Abstract
Purpose
To assess the influence of contemporary contact lens (CL) materials on human coronavirus attachment and the influence of a rub and rinse step to remove these viruses.
Methods
The binding rates of HCoV-229E and HCoV-OC43 to eight soft CL materials and four rigid gas permeable materials were analyzed. The impact of a rub and rinse step to remove these viruses from all materials was examined. The efficacy of Biotrue (Bausch & Lomb), OPTI-FREE Puremoist (Alcon), Clear Care (Alcon) and cleadew (Ophtecs) to remove virus contamination from two representative soft lens materials (etafilcon A and lotrafilcon B) was also determined.
Results
Approximately 102 to 103 infectious viral particles were recovered from each CL material. Although some materials were more prone to coronavirus adhesion, contamination of both viral types was reduced to below the limit of quantification (LQ) from all materials using a simple saline rinse step. Exposure to Clear Care and cleadew reduced the number of infectious viral particles from both etafilcon A and lotrafilcon B to below the LQ, while for Biotrue and OPTI-FREE Puremoist, infectious viral particles were reduced to below the LQ only when additional rub and rinse steps were included.
Conclusion
Human coronavirus contamination can be easily removed from CL surfaces. Although CL care products containing hydrogen peroxide and povidone-iodine efficiently removed virus contamination from CL surfaces without the need for a rub and rinse step, a full regimen including rub and rinse steps is crucial when using CL care products based on non-oxidative systems.
Keywords: Contact lens materials, Soft contact lenses, Hydrogel, Silicone hydrogel, Rigid gas permeable, Human coronavirus, HCoV-229E, HCoV-OC43
1. Introduction
The on-going coronavirus disease 2019 (COVID-19) pandemic has raised global attention with respect to viral transmission and the importance of disinfection and viral load reduction. Even though ocular complications are not a common manifestation of coronavirus infections in humans [1], [2], [3], various studies have suggested that ocular exposure may represent a potential route of entry for SARS‐CoV‐2 [4], [5], [6], [7], [8], [9]. Therefore, considering that viruses can be transferred by physical contact by the hands and fingers [10], [11], [12], it has been suggested that wearers of contact lenses (CL) are more at risk of developing COVID-19 during lens application and removal [13].
Both rigid gas permeable (RGP) and soft CL (SCL) remain a major means of refractive correction, and it is estimated that 175 million people worldwide wear CL [14], [15]. Since the development of the first SCL in the early 1960 s [16] and their commercialization in the early 1970 s [17], this market has grown exponentially, and significant technological advancements have occurred. The original polyHEMA-based conventional hydrogel (CH) SCL materials were significantly more comfortable than RGP CLs and achieved rapid growth and market share. To increase oxygen transmissibility, silicone hydrogel (SH) materials were commercialized in the late 1990 s, and have become the dominant CL material used today [14]. Although SH CLs have the highest oxygen permeability, silicone-based materials are inherently hydrophobic and various strategies have been used to decrease this hydrophobicity, such as the use of different surface treatments or incorporation of a wetting agent [18]. Nowadays, there are broadly three generations of SCL materials and over 160 different brands are available, including daily disposable (DD) and reusable options that are worn for various periods of time prior to their replacement [19].
Reusable CLs must be disinfected daily using an approved CL care product prior to reinsertion. To-date, little data exists concerning the ability of contemporary CL care products to inactivate viruses, particularly coronavirus. A recent publication and a conference abstract, using coronaviruses as a model, both demonstrate that oxidative disinfection systems have significant virucidal activity, while non-oxidative systems have minimal ability to inactivate these viruses [20], [21]. The majority of multipurpose systems (MPS) are licensed for use with a rub and rinse step prior to overnight disinfection. This rub and rinse step is highly effective at removing bacterial, fungal and amoebal bioburden from reusable CL [22], [23], [24], [25]. However, data concerning the impact of a rub and rinse step to remove coronaviruses from CLs is scarce. Currently, only one published study has evaluated the efficacy of a rub and rinse regimen to reduce the numbers of murine coronaviruses attached to etafilcon A, a CH material, and this study only reported the results from one MPS [20]. Moreover, although differences in bacteria, fungi and Acanthamoeba adhesion among different soft CL materials have been reported [22], [23], [26], [27], [28], [29], there is no data comparing the attachment of viruses to a wide variety of CL materials.
In view of this lack of data and the potential conjunctival transmission of SARS-CoV‑2 from CL materials, the main purpose of this study was to assess the influence of a variety of contemporary CL materials on human coronavirus attachment and the influence of a rub and rinse step to remove these viruses from the CL surfaces. Additionally, the efficacy of Biotrue (Bausch & Lomb), OPTI-FREE Puremoist (Alcon), Clear Care (Alcon) and cleadew (Ophtecs) to remove viral contamination from two representative soft lens materials (etafilcon A and lotrafilcon B) was also determined.
2. Materials and methods
2.1. Contact lenses
Eight unworn SCL materials and four RGP CL materials were included in this study (Table 1 ). Among the SCL materials, both SH and CH materials were examined. The RGP materials consisted of two base materials with and without a Tangible® Hydra-PEG® hydrophilic coating (Tangible Science, Redwood City, CA). The choice of materials was made to reflect a broad range of water contents, oxygen transmissibility, surface and bulk properties from contemporary contact lens materials.
Table 1.
Contact lenses evaluated in this study.
| CL material | Contact Lenses (trade name) | Manufacturer | USAN* | Surface treatment | Water content (%) |
|---|---|---|---|---|---|
| Hydrogel | Acuvue 2 | Johnson&Johnson | Etafilcon A | No | 58 |
| Proclear 1 Day | CooperVision | Omafilcon A | No | 62 | |
| Silicone Hydrogel | Acuvue Oasys | Johnson&Johnson | Senofilcon A | Internal wetting agent | 38 |
| Biofinity | CooperVision | Comfilcon A | No | 48 | |
| Clariti 1 day | CooperVision | Somofilcon A | WetLoc technology | 56 | |
| Air Optix Aqua | Alcon | Lotrafilcon B | Plasma coating | 33 | |
| Dailies Total 1 | Alcon | Delefilcon A | Water gradient technology | 33 | |
| PureVision 2 | Bausch + Lomb | Balafilcon A | Plasma oxidation | 36 |
| CL material | Contact Lenses (trade name) | Manufacturer | USAN* |
|---|---|---|---|
| Rigid Gas Permeable | Optimum Infinite | Blanchard | Tisilfocon A |
| Optimum Infinite with Hydra-PEG | Blanchard | Tisilfocon A | |
| Acuity 200 | Blanchard | Fluoroxyfocon A | |
| Acuity 200 with Hydra-PEG | Blanchard | Fluoroxyfocon A |
*USAN: United States Adopted Name.
2.2. Virus and cell lines
To-date, no accepted protocols to determine the binding of virus strains to CL materials exist. Thus, several preliminary experimental methods were examined to optimize this step. During the development of the methodologies used in this study, an insect virus vector (baculovirus vector) expressing a red fluorescent protein was used due to its ease of production, quantification, and experimentation with this vector (see Supplementary Material). Following this optimization step, using the established methodologies, two seasonal human coronaviruses, HCoV-229E and HCoV-OC43 (Risk Group 2 pathogens) were used as suitable surrogates for SARS-CoV-2 (Risk Group 3 pathogen). HCoV‐229E is an alphacoronavirus, while HCoV-OC43 is a betacoronavirus (like SARS-CoV-2). Both HCoV-229E and HCoV-OC43 cause mild upper respiratory tract infections (the common cold), while SARS-CoV-2 causes severe lower respiratory tract infection. Although there are clear differences in the pathogenicity of these viruses, they are in the same virus family, have very similar structures and are human respiratory pathogens, making them ideal surrogates for SARS-CoV-2 [30], [31], [32], [33], [34].
HCoV-229E (ATCC VR-740) and HCoV-OC43 (ATCC VR-1558) were propagated in MRC-5 (human lung epithelial cell; ATCC CCL-171) and HCT-8 (human ileocecal adenocarcinoma cell; ATCC CCL-244) cells, respectively. MRC-5 cells were maintained in Eagle's Minimum Essential Medium (EMEM) (Wisent BioProducts, Saint-Jean-Baptiste, QC, Canada) supplemented with 10% Fetal Bovine Serum (FBS), while HCT-8 cells were maintained in RPMI (Wisent BioProducts, Saint-Jean-Baptiste, QC, Canada) supplemented with 10% FBS. Both MRC-5 and HCT-8 cells were maintained in a 5% CO2 atmosphere at 37 °C and 100% humidity.
2.3. End-Point dilution assay
The end-point dilution assay was used to measure the viral load of HCoV-229E and HCoV-OC43 using MRC-5 and HCT-8 cells, respectively. It was conducted as per the Tissue Culture Infectious Dose 50 (TCID50) assay [35] and analyzed using the Most Probable Number calculator. The limit of quantification for all tested viruses was 7.8 MPN/mL.
MRC-5 and HCT-8 cells were seeded into a 96 well plate at a density of 4.5 × 104 cells/mL and 6.7 × 104 cells/mL, respectively, using their appropriate medium containing 10% FBS. Plates were incubated at 37 °C and 5% CO2 until cell monolayers reached 80–90% confluence, when their respective medium was replaced by fresh medium containing 2% FBS. Serial 10-fold dilutions of samples were prepared in cell culture medium and added to 96 well plates seeded with cells (with each sample dilution being added in each row that contains 12 wells). The plates were incubated at 33 °C and 5% CO2 for 6 or 12 days for HCoV-229E and HCoV-OC43, respectively. The wells were examined using light microscopy (Zeiss Axiovert 40C) and scored as infected or non-infected based on cytopathic effects (CPE).
2.4. Effect of rub and rinse on human coronavirus HCoV-229E and HCoV-OC43
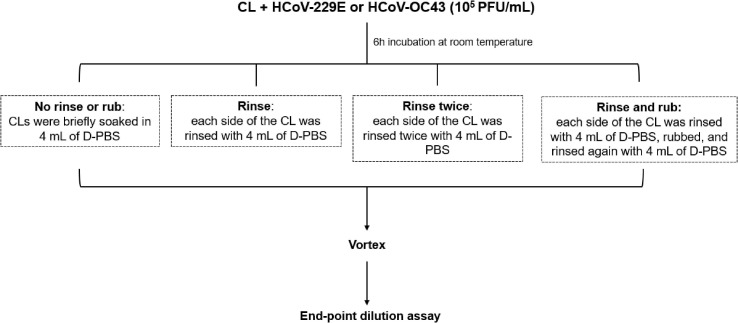
The human coronavirus binding rates to CL were tested in triplicate using three of each CL listed in Table 1. The day before each experiment, all CL were removed from their packaging and soaked in 4 mL of D-PBS overnight.
Four sets of each CL material (each set composed of three lenses) were incubated individually in 4 mL of HCoV-229E or HCoV-OC43 suspension (105 MPN/mL) for 6 h at room temperature to allow the virus to adhere to the CL surface. As a control, the virus suspension was incubated in the same condition, but with no CL. Thereafter, each set of CLs was submitted to a different treatment (Fig. 1 ). In the first condition, termed “no rub and rinse”, CLs were removed from the virus suspension and briefly soaked in 4 mL of D-PBS. In the second condition, termed “rinse”, each side of the CL was rinsed with 4 mL of D-PBS. No rub was performed. In the third condition, termed “rub and rinse”, each side of the CL was rinsed with 4 mL of D-PBS and rubbed in a circular motion (5x) using a nitrile-gloved 3D-printed “finger” of physiological proportions. In the fourth and final condition, each side of the CL was rinsed with 4 mL of D-PBS, rubbed as described above and rinsed again with 4 mL of D-PBS. After these treatments, CLs were individually transferred to a tube containing 1 mL of D-PBS, which was vigorously vortexed for 30 s to dislodge any viral particle adhered to the CL surface. Then, infectious viral particles were quantified by end-point dilution assay, as described in section 2.3.
Fig. 1.
Protocols used to test the impact of rub and rinse steps on coronaviruses removal from CL surfaces. Experiments were performed in triplicate.
2.5. Efficacy of CL care products to remove HCoV-229E from CL surfaces
Two representative CL materials were selected to test the relative efficacy of four CL care products on HCoV-229E removal. Etafilcon A and lotrafilcon A were selected because the first one is a conventional hydrogel with a negative charge and the second one is a silicone hydrogel with a neutral charge. These experiments were performed in triplicate using three CL of each material tested. CLs were removed from their blister packs and soaked in D-PBS overnight. CLs were individually soaked in 4 mL of HCoV-229E suspension (105 MPN/mL) and incubated for 6 h at room temperature. After the incubation period, each CL was disinfected using two contemporary, commercial CL care products based on non-oxidative disinfecting systems (Biotrue and OPTI-FREE Puremoist) and two based on oxidative disinfecting systems (Clear Care and cleadew). These solutions were chosen based upon their broad variety of disinfecting agents and wide commercial usage. In the first set of CLs, the CL care products were used as recommended by the manufacturers (including rub and rinse steps) and incubated for the appropriate disinfection time (Table 2 ). Moreover, the CL case supplied with Clear Care and the tablets supplied with cleadew were used according to the manufacturer’s instructions, to ensure appropriate neutralization occurred in the appropriate case. In the second set of CLs, rub and rinse steps were not included. After the incubation period with HCoV-229E, CLs were incubated with 4 mL of Biotrue or OPTI-FREE Puremoist for 4 and 6 hrs at room temperature, respectively. Then, CLs were individually vortexed in 1 mL of D-PBS and infectious viral particles were determined as described in section 2.3.
Table 2.
Contact lens care products included in this study.
| Contact lens care product | Manufacturer | Disinfectant agents | Regimen recommended by manufacturer |
|---|---|---|---|
| Biotrue | Bausch & Lomb, Rochester, NY | Polyaminopropyl biguanide 0.00013% and polyquaternium 0.0001% | 1- Place at least 3 drops of Biotrue on each side of CL and rub for 20 s 2- Rinse each side of CL for 5 s 3- Place the CL in the case with fresh Biotrue and soak for at least 4 hrs. |
|
OPTI-FREE Puremoist |
Alcon, Fort Worth, TX |
Polyquad (Polyquaternium-1) 0.001% and Aldox (Myristamidopropyl Dimethylamine) | 1- Wet each side of the CL with OPTI-FREE and rub the lens for 20 s 2- Rinse each side for 10 s 3- Fill the case with fresh OPTI-FREE and store the lens at least 6 hrs or overnight. |
| Clear Care | Alcon, Fort Worth, TX |
3% hydrogen peroxide | 1- Put CL into the special Clear Care case and rinse with Clear Care solution for at least 5 s 2- Fill the case to the line with fresh Clear Care solution and disinfect 6 hrs or overnight before use. |
| cleadew | Ophtecs, Kobe, Japan |
0.05% Povidone-iodine | 1- Add a tablet of cleadew into the case and fill with cleadew solution 2- Soak the CL at least 4 hrs 3- Rinse the CLs with cleadew before wearing them. |
2.6. Statistical analysis
Statistical analysis was conducted using GraphPad Prism v 9.2.0. Infectious virus particles are expressed as mean and standard deviation. The difference in mean infectious virus particles between test conditions was tested using unpaired t-test or one-way ANOVA with Tukey’s post-hoc multiple comparisons test. The values of infectious virus particles below the limit of quantification were assigned a ‘0′. A p-value of 0.05 or below was considered statistically significant.
3. Results
3.1. Effect of rub and rinse on human coronavirus HCoV-229E and HcoV-OC43
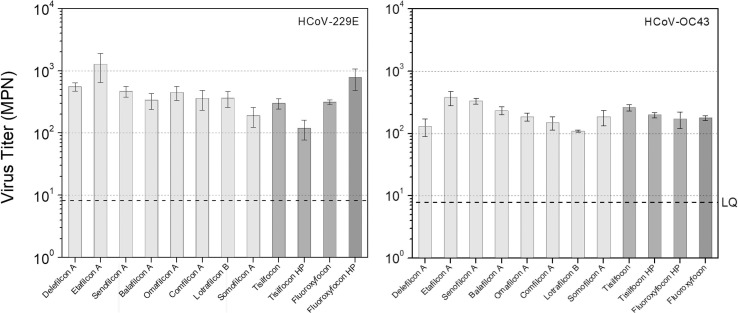
For both HCoV-229E and HCoV-OC43, approximately 102 to 103 infectious viral particles were recovered from each CL after “no rub or rinse” treatment. As observed in Fig. 2 , HCoV-229E exhibited higher adherence to the CL than HCoV-OC43.
Fig. 2.
Infectious viral particles/lens recovered from CLs after “no rub or rinse” treatment. Left panel: HCoV-229E. Right panel: HCoV-OC43. Averages and standard deviations were calculated using results of triplicate samples (3CL). As a control, virus suspension was incubated in the same conditions for each experiment, but with no CL. The titre of control was 8.79 × 104 (±2.27 × 104) MPN/mL and 8.72 x104 MPN/mL (±2.50 × 104) for HCoV-229E and HCoV-OC43, respectively. LQ: limit of quantification.
For HCoV-229E, the number of infectious virus particles recovered from fluoroxyfocon A with Hydra-PEG was significantly lower than those recovered from all other CL materials (all p values < 0.01). No statistically significant difference in the number of infectious virus particles recovered from CL surfaces was observed between all other tested CL materials after “no rub or rinse” treatment (all p values > 0.05).
For HCoV-OC43, after “no rub or rinse” treatment, the number of infectious virus particles recovered from etafilcon A was significantly higher than those recovered from other SCL, including omafilcon A, comfilcon A, somofilcon A, lotrafilcon B, and delefilcon A (all p values < 0.01), as well as from various RGP materials, including fluoroxyfocon A (p < 0.01), fluoroxyfocon A with Hydra-PEG (p < 0.01) and tisilfocon A with Hydra-PEG (p = 0.02). The number of infectious virus particles recovered from senofilcon A was also significantly higher than those recovered from comfilcon A (p = 0.01), lotrafilcon B (p < 0.01), delefilcon A (p < 0.01) and fluoroxyfocon A (p = 0.04).
No detectable virus was recovered when CLs were submitted to “rinse”, “rinse twice” and “rub and rinse” treatments, indicating that the virus titre was reduced to below the limit of quantification.
3.2. Efficacy of CL care products to remove HCoV-229E and HCoV-OC43 from SCL surface
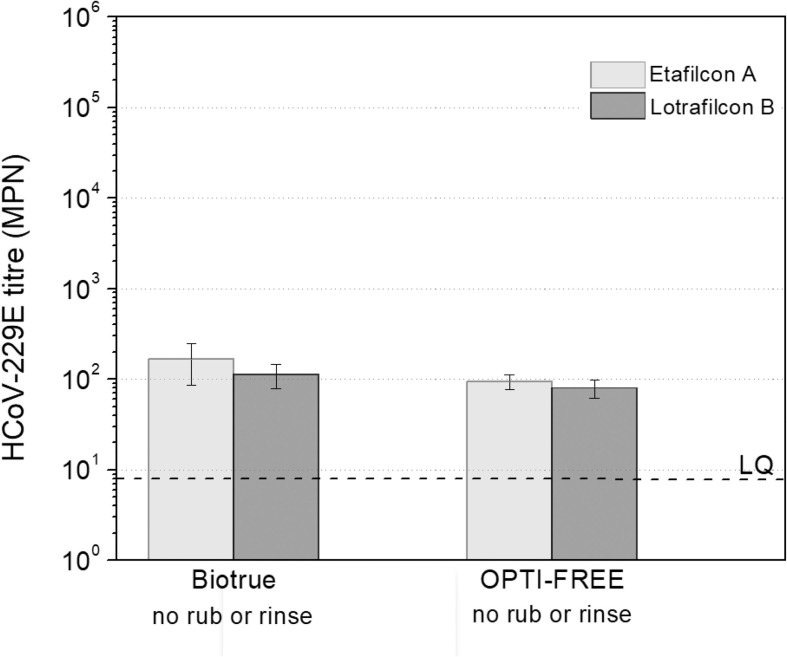
Given that a simple rinse step alone removed both virus types from all materials, only two representative soft lens materials (one CH and one SH) underwent exposure to the care systems to determine their ability to remove the bound virus particles. The CL care products based on an oxidative disinfection system (Clear Care and cleadew) were able to reduce the number of infectious viral particles that adhered to both etafilcon A (CH) and lotrafilcon B (SH) to below the limit of quantification. However, the non-oxidative disinfection systems (Biotrue and OPTI-FREE Puremoist) were able to reduce the number of infectious viral particles for both etafilcon A and lotrafilcon B to below the limit of quantification only when additional rub and rinse steps were included (Fig. 3 ). Averages of 1.12 × 102 (SD = 3.36 × 101) and 8.03 × 101 (SD = 1.84 × 101) infectious viral particles/lens were recovered from lotrafilcon B when CLs were incubated in Biotrue and OPTI-FREE (p = 0.30), respectively, without rubbing and rinsing. Similarly, 1.68 × 102 (SD = 8.23 × 101) and 9.45 × 101 (SD = 1.81 × 101) infectious viral particles/lens were recovered from etafilcon A when CLs were incubated in Biotrue and OPTI-FREE (p = 0.29), respectively, without rubbing and rinsing.
Fig. 3.
Infectious viral particles/lens recovered from etafilcon A and lotrafilcon B after disinfection with Biotrue and OPTI-FREE Puremoist with no rub and rinse steps. Averages and standard deviations were calculated using results of triplicate samples (3CL). Biotrue and OPTI-FREE Puremoist were able to reduce the number of infectious viral particles to below the limit of quantification only when rub and rinse steps were performed for both etafilcon A and lotrafilcon B. As a control, virus suspension was incubated in the same conditions, but with no CL. The titre of control was 9.27 × 104 (±4.88 × 103) MPN/mL. LQ: limit of quantification.
4. Discussion
Prior to experimentation with HCoV-229E and HCoV-OC43, it was important to develop an appropriate methodology that ensured the lenses would bind the viral particles, as no published, standard methods for this exist. Moreover, important factors that could influence the virus attachment, such as incubation time and volume of incubation solution were analyzed. It was also important to determine an appropriate rub/rinse methodology to remove the viral particles that were bound to the lenses and also determine if the vortex step efficiently recovered virus attached from CLs. For the development of methodologies, a surrogate with the lowest risk level is preferred. In this study, a modified insect virus genetically engineered to carry a gene for a fluorescent protein was chosen for development activities. The insect virus in question is part of the group of enveloped viruses known as baculoviruses. They can infect a limited number of closely related insect species and are not capable of replicating within mammalian cells, therefore, they are considered Risk Group 1. Moreover, they can be amplified to high concentration (around 108 MPN/mL) and be easily identified through the expression of a fluorescent protein. These points made them an ideal virus for the initial development methods. Therefore, various experimental conditions using baculovirus and selected types of CLs were analyzed, which are described in the Supplementary Material section. The results indicated that both CH (etafilcon A) and SH (delefilcon A) were similarly prone to baculovirus adhesion and an extended incubation period did not enhance the number of infectious viral particles recovered from the CL material (Supplementary Material – Fig. S1). Moreover, comparable numbers of infectious viral particles were recovered from the CL surfaces even when different volumes of virus suspension were used (Supplementary Material – Fig. S2). Additionally, virus was effectively recovered through vortexing the CLs in D-PBS (Supplementary Material – Fig. S4 and Table S1). After completion of these preliminary experiments, the developed methodology was then used to evaluate the attachment of both HCoV-229E and HCoV-OC43 to 12 different CL materials, including both soft and RGP materials, and to investigate the impact of a rub and rinse step on viral removal from CL surfaces.
After incubation of CLs with baculovirus and either HCoV-229E or HCoV-OC43, approximately 104 to 105 (Supplementary Material – Fig. S4) and 102 to 103 (Fig. 2) infectious viral particles, respectively, were recovered from each CL submitted to “no rub or rinse” treatment. The “no rub or rinse” treatment assesses the amount of virus that is associated with the lens as a result of incubating it in a virus containing solution. The difference in the quantity associated with the lenses can be explained by the initial titre of the virus solutions. The baculovirus suspension (107 MPN/mL) was two orders of magnitude higher than the human coronaviruses suspensions (105 MPN/mL). As mentioned previously, baculovirus is easily amplified to high concentrations, and all experiments were performed using the highest titre amplified for each virus. Therefore, the number of viruses attached to the CLs surface is almost certainly related to the initial concentration of the virus suspension the materials were exposed to.
Regarding the CL materials tested, the number of infectious virus particles recovered from all 12 materials after “no rub or rinse” treatment was relatively consistent for both HCoV-229E and HCoV-OC43. Although CH and SH were similarly prone to HCoV-229E adhesion, this virus showed a significantly lower affinity for fluoroxyfocon A with Hydra-PEG, one of the RGP materials studied. Moreover, HCoV-OC43 showed a significantly higher affinity for etafilcon A (CH) compared with omafilcon A (also a CH), and various SH (comfilcon A, somofilcon A, lotrafilcon B, and delefilcon A) and RGP materials (fluoroxyfocon A, fluoroxyfocon A with Hydra-PEG and tisilfocon A with Hydra-PEG). Additionally, HCoV-OC43 showed a significantly higher affinity for senofilcon A than other SH (comfilcon A, lotrafilcon B, delefilcon A) and RGP materials (fluoroxyfocon A). Some studies have shown that the magnitude of microorganisms’ adhesion to CLs varies according to the lens material under test. Adhesion of bacteria and Acanthamoeba to unworn CH has been shown to be significantly lower when compared to adhesion to SH lenses [22], [26], [27], [28], [29]. Moreover, differences in microorganisms’ adhesion among different SH have also been reported. Acanthamoeba and bacteria exhibited a significantly greater affinity for lotrafilcon A and B compared with galyfilcon A lenses [22], [36]. Bacteria, fungi and Acanthamoeba exhibited greater attachment to lotrafilcon B than senofilcon A lenses [23]. Consequently, contamination with the test organisms were more easily reduced from the surface of galyfilcon A and senofilcon A than from lotrafilcon B [22], [23].
However, in this study, although some materials were more prone to coronaviruses adhesion, viral contamination was easily removed from all the CL materials tested. For baculovirus, a simple rinse step with D-PBS significantly reduced the number of infectious viral particles attached to both etafilcon A (CH) and delefilcon A (SH), while a combined rub and rinse step was needed to eliminate viral particles to below the limit of quantification. However, for human coronaviruses, a single rinse step with D-PBS reduced viral particles to below the limit of quantification for all tested CLs materials, including CH, SH and RGP. This difference among the tested viruses could be due to the titre of the virus suspension: as the concentration of virus increases, more disinfection steps are needed to reduce the CL contamination. Moreover, these results indicate that viruses bind loosely to the CL materials and, when rubbed or rinsed with D-PBS, the viruses can be easily removed or detached.
As contaminations with both HCoV-229E and HCoV-OC43 were similarly reduced on all 12 tested CLs surfaces using a simple rinse regimen with D-PBS, only two CL materials (one CH and one SH) were selected to test the efficacy of the four CL care products on HCoV-229E removal. Those products based on an oxidative disinfection system, Clear Care and cleadew, were able to reduce the number of infectious viral particles adherent to both etafilcon A (CH) and lotrafilcon A (SH) to below the limit of quantification. These results are in accordance with a previous study from our group, in which oxidative CL disinfection systems showed significant virucidal activity against both HCoV-229E and HCoV-OC43 [21].
Although non-oxidative disinfection systems did not exhibit significant efficacy against viruses in previous standalone assays [20], [21], [37], some studies have demonstrated the impact of a rub and rinse step in significantly reducing the numbers of bound viral particles. In this study, Biotrue and OPTI-FREE were able to reduce the number of infectious viral particles adhered to etafilcon A (CH) and lotrafilcon A (SH) to below the limit of quantification when the lenses were rubbed and rinsed as recommended by the manufacturers. Yasir and collaborators also demonstrated the ability of Biotrue to reduce the number of murine coronaviruses adhered to etafilcon A when a rub and rinse regimen was used [20]. Similar results were obtained in previous studies when viruses other than coronaviruses were evaluated. MeniCare Soft Multipurpose Solution, that contains 0.0001% polyaminopropyl biguanide (the same as Biotrue), efficiently reduced the number of herpes simplex virus (type 1), adenovirus (type 8) and poliovirus adherent to CLs only when a rub and rinse regimen was used [37]. ReNu Multipurpose (Bausch and Lomb), that contains 0.00005% polyaminopropyl biguanide, was able to reduce the numbers of HIV on CL when a rubbing only procedure was performed [38]. Moreover, several studies have demonstrated that “rub and rinse” before disinfection is the most effective regimen to reduce microbial contaminations from CLs, including bacteria, fungi and Acanthamoeba [22], [23], [24], [25]. Rosenthal and collaborators clearly demonstrated a direct correlation between the number of steps in the cleaning regimen of non-oxidative systems and their increased disinfecting efficacy [25].
In this context, when CLs were directly incubated with non-oxidative disinfection systems without previous rinsing and rubbing, viruses were still recovered from contaminated CL [20], [37]. In this study, infectious viral particles were recovered without the addition of a rub and rinse treatment. This is a serious concern, since non-compliance with CL care disinfection regimens remains a persistent clinical problem. Non-compliance in CL wear are historically cited in the literature, reaching levels even higher than 90%, including overwear of lenses, poor hygiene, and inadequate cleaning regimes [39], [40], [41], [42], [43], [44]. Studies have shown that 40 to 75% of CL wearers fail to rub and rinse their lenses when using CL care products based on non-oxidative disinfection systems [42], [45], [46]. Dumbleton and colleagues have shown that CL wearers who did not rub and rinse their lenses have a higher rate of self-reported CL related problems than those who regularly carried out these procedures [46]. Moreover, Butcko and collaborators have reported the importance of rubbing and rinsing lenses to reduce the risk of microbial keratitis [47].
In addition, it is important to consider that certain CL care products, mainly those based on non-oxidative disinfection systems, can change the properties of the lenses, such as surface roughness [48] and hydrophobicity [49], which could potentially influence virus removal. Therefore, examination of other CL care products not tested in this study is warranted.
5. Conclusion
The results of this study have demonstrated that various CH, SH and RGP CL materials were similarly prone to human coronavirus adhesion, which can be easily removed from CL surfaces when rubbed or rinsed with only saline. This study also demonstrated the importance of following the disinfection regimens of CL care products to remove infectious coronaviruses from CL surfaces that can potentially lead to human infections. The efficacy of CL care products based on non-oxidative disinfection systems against the viruses examined can be improved by ensuring that a rub and rinse step is used prior to overnight disinfection.
6. Funding
This study was funded by Ophtecs Corp, Kobe, Japan. MS is supported by a Canadian Mitacs Accelerate grant (IT23148).
7. Ethics approval
This study received ethics approval from the Office of Research Ethics at the University of Waterloo under application number 43217: Interaction of viruses with contact lenses, spectacles and contact lens solutions.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: William Ngo, Lyndon Jones and Manish Shukla are members of the Centre for Ocular Research & Education (CORE) at the University of Waterloo. Over the past three years, members of CORE have received research funding from Alcon, Allergan, Allied Innovations, Aurinia Pharma, Azura Ophthalmics, Bausch Health Canada, BHVI, CooperVision, GL Chemtec, i-Med Pharma, J&J Vision, Lubris, Menicon, Nature’s Way, Novartis, Ophtecs, Oté Pharma, PS Therapy, Santen, SightGlass, SightSage, Topcon and Visioneering. Lyndon Jones is a consultant and/or serves on an advisory board for Alcon, CooperVision, J&J Vision, Novartis and Ophtecs. William Ngo has received consulting fees from Alcon.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clae.2022.101719.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Peng M., et al. The Role of the Ocular Tissue in SARS-CoV-2 Transmission. Clin Ophthalmol. 2020;14:3017–3024. doi: 10.2147/OPTH.S269868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocansey S., Abu E.K., Abraham C.H., Owusu-Ansah A., Boadi-Kusi S.B., Ilechie A.A., et al. Ocular Symptoms of SARS-CoV-2: Indication of Possible Ocular Transmission or Viral Shedding. Ocul Immunol Inflamm. 2020;28(8):1269–1279. doi: 10.1080/09273948.2020.1799035. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Chen X., Chen L., Deng C., Zou X., Liu W., et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18(3):360–362. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu J.Y., Xie H.T., Zhang M.C. Evidence of SARS-CoV-2 Transmission Through the Ocular Route. Clin Ophthalmol. 2021;15:687–696. doi: 10.2147/OPTH.S295283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett B.P., Wahlin K., Krawczyk M., Spencer D., Welsbie D., Afshari N., et al. Potential of Ocular Transmission of SARS-CoV-2: A Review. Vision (Basel) 2020;4(3):40. doi: 10.3390/vision4030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui K.P.Y., Cheung M.-C., Perera R.A.P.M., Ng K.-C., Bui C.H.T., Ho J.C.W., et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020;8(7):687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18(4):537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grajewski R.S., Rokohl A.C., Becker M., Dewald F., Lehmann C., Fätkenheuer G., et al. A missing link between SARS-CoV-2 and the eye?: ACE2 expression on the ocular surface. J Med Virol. 2021;93(1):78–79. doi: 10.1002/jmv.26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng W., Bao L., Gao H., Xiang Z., Qu Y., Song Z., et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C. Does hand hygiene reduce SARS-CoV-2 transmission? Graefes Arch Clin Exp Ophthalmol. 2020;258(5):1133–1134. doi: 10.1007/s00417-020-04652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przekwas A., Chen Z. Washing hands and the face may reduce COVID-19 infection. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson C.E., Boehm A.B., Elkins C.A. Transfer Rate of Enveloped and Nonenveloped Viruses between Fingerpads and Surfaces. Appl Environ Microbiol. 2021;87(22):e0121521. doi: 10.1128/AEM.01215-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones L., Walsh K., Willcox M., Morgan P., Nichols J. The COVID-19 pandemic: Important considerations for contact lens practitioners. Cont Lens Anterior Eye. 2020;43(3):196–203. doi: 10.1016/j.clae.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan P., et al. International Contact Lens Prescribing in 2021. Contact Lens Spectrum. 2022;37(1):32–38. [Google Scholar]
- 15.Akerman D. Our greatest opportunity. Cont Lens Anterior Eye. 2018;41(4):319–320. doi: 10.1016/j.clae.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Wichterle O., Lim D., Dreifus M. On the problem of contact lenses. Cesk Oftalmol. 1961;17:70–75. [PubMed] [Google Scholar]
- 17.Papas E. The history of soft contact lenses. Contact Lens Spectrum. 2021;36(11):20–26. [Google Scholar]
- 18.Musgrave C.S.A., Fang F. Contact Lens Materials: A Materials Science Perspective. Materials (Basel) 2019;12(2):261. doi: 10.3390/ma12020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalley C. Material gains: 50 years of the soft contact lens. Review of Cornea and Contact Lenses, 2021(March/April): p. 10 - 14.
- 20.Yasir M, Kumar Vijay A, Willcox M. Antiviral effect of multipurpose contact lens disinfecting solutions against coronavirus. Cont Lens Anterior Eye, 2021: p. 101513. [DOI] [PMC free article] [PubMed]
- 21.Nogueira C. et al., Antiviral activity of contemporary contact lens care solutions against human seasonal coronavirus strains. American Academy of Optometry, 2021. Boston(e-abstract #210024).
- 22.Zhu H., Bandara M.B., Vijay A.K., Masoudi S., Wu D., Willcox M.D.P. Importance of rub and rinse in use of multipurpose contact lens solution. Optom Vis Sci. 2011;88(8):967–972. doi: 10.1097/OPX.0b013e31821bf976. [DOI] [PubMed] [Google Scholar]
- 23.Kilvington S., Lonnen J. A comparison of regimen methods for the removal and inactivation of bacteria, fungi and Acanthamoeba from two types of silicone hydrogel lenses. Cont Lens Anterior Eye. 2009;32(2):73–77. doi: 10.1016/j.clae.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Shih K., Hu J., Sibley M. The microbiological benefit of cleaning and rinsing contact lenses. Int Contact Lens Clin. 1985;12(4):235–242. [Google Scholar]
- 25.Rosenthal R.A., Henry C.L., Schlech B.A. Contribution of regimen steps to disinfection of hydrophilic contact lenses. Cont Lens Anterior Eye. 2004;27(3):149–156. doi: 10.1016/j.clae.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Kodjikian L., Casoli-Bergeron E., Malet F., Janin-Manificat H., Freney J., Burillon C., et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):267–273. doi: 10.1007/s00417-007-0703-5. [DOI] [PubMed] [Google Scholar]
- 27.Santos L., Rodrigues D., Lira M., Real Oliveira M.E.C.D., Oliveira R., Vilar E.-P., et al. The influence of lens material and lens wear on the removal and viability of Staphylococcus epidermidis. Cont Lens Anterior Eye. 2008;31(3):126–130. doi: 10.1016/j.clae.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Beattie T.K., et al. Enhanced attachment of acanthamoeba to extended-wear silicone hydrogel contact lenses: a new risk factor for infection? Ophthalmology. 2003;110(4):765–771. doi: 10.1016/S0161-6420(02)01971-1. [DOI] [PubMed] [Google Scholar]
- 29.Henriques M., et al. Adhesion of Pseudomonas aeruginosa and Staphylococcus epidermidis to silicone-hydrogel contact lenses. Optom Vis Sci. 2005;82(6):446–450. doi: 10.1097/01.opx.0000168585.53845.64. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cueno M.E., Imai K. Structural Comparison of the SARS CoV 2 Spike Protein Relative to Other Human-Infecting Coronaviruses. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.594439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik Y.A. Properties of Coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42(1):3–11. [PubMed] [Google Scholar]
- 33.Enwemeka C.S., Bumah V.V., Mokili J.L. Pulsed blue light inactivates two strains of human coronavirus. J Photochem Photobiol B. 2021;222 doi: 10.1016/j.jphotobiol.2021.112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei M., Tan X. Current Strategies of Antiviral Drug Discovery for COVID-19. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.671263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed L.J., Muench H. A Simple Method of Estimating Fifty Per Cent Endpoints12. Am J Epidemiol. 1938;27(3):493–497. [Google Scholar]
- 36.Beattie T.K., Tomlinson A., McFadyen A.K. Attachment of Acanthamoeba to first- and second-generation silicone hydrogel contact lenses. Ophthalmology. 2006;113(1):117–125. doi: 10.1016/j.ophtha.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Heaselgrave W., Lonnen J., Kilvington S., Santodomingo-Rubido J., Mori O. The disinfection efficacy of MeniCare soft multipurpose solution against Acanthamoeba and viruses using stand-alone biocidal and regimen testing. Eye Contact Lens. 2010;36(2):90–95. doi: 10.1097/ICL.0b013e3181d13c2d. [DOI] [PubMed] [Google Scholar]
- 38.Amin R.M., Dean M.T., Zaumetzer L.E., Poiesz B.J. Virucidal efficacy of various lens cleaning and disinfecting solutions on HIV-I contaminated contact lenses. AIDS Res Hum Retroviruses. 1991;7(4):403–408. doi: 10.1089/aid.1991.7.403. [DOI] [PubMed] [Google Scholar]
- 39.Collins M.J., Carney L.G. Patient compliance and its influence on contact lens wearing problems. Am J Optom Physiol Opt. 1986;63(12):952–956. doi: 10.1097/00006324-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Claydon B.E., Efron N. Non-compliance in contact lens wear. Ophthalmic Physiol Opt. 1994;14(4):356–364. [PubMed] [Google Scholar]
- 41.Efron N. The truth about compliance. Cont Lens Anterior Eye. 1997;20(3):79–86. doi: 10.1016/s1367-0484(97)80002-1. [DOI] [PubMed] [Google Scholar]
- 42.Hickson-Curran S., Chalmers R.L., Riley C. Patient attitudes and behavior regarding hygiene and replacement of soft contact lenses and storage cases. Cont Lens Anterior Eye. 2011;34(5):207–215. doi: 10.1016/j.clae.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Robertson D.M., Cavanagh H.D. Non-compliance with contact lens wear and care practices: a comparative analysis. Optom Vis Sci. 2011;88(12):1402–1408. doi: 10.1097/OPX.0b013e3182333cf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan P.B., Efron N., Toshida H., Nichols J.J. An international analysis of contact lens compliance. Cont Lens Anterior Eye. 2011;34(5):223–228. doi: 10.1016/j.clae.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y., Carnt N., Stapleton F. Contact lens user profile, attitudes and level of compliance to lens care. Cont Lens Anterior Eye. 2010;33(4):183–188. doi: 10.1016/j.clae.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Dumbleton K.A., Woods C.A., Jones L.W., Fonn D. The relationship between compliance with lens replacement and contact lens-related problems in silicone hydrogel wearers. Cont Lens Anterior Eye. 2011;34(5):216–222. doi: 10.1016/j.clae.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Butcko V., McMahon T.T., Joslin C.E., Jones L. Microbial keratitis and the role of rub and rinsing. Eye Contact Lens. 2007;33(6):421–423. doi: 10.1097/ICL.0b013e318157f3df. [DOI] [PubMed] [Google Scholar]
- 48.Lira M., Franco S., Vazquez-Dorrio J.B., Real Oliveira M.E.C.D., Costa M.F.M. Surface roughness and refractive index changes in contact lens induced by lens care systems. Eye Contact Lens. 2014;40(3):140–147. doi: 10.1097/ICL.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 49.Lira M., Silva R. Effect of Lens Care Systems on Silicone Hydrogel Contact Lens Hydrophobicity. Eye Contact Lens. 2017;43(2):89–94. doi: 10.1097/ICL.0000000000000247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.