Abstract
The 5′ noncoding region of clpg2, an endopolygalacturonase gene of the bean pathogen Colletotrichum lindemuthianum, was fused to the coding sequence of a gene encoding a green fluorescent protein (GFP), and the construct was introduced into the fungal genome. Detection of GFP accumulation by fluorescence microscopy examination revealed that clpg2 was expressed at the early stages of germination of the conidia and during appressorium formation both in vitro and on the host plant.
Endopolygalacturonases (endoPGs; poly-α-1,4-galacturo-nide glycanohydrolase, EC 3.2.1.15) have long been proposed to play an important role in fungal pathogenicity by degrading the homogalacturonan regions of pectin, a major component of plant cell walls (10). In addition, research on endoPGs has been widened by the finding that these proteins are able to elicit plant defense responses through the release of active oligosaccharides and pectic fragments (1, 7). EndoPGs have been characterized and the corresponding genes have been cloned from a number of pathogenic fungi (2–4, 9, 11, 14–16). During fungal saprophytic growth, expression of endoPG genes is subjected to substrate induction as well as to catabolite repression by glucose. In Colletotrichum lindemuthianum it is also induced by the two neutral sugars arabinose and rhamnose, which are components of plant cell walls (12). Recently, the use of reverse transcription (RT)-PCR allowed the detection of endoPG transcripts during pathogenesis (4, 9, 16) and showed that endoPG genes are also induced during infection of the host.
In previous work, we cloned and characterized two endoPG genes, clpg1 and clpg2, from C. lindemuthianum, a hemibiotroph fungal pathogen that causes anthracnose on bean seedlings (3, 4). Expression studies using specific probes for each gene showed that clpg1 encodes the major produced enzyme, both in axenic culture of the fungus and at the onset of the necrotrophic stage of host colonization (4). clpg2 was shown to be induced transiently and rapidly in vitro in the presence of pectin but not during the development of the necrotrophic stage of infection, indicating that clpg1 and clpg2 are differently regulated (4). As a first step to identify the signals and transduction pathways leading to the induction of endoPG genes, we have investigated the possibility of using a gene encoding a modified version of the Aequoria victoria green fluorescent protein (SGFP-TYG; 5) as a new vital reporter gene in C. lindemuthianum.
In order to express gfp under the control of the 5′ putative regulatory sequences of clpg2, a fragment comprising 668 bp located upstream of the initiation codon and the first six codons of the coding sequence were amplified by PCR and cloned in frame with the sequence encoding the SGFP-TYG. The plasmid pPG2GFP was introduced into C. lindemuthianum via protoplast transformation along with the plasmid pAN7-1 (13), which contains a cassette conferring hygromycin resistance. For a rapid screening of GFP-expressing strains, the mycelium of each colony was grown on minimal medium containing pectin as the sole carbon source (4) and subsequently examined under fluorescence microscopy. Of the 13 colonies growing in the presence of hygromycin, 8 were fluorescent, thus showing a cotransformation rate of about 60%. No fluorescence background was detected in the wild-type strain or in a strain transformed with a promoterless vector. The presence of GFP was stable even when the transformants were subcultured on a nonselective medium, i.e., without hygromycin. The phenotypes of GFP-expressing strains were unchanged compared to the wild-type strain. The rate of growth, conidiation, and pathogenicity were also unmodified by the accumulation of GFP, suggesting that this protein does not interfere with the physiology of C. lindemuthianum. It was found that transcription of the reporter GFP gene truly reflects transcription of the wild-type clpg2 gene. Indeed, gfp transcript and fluorescence accumulation paralleled clpg2 gene expression when the mycelium was grown on pectin as the sole carbon source, whereas expression of both genes was almost undetectable when the mycelium was grown on glucose (data not shown).
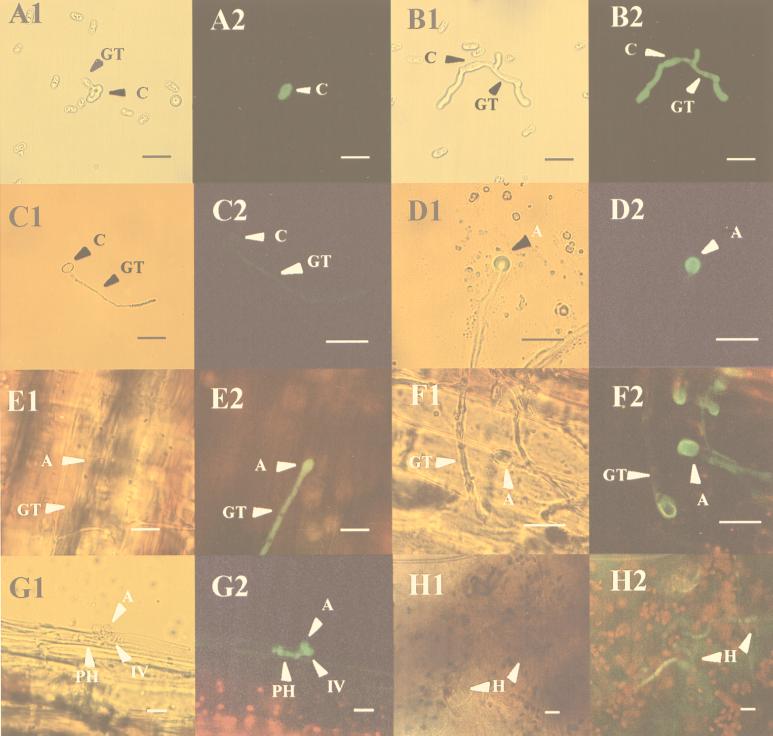
Expression of GFP under the control of the clpg2 promoter was monitored by fluorescence microscopy at different stages of development of the fungus. Since all transformants showed a similar level of fluorescence, in Fig. 1 we present the results obtained for only one of them, namely the strain H2. To study the expression of GFP during saprophytic growth, conidia from GFP-expressing strains were used to inoculate enzyme-linked immunosorbent assay plates containing 50 μl of pectin medium per well. Fluorescence was undetectable at the beginning of the experiment. However, after incubation of the plates for 12 h at 25°C, the germinating conidia but not the germ tube appeared fluorescent (Fig. 1A1 and A2), whereas at 24 h of incubation the fluorescence was easily detectable along the germ tube (Fig. 1B1 and B2). Fluorescence was very weak in conidia germinating in water on a glass slide (Fig. 1C1 and C2), except when they differentiated an appressorium in which a high level of fluorescence was detected (Fig. 1D1 and D2). In order to look for the expression of gfp during infection of the host, bean hypocotyls of the susceptible Early Wax cultivar were inoculated with H2 conidia. The epidermis was peeled off 24 h after inoculation and analyzed by fluorescence microscopy. As shown in Fig. 1, fluorescent germ tubes and swelling appressorium were detected at the surface of the infected plant tissue (Fig. 1E1 and E2). At later stages, fluorescence of the germ tube decreased whereas fluorescence in the appressorium increased, likely reflecting migration of the cytoplasm into this swelling structure (Fig. 1F1 and F2). The penetrating hyphae were also fluorescent (Fig. 1G1 and G2), whereas fluorescence was not detected at the onset of necrosis (not shown). However, after prolonged incubation, i.e., 15 days postinoculation, fluorescent hyphae could be detected in heavily macerated tissue (Fig. 1H1 and H2), possibly reflecting reinoculation of the tissue with the resident fungus.
FIG. 1.
Developmental expression of gfp under the control of the clpg2 promoter. Conidia of C. lindemuthianum H2 were allowed to germinate in vitro either on pectin medium cleared by filtration (A and B) or on a glass slide in water (C and D). They were also used to inoculate bean hypocotyls (E through H). Samples shown in panels A through D were assayed for green fluorescence after 12 h (panels A and C) and 24 h (panels B and D). Infected bean hypocotyls were examined 24 h (panels E and F), 48 h (panel G), and 15 days (panel H) after inoculation. Samples were successively analyzed by light microscopy (subpanels 1) or fluorescent light (subpanels 2). A, appressorium; C, conidium; GT, germ tube; IV, infection vesicle; PH, primary hyphae. Bar = 20 μm.
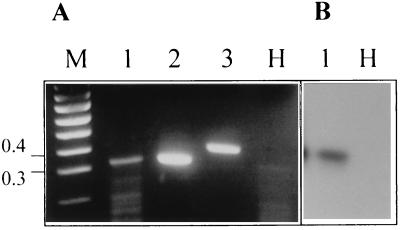
To confirm that the detection of GFP by fluorescence microscopy was correlated with the accumulation of clpg2 transcripts at the very first stages of infection, the sensitive method RT-PCR was used. Oligoprimers that span the intron-containing region of clpg2 were designed. The primers, which were tested on cloned clpg2 cDNA and on the genomic sequence of clpg2 (Fig. 2), enabled us to distinguish between RNA products (size without intron, 343 bp; lane 2) and DNA (406 bp; lane 3). Using RNA from the epidermis of infected bean hypocotyls collected 24 h after inoculation, a fragment of the right size, which was undetected in healthy tissues (Fig. 2A, lane H), was amplified (Fig. 2A, lane 1). Southern blot analysis performed by using a clpg2 probe confirmed that the amplified cDNA fragment corresponded to the transcript of clpg2 (Fig. 2B, lane 1) which accumulated early during pathogenesis.
FIG. 2.
Analysis by RT-PCR of clpg2 expression in planta. Total RNA extracted from bean hypocotyl epidermis 24 h after inoculation of the susceptible cultivar Early Wax with C. lindemuthianum race β (lanes 1) or from the corresponding healthy plant (lanes H) was used for RT-PCR analysis. To differentiate between genomic and mRNA-derived fragments, PCRs were done by using cloned cDNA (lane 2) and cloned genomic DNA (lane 3) corresponding to clpg2. The PCR products were analyzed by gel electrophoresis followed by ethidium bromide staining (panel A) and by Southern blotting by using a clpg2 probe (panel B). DNA size markers (100-bp ladder) were loaded on lane M. The sizes of two bands of the ladder, expressed in kilobase pairs, are indicated on the left.
The correlation between the early induction of gfp expression and the accumulation of clpg2 transcripts detected by RT-PCR allowed us to conclude that transcriptional activation of clpg2 occurs rapidly when the fungus enters its parasitic stage. These observations are consistent with recent reports describing early secretion of pectinases by Cochliobolus sativus, Uromyces viciae-fabae, and Claviceps purpurea during pathogenesis (6, 8, 16), suggesting that these enzymes participate in host penetration by degrading the subcuticular pectin layer. It remains to be elucidated whether induction of clpg2 in planta is mediated by pectin. Identification and deletion of pectin-responsive elements in the clpg2 promoter should help to clarify this point.
Very few investigations have relied on the use of reporter sequences to study the regulation of pathogenicity genes. Since strains of C. lindemuthianum expressing the gfp construct showed a normal phenotype, the accumulation of GFP does not seem to alter the physiology of the fungus during its life cycle and makes gfp a suitable vital marker gene for C. lindemuthianum. To our knowledge, this is the first report on the use of gfp for studying the transcriptional regulation at a single cell level of a fungal gene encoding an hydrolytic enzyme induced during interaction with the host. This should help in identifying the complex signalling pathways leading to the induction of cell wall degrading enzymes during pathogenesis.
Acknowledgments
We are indebted to Jen Sheen for the generous gift of the blue-SGFP-TYG-nos SK plasmid. We thank Arnaud Bottin for helpful discussions and Marc Buée and Guillaume Bécart for help with fluorescence microscopy.
REFERENCES
- 1.Boudart G, Lafitte C, Barthe J P, Frasez D, Esquerré-Tugayé M-T. Differential elicitation of defense responses by pectic fragments in bean seedlings. Planta. 1998;206:86–94. [Google Scholar]
- 2.Caprari C, Richter A, Bergmann C, Lo Cicero S, Salvi G, Cervone F, de Lorenzo G. Cloning and characterization of a gene encoding the endopolygalacturonase of Fusarium moniliforme. Mycol Res. 1993;97:497–505. [Google Scholar]
- 3.Centis S, Dumas B, Fournier J, Marolda M, Esquerré-Tugayé M-T. Isolation and sequence analysis of Clpg1, a gene coding for an endopolygalacturonase of the phytopathogenic fungus Colletotrichum lindemuthianum. Gene. 1996;170:125–129. doi: 10.1016/0378-1119(95)00867-5. [DOI] [PubMed] [Google Scholar]
- 4.Centis S, Guillas I, Séjalon N, Esquerré-Tugayé M-T, Dumas B. Endopolygalacturonase genes from Colletotrichum lindemuthianum: cloning of CLPG2 and comparison of its expression to that of CLPG1 during saprophytic and parasitic growth of the fungus. Mol Plant-Microbe Interact. 1997;10:769–775. doi: 10.1094/MPMI.1997.10.6.769. [DOI] [PubMed] [Google Scholar]
- 5.Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Curr Biol. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- 6.Clay R P, Bergmann C W, Fuller M S. Isolation and characterization of an endopolygalacturonase from Cochliobolus sativus and a cytological study of fungal penetration of barley. Phytopathology. 1997;87:1148–1159. doi: 10.1094/PHYTO.1997.87.11.1148. [DOI] [PubMed] [Google Scholar]
- 7.Darvill A, Augur C, Carlson R W, Cheong S J J, Eberhard S, Hahn M G, Lo V M, Marfa V, Meyer B, Mohnen D, O’Neil M A, Spiro M D, Van Halbeek H, York W S, Albersheim P. Oligosaccharins: oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology. 1992;2:181–198. doi: 10.1093/glycob/2.3.181. [DOI] [PubMed] [Google Scholar]
- 8.Deising H, Frittrang A K, Kunz S, Mendgen K. Regulation of pectin methylesterase and polygalacturonate lyase activity during differentiation of infection structures in Uromyces viciae-fabae. Microbiology. 1995;141:561–571. [Google Scholar]
- 9.Di Pietro A, Roncero I G. Cloning, expression and role in pathogenicity of pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum. Mol Plant-Microbe Interact. 1998;11:91–98. doi: 10.1094/MPMI.1998.11.2.91. [DOI] [PubMed] [Google Scholar]
- 10.Durrands P K, Cooper R M. The role of pectinases in vascular wilt disease as determined by defined mutants of Verticillium albo-altrum. Physiol Mol Plant Pathol. 1988;32:363–371. [Google Scholar]
- 11.Gao S, Choi G H, Shain L, Nuss D L. Cloning and targeted disruption of enpg-1, encoding the major in vitro extracellular endopolygalacturonase of the chestnut blight fungus, Cryphonectria parasitica. Appl Environ Microbiol. 1996;62:1984–1990. doi: 10.1128/aem.62.6.1984-1990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugouvieux V, Centis S, Lafitte C, Esquerré-Tugayé M-T. Induction by α-l-arabinose and α-l-rhamnose of endopolygalacturonase gene expression in Colletotrichum lindemuthianum. Appl Environ Microbiol. 1997;63:2287–2292. doi: 10.1128/aem.63.6.2287-2292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punt P J, Oliver R P, Dingemanse M A, Pouwels P H, Van Den Hondel C A M J J. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 14.Reymond P, Deléage G, Rascle C, Fèvre M. Cloning and sequence analysis of a polygalacturonase-encoding gene from the phytopathogenic fungus Sclerotinia sclerotiorum. Gene. 1994;146:233–237. doi: 10.1016/0378-1119(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 15.Scott-Craig J S, Panaccione D G, Cervone F, Walton J D. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell. 1990;2:1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenberge K B, Homann V, Oeser B, Tudzynski P. Structure and expression of two polygalacturonase genes of Claviceps purpurea oriented in tandem and cytological evidence for pectinolytic enzyme activity during infection of rye. Phytopathology. 1996;86:1084–1097. [Google Scholar]