Abstract
Background
Although SARS-CoV-2 vaccines immunogenicity in patients with cancer has been investigated, whether they can significantly improve the severity of COVID-19 in this specific population is undefined.
Methods
Capitalizing on OnCovid (NCT04393974) registry data we reported COVID-19 mortality and proxies of COVID-19 morbidity, including post-COVID-19 outcomes, according to the vaccination status of the included patients.
Results
2090 eligible patients diagnosed with COVID-19 between 02/2020 and 11/2021 were included, of whom 1930 (92.3%) unvaccinated, 91 (4.4%) fully vaccinated and 69 (3.3%) partially vaccinated. With the exception of a higher prevalence of patients from the UK (p = 0.0003) and receiving systemic anticancer therapy at COVID-19 diagnosis (p = 0.0082) among fully vaccinated patients, no demographics/oncological features were associated with vaccination status. The 14-days case fatality rate (CFR) (5.5% vs 20.7%, p = 0.0004) and the 28-days CFR (13.2% vs 27.4%, p = 0.0028) demonstrated a significant improvement for fully vaccinated patients in comparison with unvaccinated patients. The receipt of prior full vaccination was also associated with reduced symptomatic COVID-19 (79.1% vs 88.5%, p = 0.0070), need of COVID-19 oriented therapy (34.9% vs 63.2%, p < 0.0001), complications from COVID-19 (28.6% vs 39.4%, p = 0.0379), hospitalizations due to COVID-19 (42.2% vs 52.5%, p = 0.0007) and oxygen therapy requirement (35.7% vs 52%, p = 0.0036). Following Inverse Probability Treatment Weighting (IPTW) procedure no statistically significant difference according to the vaccination status was confirmed; however, all COVID-19 related outcomes were concordantly in favour of full vaccination. Among the 1228 (58.8%) patients who underwent a formal reassessment at participating centres after COVID-19 resolution, fully vaccinated patients experienced less sequelae than unvaccinated patients (6.7% vs 17.2%, p = 0.0320).
Conclusions
This analysis provides initial evidence in support of the beneficial effect of SARS-CoV-2 vaccines against morbidity and mortality from COVID-19 in patients with cancer.
Keywords: COVID-19, SARS-CoV-2, Vaccines, Prevention, Cancer, Clinical efficacy
1. Introduction
Patients with cancer are intrinsically more vulnerable to morbidity and mortality from Coronavirus Disease 2019 (COVID-19) [1,2]. All levels of cancer care including screening, diagnosis and treatment have been heavily impacted by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic [3,4]. In addition to the threat imposed by acute morbidity and mortality from COVID-19 in cancer patients, recent evidence highlights that oncological continuity of care can be further disrupted by long-term consequences of COVID-19, which affect approximately 15% of patients with cancer who recover from the acute phase [5].
Widespread vaccination against SARS-Cov-2 represents a highly important public health measure to reduce the severity and lethality of SARS-Cov-2.
The immunogenicity and safety profile of SARS-Cov-2 vaccines have been investigated across several types of cancers and irrespective of exposure to recent anti-cancer therapy [[6], [7], [8]]. Evidence of seroconversion following SARS-Cov-2 vaccination confirmed an antibody response in >90% of patients with solid tumours [9,10], which is comparable to the general population [11]. However, several reports highlight that a proportion of patients with cancer, such as those with haematological malignancies undergoing anti-CD20 treatments, elicit a diminished immune response to vaccines with seroconversion rates of <60% [9,10].
Evidence on the efficacy of SARS-Cov-2 vaccines from randomized clinical trials is limited to patients with stable oncological disease and off immunosuppressive anti-cancer therapy at the time of vaccination [12]. Therefore, unresolved questions exist around whether efficacy of SARS-CoV-2 vaccinaes is independent from anti-cancer treatments and whether vaccinal immunity protects from long-term consequences of COVID-19.
With these premises in mind, we performed a dedicated update of the OnCovid registry to provide initial evidence regarding the magnitude of clinical benefit of SARS-CoV-2 vaccines in influencing outcomes of COVID-19 in a large real-world oncological population.
2. Study design and outcomes
OnCovid (NCT04393974) is a European registry study that collects data from consecutive patients with solid/haematologic malignancy diagnosed with COVID-19.
By the data lock of 22/12/2021, the registry included 3237 patients diagnosed with COVID-19 between 27/02/2020 and 30/11/2021. Patients with unknown vaccination statuses were excluded. Similarly, to maintain consecutive accrual, we excluded centres that did not actively enrol subjects during the March–December accrual timeframe. A list of participating centres with eligible patients for this analysis is provided in Supplementary Table 1.
The primary objective of this study was to describe COVID-19 mortality in patients with cancer according to SARS-CoV-2 vaccination status. As secondary objectives, we estimated the impact of COVID-19 vaccines on COVID-19 symptoms and morbidity. In addition, we evaluated whether receipt of SARS-CoV-2 vaccination was associated with the occurrence of COVID-19 sequelae among patients who underwent a clinical reassessment at the participating centres.
Patients were categorized as fully vaccinated at the time of COVID-19 diagnosis if they had received two doses of the BNT162b2, mRNA-1273 and ChAdOx1-S vaccines or in case of infection diagnosed at least 28 days after a single dose of the Ad.26.COV2.S vaccine [13]. Patients who received at least one vaccination, without meeting the above-mentioned criteria, were considered partially vaccinated.
Acknowledging the competing influence of the underlying malignancy in determining clinical outcomes, we elected the all-cause 14-days CFR as the clinical end points of interest, in an attempt of differentiating early (COVID-19 related) from late (cancer-related) mortality as already done in with our registry [14]. Considering the limited number of 14-days events recorded among fully vaccinated patients, which prevented the planned Inverse Probability of Treatment Weighting (IPTW) procedure, we also evaluated the 28-days CFR.
First, we reported the distribution of key demographics and oncological characteristics consistently associated with clinical outcomes in the study population [5,[14], [15], [16], [17], [18]] across the vaccination categories, subsequently, we analyzed COVID-19 related outcomes according to the vaccination status, with a formal comparison between fully vaccinated and unvaccinated patients.
We then reported the prevalence and distribution of COVID-19 associated symptoms, and as proxiesof COVID-19 morbidity, we analyzed other COVID-19 related outcomes reproducibly described in the registry [5,[14], [15], [16], [17]], including the need of COVID-19 oriented therapy, the incidence of COVID-19 complications, the hospitalization rate and the need of oxygen therapy.
Although recognizing that the unbalanced sample size of the vaccination subgroups did not allow a powered and formal weighted comparison, we performed an exploratory IPTW procedure including key baseline demographics and oncological characteristics, to provide a preliminary adjusted estimation of the CFR and COVID-19 related outcomes.
Considering the evidence of a mild decrease over time of the antibody response to SARS-CoV-2 vaccination [19,20], we also provided a descriptive analysis of COVID-19 outcome according to vaccination timing, including only patients with an available date of vaccination. For this purpose, fully vaccinated patients were considered those who had received two doses of the BNT162b2, mRNA-1273 and ChAdOx1-S vaccines at least 7 days before the infection and those who received one dose of the Ad.26.COV2.S vaccine at least 28 days before the breakthrough infection [13,[21], [22], [23]]. Patients who received at least one vaccination, without meeting the above-mentioned criteria, were considered partially vaccinated, while those patients diagnosed with COVID-19 more than 6-months following the complete vaccination were considered separately.
Lastly, in order to describe the potential role of SARS-CoV-2 vaccines in reducing the occurrence of COVID-19 sequelae in patients with cancer, we focused on those patients who underwent a formal clinical assessment at the participating centres after COVID-19 recovery as previously done [5], and reported the incidence of COVID-19 sequelae according to their vaccination status. Sequelae were assessed by treating physicians as per local practice, and categorized according to the system/organ involved into: respiratory symptoms (including dyspnoea and chronic cough), residual fatigue, weight loss, neuro-cognitive sequelae (including cognitive, visual impairment, ano/dysosmia – age/dysgeusia, headache, confusion, lethargy) and others (including other organs dysfunctions, residual fever, muscle cramps, arthralgia, skin conditions etc). For the purpose of the analysis, COVID-19 sequelae were further clustered as: respiratory (either alone or combined with other complications) and post-COVID-19 fatigue.
A detailed description of the study methodology and statistical analysis is provided in Supplementary eMethods.
3. Results
3.1. Vaccination and patients’ characteristics
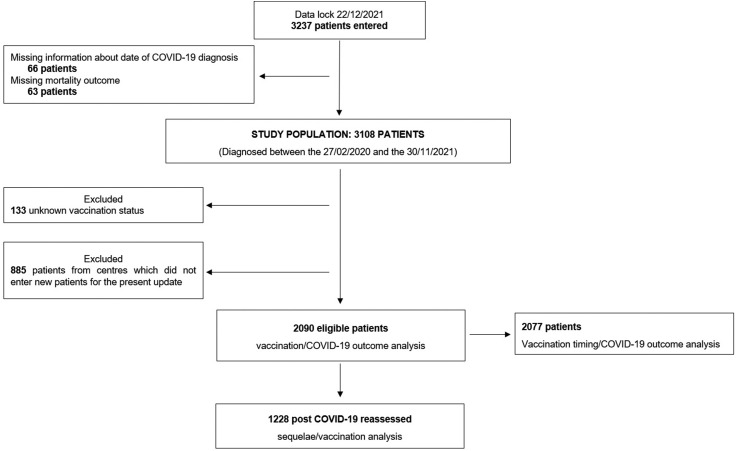
At the time of database lock, the registry included 3237 patients. A total of 129 patients were excluded due to the unconfirmed date of COVID-19 diagnosis and missing mortality outcome, another 133 patients were excluded because of unknown vaccination status. A further group of 885 patients previously entered from centres that did not enrol patients for the present update was further excluded to maintain consecutive accrual and minimize selection bias (Fig. 1 ).
Fig. 1.
Study flow diagram.
In total, 2090 eligible patients (67.2%) were included in this analysis, including 1930 (92.3%) unvaccinated, 91 (4.4%) fully vaccinated and 69 (3.3%) partially vaccinated patients. Among fully vaccinated patients 54 (59.3%) received the BNT162b2 vaccine, 18 (19.8%) received the mRNA-1273 vaccine, 16 (17.6%) received the ChAdOx1-S vaccine and 3 (3.3%) received the Ad.26.COV2.S vaccine. Among partially vaccinated patients 32 (46.4%) received the BNT162b2 vaccine, 27 (39.1%) received the mRNA-1273 vaccine and 10 (14.5%) received the ChAdOx1-S vaccine.
Table 1 reports the detailed distribution of demographics and oncological characteristics across the vaccination subgroups. As compared to unvaccinated patients, there was a higher prevalence of patients from the United Kingdom (49.5% vs 31.5%, p = 0.0003) and receiving systemic anticancer therapy at COVID-19 diagnosis (54.5% vs 40.3%, p = 0.0082) among fully vaccinated patients, while no other characteristics including sex, age, comorbidities burden and tumour features were significantly associated with vaccination status. Of note, primary tumour types were well balanced across vaccination subgroups. Importantly, the great majority of patients diagnosed after the approval of the first SARS-CoV-2 vaccine in the UK were still unvaccinated at COVID-19 diagnosis (560 vs 91/69 fully and partially vaccinated patients, respectively).
Table 1.
Demographics and oncological characteristics of eligible patients according to the vaccination status.
| Unvaccinated |
Fully vaccinated |
P-value | Partially vaccinated |
|
|---|---|---|---|---|
| N = 1930 (%) | N = 91 (%) | N = 69 (%) | ||
| Country | ||||
| United Kingdom | 607 (31.5) | 45 (49.5) | 0.0003 | 35 (50.7) |
| Spain | 712 (36.9) | 32 (35.2) | 26 (37.7) | |
| Italy | 611 (31.7) | 14 (15.4) | 8 (11.6) | |
| Sex | ||||
| Male | 1043 (54.2) | 43 (47.3) | 0.1970 | 35 (50.7) |
| Females | 883 (45.8) | 48 (52.7) | 34 (49.3) | |
| Missing | 4 | – | – | |
| Age | ||||
| <65 years | 753 (39.2) | 44 (48.4) | 0.0798 | 26 (38.8) |
| ≥65 years | 1170 (60.8) | 47 (51.6) | 41 (61.2) | |
| Missing | 6 | – | 2 | |
| Comorbidities | ||||
| 0–1 | 1031 (53.4) | 48 (52.7) | 0.9000 | 32 (46.4) |
| ≥2 | 899 (46.6) | 43 (47.3) | 37 (53.6) | |
| Smoking history | ||||
| Never smokers | 818 (50) | 34 (41) | 0.1095 | 24 (41.4) |
| Former/current smokers | 819 (50) | 49 (59) | 34 (58/6) | |
| Missing | 293 | 8 | 11 | |
| Primary Tumour | ||||
| Breast | 313 (16.4) | 16 (17.6) | 0.5284 | 13 (18.8) |
| Gastrointestinal | 385 (20.1) | 15 (16.5) | 12 (17.4) | |
| Gynaecological/Genito-Urinary | 344 (18.0) | 21 (23.1) | 13 (18.8) | |
| Thoracic | 301 (15.7) | 15 (16.5) | 13 (18.8) | |
| Others | 225 (11.8) | 13 (14.3) | 10 (14.5) | |
| Haematologic | 346 (18.1) | 11 (12.1) | 8 (11.6) | |
| Missing | 16 | – | – | |
| Tumour stage | ||||
| Local/loco-regional | 833 (47.2) | 34 (40) | 0.1959 | 31 (47) |
| Advanced | 933 (52.8) | 51 (60) | 35 (53) | |
| Missing | 164 | 6 | 3 | |
| Tumour status at COVID-19 diagnosis | ||||
| Remission/non measurable disease | 679 (35.5) | 30 (33) | 0.6249 | 27 (39.7) |
| Active malignancy | 1235 (64.5) | 61 (67) | 41 (60.3) | |
| Missing | 16 | – | 1 | |
| SACT at COVID-19 diagnosisa | ||||
| No | 1103 (59.7) | 40 (45.5) | 0.0082 | 33 (53.2) |
| Yes | 746 (40.3) | 48 (54.5) | 29 (46.8) | |
| Missing | 81 | 3 | 7 | |
| Timing of infection | ||||
| Post-vaccination phase | 560 (29.0) | 91 (100) | 69 (98.6) | |
| Pre-vaccination phase | 1370 (71.0) | – | – | |
SACT: systemic anticancer therapy.
Within 4 weeks prior to COVID-19 diagnosis.
3.2. Full vaccination is associated with improvement in COVID-19 morbidity and mortality
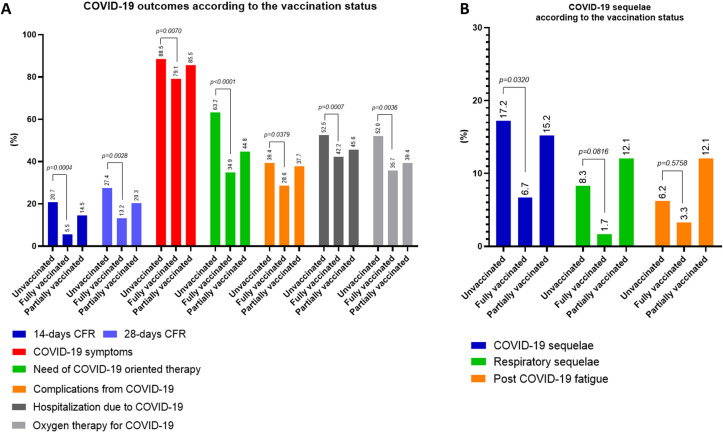
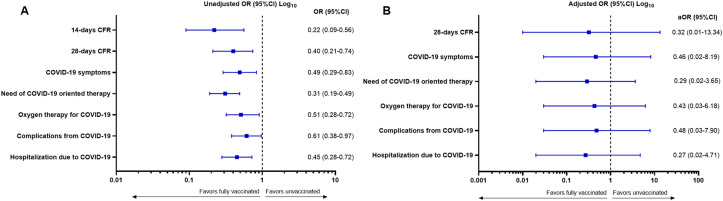
The 14-days CFR were significantly lower in fully vaccinated patients (5.5%) in comparison with unvaccinated patients (20.7%, p = 0.0004). Similarly, the 28-days CFR was significantly lower among fully vaccinated patients compared with unvaccinated patients (13.2% vs 27.4%, p = 0.0028). Compared to unvaccinated patients, the receipt of a full vaccination course was associated with improved morbidity from COVID-19, as showed by the significant reduction in the rates of symptomatic COVID-19 (79.1% vs 88.5%, p = 0.0070), provision of COVID-19-specific therapy (34.9% vs 63.2%, p < 0.0001), acute complications from COVID-19 (28.6% vs 39.4%, p = 0.0379), hospitalizations due to COVID-19 (42.2% vs 52.5%, p = 0.0007) and requirement for oxygen therapy (35.7% vs 52%, p = 0.0036). Table 2 provides a summary of COVID-19 related outcomes according to the vaccination status, also visualized in Fig. 2 A and reported as unadjusted OR in Fig. 3 A.
Table 2.
Summary of COVID-19 related outcomes according to the vaccination status.
| Unvaccinated |
Fully vaccinated |
P-value | Partially vaccinated |
|
|---|---|---|---|---|
| N = 1930 (%) | N = 91 (%) | N = 69 (%) | ||
| 14-Days case fatality rate | ||||
| Alive | 1530 (79.3) | 86 (94.5) | 0.0004 | 59 (85.5) |
| Death events | 400 (20.7) | 5 (5.5) | 10 (14.5) | |
| 28-Days case fatality rate | ||||
| Alive | 1401 (72.6) | 79 (86.8) | 0.0028 | 55 (79.7) |
| Death events | 529 (27.4) | 12 (13.2) | 14 (20.3) | |
| COVID-19 symptoms | ||||
| No | 222 (11.5) | 19 (20.9) | 0.0070 | 10 (14.5) |
| Yes | 1708 (88.5) | 72 (79.1) | 59 (85.5) | |
| Need of COVID-19 oriented therapy | ||||
| No | 676 (36.8) | 56 (65.1) | <0.0001 | 37 (55.2) |
| Yes | 1162 (63.2) | 30 (34.9) | 30 (44.8) | |
| Missing | 92 | 5 | 2 | |
| Complications from COVID-19 | ||||
| No | 1169 (60.6) | 65 (71.4) | 0.0379 | 43 (62.3) |
| Yes | 761 (39.4) | 26 (28.6) | 26 (37.7) | |
| Hospitalization | ||||
| Not required | 431 (22.5) | 36 (40.0) | 0.0007 | 15 (22.1) |
| Required due to COVID-19 | 1006 (52.5) | 38 (42.2) | 31 (45.6) | |
| Pre-existing | 479 (25.0) | 16 (17.8) | – | 22 (32.4) |
| Missing | 14 | 1 | 1 | |
| Oxygen therapy | ||||
| No | 884 (48.0) | 54 (64.3) | 0.0036 | 40 (60.6) |
| Yes | 956 (52.0) | 30 (35.7) | 26 (39.4) | |
| Missing | 90 | 7 | 3 | |
Fig. 2.
Summary of COVID-19 outcomes according to vaccination status (A). Patients were categorized as fully vaccinated at the time of COVID-19 diagnoses if they had received two doses for the BNT162b2, mRNA-1273, and ChAdOx1-S vaccines or in case of infection diagnosed at least 28 days after a single dose of the Ad.26.COV2.S vaccine. (B) Summary COVID-19 sequelae analysis according to the vaccination status.
Fig. 3.
Forest plot graph reporting the (A) unadjusted odds ratio (OR) and the (B) adjusted odds ratio (aOR) from the Inverse Probability of Treatment Weighting (IPTW) fitted multivariable logistic regression models for each COVID-19 outcomes. The following covariates were included in each model: country (United Kingdom vs Spain vs Italy), biological sex (male vs female), age (≥65 vs < 65 years), number of co-morbidities (≥2 vs 0–1), tumour status (presence of active vs non-active disease), and the receipt of systemic anticancer therapy (SACT) within 4 weeks of SARS-CoV-2 infection (yes vs no). CFR: case fatality rate. Complete multivariable models for each COVID-19 outcomes are reported in Supplementary Table 2.
IPTW analysis showed trends towards improvement in COVID-19 outcomes for fully vaccinated patients, however no statistically significant difference according to vaccination status can be confirmed for the 28-days CFR (adjusted odds ratio – AOR 0.32, 95% Confidence Intervals (CI): 0.01–13.34), COVID-19 symptoms (AOR 0.46, 95%CI: 0.02–8.19), need of COVID-19 oriented therapy (AOR 0.29, 95%CI: 0.02–3.65), oxygen therapy (AOR 0.43, 95%CI: 0.03–6.18), complications from COVID-19 (AOR 0.48, 95%CI: 0.03–7.90) and hospitalization due to COVID-19 (AOR 0.27, 95%CI: 0.02–4.71) (Fig. 3B). Multivariable logistic models for each outcome are reported in Supplementary Table 2.
3.3. Time-dependent characteristics of the relationship between SARS-Cov-2 vaccination and outcomes from COVID-19 infection
In view of the time-dependent efficacy of SARS-Cov-2 vaccines [20], we postulated whether the improvement in COVID-19 outcomes seen in fully vaccinated patients could change as a function of time. The exact dates of all vaccination doses were available for 147 patients. For this analysis 70 patients (47.6%) were considered partially vaccinated, 64 patients (43.5%) fully vaccinated ≥6 months from the infection and 13 patients (8.9%) fully vaccinated more than 6 months prior to the date of infection. Among partially vaccinated patients 33 (47.1%) received the BNT162b2 vaccine, 27 (38.6%) received mRNA-1273 and 10 (14.3%) received ChAdOx1-S. Among fully vaccinated patients, 31 (48.4%) received the BNT162b2 vaccine, 17 (26.6%) received mRNA-1273, 13 (20.3%) ChAdOx1-S and 3 (4.7%) Ad.26.COV2.S. Among the patients vaccinated ≥6 months from the infection, 11 (84.6%) received BNT162b2, 1 (7.7%) mRNA-1273 and 1 (7.7%) ChAdOx1-S. As summarized in Supplementary Table 3 and Supplementary Fig. 1, we observed a trend towards incremental improvement in all COVID-19-related outcomes across unvaccinated/partially vaccinated to fully vaccinated patients, followed by an increasing trend among patients vaccinated ≥6 months from the infection.
3.4. Vaccination against SARS-CoV-2 is associated with a lower prevalence of COVID-19 sequelae in patients with cancer
By the data lock, 1228 (58.8%) of the eligible patients underwent a formal clinical reassessment at participating centres after a median time of 40 days from COVID-19 diagnosis (Inter quartile range: 25–68). Baseline demographics and oncological characteristics stratified by vaccination status are summarized in Supplementary Table 4. Similar to what was reported for the overall population, fully vaccinated patients were more likely from the United Kingdom (46.7% vs 21.9%, p = 0.0003) and were receiving systemic anticancer therapy (SACT) at COVID-19 diagnosis (62.7% vs 47.6%, p = 0.0235). No other feature was associated with vaccination status.
Overall, 199 patients (16.2%) reported at least one COVID-19 sequela. As shown in Fig. 2B, the proportion of patients reporting at least 1 sequela from COVID-19 was significantly lower in fully vaccinated patients compared to unvaccinated controls (6.7% vs 17.2%, p = 0.0320), with no difference in the distribution of individual type of sequelae across groups (Supplementary Table 5).
4. Discussion
Vaccinal immunity to SARS-CoV-2 has radically changed the natural history of COVID-19. Whilst only partially effective in controlling viral transmission, especially after the emergence of novel variants of concern, vaccines remain widely effective in reducing the severity of COVID-19 [[21], [22], [23], [24]]. However, their remarkable clinical efficacy has been only partially demonstrated in patients with cancer [12].
Recently, data from the COVID-19 and Cancer Consortium (CCC19) on 54 fully vaccinated patients reported comparable rates of mortality and risk of adverse outcomes from COVID-19 irrespective of vaccination status, highlighting that vaccine protection vaccination may be incomplete in patients with cancer and supporting the need for further investigation in independent cohorts [25].
In this analysis of the OnCovid registry, we documented for the first time that patients who contracted SARS-Cov-2 after full vaccination were characterized by a lower probability of severe COVID-19 and mortality compared to unvaccinated controls.
Univariable analyses demonstrate a reduction in CFR 14- and 28-days post-infection for fully vaccinated patients compared to unvaccinated patients. All indices of COVID-19 morbidity showed a protective effect for fully vaccinated patients, including COVID-19 symptoms, the requirement for COVID-19-oriented therapy and oxygen therapy, complications and hospitalization rates due to COVID-19.
The retrospective design of our study and the relatively low proportion of fully vaccinated subjects by data cut-off underscores the preliminary nature of our findings. Baseline characteristics were comparable across exposed and unexposed groups, lending credence to the view that the improvement in outcomes observed in vaccinated patients may be truly due to SARS-Cov-2 immunity. While primary analyses confirmed our hypothesis, IPTW models yielded non-statistically significant trends towards improvement of outcomes, as a likely result of a largely unbalanced sample size of patients’ subgrouping.
In a clinical setting that is derived from solid level I evidence and within the limitation of a registry study, our findings provide a meaningful contribution to the growing body of knowledge demonstrating the ability of SARS-CoV-2 vaccines as a measure to reduce adverse outcomes from the disease. Compelling evidence suggests how SARS-CoV-2 vaccination induces effective immune responses across different tumour types and irrespective of recent exposure to diverse anti-cancer therapies [[6], [7], [8]]. However, protective immunity is not universal in patients with cancer, where evidence of diminished immunogenicity has been shown in patients with haematological malignancies and after treatment with CD20 inhibitors [7,9,10]: a finding that may explain the heterogeneity of results reported by the CCC19, which included 35% of patients with haematological malignancies among those fully vaccinated [25], compared to 12% of our cohort.
Whilst the retrospective nature of our study does not allow us to conclude that the association between full vaccination and improved outcome is truly causal, documentation of CFRs of 5.5% in this subgroup is highly important as it provides an important new benchmark in a more contemporary and clinically relevant estimate of lethality from COVID-19 in the post-vaccinal era [14], as it impressively diverges from the >30% estimates reported during the initial phase of the pandemic across different registries [17,26,27].
Baseline demographics and oncological characteristics, all well balanced across groups, are an unlikely source of bias. The uneven distribution of the country of origin and SACT exposure can be easily explained by country-specific differences in the delivery of immunization campaigns, where vaccines were often offered alongside SACT in some centres.
The evidence that vaccine response may decrease over time [19,20] mirrors our descriptive analysis of vaccination timing, which suggests that the protection provided by SARS-CoV-2 vaccines declines beyond the 6-months landmark. These findings may also be related to the emergence of new variants of concern, such as the B.1.617.2 (delta), which has a higher transmissibility than previous strains and proved to cause a higher rate of breakthrough infections [28,29]. Taken together, these results support the need of prioritizing frail patients for booster doses, to sustain vaccine’s immunogenicity over time [30].
Another important question that has not been addressed in any previous study in oncological patients is whether vaccinal immunity may affect the risk of developing COVID-19 sequelae. In a recent analysis of the OnCovid registry, we demonstrated that COVID-19 is detrimental to patients’ outcome even beyond the acute phase of the disease. COVID-19 sequalae can affect ∼15% of patients, with long-term consequences for their continuity of care and survival [5]. From this perspective, the additional analysis performed among COVID-19 survivors who underwent a clinical reassessment at participating centres suggests that the protection provided by vaccines extends beyond the acute phase, as supported by the reduced incidence of sequelae in fully vaccinated patients.
Our data collection relies on unplanned time intervals to capture vaccine administration, which could lead to determination bias. Moreover, breakthrough infections could have been asymptomatic with an associated risk of diminished reporting and underestimation of the effect. However, hard end points like mortality/severe disease were unlikely affected.
Notably, we did not discuss booster/third doses, as by the data lock only a small minority of breakthrough infections among patients who received it had been reported. However, considering the recently emerged B.1.1.529 (omicron) variant, its different immunogenic profile, and the wave of infections recorded in Europe between December 2021 and January 2022 [31,32], a dedicated update is currently ongoing, to produce reliable dedicated evidence.
Although preliminary, this study provides novel evidence supporting the clinical efficacy against COVID-19 morbidity, mortality and sequelae of SARS-CoV-2 vaccines in a large real-world population of patients with cancer. Universal SARS-CoV-2 vaccination should remain a goal in the management of patients with cancer during and beyond the COVID-19 pandemic.
Role of the funding source
OnCovid is sponsored by Imperial College London and received direct project funding and infrastructural support from the NIHR Imperial Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. Neither the sponsor nor the funders of the study had any role in study design, data collection, data analysis, data interpretation or writing of the report. All authors had access to all the data reported in the study.
Ethical approval and consent to participate
OnCovid was granted central approval by the United Kingdom Health Research Authority (20/HRA/1608) and by the corresponding research ethics committees at each participating institution. Full waiver of consent due to the retrospective nature of the study was granted by the UK HRA in accordance with UK law.
Authors’ contributions
All authors contributed to the publication according to the ICMJE guidelines for the authorship. All authors read and approved the submitted version of the manuscript (and any substantially modified version that involves the author's contribution to the study). Each author has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved and the resolution documented in the literature.
Consent for publication
Informed consent was waived by competent authorities due to anonymized nature of patient data and retrospective design of the study.
Availability of data and material
Study data made available upon reasonable request.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: As corresponding author of the abovementioned manuscript, I declare on behalf of my co-authors the following conflict of interests: David J Pinato received lecture fees from ViiV Healthcare, Bayer Healthcare, BMS, Roche, EISAI, Falk Foundation, travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, DaVolterra and Astra Zeneca; research funding (to institution) from MSD and BMS.Aleix Prat has declared personal honoraria from Pfizer, Roche, MSD Oncology, Eli Lilly, and Daiichi Sankyo; travel, accommodations, and expenses paid by Daiichi Sankyo; research funding from Roche and Novartis; and consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb.Matteo Lambertini acted as consultant for Roche, Novartis, Lilly, AstraZeneca, Exact Sciences, MSD, Pfizer, Seagen and received speaker honoraria from Roche, Novartis, Lilly, Pfizer, Takeda, Ipsen and Sandoz outside the submitted work.Joan Brunet has declared consulting/advisory role for MSD and Astra Zeneca.Alessandra Gennari has declared consulting/advisory role for Roche, MSD, Eli Lilly, Pierre Fabre, EISAI, and Daichii Sankyo; speakers bureau for Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, Astra Zeneca, Celgene, and Daichii Sankyo; research funds: EISAI, Eli Lilly, and Roche. CMV has received travel grants and other honoraria from BMS, MSD, Novartis and Roche.Gianluca Gaidano has declared consulting/advisory role for Janssen, Abbvie, Astra-Zeneca and BeiGene, and speaker fees from Janssen and Abbvie.Lorenza Rimassa received consulting fees from Taiho Oncology, Servier, Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi; travel expenses from Ipsen; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks.Joseph Tabernero reported consulting fees from Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho, Tessa Therapeutics and TheraMyc. He also reported speaker's fees from Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER). He also declared institutional research support from Amgen Inc, Array Biopharma Inc, AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc, Spanish Association Against Cancer Scientific Foundation and Cancer Research UK.Alessio Cortellini received consulting fees from MSD, BMS, AstraZeneca, Roche; speakers' fee from AstraZeneca, MSD, Novartis and Eisai.All remaining authors have declared no conflicts of interest.London, April 18th, 2022.
Acknowledgements
OnCovid received direct project funding and infrastructural support by the NIHR Imperial Biomedical Research Centre (BRC).
Alessio Cortellini is supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC).
David J Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and from the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG Grant ID 25697) and acknowledges support by the NIHR Imperial Biomedical Research Centre (BRC), the Imperial Experimental Cancer Medicine Centre (ECMC) and the Imperial College Tissue Bank.
G. Gaidano is supported by the AIRC 5 × 1000 Grant, No. 21198, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy. A. Gennari is supported by the AIRC IG Grant, No. 14230, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy. A. Gennari and G. Gaidano from the University of Piemonte Orientale (Novara, Italy) acknowledge support from the UPO Aging Project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2022.04.036.
Contributor Information
OnCovid study group:
David J. Pinato, Joanne S. Evans, Judith Swallow, Alessio Cortellini, Georgina Hanbury, Chris Chung, Meera Patel, Gino Dettorre, Diego Ottaviani, Amani Chowdhury, Alvin JX. Lee, Christopher CT. Sng, Tamara Yu, Marianne Shawe-Taylor, Hamish DC. Bain, Alasdair Sinclair, Lee Cooper, Lucy Rogers, Katherine Belessiotis, Cian Murphy, Samira Bawany, Saira Khalique, Ramis Andaleeb, Mark Bower, Alessia Dalla Pria, Rachel Sharkey, Thomas Newsom-Davis, Saorise Dolly, Ailsa Sita-Lumsde, Eleanor Apthorp, Eleanor Jones, Mieke Van Hemelrijck, Charlotte Moss, Beth Russell, Eleanor Apthorp, Nikolaos Diamantis, Uma Mukherjee, Sarah Townsend, Amanda Jackson, Angela Loizidou, Martine Piccart, Aleix Prat, Claudia A. Cruz, Roxana Reyes, Elia Segui, Javier Marco-Hernández, Margarita Viladot, Josep Tabernero, Juan Aguilar-Company, Isabel Ruiz-Camps, Laura Fox, David Garcia Illescas, Nadia Saoudi, Oriol Mirallas, Elisa Roldán, Joan Brunet, MCarmen Carmona Garcia, Robert Fort-Culillas, Raquel Liñan, Nadia Harbeck, Rachel Wuerstlein, Franziska Henze, Sven Mahner, Ricard Mesia, Eudald Felip, Andrea Plaja, Marc Cucurull, Ramon Salazar, Anna Sureda, Clara Maluquer, Alessandra Gennari, Federica Biello, Francesca D’Avanzo, Gianluca Gaidano, Riccardo Bruna, Andrea Patriarca, Daniela Ferrante, Lorenza Scotti, Marco Krengly, Paolo Pedrazzoli, Gianpiero Rizzo, Alexia Bertuzzi, Sabrina Rossi, Andrea Marrari, Armando Santoro, Lorenza Rimassa, Federica Grosso, Vittorio Fusco, Sara Delfanti, Antonio Maconi, Marta Betti, Bruno Vincenzi, Giuseppe Tonini, Alberto Zambelli, Carlo Tondini, Vittoria Fotia, Lorenzo Chiudinelli, Michela Franchi, Michela Libertini, Rossella Bertulli, Salvatore Provenzano, Daniele Generali, Salvatore Grisanti, Alice Baggi, Valeria Tovazzi, Corrado Ficorella, Giampiero Porzio, Alessandro Parisi, Paola Queirolo, Maristella Saponara, Raffaele Giusti, Marco Filetti, Francesca Mazzoni, Federica Zoratto, Marco Tucci, Rossana Berardi, Luca Cantini, Francesco Paoloni, Annalisa Guida, Sergio Bracarda, Clara Martinez-Vila, Maria Iglesias, Ana Sanchez de Torre, Matteo Lambertini, Marta Perachino, Fanny Pommeret, and Emeline Colomba
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Giannakoulis V.G., Papoutsi E., Siempos Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagliamento M., Agostinetto E., Bruzzone M., et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103365. doi: 10.1016/j.critrevonc.2021.103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwood E., Swanton C. Consequences of COVID-19 for cancer care - a CRUK perspective. Nat Rev Clin Oncol. 2021;18(1):3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D., Neal R.D., Duffy S.R.G., Scott S.E., Whitaker K.L., Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21(6):748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinato D.J., Tabernero J., Bower M., et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021;22(12):1669–1680. doi: 10.1016/S1470-2045(21)00573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oosting S.F., van der Veldt A.A.M., GeurtsvanKessel C.H., et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22(12):1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fendler A., Shepherd S.T.C., Au L., et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nature Cancer. 2021;2(12):1305–1320. doi: 10.1038/s43018-021-00274-w. [DOI] [PubMed] [Google Scholar]
- 8.Fendler A., Au L., Shepherd S.T.C., et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Nature Cancer. 2021;2(12):1321–1337. doi: 10.1038/s43018-021-00275-9. [DOI] [PubMed] [Google Scholar]
- 9.Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., et al. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addeo A., Shah P.K., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091–1098. doi: 10.1016/j.ccell.2021.06.009. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J., Stoesser N., Matthews P.C., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nature Microbiology. 2021;6(9):1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas S.J., Perez J.L., Lockhart S.P., et al. 1558O COVID-19 vaccine in participants (ptcpts) with cancer: subgroup analysis of efficacy/safety from a global phase III randomized trial of the BNT162b2 (tozinameran) mRNA vaccine. Ann Oncol. 2021;32:S1129. [Google Scholar]
- 13.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OnCovid Study G., Pinato D.J., Patel M., et al. Time-dependent COVID-19 mortality in patients with cancer: an updated analysis of the OnCovid registry. JAMA Oncol. 2022;8(1):114–122. doi: 10.1001/jamaoncol.2021.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinato D.J., Lee A.J.X., Biello F., et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12(7) doi: 10.3390/cancers12071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinato D.J., Scotti L., Gennari A., et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: a European study. Eur J Cancer. 2021;150:190–202. doi: 10.1016/j.ejca.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinato D.J., Zambelli A., Aguilar-Company J., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;31(10 (10)):1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettorre G.M., Dolly S., Loizidou A., et al. Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score. J Immunother Cancer. 2021;9(3) doi: 10.1136/jitc-2020-002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widge A.T., Rouphael N.G., Jackson L.A., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2020;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naaber P., Tserel L., Kangro K., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt A.L., Labaki C., Hsu C.Y., et al. COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33(3):340–346. doi: 10.1016/j.annonc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee L.Y., Cazier J.B., Angelis V., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 30.Naranbhai V., St. Denis K.J., Lam E.C., et al. Neutralization breadth of SARS-CoV-2 viral variants following primary series and booster SARS-CoV-2 vaccines in patients with cancer. Cancer Cell. 2022;40(1):103–108. doi: 10.1016/j.ccell.2021.12.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann M., Krüger N., Schulz S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–456. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araf Y., Akter F., Tang Y.D., Fatemi R., Parvez M.S.A., Zheng C., et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022 May;94(5):1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data made available upon reasonable request.