Abstract
Coronal plane deformity around the knee, also known as genu varum or genu valgum, is a common finding in clinical practice for pediatricians and orthopedists. These deformities can be physiological or pathological. If untreated, pathological deformities can lead to abnormal joint loading and a consequent risk of premature osteoarthritis. The aim of this review is to provide a framework for the diagnosis and management of genu varum and genu valgum in skeletally immature patients.
Keywords: Genu varum, Genu valgum, Pediatric deformity, Knee deformity, Guided growth, Osteotomy, Lower limb deformity
Core Tip: Physiological angulation requires no treatment other than clinical observation. If the clinical picture is doubtful, a long-standing X-ray of the lower limb is required for clarification. Once a pathological alignment is determined by X-ray, a differential diagnosis is needed to establish whether the deformity is idiopathic or a secondary condition. Furthermore, any associated deformities in other planes (e.g., rotation or length difference) should be evaluated. Pathological alignment should be treated for at least two reasons: To interrupt the vicious circle described by the Hueter-Volkmann law and to prevent premature degeneration of the joint. Both of these conditions stem from abnormal load distribution. Guided growth should be considered every time a coronal deformity of the knee is foreseen in a skeletally immature patient. Although comparative studies are lacking in the literature, this technique has a high success rate with few complications and a low impact on the patient and the family.
INTRODUCTION
Coronal angular deformities around the knee in children are a common finding in pediatric orthopedic surgery. However, a majority of these deformities, such as genu varum and genu valgum, are physiological and correspond to the normal changes in mechanical axis (MA) alignment that take place from birth to adolescence.
The purpose of this paper is to provide a review of the recent literature concerning: (1) The definition of normal (physiological) alignment and pathological genu valgum and genu varum; (2) The practical clinical approach for patients with a coronal angular deformity around the knee; and (3) A brief description of specific pathologies.
The Medline, Embase, Web of Science, and Cochrane Systematic Review databases were searched for studies published in English up to 31 August 2020. The primary search terms were “knee” AND “valgum”; “knee” AND “varum”; “normal” AND “lower” AND “limb” AND “alignment”. Papers were screened by title and abstract to identify relevant articles. Their reference lists were checked manually for additional articles. Studies involving skeletally mature patients were excluded (these studies were only included in the analysis of the lower limb alignment range).
Definition of normal alignment and the natural history of coronal angular deformity of the knee in the skeletally immature
To understand deformities of the lower extremity, it is first important to understand and establish the parameters and thresholds of normal alignment. Every long bone, such as the femur and the tibia, has a mechanical and an anatomical axis (AA) (Figure 1). The MA of the lower limb is the line connecting the center of the femoral head and the midpoint of the tibiotalar joint. The femorotibial mechanical angle (FTMA) is the angle formed by the intersection of the mechanical axes of the femur (MAF) and the tibia (MAT). The AA of the lower limb is the AA of the femur and the tibia, which forms the femorotibial anatomical angle (FTAA). The MA deviation (MAD) is defined as the distance between the center of the knee and the MA[1].
Figure 1.
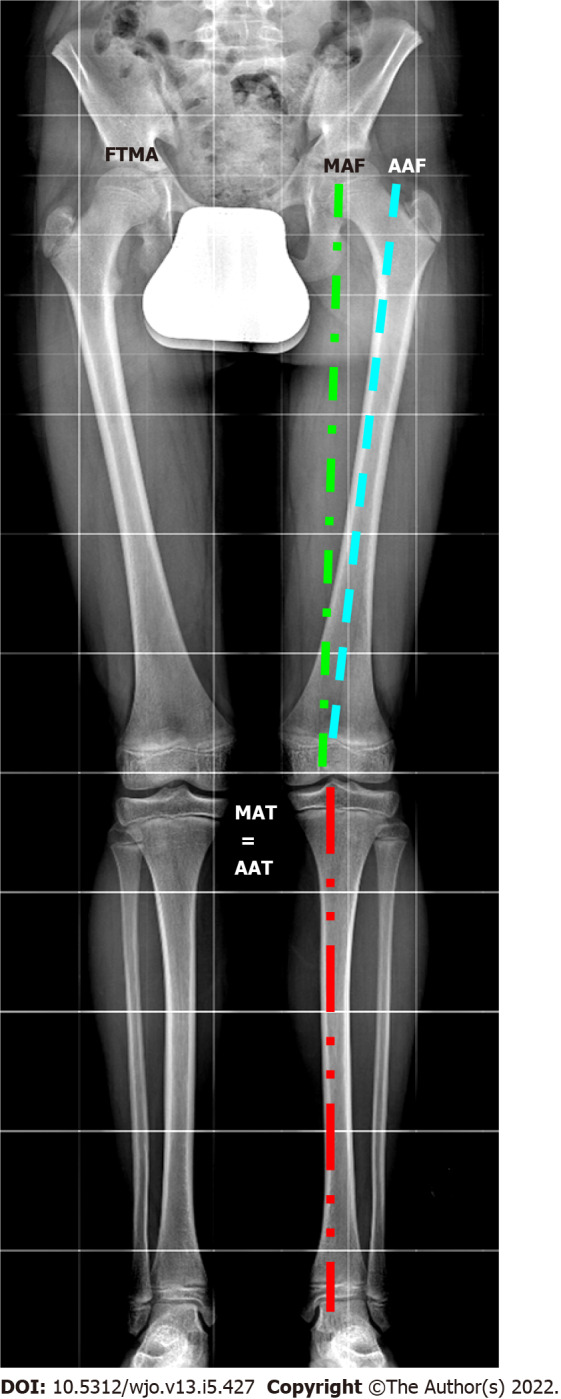
Lower limb axes evaluated in long standing X-ray. FTMA: Femorotibial mechanical axis; MAF: Mechanical axis of the femur; AAF: Anatomical axis of the femur; MAT: Mechanical axis of the tibia; AAT: Anatomical axis of the tibia.
Salenius and Vankka[2] reported that the femorotibial angle (FTA) develops physiologically from a varus alignment in the newborn (with an angulation of 10 to 15 degrees) to a neutral alignment at 18-20 mo and a valgus alignment at 3 years. Indeed, the mature FTAA is reached at approximately 8 years and is approximately 6 degrees of valgus (8 degrees in females and 7 degrees in males)[2-4]. Therefore, the persistence of genu varum beyond 2 years may be considered abnormal[5], whereas a valgus deformity greater than 15 degrees that persists after 8-10 years rarely corrects itself with growth[6].
A wide range of values for normal lower limb alignment has been reported in the literature[2,7]. However, it has been established that the FTMA tends to be neutral or slightly varus (1.3 ± 2 degrees) with the MA passing through or immediately medial to the center of the knee[1,8,9]. Indeed, the normal MAD is reported to be 8 ± 7 mm medial to the center of the knee joint line[1]. The knee joint line is reported to be approximately 3 degrees off the perpendicular line, as a consequence of a 3-degree valgus inclination of the distal femur and a 3-degree varus inclination of the proximal tibia[4]. The MAD falls away from the center of the knee in coronal angular deformities of the knee. Genu varum is defined as an increased FTA with the MA passing through the medial portion of the knee joint. Genu valgum is defined as a reduced FTA with the MA passing through the lateral portion of the knee joint[1].
ASSESSMENT
History
A careful medical history can help to differentiate between physiological and pathological coronal angular deformity (Table 1). A family history of short stature, varus or valgus alignment, and metabolic or genetic diseases should be ascertained. Personal history taking should also consider the child’s stage of development and rule out any traumatic or pathological conditions (e.g., infectious or rheumatic diseases)[5,6].
Table 1.
Principal causes of pathological genu varum and genu valgum
|
Genu varum
|
Genu valgum
|
| Idiopathic | |
| Tibia vara (blount disease) | |
| Infantile form | |
| Adolescent form | |
| Post-traumatic | |
| Physeal bar | |
| Malunion | |
| Cozen’s phenomenon | |
| Metabolic | |
| Rickets | |
| Renal disease | |
| Local disorders | |
| Infectious | |
| Tumor or tumor-like lesion | |
| Generalized disorders | |
| Metabolic (e.g., rickets, renal osteodystrophy) | |
| Constitutional disease of bone (i.e., bone dysplasia) | |
| Juvenile arthritis | |
| Neuromuscular | |
Physical examination
The clinical evaluation should include the patient’s general appearance, stature, and height percentile. Femoral anteversion, tibial torsion, and the foot’s appearance should be assessed. The evaluation of the lower limb should look for any coronal plane, sagittal plane, or translational deformities. This paper will only discuss coronal plane deformities.
The intermalleolar (IM) and intercondylar (IC) distances are used to assess coronal plane deformity, although some researchers dispute the clinical utility of this, on account of poor reproducibility[5,6,10,11]. Staheli[7] states that knee angle variations measured using FTA, IM, or IC and falling within the range of two standard deviations above or below the mean are physiological conditions. Thus, pathological genu varum and genu valgum are true frontal plane deformities that are outside the normal range of two standard deviations above or below the mean[6,12,13].
Specifically, persistent varus deformity with an IC distance greater than 5 cm in patients older than 3 years, and valgus deformity (IM distance greater than 7 cm) in patients older than 7 years should be considered abnormal[10,12,14,15]. Obesity, femoral anteversion, external rotation of the tibia, and flat foot with out-toed stance can exaggerate the IM distance and the appearance of genu valgum, whereas internal tibial torsion can give an overestimated IC distance and the appearance of genu varum[10].
Radiographs
A long-standing, weight-bearing radiograph (including the pelvis, the hips, the knees, and the ankle) should be obtained every time pathological genu valgum or genu varum is suspected. In particular, a varus deformity should be evaluated by radiograph if[16]: (1) It persists or progresses beyond 18-24 mo of age or does not resolve; and (2) There is asymmetric deformity.
Radiographs should be obtained for genu valgum if[6]: (1) The patient is older than 7 years with a tibiofemoral angle greater than 15 degrees and/or an IM distance of more than 7 cm; (2) The genu valgum is associated with short stature (below the 25th percentile) or if there are other musculoskeletal abnormalities; and (3) There is asymmetric involvement.
Radiograph parameters
Paley and Tetsworth[17], Paley et al[18] standardized the approach to the radiographic evaluation of lower limb deformities and summarized and published it as a step-by-step method called “the malalignment test”, which determines the source(s) of the malalignment (Figure 2). Long-standing films (anteroposterior and lateral) should be obtained including the hips, knees, and ankles. Proper positioning is important: The knees should be straight and the patella facing forward.
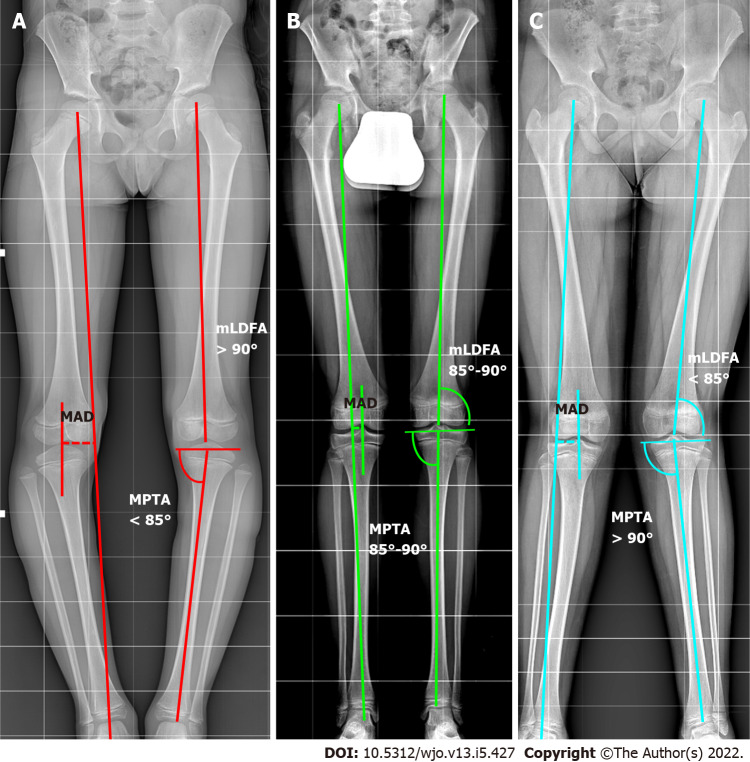
Figure 2.
The malalignment test. The mechanical axis (MA) is traced from the center of the femoral head to the center of the ankle. The metaphyseal-diaphyseal angle (MAD) is calculated in millimeters (dotted lines in the image traced from center of knee and MA). If the MAD exceeds the threshold of normality, it is necessary to find the source of the deformity. The mechanical lateral distal femur angle. Medial proximal tibial angle are evaluated. A: Varus; B: Normal; C: Valgus. MAD: Metaphyseal-diaphyseal angle; MPTA: Medial proximal tibial angle; mLDFA: Mechanical lateral distal femur angle.
TREATMENT
Observation
Patients with physiological varus or valgus knee (that is, who fall within the two standard deviations from the normal value for their age or within the second zone on the X-ray) require no treatment other than observation. Parents should be reassured that it is not a true deformity or disease, but a variant of normal lower limb alignment, which usually corrects spontaneously. These patients should be clinically evaluated every 3-6 mo to monitor the deformity. Radiographic assessment should be repeated if there is suspicion of clinical worsening[6]. Conservative treatment (e.g., shoe wedges or bracing) is not effective, is poorly tolerated, and is unnecessary in cases of physiological deviation[5-7,10].
Surgical treatment
It is generally accepted that a significant deformity that persists into preadolescence will not correct spontaneously. Physiological alignment of the lower limb is crucial for the symmetrical distribution of weight over the surfaces of the joints, especially the knee. Indeed, severe coronal malalignment has been linked to knee pain, altered gait, and occasionally patellofemoral problems[19]. Moreover, it can contribute to the development of osteoarthritis of the knee[11,20-22]. Additionally, MA deviation in the lateral or medial compartment can lead to compression in the lateral or medial physis, thereby further delaying growth as a result of the Hueter-Volkmann effect[23].
Preoperative planning
Preoperative planning could involve the malalignment test on a long-standing X-ray, as described by Paley and Tetsworth[17] (Figure 2): (1) Evaluation of MA and MAD. The first step is tracing the MA of the lower limb (e.g., from the center of the femoral head to the center of the ankle). The MA should pass through the center of the knee joint. If the MA axis does not go through the center of the knee joint, there is a MAD. Furthermore, the MA deviation can be classified into three zones as defined by Müller and Müller-Färber[24]. When the MAD exceeds the normal range (e.g., > 8 ± 7 mm medial to the center of the knee joint line[1]) or the MA passes to the first zone[23,24] a pathological malalignment is present and the following steps will determine the origin of the deformity; (2) Measurement of the mechanical lateral distal femur angle (n.v. = 87.5 ± 2.5): The lateral angle between the MAF and the line through the femoral condyles; (3) Measurement of the medial proximal tibial angle (n.v. = 87.5 ± 2.5): The medial angle between the MAT and the line through the tibial plateaus; (4) Measurement of the joint line convergence angle (n.v. = 0-2 degrees medial convergence): The angle between the femoral condyle and the tibial plateau joint line. This helps to evaluate any source from the ligament or capsular laxity or joint cartilage loss; (5) Ruling out a medial or lateral subluxation: The midpoints of the femur and the tibia should be aligned; and (6) Ruling out an intraarticular origin of the malalignment: The femoral condyles and the tibial plateaus should be aligned with respect to each other. A depressed or elevated femoral condyle or tibial plateau may indicate an intraarticular source of malorientation.
The method described evaluates deformities near the knee. Deformities away from the knee should be investigated using the center of rotation of angulation (CORA) method and other angles such as the lateral proximal femur angle and the lateral distal tibial angle[25] whose description is beyond the scope of this article.
The authors stress that these measurements are only reliable if the X-ray projection is anteroposterior with the knee in the frontal plane, which is defined as the position where the patella is centered in the femoral condyles[18,26]. This means that care must be taken to place the patient in the patella forward position, rather than in the feet forward position, as the latter is affected by tibial torsion and leads to incorrect measurement[18].
General indications
Surgical correction of coronal angular knee deformities is indicated if: (1) The MA falls within zone 2 and the patient is symptomatic; and (2) The MA is beyond zone 2[23,24]. If the deformity only involves the distal femur or the proximal tibia, the correction should only take place within the affected bone. If the deformity originates from both the femur and the tibia and is symmetrical, both bones should be treated. If, on the other hand, the deformity is asymmetrical, only the bone whose angle (LTFA and MTFA) deviates more than 5 degrees from the reference values should be treated[7,27].
How to treat pathological genu varum and genu valgum
In growing children, the treatment strategies for coronal angular deformities around the knee are: (1) Hemiepiphysiodesis; and (2) Osteotomy.
Hemiepiphysiodesis: It is well known that the physis is the area where bones grow. Epiphysiodesis is a surgical procedure that consists of the iatrogenic arrest of the growth plate to control the growth of immature bone. Epiphysiodesis can be partial (hemiepiphysiodesis) or complete and its duration can be permanent or (potentially) reversible[28].
Hemiepiphysiodesis on the convex side of the deformity should be preferred to osteotomy in skeletally immature patients with coronal angular deformity around the knee and who have reasonably sufficient growth remaining for them to reach the expected correction[28]. The prerequisites for coronal angular deformity correction include a minimum of 2 years of growth remaining and a deformity originating from or in the immediate vicinity of the growth plate[10].
Permanent hemiepiphysiodesis: Phemister[29] described an open procedure for surgical closure of the growth plate. In 1993 Terry Canale[30] reported a percutaneous epiphysiodesis technique involving mechanical disruption of the growth plate induced by a combination of drills, burrs, and/or curettes.
In 1998 Métaizeau et al[31] described percutaneous epiphysiodesis using transphyseal screws (PETS), which uses the compressive force of fully threaded cannulated screws to inhibit the growth of the physis. The screw is inserted into the metaphysis, crosswise through the growth plate and into the epiphysis on the opposite side, or in a straight direction into the epiphysis on the same side. It is reported that at least four screw threads should be engaged within the epiphysis to achieve sufficient compression[32]. PETS is sometimes referred to as reversible epiphysiodesis with screw removal. However, some authors report that the physis fuses quite quickly after screw placement and that the technique is not reliably reversible[33].
Permanent epiphysiodesis for coronal deformity is associated with low cost, minimally invasive procedures, and low cosmetic impact. It is an attractive option for patients who are near skeletal maturity because it eliminates implant-related complications and the unpredictability of the rebound effect. However, this technique requires accurate preoperative planning in order to time the procedure precisely, which is essential for achieving the exact estimated correction[11]. Inappropriate timing of surgery could lead to under- or overcorrection of the deformity[34]. Ferrick et al[35] suggest performing permanent hemiepiphysiodesis if there are 1 or 2 years of growth remaining and stress the importance of close follow-up. If correction is achieved before maturity, epiphysiodesis should be completed with or without contralateral epiphysiodesis to control leg length equality.
Temporary (or reversible) hemiepiphysiodesis (growth plate modulation)
The longitudinal growth of long bones occurs at the level of the growth plates, which are affected by mechanical loading as a result of the Hueter-Volkmann law. Summarizing, the tensile forces that increase growth rate and compression decrease activity in the growth plate[36,37]. Growth plate modulation is induced by a surgical device tethered to one side of a growing physis, thereby allowing differential growth[36].
Staples: Haas[38] was the first to describe a reversible epiphysiodesis technique using wires over the growth plate. However, this technique became more popular after the studies of Blount and Clarke[39], who controlled growth by epiphyseal stapling (Figure 3). Satisfactory results with correction of the deformity are reported in 80%-85% of cases. Implant failure with staple extrusion or breakage is observed in approximately 10% of treated physes.
Figure 3.
Staple hemiepiphysiodesis.
Tension band plate (guided growth): Stevens[40] coined the term “guided growth” and proposed an alternate device consisting of a small, non-rigid extraperiosteal plate locked by two screws - one proximal and one distal to the physis - serving as a focal hinge at the perimeter of the physis (Figure 4). The critical difference between Blount’s staple and Stevens’ plate is that the former is rigid and compresses the physis, whereas the latter is a non-rigid device and acts as a tension band plate (TBP)[40]. This difference means a lower risk of plate extrusion and the need to insert only one plate.
Figure 4.
Tension band plate hemiepiphysiodesis for correcting genu valgum deformity.
Stevens’ classic TBP, known as the 8-plate, consists of a non-locking plate and two screws. Owing to the high cost of these classic plates, particularly in developing countries, some authors report good results using 2-hole 3.5 mm stainless steel reconstruction plates and 4 mm non-cannulated cancellous screws[41].
Steven[40] reports a faster correction rate using the TBP instead of staples. However, several other studies show faster correction with staples, suggesting that the latter implant causes immediate compression to the physis, whereas the tension plate becomes effective a few months after insertion[42]. There is some debate in the literature about the angle of the screws during implantation. In a recent clinical study, Eltayeby et al[43] show that the initial screw angle (0-30 degrees) does not significantly affect the rate of correction, and they recommend inserting screws taking account of anatomical restrictions to avoid the physis, rather than favoring parallel, divergent or widely divergent configurations.
Some authors report a modified surgical technique called the “sleeper plate”[44,45]. In this modified TBP, when the deformity is corrected, the metaphyseal screw is removed and the plate with the epiphyseal screw is left in place. If the deformity recurs, only the metaphyseal screw needs to be reinserted.
Outcomes and complications of reversible hemiepiphysiodesis
The success rate of growth plate modulation in the literature is reported to be close to 100% in idiopathic cases with a low complication rate[11,19,46]. Pathological cases have a slightly lower success rate and a higher complication rate[19,46]. Adverse events can be divided into: (1) Failure to achieve the correction: This is defined as the failure of the screws to diverge during treatment and to correct the deformity. The risk factors for failure are related to body mass index (BMI), age, and underlying etiology[46]. The rate of this complication differs in the studies present in the literature. It has been reported as being close to 0% for idiopathic cases. In pathological physes, the failure rate is reported to be as high as 45% in patients with pseudoachondroplasia and 36.8% in those with Blount disease[47]. Boero et al[19] reported no correction failure in the idiopathic deformity group, while in the non-idiopathic deformity group the complete correction rate was 78.5%, the partial correction rate 17.9%, and the no correction rate 3.6%. Joeris et al[48] published an international multicenter study in which a 66% success rate was reported for varus/valgus deformity correction using TBPs. In this paper, patients with idiopathic and secondary deformities were not analyzed separately; (2) Overcorrection: Precise timing of implant or hardware removal and routine post-operative follow-up is essential for avoiding this complication. Overcorrection is usually associated with low patient compliance and failure to attend clinical or radiological monitoring after implantation[49]; (3) Hardware failure: Implant breakage, loosening, migration or extrusion were reported for both staples and TBPs[50,51]. The original TBP technique involves the use of cannulated screws. Several reports describe broken screws in this type of implant, particularly in obese patients. The use of solid screws should be considered in patients with a high BMI in order to increase the strength and resistance of the implants[52]; (4) Premature epiphyseal closure: This is a theoretical complication, but an extremely rare one as long as the hardware is removed within 2 or 3 years of insertion[19,46]. To avoid this complication, the entire procedure should be extraperiosteal and care must be taken to preserve the physis, the zone of Ranvier, and the perichondrial ring of LaCroix during hardware implantation[11,44]. This complication is reported to be higher in cases using the sleeper plate technique[45]; and (5) Rebound: This is defined as a recurrence of malalignment (at least 5 degrees of varus or valgus) after the restoration of physeal growth on removal of the implanted hardware and is caused by growth acceleration on the previously tethered physis[4,11,13,46]. This phenomenon is theoretically possible in all patients, but is not always present and cannot be predicted with certainty. Leveille et al[53] report that patients at high risk of rebound are those with severe initial deformity (> 20 degrees) who undergo early hemiepiphysiodesis (< 10 years for girls and < 12 years for boys). Some authors routinely perform an overcorrection of 5 degrees[51]. However, Leveille et al[53] recommend only overcorrecting patients at high risk of rebound, to avoid creating a new deformity in patients who do not experience a rebound.
Timing of hemiepiphysiodesis
When hemiepiphysiodesis is timed perfectly, the deformity is corrected in step with skeletal maturity. The timing of this procedure is therefore crucial in cases of permanent hemiepiphysiodesis. If this procedure is performed too early, it could lead to overcorrection of the deformity and require circular epiphysiodesis (with or without epiphysiodesis of the contralateral limb to counteract any potential length discrepancies). If performed too late, it may not fully correct the deformity. Temporary hemiepiphysiodesis is theoretically reversible and therefore enables growth to resume once the deformity is corrected.
Numerous methods are reported in the literature for predicting growth, particularly correction of limb-length discrepancy (LLD). Only a few articles test and report the reliability of these methods, in cases where epiphysiodesis is used to correct angular deformities of the knee.
Bowen et al[54] and Inan et al[55] chart angular deformity against remaining growth potential calculated using a formula and combining the Green-Anderson growth remaining graph. The authors summarize that, as a general rule, a correction of about 7 degrees can be estimated in cases of epiphysiodesis of the distal femur and 5 degrees in cases of epiphysiodesis of the proximal tibia. The authors also state that the prediction was accurate for normal physes but inaccurate in cases of abnormal physes[54].
Paley and Tetsworth[17], and Paley et al[18] introduced the Multiplier Method (MM) to predict various parameters of growth, the timing for epiphysiodesis, and timing for hemiepiphysiodesis, using both skeletal age and chronological age when calculating LLD at maturity. Some authors report greater accuracy using the skeletal age, but Eltayeby et al[56] report the same prediction accuracy using skeletal and chronological age. They also show that MM tends to underpredict and so suggest that guided growth procedures performed right before skeletal maturity should be started 2 to 4 mo earlier than suggested by MM[56]. In their systematic review of the literature Wu et al[57] report poor reliability with MM for angular deformity prediction. It is more reliable for younger patients with idiopathic deformity and shows less prediction accuracy for patients older than 10 years or with non-idiopathic deformities.
Osteotomy
The use of corrective osteotomy is indicated in patients close to or at skeletal maturity, or in those whose growth cartilages are not functional (e.g., after an infection, or in the presence of a physeal bar). The specifics of realignment osteotomies are beyond the scope of this article and have been reported in articles on this theme and summarized by Paley[58]. However, it is necessary to introduce the fundamental concept of the CORA, which can be summarized as the point of maximum deformity. When a corrective osteotomy is planned, the correction should be established close to the CORA to avoid introducing translation deformity[18]. In varus and valgus deformities of the knee, the CORA is adjacent to the articular surface and the physis. For this reason, osteotomy, whether of the distal femur or the proximal tibia, is generally not feasible in skeletally immature patients. This is to avoid iatrogenic damage to the growth plate. Thus, in order to achieve realignment with corrective osteotomy, preoperative planning should take account of both the original angular deformity and any translation deformity introduced[18].
Correction through osteotomy can be acute, achieved using internal fixation devices (e.g., Kirschner wires, intramedullary nail, plates) or gradual, using an external fixator and distraction osteogenesis[59]. Gradual correction is attractive in cases of multiplanar deformity and modern hexapod systems are particularly useful in these situations[60].
The different types of osteotomy used to correct a deformity acutely are[10,61]: (1) Opening wedge; (2) Closing wedge; (3) Reverse wedge; and (4) Dome osteotomy. Acute deformity correction predisposes the patient to certain risks that should be taken into consideration during planning. Non-union or delayed union should be considered in opening wedge osteotomies greater than 20 degrees[62].
Neurovascular structures risk being stretched during acute correction. It is reported that the risk of injury to neurovascular structures is related to the magnitude of correction, but the limit is not well defined. Other factors that add to this risk are the site and type of osteotomy and the direction of correction. For example, a correction of a valgus to varus deformity of the knee by osteotomy of the distal femur or proximal tibia puts the common peroneal nerve (CPN) at risk, even if the correction is small (about 5 degrees)[63]. Conversely, a correction of a varus deformity releases the CPN. Further-more, the deep peroneal nerve (DPN), which passes under the intermuscular septum between the lateral and anterior compartment of the leg, is more at risk of injury than the superficial peroneal nerve (SPN)[25]. For the same reason, internal or external rotation osteotomies involving tensioning of the intermuscular septum create more risk for the DPN and less for the SPN. For these reasons, some authors suggest performing prophylactic peroneal nerve decompression before acute correction[63]. Additionally, the motor branch to the extensor hallucis longus is particularly at risk during fibular osteotomy[64].
SPECIFIC CONDITIONS
Idiopathic pathological genu valgum and genu varum
It is generally established that growth plate modulation with staples or TBPs determines less morbidity than osteotomy. However, it is essential to evaluate the timing of epiphysiodesis and to schedule close clinical monitoring to avoid overcorrection.
Post-traumatic
Trauma is one of the most frequent causes of pathological coronal deformity around the knee. The deformity may be a result of inadequate reduction or injury to the growth cartilage with a consequent alteration or arrest in growth (e.g., physeal bar). In the latter case, some authors report a high risk in cases of type 3 Salter-Harris (SH) fracture of the proximal tibia, whereas the type of SH fracture in the distal femur is poorly predictive[6]. In some cases, the physeal injury may be misdiagnosed if concomitant with another fracture of the femur or tibia. Therefore, some authors recommend knee X-rays in all patients with a traumatic lower limb injury[65].
Depending on the age of the patient, the location, the cause and the extent of the deformity, treatment may involve observation, physeal bar resection, epiphysiodesis, chondrodiastasis, or corrective osteotomy. Physeal bar resection consists of removing the bone bridging the metaphysis and the epiphysis and filling the gap with interposition material (e.g., fat, methyl methacrylate or polymeric silicone) to prevent the bony bar from reforming. This is indicated when there are at least 1 or 2 years of remaining growth, and when the bar involves < 50% of the growth plate. If a clinically unacceptable deformity is present at the time of physeal bar resection, an osteotomy or hemiepiphysiodesis is indicated to realign the lower limb. In fact, a successful physeal bar resection alone would not be able to fully correct the deformity[66].
A frequent form of post-traumatic knee valgus is tibia valga following fracture of the proximal metaphysis of the tibia, also known as Cozen’s phenomenon. The exact etiology is still under debate. In these cases, the maximum magnitude of deformity is variable, and is reached approximately 12 mo after injury. Parents should be advised of this eventuality and be informed that the deformity tends to resolve spontaneously within 2-4 years and only requires observation. Surgical treatment should be reserved for severe and symptomatic cases or for patients close to skeletal maturity with residual deformity[67]. Some authors report that, to prevent this deformity, the proximal tibial fracture should be treated with a varus-molded long-leg cast, although the efficacy of this procedure has been disputed in the literature[68]. Hemiplateau elevation (HE) is the treatment of choice for growing children with persistent deformity requiring surgery[69]. This must be performed within about three years of the trauma, since deformities tend to migrate distally at the level of the diaphysis during growth. Therefore, delayed HE could lead to a secondary “Z”-shaped deformity of the tibia (varus deformity proximal to the valgus deformity of the diaphysis)[16]. Corrective osteotomy should be avoided in growing children, as it can produce effects similar to the traumatic event itself and accentuate valgus deformity. It may be indicated in patients close to skeletal maturity with residual deformity[16].
TIBIA VARA
Introduction
Tibia vara is a developmental condition characterized by multiplanar deformities dominated by progressive genu varum malalignment caused by a growth alteration of the medial proximal metaphysis, physis and epiphysis of the tibia[70-72]. In 1937 Blount[73] published the first detailed description of this pathology, introducing the term “osteochondrosis deformans tibiae”. He was the first to identify two forms based on the age of onset: The infantile form and the adolescent form (Table 2). These two forms of tibia vara are clinically and radiographically distinct. The infantile form is when the deformity is noted before the age of 4 and the adolescent form is when the diagnosis is made after the age of 10.
Table 2.
Main differences between infantile and adolescent forms of Blount disease
|
|
Infantile form
|
Adolescent form
|
|
| Age (yr) | < 5 | > 10 | |
| Clinical features | 50% bilateral, overweight, lateral thrust during gait, possible internal rotation of the tibia | Usually unilateral, overweight/obese, male predominance | |
| X-RAY appearance | Varus angulation at the epiphyseal-metaphyseal junction, metaphyseal beaking with apparent fragmentation, medial physeal line widening and irregularity; lateral tibial subluxation; possible compensatory distal femur valgus | Widening of the proximal medial physeal line, normal shape of the proximal tibial metaphysis; possible presence of distal femur varus and compensatory distal tibial valgus | |
| Natural history | Depending on the stage (spontaneous resolution is possible) | Usually progressive without spontaneous resolution | |
| Treatment options | Depending on the stage | Surgery only | |
Infantile tibia vara
Clinical findings: Infantile tibia vara is a developmental condition causing progressive varus deformity of the knee in young children. Its prognosis appears to be worse predominantly in the non-white population. The clinical picture of a patient with confirmed Blount disease includes a frequently bilateral (50%), though rarely symmetrical, varus, and variable internal tibial rotation[72]. A positive “cover up test” is also reported in the literature[74]. In some cases, lateral ligament instability with a “varus thrust gait” may be present[75].
Radiographic classification
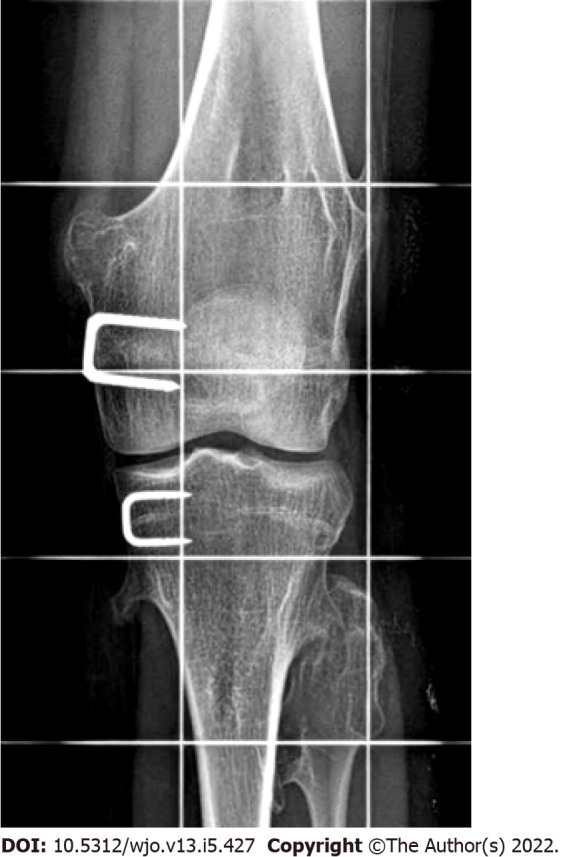
On a long-leg anteroposterior radiograph, Blount disease typically appears as a varus deformity of the lower limb, presenting lack of ossification of the medial tibial plateau. In the early stages of the disease, the characteristic findings may not be present and it may be difficult to differentiate an idiopathic varus from Blount disease. Levine and Drennan[76] measured the metaphyseal-diaphyseal angle (MDA) on anteroposterior radiographs (Figure 5) for early detection of infantile tibia vara, and reported that an MDA > 10 degrees is suggestive of infantile Blount disease. Although there are discordant data in the literature regarding the usefulness of the MDA, mainly due to poor accuracy of measurement, some authors believe that a toddler may be at risk of Blount disease with a FTA > 20 degrees and an MDA greater than 10 degrees. However, the definitive diagnosis should only be made if the characteristic lesions are present[16]. Infantile tibia vara is traditionally staged using Langenskiöld’s classification with six progressive stages, based on the radiographic aspects of the medial proximal tibia and epiphyseal-physeal-metaphyseal alterations[77].
Figure 5.
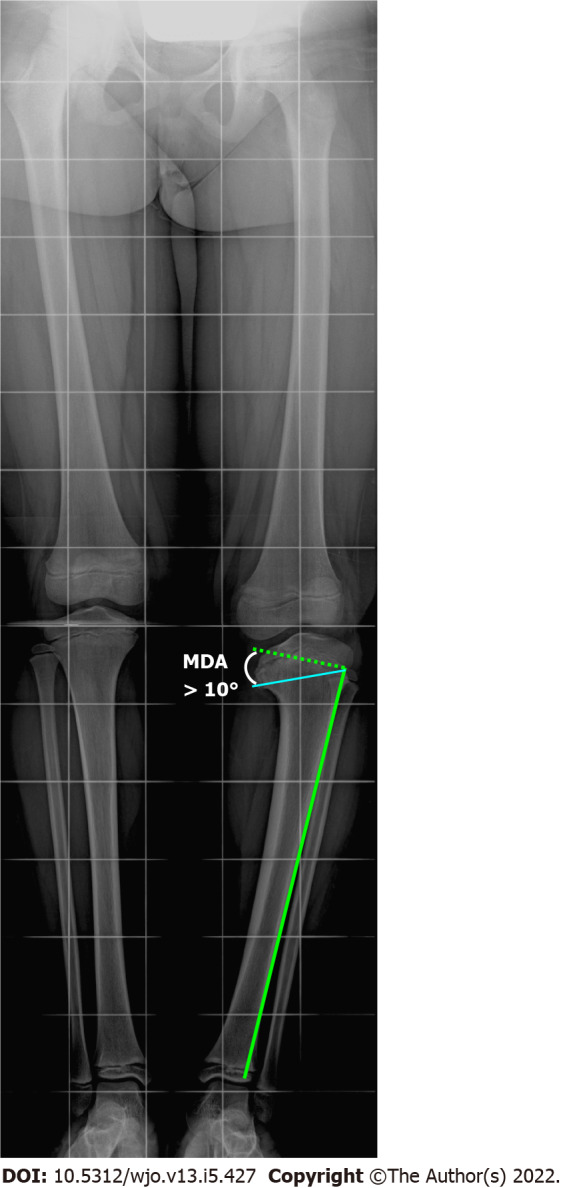
Radiographic measurement of Drennan’s metaphyseal-diaphyseal angle. The metaphyseal-diaphyseal angle (MDA) is measured from a perpendicular line to the tibial diaphyseal axis and a line passing through the axial plane of the proximal tibial metaphysis. An MDA > 10 degrees associated with a tibiofemoral angle > 20 degrees indicates a toddler at risk. MDA: Metaphyseal-diaphyseal angle.
Non-operative treatment
The effectiveness of full-time (23 h a day) long-leg antivarus bracing (e.g., knee-ankle-foot orthosis - KAFO -, elastic KAFO and conventional hip-knee-ankle-foot orthoses) has been reported in certain patients. Patients should be ≤ 3 years old with unilateral involvement and stage ≤ II in Langenskiöld’s classification. Patients with bilateral involvement may also benefit from bracing, but it is reported that there is a greater risk of progression and need for surgical correction[78]. However, the effectiveness of bracing has not been demonstrated. Indeed, some researchers advise clinical observation at stages II and III up to the age of 4[79,80].
Surgical treatment
The goals of surgical treatment can be summarized as: (1) Restoring the normal alignment of the proximal tibia by correcting the multiplanar deformity, in order to disrupt the vicious circle of proximal medial tibial alteration and prevent the onset of degenerative arthritis; (2) Preventing physeal damage; and (3) Preventing recurrence in order to limit the number of surgical procedures. It has been shown that surgical treatment in the early stages of the disease (stage II) is crucial to reduce the risk of deformity recurrence and physeal damage. There is strong consensus in the literature on not delaying surgery and on proceeding in cases of: (1) Patients < 4 years with progressive clinical and radiographic evidence of Blount disease; (2) Patients ≥ 4 years, even in stage I and II without signs of spontaneous correction, especially if the deformity exceeds 10 degrees of FTA varus; and (3) Patients in stage ≥ III in Langenskiöld’s classification. The main surgical procedures for infantile Blount are as follows.
High tibial and fibular osteotomy: High tibial and fibular osteotomy (HTO) is the treatment that is generally defined as being the choice for stage I to III in Langenskiöld’s classification. It is reported that, if HTO is performed in stage I and II, the risk of recurrence is significantly lower than in stage III, where recurrence is not uncommon. The goal of HTO is to fully correct or overcorrect the multiplanar deformity (varus, flexion, and internal rotation) of the proximal tibia (Figure 6). The aim is to interrupt the vicious circle caused by varus alignment and medial compartment overload, and enhance spontaneous recovery from the local disturbance of growth. For this reason, several authors recommend an overcorrection to 5 degrees of valgus alignment, along with lateral translation of the distal segment in order to unload the medial compartment and lateralize the MA.
Figure 6.
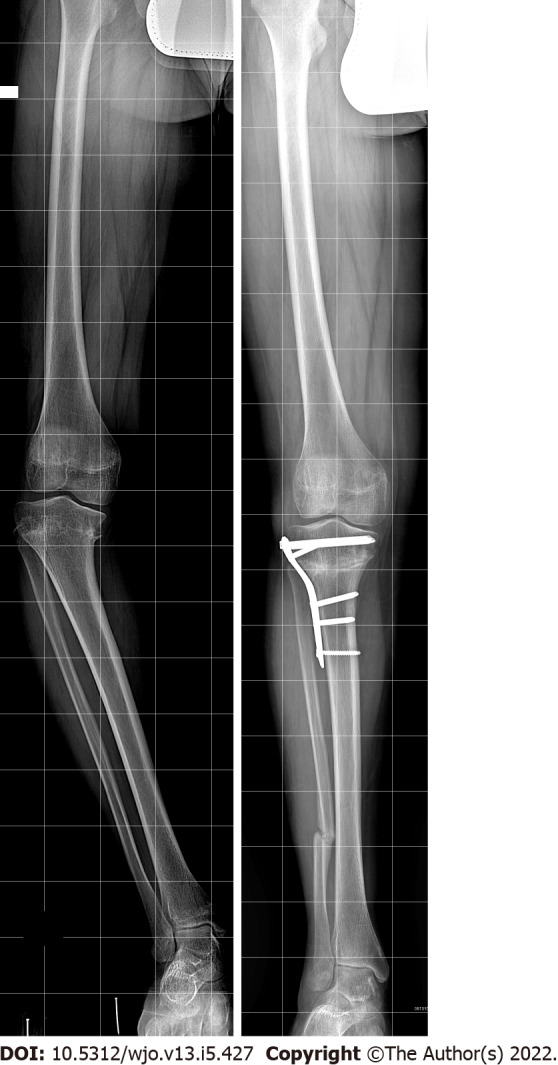
High tibial osteotomy for genu varum correction.
The correction can be achieved either acutely, using internal fixation (e.g., Steinmann pins or Kirschner wires), or gradually, using an external fixator. Tibial osteotomy should be performed just distal to the insertion of the patellar tendon and in combination with fibular osteotomy of the proximal third of its diaphysis. The internal rotation deformity of the tibia may be corrected through external rotation of the distal fragment. Some authors suggest prophylactic fasciotomy of the anterior, lateral, and posterior compartments of the leg to prevent compartment syndrome, which has been reported as a complication.
Growth plate modulation: Growth modulation using a TBP is reported to be a valid alternative to HTO. The main indication is stage < II (prior to physeal bar formation) usually in patients younger than 6 years[81-83]. The internal tibial rotational deformity cannot be corrected using growth modulation. Several studies report a high rate of implant failure with breakage of titanium/cannulated screws and some authors recommend using non-cannulated stainless steel screws[84].
Physeal arrest resection: The main indication is the documented presence of a physeal bar prior to frank bony bridge formation (stage IV and V) in patients with at least 2-4 years of growth remaining. The interposition of material (e.g., fat or methyl methacrylate) is a crucial step after medial epiphysiolysis to prevent rebridging. The position and size of the physeal bar should be evaluated preoperatively using advanced imaging [e.g., computed tomography scan or magnetic resonance imaging (MRI)]. Physeal arrest resection is typically combined with HTO to achieve valgus alignment beyond 0 degrees. Andrade et al[85] report a success rate of more than 80% for physeal arrest resection in children < 7 years old. The results were less predictable in patients older than 7 years or who had previously undergone surgery, and the authors recommend alternative treatment methods for these patients.
HE: The main indication is patients older than 10 years with high-grade deformity associated with intraarticular deformity and joint incongruence caused by medial tibial plateau depression. Preoperative advanced imaging (e.g., MRI) is mandatory to confirm joint incongruence. Indeed, X-ray may overestimate the intraarticular deformity, showing a severe medial ossification defect and depression, and the joint cartilage can maintain joint congruity. Intraoperative arthrography may also provide a better picture of the actual geometry of the joint surfaces. HE can be combined with metaphyseal tibial osteotomy to address the remaining long-bone deformity. Lateral proximal tibial and proximal fibular epiphysiodesis are recommended in combination with HE to prevent recurrent deformity.
Limb-length equalization procedures: LLD can be a component of the Blount deformity. Correction of the angular deformity using open-wedge HTO may equalize the LLD. In other cases, lengthening by distraction osteogenesis can be combined with gradual deformity correction using an external fixation device, performed alone as necessary, or limb-length equalization can be achieved by performing contralateral epiphysiodesis.
Adolescent tibia vara
Observation is usually considered for mild cases only and there are no data available on the use of an orthosis. Opening or closing wedge HTO is the treatment of choice in cases of severe (> 20 degrees) varus deformity or in cases of multiplanar deformity (varus, procurvatum and internal rotation). Acute deformity correction is usually suggested in uniplanar or milder cases, whereas gradual correction using an external fixator, particularly a hexapod system, is useful in cases of severe varus deformity or complex multiplanar deformity.
Lateral hemiepiphysiodesis. Good results have been reported, but patients should be carefully selected to avoid failure. The main indications are sufficient remaining growth (< 14 years old), BMI < 40 kg/m2, body weight < 100 kg, and less than 15 degrees of varus malalignment[82].
Medial hemichondrodiastasis. This is reported to be effective in patients with moderate varus deformity (< 20 degrees) who are nearing skeletal maturity. This technique has two main advantages: It corrects the varus and the LLD simultaneously, and it entails permanent epiphysiodesis, which reduces the risk of recurrence.
CONCLUSION
Coronal plane deformities around the knee are common during childhood and are usually a cause of serious concern for parents. Physiological variations in the growth and development of children need no treatment other than observation. True deformities need proper management to avoid consequences. Treatment should be tailored to the underlying disease and associated deformities on the other axis (e.g., rotation or shortening) should be ruled out. As a general rule, modulation surgery should be the treatment of choice in patients with sufficient residual growth, whereas deformities in skeletally mature patients require osteotomy or distraction osteogenesis.
Footnotes
Conflict-of-interest statement: Authors declare they have not conflict of interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 25, 2021
First decision: July 28, 2021
Article in press: April 8, 2022
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abulsoud MI, Egypt S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
Contributor Information
Valentino Coppa, Clinical Orthopaedics, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona 60121, Italy.
Mario Marinelli, Clinical Orthopaedics, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona 60121, Italy.
Roberto Procaccini, Clinical Orthopaedics, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona 60121, Italy.
Danya Falcioni, Clinical Orthopaedics, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona 60121, Italy.
Luca Farinelli, Clinical Orthopaedics, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona 60121, Italy.
Antonio Gigante, Clinical Orthopaedics, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona 60121, Italy. scienza.clinortop@gmail.com.
References
- 1.Paley D. Principles of Deformity Correction. In: Paley D. Normal Lower Limb Alignment and Joint Orientation. Heidelberg: Springer, 2002: 1-18. [Google Scholar]
- 2.Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259–261. [PubMed] [Google Scholar]
- 3.Sabharwal S, Zhao C, Edgar M. Lower limb alignment in children: reference values based on a full-length standing radiograph. J Pediatr Orthop. 2008;28:740–746. doi: 10.1097/BPO.0b013e318186eb79. [DOI] [PubMed] [Google Scholar]
- 4.Moreland JR, Bassett LW, Hanker GJ. Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987;69:745–749. [PubMed] [Google Scholar]
- 5.Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326–335. doi: 10.5435/00124635-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 6.White GR, Mencio GA. Genu Valgum in Children: Diagnostic and Therapeutic Alternatives. J Am Acad Orthop Surg. 1995;3:275–283. doi: 10.5435/00124635-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Staheli LT. Practice of Pediatric Orthopaedic. In: Mosca V. Lower Limb. Seattle: Lippincott Williams & Wilkins, 2006: 77-104. [Google Scholar]
- 8.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990:215–227. [PubMed] [Google Scholar]
- 9.Cahuzac JP, Vardon D, Sales de Gauzy J. Development of the clinical tibiofemoral angle in normal adolescents. A study of 427 normal subjects from 10 to 16 years of age. J Bone Joint Surg Br. 1995;77:729–732. [PubMed] [Google Scholar]
- 10.Gupta P, Gupta V, Patil B, Verma V. Angular deformities of lower limb in children: Correction for whom, when and how? J Clin Orthop Trauma. 2020;11:196–201. doi: 10.1016/j.jcot.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saran N, Rathjen KE. Guided growth for the correction of pediatric lower limb angular deformity. J Am Acad Orthop Surg. 2010;18:528–536. doi: 10.5435/00124635-201009000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Heath CH, Staheli LT. Normal limits of knee angle in white children--genu varum and genu valgum. J Pediatr Orthop. 1993;13:259–262. [PubMed] [Google Scholar]
- 13.Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974:12–17. doi: 10.1097/00003086-197403000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Yeo A, James K, Ramachandran M. Normal lower limb variants in children. BMJ. 2015;350:h3394. doi: 10.1136/bmj.h3394. [DOI] [PubMed] [Google Scholar]
- 15.Kaspiris A, Zaphiropoulou C, Vasiliadis E. Range of variation of genu valgum and association with anthropometric characteristics and physical activity: comparison between children aged 3-9 years. J Pediatr Orthop B. 2013;22:296–305. doi: 10.1097/BPB.0b013e328360f9a5. [DOI] [PubMed] [Google Scholar]
- 16.Herring JA. Tachdjian’s Pediatric Orthopaedics: From the Texas Scottish Rite Hospital for Children. 5th ed. In: Johnston CE, Young M. Disorders of the Leg. Philadelphia: Elsevier, 2013: 713-761. [Google Scholar]
- 17.Paley D, Tetsworth K. Mechanical axis deviation of the lower limbs. Preoperative planning of uniapical angular deformities of the tibia or femur. Clin Orthop Relat Res. 1992:48–64. [PubMed] [Google Scholar]
- 18.Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25:425–465. [PubMed] [Google Scholar]
- 19.Boero S, Michelis MB, Riganti S. Use of the eight-Plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5:209–216. doi: 10.1007/s11832-011-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT, Niu J, Gross KD, Englund M, Sharma L, Cooke TD, Guermazi A, Roemer FW, Segal N, Goggins JM, Lewis CE, Eaton C, Nevitt MC. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum. 2013;65:355–362. doi: 10.1002/art.37726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janakiramanan N, Teichtahl AJ, Wluka AE, Ding C, Jones G, Davis SR, Cicuttini FM. Static knee alignment is associated with the risk of unicompartmental knee cartilage defects. J Orthop Res. 2008;26:225–230. doi: 10.1002/jor.20465. [DOI] [PubMed] [Google Scholar]
- 22.Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367–377. [PubMed] [Google Scholar]
- 23.Stevens PM, Maguire M, Dales MD, Robins AJ. Physeal stapling for idiopathic genu valgum. J Pediatr Orthop. 1999;19:645–649. [PubMed] [Google Scholar]
- 24.HierholzerK G, Müller H. Corrective Osteotomies of the Lower Extremity after Trauma. In: Müller KH, Müller-Färber J. Indications, Localization and Planning of Posttraumatic Osteotomies about the Knee. Heidelberg: Springer, 1985: 195-223. [Google Scholar]
- 25.Paley D. Principles of Deformity Correction. Heidelberg: Springer, 2002. [Google Scholar]
- 26.Wright JG, Treble N, Feinstein AR. Measurement of lower limb alignment using long radiographs. J Bone Joint Surg Br. 1991;73:721–723. doi: 10.1302/0301-620X.73B5.1894657. [DOI] [PubMed] [Google Scholar]
- 27.Sabharwal S. Pediatric Lower Limb Deformities. In: Miller ML, Eric Gordon J. Decision making in lower extremity deformity correction. New York: Springer, 2016: 37-50. [Google Scholar]
- 28.Ghanem I, Karam JA, Widmann RF. Surgical epiphysiodesis indications and techniques: update. Curr Opin Pediatr. 2011;23:53–59. doi: 10.1097/MOP.0b013e32834231b3. [DOI] [PubMed] [Google Scholar]
- 29.Phemister DB. Operative arrestment of longitudinal growth of bones in the treatment of deformities. J Bone Joint Surg Br. 1933;15:1–15. [Google Scholar]
- 30.Terry Canale S. Percutaneous epiphysiodesis. Oper Tech Orthop. 1993;3:161–165. [Google Scholar]
- 31.Métaizeau JP, Wong-Chung J, Bertrand H, Pasquier P. Percutaneous epiphysiodesis using transphyseal screws (PETS) J Pediatr Orthop. 1998;18:363–369. [PubMed] [Google Scholar]
- 32.Dodwell ER, Garner MR, Bixby E, Luderowski EM, Green DW, Blanco JS, Widmann RF. Percutaneous Epiphysiodesis Using Transphyseal Screws: a Case Series Demonstrating High Efficacy. HSS J. 2017;13:255–262. doi: 10.1007/s11420-017-9549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruzbarsky JJ, Goodbody C, Dodwell E. Closing the growth plate: a review of indications and surgical options. Curr Opin Pediatr. 2017;29:80–86. doi: 10.1097/MOP.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 34.Woo K, Lee YS, Lee WY, Shim JS. The Efficacy of Percutaneous Lateral Hemiepiphysiodesis on Angular Correction in Idiopathic Adolescent Genu Varum. Clin Orthop Surg. 2016;8:92–98. doi: 10.4055/cios.2016.8.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrick MR, Birch JG, Albright M. Correction of non-Blount's angular knee deformity by permanent hemiepiphyseodesis. J Pediatr Orthop. 2004;24:397–402. doi: 10.1097/00004694-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Gottliebsen M, Shiguetomi-Medina JM, Rahbek O, Møller-Madsen B. Guided growth: mechanism and reversibility of modulation. J Child Orthop. 2016;10:471–477. doi: 10.1007/s11832-016-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frost HM. A chondral modeling theory. Calcif Tissue Int. 1979;28:181–200. doi: 10.1007/BF02441236. [DOI] [PubMed] [Google Scholar]
- 38.Haas SL. Retardation of bone growth by a wire loop. J Bone Jt Surg - Am Vol. 1945;27:25–36. [Google Scholar]
- 39.Blount WP, Clarke GR. Control of bone growth by epiphyseal stapling; a preliminary report. J Bone Joint Surg Am. 1949;31A:464–478. [PubMed] [Google Scholar]
- 40.Stevens PM. Guided growth for angular correction: a preliminary series using a tension band plate. J Pediatr Orthop. 2007;27:253–259. doi: 10.1097/BPO.0b013e31803433a1. [DOI] [PubMed] [Google Scholar]
- 41.Narayana Kurup JK, Shah HH. Hemiepiphysiodesis using 2-holed reconstruction plate for correction of angular deformity of the knee in children. J Orthop. 2020;20:54–59. doi: 10.1016/j.jor.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goyeneche RA, Primomo CE, Lambert N, Miscione H. Correction of bone angular deformities: experimental analysis of staples versus 8-plate. J Pediatr Orthop. 2009;29:736–740. doi: 10.1097/BPO.0b013e3181b529fc. [DOI] [PubMed] [Google Scholar]
- 43.Eltayeby HH, Iobst CA, Herzenberg JE. Hemiepiphysiodesis using tension band plates: does the initial screw angle influence the rate of correction? J Child Orthop. 2019;13:62–66. doi: 10.1302/1863-2548.13.180086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadhim M, Hammouda AI, Herzenberg JE. The “Sleeper” plate: A technical note. J Limb Lengthen Reconstr. 2019;5:27–32. [Google Scholar]
- 45.Keshet D, Katzman A, Zaidman M, Eidelman M. Removal of Metaphyseal Screw Only After Hemiepiphysiodesis Correction of Coronal Plane Deformities Around the Knee Joint: Is This a Safe and Advisable Strategy? J Pediatr Orthop. 2019;39:e236–e239. doi: 10.1097/BPO.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 46.Shabtai L, Herzenberg JE. Limits of Growth Modulation Using Tension Band Plates in the Lower Extremities. J Am Acad Orthop Surg. 2016;24:691–701. doi: 10.5435/JAAOS-D-14-00234. [DOI] [PubMed] [Google Scholar]
- 47.Oto M, Yılmaz G, Bowen JR, Thacker M, Kruse R. Adolescent Blount disease in obese children treated by eight-plate hemiepiphysiodesis. Eklem Hastalik Cerrahisi. 2012;23:20–24. [PubMed] [Google Scholar]
- 48.Joeris A, Ramseier L, Langendörfer M, von Knobloch M, Patwardhan S, Dwyer J, Slongo T. Paediatric lower limb deformity correction with the Eight Plate: adverse events and correction outcomes of 126 patients from an international multicentre study. J Pediatr Orthop B. 2017;26:441–448. doi: 10.1097/BPB.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 49.Jelinek EM, Bittersohl B, Martiny F, Scharfstädt A, Krauspe R, Westhoff B. The 8-plate versus physeal stapling for temporary hemiepiphyseodesis correcting genu valgum and genu varum: a retrospective analysis of thirty five patients. Int Orthop. 2012;36:599–605. doi: 10.1007/s00264-011-1369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosseinzadeh P, Ross DR, Walker JL, Talwalkar VR, Iwinski HJ, Milbrandt TA. Three Methods Of Guided Growth For Pediatric Lower Extremity Angular Deformity Correction. Iowa Orthop J. 2016;36:123–127. [PMC free article] [PubMed] [Google Scholar]
- 51.Zuege RC, Kempken TG, Blount WP. Epiphyseal stapling for angular deformity at the knee. J Bone Joint Surg Am. 1979;61:320–329. [PubMed] [Google Scholar]
- 52.Kadhim M, Hammouda AI, Herzenberg JE. Solid screw insertion for tension band plates: a surgical technique tip. J Child Orthop. 2016;10:307–311. doi: 10.1007/s11832-016-0748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leveille LA, Razi O, Johnston CE. Rebound Deformity After Growth Modulation in Patients With Coronal Plane Angular Deformities About the Knee: Who Gets It and How Much? J Pediatr Orthop. 2019;39:353–358. doi: 10.1097/BPO.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 54.Bowen JR, Leahey JL, Zhang ZH, MacEwen GD. Partial epiphysiodesis at the knee to correct angular deformity. Clin Orthop Relat Res. 1985:184–190. [PubMed] [Google Scholar]
- 55.Inan M, Chan G, Bowen JR. Correction of angular deformities of the knee by percutaneous hemiepiphysiodesis. Clin Orthop Relat Res. 2007;456:164–169. doi: 10.1097/01.blo.0000246560.65714.c8. [DOI] [PubMed] [Google Scholar]
- 56.Eltayeby HH, Gwam CU, Frederick MM, Herzenberg JE. How Accurate is the Multiplier Method in Predicting the Timing of Angular Correction After Hemiepiphysiodesis? J Pediatr Orthop. 2019;39:e91–e94. doi: 10.1097/BPO.0000000000001278. [DOI] [PubMed] [Google Scholar]
- 57.Wu Z, Ding J, Zhao D, Zhao L, Li H, Liu J. Multiplier method may be unreliable to predict the timing of temporary hemiepiphysiodesis for coronal angular deformity. J Orthop Surg Res. 2017;12:104. doi: 10.1186/s13018-017-0604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paley D. Principles of Deformity Correction. In: Osteotomy Concepts and Frontal Plane Realignment. Heidelberg: Springer, 2002: 99-154. [Google Scholar]
- 59.Paley D. Principles of Deformity Correction. In: Length Considerations: Gradual Versus Acute Correction of Deformities. Heidelberg: Springer, 2002: 269-289. [Google Scholar]
- 60.Riganti S, Nasto LA, Mannino S, Marrè Brunenghi G, Boero S. Correction of complex lower limb angular deformities with or without length discrepancy in children using the TL-HEX hexapod system: comparison of clinical and radiographical results. J Pediatr Orthop B. 2019;28:214–220. doi: 10.1097/BPB.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 61.Smith JO, Wilson AJ, Thomas NP. Osteotomy around the knee: evolution, principles and results. Knee Surg Sports Traumatol Arthrosc. 2013;21:3–22. doi: 10.1007/s00167-012-2206-0. [DOI] [PubMed] [Google Scholar]
- 62.Kamegaya M, Shinohara Y, Shinada Y. Limb lengthening and correction of angulation deformity: immediate correction by using a unilateral fixator. J Pediatr Orthop. 1996;16:477–479. doi: 10.1097/00004694-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Nogueira MP, Paley D. Prophylactic and Therapeutic Peroneal Nerve Decompression for Deformity Correction and Lengthening. Oper Tech Orthop. 2011;21:180–183. [Google Scholar]
- 64.Tunggal JA, Higgins GA, Waddell JP. Complications of closing wedge high tibial osteotomy. Int Orthop. 2010;34:255–261. doi: 10.1007/s00264-009-0819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71:698–703. [PubMed] [Google Scholar]
- 66.Dabash S, Prabhakar G, Potter E, Thabet AM, Abdelgawad A, Heinrich S. Management of growth arrest: Current practice and future directions. J Clin Orthop Trauma. 2018;9:S58–S66. doi: 10.1016/j.jcot.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papamerkouriou YM, Tsoumpos P, Tagaris G, Christodoulou G. Does Cozen's phenomenon warrant surgical intervention? J Child Orthop. 2020;14:213–220. doi: 10.1302/1863-2548.14.190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burton A, Hennrikus W. Cozen's phenomenon revisited. J Pediatr Orthop B. 2016;25:551–555. doi: 10.1097/BPB.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 69.Morin M, Klatt J, Stevens PM. Cozen's deformity: resolved by guided growth. Strategies Trauma Limb Reconstr. 2018;13:87–93. doi: 10.1007/s11751-018-0309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabharwal S. Blount disease. J Bone Joint Surg Am. 2009;91:1758–1776. doi: 10.2106/JBJS.H.01348. [DOI] [PubMed] [Google Scholar]
- 71.Sabharwal S. Blount disease: an update. Orthop Clin North Am. 2015;46:37–47. doi: 10.1016/j.ocl.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Janoyer M. Blount disease. Orthop Traumatol Surg Res. 2019;105:S111–S121. doi: 10.1016/j.otsr.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Blount WP. Tibia Vara. J Bone Joint Surg. 1937;19:1–29. [Google Scholar]
- 74.Davids JR, Blackhurst DW, Allen BL Jr. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278–284. doi: 10.1097/01202412-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 75.Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408–418. doi: 10.5435/JAAOS-21-07-408. [DOI] [PubMed] [Google Scholar]
- 76.Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158–1163. [PubMed] [Google Scholar]
- 77.Langenskiold A. Tibia vara; (osteochondrosis deformans tibiae); a survey of 23 cases. Acta Chir Scand. 1952;103:1–22. [PubMed] [Google Scholar]
- 78.Richards BS, Katz DE, Sims JB. Effectiveness of brace treatment in early infantile Blount's disease. J Pediatr Orthop. 1998;18:374–380. [PubMed] [Google Scholar]
- 79.Shinohara Y, Kamegaya M, Kuniyoshi K, Moriya H. Natural history of infantile tibia vara. J Bone Joint Surg Br. 2002;84:263–268. doi: 10.1302/0301-620x.84b2.11821. [DOI] [PubMed] [Google Scholar]
- 80.Vasiliadis AV, Maris A, Gadikoppula S. Tibia vara or Blount's disease: Why an early diagnosis and treatment are important? Clin Pract. 2020;10:1222. doi: 10.4081/cp.2020.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heflin JA, Ford S, Stevens P. Guided growth for tibia vara (Blount's disease) Medicine (Baltimore) 2016;95:e4951. doi: 10.1097/MD.0000000000004951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Danino B, Rödl R, Herzenberg JE, Shabtai L, Grill F, Narayanan U, Gigi R, Segev E, Wientroub S. The efficacy of guided growth as an initial strategy for Blount disease treatment. J Child Orthop. 2020;14:312–317. doi: 10.1302/1863-2548.14.200070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Griswold BG, Shaw KA, Houston H, Bertrand S, Cearley D. Guided growth for the Treatment of Infantile Blount's disease: Is it a viable option? J Orthop. 2020;20:41–45. doi: 10.1016/j.jor.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plateguided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30:594–597. doi: 10.1097/BPO.0b013e3181e4f591. [DOI] [PubMed] [Google Scholar]
- 85.Andrade N, Johnston CE. Medial epiphysiolysis in severe infantile tibia vara. J Pediatr Orthop. 2006;26:652–658. doi: 10.1097/01.bpo.0000230338.03782.75. [DOI] [PubMed] [Google Scholar]