Abstract
Exosomes are extracellular vesicles, 50–150 nm in diameter, released by most cells. Exosomes contain several intracellular components, including DNA, RNA, and proteins, which reflect the parent cell’s status and contribute to intercellular communication. Cancers are associated with high morbidity and mortality rates worldwide. Owing to a high survival rate, cancer treatment by immune modulation of the tumor microenvironment has recently received a lot of attention. Exosomes’ role in immunological control is also being studied extensively. Exosomes play a role in cancer-immune cell communication. Through intracellular communication, exosomes promote tumor growth, metastasis, angiogenesis, and drug resistance. In addition, innate immune cell-derived exosomes and adaptive immune cell exosomes have an anti-tumorigenic activity. Exosome-related tumor microenvironment drugs are being developed, including inhibitors of exosomal release, tumor-derived exosomes, and immune cell-derived exosome engineering, although there are still some obstacles to overcome. We describe in this review the significance of exosomes in the tumor microenvironment. We also summarize current studies on anticancer immune drug development and the challenges in developing exosome-related drugs.
Keywords: Exosome, Drug development, Cancer therapy, Pro-tumorigenic function, Anti-tumorigenic function
Introduction
Cancer remains the leading cause of death worldwide. There are currently over 200 different forms of cancer, with over 18 million new cases and 9 million deaths per year (Hannafon and Ding 2013; Bray et al. 2018). Several treatments have been developed, including surgery, radiation, chemotherapy, targeted therapy, and immunotherapy, to overcome these tumors. Although targeted therapy reduces the systemic adverse effects of previously used chemotherapy and radiation therapies, the problems of drug resistance and long-term effects remain (Yu et al. 2019). Immunotherapy is a method of suppressing or eliminating cancer by regulating the immune system, which has lately been highlighted. Immunotherapy functions by reactivating a weakened immune system or inducing an immune response by infusing cancer-fighting immune cells (Esfahani et al. 2020; Kennedy and Salama 2020). Immune checkpoint inhibitors (ICIs), immune stimulators, adoptive cell treatment, and cancer vaccines are types of immunotherapies (Waldman et al. 2020). Immunotherapy, particularly ICIs, has a disadvantage in that it has a low response rate, which has been linked to a low immune activity around the developing cancer (Rotte et al. 2018). New treatments are required to solve the problem of cancer therapies.
Exosomes are extracellular vesicles enveloped by a lipid bilayer membrane and are secreted by most cells, with sizes ranging from 20 to 150 nm. Exosomes were previously thought to play a role in waste transport within the cell; however, recent research has revealed that exosomes represent the state of the secretory cell and play an important role in cell–cell communication by regulating the role and function of the recipient cell, and contributing to the biological processes of various diseases, including cancer (Harding et al. 1983; Pan and Johnstone 1983; Vlassov et al. 2012; Lopez-Verrilli and Court 2013; Dai et al. 2020).
The exosomal functions are determined by exosomal proteins, lipids, and nucleic acids, such as DNA and RNA, derived from parent cells (Fig. 1). The types of DNA identified in exosomes include single-stranded DNA, double-stranded DNA, and mitochondrial DNA. RNA identified in exosomes includes microRNA (miRNA), mRNA, circular RNA (circRNA), long non-coding RNA (lncRNA), and other non-coding RNAs. Each of these proteins, lipids, and nucleic acids is delivered to the target cell and regulates cellular activities critical for exosome function (Thery et al. 2002b; Valadi et al. 2007; Skog et al. 2008; Zhang et al. 2017). Exosomes, which are structurally similar to lipid nanoparticles, can protect and deliver internal components. These properties facilitate the use of exosomes as a therapeutic material. Exosomes possess pharmacological potency, allowing them to be employed to carry drugs, proteins, and nucleic acids (Luan et al. 2017; Maia et al. 2018; Kalluri and LeBleu 2020). Other secreted vesicles include microvesicles and apoptotic blebs, which are distinguished by their diameters. Microvesicles and apoptotic blebs are typically 200–1000 nm and 500–2000 nm in size, respectively (Vlassov et al. 2012; Akers et al. 2013; Schageman et al. 2013; Doyle and Wang 2019). Therefore, developing a technology capable of separating homogeneous exosomes from these extracellular vesicles at a high purity is necessary to perform basic or developmental research on exosomes or utilize them as drugs or drug carriers. Ultracentrifugation and affinity-based commercial kits are currently the most commonly used exosome separation techniques. Additionally, tangential flow filtration (TFF) has been established as a separation technique. Nonetheless, homogeneously separating exosomes from several extracellular vesicles remains difficult. As a result, research is needed to develop new exosome isolation techniques and biomarkers.
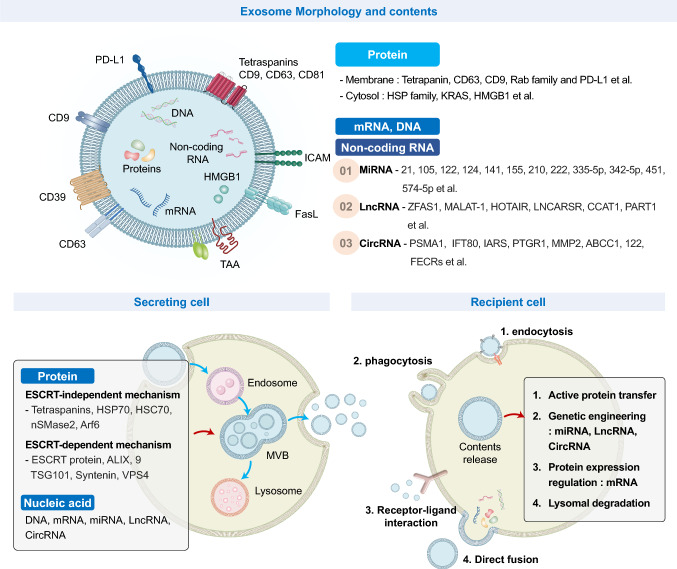
Fig. 1.
Exosome contents, biogenesis, and cell–cell communication. Exosomes contain various biomolecules such as membrane proteins (e.g., CD63, CD9, Rab family), cytosolic proteins (e.g., heat shock protein (HSP) family, oncogenic KRAS, HMGB1), mRNA, DNA, and non-coding RNAs (miRNA, lncRNA, circRNA), and contribute to cell–cell communication by delivering biomolecules to the recipient cell. During exosome biogenesis, an intraluminal vesicle (ILV) and a multivesicular body (MVB) are formed from the endosome. In MVB formation, cargo sorting has an endosomal sorting complex required for transport (ESCRT)-dependent and -independent pathways. MVBs translocate and fuse to the plasma membrane (PM), releasing exosomes into the extracellular space. For regulation of cellular functions, exosomes interact with the PM of recipient cells via the exosomal membrane or deliver biomolecules into the cells. Exosomes communicate with the recipient cell through: (1) endocytosis, (2) phagocytosis, (3) receptor-ligand interactions, and (4) direct fusion
In this review, we investigate the pro-tumorigenic and anti-tumorigenic properties of exosomes via cell–cell communication. We also discuss the application of exosome drug development based on these properties and the challenges associated with drug development.
Function of exosomes in the tumor microenvironment
Cancer progression is determined via cell–cell communication in the tumor microenvironment. Exosomes regulate the metabolic state of surrounding cells in the cancer environment. Through metabolic reprogramming, exosomes promote cancer growth, metastasis, angiogenesis, and drug resistance. Exosomes can be administered directly to cancer cells to suppress tumor progression or delivered to immune cells in the tumor microenvironment, inducing an anti-tumorigenic function via immune activation. Exosomes also enhance pro-tumorigenic functions by suppressing the immune system (Maia et al. 2018; Dai et al. 2020; Li and Simon 2020).
Pro-tumorigenic function of exosomes
Tumor proliferation
Since cancer cell proliferation is uncontrolled, it necessitates various intracellular components, such as proteins and lipids, as well as metabolic rewiring to maintain the growth rate. Additionally, cancer metabolism exhibits high aerobic glycolysis and macromolecule synthesis due to the amount of energy required and the abnormal tumor microenvironment (Zhu and Thompson 2019).
When exosomes are produced from parent cells, they carry several biological contents required for cancer cell division and metabolic regulation, such as the transport of the oncogenic form of epidermal growth factor receptor (EGFRvIII) mRNA via the exosomes. Glioblastoma patient serum contains EGFRvIII mRNA-positive exosomes, which transfer EGFRvIII mRNA to glioma cells, thus activating transforming signaling pathways (MAPK and AKT) to promote anchorage-independent growth capability and cell proliferation (Al-Nedawi et al. 2008). Additionally, exosomes secreted from glioma, NSCLC, and GC cell lines increase cancer cell proliferation by activating the AKT and ERK pathways (Skog et al. 2008). DNp73 mRNA is abundantly expressed in colon cancer exosomes and transported to recipient cells, wherein it suppresses p53, a tumor suppressor gene, resulting in a change in cellular signaling and increased cell proliferation (Soldevilla et al. 2014). Exosomes released by other cells in the microenvironment can affect cancer cell metabolism and proliferation. Zhao et al. (2016) demonstrated that exosomes secreted from cancer-associated fibroblasts (CAFs) regulate cancer cell metabolism. CAF-derived exosomes boost glycolysis and glutamine-dependent reductive carboxylation while decreasing mitochondrial oxidative phosphorylation (OXPHOS). (Zhao et al. 2016).
Exosomes can enhance tumor growth under stressful situations by transporting amino acids, TCA cycle intermediates, and lipids. By suppressing OXPHOS and increasing glycolysis, the exosomal lncRNA snhg3 can inhibit miRNA-330-5p, enhance pyruvate kinase (PKM) expression, and increase breast cancer cell proliferation (Li et al. 2020).
Together, these studies show that exosomes can promote tumor proliferation through metabolic reprogramming while providing sufficient material for cancer growth.
Tumor metastasis
Tumor metastasis occurs when cancer cells move away from their original locations and colonize distant organs. During tumor metastasis, cancer cells separate from the tumor into the blood and lymphatic vessels and move to other organs. As cancer cells arrive at the distant organs, they settle and populate, and tumor metastasis is completed (Zhang and Yu 2019; Fares et al. 2020). Therefore, tumor angiogenesis is a critical step in the early stages of metastasis, as it involves the formation of new blood vessels from existing ones. Tumor angiogenesis happens after a tumor has developed and can provide the nutrition and oxygen required for rapid growth (Folkman 2002; Gordon et al. 2010; Bielenberg and Zetter 2015).
Tumor-released exosomes are involved in angiogenesis and metastasis (Ahmadi and Rezaie 2020). Exosomes stimulate angiogenesis in endothelial cells, and angiogenesis-related proteins and nucleosides have been discovered inside exosomes (Ludwig and Whiteside 2018; Zhou et al. 2018). Exosomes containing VEGF can be transported to endothelial cells and activate the VEGF signaling pathway to promote angiogenesis. A recent study revealed that retinoblastoma cell-derived exosomes induce angiogenesis via miRNAs. miRNA-92a-3p, which is transported to human endothelial cells via exosomes, causes angiogenesis by increasing endothelial cell migration and tube formation (Chen et al. 2021).
In addition to angiogenesis, factors that increase tumor metastasis include an increase in cancer cell invasion and migration and the establishment of a pre-metastatic niche, an environment in which circulating tumor cells establish. Exosomes exert a direct effect on tumor cells and surrounding cells, thus increasing tumor migration, invasion, and the pre-metastatic niche. Increased epithelial-mesenchymal transition (EMT) of target tumor cells via exosomes is one approach that directly affects tumor cells. EMT is a dynamic process whereby cells change their phenotype from epithelial to mesenchymal state to further the cell migration, invasion, and formation of a pre-metastatic niche (Cho et al. 2019; Lebelo et al. 2019; Seo et al. 2021; Yang et al. 2020a). Exosomal miRNAs and lncRNAs are the key transcriptional regulators of EMT. LncRNA-PNUTS binds to miRNA-205, controls EMT, and promotes tumor metastasis by enhancing cell migration and invasion (Grelet et al. 2017; Hwang and Yang 2021).
There have also been reports on exosome functions, such as immune response, angiogenesis, and matrix remodeling, which are important in establishing a pre-metastatic niche for tumor metastasis (Guo et al. 2019). The immune system’s control and inflammation are key to the formation of a pre-metastatic niche for tumor metastasis. The expression of the S100 family, a pro-inflammatory protein, was increased in the pre-metastatic niche by exosomes containing various integrins (Hoshino et al. 2015). For cancer cells to settle in distant organs and evade immune surveillance, immune suppression in the pre-metastatic niche is essential. Exosomes express PD-L1 for immunological evasion. Cancer cell death and T cell activation are inhibited when PD-L1 interacts with PD-1 (Akinleye and Rasool 2019). Chen et al. found that exosomal PD-L1 promotes cancer growth through immunosuppression and decreases T cell responses in the spleen and lymph; in addition, circulating exosomal PD-L1 was identified in a patient with metastatic melanoma. As a result, immune suppression causes metastasis when exosomal PD-L1 reaches distant organs (Chen et al. 2018). Exosome immunosuppression is caused by the malfunctioning of CD4+ and CD8+T cells, as well as NK cells, and a decline in immune cell recruitment. By controlling the distant organ environment, exosomes with immunosuppressive properties can be transformed into a pre-metastatic niche (Clever et al. 2016; Czernek and Duchler 2017; Ludwig et al. 2017).
Collectively, the regulation of the tumor microenvironment through exosomes (cell migration/invasion and angiogenesis) and immunosuppression around the pre-metastatic niche are important mechanisms to accelerate cancer metastasis.
Tumor drug resistance
Several mechanisms underlie drug resistance, including drug efflux, drug metabolism mutations, DNA damage repair, and epigenetic alterations, which inhibit the ineffectiveness of anticancer drugs (Mashouri et al. 2019; Guo et al. 2020). Recent studies on the role of exosomes in drug resistance suggest that exosomes released by drug-resistant cancer cells generate drug resistance by transmitting drug resistance to other cells (Wang et al. 2020b). In CRC, high PKM2 levels are associated with oxaliplatin resistance. Drug resistance is acquired through an increase in PKM2 expression when circRNA-122 targeting miRNA targeting PKM is used to treat drug-sensitive cells via exosomes. Exosomes also contribute to the acquisition of treatment resistance in lung cancer patients. Exosomes isolated from chemoresistant lung cancer patients not only enhance pre-metastatic niche formation, but also diminish BACH2/GATA-3 expression, a transcription factor that promotes PKM2-dependent metabolism, resulting in drug resistance (Petanidis et al. 2020).
Exosomes secreted from fibroblasts promote CRC chemoresistance through exosomal Wnts. In cancer stem cells, exosomal Wnt stimulates Wnt activity and drug resistance (Hu et al. 2019). Exosomes, which are secreted by cancer stem cells, also increase drug resistance. The stimulation of the mTOR pathway by the transfer of miRNA-210 found in cancer stem cell exosomes causes pancreatic cancer resistance to gemcitabine (Yang et al. 2020b).
Thus, targeting exosomes to overcome drug resistance could be a reliable method; however, further studies are needed before specific therapies can be developed.
Anti-tumorigenic function of exosomes
Compared to the pro-tumorigenic function of exosomes, healthy cells and specific cancer cells exhibit anti-tumorigenic functions through immune activation. A biological response that identifies and suppresses foreign substances or autologous mutations is known as the immune response. The immune response can be divided into innate (an early recognition of foreign substances) and adaptive (activation of specific immune responses) responses. Exosomes produced by immune cells, particularly dendritic cells for innate immunity and T cells for adaptive immunity, inhibit cancer progression (Utsugi-Kobukai et al. 2003; Admyre et al. 2006; Pitt et al. 2014; Xu et al. 2020). Furthermore, tumor-secreted exosomes show anti-tumorigenic properties and anticancer efficacy by modulating the immune system via tumor-related antigens and immune stimulators (Rao et al. 2016).
Tumor-released exosomes
Exosomes are secreted by most cancer cells and have pro-tumorigenic properties such as tumor development, metastasis, angiogenesis, and drug resistance. However, tumor-released exosomes play an essential role in expressing an anti-tumorigenic function through an innate immune response.
First, exosomes generated by hepatocellular carcinoma (HCC) cells deliver antigens from parent cells to innate immune cells, eliciting a stronger immune response than tumor cell lysates. In animal experiments using HCC-released exosome-pulsed DCs, sufficient anti-tumor activity has been confirmed (Rao et al. 2016). HCC-released exosome-pulsed DCs increased the number of T lymphocytes around the tumor microenvironment, increased the levels of anti-tumor cytokine interferon-γ (IFN- γ), and decreased the amount of anti-immune cytokines IL-10 and tumor growth factor-β (TGF-β). Zech et al. (2012) discovered that tumor-released exosomes promote leukocyte proliferation and tumor antigen-specific cytotoxic T lymphocyte (CTL) responses in rat pancreatic adenocarcinoma Bsp73ASML (ASML)-derived exosomes (Zech et al. 2012). Another study suggests that a protein in exosomes generated by pancreatic cancer cells can activate DCs/cytokine-induced killer cells, resulting in anticancer effects. This raised the possibility of using the proteins from tumor-released exosomes as a cancer vaccine that inhibits cancer by transferring the parent cell’s protein to the immune system (Que et al. 2016). As a result, tumor-released exosomes could efficiently stimulate anti-tumor immunity. When tumor cell-released exosomes were treated with DCs and utilized to activate the DC cell vaccination in mice, the exosomes elicited an anti-tumor immune response and improved survival rates more efficiently than tumor cell lysates. Thus, the development of an anticancer vaccine based on exosomes will be effective, as demonstrated by the model (Li et al. 2013; Wang et al. 2020a). Hence, tumor-released exosomes have anticancer efficacy via tumor antigen and immune stimulation functions, and can be employed for cancer treatment or DC cell therapy to activate an effective anticancer immune system.
DC-derived exosome (DEX)
Relative to tumor-released exosomes that perform dual functions in the tumor microenvironment, DEX mainly exhibits anti-tumorigenic functions (Escudier et al. 2005; Pitt et al. 2016; Yao et al. 2021). DCs serve as antigen presenters in the early stages of anti-tumor immunity, triggering T-cell and B-cell adaptive immune systems to exert anticancer effects. DCs are activated via antigens in tumor-released exosomes, thereby inhibiting tumor growth, and also possess anticancer efficacy by secreting large amounts of exosomes (Lindenbergh et al. 2020).
Like the parental DCs, DEX are involved in T cell activation directly or indirectly, resulting in anticancer effects. DEX contains enough MHC-I, MHC-II, CD80, CD86, and HSP family of proteins to activate T cells. Zitvogel et al. (1998) used this feature of DEX to collect exosomes from tumor peptide-pulsed DCs, confirming T cell-dependent anticancer activity through CTL activation, demonstrating complete eradication in an in vivo model with a single injection (Zitvogel et al. 1998). DEX overexpression of α-fetoprotein reduced tumor progression in the HCC mouse model by boosting the amount of IFN-expressing CD8+T cells while decreasing regulatory T cell counts (Lu et al. 2017).
In previous studies, DEX’s direct T cell activation ability was shown to be effective for re-stimulation of activated or memory T cells but not for priming naive T cells (Thery et al. 2002a; Vincent-Schneider et al. 2002). Transferring an antigenic peptide/MHC complex to the surrounding antigen-presenting cells (APCs) utilizing DEX is a more effective technique to activate naive T cells. APCs activated by DEX are capable of naive T-cell priming. This kind of indirect naive T-cell activation was more efficient than DEX-mediated direct T-cell activation. The surface expression of ICAM-1 increases due to the exosomes produced by activated DCs, which further improves its binding to other APCs. In addition, increased expression of MHC and CD86 enables T cell priming by delivering antigens to bystander APCs and activating them sufficiently (Segura et al. 2005a, 2005b).
T cell-derived exosomes
In the immune system, T cells receive information about cancer antigens from APCs, such as DCs, and are activated to suppress cancer. T cells can be divided into the following two types: CD8+CTLs that express CD8 proteins and directly eliminate cancer, and CD4+helper T cells that express CD4 proteins and activate cytotoxic T and B cells (Lu et al. 2018).
CD8+T cell exosomes are released into numerous cells in the tumor microenvironment and possess anticancer properties. Exosomes released by active CD8+T lymphocytes inhibit cancer progression by eliminating mesenchymal cells from around the malignancy and destroying the tumor stroma (Seo et al. 2018). Exosomes secreted from CD8+T cells also stimulate other CD8+T cells exhibiting anticancer effects. Exosomes from IL-12-stimulated CD8+T cells are transported to bystander CD8+T cells to boost interferon-γ and granzyme B production. IL-12 stimulation changes the size and number of exosomes produced by CD8+T cells, thereby regulating bystander CD8 T cells. Additionally, T cell exosomes directly target tumors and exhibit anticancer effects (Li et al. 2017). In a recent study, Qiu et al. (2021) discovered that exosomal PD-1 released by activated T cells binds to PD-L1 in cancer cells and suppresses the immune response. PD-1 is a protein present on the surface of T cells, which binds to PD-L1 and inhibits the T cell-mediated immune response. The survival rate of TNBC patients with tumor-infiltrating lymphocytes (TILs) showing a strong PD-1 expression improved. This phenomenon occurs because the PD-1 exosome secreted from PD-1 positive TIL binds to cancer PD-L1 and suppresses its immunosuppressive action (Qiu et al. 2021). Therefore, exosomes secreted from activated and cancer-suppressing CD8+T cells can regulate the surrounding cells to eliminate cancer.
Cancer drug development using exosomes
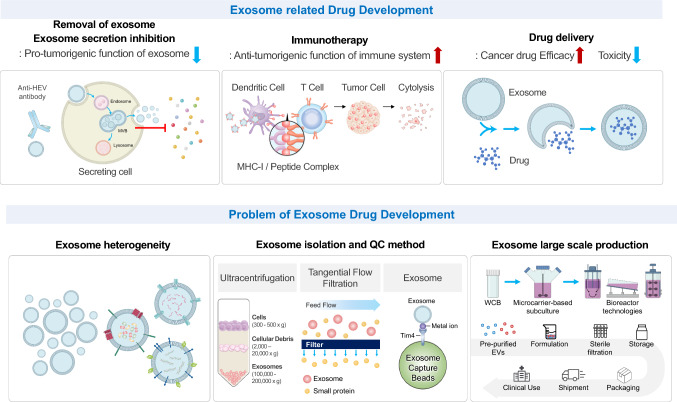
Exosomes are released by cells and include bioactive molecules with pro-tumorigenic and anti-tumorigenic properties. There are three methods for developing anticancer drugs using exosomes (Fig. 2). The first is the use of exosomes to kill cancer cells directly. Second, exosomes are used as drug carriers like siRNAs, miRNAs, chemotherapeutics, and proteins. The antigen delivery technology and immune simulator can be used as cancer vaccines or adjuvants. Finally, because exosomes have pro-tumorigenic activity, blocking their production is a way to suppress tumor progression and metastasis.
Fig. 2.
Advantages and problems of exosome drug development. Exosome-related drug development focuses on (1) inhibiting the pro-tumorigenic function of exosome secretions, (2) cancer vaccines, and (3) drug delivery systems. The problem with exosome drug development is that homogeneous exosome isolation technology has not yet been developed. In addition, the gold-standard method for isolating exosomes and an appropriate quality control method need to be established
Drug delivery via exosomes
Chemotherapeutics are used to treat cancer cells that divide rapidly but exert serious side effects as they kill healthy cells (Alfarouk et al. 2015; Zhao and Zhang 2017; Nurgali et al. 2018). Drug delivery systems based on nanoparticles have been developed to overcome these side effects. However, just 0.7% of nanoparticles reach tumors in previous in vivo experiments using nanoparticles, making their usage difficult. Therefore, a novel drug delivery system is necessary (Wilhelm et al. 2016; He et al. 2019).
Exosomes are employed to deliver anticancer drugs and other bioactive molecules (Walker et al. 2019; Chen et al. 2020). Various strategies for loading drugs into exosomes have been established. There are two methods for loading drugs into exosomes: 1. loading drugs when exosomes are being created, 2. directly injecting drugs into exosomes (for example, via electroporation, sonication, and incubation). Exosome-loaded drugs successfully inhibit cancer or reduce side effects. For example, when exosomes are utilized to carry doxorubicin, cardiac toxicity reduces by approximately 40%, while the anticancer effect is maintained. Furthermore, the low toxicity of exosomal doxorubicin allows for higher doses in mouse breast and ovarian cancer models, resulting in a more effective anticancer response (Toffoli et al. 2015; Hadla et al. 2016). Drug loading into exosomes has a more effective anticancer activity than a single treatment. In a breast tumor model using NOD/SCID mice, doxorubicin- and taxol-loaded mesenchymal stroma/stem-like cell (MSC)-derived exosomes showed more potent anticancer and metastasis inhibitory effects than the controls (Melzer et al. 2019). Finally, exosomes carrying paclitaxel show a stronger anticancer activity in prostate cancer (Saari et al. 2015). Therefore, exosome-based chemotherapeutic administration can lessen side effects while increasing anticancer potency.
Drug development via secretion, inhibition, and removal of exosomes
Tumor-released exosomes are involved in tumor progression, metastasis, angiogenesis, formation of a pre-metastatic niche, and drug resistance, thus performing pro-tumorigenic functions (Whiteside 2016; Dai et al. 2020). Exosomes generated by tumors decrease DC maturation and NK cell activation, as well as enhance macrophage differentiation into tumor-promoting macrophages (Chen et al. 2017; Olejarz et al. 2020). Therefore, inhibiting tumor-released exosome secretion would be an effective anticancer strategy.
Blocking tumor-released exosome secretion is accomplished by inhibiting exosome production and eliminating the release of exosomes. Rab27a is an essential factor in exosome secretion, and the delivery of miRNA-494 to inhibit Rab27a in nude mice with melanoma decreased primary tumor progression and metastasis (Li et al. 2019). Im et al. (2019) recently showed that the FDA-approved antibiotic sulfisoxazole (SFX), suppresses tumor progression and metastasis by inhibiting the secretion of small extracellular vesicles (sEVs). Through the binding of MVB and lysosomes, SFX restricts the secretion of sEVs via mechanical destruction (Im et al. 2019). Exosomes generated by tumors are found in the blood of patients and have been shown to increase tumor growth and metastasis. Exosome elimination can thereby decrease the pro-tumorigenic activity of the exosome. A hemofiltration system (such as the Aethlon ADAPTtm system) has been developed for this purpose (Marleau et al. 2012). Blocking exosome release or decreasing exosomes in the blood of cancer patients may be a good strategy for suppressing cancer since cancer exosomes typically augment pathogenesis by tumor progression and metastasis.
Cancer vaccine: tumor antigen and adjuvant
A cancer vaccine (composed of antigen and adjuvant) is a drug for controlling the immune system to treat cancer. Cancer vaccines contain antigens that are either tumor-specific proteins found solely in cancer cells or tumor-associated proteins, which are found in a variety of cells, especially in cancer. An adjuvant can boost immunological activation in the absence of an antigen.
A cancer vaccine acts in the early stages of the cancer immunity cycle. Antigens and adjuvants are intentionally included in cancer vaccines to mimic antigen release during early cancer cell death and stimulate anticancer immunity. Recently, it was demonstrated that exosomes could act as antigens and adjuvants to activate the cancer immunity cycle (Dutta 2021). Cancer vaccines using tumor-released exosomes have the advantage of being cancer-specific. Recently, several attempts have been made to treat cancer by activating anticancer immunity via cancer vaccines using cancer antigens. However, identifying cancer-specific antigens takes time, and ensuring adequate T cell activation to treat cancer is challenging (Jeanbart et al. 2014; Ott et al. 2017; Sahin and Tureci 2018).
Exosomes preserve their parent cell characteristics. Therefore, they can be utilized as a source of cancer antigens. The first report of a cancer vaccine using a tumor-released exosome eliciting a T cell immune response against specific tumors was published in 2001. (Wolfers et al. 2001; Vincent-Schneider et al. 2002; Whiteside 2017). Because tumor-released exosomes contain tumor antigens and immunostimulatory molecules, such as CD70, CD80, OX40, MHC molecules, and HSPs, these can directly activate innate immune cells. For example, HSP70 in the exosome is a DAMP protein that acts on innate immune cells to increase pro-inflammatory cytokine secretion (Graner et al. 2007). Exosomes containing both tumor antigens and immunostimulatory molecules can be exploited as cancer vaccines, and clinical trials on exosomes released from tumors are ongoing (NCT02657460 and NCT01854866). However, as tumor-released exosomes have a pro-tumorigenic role, further research is needed before they can be used effectively.
Exosomes secreted by immune cells can also be used as cancer vaccines. Through MHC-I and MHC-II molecules, as well as co-stimulatory molecules CD40, CD80, and CD86, DEX has anti-tumorigenic activities and activate other immune cells, including DCs or parent cells (Admyre et al. 2006; Pitt et al. 2016). A single intradermal injection of tumor peptide-pulsed DEX could suppress and eradicate tumor development (Zitvogel et al. 1998). DEX can also stimulate tumor-specific CD8+T cells in combination with synthetic peptides to inhibit the tumor (Andre et al. 2004).
Engineered exosome is another strategy to utilize exosomes as cancer vaccines. It was confirmed that purified exosomes from Lewis lung tumor cells overexpressing CD40L have a DC maturation effect in vivo. It can suppress Lewis lung tumors by activating anticancer immunity (Wang et al. 2014). CD47 is a "don’t eat me" signal that is overexpressed in tumor cells and serves as a myeloid immune checkpoint that regulates cancer cell phagocytosis by binding to SIRP-α. Exosomes overexpressing SIRP-α interact with the CD47 of tumor cell membrane, thus enhancing macrophage phagocytization, increasing T cell infiltration around the tumor cell, and suppressing tumor growth (Koh et al. 2017; Cho et al. 2018). Codiak Biosciences (Cambridge, MA, USA), an exosome research and development company, has developed exoSTING, exosome including the STING agonist, a TLR agonist. ExoSTING overexpressing PTGFRN shows that boosting IFN genes (STING) levels in APCs using CDN, a small molecule STING agonist, thus preventing tumor progression (Dooley et al. 2021).
Challenges in exosome for drug development
Exosome-based drug development and clinical trials are ongoing (Table 1). A clinical trial using MSC exosomes to treat COVID-19, which has recently caused a pandemic, is currently underway (NCT04798716) (Chen et al. 2020; Perocheau et al. 2021). However, the following issues need to be addressed in the development of exosome drugs and therapeutics: exosome heterogeneity and the development of a gold-standard method for exosome isolation, as well as large-scale exosome manufacturing, purification, and quality control (Fig. 2).
Table 1.
Clinical trials based on exosome and micro particle for cancer treatment
| ID | Indication | Usage | Administration | Dose | Phase | Purification | Exosome Manipulation |
|---|---|---|---|---|---|---|---|
| NCT01668849 | Head and neck cancer | Single immunotherapy | Grape extract selfadministered by mouth daily for 35 days | Not available | Phase 1 | Not available | Unmodified |
| NCT01294072 | Colon cancer | Single immunotherapy | Tablets-taken daily for 7 days | 3.6 g | Phase 1 | Not available | Curcumin conjugated plant exosome |
| NCT01159288 | Non-small cell lung cancer | Tumor antigen delivery | Intradermal injection a weekly interval during consecutive weeks and intradermal injection every 2 weeks during 6 weeks |
8.5 × 1011 – 1.0 × 10.13 MHC Class II molecules |
Phase 2 | 500-kDa concentration and UC with D2O/sucrose cushion |
peptide pulsed on to DC, EBV, MAGE-A3 DP04, A1, -A3, NY-ESO-1, Melan-A/MART-1 |
| NCT03608631 | Metastatic pancreatic adenocarcinoma | siRNA delivery | Intravenous injection: 1, 4, 10 days and repeat every 14 days for up to 3 courses | Not available | Phase 1 |
Differential centrifugations + sucrose density gradient UC |
Electroporation with KrasG12D siRNA |
| NCT01854866 | Malignant pleural effusion, malignant ascites | Drug delivery | Locally injected 4 times a week | Not available | Phase 2 | Differential centrifugations | Added cisplatin |
| NCT02657460 | Malignant pleural effusion | Drug delivery | Injected once in 2 days until malignant pleural effusion are disappeared or the treatment cycle has been six times | Not available | Phase 2 | Differential centrifugations | Added methotrexate (MTX) |
|
REF (Escudier et al.) |
Melanoma | Vaccination | Every week vaccination intradermal, subcutaneous injection during 4 weeks |
4 × 1012 or 1.3 × 1013 MHC Class II molecules |
Phase 1 | 500-kDa ultrafiltration (UF), ultracentrifugation with sucrose cushion | Pulsed with MAGE tumor peptides |
|
REF (Morse et al.) |
Non-small cell lung cancer | Vaccination | Combination of subcutaneous and intradermal injection weekly for 4 weeks | 1.3 × 1013 MHC Class II molecule | Phase 1 | Ultracentrifugation with D2O/sucrose cushion | Pulsed with MAGE-A3, A4, -A10, -3DPO4 tumor peptide |
|
REF (Dai et al.) |
Colorectal cancer | Vaccination | 4 Subcutaneous injection at weekly intervals | 100–500 μg of protein | Phase 1 | Differential centrifugation with D2O/sucrose cushion | With GM-CSF |
Exosome heterogeneity and exosome isolation methods
The exosome population is heterogeneous, which causes problems in exosome biogenesis research, development of therapeutic and diagnostic indications using exosomes, as well as the approval of therapeutic agents. In a recent review, exosome heterogeneity was classified into size, content, functional, and source heterogeneity. Exosomes are classified into three groups based on their sizes: 40–75 nm Exosome A, 75–100 nm Exosome B, and 100–160 nm Exosome C. The representative exosome surface markers CD9, CD63, and CD81 are used to classify exosomes into three categories. Heterogeneity is caused by the unequal production of MVBs during exosome biogenesis (Kalluri and LeBleu 2020).
The contents of purified exosomes are different when exosomes are isolated through different exosome purification methods (Kowal et al. 2016). Such exosome heterogeneity represents limitations of the current exosome separation technology in terms of size-dependence and marker-dependence. The primary requirement of the innovative exosome purification technology is to overcome exosome heterogeneity through more precise size-specific separation. It should be possible to separate more homogeneous exosomes by separating and purifying 40–75 nm Exosome A and 75–100 nm Exosome B. Therefore, a filter for size-dependent exosome purification must be developed, and the TFF approach, which uses a filter with 10 nm units, has the advantages of better homogeneity of exosome classification, less time, and a large workforce. Additionally, TFF has been proven to purify exosomes more accurately compared to UC (Bari et al. 2019).
The second requirement is to develop a novel exosome marker and isolate homogeneous exosomes. Exoquick, an affinity capture technology for exosome purification, is already on the market. Exosome purification techniques have been compared in previous publications, and affinity-based exosome purification has been demonstrated to possess a greater ability to purify homogeneous exosomes than UC or differential centrifugation. The possibility of homogeneous exosome purification utilizing affinity capture technology based on a specific exosome marker has been confirmed (Tauro et al. 2012). Therefore, the exosome purification method is expected to be developed by the fusion of size-dependent and marker-dependent purification techniques, and this novel technology will be useful for basic research and development through the isolation of highly homogeneous exosomes.
Large-scale production, purification, and quality control
Each clinical trial utilizes a different large-scale manufacturing method (Table 1). One of the main challenges to manufacturing exosomes for clinical trials is the development of large-scale cell culture techniques for exosome purification. Recent clinical trials have primarily used MSC-based exosomes or immune cell-based exosomes; however, large-scale stem cell culture limited by high cost and low-quality control (Kirouac and Zandstra 2008; Ahrlund-Richter et al. 2009; Isasi et al. 2016).
Various approaches to resolving this problem are being investigated. A study was conducted to produce exosomes utilizing a serum-free medium without fetal bovine serum (FBS) to improve economic feasibility and obtain high purity exosomes from parental cells. A study using neuroblastoma cells suggests that converting from an FBS-containing medium to a serum-free medium had no effect on exosome size or biophysical tendency. In fact, the quantity of exosomes increases in the serum-free medium. Thus, the possibility of exosome production in the serum-free medium without FBS was verified (Kordelas et al. 2014).
Another major challenge to the efficient production of exosomes is the gold standard method of large-scale exosome purification. Size exclusion, sedimentation force or flotation density, precipitation-based procedures, and affinity-based methods are the four strategies used for exosome purification. Exosomes are currently isolated using filtration and ultracentrifugation. This strategy, however, has its limitations of being time and labor-intensive requiring a scale-out rather than a scale-up. Furthermore, as the exosome purification procedure is comparable to the initial virus purification process, removing virus contamination during clinical-grade exosome isolation is difficult. Therefore, a new type of exosome purification technology for therapeutic use is required (Colao et al. 2018).
The ideal exosome purification method is the sequential purification of exosomes. For example, filtration-based recovery, followed by chromatography-based purification. TFF is a powerful exosome purification technique that may concentrate MSC-released exosomes by up to 125 times (Lai et al. 2010). Combining size-exclusion liquid chromatography with ultrafiltration is capable of separating exosomes with a higher yield than differential ultracentrifugation without affecting biophysical properties. This approach is also ideal for exosome purification on a larger scale (Nordin et al. 2015).
A recent study presented an exosome purification method through a selection using TFF, affinity capture, and additional charge or other chemical properties. This method is scalable and can purify specific exosomes (Colao et al. 2018).
Conclusions
Exosomes have been studied and developed extensively worldwide. Additionally, several preclinical and clinical trials, as well as laboratory-level research, have been conducted to develop therapeutic agents using exosomes.
Despite various studies conducted on cancer treatment, cancer remains the leading cause of death globally. Chemotherapy, targeted therapy, and ICI have not effectively controlled cancer progression. Because chemotherapy has several negative effects, targeted therapy has a limitation of indication, and ICI shows a low response rate, improved cancer treatment strategies are needed. Exosome-based immunotherapy can improve the low response rate of ICIs. It has also been used as a chemotherapeutic drug delivery technique and has the potential to reduce the side effects of standard chemotherapy while also improving its efficacy.
However, because exosomes, particularly tumor-released exosomes, have a pro-tumorigenic proclivity and show excessive immune responses, further studies on their pro-tumorigenic and anti-tumorigenic functions are required. Establishing a gold-standard for large-scale manufacturing procedure to produce scalable and pure exosomes for clinical trials is imperative. Despite several challenges, exosomes show great potential as a drug candidate and drug delivery tool to treat cancer.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIT) (Grant Nos. 2021R1F1A1060166, 2022R1C1C1013481).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Rezaie J. Tumor cells derived-exosomes as angiogenenic agents: possible therapeutic implications. J Transl Med. 2020;18:249. doi: 10.1186/s12967-020-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrlund-Richter L, De Luca M, Marshak DR, Munsie M, Veiga A, Rao M. Isolation and production of cells suitable for human therapy: challenges ahead. Cell Stem Cell. 2009;4:20–26. doi: 10.1016/j.stem.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. doi: 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- Bari E, Perteghella S, Catenacci L, Sorlini M, Croce S, Mantelli M, Avanzini MA, Sorrenti M, Torre ML. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (Lond) 2019;14:753–765. doi: 10.2217/nnm-2018-0240. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Zetter BR. The contribution of angiogenesis to the process of metastasis. Cancer J. 2015;21:267–273. doi: 10.1097/PPO.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Chen W, Jiang J, Xia W, Huang J. Tumor-related exosomes contribute to tumor-promoting microenvironment: an immunological perspective. J Immunol Res. 2017;2017:1073947. doi: 10.1155/2017/1073947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, Mcgettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2020;32:113–120. doi: 10.4103/tcmj.tcmj_182_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Chen X, Luo Q, Liu X, Wang X, Cui Z, He A, He S, Jiang Z, Wu N, Chen P, Yu K, Zhuang J. Retinoblastoma cell-derived exosomes promote angiogenesis of human vesicle endothelial cells through microRNA-92a-3p. Cell Death Dis. 2021;12:695. doi: 10.1038/s41419-021-03986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Nam GH, Hong Y, Kim YK, Kim DH, Yang Y, Kim IS. Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J Control Release. 2018;279:326–335. doi: 10.1016/j.jconrel.2018.04.037. [DOI] [PubMed] [Google Scholar]
- Cho ES, Kang HE, Kim NH, Yook JI. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT) Arch Pharm Res. 2019;42:14–24. doi: 10.1007/s12272-018-01108-7. [DOI] [PubMed] [Google Scholar]
- Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell. 2016;166(1117–1131):e14. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24:242–256. doi: 10.1016/j.molmed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Czernek L, Duchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp (Warsz) 2017;65:311–323. doi: 10.1007/s00005-016-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley K, Mcconnell RE, Xu K, Lewis ND, Haupt S, Youniss MR, Martin S, Sia CL, Mccoy C, Moniz RJ, Burenkova O, Sanchez-Salazar J, Jang SC, Choi B, Harrison RA, Houde D, Burzyn D, Leng C, Kirwin K, Ross NL, Finn JD, Gaidukov L, Economides KD, Estes S, Thornton JE, Kulman JD, Sathyanarayanan S, Williams DE. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther. 2021;29:1729–1743. doi: 10.1016/j.ymthe.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A. Exosomes-based cell-free cancer therapy: a novel strategy for targeted therapy. Immunol Med. 2021;44:116–123. doi: 10.1080/25785826.2020.1818482. [DOI] [PubMed] [Google Scholar]
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH., Jr. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27:S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Mendelson DS, Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer. 2010;126:1777–1787. doi: 10.1002/ijc.25026. [DOI] [PubMed] [Google Scholar]
- Graner MW, Cumming RI, Bigner DD. The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences. J Neurosci. 2007;27:11214–11227. doi: 10.1523/JNEUROSCI.3588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelet S, Link LA, Howley B, Obellianne C, Palanisamy V, Gangaraju VK, Diehl JA, Howe PH. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19:1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W, Ji Z, Sun Z. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39. doi: 10.1186/s12943-019-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo QR, Wang H, Yan YD, Liu Y, Su CY, Chen HB, Yan YY, Adhikari R, Wu Q, Zhang JY. The role of exosomal microRNA in cancer drug resistance. Front Oncol. 2020;10:472. doi: 10.3389/fonc.2020.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, Rizzolio F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (Lond) 2016;11:2431–2441. doi: 10.2217/nnm-2016-0154. [DOI] [PubMed] [Google Scholar]
- Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc Chem Res. 2019;52:2445–2461. doi: 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, De Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H, Li XL, Tao DD, Wu YQ, Gong JP, Qin JC. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. 2019;38:1951–1965. doi: 10.1038/s41388-018-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Yang YM. Exosomal microRNAs as diagnostic and therapeutic biomarkers in non-malignant liver diseases. Arch Pharm Res. 2021;44:574–587. doi: 10.1007/s12272-021-01338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im EJ, Lee CH, Moon PG, Rangaswamy GG, Lee B, Lee JM, Lee JC, Jee JG, Bae JS, Kwon TK, Kang KW, Jeong MS, Lee JE, Jung HS, Ro HJ, Jun S, Kang W, Seo SY, Cho YE, Song BJ, Baek MC. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat Commun. 2019;10:1387. doi: 10.1038/s41467-019-09387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isasi R, Rahimzadeh V, Charlebois K. Uncertainty and innovation: Understanding the role of cell-based manufacturing facilities in shaping regulatory and commercialization environments. Appl Transl Genom. 2016;11:27–39. doi: 10.1016/j.atg.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanbart L, Ballester M, De Titta A, Corthesy P, Romero P, Hubbell JA, Swartz MA. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2:436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Lebleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang Y, Kim IS. Exosome-SIRPalpha, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, De Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Arch Pharm Res. 2019;42:833–847. doi: 10.1007/s12272-019-01185-2. [DOI] [PubMed] [Google Scholar]
- Li F, Simon MC. Cancer cells don’t live alone: metabolic communication within tumor microenvironments. Dev Cell. 2020;54:183–195. doi: 10.1016/j.devcel.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X. Exosomes derived from Rab27aoverexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep. 2013;8:1876–1882. doi: 10.3892/mmr.2013.1738. [DOI] [PubMed] [Google Scholar]
- Li L, Jay SM, Wang Y, Wu SW, Xiao Z. IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8(+) T cells. Sci Rep. 2017;7:13365. doi: 10.1038/s41598-017-14000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Wang S, Li P, Zheng C, Zhou X, Tao Y, Chen X, Sun L, Wang A, Cao K, Tang S, Zhou J. Blockage of transferred exosome-shuttled miR-494 inhibits melanoma growth and metastasis. J Cell Physiol. 2019 doi: 10.1002/jcp.28234. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao Z, Liu W, Li X. SNHG3 functions as miRNA sponge to promote breast cancer cells growth through the metabolic reprogramming. Appl Biochem Biotechnol. 2020;191:1084–1099. doi: 10.1007/s12010-020-03244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbergh MFS, Wubbolts R, Borg EGF, van Van’t Veld EM, Boes M, Stoorvogel W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J Extracell Vesicles. 2020;9:1798606. doi: 10.1080/20013078.2020.1798606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verrilli MA, Court FA. Exosomes: mediators of communication in eukaryotes. Biol Res. 2013;46:5–11. doi: 10.4067/S0716-97602013000100001. [DOI] [PubMed] [Google Scholar]
- Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, Qi H, Guo H, Yin H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu J, Tian J, Wang S. Role of T cell-derived exosomes in immunoregulation. Immunol Res. 2018;66:313–322. doi: 10.1007/s12026-018-9000-0. [DOI] [PubMed] [Google Scholar]
- Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22:409–417. doi: 10.1080/14728222.2018.1464141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23:4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell–cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer C, Rehn V, Yang Y, Bahre H, Von Der Ohe J, Hass R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers (Basel) 2019 doi: 10.3390/cancers11060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin JZ, Lee Y, Vader P, Mager I, Johansson HJ, Heusermann W, Wiklander OP, Hallbrink M, Seow Y, Bultema JJ, Gilthorpe J, Davies T, Fairchild PJ, Gabrielsson S, Meisner-Kober NC, Lehtio J, Smith CI, Wood MJ, El Andaloussi S. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Nurgali K, Jagoe RT, Abalo R. Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol. 2018;9:245. doi: 10.3389/fphar.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejarz W, Dominiak A, Zolnierzak A, Kubiak-Tomaszewska G, Lorenc T. Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res. 2020;2020:6272498. doi: 10.1155/2020/6272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Perocheau D, Touramanidou L, Gurung S, Gissen P, Baruteau J. Clinical applications for exosomes: are we there yet? Br J Pharmacol. 2021;178:2375–2392. doi: 10.1111/bph.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanidis S, Domvri K, Porpodis K, Anestakis D, Freitag L, Hohenforst-Schmidt W, Tsavlis D, Zarogoulidis K. Inhibition of kras-derived exosomes downregulates immunosuppressive BACH2/GATA-3 expression via RIP-3 dependent necroptosis and miR-146/miR-210 modulation. Biomed Pharmacother. 2020;122:109461. doi: 10.1016/j.biopha.2019.109461. [DOI] [PubMed] [Google Scholar]
- Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, Zitvogel L. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Yang Y, Yang R, Liu C, Hsu JM, Jiang Z, Sun L, Wei Y, Li CW, Yu D, Zhang J, Hung MC. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40:4992–5001. doi: 10.1038/s41388-021-01896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que RS, Lin C, Ding GP, Wu ZR, Cao LP. Increasing the immune activity of exosomes: the effect of miRNA-depleted exosome proteins on activating dendritic cell/cytokine-induced killer cells against pancreatic cancer. J Zhejiang Univ Sci B. 2016;17:352–360. doi: 10.1631/jzus.B1500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29:71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Sahin U, Tureci O. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- Schageman J, Zeringer E, Li M, Barta T, Lea K, Gu J, Magdaleno S, Setterquist R, Vlassov AV. The complete exosome workflow solution: from isolation to characterization of RNA cargo. Biomed Res Int. 2013;2013:253957. doi: 10.1155/2013/253957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- Seo N, Shirakura Y, Tahara Y, Momose F, Harada N, Ikeda H, Akiyoshi K, Shiku H. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435. doi: 10.1038/s41467-018-02865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Ha J, Kang E, Cho S. The role of epithelial-mesenchymal transition-regulating transcription factors in anti-cancer drug resistance. Arch Pharm Res. 2021;44:281–292. doi: 10.1007/s12272-021-01321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, Van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldevilla B, Rodriguez M, San Millan C, Garcia V, Fernandez-Perianez R, Gil-Calderon B, Martin P, Garcia-Grande A, Silva J, Bonilla F, Dominguez G. Tumor-derived exosomes are enriched in DeltaNp73, which promotes oncogenic potential in acceptor cells and correlates with patient survival. Hum Mol Genet. 2014;23:467–478. doi: 10.1093/hmg/ddt437. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S, Gamini A, Canzonieri V, Rizzolio F. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine (Lond) 2015;10:2963–2971. doi: 10.2217/nnm.15.118. [DOI] [PubMed] [Google Scholar]
- Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX, Wolfram J. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol Med Rep. 2014;9:125–131. doi: 10.3892/mmr.2013.1759. [DOI] [PubMed] [Google Scholar]
- Wang C, Huang X, Wu Y, Wang J, Li F, Guo G. Tumor cell-associated exosomes robustly elicit anti-tumor immune responses through modulating dendritic cell vaccines in lung tumor. Int J Biol Sci. 2020;16:633–643. doi: 10.7150/ijbs.38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T, Liu R, Fan Q, Zhu K, Li J, Zhan Y, Ying G, Ba Y. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14:539–555. doi: 10.1002/1878-0261.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Stimulatory role of exosomes in the context of therapeutic anti-cancer vaccines. Biotarget. 2017 doi: 10.21037/biotarget.2017.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, Garcia De Herreros A, Goodall GJ, Hadjantonakis AK, Huang RYJ, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massague J, Mayor R, Mcclay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G, Association EMTI. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–352. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (Dordr) 2020;43:123–136. doi: 10.1007/s13402-019-00476-6. [DOI] [PubMed] [Google Scholar]
- Yao Y, Fu C, Zhou L, Mi QS, Jiang A. DC-derived exosomes for cancer immunotherapy. Cancers (Basel) 2021 doi: 10.3390/cancers13153667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WD, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66–70. doi: 10.1016/j.canlet.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Zech D, Rana S, Buchler MW, Zoller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10:37. doi: 10.1186/1478-811X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J, Ning T, Huang D, Li H, Zhang L, Ying G, Ba Y. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep. 2017;7:44735. doi: 10.1038/srep44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Chen KK, Zhang J, Xiao B, Huang Z, Ju C, Sun J, Zhang F, Lv XB, Huang G. The decade of exosomal long RNA species: an emerging cancer antagonist. Mol Cancer. 2018;17:75. doi: 10.1186/s12943-018-0823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20:436–450. doi: 10.1038/s41580-019-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]