Abstract
Pseudomonas putida GB-1-002 catalyzes the oxidation of Mn2+. Nucleotide sequence analysis of the transposon insertion site of a nonoxidizing mutant revealed a gene (designated cumA) encoding a protein homologous to multicopper oxidases. Addition of Cu2+ increased the Mn2+-oxidizing activity of the P. putida wild type by a factor of approximately 5. The growth rates of the wild type and the mutant were not affected by added Cu2+. A second open reading frame (designated cumB) is located downstream from cumA. Both cumA and cumB probably are part of a single operon. The translation product of cumB was homologous (level of identity, 45%) to that of orf74 of Bradyrhizobium japonicum. A mutation in orf74 resulted in an extended lag phase and lower cell densities. Similar growth-related observations were made for the cumA mutant, suggesting that the cumA mutation may have a polar effect on cumB. This was confirmed by site-specific gene replacement in cumB. The cumB mutation did not affect the Mn2+-oxidizing ability of the organism but resulted in decreased growth. In summary, our data indicate that the multicopper oxidase CumA is involved in the oxidation of Mn2+ and that CumB is required for optimal growth of P. putida GB-1-002.
The Mn cycle in nature is strongly determined by the redox state of the metal. Generally, reduced Mn [Mn(II)] forms soluble salts, and oxidized Mn [Mn(III and IV)] precipitates as highly insoluble oxides and oxyhydroxides. Interconversions between reduced and oxidized forms are usually catalyzed by microorganisms, which strongly influence the Mn cycle (20).
Work in our laboratory has focused on the process of bacterial Mn2+ oxidation, which is a widespread phenomenon that occurs in many different environments and is catalyzed by a variety of microbial species. Many different oxidation mechanisms are recognized, including indirect mechanisms (which act through changes in the pH or Eh of the environment) and direct mechanisms (which involve the mediation of macromolecules, such as proteins or protein-polysaccharide complexes). The fact that macromolecules are directly involved in Mn2+ oxidation suggests that this process has a physiological function, but its functional significance remains unclear, even though several possibilities have been suggested. For example, Mn2+ oxidation may supply energy for growth (19, 28) or may be involved in scavenging of harmful oxygen species (4, 29), or accumulated Mn oxides may serve as terminal electron acceptors that support anaerobic growth (15, 16, 18). Other possible functions of Mn2+ oxidation include improving competition for dissolved Mn2+, prolonging the viability of manganese-encrusted cells (1), and lysing of complex humic substances in order to provide small organic substrates for growth (35). A major difficulty in elucidating the mechanisms and functional significance of bacterial Mn2+ oxidation stems from the diversity of the species capable of Mn2+ oxidation. Common properties and unifying principles that underlie the oxidizing processes were not found until recently.
Recent application of molecular genetic techniques appears to have revealed a common element of the Mn2+-oxidizing systems of two bacterial species. Leptothrix discophora SS-1, a freshwater proteobacterium, secretes an Mn2+-oxidizing factor into media (2, 7). A putative L. discophora SS-1 operon was identified in which one of the genes, which supposedly encodes a structural component of the oxidizing factor, encodes a protein homologous to multicopper oxidases (13). Bacillus sp. strain SG-1, a marine gram-positive spore-forming bacterium, produces spores that are capable of oxidizing Mn2+ (16, 33). A sporulation-dependent operon of SG-1 was found to encode, inter alia, a protein homologous to several multicopper oxidases, and transposon mutagenesis of this operon resulted in a non-Mn-oxidizing phenotype (38).
Pseudomonas putida is a freshwater proteobacterial species, and two strains of this species (MnB1 and GB-1) have been shown to oxidize Mn2+ (14, 22, 31). When supplied with Mn2+, the cells deposit Mn oxide outside the outer membrane in the early stationary growth phase (31). The oxidation appears to be catalyzed by an enzyme (31). Transposon mutagenesis of these two P. putida strains has yielded several mutants that are defective in Mn2+ oxidation or in secretion of the oxidizing factor(s) across the outer membrane (8, 10, 17). An analysis of a number of these mutants indicated that cytochrome c is involved in the oxidation of Mn2+ and that the specific protein secretion pathway is involved in transport of the oxidizing factor.
Analysis of another nonoxidizing transposon mutant of strain GB-1 localized the mutation in a partially sequenced open reading frame (ORF) encoding, inter alia, two consensus Cu2+-binding regions (17). This finding suggests that GB-1 and other Mn2+-oxidizing pseudomonads also depend on a Cu2+-binding protein for Mn2+ oxidation. In this paper we describe a detailed analysis of the mutation site in the transposon mutant and the effect of Cu2+ on the Mn2+-oxidizing activity of P. putida GB-1.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. P. putida GB-1 (which was first described by Corstjens et al. [11]) was kindly provided by K. H. Nealson (Jet Propulsion Laboratory, Pasadena, Calif.). This organism is resistant to ampicillin (17) and chloramphenicol. P. putida GB-1-002 is a spontaneous streptomycin-resistant (Smr) mutant of GB-1 (17) that was used to generate transposon mutants with Smr as an extra phenotypic marker. The transposon-containing plasmid pBR322::Tn5 was constructed by T. Goosen (Department of Genetics, Wageningen Agricultural University, Wageningen, The Netherlands). This construct does not replicate in P. putida GB-1.
TABLE 1.
Bacterial strains and plasmids
| P. putida strains GB-1 | Relevant characteristicsa | Reference |
|---|---|---|
| P. putida strains | ||
| GB-1 | Wild type, Apr Cmr | 11 |
| GB-1-002 | Spontaneous mutant of GB-1, Apr Cmr Smr | 17 |
| GB-1-007 | Non-Mn2+-oxidizing Tn5 mutant of GB-1-002, Apr Cmr Smr Kmr | 17 |
| GB-1-010 | Site-directed cumB gene replacement mutant of GB-1-002, Apr Cmr Smr Kmr | This study |
| E. coli strains | ||
| DH5α | ΔlacU169 (F80lacZM15) recA1 | 34 |
| GJ23 | recA derivative of AB1157 + pGJ28 | 37 |
| Plasmids | ||
| pBR322 | ori ColE1, Apr Tcr, narrow host range | 6 |
| pBR322::Tn5 | ori ColE1, Apr Tcr Kmr, narrow host range | 17 |
| pUC19 | ori ColE1, Apr, lacI 80dlacZ, narrow host range | 40 |
| pPLH7 | 11.3-kb EcoRI fragment (Kmr, Tn5) from GB-1-007 cloned in pUC19 | This study |
| pPLH114A+B | EcoRI-BamHI fragments from pPLH7 cloned in pUC19 | This study |
| pPLH70 | 5.2-kb EcoRI fragment containing cumA and cumB cloned in pUC19 | This study |
| pPLH75 | 5.2-kb EcoRI fragment from pPLH70 containing kanamycin cassette inserted in BsaAI site of cumB, cloned in pBR322 | This study |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; Smr, streptomycin resistance; Cmr, chloramphenicol resistance.
Media and culture conditions.
P. putida GB-1 was grown at room temperature in LD (L. discophora) medium as described previously for L. discophora SS-1 (7). To measure the effects of copper, nickel, and zinc on growth and/or Mn2+-oxidizing activity, CuCl2, ZnCl2, and NiCl2 were added at concentrations ranging from 0 to 100 μM. Cell growth was monitored by determining the optical density at 600 nm. Escherichia coli DH5α and GJ23 were cultured in Luria-Bertani medium (27) at 37°C. Solid media contained 1.8% (wt/vol) agar (Gibco BRL).
Selection markers were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; tetracycline, 25 μg/ml; streptomycin, 100 μg/ml; and chloramphenicol, 50 μg/ml.
Determination of Mn2+-oxidizing activity.
The Mn2+-oxidizing activity of P. putida GB-1-002 was determined quantitatively with the redox dye Leucoberbelin blue (LBB) as described previously for L. discophora SS-1 (7). LBB is oxidized by Mn with valences of +3 or higher, which results in a blue product. P. putida cells were harvested from a liquid culture by centrifugation. They were rinsed once with 1 volume of 10 mM HEPES buffer (pH 7.5). Eventually, the cells were resuspended in 1 volume of 10 mM HEPES (pH 7.5). Oxidation reactions were started by adding MnCl2 (final concentration, 100 μM) to equal amounts of cells or lysate. At regular intervals 100-μl samples were added to 500 μl of LBB. The cell material was removed by centrifugation, and 200-μl aliquots of the supernatants were transferred to a microtiter plate. The absorbance at 620 nm was measured with a Titertek Multiskan apparatus. KMnO4 was used as the standard. In the LBB assay, 240 μM KMnO4 is equivalent to 600 μM MnO2. In all cases, LBB was oxidized only after Mn2+ was added to the samples.
All Mn2+ oxidation assays were performed at room temperature.
Molecular genetic techniques.
Transposon mutagenesis of P. putida GB-1-002 was performed as described previously (17). One of the nonoxidizing mutants obtained was designated GB-1-007. An 11.0-kb Tn5-containing EcoRI fragment of the genomic DNA of mutant GB-1-007 was cloned in pUC19, resulting in plasmid pPLH7 (Fig. 1). A 5.9-kb BamHI-EcoRI fragment and a 5.1-kb BamHI fragment (3.1 kb of Tn5 plus 2.8 kb of Pseudomonas DNA and 2.7 kb of Tn5 plus 2.4 kb of Pseudomonas DNA, respectively) were cloned in vector pUC19, yielding plasmids pPLH114A and pPLH114B, respectively. The subcloning procedure allowed the nucleotide sequences adjacent to Tn5 to be determined with a primer (5′-CCG-TTC-AGG-ACG-CTA-CTT-GT-3′) specific for the inverted repeats of Tn5. Subsequently, sequences were determined by using M13/pUC19 forward and reverse primers and primer walking. A sequence analysis was performed by using automated dideoxy chain termination technology. The resulting nucleotide sequences were analyzed further with programs of the Wisconsin Genetics Computer Group (version 8.1) and the Pseudomonas Genome Project.
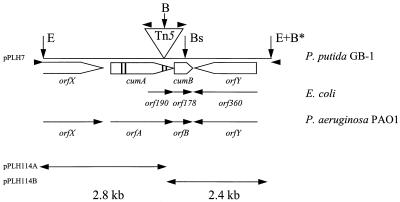
FIG. 1.
Map of the Tn5-containing EcoRI fragment from the P. putida mutant GB-1-007. The triangle indicates the site of the Tn5 insertion (length of Tn5, 5.8 kb). The open arrows indicate predicted gene locations and orientations. The vertical bars in cumA indicate consensus Cu2+-binding regions. The solid arrowheads indicate the locations of the forward and reverse M15/pUC19 primers and the Tn5 sequence primer. The small vertical arrows indicate restriction enzyme sites used in constructing pPLH7 and pPLH114A+B. Abbreviations for restriction enzymes: E, EcoRI; B, BamHI; Bs, BsaAI (site of kanamycin cassette insertion). The BamHI restriction site in the MCS of pUC19 is indicated by B*. The GenBank accession number of the E. coli sequence is X61396.
Construction, screening of the genomic library of P. putida GB-1-002 to select constructs that hybridized with digoxigenin-labeled pPLH7, and complementation were performed as described previously (8, 17). To obtain a P. putida GB-1 cumB mutant (Fig. 1), the 5.2-kb hybridizing EcoRI fragment from one of the constructs selected was cloned in pUC19, resulting in pPLH70 (which was identical to pPLH7 lacking Tn5 [Fig. 1]). A unique BsaAI site in cumB was used to insert a 1.2-kb kanamycin cassette (Pharmacia Biotech) after the 3′ overhang was removed by using T4 DNA polymerase. The EcoRI fragment containing the kanamycin cassette was subcloned in pBR322, resulting in pPLH75. This construct was mobilized to P. putida GB-1-002 by using E. coli GJ23 as described by Van Haute et al. (37). The P. putida GB-1-002 cumB mutants were selected on the basis of kanamycin resistance (recombination) and chloramphenicol resistance (loss of E. coli GJ23). Tetracycline sensitivity was used to select for double recombination events. cumB gene replacement was confirmed by PCR by using cumB-specific primers.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the GenBank database under accession no. AF086638.
RESULTS
Analysis of the Tn5 insertion site in the nonoxidizing mutant GB-1-007.
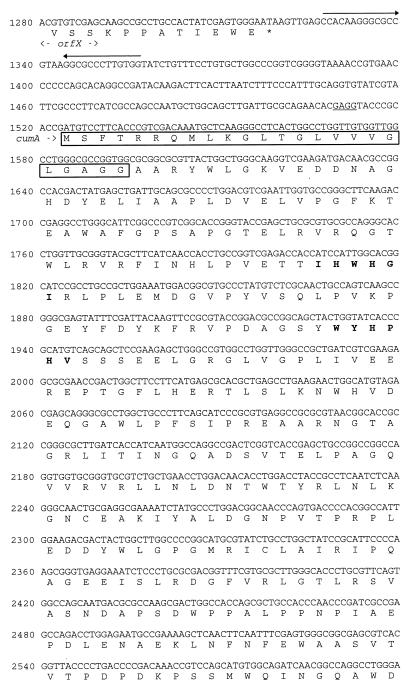
Transposon mutagenesis of P. putida GB-1-002 resulted in several classes of nonoxidizing phenotypes, some of which were defective in secretion of the Mn2+-oxidizing factor and some of which were completely devoid of Mn2+-oxidizing activity (17). One of the latter mutants was designated GB-1-007. The location of Tn5 in GB-1-007 and the nucleotide sequences of the adjacent regions were determined (Fig. 1). The translation products of some of the ORFs detected are shown in Fig. 2. The transposon was inserted in an ORF identified as a gene encoding a protein homologous to multicopper oxidases based on the presence of predicted Cu2+-binding regions (Fig. 2 and 3). This gene was designated cumA (Cu protein involved in manganese oxidation). Multicopper oxidases are found in a wide variety of organisms and are characterized by their conserved Cu2+-binding sites (Fig. 3). The predicted cumA translation product is a 459-amino-acid protein that has a molecular weight of approximately 50,500 and contains an N-terminal signal peptide (Fig. 2). The overall level of identity with an ORF of Pseudomonas aeruginosa (referred to below as orfA), as determined on the basis of the amino acid sequence, was 67%. cumA is preceded by a possible Shine-Dalgarno sequence (Fig. 2). Upstream of cumA an ORF designated orfX was detected. orfX exhibited homology to guaA, which codes for GMP synthase (level of identity with 400 amino acids encoded by the 3′ end of the gene encoding the Bacillus subtilis GMP synthase [GenBank accession no. P29727] [25], 55%). Immediately downstream from orfX strong secondary structures indicate that a transcription termination site is present (Fig. 2).
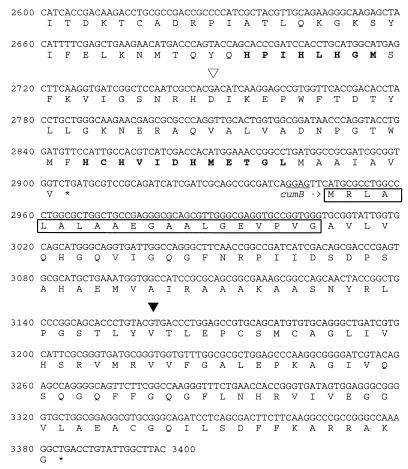
FIG. 2.
Nucleotide sequence of part of the 11.0-kb pPLH7 EcoRI fragment. The amino acid sequences of the potential gene products are also shown. Possible Shine-Dalgarno sequences are underlined. Inverted repeats which might represent transcription terminators are indicated by arrows. The predicted CumA and CumB signal peptides are enclosed in boxes, and the predicted copper-binding regions are indicated by boldface type. The site of transposon insertion is indicated by an open arrowhead, and the kanamycin cassette insertion site in cumB is indicated by a solid arrowhead.
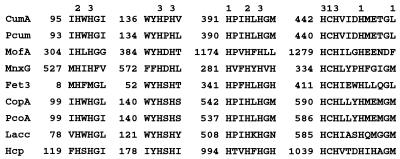
FIG. 3.
Alignment of the amino acid sequences of the copper-binding sites in CumA, the P. aeruginosa PAO1 homologue, and several other multicopper oxidases. The copper-binding residues are designated 1, 2, and 3 on the basis of the types of copper which they potentially bind. Abbreviations: CumA, P. putida GB-1 CumA (this study); Pcum, P. aeruginosa PAO1 CumA homologue (Pseudomonas Genome Project); MofA, L. discophora SS-1 MofA (GenBank accession no. Z25774) (13); MnxG, marine Bacillus sp. strain SG-1 MnxG (GenBank accession no. U31081) (38); Fet3, S. cerevisiae ferroxidase (GenBank accession no. P38993) (5); CopA, P. syringae copper resistance protein (GenBank accession no. M19930) (26); PcoA, E. coli plasmid pRJ1004 copper resistance protein (GenBank accession no. X83541) (9); Lacc, Neurospora crassa laccase (GenBank accession no. P10574) (21); Hcp, human ceruloplasmin (GenBank accession no. M13699) (23).
Downstream from cumA, two other ORFs, designated cumB and orfY, were identified (Fig. 1 and 2). cumB, which is preceded by a possible Shine-Dalgarno sequence, is located next to cumA. Since cumB and orfY have the same orientation and because the short intergenic region does not contain a transcription termination site, these genes are assumed to be part of an operon (designated Cum). The predicted cumB translation product is a 145-amino-acid protein that has an estimated molecular weight of 16,000 and contains a potential signal peptide (Fig. 2). CumB exhibited homology (level of identity, 67%) with the protein encoded by the ORF (orfB) downstream from orfA in P. aeruginosa (Fig. 1). It also exhibited homology with Orf178 of E. coli (level of identity, 52% [32]) (Fig. 1) and Orf74 of Bradyrhizobium japonicum (level of identity, 45%; GenBank accession no. L34743 [39]). The orientation of orfY is opposite that of cumA and cumB, and its predicted translation product (length, 485 amino acids; estimated molecular weight, 53,500) exhibited homology (level of identity, 65%) with the product of the P. aeruginosa ORF (orfY) downstream from orfB (Fig. 1). This protein also exhibited homology with Orf360 of E. coli (level of identity, 38% [32]), but in contrast to both P. putida and P. aeruginosa, E. coli does not contain a cumA homologue preceding orf178.
Screening of the P. putida genomic library with digoxigenin-labeled pPLH7 resulted in isolation of 12 positive clones. The DNA inserts of nine of these clones contained a 5.2-kb EcoRI fragment that hybridized with pPLH7. Mobilization of these nine constructs to mutant GB-1-007 did not result in restoration of Mn2+-oxidizing activity.
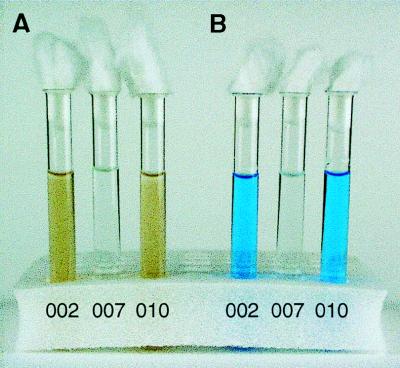
As complementation did not succeed, we could not eliminate the possibility that inhibition of Mn2+ oxidation in the mutant GB-1-007 resulted from a polar effect of the Tn5 insertion in cumA on cumB. A gene replacement study was performed to eliminate the possibility that cumB is involved in the oxidation of Mn2+. The mutant obtained (designated GB-1-010) was tested for Mn2+-oxidizing activity, and the growth rate was compared to the growth rates of the wild type and mutant GB-1-007. GB-1-010 retained the ability to oxidize Mn2+ (Fig. 4), but growth defects similar to those of mutant GB-1-007 were observed (data not shown).
FIG. 4.
(A) Manganese-oxidizing activities of P. putida GB-1-002 and GB-1-007 and cumB mutant GB-1-010 in LD medium containing 100 μM MnCl2. (B) Same three samples after the redox dye LBB was added.
Effect of Cu2+ on the Mn2+-oxidizing activity of P. putida GB-1-002 and on the growth rates of P. putida GB-1-002 and GB-1-007.
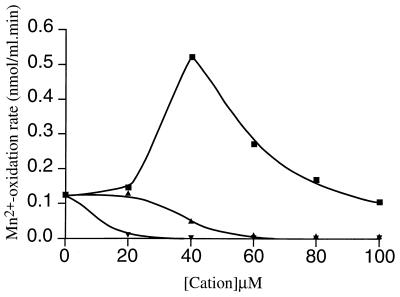
The effect of a mutation in a gene (cumA) encoding a multicopper oxidase on Mn2+ oxidation in P. putida may indicate that Mn2+ oxidation is Cu2+ dependent. Therefore, we cultured cells with different concentrations of exogenously added Cu2+ and determined the Mn2+-oxidizing activities of the cultures in the early stationary growth phase. We observed that Cu2+ had a clear stimulating effect on the oxidation of Mn2+ (Fig. 5). A maximum Mn2+ oxidation rate of 0.52 nmol/ml · min was observed in the presence of 40 μM Cu2+, which was approximately fivefold greater than the rate observed in medium without extra Cu2+. At Cu2+ concentrations greater than 40 μM the stimulating effect decreased.
FIG. 5.
Effects of different Cu2+ (■), Zn2+ (▴), and Ni2+ (▾) concentrations on the Mn2+ oxidation rate of P. putida GB-1, as determined by the LBB assay. For experimental details see the text.
To determine whether stimulation of the oxidizing activity was specific for Cu2+ ions and not for addition of divalent cations in general, the effects of Zn2+ and Ni2+ were studied (Fig. 5). Stimulation of the oxidation of Mn2+ was not observed. In contrast, at Zn2+ concentrations greater than 20 μM a decrease in oxidation was observed. The effect of Ni2+ was even more pronounced. A decrease in Mn2+ oxidation was observed at an Ni2+ concentration of 10 μM. These results indicate that Cu2+ is specifically involved in oxidation of Mn2+ in P. putida.
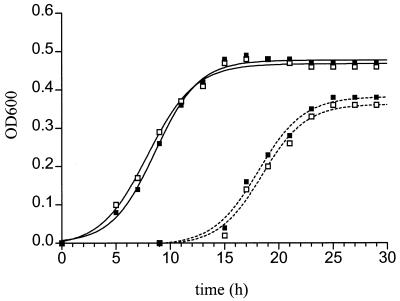
Because the Mn2+-oxidizing activity of P. putida is growth phase dependent (31), the effects of Cu2+ on the growth rates of both the P. putida wild type and the mutant were determined (Fig. 6). No significant differences in the growth rate of the wild type were detected with Cu2+ concentrations up to 100 μM. Cells entered the logarithmic growth phase at approximately the same time after inoculation and reached the stationary growth phase simultaneously. Similar results were obtained with the mutant. However, compared to the wild type, the mutant had a longer lag phase and reached a lower maximum cell density (independent of the Cu2+ added). Addition of Zn2+ or Ni2+ at concentrations up to 100 μM did not have any effect on the growth rate of either the wild type or the mutant (data not shown).
FIG. 6.
Effects of no Cu2+ (■) and 100 μM Cu2+ (□) on the growth rates of P. putida GB-1-002 (——) and GB-1-007 (–––). For experimental details see the text. OD600, optical density at 600 nm.
DISCUSSION
Previous studies have indicated that Mn2+ oxidation in P. putida GB-1 is catalyzed by an outer membrane enzyme or, more likely, an enzyme complex (8, 17, 31). Electrophoretic analyses of cell extracts (12, 31) revealed the presence of Mn2+-oxidizing factors with molecular weights ranging from 250,000 to 130,000, which were assumed to represent the oxidizing complex or parts of the oxidizing complex. We propose that an important constituent of the oxidizing complex is a Cu-dependent oxidase, the product of cumA. The genomic organization of the regions adjacent to cumA indicates that this gene constitutes an operon with the downstream ORF cumB. cumA is preceded by inverted repeat sequences that are able to form a stem-loop structure, the transcription termination site of the preceding gene, orfX, which is a homologue of the GMP synthase gene. We propose that the DNA region between orfX and cumA contains the promoter sequence. As the short intergenic region between cumA and cumB does not contain transcription termination sequences, these genes are probably transcribed from the same promoter. cumB is followed by an ORF (orfY) that clearly belongs to another operon in view of its opposite orientation. Transposon insertion in cumA abolished the Mn2+-oxidizing activity of the organism, whereas mutation of cumB had no such effect, clearly indicating that cumA is involved in Mn2+ oxidation. The gene product CumA contains a signal sequence, in accordance with the outer membrane location of the oxidizing factor. We found that growth of cells on media with exogenously added Cu2+ stimulated the Mn2+-oxidizing activity compared to the activity of cells grown with no Cu2+ addition, whereas neither Zn2+ nor Ni2+ enhanced the activity. The suggestion that a Cu2+-dependent oxidase is involved in Mn2+ oxidation in P. putida is supported by evidence that the multicopper oxidases MofA and MnxG are involved in Mn2+ oxidation in two other oxidizing organisms, L. discophora SS-1 and Bacillus sp. strain SG-1, respectively (13, 38). In the latter organism the oxidizing activity could also be stimulated by adding Cu2+ (38). In L. discophora SS-1, the effect of Cu2+ on Mn2+ oxidation has not been studied yet.
We demonstrated that the opportunistic pathogen P. aeruginosa PAO1 contains an ORF (orfA) that is very similar to cumA of P. putida GB-1. Preliminary experiments in our laboratory showed that in principle, logarithmic liquid cultures of P. aeruginosa are able to oxidize Mn2+ (data not shown), although it was difficult to reproducibly demonstrate this activity. In spite of the uncertainty, it is tempting to correlate this oxidizing activity with the presence of the cumA homologue orfA.
The data obtained in this study strongly support the notion that involvement of multicopper oxidases in Mn2+ oxidation is common in Mn2+-oxidizing bacteria (36). However, several questions remain to be answered. The first question is related to the fact that complementation of the mutant has not been successful so far. In a previous study (17) we found a single transposon insertion in the mutant GB-1-007, which showed that the lack of complementation cannot be due to other possible insertions. The transposon insertion is located near the 3′ end of cumA between two copper-binding regions. It is possible that this location of the transposon still allows production of large amounts of almost complete CumA which may compete with CumA expressed from the complementing fragment (for instance, in the formation of the oxidizing complex). Because the essential fourth copper-binding region is missing in mutated CumA, a nonfunctional Mn2+-oxidizing complex should be formed. We will use site-specific gene replacement in cumA to resolve this question.
The site-directed gene replacement in cumB eliminated the possibility that cumB is involved in Mn2+ oxidation and confirmed that the decreased growth rate of the mutant GB-1-007 was the result of a polar effect of the transposon on cumB transcription, which supported the suggestion that cumA and cumB are cotranscribed from the same promoter. The involvement of cumB in growth is consistent with the observation that a cumB homologue in B. japonicum, orf74, is required for optimal free-living growth (39). Like the mutant GB-1-007 and the cumB mutant, mutants in which orf74 was disrupted had a longer lag phase and reached a lower cell density than the wild type. In E. coli, another cumB homologue (orf178) seems to be involved in the cell-killing function of members of the gef gene family in a manner that so far is not known (32). P. putida GB-1 is sensitive to the gef gene family (32), which may be the result of the product of cumB. This gene has not been found in P. putida GB-1 previously.
A second question to be resolved concerns the stimulating effect of Cu2+ on Mn2+ oxidation in P. putida. We found that the presence of low amounts of Cu2+ in the culture medium specifically enhanced the oxidizing activity of the cells. However, it is not clear yet whether the stimulating effect should be ascribed to Cu2+-enhanced transcription of the oxidizing factor (presumed to be encoded by cumA), to production of a more active factor as a result of optimal Cu2+ incorporation, or to a combination of these effects. Why stimulation of Mn2+ oxidation decreased at Cu2+ concentrations higher than the optimum concentration (40 μM) also must be explained. It is possible that at supraoptimal concentrations, Cu2+ ions also occupy Mn2+-binding sites. Competition for Mn2+-binding sites and/or Cu2+-binding sites may explain the inhibiting effects of Zn2+ and Ni2+ on Mn2+ oxidation. Studies of the effect of Cu2+ on the expression of cumA and the effects of specific Cu2+-chelating agents on Mn2+ oxidation followed by reconstitution experiments should provide more insight into these questions.
Finally, it is possible to speculate about the physiological functions of multicopper oxidases in Mn2+-oxidizing bacteria. Multicopper oxidases occur in a wide variety of organisms and can have different cellular functions. The multicopper oxidase family includes the CopA proteins of Pseudomonas syringae (26) and Xanthomonas campestris (24) and the PcoA protein of the E. coli plasmid pRJ1004 (9), all of which are involved in Cu2+ resistance. The P. putida GB-1 CumA protein probably is not involved in Cu2+ resistance, as mutation of the corresponding gene did not result in Cu2+-sensitive growth of mutant cells. Other members of the multicopper oxidase family are the Saccharomyces cerevisiae Fet3 protein (5) and the human ceruloplasmin (23), both of which act as ferroxidases involved in high-affinity iron uptake. White rot fungi produce the multicopper oxidase laccase, which produces strongly oxidizing Mn(III) chelates that are used in the oxidation of lignin compounds (3). Production of strong oxidizing agents that release nutrients from resistant organic compounds may be an important function of Mn2+-oxidizing multicopper oxidases in nutrient-poor environments (35). However, metabolically inert structures, like the Mn2+-oxidizing spores of Bacillus sp. strain SG-1, do not obviously benefit from such a process. It is possible that the multicopper oxidases of Mn2+-oxidizing bacteria have primary cellular functions other than Mn2+ oxidation. These oxidases may have the ability to oxidize Mn2+, which allows the bacteria to use the products depending on the organism and circumstances and thus to gain a selective advantage. Such an advantage may be exploited at the cellular level (by generation of nutrients, production of alternative electron acceptors, etc. [see above]), but an advantage at the ecological level may also be envisaged. In some environments, oxidation and reduction of Mn2+ (and Fe2+) are coupled to oxidation and reduction of carbon, which permits efficient cycling of nutrients and reduction equivalents in stable ecosystems (30). Mn2+-oxidizing bacteria play an important role in such ecosystems by guaranteeing the supply of an electron sink to the reducing zones. This may contribute to the widespread occurrence of Mn2+-oxidizing bacterial species in nature.
ACKNOWLEDGMENTS
We thank Theo Goosen for supplying pBR322::Tn5 and Nora Goosen and Eric Vijgenboom for fruitful discussions during the course of this work.
REFERENCES
- 1.Adams L F, Ghiorse W C. Influence of manganese on growth of a sheathless strain of Leptothrix discophora. Appl Environ Microbiol. 1985;49:556–562. doi: 10.1128/aem.49.3.556-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams L F, Ghiorse W C. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J Bacteriol. 1987;169:1279–1285. doi: 10.1128/jb.169.3.1279-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald F, Roy B. Production of manganic chelates by laccase from the lignin-degrading fungus Trametes (Coriolus) versicolor. Appl Environ Microbiol. 1992;58:1496–1499. doi: 10.1128/aem.58.5.1496-1499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald F S, Fridovich I. Manganese and defense against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askwith C, Eide D, van Ho A, Bernard P S, Li L, Davis-Kaplan S, Sipe D M, Kaplan J. The Fet3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 6.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multiple cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 7.Boogerd F C, de Vrind J P M. Manganese oxidation by Leptothrix discophora. J Bacteriol. 1987;169:489–494. doi: 10.1128/jb.169.2.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwers G J, de Vrind J P M, Corstjens P L A M, de Vrind-de Jong E W. Genes of the two-step protein secretion pathway are involved in the transport of the manganese-oxidizing factor across the outer membrane of Pseudomonas putida strain GB-1. Am Mineral. 1998;83:1573–1582. [Google Scholar]
- 9.Brown N L, Barrett S R, Camakaris J, Lee T O, Rouch D A. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol Microbiol. 1995;17:1153–1166. doi: 10.1111/j.1365-2958.1995.mmi_17061153.x. [DOI] [PubMed] [Google Scholar]
- 10.Caspi R, Tebo B M, Haygood M G. c-Type cytochromes and manganese oxidation in Pseudomonas putida MnB1. Appl Environ Microbiol. 1998;64:3549–3555. doi: 10.1128/aem.64.10.3549-3555.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corstjens P L A M, de Vrind J P M, Westbroek P, de Vrind-de Jong E W. Enzymatic iron oxidation by Leptothrix discophora: identification of an iron-oxidizing protein. Appl Environ Microbiol. 1992;58:450–454. doi: 10.1128/aem.58.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corstjens P L A M. Bacterial oxidation of iron and manganese, a molecular-biological approach. Ph.D. dissertation. Leiden, The Netherlands: Leiden University; 1993. [Google Scholar]
- 13.Corstjens P L A M, de Vrind J P M, Goosen T, de Vrind-de Jong E W. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese oxidizing protein with copper domains. Geomicrobiol J. 1997;14:91–108. [Google Scholar]
- 14.DePalma S R. Manganese oxidation by Pseudomonas putida. Ph.D. dissertation. Cambridge, Mass: Harvard University; 1993. [Google Scholar]
- 15.de Vrind J P M, Boogerd F C, de Vrind-de Jong E W. Manganese reduction by a marine Bacillus species. J Bacteriol. 1986;167:30–34. doi: 10.1128/jb.167.1.30-34.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vrind J P M, de Vrind-de Jong E W, de Voogt J-W H, Westbroek P, Boogerd F C, Rosson R A. Manganese oxidation by spores and the spore coats of a marine Bacillus species. Appl Environ Microbiol. 1986;52:1096–1100. doi: 10.1128/aem.52.5.1096-1100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vrind J P M, Brouwers G J, Corstjens P L A M, den Dulk J, de Vrind-de Jong E W. The cytochrome c maturation operon is involved in manganese oxidation in Pseudomonas putida GB-1. Appl Environ Microbiol. 1998;64:3556–3562. doi: 10.1128/aem.64.10.3556-3562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vrind-de Jong E W, de Vrind J P M, Boogerd F C, Westbroek P, Rosson R A. Manganese transformations by marine Bacillus species. In: Crick R E, editor. Origin, evolution and modern aspects of biomineralization in plants and animals. New York, N.Y: Plenum Press; 1990. pp. 489–496. [Google Scholar]
- 19.Ehrlich H L. Different forms of bacterial manganese oxidation. In: Strohl W R, Tuovinen H, editors. Microbial chemoautotrophy. Ohio State University Colloquium no. 8. Columbus: Ohio State University Press; 1984. pp. 47–56. [Google Scholar]
- 20.Ehrlich H L. Geomicrobiology. New York, N.Y: Marcel Dekker; 1996. [Google Scholar]
- 21.German U A, Muller G, Hunziker P E, Lerch K. Characterization of two allelic forms of Neurospora crassa laccase: amino- and carboxy-terminal processing of a precursor. J Biol Chem. 1988;263:885–896. [PubMed] [Google Scholar]
- 22.Jung W K, Schweisfurth R. Manganese oxidation by an intracellular protein of a Pseudomonas species. Z Allg Microbiol. 1979;19:107–115. [PubMed] [Google Scholar]
- 23.Koschinsky M L, Funk W D, van Oost B A, MacGillivray R T. Complete cDNA sequence of human preceruloplasmin. Proc Natl Acad Sci USA. 1986;83:5086–5090. doi: 10.1073/pnas.83.14.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y-A, Hendson M, Panopoulos N J, Schroth M N. Molecular cloning, chromosomal mapping, and sequence analysis of copper resistance genes from Xanthomonas campestris pv. juglandis: homology with small blue copper proteins and multicopper oxidase. J Bacteriol. 1994;176:173–188. doi: 10.1128/jb.176.1.173-188.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mäntsälä P, Zalkin H. Cloning and sequencing of Bacillus subtilis purA and guaA, involved in the conversion of IMP to AMP and GMP. J Bacteriol. 1992;174:1883–1890. doi: 10.1128/jb.174.6.1883-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellano M A, Cooksey D A. Nucleotide sequencing and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1988;170:2879–2883. doi: 10.1128/jb.170.6.2879-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 28.Nealson K H, Tebo B M, Rosson R A. Occurrence and mechanisms of microbial oxidation of manganese. Adv Appl Microbiol. 1988;33:299–318. [Google Scholar]
- 29.Nealson K H, Rosson R A, Myers C R. Mechanisms of oxidation and reduction of manganese. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley & Sons; 1989. pp. 383–411. [Google Scholar]
- 30.Nealson K H, Myers C R. Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol. 1992;58:439–443. doi: 10.1128/aem.58.2.439-443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazaki M, Sugita T, Shimizu M, Ohode Y, Iwamoto K, de Vrind-de Jong E W, de Vrind J P M, Corstjens P L A M. Partial purification and characterization of manganese-oxidizing factors of Pseudomonas fluorescens GB-1. Appl Environ Microbiol. 1997;63:4793–4799. doi: 10.1128/aem.63.12.4793-4799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulsen L K, Larsen N W, Molin S, Andersson P. Analysis of an Escherichia coli mutant strain resistant to the cell-killing function encoded by the gef gene family. Mol Microbiol. 1992;6:895–905. doi: 10.1111/j.1365-2958.1992.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosson R A, Nealson K H. Manganese binding and oxidation by spores of a marine Bacillus. J Bacteriol. 1982;151:1027–1034. doi: 10.1128/jb.151.2.1027-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sunda W G, Kieber D J. Oxidation of humic substances by manganese oxides yields low-molecular weight organic substrates. Nature. 1994;367:62–64. [Google Scholar]
- 36.Tebo B M, Ghiorse W C, van Waasbergen L G, Siering P L, Caspi R. Bacterial mediated mineral formation: insights into manganese(II) oxidation from molecular genetic and biochemical studies. In: Banfield J F, Nealson K H, Ribbe P H, editors. Reviews in mineralogy. Vol. 35. Washington, D.C: Mineral Society of America; 1997. pp. 225–266. [Google Scholar]
- 37.Van Haute E, Joos H, Maes M, Warren G, Van Montagu, Schell J. Intergenic transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2:411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Waasbergen L G, Hildebrand M, Tebo B M. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J Bacteriol. 1996;178:3517–3530. doi: 10.1128/jb.178.12.3517-3530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weidenhaupt M, Schmid-Appert M, Thöny B, Hennecke H, Fischer H-M. A new Bradyrhizobium japonicum gene required for free-living growth and bacteroid development is conserved in other bacteria and in plants. Mol Plant Microbe Interact. 1995;8:454–464. doi: 10.1094/mpmi-8-0454. [DOI] [PubMed] [Google Scholar]
- 40.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]