Abstract
Background:
Stroke persists as an important cause of long-term disability world-wide with the need for rehabilitation strategies to facilitate plasticity and improve motor function in stroke survivors. Rhythm-based interventions can improve motor function in clinical populations. This study tested a novel music-motor software application ‘GotRhythm’ on motor function after stroke.
Methods:
Participants were 22 stroke survivors undergoing inpatient rehabilitation in a subacute stroke ward. Participants were randomised to the GotRhythm intervention (combining individualised music and augmented auditory feedback along with wearable sensors to deliver a personalised rhythmic auditory stimulation training protocol) or usual care. Intervention group participants were offered 6-weeks of the GotRhythm intervention, consisting of a supervised 20-minute music-motor therapy session using GotRhythm conducted 3 times a week for 6 weeks. The primary feasibility outcomes were adherence to the intervention and physical function (change in the Fugl-Meyer Assessment of Motor Recovery score) measured at baseline, after 3-weeks and at end of the intervention period (6-weeks).
Results:
Three of 10 participants randomised to the intervention did not receive any of the GotRhythym music-motor therapy. Of the remaining 7 intervention group participants, only 5 completed the 3-week mid-intervention assessment and only 2 completed the 6-week post-intervention assessment. Participants who used the intervention completed 5 (IQR 4,7) sessions with total ‘dose’ of the intervention of 70 (40, 201) minutes.
Conclusion:
Overall, adherence to the intervention was poor, highlighting that application of technology assisted music-based interventions for stroke survivors in clinical environments is challenging along with usual care, recovery, and the additional clinical load.
Keywords: Stroke, rehabilitation, music-motor therapy
Introduction
Stroke is the third most common cause of death in Australia and a leading cause of long-term disability. 1 More than 50% of stroke survivors report some degree of disability and require long-term assistance and support after discharge from hospital. 2 Clinical and animal studies have shown extensive cortical reorganisation and injury-induced plasticity occurs in the motor areas and corticospinal tract after stroke.2-4 Harnessing these plasticity processes may provide a means of maximising recovery of stroke survivors.5,6
Currently, rehabilitation and interventions for stroke recovery are focussed on intensive motor training, exercise and physiotherapy. Research shows rehabilitation programmes that incorporate the principles of motor learning, such as mass repetition of goal-oriented and task-specific exercises performed at high intensity can induce beneficial plasticity and produce long-term changes in motor function in stroke survivors.7,8
Although beneficial effects have been observed in stroke survivors with intensive motor training, there are several limitations of current therapies and rehabilitation approaches. Chronic stroke survivors are a heterogenous population presenting with a variety of upper and lower extremity impairments and treatment must be tailored to the needs of individual stroke patients. 2 Clinical guidelines now also recommend larger doses of motor training (>2 hours per day), which can be difficult for those with severe impairments and older stroke survivors.9-11 Finally, reduced motivation and low treatment adherence to rehabilitation programmes has been observed in stroke survivors both in hospital and in the community and this is a significant barrier to recovery.12,13 Therefore, there is a need to develop novel therapies that incorporate the effective principles of current treatments to augment training induced plasticity, while maintaining patient motivation and adherence.
Rhythm can entrain and prime neurons in the motor cortex via the rich neural connections between auditory and motor regions,14-21 and numerous studies have evaluated the therapeutic effectiveness of Rhythmic Auditory Stimulation (RAS) and music-based therapies in various clinical populations, with growing evidence that RAS can improve motor outcomes in stroke survivors.22-31 RAS, in addition to other benefits, can improve different gait parameters such as stride length and walking symmetry in stroke survivors.24,25,28,32-39 RAS has also been shown to improve neuromotor control, enhance stability and increase walking speed in people with chronic stroke.29,33,40 Meta-analyses on RAS for post-stroke recovery of upper-extremity function has demonstrated rhythmic cueing can enhance strength, elbow range of motion, synchrony, finger dexterity and co-ordination, and overall upper limb function in stroke survivors.41,42 The psychosocial benefits of listening to music during exercise and rehabilitation have been well documented, with many studies reporting the positive impact of music on motivation and endurance in exercise regimes for both healthy and stroke-affected populations.43,44 Music within rehabilitation can also improve patient mood, enhance the affective experience of therapy and increase enjoyment of exercise, which may allow patients to maintain motivation.32,45-47
Digital therapeutic technology has the potential to improve long-term motor outcomes after stroke by providing stroke survivors with independent access to individualised and targeted interventions after discharge from hospital and other rehabilitation services, or to use as an adjunct therapy in addition to physiotherapy and other rehabilitation sessions.31,48 Currently, few mobile software applications have been developed, and researchers cite multiple barriers to integration into rehabilitation protocols.49,50 We therefore developed a low-cost and accessible iPhone software application (app) that can be combined with wearable sensors to address this gap: ‘GotRhythm’. GotRhythm is a novel music-motor training app that integrates personal music, wireless wearable sensors and real-time auditory feedback via a metronome to deliver a tailored RAS protocol. GotRhythm also provides high-resolution recording of each person’s motor performance throughout training that can be used to monitor progress during rehabilitation. This randomised controlled trial was designed to test the feasibility and effectiveness of a 6-week GotRhythm intervention on motor function compared to conventional rehabilitation in sub-acute stroke survivors undergoing rehabilitation on a stroke rehabilitation ward.
Methods
Ethics
The research study was approved by the Royal Perth Hospital Human Research Ethics Committee (2017/188; RGS0000000044). All participants (and next of kin or people responsible for participants) were provided with a written information sheet, a simplified written summary of the information sheet designed for people experiencing communication impairments, and verbal information about the study. The study was prospectively registered (ACTRN12617000488303). All participants provided written informed consent.
Design
Single blind randomised controlled trial.
Setting and participants
Participants were recruited from a public sub-acute stroke rehabilitation unit at a hospital in Perth, Australia. Inclusion criteria included participants >18 years undergoing rehabilitation after stroke (subacute stage: 0-180 days following initial stroke) and whose clinical staff judged were likely to be inpatients ⩾6-weeks. Exclusion criteria included severe joint pain, impairment, other than from stroke, that prevented participant’s ability to complete the protocol, or unstable co-morbid medical or psychiatric disease. Patients had no severe cognitive impairment as assessed by clinicians on the stroke rehabilitation unit.
Randomisation
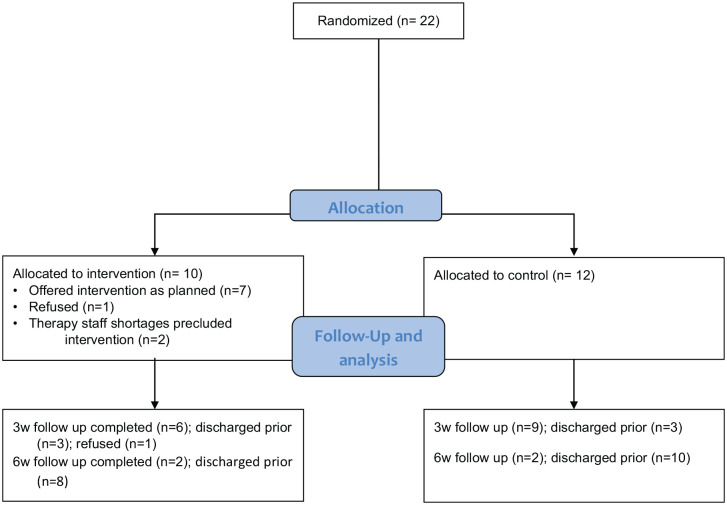
Consenting participants were randomised using a computer-generated sequence of numbers and sealed opaque envelopes (Figure 1).
Figure 1.
Participants were randomly allocated to an intervention (n = 10) or control (n = 10) group.
Intervention
GotRhythm is a mobile phone-based app used to deliver individualised music-motor therapy to stroke survivors and provides feedback on motor performance. GotRhythm was provided on an iOS device (iPhone 5, Apple Inc., CA). GotRhythm training was tailored to a participant’s specific injury in consultation with their hospital clinicians. The training took place under supervision at the hospital’s stroke rehabilitation clinic, and training was in addition to the usual stroke rehabilitation programme recommended by their clinician. GotRhythm supports a wide array of sensors to detect a variety of physical activities, including both upper and lower limb movements, as well as gross and fine motor skills. A series of participant-specific movement tasks focussing on different affected body parts were selected to match the rehabilitation goals of each patient.
Patients wore non-intrusive sensors (wireless inertial motion units [IMU]; Mbientlab Inc., San Francisco, CA) on the relevant body part that link wirelessly to the app. The IMUs wirelessly transmit acceleration, gyroscope and magnetometer data at a rate of 100 Hz to the GotRhythm app. GotRhythm converts the raw sensor data into attitude angles (yaw, pitch and roll) in real time. Clinical staff configured the start and end point angles in GotRhythm for the chosen attitude (eg, yaw). For example, GotRhythm is configured for periodic upper arm movement from 0° (parallel to the ground, start point) to 45° (end point). One complete cycle through this range of movement corresponds to a ‘beat’, and the aim is to match the beats to the target beats per minute (BPM). The allied health professional in charge of the patient’s rehabilitation prescribed the GotRhythm training by assigning a suitable target rhythm using BPM for each patient. The initial tempo was matched to the patient’s baseline movement frequency where patients were instructed to move at a comfortable pace. Tempo was adjusted in each session to suit each patient’s abilities, such as increasing the BPM as patients’ function improved, and progressively attuned to any changes in the patient’s rehabilitation needs over the intervention period. The tolerance band (the acceptable tempo range the participant could move within) was also adjusted to suit the patient’s abilities. For example, a target BPM of 60 with a tolerance of 3+/−, results in an acceptable tolerance range of 57 to 63. Music was selected based on participant’s personal preferences: 8 to 10 songs were selected with an approximate overall playtime of 30-minutes. GotRhythm changes the tempo of the music to the defined target BPM in real time without any change in pitch. During each intervention session, participants were instructed to move to the beat of the music. When the participant’s movements deviated from the selected tempo range, the music was silenced and replaced with a metronome playing at the pre-selected target tempo. The metronome provided real-time auditory feedback until the participant moved again within the target tempo range.
Intervention group participants were offered 6-weeks of the GotRhythm intervention, consisting of a supervised 20-minute music-motor therapy session using GotRhythm conducted 3 times a week for 6 weeks. The GotRhythm programme consisted of a warm-up, preparatory activities (BPM/movement selection), main activities and cool down led by their clinician. Participants were able to stop, and rest whenever required.
The target for total sessions was 18 sessions (ie, 360 minutes of intervention in total). These sessions were planned to be conducted on Monday, Wednesday and Friday of each week.
Control group participants’ usual care stroke rehabilitation was unchanged during the study, with no additional music-motor therapy.
Outcomes
Adherence to intervention. Clinicians recorded the length of time each patient engaged with the app in logbooks provided by the research team.
Change in Fugl-Meyer Assessment (FMA) of Motor Recovery score from baseline.
The FMA is a stroke-specific, performance-based impairment index that evaluates upper and lower limb motor function, balance, sensation and joint functioning in patients following stroke with excellent inter- and intra-rater reliability.51-54 The motor domain includes items measuring movement, co-ordination and speed and reflex action of the shoulder, elbow, forearm, wrist, hand, hip, knee and ankle. Items were scored on an ordinal scale of 0 (cannot perform, absent), 1 (partial impairment), and 2 (no impairment). Overall motor scores range from 0 (hemiplegia) to maximum of 100 (normal motor performance), with 66 points assigned for the upper extremity and 34 points for lower extremity. Participants were assessed with the FMA at baseline, after 3-weeks and at end of the intervention period (6-weeks). Assessments were conducted by a trained member of research team. Assessors were masked to participant group allocations.
Sample size and data analyses
For this feasibility pilot study a sample size of 20 participants (n = 10 intervention group) was sought, informed by previous stroke intervention studies,55-58 as this would be likely to be adequate to assess the primary outcome of adherence to the intervention.
Results
Adherence to the intervention
Overall, adherence to the GotRhythm intervention was limited. Three participants randomised to the intervention did not receive GotRhythm. Despite providing informed consent 1 participant declined to engage with the intervention and no intervention sessions were attempted. For 2 participants clinical staffing shortages precluded provision of the GotRhythm intervention as planned.
Of the 7 participants who engaged in the intervention in the first 3-week intervention block, only 5 completed the 3-week mid-intervention assessment (1 participant was discharged before undergoing their 3-week assessment and 1 refused). Only 2 of the 10 intervention participants were still inpatients, completed some intervention for the second 3-week intervention block, and completed the 6-week post-intervention assessment.
Recording of data in the app, which requires recordings to be manually started and stopped, also appeared to be inconsistent. For 5 of the 7 participants who received the GotRhythm intervention, use of the app according to the study logs exceeded actual recordings in the app, suggesting that the manual step required to initiate recording in the app had not been completed.
The intervention was intended to deliver three 20 minute sessions in each of two 3-week blocks, that is a total of 180 minutes across 9 sessions for block 1, and a further 180 minutes across 9 sessions in block 2. Overall, the 7 participants were logged to use the GotRhythm app for 70 (IQR 40, 201) minutes in 5 (4, 7) sessions however app recordings only validated use for 19 (0, 34) minutes in 3 (0, 4) sessions.
The actual number of sessions attempted varied widely – from minimum 3 to maximum 8 (of 9 intended) sessions in the first 3-week block and from minimum 1 to maximum 6 (of 9 intended) for the 2 participants continuing in the second 3-week block. The 2 participants who remained inpatients and engaged in both blocks of the intervention completed 14 sessions (201 minutes) and 7 sessions (142 minutes) of the intended ‘dose’ of 18 sessions and 360 minutes. The duration of sessions was also very variable ranging from 4 minutes (of the intended 20 minutes) up to 30 minutes.
Change in FMA scores
Fugl-Meyer assessment results are presented in Table 1. Upper limb scores improved at 3-week follow-up in both intervention and control groups.
Table 1.
Participant characteristics and Fugl-Meyer Assessment scores at baseline, 3 and 6 weeks.
| Gender | Intervention | Control | |
|---|---|---|---|
| Male 5, female 5 | Male 8, female 4 | ||
| Fugl Meyer Upper limb |
Baseline | n = 10 37 (32-49) |
n = 12 15 (4-49) |
| 3 wk | n = 6 46 (39-60) |
n = 9 26 (5-56) |
|
| 6 wk | n = 2 48 (44-52) |
n = 2 29 (14-44) |
|
| Fugl Meyer Lower Limb |
Baseline | n = 10 15 (10-26) |
n = 12 17 (5-26) |
| 3 wk | n = 6 22 (19-30) |
n = 9 22 (8-30) |
|
| 6 wk | n = 2 15 (9-21) |
N = 2 5 (3-7) |
Clinician feedback
Feedback provided by 2 clinicians (ward therapists) suggested it may be easier to have a shorter intervention session every day (rather than a 20 minutes session on 3 days of the week). Time required for study assessments was perceived to be at the expense of ‘usual’ therapy time. Therapists cited difficulty ensuring availability of clinical staff to implement the intervention and assist research staff and participants. It was also perceived that the research intervention tended to be added onto usual therapy and thus performed later in the day when participants may have been more fatigued. Participants were perceived to enjoy the music but, despite that, to become bored by repetitive movements.
Discussion
Main findings
Overall, adherence to the intervention in practice was poor. Although the concept of rehabilitation using RAS for stroke survivors remains attractive, in practice, designing an effective and engaging intervention has many challenges, even when supported by clinical staff.
Results in context
Studies of music-motor therapy have occurred in a range of settings and with heterogenous goals. The dosage we chose (3 sessions per week for 6 weeks) in the current study is lower than some previous studies, although interventions delivered 3 days a week for 3 to 6 weeks have led to small but significant improvements in movement.59,60 The clinicians’ suggestion for daily therapy is consistent with previous research that has primarily used higher dosage with daily application, 5 days per week for 3 to 6 weeks, and led to statistically significant changes in motor function.22,24,26,33,39,61-64
Strengths/limitations
Interpretation of our data is limited by the small number of participants, frequent losses to follow up and incomplete recording of data in the app itself, limiting the conclusions that can be drawn. Future revisions of the app should avoid the requirement for manual initiation of recording of data in the app to ensure the total time spent training during each session and participants’ performance across the training period can be assessed. Participants were heterogeneous (with varied levels of impairment) and future studies may more clearly define a target population.
Conclusions
This pilot suggests that future studies of RAS in inpatient stroke survivors will require resourcing for delivery of the intervention, and recording of data, by research, rather than clinical, staff and be carefully designed to maximise adherence to the intervention.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Neurological Research Program (NRP) and Royal Perth Hospital Medical Research Foundation.
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to study conception and design and provided critical revisions. CEB analysed the data. KH was involved in data acquisition, data intepretation and drafted the manuscript.
ORCID iDs: Katherine Hankinson  https://orcid.org/0000-0002-6609-3997
https://orcid.org/0000-0002-6609-3997
Christopher Etherton-Beer  https://orcid.org/0000-0001-5148-0188
https://orcid.org/0000-0001-5148-0188
References
- 1. WHO. World report on ageing and health. World Health Organization; 2015. 9241565047. [Google Scholar]
- 2. Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci. 2011;29:395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ward NS. Mechanisms underlying recovery of motor function after stroke. Postgrad Med J. 2005;81:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones TA, Adkins DL. Motor system reorganization after stroke: stimulating and training toward perfection. Physiology. 2015;30:358-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nudo RJ. Plasticity. NeuroRx. 2006;3:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang Y. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front Hum Neurosci. 2014;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84-90. [DOI] [PubMed] [Google Scholar]
- 9. Adey-Wakeling Z, Crotty M. Upper limb rehabilitation following stroke: current evidence and future perspectives. Aging Health. 2013;9:629-647. [Google Scholar]
- 10. Lang CE, Strube MJ, Bland MD, et al. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann Neurol. 2016;80:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mead GE. Post-stroke fatigue: new evidence of a possible biological cause. J Neurol Neurosurg Psychiatry. 2015;86:824-824. [DOI] [PubMed] [Google Scholar]
- 12. Jin J, Sklar GE, Min Sen Oh V, Chuen LIS. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008;4:269-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: A systematic review. Man Ther. 2010;15:220-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grahn JA, Brett M. Rhythm and beat perception in motor areas of the brain. J Cogn Neurosci. 2007;19:893-906. [DOI] [PubMed] [Google Scholar]
- 15. Chen JL, Penhune VB, Zatorre RJ. Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex. 2008;18:2844-2854. [DOI] [PubMed] [Google Scholar]
- 16. Chen JL, Penhune VB, Zatorre RJ. Moving on time: brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. J Cogn Neurosci. 2008;20:226-239. [DOI] [PubMed] [Google Scholar]
- 17. Chen JL, Zatorre RJ, Penhune VB. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. Neuroimage. 2006;32:1771-1781. [DOI] [PubMed] [Google Scholar]
- 18. Crasta JE, Thaut MH, Anderson CW, Davies PL, Gavin WJ. Auditory priming improves neural synchronization in auditory-motor entrainment. Neuropsychologia. 2018;117:102-112. [DOI] [PubMed] [Google Scholar]
- 19. Bengtsson SL, Ullén F, Ehrsson HH, et al. Listening to rhythms activates motor and premotor cortices. Cortex. 2009;45:62-71. [DOI] [PubMed] [Google Scholar]
- 20. Thaut MH. The discovery of human auditory-motor entrainment and its role in the development of neurologic music therapy. Prog Brain Res. 2015;217:253-266. [DOI] [PubMed] [Google Scholar]
- 21. Thaut MH. Entrainment and the motor system. Music Ther Perspect. 2013;31:31-34. [Google Scholar]
- 22. Thaut MH, McIntosh GC, Rice RR. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. 1997;151:207-212. [DOI] [PubMed] [Google Scholar]
- 23. Thaut MH, Kenyon GP, Hurt CP, McIntosh GC, Hoemberg V. Kinematic optimization of spatiotemporal patterns in paretic arm training with stroke patients. Neuropsychologia. 2002;40:1073-1081. [DOI] [PubMed] [Google Scholar]
- 24. Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: A single-blind, randomized trial. Neurorehabil Neural Repair. 2007;21:455-459. [DOI] [PubMed] [Google Scholar]
- 25. Lee S, Lee K, Song C. Gait training with bilateral rhythmic auditory stimulation in stroke patients: A randomized controlled trial. Brain Sci. 2018;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malcolm MP, Massie C, Thaut M. Rhythmic auditory-motor entrainment improves hemiparetic arm kinematics during reaching movements: A pilot study. Top Stroke Rehabil. 2009;16:69-79. [DOI] [PubMed] [Google Scholar]
- 27. Mainka S, Wissel J, Völler H, Evers S. The use of rhythmic auditory stimulation to optimize treadmill training for stroke patients: A randomized controlled trial. Front Neurol. 2018;9:755-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeong S, Kim MT. Effects of a theory-driven music and movement program for stroke survivors in a community setting. Appl Nurs Res. 2007;20:125-131. [DOI] [PubMed] [Google Scholar]
- 29. Suh JH, Han SJ, Jeon SY, et al. Effect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patients. NeuroRehabilitation. 2014;34:193-199. [DOI] [PubMed] [Google Scholar]
- 30. Street AJ, Magee WL, Odell-Miller H, Bateman A, Fachner JC. Home-based neurologic music therapy for upper limb rehabilitation with stroke patients at community rehabilitation stage - a feasibility study protocol. Front Hum Neurosci. 2015;9:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hutchinson K, Sloutsky R, Collimore A, et al. A music-based digital therapeutic: Proof-of-concept automation of a progressive and individualized rhythm-based walking training program after stroke. Neurorehabil Neural Repair. 2020;34:986-996. [DOI] [PubMed] [Google Scholar]
- 32. Magee WL, Clark I, Tamplin J, Bradt J. Music interventions for acquired brain injury. Cochrane Database Syst Rev. 2017;1:CD006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cha Y, Kim Y, Hwang S, Chung Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: A pilot randomized controlled study. NeuroRehabilitation. 2014;35:681-688. [DOI] [PubMed] [Google Scholar]
- 34. Roerdink M, Lamoth CJ, Kwakkel G, van Wieringen PC, Beek PJ. Gait coordination after stroke: benefits of acoustically paced treadmill walking. Phys Ther. 2007;87:1009-1022. [DOI] [PubMed] [Google Scholar]
- 35. Chouhan S, Kumar S. Comparing the effects of rhythmic auditory cueing and visual cueing in acute hemiparetic stroke. Int J Ther Rehabil. 2012;19:344-351. [Google Scholar]
- 36. Ko B-W, Lee H-Y, Song W-K. Rhythmic auditory stimulation using a portable smart device: short-term effects on gait in chronic hemiplegic stroke patients. J Phys Ther Sci. 2016;28:1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kobinata N, Ueno M, Imanishi Y, Yoshikawa H. Immediate effects of rhythmic auditory stimulation on gait in stroke patients in relation to the lesion site. J Phys Ther Sci. 2016;28:2441-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oh Y-S, Kim H-S, Woo Y-K. Effects of rhythmic auditory stimulation using music on gait with stroke patients. Phys Ther Korea. 2015;22:81-90. [Google Scholar]
- 39. Schauer M, Mauritz K-H. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clin Rehabil. 2003;17:713-722. [DOI] [PubMed] [Google Scholar]
- 40. Ghai S, Ghai I. Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: A systematic review & dose-response meta-analysis. Sci Rep. 2019;9:2183-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoo GE, Kim SJ. Rhythmic auditory cueing in motor rehabilitation for stroke patients: systematic review and meta-analysis. J Music Ther. 2016;53:149-177. [DOI] [PubMed] [Google Scholar]
- 42. Ghai S. Effects of real-time (sonification) and rhythmic auditory stimuli on recovering arm function post stroke: a systematic review and meta-analysis. Front Neurol. 2018;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stork MJ, Kwan MY, Gibala MJ, Martin Ginis KA. Music enhances performance and perceived enjoyment of sprint interval exercise. Med Sci Sports Exerc. 2015;47:1052-1060. [DOI] [PubMed] [Google Scholar]
- 44. Maclean N, Pound P, Wolfe C, Rudd A. Qualitative analysis of stroke patients’ motivation for rehabilitation. BMJ. 2000;321:1051-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Särkämö T, Tervaniemi M, Huotilainen M. Music perception and cognition: development, neural basis, and rehabilitative use of music. Wiley Interdiscip Rev Cogn Sci. 2013;4:441-451. [DOI] [PubMed] [Google Scholar]
- 46. Särkämö T, Tervaniemi M, Laitinen S, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain. 2008;131:866-876. [DOI] [PubMed] [Google Scholar]
- 47. Sihvonen AJ, Särkämö T, Leo V, Tervaniemi M, Altenmüller E, Soinila S. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017;16:648-660. [DOI] [PubMed] [Google Scholar]
- 48. Nussbaum R, Kelly C, Quinby E, Mac A, Parmanto B, Dicianno BE. Systematic review of mobile health applications in rehabilitation. Arch Phys Med Rehabil. 2019;100:115-127. [DOI] [PubMed] [Google Scholar]
- 49. Lopez WO, Higuera CA, Fonoff ET, Souza Cde O, Albicker U, Martinez JA. Listenmee and Listenmee smartphone application: synchronizing walking to rhythmic auditory cues to improve gait in Parkinson’s disease. Hum Mov Sci. 2014;37:147-156. [DOI] [PubMed] [Google Scholar]
- 50. Muto T, Herzberger B, Hermsdoerfer J, Miyake Y, Poeppel E. Interactive cueing with walk-mate for hemiparetic stroke rehabilitation. J Neuroeng Rehabil. 2012;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606-1610. [DOI] [PubMed] [Google Scholar]
- 52. Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447-454. [DOI] [PubMed] [Google Scholar]
- 53. Hernández ED, Galeano CP, Barbosa NE, et al. Intra- and inter-rater reliability of Fugl-Meyer assessment of upper extremity in stroke. J Rehabil Med. 2019;51:652-659. [DOI] [PubMed] [Google Scholar]
- 54. Sullivan KJ, Tilson JK, Cen SY, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42:427-432. [DOI] [PubMed] [Google Scholar]
- 55. Chang MC, Kim DY, Park DH. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. 2015;8:561-566. [DOI] [PubMed] [Google Scholar]
- 56. Madhavan S, Stinear JW, Kanekar N. Effects of a single session of high intensity interval treadmill training on corticomotor excitability following stroke: Implications for therapy. Neural Plast. 2016;2016:1686414-1686418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kwon TG, Park E, Kang C, Chang WH, Kim YH. The effects of combined repetitive transcranial magnetic stimulation and transcranial direct current stimulation on motor function in patients with stroke. Restor Neurol Neurosci. 2016;34:915-923. [DOI] [PubMed] [Google Scholar]
- 58. Singer BJ, Vallence AM, Cleary S, Cooper I, Loftus AM. The effect of EMG triggered electrical stimulation plus task practice on arm function in chronic stroke patients with moderate-severe arm deficits. Restor Neurol Neurosci. 2013;31:681-691. [DOI] [PubMed] [Google Scholar]
- 59. Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whitall J, Waller SM, Sorkin JD, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: A single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25:118-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schneider S, Münte T, Rodriguez-Fornells A, Sailer M, Altenmüller E. Music-supported training is more efficient than functional motor training for recovery of fine motor skills in stroke patients. Music Percept. 2010;27:271-280. [Google Scholar]
- 62. Altenmüller E, Marco-Pallares J, Münte TF, Schneider S. Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy. Ann N Y Acad Sci. 2009;1169:395-405. [DOI] [PubMed] [Google Scholar]
- 63. Rojo N, Amengual J, Juncadella M, et al. Music-supported therapy induces plasticity in the sensorimotor cortex in chronic stroke: a single-case study using multimodal imaging (fMRI-TMS). Brain Inj. 2011;25:787-793. [DOI] [PubMed] [Google Scholar]
- 64. Amengual JL, Rojo N, Veciana de Las Heras M, et al. Sensorimotor plasticity after music-supported therapy in chronic stroke patients revealed by transcranial magnetic stimulation. PLoS One. 2013;8:e61883. [DOI] [PMC free article] [PubMed] [Google Scholar]