Abstract
Wnt–β-catenin signaling plays a key role in orthodontic tooth movement (OTM), a common clinical practice for malocclusion correction. However, its targeted periodontal ligament (PDL) progenitor cells remain largely unclear. In this study, we first showed a synchronized increase in Wnt–β-catenin levels and Axin2+ PDL progenitor cell numbers during OTM using immunostaining of β-catenin in wild-type mice and X-gal staining in the Axin2-LacZ knock-in line. Next, we demonstrated time-dependent increases in Axin2+ PDL progenitors and their progeny cell numbers within PDL and alveolar bones during OTM using a one-time tamoxifen-induced Axin2 tracing line (Axin2CreERT2/+; R26RtdTomato/+). Coimmunostaining images displayed both early and late bone markers (such as RUNX2 and DMP1) in the Axin2Lin PDL cells. Conversely, ablation of Axin2+ PDL cells via one-time tamoxifen-induced diphtheria toxin subunit A (DTA) led to a drastic decrease in osteogenic activity (as reflected by alkaline phosphatase) in PDL and alveolar bone. There was also a decrease in new bone mass and a significant reduction in the mineral apposition rate on both the control side (to a moderate degree) and the OTM side (to a severe degree). Thus, we conclude that the Axin2+ PDL cells (the Wnt-targeted key cells) are highly sensitive to orthodontic tension force and play a critical role in OTM-induced PDL expansion and alveolar bone formation. Future drug development targeting the Axin2+ PDL progenitor cells may accelerate alveolar bone formation during orthodontic treatment.
Keywords: periodontal ligament, Wnt signaling pathway, osteogenesis, cellular mechanotransduction, orthodontics, cell lineage
Introduction
Orthodontic tooth movement (OTM) is initiated by external mechanical forces and mediated by the remodeling of the periodontal ligament (PDL) and alveolar bone. The PDL is a fiber-reinforced tissue that contains an abundant vascular network and heterogeneous cell populations. This tissue plays a critical role in transducing mechanical stress and maintaining periodontal homeostasis (Feng et al. 2016; Zhang et al. 2016). OTM occurs when orthodontic forces are transduced into biological events. The tension side exhibits stretched PDL fibers and new alveolar bone formation. Meanwhile, the pressure side is characterized by compressed and disorganized PDL and subsequent alveolar bone resorption (Krishnan and Davidovitch 2006). Notably, the type of alveolar bone resorption depends on the magnitude of forces applied on the tooth; light forces are preferable as they evoke frontal resorption of bone, whereas heavy forces often cause necrosis (hyalinization) of the PDL and undermining bone resorption (Reitan 1957; Krishnan and Davidovitch 2006).
It has long been postulated that a PDL stem/progenitor cell (PDLSC) population supports the homeostasis of the periodontium with its capability of self-renewal and multilineage differentiation (Seo et al. 2004; Sonoyama et al. 2006; Feng et al. 2010). Our lab has demonstrated that PDL provides a key contribution during normal alveolar bone formation. Our research shows 2- and 3-fold higher mineral apposition rates at the PDL–alveolar bone interface than those on periosteal and endosteal surfaces (Ren et al. 2015).
It has been well documented that mechanical loading (such as occlusion, mastication, and orthodontic forces) directly regulates PDL homeostasis by inducing the proliferation and differentiation of PDLSCs (Tang et al. 2012; Chang et al. 2017). Critical osteogenic factors including runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), and osteocalcin (OCN) are upregulated in human PDLSCs under cyclic tensile strain in vitro (Tang et al. 2012; Shen et al. 2014), as well as in PDL during OTM (Kawarizadeh et al. 2005; Takimoto et al. 2015; Fu et al. 2016). To date, many factors and signaling pathways have been reported to be involved in osteogenesis in response to orthodontic tensile force, including Wnt/β-catenin pathway, Hippo-Yes–associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) signaling, and periostin (Fu et al. 2016; Rangiani et al. 2016; Zhang et al. 2016; Huelter-Hassler et al. 2017; Xu et al. 2017; Sun et al. 2018). Sclerostin is known to be a key negative regulator of bone mass by antagonizing canonical Wnt signaling via binding to LRP5/6 on the surface of osteoblasts (Li et al. 2005). Previous studies have reported a biphasic change in SOST expression during OTM: there was an increase in the compression site while a brief downregulation occurred on the tension side (Rangiani et al. 2016; Odagaki et al. 2018), indicating that SOST has a critical role in regulating periodontal remodeling during OTM. Periostin (an extracellular matrix protein that is highly expressed in the PDL) is known to be responsive to mechanical stimulation in maintaining the integrity of periodontal tissues. Conventional knockout of periostin leads to a sharp reduction in new alveolar bone formation on the OTM-tension side, indicating a critical role of periostin during OTM (Rangiani et al. 2016). However, the underlying mechanism by which PDL progenitors contribute to periodontal homeostasis upon mechanical force has not yet been elucidated.
Axin2 is one of the key targeting molecules of Wnt/β-catenin signaling, which is mediated through T-cell factor/lymphoid enhancer factor (TCF/LEF) (Jho et al. 2002). Recent studies suggest that Axin2+ cells are stem/progenitor cells in various types of tissues (Van Amerongen et al. 2012; Bowman et al. 2013; Lim et al. 2013; Wang et al. 2015). Axin2+ PDL cells play critical roles in postnatal cementogenesis (Xie et al. 2019), the healing of extraction sockets (Yuan et al. 2018), and adaptative responses to hyperloading (Xu et al. 2019) and underloading during mastication (Zhang et al. 2019). However, their role in OTM is largely unknown.
The goal of this study was to investigate whether Axin2+ PDL progenitor cells directly contribute to OTM-induced alveolar bone formation using multiple cell lineage tracing lines. The Axin2-lacZ line was used to reveal the temporal and spatial expression patterns of the endogenous Axin2+ progenitor cells during normal and OTM: Axin2CreERT2/+; R26RtdTomato/+ to trace Axin2+ progeny and Axin2-CreERT2/+; R26RtdTomato/DTA+ for loss function studies. Our findings demonstrate the vital role of Axin2+ PDL progenitor cells and their progeny during OTM-induced alveolar bone formation, which may facilitate future drug development aimed at accelerating OTM-induced alveolar bone formation in patients.
Materials and Methods
Transgenic Mice and Orthodontic Tooth Movement
All experimental protocols followed ARRIVE (Animal Research Reporting of In Vivo Experiments) guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University College of Dentistry (IACUC number: 2021-0134-COD).
Wild-type (WT, stock number: 000664), Axin2lacZ/+ (stock number: 009120), Axin2CreERT2/+ (stock number: 018867), R26RtdTomato/+ (stock number: 007905), and R26RDTA/+ (stock number: 006331) mice were purchased from Jackson Laboratory. The mice were housed in a temperature-controlled environment with 12-h light/dark cycles. WT mice were used to assess β-catenin expression in periodontium. Axin2lacZ/+ heterozygous mice had a nuclear-localized β-galactosidase (NLS-lacZ) gene under the control of the endogenous Axin2 promoter/enhancer regions and were used to indicate endogenous Axin2 expression. Meanwhile, Axin2-CreERT2/+; R26RtdTomato/+ mouse lines were used to trace Axin2+ progenitors in PDL during orthodontic tooth movement. In addition, R26RDTA/+ mice had a loxP-flanked STOP cassette preventing expression of diphtheria toxin fragment A (DTA). Exposure to Cre recombinase removed the lox-stop fragment and resulted in ablation of the Cre-expressing cells (Ivanova et al. 2005). By crossing Axin2-CreERT2/+; R26RtdTomato/+ mice with R26RDTA/+ mice, Axin2-CreERT2/+; R26RtdTomato/DTA+ mouse line was generated to further determine the role of Axin2+ lineage by specifically ablating the Axin2+ cells. An OTM model was established at the age of postnatal day 28 (P28). Cell lineage tracing lines received a single intraperitoneal injection of tamoxifen (Sigma, T5648, 75 mg/kg body weight) at P28 immediately after OTM started. The animals were sacrificed after 3 d (P31), 8 d (P36), or 14 d (P42) of tooth movement, respectively.
The OTM model was established following a previously published method (Rangiani et al. 2016). Briefly, a customized coil spring was made of superelastic nickel-titanium (NiTi) with 0.003 inches of pitch and 0.019 inches for the inside diameter (Motion Dynamics Corporation). To obtain a 0.5-N force with 4 mm of activation, the springs were cut to a 2.5-mm length and bonded with light-cured resin between the mesial surface of the maxillary left first molar (M1) and lingual surface of maxillary incisors. The maxillary left first molars were used for OTM, and the maxillary right first molars in the same animals were used for our control. Because of food intake limitations due to this procedure, all animals in this study lost body weight. However, none of the mice lost more than 20% of their body weight.
5-Ethynyl-2′-Deoxyuridine Injection and Fluorochrome Labeling
To test the mitotic activity of Axin2+ PDL progenitor cells, 5-ethynyl-2′-deoxyuridine (EdU; Carbosynth, NE08701, 20 mg/kg body weight) was administrated intraperitoneally in Axin2-CreERT2/+; R26RtdTomato/DTA+ mice 24 h prior to euthanasia. A Click-iT Imaging Kit with Alexa Flour 488-azide (Invitrogen, C10337) was used to detect EdU in cryosections.
To determine how ablation of Axin2+ PDL cells affected the new alveolar bone formation, calcein (Fluka, 21030, 10 mg/kg body weight) was injected intraperitoneally in Axin2-CreERT2/+; R26RtdTomato/+ mice and Axin2-CreERT2/+; R26RtdTomato/DTA+ mice after 1 d of OTM (P29), followed by alizarin red (Sigma, A3882, 20 mg/kg body weight) 5 d later (P34). Mice were sacrificed after 8 d of OTM (P36). The mineral apposition rate was calculated with the distance between 2 labels divided by 5 d.
Statistical Analysis
Results are presented as the mean ± standard deviation of independent replicates (n = 4). An independent t test was used to quantify the differences between the OTM side and control side at the same time point. Then, we performed a one-way analysis of variance (ANOVA) with Tukey’s post hoc test to quantify differences among the 3 different time points within the same side. A two-way ANOVA with Tukey’s post hoc test was then used to quantify the differences in OTM and control sides within 2 different mice groups. P ≤ 0.05 was considered significant. GraphPad Prism (GraphPad Software) was used for these analyses.
Results
A Synchronized Increase in Wnt–β-catenin and Axin2LacZ Expressions in PDL Progenitor Cells during OTM
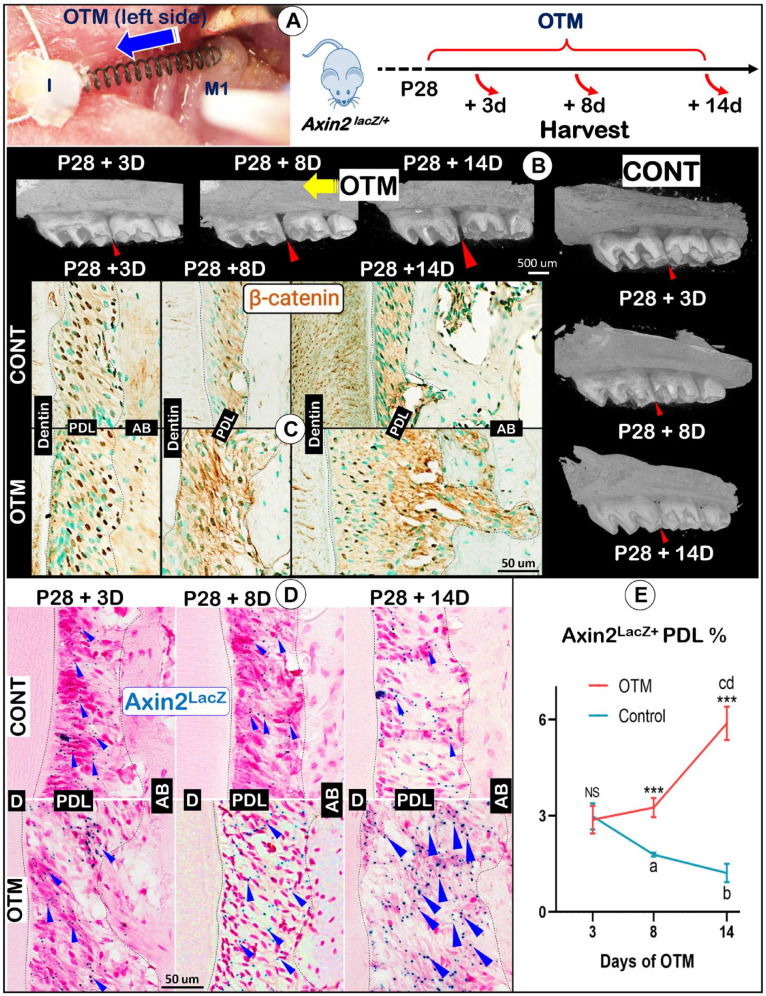
Although the Wnt/β-catenin pathway plays a critical role in maintaining periodontal homeostasis, it is largely unclear whether and how Wnt-responsive PDL cells contribute to alveolar bone formation during OTM. To address these questions, we established OTM models using the noninducible Axin2lacZ/+ (reflecting endogenous Axin2, a key downstream transcriptional factor of Wnt–β-catenin) knock-in reporter line to trace changes of Axin2+ progenitor numbers during OTM. Briefly, the OTM was started at P28 by bonding a customized coil spring between the maxillary left first molars and maxillary incisors for generation of a constant force of 0.5 N (Fig. 1A, left). The mice were sacrificed at 3 d, 8 d, or 14 d after OTM initiation (Fig. 1A, right). Based on micro–computed tomography (CT) images, OTM led to a gradual increase in the space between first and second molars (Fig. 1B, left, arrows), which agrees with our previous report (Rangiani et al. 2016).
Figure 1.
A coordinated response between β-catenin and Axin2 expression patterns during orthodontic tooth movement (OTM). (A) The OTM procedure was established in Axin2lacZ/+ mice. OTM started at postnatal day 28 (P28), and mice were harvested at P31 (+3 d), P36 (+8 d), or P42 (+14 d). (B) Representative 3-dimensional micro–computed tomography views of changes of the gap between first and second molars over time on OTM sides (arrowheads; left panel) compared to the control side (small arrowheads; lower right panel). (C) The immunohistochemical images displayed changes in β-catenin levels on the distal area of maxillary first molar (M1) mesial roots. (D) X-gal staining images identified changes in Axin2LacZ signals with the OTM sides at lower panels and control sides on upper panels. (E) Quantification of Axin2LacZ+ periodontal ligament (PDL) cells at various time points after initiation of OTM. n = 4; independent t test between OTM and control (CONT) at each time point. NS, not significant; ***P < 0.001. One-way analysis of variance with Tukey’s post hoc test among different time points within the same side: within control side, aP < 0.001, bP < 0.001 versus +3 d group; within OTM side, cP < 0.001 versus +3 d group; dP < 0.001 versus +8 d group. AB, alveolar bone.
To determine the temporal and spatial expression patterns of β-catenin and Axin2 under OTM tension force, the region of interest (ROI) was selected at the middle third fraction of the distal area of the maxillary first molar mesial root, which was based on the mesially moved direction (Fig. 1B). Immunostaining of β-catenin in WT mice and X-gal staining (which reflects Axin2 signal) in Axin2lacZ/+ mice showed that both β-catenin (Fig. 1C, upper panels) and Axin2-lacZ (Fig. 1D, upper panels) signals were dispersed throughout the PDL and gradually decreased over time in the control group. In contrast, both β-catenin level (Fig. 1C, lower panels) and Axin2LacZ+-PDL cell number (Fig. 1D, lower panels) were gradually increased under orthodontic tension force, especially at +8 d and +14 d stages. Our quantitative data showed those increased lacZ+ areas in the PDL at P36 (+8 d) and P42 (+14 d) were significantly different from the control groups (Fig. 1E; ***P < 0.001, n = 4). Taken together, these data support the notion that a low level of Wnt signaling activity (reflected by β-catenin and nuclear Axin2 signals) is required for the homeostasis of adult periodontium (Yuan et al. 2018; Zhang et al. 2019); however, there is a synchronized increase in β-catenin and Axin2 signals under orthodontic tension force, indicating a likely role of β-catenin and Axin2+ PDL cells in the periodontal remodeling during OTM.
A Significant Increase in Axin2+ Lineage PDL Cells during OTM
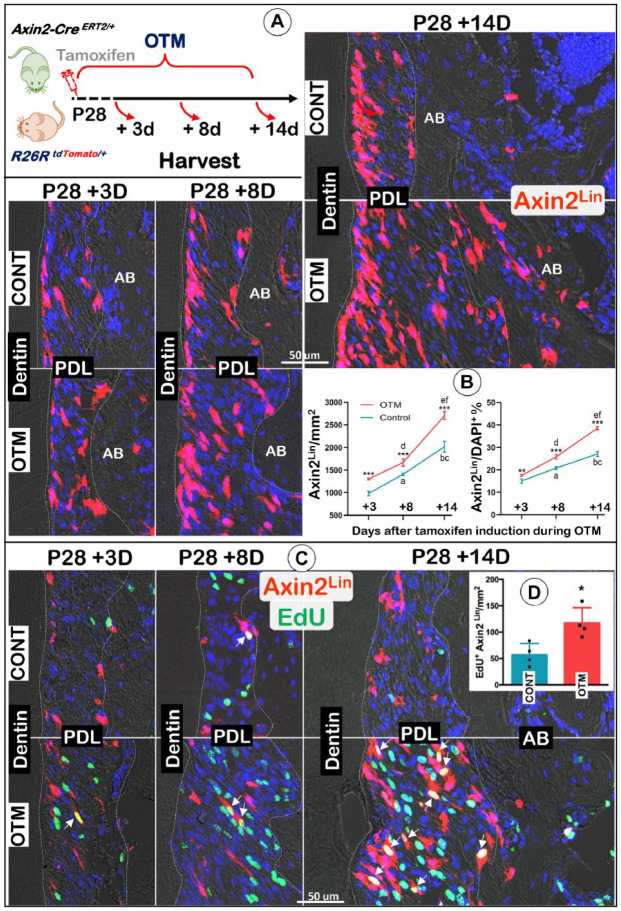
To trace the fate of Axin2+ PDL progenitors in response to orthodontic tension force, we crossed Axin2-CreERT2/+ mice with R26RtdTomato/+ (named Axin2Lin) mice. We administered a one-time dose of tamoxifen to the P28 tracing mice at the same time OTM was induced. The mice were harvested at P31 (+3 d), P36 (+8 d), or P42 (+14 d), respectively (Fig. 2A, left panel). Our confocal images showed a progressive increase in Axin2Lin PDL cell numbers on both the control and OTM sides over time, with higher Axin2Lin PDL cell numbers on the OTM side (Fig. 2A). The quantification of Axin2Lin cells numbers/mm2 and the ratio of Axin2Lin cells/DAPI+ cells showed that the differences between the OTM and the control side are statistically significant (Fig. 2B; n = 4; P < 0.01). Furthermore, colocalization of Axin2Lin cells and EdU staining confirmed a much higher proliferation level on the OTM side compared to the control side (Fig. 2C, D). Together, these data indicate that Axin2+ progenitor cells maintain a low level of proliferation, but their progeny cells are active over time, and Axin2Lin PDL cells are highly sensitive to tension force during OTM.
Figure 2.
Orthodontic tooth movement (OTM) triggers a great increase in Axin2+ progeny cell numbers. (A) A schematic diagram of OTM and one-time injection of tamoxifen in the tracing line (Axin2CreERT2/+; R26RtdTomato/+) started at postnatal day 28 (P28) and harvested at P31 (+3 d), P36 (+8 d), or P42 (+14 d), respectively (left panel), and confocal imaging views of changes in Axin2 progeny cells on both control and OTM sides. (B) Quantification of changes in Axin2+ progeny cell numbers in the periodontal ligament (PDL) space at various time points after initiation of OTM, including Axin2Lin cells/mm2 (left panel) and the ratio of Axin2Lin cells/DAPI+ cells (right panel). n = 4; independent t test between OTM and control (CONT) at each time point: ***P < 0.001. One-way analysis of variance with Tukey’s post hoc test among different time points within the same side: within the control side, aP < 0.001, bP < 0.001 versus +3 d group; cP < 0.001 versus +8 d group; within the OTM side, dP < 0.001, eP < 0.001 versus +3 d group; fP < 0.001 versus +8 d group. (C) EdU staining showed high proliferation activity in PDL cells with some overlapped with Axin2 progeny cells (white arrows) on the OTM-tension side. AB, alveolar bone. (D) Quantification of EdU+ Axin2Lin/mm2 in the PDL space at P42 (+14 d). Independent t test between CONT and OTM. n = 4; *P < 0.05.
Axin2Lin PDL Cells Directly Contribute to Newly Formed Alveolar Bone during OTM
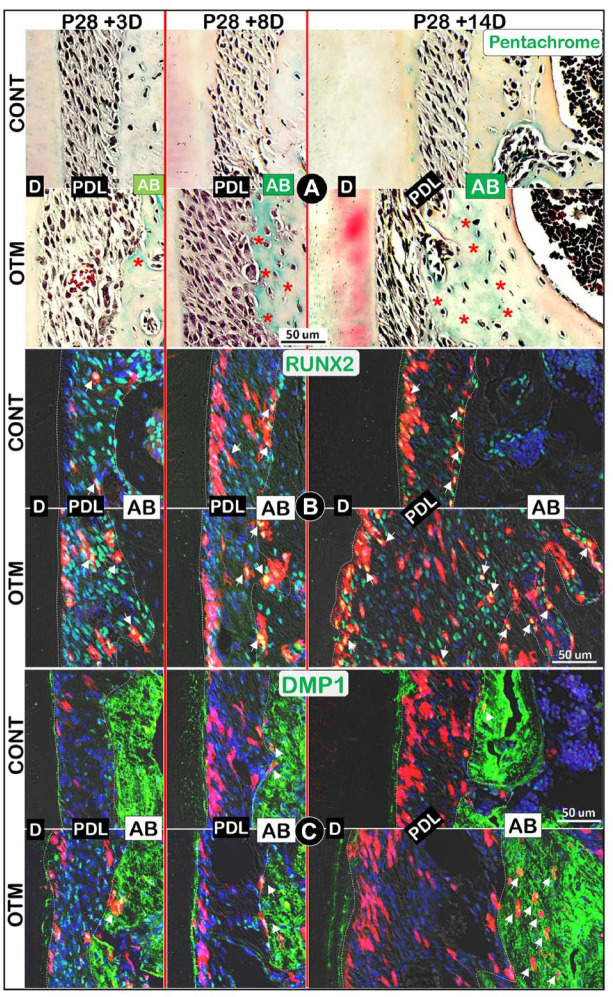
One of the key OTM responses is an increase in alveolar bone formation on the tension side. Here, we first showed a gradual increase in the newly formed bone matrices on the OTM side compared to the control side using Pentachrome staining images, in which the newly formed bone matrix was indicated by the color green (Fig. 3A, lower panels).
Figure 3.
Orthodontic tooth movement (OTM) increases both Axin2+ progeny cell numbers and new bone formation. (A) Pentachrome staining images revealed changes in alveolar bone areas during OTM (immature matrix in green, red asterisks). (B) Colocalization of Axin2+ red progeny cells with RUNX2 immunostaining (green, white arrows). (C) Colocalization of Axin2+ red progeny cells with DMP1 immunostaining (green, white arrows). AB, alveolar bone; CONT, control; D, dentin; PDL, periodontal ligament.
To test whether Axin2+ PDL progenitor cells directly contribute to the newly increased alveolar bone during OTM, we performed a combination of Axin2Lin tracing plus coimmunostaining with key bone markers. The confocal images in Fig. 3B (left panels) showed an initial increase in Axin2+ cells and RUNX2+ expressions (in red and yellow) within the PDL at P31 (+3 d). At P36 (+8 d), more positive signals were observed in alveolar bone cells on the OTM side compared to that of the control side (Fig. 3B, middle panels). By P42 (+14 d), a drastic increase in Axin2+ PDL cells and Axin2+/RUNX2+ bone cells was observed on the OTM side (Fig. 3B, right panels), although the quantification data did not show statistical significance (Appendix Fig. 1B). Similarly, a gradual increase in DMP1+ osteocytes (Fig. 3C, lower panels) or SOST+ osteocytes (Appendix Fig. 1A, lower panels) from the Axin2 lineage was documented on the OTM side. At P42 (+14 d), the DMP1+ Axin2Lin/mm2 and SOST+ Axin2Lin/mm2 on the OTM side were significantly higher than the control side (Appendix Fig. 1C, D). Together, these tracing data support the notion that Axin2+ PDL progenitor cells directly contribute to the newly increased PDL and alveolar bone cells during OTM.
Ablation of Axin2 Lineage PDL Cells Significantly Reduced Osteogenesis on Both Control (Moderate) and OTM (Severe) Sides
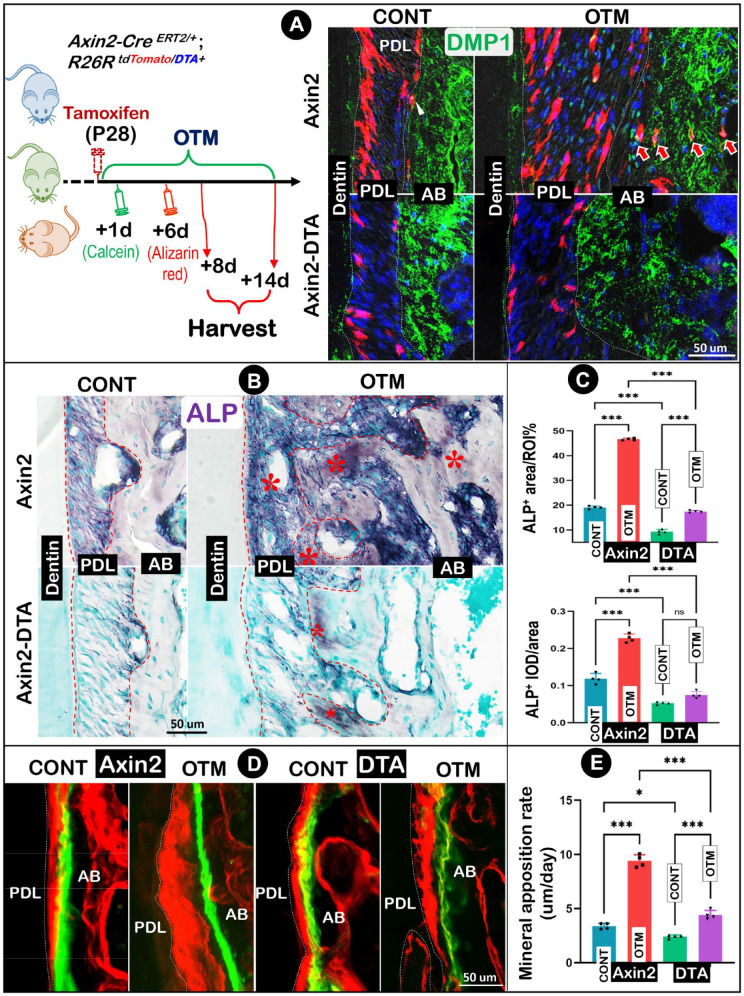
To further support the role of Axin2+ PDL cells in osteogenesis during normal development and OTM response, we studied the impact of ablating Axin2 lineage PDL cells during osteogenesis using the compound tracing line of Axin2-CreERT2/+; R26RtdTomato/DTA+ mice (named the Axin2-DTA line). The animals received a one-time tamoxifen induction and then underwent double labeling (Fig. 4A, left panel). Mice were harvested at P42 (+14 d; for tracing and ALP studies) or P36 (+8 d; for bone formation rate studies), respectively. Notably, the Axin2-DTA–treated group exhibited a similar tooth movement rate (Appendix Fig. 2A, B) and osteoclastic activity on the compression side (Appendix Fig. 2C) when compared to the nontreated normal line (named the Axin2 line). As shown in Fig. 4A (lower right panels), Axin2+ PDL cell numbers in the Axin2-DTA–treated group greatly reduced in both the control and OTM PDL, with essentially no detectable tdTomato+/DMP1+ cells in the alveolar bone compared to the Axin2 line (Fig. 4A, upper right panels, quantification in Appendix Fig. 3).
Figure 4.
Ablation of Axin2 lineage cells leads to a drastic decrease in osteogenesis on both control and orthodontic tooth movement (OTM) sides. (A) A schematic diagram of OTM, one-time injection of tamoxifen plus injections of calcein (at +1 d) and alizarin red (at +6 d) in the tracing line (Axin2CreERT2/+; R26RtdTomato/DTA+, named Axin2-DTA line) started at postnatal day 28 (P28) and harvested at P36 (+8 d) and P42 (+14 d), respectively (left panel) and the confocal image views of changes in Axin2 progeny cells on both control (CONT) and OTM sides with and without diphtheria toxin fragment A (DTA) activation. (B) Changes in alkaline phosphatase (ALP) (red asterisks) with and without DTA activation. (C) Quantitative analyses of ALP+ area ratio and mean integral optical density (IOD) in mice with and without DTA activation. (D) Fluorochrome labeling assays on both control and OTM sides with and without DTA activation. (E) Quantification of mineral apposition rates on both control and OTM sides with and without DTA activation. Two-way analysis of variance with Tukey’s post hoc test was used for statistical analyses. n = 4; NS, not significant; *P < 0.05, ***P < 0.001. AB, alveolar bone; PDL, periodontal ligament.
Next, we examined ALP activities in PDL and alveolar bone areas (Fig. 4B). In the normal group, the control side displayed a high basic level in both PDL and alveolar bone areas (Fig. 4B, upper left), while the OTM side showed a drastic increase in ALP level (Fig. 4B, upper right). However, ALP was largely undetectable within the alveolar bone of the Axin2-DTA group, with a very low level in the unloaded PDL (Fig. 4B, lower left). Furthermore, there was only a marginal increase of ALP during OTM (Fig. 4B, lower right). Our quantitative data showed a 2-fold increase in the ALP+ area induced by the tension force in the normal line, whereas DTA treatment led to a decrease of 51% and 63% of the ALP+ area ratio on the control side and OTM side, respectively (Fig. 4C, upper panel, n = 4; P < 0.001). Similarly, the ALP mean integral optical density (IOD) was doubled on the OTM side in the Axin2 line, yet the Axin2-DTA line had a decrease of 56% and 67% on the nonloaded and the OTM side, respectively (Fig. 4C, lower panel, n = 4; P < 0.001).
To address the early change in the mineral apposition rate, we sacrificed mice at P36 (8 d after induction of OTM). Our confocal images showed a great increase in the mineral apposition rate on the OTM side in the normal line (Fig. 4D, left panel). In the DTA-treated group, there was only a mild increase on the loaded side (Fig. 4D, right panel). The quantitative data showed a nearly 3-fold increase in mineral apposition rates within the OTM, indicating accelerated new bone formation in response to orthodontic tension force (Fig. 4E; left panel; n = 4; P < 0.001). In contrast, there was only a less than 50% increase in mineral apposition rate on the OTM side in the Axin2-DTA group (Fig. 4E; right panel; n = 4; P < 0.001) compared to the unloaded side. Overall, genetic ablation of the Axin2 lineage PDL cells resulted in a sharp reduction of new bone formation from both the basic level and the loading response.
Discussion
From a development perspective, both PDL progenitor cells and their progeny would proportionally increase during PDL expansion. Interestingly, we observed an opposite developmental direction with a moderate decrease in Axin2LacZ+ progenitor cell numbers (Fig. 1D, E) but a robust and steady increase in Axin2Lin progeny cell numbers (Fig. 2A, B). This information supported the notion that most of Axin2 progenitor cells are largely quiescent and that their progeny cells actively contribute to alveolar bone expansion during normal development. A recent report showed a low level of Wnt signaling activity in PDL (Yuan et al. 2018; Zhang et al. 2019), which agrees with our finding. In contrast, there is a drastic increase in Axin2LacZ+ PDL cell numbers plus a sharp increase in Axin2Lin progeny cell numbers during OTM, which are responsible for an increase in PDL space and alveolar bone volume. This finding suggests that Axin2LacZ+ PDL progenitor cells are highly sensitive to mechanical force and robustly activated by OTM.
In this study, we showed that DTA-ablated Axin2+ progenitors in the PDL led to a sharp reduction of Axin2Lin progeny cell numbers, alveolar bone mass, and bone formation rates (Fig. 4), although a low baseline ALP activity in the DTA-targeted mice remained. The following 3 facts count for this unexpected result: 1) we only targeted the Axin2Lin progeny at P28 or after, and the early intact Axin2Lin progeny population “escaped” the impact of DTA treatment; 2) the tamoxifen-induced DTA action is not 100% efficient; and 3) there might be non-Axin2+ PDL progenitor cells, which effectively respond to OTM.
Although this study mainly focused on the OTM-tension side, we also noticed a biphasic OTM response on the compression side: a sharp reduction in RUNX2+-PDL cells at P31 (+3 d) and P36 (+8 d) but a great expansion of the RUNX2+-PDL cells on the OTM side at P28 + 14 d (Appendix Fig. 4, lower left panels). In addition, more Axin2Lin cells and strong DMP1 expression were observed on the compression side at P28+14 d (Appendix Fig. 4, lower right panels). Several DMP1+ Axin2Lin cells were located on the alveolar bone surface (Appendix Fig. 4, lower right panel). Together, these preliminary data support the role of Axin2+ PDL cells in PDL and bone recovery on the compression side.
Overall, the current study demonstrated that OTM induces a time-dependent increase in the Axin2+ PDL progenitors and their progeny, which are responsible for a robust expansion in the PDL space and alveolar bone osteogenesis (Fig. 5). Identification of the key function of Axin2+ PDL progenitors and their progeny in response to OTM-induced new bone formation not only fills the knowledge gap in understanding the cell origins and mechanisms underlying these changes but also provides a new targeted cell population (Axin2+ PDL progenitors/their progeny) for future drug development to prevent relapse following OTM.
Figure 5.
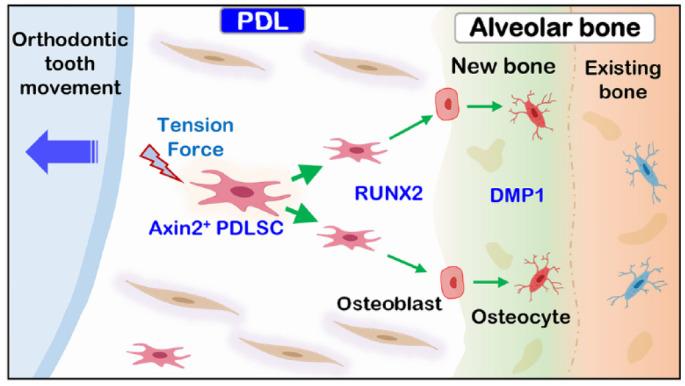
Schematic diagram depicting critical roles of Axin2+ progenitor cells and their progeny in orthodontic tension–induced new bone formation. Upon the stimulation of tension force, the quiescent Axin2+ periodontal ligament (PDL) cells (as a key progenitor cell source) became mitotically active and directly differentiated into osteoblasts (expressing RUNX2); they then became osteocytes (expressing DMP1), contributing to the new bone formation on the tension side.
Author Contributions
K. Wang, C. Xu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; X. Xie, Y. Jing, P.J. Chen, Z. Wang, contributed to data acquisition, critically revised the manuscript; S. Yadav, R.W. Taylor, contributed to data analysis and interpretation, critically revised the manuscript; J. Wang, J.Q. Feng, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211062585 for Axin2+ PDL Cells Directly Contribute to New Alveolar Bone Formation in Response to Orthodontic Tension Force by K. Wang, C. Xu, X. Xie, Y. Jing, P.J. Chen, S. Yadav, Z. Wang, R.W. Taylor, J. Wang and J.Q. Feng in Journal of Dental Research
Acknowledgments
We thank Dr. Heather M. O’Brien and Ms. Meghann Holt for their assistance with the editing of this article.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by US National Institutes of Health grants DE030643 and DE025659 to J.Q. Feng.
ORCID iDs: K. Wang  https://orcid.org/0000-0002-1866-0678
https://orcid.org/0000-0002-1866-0678
P.J. Chen  https://orcid.org/0000-0002-8875-432X
https://orcid.org/0000-0002-8875-432X
J.Q. Feng  https://orcid.org/0000-0003-1508-5038
https://orcid.org/0000-0003-1508-5038
A supplemental appendix to this article is available online.
References
- Bowman AN, Van Amerongen R, Palmer TD, Nusse R. 2013. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin–responsive neural stem cells. Proc Natl Acad Sci. 110(18):7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Lin H, Fu H, Wang B, Han G, Fan M. 2017. Microrna-195-5p regulates osteogenic differentiation of periodontal ligament cells under mechanical loading. J Cell Physiol. 232(12):3762–3774. [DOI] [PubMed] [Google Scholar]
- Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S. 2010. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 16(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Yang R, Liu D, Wang X, Song Y, Cao H, He D, Gan Y, Kou X, Zhou Y. 2016. PDL progenitor-mediated PDL recovery contributes to orthodontic relapse. J Dent Res. 95(9):1049–1056. [DOI] [PubMed] [Google Scholar]
- Fu HD, Wang BK, Wan ZQ, Lin H, Chang ML, Han GL. 2016. Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J Mol Histol. 47(5):455–466. [DOI] [PubMed] [Google Scholar]
- Huelter-Hassler D, Tomakidi P, Steinberg T, Jung BA. 2017. Orthodontic strain affects the Hippo-pathway effector YAP concomitant with proliferation in human periodontal ligament fibroblasts. Eur J Orthod. 39(3):251–257. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. 2005. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 43(3):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. 2002. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 22(4):1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarizadeh A, Bourauel C, Gotz W, Jager A. 2005. Early responses of periodontal ligament cells to mechanical stimulus in vivo. J Dent Res. 84(10):902–906. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Davidovitch Z. 2006. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 129(4):469.e1–469.e32. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. 2005. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 280(20):19883–19887. [DOI] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WLC, Chau RMW, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. 2013. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 342(6163):1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odagaki N, Ishihara Y, Wang Z, Ei Hsu Hlaing E, Nakamura M, Hoshijima M, Hayano S, Kawanabe N, Kamioka H. 2018. Role of osteocyte-PDL crosstalk in tooth movement via SOST/sclerostin. J Dent Res. 97(12):1374–1382. [DOI] [PubMed] [Google Scholar]
- Rangiani A, Jing Y, Ren Y, Yadav S, Taylor R, Feng JQ. 2016. Critical roles of periostin in the process of orthodontic tooth movement. Eur J Orthod. 38(4):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan K. 1957. Some factors determining the evaluation of forces in orthodontics. Am J Orthod. 43(1):32–45. [Google Scholar]
- Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN, Qin C, Ke H, Liu M, Feng JQ. 2015. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J. 29(7):2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B-M, Miura M, Gronthos S, Mark Bartold P, Batouli S, Brahim J, Young M, Gehron Robey P, Wang CY, Shi S. 2004. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 364(9429):149–155. [DOI] [PubMed] [Google Scholar]
- Shen T, Qiu L, Chang H, Yang Y, Jian C, Xiong J, Zhou J, Dong S. 2014. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int J Clin Exp Pathol. 7(11):7872. [PMC free article] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, et al. 2006. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Wen Y, Wu X, Zhang Y, Qiao X, Xu X. 2018. Expression pattern of YAP and TAZ during orthodontic tooth movement in rats. J Mol Histol. 49(2):123–131. [DOI] [PubMed] [Google Scholar]
- Takimoto A, Kawatsu M, Yoshimoto Y, Kawamoto T, Seiryu M, Takano-Yamamoto T, Hiraki Y, Shukunami C. 2015. Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development. 142(4):787–796. [DOI] [PubMed] [Google Scholar]
- Tang N, Zhao Z, Zhang L, Yu Q, Li J, Xu Z, Li X. 2012. Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch Med Sci. 8(3):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Amerongen R, Bowman AN, Nusse R. 2012. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 11(3):387–400. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhao L, Fish M, Logan CY, Nusse R. 2015. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 524(7564):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang J, Wang K, Li C, Zhang S, Jing D, Xu C, Wang X, Zhao H, Feng JQ. 2019. Axin2+-mesenchymal pdl cells, instead of k14+ epithelial cells, play a key role in rapid cementum growth. J Dent Res. 98(11):1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HY, Nie EM, Deng G, Lai LZ, Sun FY, Tian H, Fang FC, Zou YG, Wu BL, Ou-Yang J. 2017. Periostin is essential for periodontal ligament remodeling during orthodontic treatment. Mol Med Rep. 15(4):1800–1806. [DOI] [PubMed] [Google Scholar]
- Xu Q, Yuan X, Zhang X, Chen J, Shi Y, Brunski JB, Helms JA. 2019. Mechanoadaptive responses in the periodontium are coordinated by Wnt. J Dent Res. 98(6):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Pei X, Zhao Y, Tulu US, Liu B, Helms JA. 2018. A Wnt-responsive PDL population effectuates extraction socket healing. J Dent Res. 97(7):803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu W, Zhao J, Ma X, Shen L, Zhang Y, Jin F, Jin Y. 2016. Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/β-catenin pathway. Biochim Biophys Acta. 1860(10):2211–2219. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan X, Xu Q, Arioka M, Van Brunt LA, Shi Y, Brunski J, Helms JA. 2019. Molecular basis for periodontal ligament adaptation to in vivo loading. J Dent Res. 98(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211062585 for Axin2+ PDL Cells Directly Contribute to New Alveolar Bone Formation in Response to Orthodontic Tension Force by K. Wang, C. Xu, X. Xie, Y. Jing, P.J. Chen, S. Yadav, Z. Wang, R.W. Taylor, J. Wang and J.Q. Feng in Journal of Dental Research