Abstract
Disruption of quorum sensing (QS) system, which is a central regulator for pathogenesis of Pseudomonas aeruginosa, is referring to as quorum quenching (QQ). This study was undertaken to evaluate and enhance the anti-quorum sensing (AQS) potential of probiotic strain Lactobacillus rhamnosus GG. The cell-free supernatant (CFS) of this probiotic strain showed anti-quorum sensing activity against Pseudomonas aeruginosa, which was determined using well-diffusion agar-plate assay. Anti-quorum sensing potential of L. rhamnosus GG was enhanced by optimization of various cultural conditions using classical and statistical optimization approaches. Six variables were optimized using one-variable-at-a-time (OVAT) method. Four significant variables, viz., temperature, pH, incubation time, metal ion, and its concentration, were chosen for further optimization by response surface methodology (RSM) using central composite design (CCD). Analysis of variance (ANOVA) demonstrated that the regression model is highly significant, as indicated by F test with a low probability value (p < 0.0002) and high value of coefficient of determination (0.8738) and also had significant influence on the generation of anti-quorum sensing effector molecules. Maximum production of anti-quorum sensing activity, in terms of zones of inhibition, was achieved under the following optimized conditions such as 37 °C temperature, pH 6.5, incubation time 24 h, and 2.5 mM concentration of zinc sulfate (ZnSO4). The quadratic model predicted 1.3-fold increase anti-quorum sensing activity production over un-optimized cultural conditions. The present research is the first report representing the enhancement of anti-quorum sensing potential of L. rhamnosus GG.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03187-2.
Keywords: Quorum sensing signal molecules, Anti-quorum sensing activity, Probiotic strain, Pseudomonas aeruginosa, Classical optimization, Response surface methodology
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that causes a significant morbidity and mortality due to severe infections in the respiratory, bloodstream, gastrointestinal, and urinary tract (Olejnickova et al. 2014; Alexandre et al. 2014; Chatterjee et al. 2015). This bacterium is involved in both community-acquired and hospital-acquired infections (Fujii et al. 2014; Kaier et al. 2019), due to which its prevalence is emerging as a serious problem worldwide (Morkunas et al. 2012; Baker and Satlin 2016). The World Health Organization (WHO) has recognized P. aeruginosa as serious threat for human health (Tacconelli and Magrini 2017). Pathogenesis of this bacterium is multifactorial, because it depends on production of cell associated and extracellular virulence factors as well as formation of antibiotic resistant biofilm and all these are regulated by QS system (Todar 2009; Pobiega et al. 2018).
Bacterial pathogen displays the most prominent social behavior known as quorum sensing (QS) in competitive environment. QS is the molecular mechanism by which bacteria sense their overall population density and P. aeruginosa employ AHL-based QS mechanisms. This bacterium produces small QS signal molecules called N-acyl homoserine lactones (AHLs) which regulate different virulence factors, biofilm formation, antibiotic resistance leading to increased pathogenicity (Zhu et al. 2002; Liu et al. 2007; Alexandre et al. 2014; Borges et al. 2016). Hence, the disruption of QS mechanism could be developed into a promising and novel strategy to attenuate pathogenesis, since antibiotic resistance in P. aeruginosa is increasing very rapidly and worldwide posing a major threat to human health.
Disruption of QS system is known as “Quorum quenching” (QQ) or anti-quorum sensing (AQS). This is an attractive strategy to diminish the capability of virulent expression. Recently, many bacterial species having the ability to produce AHL degrading components or enzymes have been successfully used against QS system of P. aeruginosa in-vitro as well as in-vivo (Chang et al. 2017; See-Too et al. 2017; Guendouze et al. 2017; Bergonzi et al. 2018; Rehman and Leiknes 2018; Fong et al. 2018; Malesevic et al. 2019; Mion et al. 2019). Yang et al. (2020) described the effective role of natural products on regulation of QS system and proved their therapeutic potential against different pathogenic microorganisms. These studies strongly indicate that anti-quorum sensing potential of bacteria could be used to develop a potent tool for control of infectious diseases and therefore establish a new generation of therapeutic agents that would be useful in medical field.
In this context, many probiotic bacteria have also been used in the treatment of many infections caused by P. aeruginosa such as gastrointestinal tract (Hwang et al. 2017), pulmonary (Alexandre et al. 2014), and burn wound (Valdez et al. 2005; Ramos et al. 2012; Onbas et al. 2019). They have also been used as antibacterial agents (Al-Malkey et al. 2017) and also help to weaken the resistance for antibiotics in this bacterium (Han et al. 2015). All these studies have focused on the inhibition of virulence factors, biofilm eradication, and reduction of bio burden as well as killing of the pathogen. L. rhamnosus GG recognized as one of the best probiotic strains due to its potential health benefits. This probiotic strain is well-established and widely used in clinical trials and scientific research (Segers and Lebeer 2014). It is well-known and most frequently used probiotic strain in the treatment as well as prevention of various gastrointestinal tract infections, cancer and also stimulates the immune responses (Banna et al. 2017; Liu et al. 2019). The quorum quenching potential of probiotic strain has been studied against methicillin resistant Staphylococcus aureus (Boopathi et al. 2017) as well as a biocontrol agent against bacterial fish diseases in aquaculture (Novita et al. 2015; Ghanei-Motlagh et al. 2020). These studies showed that probiotic strains have enormous health benefits and can attenuate the virulence phenotypes without killing the pathogenic bacteria. Hence, probiotic strain with anti-quorum sensing potential can be considered as a unique anti-virulence strategy that will not only inhibit virulence factors without interfering with growth of P. aeruginosa, but also maintain and enhance the efficacy of host immune system.
Several studies revealed that classical as well as statistical approaches have been used for optimization of probiotic strains for postbiotics (Sharma et al. 2018), bacteriocin (Malheiros et al. 2015; Mathew and Augustine 2018), enzymes (Naganandhini et al. 2014; Deng et al. 2020), and antimicrobial production (Lin and Pan 2015; Rohmatussolihat et al. 2018). Some reports in the literature revealed that application of response surface methodology has been used to enhance production in L. rhamnosus GG for its growth and biomass (Liew et al. 2005; Polak-Berecka et al. 2011) and lactic acid (Mel et al. 2008), and to improve traditional food (Yahyaoui et al. 2017). To the best of our knowledge, there is no report on optimization of culture conditions for enhancement of anti-quorum sensing potential of L. rhamnosus GG. Since, cultural conditions play a major role in maximizing the production of any kind of components and activity in bacterial strains; it will be worthwhile to study the L. rhamnosus GG probiotic strain for anti-quorum sensing ability and its enhanced production.
In the light of these findings, the present work is designed to investigate the probiotic strain L. rhamnosus GG for its anti-quorum sensing potential and optimization for enhancement of anti-quorum sensing activity using classical as well as statistical approaches. The present study is first attempt of its kind and the product of probiotic strain might prove to be effective anti-quorum sensing candidate against P. aeruginosa.
Materials and methods
Bacterial strains and culture conditions
Standard strain of P. aeruginosa PAO1 was obtained from Dr. Barbara H. Iglewski, University of Rochester, New York, USA. It was originally isolated by Iglewski and Kabat in (1975). This strain was maintained on Luria agar slants and stabs kept at 4 °C and also as 20% skim milk and glycerol stocks at –20 °C. Biosensor strain Agrobacterium tumefaciens NT1 was grown in Luria Bertani media in the presence of specified antibiotic for maintenance of reporter plasmids. Antibiotic was purchased from Sigma-Aldrich chemical company (St. Louis, MO, USA). Probiotic strain L. rhamnosus GG was cultured in de Man, Rogosa and Sharpe (MRS) broth at 37 °C. This strain was maintained on MRS agar slants and stabs kept at 4 °C and also as 20% skim milk and glycerol stocks at –20 °C.
Determination of anti-quorum sensing (AQS) potential of probiotic strain Lactobacillus rhamnosus GG
Determination of anti-quorum sensing potential of L. rhamnosus GG was carried out using well-diffusion agar-plate assay (Chen et al. 2010) with some modification. Pure colonies of L. rhamnosus GG were inoculated into MRS broth and incubated at 37 °C for 18–24 h under static conditions. After incubation, cell-free supernatant was collected by centrifuging the culture at 8000×g for 10 min at 4 °C. The cell pellet was suspended in phosphate buffer saline (PBS; 0.01 M, pH 7.2) and termed as cell suspension. Cell-free supernatant (CFS) and cell suspension were then examined for anti-quorum activity using qualitative bioassay. Before this experiment, AHLs from P. aeruginosa were extracted using methodology described by Shaw et al. (1997). For AHLs extraction, the culture supernatant was obtained by removal of bacterial cells using centrifugation from overnight grown culture of P. aeruginosa. Cell-free supernatant was extracted twice with equal volume of acidified ethyl acetate (0.1% acetic acid). After that, both the extracts were pooled in a round-bottom flask and combined extract was concentrated using a rotatory evaporator. This extract was then used as a source of AHLs in well-diffusion agar plate assay. Fresh Luria agar plates covered with 40 μL of X-gal (also abbreviated BCIG for 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (20 mg/mL stock solution in dimethylformamide) were allowed to dry and 100 μL AHLs extracted from P. aeruginosa PAO1 were spread on these plates. Plates were kept at room temperature for 30 min for complete absorption. Biosensor strain (A. tumefaciens NT1) was spread evenly on the prepared plates and kept under sterile conditions for 5–10 min at room temperature. The 100 µL of cell-free supernatant, cell suspension of L. rhamnosus GG, and controls (MRS broth and PBS) were loaded in 7 mm well bored in Luria agar plates and incubated at 30 °C for 24 h. Plates were then observed for the development of colorless zones of quorum sensing inhibition. The anti-quorum sensing was determined by measuring the diameter of colorless zone (in mm) surrounding the wells.
Quantitative estimation of AHL molecules using β-galactosidase assay
The amount of AHLs in the cell-free supernatant of P. aeruginosa PAO1 was estimated by examining the β-galactosidase activity as predicted previously (Kumar et al. 2015). Briefly, 20 mL overnight culture of biosensor strain (A. tumefaciens NT1) was mixed with 5 mL of AHLs extracted from treated (1% cell-free supernatant of L. rhamnosus GG) and untreated P. aeruginosa PAO1 (control). This reaction mixture was incubated at 30 °C with continuously shaking at 100 rpm for 5 h. Cell lysate was prepared and the β-galactosidase activity was then measured as described by Miller (1972). The Units of β-galactosidase expressed as Miller Units (MU) and were calculated using the formula
Determination of minimum inhibitory concentration and growth curve analysis
Minimum inhibitory concentration (MIC) of cell-free supernatant of L. rhamnosus GG was evaluated against A. tumefaciens NT1 using resazurin microtiter plate assay as described by Webster et al. (2010). Furthermore, the growth profile of A. tumefaciens NT1 was analyzed to examine the inhibitory effect of CFS of L. rhamnosus GG according to the method described by Bose et al. (2020). Briefly, early log phase cells of biosensor strain (1 × 106 CFU/mL) were inoculated into 100 mL of LB broth medium containing 1 and 5% of CFS. LB broth medium inoculated with A. tumefaciens NT1 only served as positive control. The flasks were incubated at 28 °C and the OD600 was monitored at 2 h intervals for up to 24 h.
Optimization of cultural conditions for anti-quorum sensing activity production in Lactobacillus rhamnosus GG using one-variable-at-a-time (OVAT) approach
For the selection of initial parameters, one variable at a time method was employed. This method was used to change one variable at a time, while other variables remaining constant. The effect of inoculum age, inoculum volume, temperature, pH, incubation time, and metal ions were optimized for maximum production of anti-quorum sensing activity. In this study, cell-free supernatant of L. rhamnosus GG was evaluated for maximum production of anti-quorum sensing activity using well-diffusion agar plate method by measuring the colorless zone size (mm) around the well and then expressed in terms of zone of inhibition.
Effect of inoculum age and inoculum volume
To study the effect of inoculum age and size, MRS broth was inoculated with different inoculum age from 16–48 h and inoculum size from 0.5–3.5% (v/v). Cell-free supernatant was investigated for maximum anti-quorum sensing activity production.
Effect of incubation temperature
To study the effect of incubation temperature, MRS broth was incubated at different temperature such as 20, 25, 30, 35, 37, 40, 45, 50, 55, and 60 °C keeping all other conditions constant. Temperature with highest anti-quorum sensing activity production was optimized and used for further study.
Effect of pH
The effect of pH on anti-quorum sensing acitivity production was assessed by varying the pH of MRS broth (pH 2.5–8). Thereafter, MRS broth was inoculated with L. rhamnosus GG culture and incubated under optimized production conditions. Cell-free supernatant was analyzed for maximum production of anti-quorum sensing activity.
Effect of incubation time
Optimal incubation time for the production of anti-quorum activity was determined by incubating the production medium (MRS broth; pH, 6.5) at 37 °C for 48 h. Samples were withdrawn after every 6 h of incubation and cell-free supernatant was assayed for maximum anti-quorum sensing activity production.
Effect of metal ions
To check the effect of metal ions on the production of anti-quorum sensing activity, different metal ions (ZnSO4, FeSO4, NiSO4, CuCl2, CaCl2, and CdCl2) were used. Each of 50 mL of MRS broth (pH 6.5) was separately supplemented with 1 mM of different metal ions, while MRS broth without metal ions was used as control. Following the incubation at 37 °C for 24 h, cell-free supernatant was observed for maximum production of anti-quorum sensing activty.
Effect of optimized metal ion concentration
To study the effect of different concentrations of optimized ZnSO4 metal ion, production media (MRS broth) were supplemented with different concentrations (1, 2.5, 5, 7, and 10 mM) of ZnSO4 metal ion. MRS broth without ZnSO4 metal ion concentrations was used as control. After incubation at 37 °C for 24 h, cell-free supernatant was checked for maximum anti-quorum sensing activity production.
Response surface methodology (RSM)
On the basis of one-variable-at-a-time (OVAT) experiment for production of anti-quorum sensing activity, four variables such as temperature, pH, incubation time, metal ion, and its concentration (ZnSO4, 2.5 mM) were selected for further optimization by response surface methodology. The Central Composite Design (CCD) was employed using Design Expert 8.0.4 trial Version (Stat-Ease Inc., Minneapolis, USA) to optimize the level of the significant variables. Anti-quorum sensing activity in terms of zone of inhibition was recorded as response. A set of 30 experiments were carried out and 6 replications at center point were designed for each variables at 5 different levels (– α, – 1, 0, + 1, and + α). All the experiments were performed in replicates and average values are presented in Table 2. Regression analysis was performed on the data obtained. Experimental data of RSM were fitted via multiple regression procedure, using the following second-order polynomial equation:
| 1 |
where Yi is predicted response, XiXj are the independent variable, β0 is the intercept coefficient, βi is the linear coefficient, βii is the quadratic coefficient, and βij is the interaction factors. The well-diffusion agar plate assay (Chen et al. 2010) with some modification was used to estimate the response (production of anti-quorum sensing activity in term of zone of inhibition) in cell-free supernatant of L. rhamnosus GG.
Table 2.
Central composite design (CCD) for the four variables and experimentally determined actual value and predicted value for anti-quorum sensing activity in terms of zone of inhibition by RSM
| Run | Factor 1 Temperature ( °C) |
Factor 2 pH |
Factor 3 Incubation time (h) |
Factor 4 metal ion (mM) |
Actual value (mm) |
Predicted value (mm) |
Residual value (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 32 | 7 | 12 | 4 | 0.0000 | 0.6272 | – 0.6272 |
| 2 | 37 | 6.5 | 48 | 2.5 | 10.00 | 8.03 | 1.97 |
| 3 | 37 | 6.5 | 24 | 0 | 9.50 | 8.95 | 0.5540 |
| 4 | 32 | 6 | 36 | 4 | 8.00 | 8.98 | – 0.9772 |
| 5 | 37 | 6.5 | 24 | 2.5 | 13.00 | 11.48 | 1.52 |
| 6 | 37 | 5.5 | 24 | 2.5 | 5.90 | 5.82 | 0.0828 |
| 7 | 42 | 6 | 12 | 4 | 6.00 | 4.93 | 1.07 |
| 8 | 42 | 7 | 12 | 4 | 5.30 | 2.96 | 2.34 |
| 9 | 42 | 7 | 36 | 4 | 5.40 | 5.19 | 0.2062 |
| 10 | 37 | 6.5 | 24 | 2.5 | 10.50 | 11.48 | – 0.9782 |
| 11 | 37 | 6.5 | 24 | 2.5 | 10.70 | 11.48 | – 0.7782 |
| 12 | 32 | 7 | 12 | 1 | 5.00 | 4.24 | 0.7610 |
| 13 | 37 | 6.5 | 24 | 2.5 | 11.50 | 11.48 | 0.0218 |
| 14 | 32 | 7 | 36 | 4 | 5.10 | 4.91 | 0.1895 |
| 15 | 32 | 6 | 12 | 1 | 6.80 | 6.81 | – 0.0057 |
| 16 | 47 | 6.5 | 24 | 2.5 | 7.00 | 9.23 | – 2.23 |
| 17 | 42 | 6 | 12 | 1 | 6.60 | 6.19 | 0.4110 |
| 18 | 37 | 6.5 | 24 | 2.5 | 12.00 | 11.48 | 0.5218 |
| 19 | 37 | 6.5 | 24 | 2.5 | 11.00 | 11.48 | – 0.4782 |
| 20 | 27 | 6.5 | 24 | 2.5 | 11.00 | 9.57 | 1.43 |
| 21 | 37 | 6.5 | 0 | 2.5 | 0.0000 | 2.77 | – 2.77 |
| 22 | 32 | 7 | 36 | 1 | 7.00 | 7.87 | – 0.8723 |
| 23 | 42 | 7 | 36 | 1 | 7.30 | 7.91 | – 0.6057 |
| 24 | 37 | 7.5 | 24 | 2.5 | 1.0000 | 1.88 | – 0.8838 |
| 25 | 32 | 6 | 12 | 4 | 6.50 | 5.29 | 1.21 |
| 26 | 42 | 6 | 36 | 1 | 8.00 | 7.17 | 0.8277 |
| 27 | 42 | 6 | 36 | 4 | 6.40 | 6.56 | – 0.1605 |
| 28 | 42 | 7 | 12 | 1 | 7.50 | 6.32 | 1.18 |
| 29 | 37 | 6.5 | 24 | 5.5 | 2.00 | 3.19 | – 1.19 |
| 30 | 32 | 6 | 36 | 1 | 8.10 | 9.84 | – 1.74 |
Statistical analysis
The experimental data obtained from the central composite design (Table 2) was employed for regression analysis. The statistical significance and goodness-of-fit of regression model was assessed by F test and the coefficient of determination R2, respectively. The t test was employed to determine the significance of the regression coefficients and p values were used as a tool to assess the significance of each coefficient (Myers and Montgomery 2002). All these results were evaluated by the analysis of variance (ANOVA, Table 3). Response data were fed and analyzed by the software to generate 3D plots indicating the optimum conditions and interactions among these variables. The statistical analysis was accomplished using Design Expert 8.0.4 trial Version (Stat-Ease Inc., Minneapolis, USA).
Table 3.
Analysis of variance (ANOVA) for composite central design for production of anti-quorum sensing activity in terms of zone of inhibition
| Source | Sum of squares | Degree of freedom | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Model | 290.56 | 14 | 20.75 | 7.42 | 0.0002 | Significant |
| A—Temp | 0.1667 | 1 | 0.1667 | 0.0596 | 0.8104 | |
| B—pH | 23.21 | 1 | 23.21 | 8.30 | 0.0114 | |
| C—Incubation time | 41.61 | 1 | 41.61 | 14.88 | 0.0016 | |
| D—Metal ion | 24.91 | 1 | 24.91 | 8.91 | 0.0093 | |
| AB | 7.29 | 1 | 7.29 | 2.61 | 0.1272 | |
| AC | 4.20 | 1 | 4.20 | 1.50 | 0.2392 | |
| AD | 0.0625 | 1 | 0.0625 | 0.0223 | 0.8832 | |
| BC | 0.3600 | 1 | 0.3600 | 0.1287 | 0.7248 | |
| BD | 4.41 | 1 | 4.41 | 1.58 | 0.2284 | |
| CD | 0.4225 | 1 | 0.4225 | 0.1511 | 0.7030 | |
| A2 | 7.46 | 1 | 7.46 | 2.67 | 0.1232 | |
| B2 | 100.55 | 1 | 100.55 | 35.95 | 0.0001 | |
| C2 | 63.83 | 1 | 63.83 | 22.83 | 0.0002 | |
| D2 | 50.99 | 1 | 50.99 | 18.23 | 0.0007 | |
| Residual | 41.95 | 15 | 2.80 | |||
| Lack of fit | 37.58 | 10 | 3.76 | 4.29 | 0.0607 | Not significant |
| Pure error | 4.38 | 5 | 0.8750 | |||
| Cor total | 332.51 | 29 |
Note: p > 0.05, insignificant difference; p < 0.05, significant difference; p < 0.01, significant difference. Standard deviation = 1.67; mean = 7.14; CV = 23.43%; R2 = 0.8738; adjusted R2 = 0.7561; predicted R2 = 0.3110; Adeq precision = 9.1763.
Validation of statistical model
The statistical model was validated taking anti-quorum sensing activity production under the optimum conditions predicted by the model in shake flask level. Anti-quorum sensing activity in terms of zone of inhibition was determined on plate bioassy in three replicates. The observed experimental values were compared with the values as predicted by the RSM study.
Results and discussion
In recent years, the prevalence of nosocomial infections due to Multi Drug Resistant (MDR) P. aeruginosa has raised in tertiary care hospitals in India as well as on a global scale (Oliver et al. 2015; Baker and Satlin 2016). Pathogenicity of this bacterium directly depends on production of virulence factors and formation of antibiotic-resistant biofilm regulated by quorum sensing system (Borges et al. 2016). Today, the researchers around the globe have paid attention to discovery of natural and safe antibiotic alternative strategies against quorum sensing system of P. aeruginosa. In this context, anti-quorum sensing potential of probiotic strain could be a useful alternative strategy against these problems. Hence, this study reported the anti-quorum sensing ability and optimization for its maximum production from probiotic strain L. rhamnosus GG.
Anti-quorum sensing activity (AQS) of Lactobacillus rhamnosus GG
The anti-quorum sensing potential of L. rhamnosus GG was evaluated by performing well-diffusion agar-plate assay using biosensor strain A. tumefaciens NT1 and AHLs extracted from P. aeruginosa. In the present study, A. tumefaciens NT1 (traR, tra::lacZ749) was selected as a biosensor strain, because it contains a plasmid with lacZ fusion to traG, a gene which requires TraR transcriptional activator and acyl-homoserine lactone for expression (Piper et al. 1993). The traG::lacZ reporter of this strain gets activated when supplied with an exogenous active AHL molecules leading to formation of blue color due to hydrolysis of X-gal resulting from the induction of β-galactosidase gene (Joshi et al. 2014). The anti-quorum sensing activity due to the degradation of AHL was observed as a colorless zone around the wells in Luria agar plate. QS inhibition although allows the growth of biosensor strain, but constrains the induction of β-galactosidase and ultimately inhibits the hydrolysis of X-gal or pigmentation, due to which a turbid zone is formed around the well in plates (Weng et al. 2012; Joshi et al. 2014).
In this study, anti-quorum sensing activity in terms of zone of inhibition (10 mm) was found only in cell-free supernatant (CFS) and not in cell suspension of L. rhamnosus GG (Fig. 1a). The zone of inhibition was not observed in a Luria agar plate inoculated with controls, i.e., MRS broth and PBS (Fig. 1b), indicating no inhibition of QS signaling molecule (AHL). Based on these observations, it was concluded that anti-quorum sensing activity of L. rhamnosus GG is extracellular and not intracellular as cell suspension did not show inhibition of signal molecule of quorum sensing. De Marco et al. (2017) reported that the cell free supernatant of synbiotics (combination of probiotic and prebiotic in the form of synergism) has anti-virulence activity. However, the anti-quorum sensing activity observed in extracellular substance produced by probiotic strain, i.e., Bacillus sp., is comparable to the present study (Novita et al. 2015). Furthermore, the effect of CFS of L. rhamnosus GG on the AHLs production in P. aeruginosa PAO1 was studied using β-galactosidase assay. The results revealed that β-galactosidase activity was significantly inhibited (70 MU) following treatment with 1% CFS as compared to the untreated control (108 MU) (Fig. 2).
Fig.1.
Screening of anti-quorum sensing potential of L. rhamnosus GG using biosensor strain A. tumefaciens NT1. a Colorless zone of inhibition around the well indicating anti-quorum sensing activity observed in CFS (cell-free supernatant) and no zone observed in CS (cell suspension). b In control of PBS and MRS broth, no zone of inhibition around the well indicating no anti-quorum sensing activity
Fig.2.
β-Galactosidase assay for quantitative estimation of acyl homoserine lactones (AHLs) production by P. aeruginosa PAO1 in the absence (control) and in the presence of 1% cell-free supernatant (CFS) of L. rhamnosus GG
This reduction in enzyme activity can be correlated with the reduction in AHL production in CFS-treated PAO1 (Bai and Rai 2011; Husain et al. 2013). These findings strongly suggest that the CFS of L. rhamnosus GG inhibit quorum sensing system by abrogating the production of AHL molecules in P. aeruginosa PAO1.
Since, probiotic strain also produces various secondary metabolites that show antimicrobial activity (Chen et al. 2019), and therefore, the CFS of L. rhamnosus GG was investigated for the MIC as well as its effect on the growth profile of A. tumefaciens NT1. MIC value of CFS was determined by employing the resazurin dye reduction assay in 96-well microtiter plates (Fig. S1). Cell viability was determined on the basis of reduction of resazurin dye (blue) to (pink) by viable bacterial cells, while non-viable bacterial cells fail to reduce resazurin dye (Guerin et al. 2001). This results of the present study revealed that CFS of L. rhamnosus GG did not inhibit the growth of the biosensor strain at high concentrations.
These findings were further confirmed by monitoring the growth profile of biosensor strain (A. tumefaciens NT1) in the absence and presence of CFS (1 and 5% concentrations) of L. rhamnosus GG (Fig. 3). It was observed that both the concentrations of CFS had no inhibitory effects on the growth of A. tumefaciens NT1 in comparison to untreated control (MRS broth without CFS). Therefore, the zone of inhibition obtained with biosensor strain in agar well-plate assay (Fig. 1) can be purely attributed to the anti-quorum sensing activity of CFS and not by its antimicrobial nature. Hence, the probiotic strain L. rhamnosus GG showed promising anti-quorum sensing activity against quorum sensing signaling molecule (i.e., AHL) of P. aeruginosa.
Fig.3.
Growth profile of biosensor strain A. tumefaciens NT1 in absence (control) and presence of different concentrations of Cell Free Supernatant (CFS) of L. rhamnosus GG
Optimization of cultural conditions for anti-quorum sensing activity production in Lactobacillus rhamnosus GG using one-variable-at-a-time (OVAT) approach
Different cultural conditions for production of anti-quorum sensing activity were optimized and selected by OVAT method (Fig. 4a–g).
Fig.4.
Effect of different cultural conditions on production of anti-quorum sensing activity: a inoculum age, b initial inoculum volume, c temperature, d pH, e metal ions, f metal ion (ZnSo4) concentrations, and g incubation time
Inoculum age and inoculum volume
The results of OVAT approach revealed that the maximum production of anti-quorum sensing activity by L. rhamnosus GG was recorded in the production media (MRS broth) inoculated with 1% volume of 18 h of inoculum (Fig. 4a–b). Jin et al. (1998), showed that in the low ranges of inoculum age and volume, longer time was required for cells to multiply and produce sufficient desired product responsible for any activity. Furthermore, the production of anti-quorum sensing activity was reduced with increase in the age and volume of inoculum. Decline in activity with the increase in the age and size of inoculum might be due to depletion of all nutrients creating nutrient imbalance and increased competition for nutrient uptake in culture media (Roopesh et al. 2006). In the present study, similar results were observed in which very less anti-quorum sensing activity was found at lower and higher ranges of age and volume of inoculum.
Incubation temperature
The production of anti-quorum sensing activity increased at temperature 25–37 °C, while activity decreased as temperature increased from 37 to 60 °C. The optimum temperature was found to be 37 °C for maximum production of anti-quorum sensing activity in L. rhamnosus GG (Fig. 4c). The research work of Souza et al. (2017) revealed that the temperature range between 30 and 37 °C allowed maximum production of metabolites like bacteriocin, biosurfactant, as well as lactic acid by bacterial strain Lactococcus lactis subsp. lactis CECT-4434. Production of anti-quorum sensing activity was observed up to 40 °C; after that, a sharp decline was observed and there was complete loss at 55–60 °C temperatures, while at lower temperature 20 °C, activity was not detected. The results of this study accorded well with the fact that extreme temperatures are unfavorable for bacterial growth and their metabolite production (Ripa et al. 2009). Kok and Papert (2002) also proved that temperature is one of the major factors which affect the growth rate of antagonist or metabolites.
pH
The pH of the growth medium has a major effect on the growth of microorganisms and their metabolites production processes (Lilly and Barnett 1951; Thongwai and Kunopakarn 2007). Some studies described that the metabolic processes and biodegradation ability of microbial populations are highly susceptible to a change in pH and showed inferior results (San Martin 2011; Wang et al. 2011). Higher or lower pH values, i.e., extremes in pH, were also shown to have a negative influence on the growth of microbial populations (Meredith et al. 2000; Rahman et al. 2003). In this study, the production of anti-quorum sensing activity was found to be increased from pH 4.0–6.5, while activity decreased when pH was increased further from pH 6.5–8. Thus, pH 6.5 was found to be optimum for the maximum production of anti-quorum sensing activity (Fig. 4d). These results are in accordance with the fact that most of the microbes have the ability to produce their microbial products at pH ranging from 5.5–8.5 (Thongwai and Kunopakarn 2007; Jain and Pudir 2011). Anti-quorum sensing activity was not detected at pH 2.5–3.0, and this might be due to the reason that bacterium was not able to reach a sufficient amount of biomass required for the activity. LeBlanc et al. (2004) and Mataragas et al. (2003) reported that the production of biomass and bacteriocin activity of LAB (L. fermentum CRL 722, Luconostoc mesenteroides L124 and L442) were less at pH 4.5. These studies showed that pH between 6.0 and 6.5 were optimum for the growth of LAB and bacteriocin activity and further indicated that the LAB growth was suppressed at pH < 5.0.
Incubation time
The results revealed that the production of anti-quorum sensing activity initiates at 12 h of incubation, and then, activity increased with increasing biomass of the L. rhamnosus GG. The maximum activity was recorded at 24 h of incubation and bacterium attained its stationary phase. It was observed that the anti-quorum sensing activity started to decline after 30 h of incubation (Fig. 4g). The loss of activity of metabolites (enzyme) might be due to the depletion of the nutrients in culture medium and production of toxic metabolites with increased biomass production (Trivedi et al. 2017).
Metal ion and its concentration
Among the differernt metals ions, i.e., (ZnSO4, FeSO4, NiSO4, CuCl2, CaCl2, and CdCl2), ZnSO4 was optimized for maximum production of anti-quorum sensing activity (Fig. 4e). Furthermore, 2.5 mM concentration of ZnSO4 (Fig. 4f) was found to be best, while less activity production was observed in controls (L. rhamnosus GG in MRS broth without any metal ions). The production of anti-quorum sensing activity was found to be moderate in CuCl2, CaCl2, and FeSO4 very less in NiSO4 and was not found in CdCl2. The metal ion ZnSO4 with 2.5 mM concentration was chosen for further study. These obtained results might be due to that the optimized concentration of zinc protects the cells from oxidative stress. Zinc is considered as an important micronutrient, and its appropriate concentration protects the cells from oxidative damage and also essential for proper functioning of the microbial system (Marreiro et al. 2017). Table 1 showing all the parameters with their optimized range and maximum zone of inhibition.
Table 1.
Standardization of different parameters for anti-quorum sensing activity in terms of zone of inhibition
| S. no. | Parameters | Variables | Optimized range | Zone of inhibition (mm) |
|---|---|---|---|---|
| 1 | Inoculum age (h) | 16–48 | 18 | 10 |
| 2 | Inoculum volume (%v/v) | 0.5–3.5 | 1 | 10 |
| 3 | Temperature ( °C) | 20–60 | 37 | 10 |
| 4 | pH | 2.5–8 | 6.5 | 10 |
| 5 | Incubation time (h) | 6–48 | 24 | 10.5 |
| 6 | Metal ions (mM) | Cd2+, Ni2+, Fe2+, Cu2+, Zn2+, Ca2+ | Zn2+ (2.5 mM) | 10.5 |
Response surface methodology (RSM)
Response surface methodology (RSM) is a combination of mathematical and statistical techniques. It is a statistical tool used to design experiments, modeling, estimates the effects of variables, assesses significant relationship between the variables, and evaluates optimal conditions of variables for desirable response (Montgomery 1991, 1997).
Central composite design (CCD) analysis
CCD was employed for evaluation of interaction between the selected variables, which ultimately affect the production of anti-quorum sensing activity in terms of zone of inhibition. Table 2 represents the design matrix for CCD analysis and illustrates the coded values of independent variables, experimentally determined actual values, and predicted values. Experimental data obtained from CCD were evaluated using regression analysis. On the basis of regression coefficient, the response variable, and the test variables, data were fitted to second-order polynomial equation. This equation explain the dependence of anti-quorum sensing activity production on the medium components.
Final equation in terms of coded factors given below:
| 2 |
where Y is the response value (anti-quorum sensing activity in terms of zone of inhibition) and A, B, C, and D are temperature, pH, incubation time, and metal ion, respectively. The terms AB, AC, AD, BC, BD, and CD are temperature and pH, temperature and incubation time, temperature and metal ion, pH and incubation time, pH and metal ion, and incubation time and metal ion, respectively.
Analysis of variance (ANOVA) for composite central design
The analysis of variance (ANOVA) was used to evaluate the statistical significance of the composite central design experiment (Table 3). The effect of each variable on production of anti-quorum sensing activity in terms of zone of inhibition was identified by F values and p values. ANOVA demonstrated that the regression model is highly significant, as indicated by F test with a low p value. p values (0.0002) less than 0.05 indicate that model terms are significant. The p values of the linear coefficients B, C, and D were less than 0.05, indicating that linear effects on production of anti-quorum sensing activity were significant, while the linear coefficient A had insignificant effects, because its p value was greater than 0.05.
The p values of squared coefficients B2, C2, and D2 were less than 0.05, indicating that they all had significant influence on production of anti-quorum sensing activity, while A2 was found to be insignificant, because its p value was greater than 0.05. The p value of all interactive coefficients was greater than 0.05 indicating insignificant influence on production of anti-quorum sensing activity.
Coefficient of determination (R2) was used to investigate the adequacy of model. The R2 value higher than 0.9 represents the better adequacy of the regression model. In this study, the value of R2 0.8738 indicated coherence between predicted and experimental values predicted by the model equation. The value of adjusted R2 is (0.8738), suggesting that only 12.62% of the total variation could not be explained on the basis of this model. The p value for the lack of fit was found to be 0.0607 which is not significant, indicating that this quadratic model adequately fit into the data.
Interaction between variables
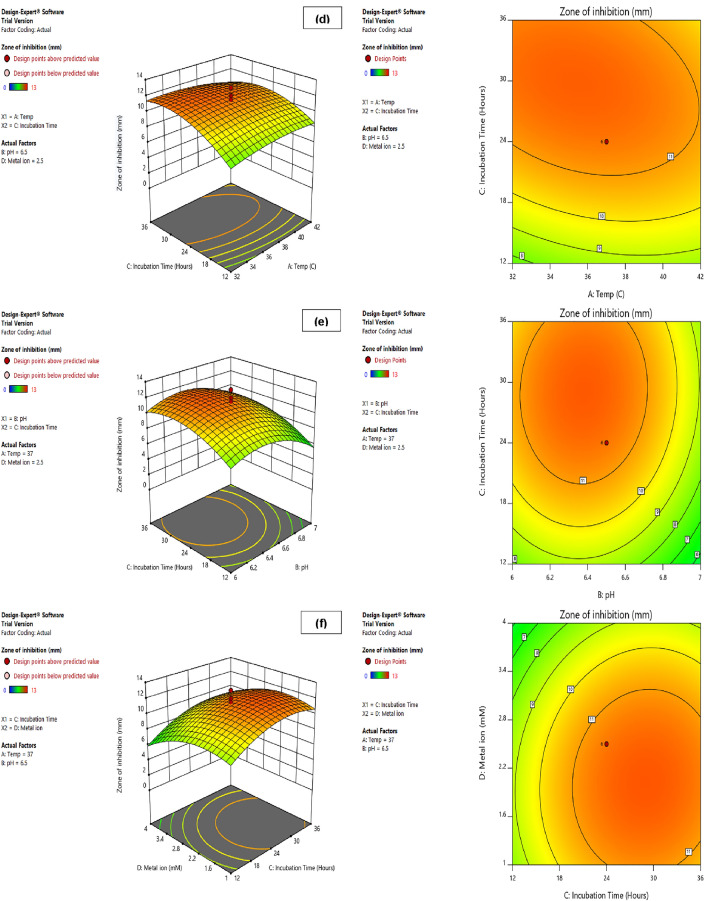
Regression equation is represented graphically by three-dimensional response surface plots and corresponding contour plots (Fig. 5a–f). These plots illustrated the effect of two independent variables within the experimental range, while keeping the other two variables constant at the middle value. Three-dimensional response surface and contour plots were generally used to predict the relationship between the response and experimental level of each independent and dependent variables.
Fig.5.
Three-dimensional response surface and Contour plots illustrating the effect of independent variables on the production of maximum anti-quorum sensing activity in terms of zone of inhibition. a temperature and pH; b temperature and metal ion; c pH and metal ion; d temperature and incubation time; e pH and incubation time; f incubation time and metal ion
The relationship between temperature and pH for the production of anti-quorum sensing activity in terms of zone of inhibition was illustrated at a time in 3D response surface and contour plots (Fig. 5a), while keeping the other variables constant at their levels. These plots show that anti-quorum sensing activity increased with increase in temperature up to the optimum level of 37 °C and further increase in temperature leads to fall in the activity production. Similarly, production of anti-quorum sensing activity rises with an increase in pH up to optimum level of 6.5, while the activity started to decline when pH was increased further.
Figure 5b illustrates the 3D response surface and contour plots and shows the effect of temperature and ZnSO4 metal ion concentration on the production of anti-quorum sensing activity in terms of zone of inhibition. The results showed that concentration of ZnSO4 metal ion in media increased the production of anti-quorum sensing activity up to 2.5 mM, whereas higher concentration resulted in decreased the activity production. On the other hand, anti-quorum sensing activity was increased with rise in temperature up to 37 °C, after that rise in temperature resulted in decreased production of anti-quorum sensing activity. Furthermore, the 3D response surface and contour plots in Fig. 5c depicted the relationship of anti-quorum sensing activity production with the pH and metal ion and results showed the significant interaction between these two variables for the production of activity.
Figure 5d, e, and f show the interaction between temperature and incubation time, pH and incubation time, and incubation time and metal ion in 3D response surface and contour plots, respectively. The results of these plots showed that production of anti-quorum sensing activity increased with increase in temperature, pH and ZnSO4 metal ion concentration up to optimal level of 37 °C, 6.5 and 2.5 mM, respectively. Anti-quorum sensing activity production increased with raise in incubation time (24 h), whereas further escalation in incubation time leads to decrease in activity in all these cases.
The optimal values of the test variables were predicted by quadratic model. Highest production of anti-quorum sensing activity in terms of zone of inhibition, i.e., 13 mm, was obtained when production medium supplemented with these optimal values such as 2.5 mM concentration of ZnSO4 with pH 6.5 and incubated at 37 °C for 24 h. The enhanced anti-quorum sensing activity in term of zone of inhibition from 10 to 13 mm indicating 1.3-fold increase in activity after optimization. Rajesh and Rai (2015), reported that the response surface methodology using CCD analysis yielded a 1.33-fold increase in AHL-lactonase enzyme from endophytic bacteria Enterobacter aerogenes VT66.
Validation of the mathematical model
To evaluate the validity of statistical model, the production of anti-quorum sensing activity in terms of zone of inhibition was analyzed under optimized conditions. The experimental value of average zone of inhibition was 13 mm, which was very closer to the predicted value 11.48 mm (Table 4). The results revealed that the predicted values matched well with experimental values in optimal conditions and strongly support the RSM model with good correlation.
Table 4.
Validation of statistical model showing the production of anti-quorum sensing activity in terms of zone of inhibition at optimal conditions
| Parameters | Optimized range | Predicted value | Observed value |
|---|---|---|---|
| Temperature ( °C) | 37 | 11.48 mm | 13 mm |
| pH | 6.5 | ||
| Incubation time (h) | 24 | ||
| Metal ions (mM) | ZnSO4 (2.5 mM) |
Conclusion
The results of this study confirmed that probiotic strain L. rhamnosus GG possessed anti-quorum sensing potential. Classical and statistical optimization for physiochemical conditions are one of the best tools for obtaining enhanced production of any activity. Four variables, viz., temperature, pH, incubation time, and ZnSO4 metal ion (2.5 mM), were optimized through OVAT method for maximum production of anti-quorum sensing. Response surface methodology further showed that these optimized variables have a significant influence on L. rhamnosus GG for anti-quorum sensing production and exhibited 1.3-fold rises in activity. The study proposes that L. rhamnosus GG with enhanced anti-quorum sensing potential, under standardized conditions, can be efficiently employed against P. aeruginosa to reduce its virulence. Hence, the data obtained from this work seem promising and deserve to be studied further especially for subsequent purification, characterization, and identification of bioactive by-products/metabolites attributing for the anti-quorum sensing activity of L. rhamnosus GG.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Financial assistance provided by University Grant Commission-National Fellowship for Higher Education (NFHE), New Delhi, India to SD (NFST-2015-17-ST-HIM-3198) and UGC-SAP is highly acknowledged.
Author contributions
S Devi, K Harjai, and S Chhibber conceptualized and designed the study. S Devi did the literature survey, laboratory experiments, and made the first draft of manuscript. S Devi, K Harjai, and S Chhibber were responsible for article drafting and critical revision.
Availability of data and materials
All data have been included in the manuscript.
Code availability
Not applicability.
Declarations
Conflict of interest
The authors have no conflict of interest.
Ethic approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Alexandre Y, Le Berre R, Barbier G, Le Blay G. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol. 2014;14(1):1–10. doi: 10.1186/1471-2180-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Malkey MK, Ismeeal MC, Al-Hur FJA, Mohammed SW, Nayyef HJ. Antimicrobial effect of probiotic Lactobacillus spp. on Pseudomonas aeruginosa. J Contemp Med Sci. 2017;3:218–223. doi: 10.22317/jcms.06201704. [DOI] [Google Scholar]
- Bai AJ, Rai VR. Bacterial quorum sensing and food industry. Compr Rev Food Sci Food Saf. 2011;10:183–193. doi: 10.1111/j.1541-4337.2011.00150.x. [DOI] [Google Scholar]
- Baker TM, Satlin MJ. The growing threat of multidrug-resistant Gram-negative infections in patients with hematologic malignancies. Leuk Lymp. 2016;57:2245–2258. doi: 10.1080/10428194.2016.1193859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferrau F, Libra M. Lactobacillus rhamnosus GG: an overview to explore the rationale of its use in cancer. Front Pharmacol. 2017;8:603. doi: 10.3389/fphar.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergonzi C, Schwab M, Naik T, Daude D, Chabriere E, Elias M. Structural and biochemical characterization of Aal, a quorum quenching lactonase with unusual kinetic properties. Sci Rep. 2018;8:11262. doi: 10.1038/s41598-018-28988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathi S, Selvakumar G, Sivakumar N. Quorum quenching potentials of probiotic Enterococcus durans lab38 against methicillin resistant Staphylococcus aureus. Asian J Pharm Clin Res. 2017;10(4):445–450. doi: 10.22159/ajpcr.2017.v10i4.17039. [DOI] [Google Scholar]
- Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simoes M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules. 2016;21:877. doi: 10.3390/molecules21070877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose SK, Chauhan M, Dhingra N, Chhibber S, Harjai K. Terpinen-4-ol attenuates quorum sensing regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Future Microbiol. 2020;15:127–142. doi: 10.2217/fmb-2019-0204. [DOI] [PubMed] [Google Scholar]
- Chang H, Zhou J, Zhu X, Yu S, Chen L, Jin H, Cai Z. Strain identification and quorum sensing inhibition characterization of marine-derived Rhizobium sp. NAO1. R Soc Open Sci. 2017;4:170025. doi: 10.1098/rsos.170025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Anju C, Biswas L, Kumar VA, Mohan CG, Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol. 2015;306:48–58. doi: 10.1016/j.ijmm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lai CC, Huang HL, Huang WY, Toh HS, Weng TC, Chuang YC, Lu YC, Tang HJ. Antimicrobial activity of Lactobacillus species against carbapenem-resistant Enterobacteriaceae. Front Microbiol. 2019;10:789. doi: 10.3389/fmicb.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RD, Zhou ZG, Cao YA, Bai YG, Yao B. High yield expression of an AHL-lactonase from Bacillus sp B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb Cell Fact. 2010;9:39. doi: 10.1186/1475-2859-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco S, Piccioni M, Muradyan D, Zadra C, Pagiotti RD, Pietrella D. Antibioflm and antiadhesive activities of different synbiotics. J Prob Health. 2017;5:1000182. doi: 10.4172/2329-8901.1000182. [DOI] [Google Scholar]
- Deng Y, Xu M, Ji D, Agyei D. Optimization of β-galactosidase production by batch cultures of Lactobacillus leichmannii 313 (ATCC 7830™) Fermentation. 2020;6:27. doi: 10.3390/fermentation6010027. [DOI] [Google Scholar]
- Fong J, Zhang C, Yang R, Boo ZZ, Tan SK, Nielsen TE, Givskov M, Liu XW, Bin W, Su H, Yang L. Combination therapy strategy of quorum quenching enzyme and quorum sensing inhibitor in suppressing multiple quorum sensing pathways of P. aeruginosa. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-19504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii A, Seki M, Higashiguchi M, Tachibana I, Kumanogoh A, Tomono K. Community-acquired, hospital-acquired, and healthcare-associated pneumonia caused by Pseudomonas aeruginosa. Respir Med Case Rep. 2014;12:30–33. doi: 10.1016/j.rmcr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanei-Motlagh R, Mohammadian T, Gharibi D, Menanteau-Ledouble S, Mahmoudi E, Khosravi M, Zarea M, El-Matbouli M. Quorum quenching properties and probiotic potentials of intestinal associated bacteria in Asian sea bass Lates calcarifer. Mar Drugs. 2020;18:23. doi: 10.3390/md18010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendouze A, Plener L, Bzdrenga J, Jacquet P, Remy B, Elias M, Lavigne JP, Daude D, Chabriere E. Effect of quorum quenching lactonase in clinical isolates of Pseudomonas aeruginosa and comparison with quorum sensing inhibitors. Front Microbiol. 2017;8:227. doi: 10.3389/fmicb.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin TF, Mondido M, McClenn B, Peasley B. Application of resazurin for estimating abundance of contaminant-degrading micro-organisms. Lett Appl Microbiol. 2001;32:340–345. doi: 10.1046/j.1472-765x.2001.009. [DOI] [PubMed] [Google Scholar]
- Han HZ, Zhao YJ, Shi CZ, Liang Y, Yang J. Effect of Lactobacillus plantarum on intestinal infection in multiple drug-resistant bacteria mice. Surg Infect. 2015;16:762–768. doi: 10.1089/sur.2014.222. [DOI] [PubMed] [Google Scholar]
- Husain FM, Ahmad I, Asif M, Tahseen Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J Biosci. 2013;38:835–844. doi: 10.1007/s12038-013-9385-9. [DOI] [PubMed] [Google Scholar]
- Hwang IY, Koh E, Wong A, March JC, Bentley WE, Lee YS, Chang MW. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat Commun. 2017;8:15028. doi: 10.1038/ncomms15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Porc Natl Acad Sci USA. 1975;72:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Pundir RK. Effect of fermentation medium, pH and temperature variations on antibacterial soil fungal metabolite production. J Agric Technol. 2011;7:247–269. [Google Scholar]
- Jin B, Van Leeuwen HJ, Patel B, Yu Q. Utilization of starch processing waste water for production of biomass protein and fungal α-amylase by Aspergillus oryzae. Bioresour Technol. 1998;66:201–206. doi: 10.1016/S0960-8524(98)00060-1. [DOI] [Google Scholar]
- Joshi S, Kaur A, Sharma P, Harjai K, Capalash N. Lactonase-expressing Lactobacillus plantarum NC8 attenuates the virulence factors of multiple drug resistant Pseudomonas aeruginosa in co-culturing environment. World J Microbiol Biotechnol. 2014;30:2241–2249. doi: 10.1007/s11274-014-1645-9. [DOI] [PubMed] [Google Scholar]
- Kaier K, Heister T, Gotting T, Wolkewitz M, Mutters NT. Measuring the in-hospital costs of Pseudomonas aeruginosa pneumonia: methodology and results from a German teaching hospital. BMC Infect Dis. 2019;19:1028. doi: 10.1186/s12879-019-4660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok CJ, Papert A. Effect of temperature on in vitro interactions between Verticillium chlamydosporium and other Meloidogyne-associated microorganisms. Biocontrol. 2002;47:603–606. doi: 10.1023/A:1016516505002. [DOI] [Google Scholar]
- Kumar L, Chhibber S, Kumar R, Kumar M, Harjai K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia. 2015;102:84–95. doi: 10.1016/j.fitote.2015.02.002. [DOI] [PubMed] [Google Scholar]
- LeBlanc JG, Garro MS, Savoy de Giori G. Efect of pH on Lactobacillus fermentum growth, rafnose removal, α-galactosidase activity and fermentation products. Appl Microbiol Biotechnol. 2004;65:119–123. doi: 10.1007/s00253-003-1532-z. [DOI] [PubMed] [Google Scholar]
- Liew SL, Ariff A, Raha AR, Ho YW. Optimization of medium composition for the production of a probiotic microorganism, Lactobacillus rhamnosus, using response surface methodology. Int J Food Microbiol. 2005;102:137–142. doi: 10.1016/j.ijfoodmicro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lilly VG, Barnett HL. Physiology of Fungi. New York: McGraw Hill Book Company Inc.; 1951. p. 464. [Google Scholar]
- Lin TH, Pan TM. Optimization of antimicrobial substances produced from Lactobacillus paracasei subsp. paracasei NTU 101 (DSM 28047) and Lactobacillus plantarum NTU 102 by response surface methodology. J Food Sci Technol. 2015;52:6010–6016. doi: 10.1007/s13197-014-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gu Z, Song F, Zhang H, Zhao J, Chen W. Lactobacillus plantarum ZS2058 and Lactobacillus rhamnosus GG use different mechanisms to prevent Salmonella infection in vivo. Front Microbiol. 2019;10:299. doi: 10.3389/fmicb.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wang H, Griffiths MW. Regulation of alkaline metalloprotease promoter by N-acylhomoserine lactone quorum sensing in Pseudomonas fluorescens. J Appl Microbiol. 2007;103:2174–2184. doi: 10.1111/j.1365-2672.2007.03488.x. [DOI] [PubMed] [Google Scholar]
- Malesevic M, Di Lorenzo F, Filipic B, Stanisavljevic N, Novovic K, Enerovic L, Polovic N, Molinaro A, Kojic M, Jovcic B. Pseudomonas aeruginosa quorum sensing inhibition by clinical isolate Delftia tsuruhatensis 11304: involvement of N octadecanoyl homoserine lactones. Sci Rep. 2019;9:16465. doi: 10.1038/s41598-019-52955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malheiros PS, Sant’Anna V, Todorov SD, Franco BDGM, Optimization of growth and bacteriocin production by Lactobacillus sakei subsp. sakei 2a. Braz J Microbiol. 2015;46:825–834. doi: 10.1590/S1517-838246320140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiro DDN, Cruz KJC, Morais JBS, Beserra JB, Severo JS, de Oliveira ARS. Zinc and oxidative stress: current mechanisms. Antioxidants. 2017;6(2):24. doi: 10.3390/antiox6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataragas M, Metaxopoulos J, Galiotou M, Drosinos EH. Infuence of pH and temperature by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442. Meat Sci. 2003;64:265–271. doi: 10.1016/S0309-1740(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Mathew S, Augustine A. Isolation of probiotic bacteria and optimization of physical and nutrition parameters for bacteriocin production. Int J Curr Microbiol App Sci. 2018;7(10):2717–2726. doi: 10.20546/ijcmas.2018.710.316. [DOI] [Google Scholar]
- Mel M, Karim MIA, Salleh MRM, Amin NAM. Optimizing media of Lactobacillus rhamnosus for lactic acid fermentation. J Appl Sci. 2008;8:3055–3059. doi: 10.3923/jas.2008.3055.3059. [DOI] [Google Scholar]
- Meredith W, Kelland S, Jones D. Influence of biodegradation on crude oil acidity and carboxylic acid composition. Org Geochem. 2000;31(1059):1073. doi: 10.1016/S0146-6380(00)00136-4. [DOI] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Mion S, Remy B, Plener L, Bregeon F, Chabriere E, Daude D. Quorum quenching lactonase strengthens bacteriophage and antibiotic arsenal against Pseudomonas aeruginosa clinical isolates. Front Microbiol. 2019;10:2049. doi: 10.3389/fmicb.2019.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery DC. Design and analysis of experiments. 3. New York, USA: John Wiley and Sons; 1991. [Google Scholar]
- Montgomery DC. Design and analysis of experiments. 4. New York, USA: John Wiley and Sons; 1997. [Google Scholar]
- Morkunas B, Galloway WRJD, Wright M, Ibbeson BM, Hodgkinson JT, O'Connell KMG, Bartolucci N, Della Valle M, Welch M, Spring DR. Inhibition of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autouinducer-mimics. Org Biomol Chem. 2012;10:8452–8464. doi: 10.1016/j.ijfoodmicro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology: process and product optimization using designed experiments. 2. New York, USA: John Wiley and Sons; 2002. [Google Scholar]
- Naganandhini S, Vijila K, Gunasekaran S. Optimizing fermentation conditions for fructosyltransferase enzyme production by Lactobacillus plantarum. Afr J Microbiol Res. 2014;8:2429–2435. doi: 10.5897/AJMR12.967. [DOI] [Google Scholar]
- Novita H, Rusmana I, Yuhana M, Pasaribu FH. Potential of Bacillus sp., as a producer of AHL lactonase and its application as a probiotic for the preservation of Mas in Catfish (Clarias gariepinus) J Fish Aquat Sci. 2015;10:464–476. doi: 10.3923/jfas.2015.464.476. [DOI] [Google Scholar]
- Olejnickova K, Hola V, Ruzicka F. Catheter-related infections caused by Pseudomonas aeruginosa: virulence factors involved and their relationships. Pathog Dis. 2014;72:87–94. doi: 10.1111/2049-632X.12188. [DOI] [PubMed] [Google Scholar]
- Oliver A, Mulet X, Lopez-Causape C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat. 2015;22:41–59. doi: 10.1016/j.drup.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Onbas T, Osmanagaoglu O, Kiran F. Potential properties of Lactobacillus plantarum F-10 as a Bio- control strategy for wound infections. Probiotics Antimicrob Prot. 2019;11:1110–1123. doi: 10.1007/s12602-018-9486-8. [DOI] [PubMed] [Google Scholar]
- Piper KR, Vonbodman SB, Farrand SK. Conjugation factor of Agrobacterium-tumefaciens regulates Ti-plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- Pobiega M, Chmielarczyk A, Koziol J, Wesolowska MP, Ziolkowski G, Romaniszyn D, Bulanda M, Mach JW. Virulence factors genes and drug resistance in Pseudomonas aeruginosa strains derived from different forms of community and healthcare associated infections. Postepy Hig Med Dosw. 2018;72:751–759. doi: 10.5604/01.3001.0012.2426. [DOI] [Google Scholar]
- Polak-Berecka M, Wasko A, Kordowska-Wiater MK, Targonski Z, Kubik-Komar AK. Application of response surface methodology to enhancement of biomass production by Lactobacillus rhamnosus E/N. Braz J Microbiol. 2011;42:1485–1494. doi: 10.1590/S1517-83822011000400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Rahman T, Kourkoutas Y, Petsas I, Marchant R, Banat M. Enhanced bioremediation of n- alkane in petroleum sludge using bacterial consortium amended with rhamnolipids and micronutrients. Bioresour Technol. 2003;90:159–168. doi: 10.1016/s0960-8524(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Rajesh PS, Rai VR. Use of aiiA gene amplification for AHL-lactonase production from endophytic bacterium Enterobacter species. Int J Biol Macromol. 2015;72:1013–1019. doi: 10.1016/j.ijbiomac.2014.09.049. [DOI] [PubMed] [Google Scholar]
- Ramos AN, Cabral ME, Noseda D, Bosch A, Yantorno OM, Valdez JC. Antipathogenic properties of Lactobacillus plantarum on Pseudomonas aeruginosa: the potential use of its supernatants in the treatment of infected chronic wounds. Wound Repair Regen. 2012;20:552–562. doi: 10.1111/j.1524-475X.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- Rehman ZU, Leiknes T. Quorum-quenching bacteria isolated from red sea sediments reduce biofilm formation by Pseudomonas aeruginosa. Front Microbiol. 2018;9:1354. doi: 10.3389/fmicb.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa FA, Nikkon F, Zaman S, Khondkar P. Optimal conditions for antimicrobial metabolites production from a new Streptomyces sp. RUPA-08PR isolated from Bangaladeshi soil. Mycobiolgy. 2009;37:211–214. doi: 10.4489/MYCO.2009.37.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmatussolihat Lisdiyanti P, Yopi Widyastuti Y, Sukara E. Medium optimization for antimicrobial production by newly screened lactic acid bacteria. Ann Bogor. 2018;22(1):1–11. doi: 10.14203/ab.v22i1.322. [DOI] [Google Scholar]
- Roopesh K, Ramachandran S, Nampoothiri KM, Szakacs G, Pandey A. Comparison of phytase production on wheat bran and oil cakes in solid-state fermentation by Mucor racemosus. Biores Technol. 2006;97:506–511. doi: 10.1016/j.biortech.2005.02.046. [DOI] [PubMed] [Google Scholar]
- San Martin YB. Bioremediation: a tool for the management of oil pollution in marine ecosystems. Biotecnol Aplicada. 2011;28:69–76. [Google Scholar]
- See-Too WS, Ee R, Lim YL, Convey P, Pearce DA, Yin WF. Chan KG (2017) AidP, a novel N-Acyl homoserine lactonase gene from Antarctic Planococcus sp. Sci Rep. 2017;7(1):1–11. doi: 10.1038/srep42968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb Cell Fact. 2014;13(Suppl 1):S7. doi: 10.1186/1475-2859-13-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Harjai K, Shukla G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P. aeruginosa PAO1 biofilm. Folia Microbiol. 2018;63:181–190. doi: 10.1007/s12223-017-0545-4. [DOI] [PubMed] [Google Scholar]
- Shaw PD, Ping G, Daly SL, Cha C, Cronan JE. Detecting and characterizing N- acyl- homoserine lactone signal molecules by thin layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza EC, de Azevedo POS, Dominguez JM, Converti A, de Souza Oliveira RP. Influence of temperature and pH on the production of biosurfactant, bacteriocin and lactic acid by Lactococcus lactis CECT-4434. CYTA J Food. 2017;15:525–530. doi: 10.1080/19476337.2017.1306806. [DOI] [Google Scholar]
- Tacconelli E, Margrini N (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization.http://www.who.int/entity/medicines/publications/WHO-PPLShort_Summary_25Feb
- Thongwai N, Kunopakarn J. Growth inhibition of Ralstonia solanacearum PT1J by antagonistic bacteria isolated from soils in the Northern part of Thailand. Chiang Mai J Sci. 2007;34:345–354. [Google Scholar]
- Todar K (2009) Opportunistic infections caused by Pseudomonas aeruginosa. In: Kenneth Todar’s online textbook of bacteriology. University of Wisconsin-Madison, Department of Bacteriology, http://www.textbookofbacteriology.net/
- Trivedi S, Sharma A, Jain P. Enhancement of phytase production from a new probiotic strain Bacillus subtilis P6. Int J Curr Microbiol App Sci. 2017;6(6):2744–2759. doi: 10.20546/ijcmas.2017.606.328. [DOI] [Google Scholar]
- Valdez J, Peral M, Rachid M, Santana M, Perdigon G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005;11:472–479. doi: 10.1111/j.1469-0691.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang S, Li Y, Klassen W. Potential approaches to improving biodegradation of hydrocarbons for bioremediation of crude oil pollution. J Environ Prot. 2011;2:47–55. doi: 10.4236/jep.2011.21005. [DOI] [Google Scholar]
- Webster D, Lee TDG, Moore J, Manning T, Kunimoto D, Leblanc D, Johnson JA, Gray CA. Antimycobacterial screening of traditional medicinal plants using the microplate resazurin assay. Can J Microbiol. 2010;56:487–494. doi: 10.1139/w10-035. [DOI] [PubMed] [Google Scholar]
- Weng LX, Zhang YQ, Meng H, Yang YX, Quan ZX, Zhang YY, Wang LH. Screening and isolating quorum sensing inhibitor from bacteria. Afr J Microbiol Res. 2012;6:927–936. doi: 10.5897/AJMR11.813. [DOI] [Google Scholar]
- Yahyaoui AG, Bouzaiene T, Aouidi F, Aydi A, Hamdi M. Traditional cereal food as container of probiotic bacteria “Lb. rhamnosus GG”: optimization by response surface methodology. J Food Qual. 2017;2017:1–12. doi: 10.1155/2017/1742143. [DOI] [Google Scholar]
- Yang M, Meng F, Gu W, Li F, Tao Y, Zhang Z, Zhang F, Yang X, Li J, Yu J. Effects of natural products on bacterial communication and network-quorum sensing. BioMed Res Int. 2020;2020:8638103. doi: 10.1155/2020/8638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Thuruthyil SJ, Willcox MDP. Determination of quorum-sensing signal molecules and virulence factors of Pseudomonas aeruginosa isolates from contact lens-induced microbial keratitis. J Med Microbiol. 2002;51:1063–1070. doi: 10.1099/0022-1317-51-12-1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have been included in the manuscript.
Not applicability.