L, Kostopoulos E. Retrospective analysis of outcomes from two intensive comprehensive aphasia programs. Top Stroke Rehabil. 2013;20:388–397. [DOI] [PubMed] [Google Scholar]
- [47].Rodriguez AD, Worrall L, Brown K, et al. Aphasia LIFT: Exploratory investigation of an intensive comprehensive aphasia programme. Aphasiology. 2013;27:1339–1361. [Google Scholar]
- [48].Babbitt EM, Worrall L, Cherney LR. Structure, Processes, and Retrospective Outcomes From an Intensive Comprehensive Aphasia Program. Am J Speech Lang Pathol. 2015;24:S854. [DOI] [PubMed] [Google Scholar]
- [49].Winans-Mitrik RL, Hula WD, Dickey MW, et al. Description of an intensive residential aphasia treatment program: Rationale, clinical processes, and outcomes. Am J Speech Lang Pathol. 2014;23:S330–S342. [DOI] [PubMed] [Google Scholar]
- [50].Malec JF. Goal Attainment Scaling in Rehabilitation. Neuropsychol Rehabil. 1999;9:253–275. [Google Scholar]
- [51].Randolph C Repeatable Battery for the Assessment of Neuropsychological Status Update. Bloomington, MN: PsychCorp; 1998. [Google Scholar]
- [52].Holland AL, Milman LH. Scales of Cognitive and Communicative Ability for Neurorehabilitation (SCCAN). Austin, Tx: PRO-ED, Inc.; 2012. [Google Scholar]
- [53].Bedell G Further validation of the Child and Adolescent Scale of Participation (CASP). Dev Neurorehabilitation. 2009;12:342–351. [DOI] [PubMed] [Google Scholar]
- [54].McDougall J, Bedell G, Wright V. The youth report version of the Child and Adolescent Scale of Participation (CASP): assessment of psychometric properties and comparison with parent report. Child Care Health Dev. 2013;39:512–522. [DOI] [PubMed] [Google Scholar]
- [55].Tulsky DS, Kisala PA, Victorson D, et al. TBI-QOL: Development and Calibration of Item Banks to Measure Patient Reported Outcomes Following Traumatic Brain Injury. J Head Trauma Rehabil. 2016;31:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cella D, Lai J-S, Nowinski CJ, et al. Neuro-QOL. Neurology. 2012;78:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gershon RC, Lai JS, Bode R, et al. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual Life Res. 2012;21:475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Johnston MV, Miklos CS. Activity-related quality of life in rehabilitation and traumatic brain injury. Arch Phys Med Rehabil. 2002;83:S26–S38. [DOI] [PubMed] [Google Scholar]
- [59].Brown M, Vandergoot D. Quality of life for individuals with traumatic brain injury: comparison with others living in the community. J Head Trauma Rehabil. 1998;13:1–23. [DOI] [PubMed] [Google Scholar]
- [60].Hilari K, Cruice M, Sorin-Peters R, et al. Quality of Life in Aphasia: State of the Art. Folia Phoniatr Logop. 2016;67:114–118. [DOI] [PubMed] [Google Scholar]
- [61].Svendsen HA, Teasdale TW. The influence of neuropsychological rehabilitation on symptomatology and quality of life following brain injury: A controlled long-term follow-up. Brain Inj. 2006;20:1295–1306. [DOI] [PubMed] [Google Scholar]
- [62].Theodoros D Telepractice in Speech-Language Pathology: The Evidence, the Challenges, and the Future. Perspect Telepractice. 2011;1:10–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


