Abstract
Oxytocin (OT), a nonapeptide, has a variety of functions. Despite extensive studies on OT over past decades, our understanding of its neural functions and their regulation remains incomplete. OT is mainly produced in OT neurons in the supraoptic nucleus (SON), paraventricular nucleus (PVN) and accessory nuclei between the SON and PVN. OT exerts neuromodulatory effects in the brain and spinal cord. While magnocellular OT neurons in the SON and PVN mainly innervate the pituitary and forebrain regions, and parvocellular OT neurons in the PVN innervate brainstem and spinal cord, the two sets of OT neurons have close interactions histologically and functionally. OT expression occurs at early life to promote mental and physical development, while its subsequent decrease in expression in later life stage accompanies aging and diseases. Adaptive changes in this OT system, however, take place under different conditions and upon the maturation of OT release machinery. OT can modulate social recognition and behaviors, learning and memory, emotion, reward, and other higher brain functions. OT also regulates eating and drinking, sleep and wakefulness, nociception and analgesia, sexual behavior, parturition, lactation and other instinctive behaviors. OT regulates the autonomic nervous system, and somatic and specialized senses. Notably, OT can have different modulatory effects on the same function under different conditions. Such divergence may derive from different neural connections, OT receptor gene dimorphism and methylation, and complex interactions with other hormones. In this review, brain functions of OT and their underlying neural mechanisms as well as the perspectives of their clinical usage are presented.
Keywords: functions, hypothalamus, neurohumoral reflex, oxytocin, supraoptic nucleus
Introduction
Hormones are important factors for body development and adaptive responses to environmental changes. Among many hormones, oxytocin (OT) is not only the key factor in the milk-ejection reflex, the most classical neural humoral reflex (Wakerley et al., 1994), but also possesses a variety of functions such as regulation of immunological, metabolic and endocrine activity and many other peripheral functions (Liu et al., 2022; Wang et al., 2015; Wang et al., 2019b; Wang et al., 2022; Yang et al., 2013) as outlined in Figure 1. OT is mainly synthesized in OT neurons in the supraoptic nucleus (SON), paraventricular nucleus (PVN) and several accessory nuclei in the hypothalamus. In the SON and lateral and peripheral PVN, OT neurons, as magnocellular neuroendocrine cells, co-exist with vasopressin (VP) neurons as well as astrocytes (Wang et al., 2021a) and project to many hypothalamic sites, forebrain structures, periventricular area in addition to the posterior pituitary (Figure 2A). In the medial PVN, there is a group of non-neuroendocrine parvocellular OT neurons that innervate varieties of brainstem and spinal cord sites (Figure 2B). There are mutual innervations among the SON and PVN. For example, some spinal cord-projecting parvocellular OT neurons send collateral projections onto magnocellular OT neurons (Eliava et al., 2016). The inherent connections among OT neurons within different hypothalamic nuclei form a network (Figure 2C), which is an important structural basis for coordinated activity of OT neurons at different sets in response to external stimuli. In addition, many extrahypothalamic regions also contain OT neurons or receive neural innervations from OT neurons (Knobloch & Grinevich, 2014), and they may exert local regulatory roles. OT functions are mediated by OT receptor (OTR) that is present on the target tissue throughout the mammalian body (Assinder, 2022; Gimpl & Fahrenholz, 2001). Thus, OT can modulate higher brain functions, instinctive behaviors, somatic and visceral sensations, visceral motor activity, etc. In this review, OT functions in the central nervous system are discussed to aid readers in understanding OT functions as they face abundant, sometime contradictory reports. Table 1 is a summary of OT higher brain functions and the underlying mechanisms.
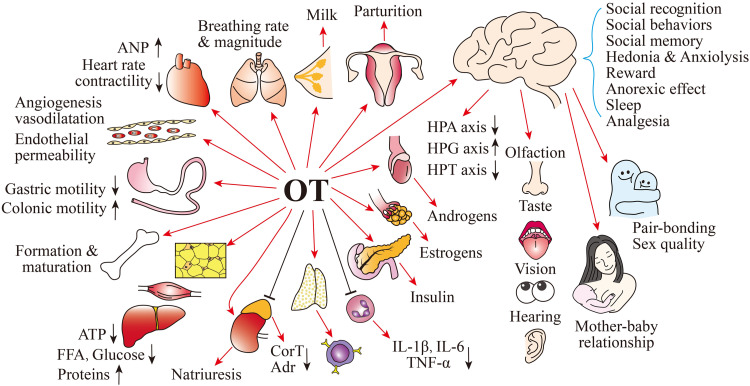
Figure 1.
Summary of hormonal functions of oxytocin (OT). Abbreviations: Adrenaline (Adr), Atrial natriuretic peptide (ANP), Corticosteroids (CorT), Free fatty acids (FFA), Gonadotropin-releasing hormone (GnRH), Hypothalamic-pituitary-adrenal (HPA) axis, Hypothalamic pituitary gonad (HPG) axis, Hypothalamic pituitary thyroid (HPT) axis, Interleukin (IL), and Tumor necrosis factor (TNF)-α.
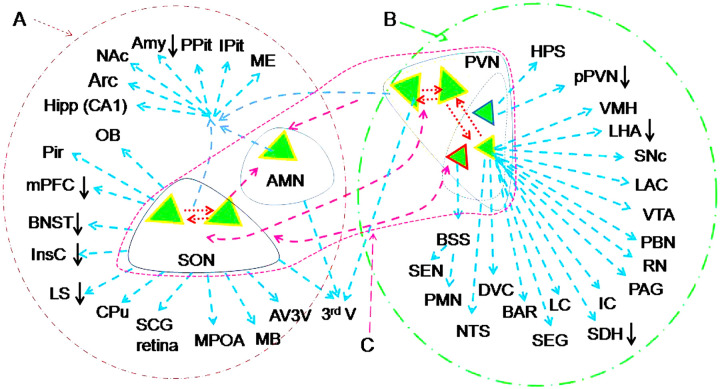
Figure 2.
Outline of neural innervations of OT neurons in the central nervous system. A-C. Magnocellular OT neurons (A), parvocellular OT neurons (B) and their interconnections (C). Note that: Oxytocin (OT, green triangles) neurons in the parvocellular division (smaller triangles) of the paraventricular nucleus (pPVN) likely belong to different functional groups; OT neurons in the supraoptic nucleus (SON) and PVN connect mutually through axon/axon collaterals (dashed blue arrows) or dendrites (red dashed arrows); the downward arrows indicate inhibition evoked by OT activation of GABAergic interneurons. Abbreviations of neural structures: The third ventricle (3rdV), Accessory magnocellular nuclei (AMN), Anterior cingulate cortex (ACC), Amygdala (Amy), Arcuate nucleus (Arc), Anteroventral third ventricle region (AV3 V), Barrington's nucleus (BAR), Bed nuclei of the stria terminalis (BNST), Bulbospinal system (BSS), Caudate putamen (CPu), Dorsal vagal complex (DVC), Hippocampus (Hipp), Hypophyseal portal system (HPS), Inferior colliculus (IC), Insular cortex (InsC), Intermediate lobe of the pituitary (IPit), Lateral hypothalamus area (LHA), Lateral septum (LS), Left auditory cortex (LAC), Locus coeruleus (LC), Mammillary body complex (MB), Median eminence (ME), Medial prefrontal cortex (mPFC), Medial preoptic area (MPOA), Nucleus accumbens (NAc), Nucleus tractus solitarius (NTS), Olfactory bulb (OB), Parabrachial nucleus (PBN), Phrenic motor nucleus (PMN), Periaqueductal gray (PAG), Piriform cortex (Pir), Posterior pituitary (PPit), Raphe nucleus (RN), Spinal dorsal horn (SDH), Spinal ejaculation generator (SEG). Substantia nigra compact part (SNc), Superior cervical ganglion (SCG), Sympathetic excitatory neuron (SEN), Ventromedial hypothalamic nucleus (VMH), Ventral tegmental area (VTA). Neural connections of OT neurons refer to (Knobloch & Grinevich, 2014; Liao et al., 2020; Zhang et al., 2021).
Table 1.
OT Higher Brain Functions and the Underlying Mechanisms.
| Category | Physiologic effects | Neural mechanisms | Pharmacological effects | References |
|---|---|---|---|---|
| Social cognition | (1)Promote their sociability, learning and memory, and locomotor activity while decreasing plasma corticosterone and anxiety. | (2)Desensitizing amygdala by increasing 5-HT in RN, NA in LC, GABA in mPFC and DA in VTA, NAc, and ACC. | (3) IAO can facilitate social recognition and inter-personal synchrony, but alleviate negative symptoms in schizophrenia | (1) (Cuneo et al., 2021; Jahangard et al., 2020; Meixner et al., 2019); (2) (Jung et al., 2021; Liu et al., 2021a; Maier et al., 2019; Wang et al., 2021b); (3) (Runyan et al., 2021; Sabe et al., 2021; Zheng et al., 2021b) |
| Pro-social behavior | (4)Promote parenting behavior, pair-bonding, social interaction, and affiliative behavior as well as neonate brain development; reducing fear. | (5) Activating GABA inhibition during delivery; promoting neurite outgrowth and synapse formation; holding normal OT/OTR signaling; inhibiting amygdala. | (6) IAO improves social functioning in ASD patients; activating GABAergic signal in BNST; activating PVN-social brain routes; optimizing OT neuronal activity. | (4) (John & Jaeggi, 2021; Shishido et al., 2021; Yoshihara et al., 2018; Yuan et al., 2019); (5) (Reichova et al., 2021; Tyzio et al., 2014); (6) (Francesconi et al., 2021; Huang et al., 2021; Jimenez et al., 2015; Li et al., 2020; Li et al., 2021e) |
| Learning and memory | (7) Enhance positive social memory and attenuate memory consolidation and retrieval of non-social stimuli. | (8) Increase OT release in the OBs; enhance cortical information transfer; increase signal-to-noise ratio via GABA action; inhibit neuronal death. | (9) IAO can reduce deficits in safety learning, contextual fear and levels of glucocorticoid; reverse amyloid β-induced hippocampal impairment. | (7) (Feifel et al., 2012; Guastella et al., 2008; Latt et al., 2018; Plasencia et al., 2019; Tse et al., 2018); (8) (Bertoni et al., 2021; Hu et al., 2015; Larrazolo-Lopez et al., 2008); (9) (Kreutzmann & Fendt, 2021; Takahashi et al., 2020). |
| Anti-depressive & anxiolysis | (10) Increase self-confidence and inter-personal relationship; decrease cognitive anxiety, social stress, depression and suicidality. | (11) Inhibit amygdala and HPA-axis by activation of PVN GABA action; protect the hippocampus; increase 5-HT release in RN and RN-hippocampal activity. | (12) Cure moderate sub-clinical depression; reverse maternal depression; enhance connectivity of amygdala and bilateral insula and middle CG genotype in patients. | (10) (Fisher et al., 2021; La Fratta et al., 2021; Torres et al., 2022; Warrener et al., 2021); (11) (Borroto-Escuela et al., 2021; Matsushita et al., 2019; Xie et al., 2022; Yoshida et al., 2009); (12) (Li et al., 2021e; Lindley Baron-Cohen et al., 2021) |
| Reward | (13) Produce enjoying experience and motivation to rewarding stimuli like pair bonding, prize, gambling, delicious meals; sexual contact, and positive social interaction; promote sociability, suppress social stress, depression and anxiety. | (14) Activate 5-HT and Glu transmission from RN to VTA and VTA to reinforce learning by serotonergic neuron co-transmitter glutamate mesocorticolimbic DA pathway from the VTA to the NAc and PVN; inhibit activities of the HPA axis, amygdala and endogenous opioid system | (15) OT-driven social signals compete with drug-elicited reinforcing signals in brain reward circuitry and enable the rewarding effects of prosocial behavior at the expense of drug-related rewards. It involves DA and glutamatergic systems in NAc, GABA and Glu signals in the CeA, and opioid effect in the VTA. | (13) (Cuneo et al., 2021; Dolen et al., 2013; Horrell et al., 2019; La Fratta et al., 2021; Siu et al., 2021); (14) (Courtiol et al., 2021; Hung et al., 2017; Sundar et al., 2021); (15) (Bowen & Neumann, 2017; Logan et al., 2021; Pedersen, 2017; Tops et al., 2014) |
| Negative impacts | (16) Create intergroup conflict and violence as well as maternal attacks; postpartum maternal depression following social stress. | (17) Prolonged increase in somatodendritic OT secretion causes post-excitation inhibition of OT neurons, which results in -abnormality of brain and peripheral functions; it is often associated with increase VP secretion and HPA-axis activity | (18) More anhedonia in individuals low in extraversion, trust-altruism, and openness to experience, elevated depressive symptoms recall memories, particularly in non-social context; intrapartum OT application increase morbidity of postnatal depressive symptoms in pregnant women | (16) (De Dreu et al., 2011; Li et al., 2020; Li et al., 2021e); (17) (Li et al., 2020; Li et al., 2021c; Li et al., 2021e; Liu et al., 2019; Wang et al., 2006) (18) (Tichelman et al., 2021; Wong et al., 2021a; Wong et al., 2021c) |
Abbreviations: 5-HT, serotonin; ACC, anterior cingulate cortex; ASD, autism spectrum disorder; CeA, central nucleus of the amygdala; DA, dopamine; GABA, ɣ-aminobutyric acid; Glu, glutamate; HPA axis, hypothalamic-pituitary-adrenal (HPA) axis; IAO, intranasal application of OT; LC, Locus coeruleus; mPFC, medial prefrontal cortex; NA, norepinephrine; NAc, nucleus accumbens; OBs, olfactory bulb; OT, oxytocin; OTR, OT receptor; PVN, paraventricular nucleus; RN, raphe nuclei; VP, vasopressin; VTA.
Higher Brain Functions
In humans, OT is essential for brain functioning during development. OT roles have been well recorded in at-birth anti-inflammation and protection of the developing fetal brain (Kingsbury & Bilbo, 2019), neonate preference for the mother (Galbally et al., 2011) and social functioning of children and adolescents (Ellis et al., 2021; John & Jaeggi, 2021). OT is also involved in maintenance of normal brain functions during aging (Rocha-Santos et al., 2020; Sakakibara et al., 2010). A dramatic OT brain function is its modulation of cognition and behavior in social activity. As reported, OTRs are present in extensive brain areas in human, particularly the olfactory bulbs (OBs), globus pallidum, caudate, putamen, thalamus, hippocampus, amygdala and temporal pole (Quintana et al., 2019). Thus, OT can influence cortical organization and regulate higher brain functions through many approaches as outlined in Figure 3.
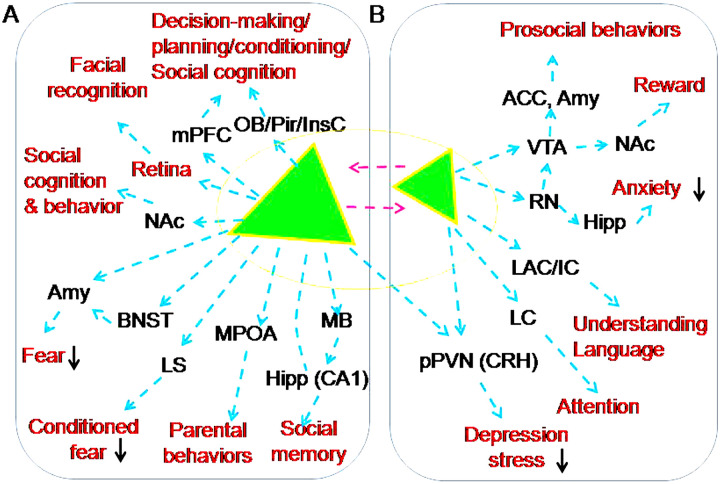
Figure 3.
Approaches of OT modulation of the higher brain functions. A-B. Magnocellular OT neurons (A) and parvocellular OT neurons (B). The red font indicates a variety of OT effects (please refer to the text for details). Signs and abbreviations are the same as Figure 2.
Social Cognition
Abundant evidence indicates that OT can promote social recognition. In patients with ovarian cancer, higher levels of positive affection, purpose in life and social nurturance are related to higher levels of OT in ascites (Cuneo et al., 2021). Suicide survivors have significantly lower serum OT concentrations, which are associated with negative perceptions of the quality of social lives (Jahangard et al., 2020). OT levels in cerebrospinal fluid are increased in individuals with schizophrenia but decreased in their serum (Hernandez-Diaz et al., 2021). Moreover, single nucleotide polymorphism (SNP)? rs53576 and functional polymorphism rs2268498 of OTR gene influence language functions by reducing an interpretation bias in the spontaneous appraisal of neutral words (Meixner et al., 2019). Malfunctions of OT-OTR signaling processes can significantly disturb social interaction capabilities and cope with daily life activities in autism spectrum disorders (ASDs), major depression, Williams syndrome, sexual offenses, fragile X syndrome and posttraumatic stress disorder (Jurek & Neumann, 2018). Thus, a balanced OT/OTR signaling process is pivotal for mental health.
Effect of OT on social recognition is mediated by multiple neurotransmitter systems, such as brainstem serotonin (5-HT), noradrenaline and dopamine and different brain structures. In 121 healthy subjects, OT switched bilateral amygdala threat sensitization to desensitization, and this effect was significantly attenuated during decreased central serotonin signaling via pretreatment with acute tryptophan depletion (Liu et al., 2021a). Disturbing OT neuronal activity in the SON can activate the amygdala in rats (Liu et al., 2017), a brain region facilitating social anxiety and fear in humans (Meixner et al., 2019). Optogenetic excitation of PVN OT fibers excites noradrenergic neurons in the locus coeruleus and augments attention to novel objects by co-release of OT and glutamate in male rats (Wang et al., 2021b). Fear-related behaviors are rigidly controlled by the medial prefrontal cortex (mPFC) while OT-mediated decrease in neuronal activity in the mPFC could facilitate social buffering in rats (Jung et al., 2021). In addition, corticostriatal connectivity in women (Bethlehem et al., 2017) and additional brain regions such as the amygdala and anterior cingulate cortex in both women and men (Maier et al., 2019), are also involved in OT effects on social recognition. It is likely that biochemical activity of OT neurons in the PVN and SON during social interactions increases significantly and that OT release in their terminals in the ventral tegmental area (VTA) increases excitatory drive onto reward-specific VTA dopamine neurons in the amygdala and anterior cingulate cortex to reduce stress and fear, and thus enhances individuals’ prosocial behaviors as well as language functions.
OT functions are also manifested when intranasal administration of OT (IAO) is used in patients and in animal models. Pharmacologically, IAO can facilitate adaptive individuation/categorization face-processing in men (Liu et al., 2021b), detection accuracy of briefly-presented emotional stimuli including facial emotion recognition (Schulze et al., 2011), and interpersonal synchrony during dance (Josef et al., 2019), but reduce the negative emotional impact of jealousy in established romantic partners and thus, help maintenance of positive inter-personal relationships (Zheng et al., 2021b). Consistently, IAO can increase the pleasantness of social touch massage and neural responses in key regions involved in reward, social cognition, emotion and salience and in default mode networks in men (Chen et al., 2020; Zheng et al., 2021a). In animal studies, the underlying neurochemical processes are further explored. IAO can reduce the impairments in social novelty recognition and increase inhibitory synaptic inputs in the mPFC in rats with traumatic brain injury (Runyan et al., 2021). Notably, high doses of IAO may be more efficacious in alleviating negative symptoms in schizophrenia; however, that could be a pharmacological effect only since salivary OT levels have no clear correlation with the negative symptoms in humans (Sabe et al., 2021). Thus, studying on OT effects on higher brain functions should consider brain OT levels and OTR signaling efficiency rather than peripheral levels only, especially under pathological conditions.
Pro-Social Behavior
OT can influence parenting behavior, pair-bonding and affiliative behavior (de Jong & Neumann, 2018). People who grow up under conditions that are more adverse tend to have lower endogenous OT (Ellis et al., 2021). Thus, OT can increase social closeness, attachment and prosocial behaviors.
Parental behaviors are specific form of social behaviors. While OT is well-known as a key hormone in the establishment and maintenance of maternal behavior, it also promotes paternal behaviors (Yoshihara et al., 2018). Maternal behavior begins at the end of pregnancy before breastfeeding (Bridges, 2015) and is clearly associated with OT actions. In women who received epidural anesthesia, significantly higher mean salivary OT level appeared at 1–2 days after giving a birth than at 36–37 gestational weeks and OT levels became significantly lower at 4–5 days postpartum than that at 1–2 days postpartum. Postpartum maternity blues and fatigue were negatively correlated with salivary OT level at 4–5 days postpartum women (Shishido et al., 2021) and in primiparous postpartum California mice (Guoynes & Marler, 2021). In mandarin voles, microinjection of an OTR antagonist into the medial optic area (MPOA) of fathers can significantly reduce the total duration of paternal behavior, increase the latency to approach the pup and initiate paternal behavior (Yuan et al., 2019). Different from maternal behaviors, paternal behaviors rely on additional sensory signals from their mates, environment, and/or offspring to present paternal behavior (Horrell et al., 2019).
Disorders in OT/OTR signaling cause aberrant social behaviors as represented by ASDs, which has been illustrated in children (John & Jaeggi, 2021; Yang et al., 2017) and in mice (Kitagawa et al., 2021). OT-mediated GABA inhibition during the delivery process can protect individuals from chronic Cl− deficiency-associated ASDs in rodent offspring (Tyzio et al., 2014). Moreover, OT stimulates the expression of numerous genes involved in neurite outgrowth and glutamatergic synapse formation in mouse early development stages (Reichova et al., 2021), and thus the decreased OT actions could cause ASDs. Correspondingly, OT is accepted as an effective treatment for some core symptoms of ASDs, especially in the domain of social functioning revealed in a multilevel meta-analytic model of human data (Huang et al., 2021).
Paradoxically, many ASD subjects exhibit elevated but not decreased serum OT levels, which are correlated with childhood Autism Rating Scale score (Yang et al., 2017). This fact suggests that ASDs are associated with reduced OT/OTR signaling process but not blood OT levels. This finding is consistent with the fact that many neurodevelopmental disorders share common learning and behavioral impairments as well as dysregulation of OTR, such as abnormal DNA methylation in the OTR gene (Siu et al., 2021),
It adults with ASDs, there are higher levels of OTR gene methylation in the intron 1 area. This hypermethylation is related to clinical symptoms and to a hypoconnectivity between cortico-cortical areas involved in the theory of mind. The hypermethylation is also positively correlated to social responsiveness deficits in ASDs and to a hyperconnectivity between striatal and cortical brain areas in humans (Andari et al., 2020). Moreover, ASD severity is also associated with OTR SNP rs2254298 (Yang et al., 2017). In two ASD-associated OTR SNPs, rs2254298 and rs53576 have significant association with altered brain activity in the right supramarginal gyrus, which can be an important etiology of ASDs in adolescents (Uzefovsky et al., 2019). It was also reported that disease severity of the patients carrying GA and AA genotypes (GA/AA) of the SNP rs237902 is more severe compared to severity of GG genotype in ASD children (Ocakoglu et al., 2018). Thus, abnormal OTR signals in brain regions regulating social behaviors may underlie the etiology of ASDs. Taken together, disorders in OT/OTR signaling can cause ASD-associated aberrant social behaviors.
Effects of OT on social behaviors are related to OT actions on social brains directly or through the circulation. OT can increase the firing activity of GABAergic neurons in the dorsolateral bed nucleus of the stria terminalis (BNST) by activation of OTR, which in turn inhibits output neurons from the BNST to central amygdala in male rats (Francesconi et al., 2021), thereby inhibiting cued fear. Moreover, circulating OT increases social interaction and maternal behaviors by acting on the mPFC after being taken up into the brain via the receptor for advanced glycation end-products, an OT-binding protein in male mice (Munesue et al., 2021). In the establishment of parental behaviors, the PVN likely occupies a central position in female rats (Munetomo et al., 2016) and male mice (Shabalova et al., 2020). It is known that PVN OT neurons project to the hippocampus, amygdala, ventral striatum, hypothalamus, nucleus accumbens (NAc, in the ventral striatum) and brainstem nuclei that are known as the key structures modulating social behaviors in rats (Ruthschilling et al., 2012) and in female rabbits (Jimenez et al., 2015). This histology feature sets a solid neural basis for OT to modulate parental behaviors. Notably, OT neurons in the SON are also implicated in maintenance of maternal behaviors as highlighted in a series of observations recently in rats (Li et al., 2020; Li et al., 2021a; Li et al., 2021b; Li et al., 2021c). This is consistent with the structural connection of the SON neurons with the brain regions in rats (Zhang et al., 2021) that regulate maternal behaviors.
IAO but not intranasal VP promotes cooperative behaviors shown in a meta-analysis of human data (Yang et al., 2021), friendliness in horses (Lee & Yoon, 2021), and patience, cognitive flexibility in reversal learning and generosity under advantageous conditions in humans (Kapetaniou et al., 2021). OT also alleviates negative consequences of isolation in humans (Pfundmair & Echterhoff, 2021). In animal experiments, urinary OT concentrations following IAO are positively associated with dogs’ duration of social proximity and looking towards their owners (Pedretti et al., 2021). As a whole, OT can promote social behaviors directly or indirectly.
Learning and Memory
Learning and memory are modulated by OT. In humans, OT can enhance positive social memory while attenuating memory consolidation and retrieval of non-social stimuli like words and visual objects in humans (Guastella et al., 2008). Mothers with high salivary OT responsiveness recall previous positive social events with great detail and use uncontrollability attribution to explain such positive events (Tse et al., 2018), which may enhance the sensitivity and positive behaviors of parenting. Prenatal and early postnatal periods appear to be the most sensitive periods of brain development in mammals; action of various factors at these stages of brain development can result in neurodegeneration, memory impairment, and mood disorders at later periods of life. OT serves as an anti-stress, pro-angiogenic, and neurogenesis-controlling molecule contributing to dramatic changes in brain plasticity in early life stress, and thus prevents aberrant memory and behaviors (Lopatina et al., 2021). Women have higher plasma OT levels than men; older adults have higher plasma VP levels than young adults. Functionally, higher OT and lower VP levels are associated with faster sensorimotor processing speed, with sex and age moderating these effects including verbal memory (Plasencia et al., 2019). This integrated approach identifies variations in endogenous peripheral neuropeptide levels in humans, supporting their sex- and age-specific role as “difference makers” in attachment and cognition. In animal studies, vaginocervical stimulation promotes olfactory social recognition memory in female rats due to release of OT in the OBs during proestrus period (Larrazolo-Lopez et al., 2008). IAO has the capacity to compensate deficits in safety learning, along with a reduction in contextual fear and corticosterone levels shown in rats (Kreutzmann & Fendt, 2021). Obviously, the activity of the OT system is highly correlated with learning and memory in humans and animals. Notably, facilitatory effects of OT on long-term memory processes in healthy humans are based on emotional but not non-emotional stimuli (Brambilla et al., 2016).
OT selective facilitation of learning with social feedback is likely associated with enhanced responses and functional connectivity in emotional memory and reward processing regions in humans (Hu et al., 2015). As an essential carrier of memory process, the septohippocampal pathway is also the target of OT as shown in animal studies. OT can enhance cortical information transfer while simultaneously lowering background activity by increasing fast-spiking inhibitory interneuronal activity in the hippocampus shown in a mouse model of ASDs (Bertoni et al., 2021). In mice, OT can reverse amyloid-β-induced impairment of synaptic plasticity in hippocampus trough phosphorylation of extracellular signal-regulated protein kinase 1 and 2 (pERK1/2) and Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Takahashi et al., 2020). OT can alleviate stress-evoked hippocampal dysfunction and neuronal loss by inhibiting glucocorticoids-induced neuronal death in mouse primary hippocampal neurons (Latt et al., 2018).
In patients with schizophrenia, after 3 weeks of IAO treatment, there is no evidence for an amnestic effect; however, there is significantly better performance with IAO on several subtests of the California Verbal Learning Test (CVLT); namely total Recall trials, short delayed free recall and total recall discrimination (Feifel et al., 2012). IAO attenuates the normal bias in selecting the happy face accompanied by reduced activation in a network of brain regions that support mentalizing and processing of facial emotion, salience, aversion, uncertainty and ambiguity in social stimuli, including the amygdala, temporo-parietal junction, posterior cingulate cortex, precuneus and insula (Wigton et al., 2022). IAO can increase ratings of the vividness of recalled memories during the social context only; however, individuals with elevated depressive symptoms also recall memories that are more negative following IAO relative to placebo and only in the non-social context (Wong et al., 2021a). This is consistent with previous proposal that OT has no effect on or even worsening of memory in non-emotional stimuli (Brambilla et al., 2016). In animal studies, IAO can compensate deficits in safety learning, along with a reduction in contextual fear and corticosterone levels as shown in rats under acoustic startle test (Kreutzmann & Fendt, 2021). IAO also overcomes maternal separation-induced impairment in long-term potentiation generation in the CA1 area of the hippocampus in rats (Joushi et al., 2021). These memory enhancing effects of IAO may contribute to the facilitation of social engagement and social interactions in patients with schizophrenia.
Anti-Depression and Anxiolysis
Disorders in OT secretion involve many psychiatric disorders including depression, anxiety, schizophrenia, and ASDs (Yoon & Kim, 2020), which have been recognized in many studies of animals and humans. In young male soccer players, winners with higher OT levels have significantly lower cognitive anxiety and higher self-confidence scores than losers who have higher cortisol levels (La Fratta et al., 2021). In patients of premanifest and manifest Huntington's disease, OT levels are negatively associated with depressive symptoms (Fisher et al., 2021). Veterans who socialized with comrades more frequently have higher levels of urinary OT as well as less depressive symptoms. Social connectedness is a strong negative predictor of symptoms of both depression and suicidality (Warrener et al., 2021). Salivary OT after an episode of playing with parents has better reliabilities than baseline in children's social behavior (Torres et al., 2022). Numerous studies from humans and animals also indicate that OT uniquely facilitates adaptive fear but reduces maladaptive anxiety (Janecek & Dabrowska, 2019).
In general, the anti-depressive effect of OT is believed due to its regulating neuronal activity, influencing neuroplasticity and regeneration, altering neurotransmitter release, down-regulating hypothalamic-pituitary-adrenal (HPA) axis, anti-inflammation, antioxidation, and genetic effects (Xie et al., 2022). As shown in studies on rats, excessive increase in glucocorticoids in chronic stress causes atrophy of the hippocampus (Warfvinge et al., 2020), while central OT can activate GABAA-receptors in the PVN and thus inhibit acute restraint stress-induced corticotrophin-releasing hormone mRNA expression (Bulbul et al., 2011) and the toxic effects of glucocorticoids (Matsushita et al., 2019). Interaction between 5-HT and OT systems is also important in these processes. The anxiolytic effects of OT in preclinical and clinical findings are not only related to the HPA axis, but also to the 5-HT system (Yoon & Kim, 2020). OT in the brain can facilitate 5-HT release within the median raphe nucleus and reduce anxiety-related behavior via OTRs in mice (Yoshida et al., 2009). This effect is associated with activation of 5-HT2A receptor (5-HT2A-R) and 5-HT2C-R heteroreceptor complexes in the raphe-hippocampal 5--HT neuron systems. This is because pathological blunting of the OTR promoters in 5-HT2A-R and especially in 5-HT2C-R heteroreceptor complexes promotes the development of depression and other types of psychiatric diseases involving disturbances in social behaviors in major depression (Borroto-Escuela et al., 2021). Recent studies in rats further highlight that social stress-evoked increase in hypothalamic OT secretion can account for maternal depression (Li et al., 2020; Li et al., 2021f). Notably, IAO can benefit new mothers who report moderate sub-clinical levels of depression (Lindley Baron-Cohen et al., 2021). In rats, social stress-elicited maternal depression is reversible upon IAO (Li et al., 2020; Li et al., 2021f). Functional magnetic resonance imaging investigation revealed that IAO can reduce amygdala hyper-activity in subjects with anxiety, and enhance connectivity between the amygdala and bilateral insula and middle cingulate gyrus in patients but not in healthy controls. Thus, the occurrence of depression and anxiety are due to disorders in OT-associated signaling processes while IAO has the potential to treat depression and anxiety.
Reward and Addiction
Social cognition and prosocial behaviors are closely associated with the rewarding mechanism. The reward system is mainly made up of the main dopamine pathways of the brain, especially the mesolimbic pathway from the VTA to NAc. This system is activated by rewarding (e.g. pair bonding, sexual contact, and positive social interaction) or reinforcing stimuli (e.g. addictive drugs). OT interacts with the mesocorticolimbic circuit and produces rewarding effects in response to rewarding stimuli (Dolen et al., 2013). It is likely that OT release from parvocellular division of the PVN and axonal collaterals of magnocellular OT neurons (Strathearn et al., 2019; Sundar et al., 2021) causes release of dopamine in the NAc and PVN while suppressing activity of the HPA axis and brain endogenous opioid systems (Hung et al., 2017), thereby facilitating rewards. This process can be enforced by serotonin transmission from raphe neurons to VTA to reinforce learning by serotonergic neuron co-transmitter glutamate (Courtiol et al., 2021). The rewarding functions of OT result from its acting within the NAc and VTA, and facilitate social reward and approach behavior. However, in aversive social contexts, additional pathways in the BNST play critical roles in mediating the effects of OT induction of avoidance of potentially dangerous social contexts (Steinman et al., 2019).
Addiction occurrence correlates with a hypodopaminergic dysfunction within the reward circuitry of the brain (Gardner, 2011) as well as malfunctions of the OT system. Acute cocaine treatment decreases OT levels in the hippocampus of rats (Johns et al., 1993). In mice, 14-day escalating-dose cocaine administration and 14-day withdrawal increase plasma corticosterone levels and OTR binding in the piriform cortex, lateral septum and amygdala. A specific cocaine withdrawal-induced increase in OTR binding is present in the medial septum (Georgiou et al., 2016). OT has protective effects against drug addiction by modulation of the initial response to drugs of abuse via shifting novelty processing from ventral to dorsal striatal structure (Tops et al., 2014), and attenuation of the development of dependence by reducing drug-elicited dopamine neurotransmission in the mesocorticolimbic circuit (Pedersen, 2017). OT also decreases withdrawal symptoms like facial fasciculation, convulsion, hypothermia, and anxiety-like and depression-like behavior during morphine, cocaine, nicotine, and alcohol abstinence (Bowen & Neumann, 2017). In addition, OT blocks drug reinstatement by blocking exposure to drug cues, drug priming, or stressful experience-evoked activation of the NAc (Cox et al., 2017) and exerts a general anti-stress effect (Ferrer-Perez et al., 2019). These views have gained supports from animal studies. IAO can decrease craving and relapse related to alcohol, cannabis, opioids, cocaine, or nicotine, etc (Houghton et al., 2021; Van Hedger et al., 2020). Activation of OTRs during recovery from opioid overdose could also eliminate or attenuate negative side effects associated with traditional opioid receptor antagonism (Brackley & Toney, 2021). These effects are associated with increased release of dopamine and glutamate and the activation of cortical and basal ganglia sites, which modulates the activity of mesolimbic dopaminergic neurons (Koob & Volkow, 2016). OT-reduced addictive behaviors is also related to restoring abnormal drug-induced changes in the glutamatergic system in NAc (Logan et al., 2021; Sundar et al., 2021). In addition, changes in synaptic transmission in the central nucleus of the amygdala (CeA) are an important target in mediating alcohol dependence. Acute alcohol consumption facilitates GABAergic transmission in the CeA via both pre- and postsynaptic mechanisms while decreasing ionotropic glutamate receptors-mediated transmissions; chronic alcohol drinking increases baseline GABAergic transmission and upregulatesN-methyl-D-aspartate (NMDA) receptor-mediated transmission (Roberto et al., 2021). It is likely that drug-mediated and OT-driven social reward signals compete within the brain reward circuitry; while drugs suppress the activity of OT system, OT may enable the rewarding effects of prosocial behavior at the expense of drug-related rewards (Kovatsi & Nikolaou, 2019; Sundar et al., 2021). It is likely that under physiological condition, OT activates dopamine release while suppressing endogenous opioid release, and thus facilitation of opioids on dopamine release is negligible. However, drug-evoked addiction is accompanied with strong dopamine release while inhibiting OT neuronal activity. Once endogenous OT system is reactivated or exogenous OT is supplied, the rewarding effects of OT surpasses that of the additive substances, stable dopamine release is restored without the need of other substances. As a whole, OT can prevent stress and priming-induced relapse to drug seeking while inspiring rewarding to replace the impulse of addiction.
Negative Impacts on Higher Brain Functions
While OT is generally considered as a “cuddle chemical”, its adverse effects on social behaviors have also been identified. For example, OT may create intergroup conflict and violence because OT motivates in-group favoritism in humans (De Dreu et al., 2011) and in wild chimpanzees (Samuni et al., 2017). It is typically observed in maternal attacks toward unfamiliar male intruder in lactating rats (Li et al., 2021d). Individuals low in extraversion, trust-altruism, and openness to experience become significantly more anhedonia after receiving IAO relative to placebo, following imagery training of positive social outcomes in humans (Wong et al., 2021c). Similarly, individuals with elevated depressive symptoms recall memories that are more negative following IAO in the non-social context (Wong et al., 2021b). In addition, intrapartum OT application is positively associated with postnatal depressive symptoms in pregnant women (Tichelman et al., 2021). These findings implicate that abnormally increased OT bioavailability in vulnerable persons can evoke or enforce some negative social activities.
Mechanisms underlying differential effects of OT on intra-group cooperation and inter-group hostility have not been identified neurophysiologically. However, destructive effects of overly-produced OT in the hypothalamus could account for many of these adverse OT effects. Excessive OT in the hypothalamus can reduce OT release from axon terminals including the posterior pituitary and possibly in the brain identified in rat dams deprived of pups, which causes hypogalactia and maternal depression (Li et al., 2020; Li et al., 2021f). This is because excessive OT causes post-excitation inhibition of OT neurons as shown in rats (Wang et al., 2006) and reduced OT neuronal excitability, which in turn reduces OT release into the blood and the brain via axonal terminals (Zhang et al., 2021). Thus, when cytosolic Ca2+-dependent OT release from the somata and dendrites of OT neurons increases (Ludwig et al., 2002), brain OT release through axon collaterals could be reduced, thereby causing depression and anxiety. Notably, IAO does not worsen maternal behaviors but alleviates maternal depression in rats (Li et al., 2021c; Li et al., 2021e; Liu et al., 2019). Since IAO tends to increase brain OT levels, these findings indicate that social stress does reduce extrahypothalamic OT release despite the increase in hypothalamic OT levels.
Instinctive Behaviors
OT can regulate defence, eating and drinking, sexual behavior, female reproduction, sleep, and other instinctive behaviors directly or by modulating activity of limbic system and other brain structures. Approaches mediating OT effects on the instinctive behaviors and somatosensory processes are presented in Figure 4 and Table 2.
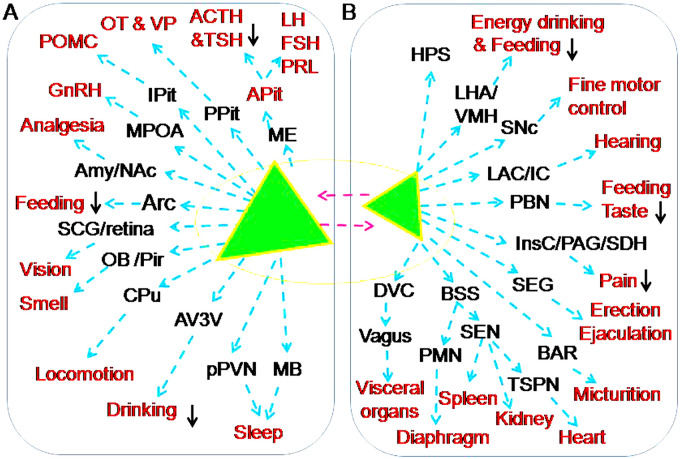
Figure 4.
Approaches mediating OT effects on the instinctive behaviors and somatosensory processes. A-B. Magnocellular OT neurons (A) and parvocellular OT neurons (B). The red font indicates a variety of targets of OT actions (please refer to the text for details). Abbreviations: Adrenocorticotropic hormone (ACTH), Anterior pituitary (APit), Corticotrophin-releasing hormone (CRH), Follicle-stimulating hormone (FSH), Gonadotropin-releasing hormone (GnRH), Luteinizing hormone (LH), Proopiomelanocortin (POMC), Prolactin (PRL), Thoracic sympathetic preganglionic neurons (TSPN), Thyroid-stimulating hormone (TSH) and Vasopressin (VP). Signs and other abbreviations are the same as Figure 2.
Table 2.
OT Effect on Instinctive Behavior and the Underlying Mechanisms.
| Category | Physiologic effects | Neural mechanisms | Pharmacological effects | References |
|---|---|---|---|---|
| Eating and drinking | (1) Decrease food and drinking intake driven by energy; block consumption of toxic food and increase proactive control over feeding with overweight. | (2) Activate VMH and DVC; change activity of the LH and AN; block alcohol-induced DA release within the NAc; activate paraventricular thalamic neurons and suppress HPA axis. | (3) Improve feeding and social skills of infants with Prader-Willi syndrome; improves feeding of patients with anorexia nervosa and inhibit MCH neuronal activity in the LH. | (1) (Bowen et al., 2011; Gartner et al., 2018; Klockars et al., 2017; Pirnia et al., 2020; Plessow et al., 2021); (2) (Althammer & Grinevich, 2017; Klockars et al., 2017; McConn et al., 2019b; Peters et al., 2017; Yao et al., 2012); (3) (Russell et al., 2018; Tauber et al., 2017) |
| Reproduction | (4) Promote sex processes; stimulate HPG axis activity and ovulation; maintain normal menstruation and pregnancy; determine pulsatile OT release during parturition and breastfeeding and maternal behavior. | (5) Activate OT-DAergic neural pathways; increase GnRH release; activate PVN-VTA/SEG pathways; promote maturation of pulsatile OT release machinery involving the SON, PVN and MB as well as OT neurons-maternal behavior-regulating brain regions. | (6) Improve sexuality and its quality; accelerate parturition; facilitate conditional milk letdown; improve erectile dysfunction in aged people with Parkinson's disease; alleviate menstrual migraine and postpartum mood disorders. | (4) (Cera et al., 2021; Dekker et al., 2021; Evans et al., 2003; Higuchi & Okere, 2002); (5) (Bealer et al., 2010; Hou et al., 2016; Li et al., 2021d; Selvage & Johnston, 2004; Uta et al., 2021; Wang et al., 2013); (6) (Bealer et al., 2010; Bharadwaj et al., 2021; Cowley, 2005; Lassey et al., 2021; Sakakibara et al., 2010) |
| Anti-aging | (7) Reduction of OT secretion is accompanied with reduced general health levels, immunity, sexuality and cognitive activity. | (8) Reduced number of OT neurons makes all OT-associated brain functions decrease over time. | (9) Increase functional connectivity of neural circuits involved in social perception; improve emotional stability and body functions | (7) (Maestrini et al., 2018; Orihashi et al., 2020); (8) (Sannino et al., 2017; Zohrabi et al., 2020); (9) (Crowley et al., 2015; Sakakibara et al., 2010; Valdes-Hernandeza et al., 2021; Wang et al., 2019b). |
| Nociception and analgesia | (10) OTR SNP increases pain; OT inhibits chronic somatic pains and visceral pain while reducing panic emotions to injury. | (11) Increase neural activity and neural connections between different brain areas; increase GABAergic signals in the brain and NA signals in spinal cord. | (12) Improve chronic pelvic and low back pains; inhibit the DRG, ACC, PAG, amygdala and NAc regions; increase social proximity and support and anxiolysis. | (10) (Lucas et al., 2021; Tsushima et al., 2021); (11) (Gamal-Eltrabily et al., 2020; Schneider et al., 2020; Yang et al., 2011; Zhu et al., 2021); (12) (Flynn et al., 2021; Kreuder et al., 2019; Riem et al., 2021). |
| Sleep | (13) Improve sleep quality in postpartum women, cancer survivors, patients with ALS and women with a history of sexual assault. | (14) Inhibit HPA axis and melatonin secretion; cause anxiolytic, anti-depression, sedating effects and emotional stability; regulate MB activity. | (15) Increase respiratory rate and reduce obstructive event duration and oxygen desaturation in patients with sleep apnea. | (13) (Comasco et al., 2016; Gabery et al., 2021; Kendall-Tackett et al., 2013; Lipschitz et al., 2015); (14) (Comasco et al., 2016; Bulbul et al., 2011; Teclemariam-Mesbah et al., 1997; (15) (Jain et al., 2017; Jain et al., 2020). |
Abbreviations: ACC, anterior cingulate cortex; ALS, amyotrophic lateral sclerosis;AN, arcuate nucleus; DA, dopamine; DRG, dorsal root ganglion; DVC, dorsal vagal complex; GABA, ɣ-aminobutyric acid; GnRH, gonadotropin-releasing hormone; HPA axis, hypothalamic-pituitary-adrenal axis; HPG, hypothalamic-pituitary-gonadal axis; LH, lateral hypothalamus; MCH, melanin-concentrating hormone; MB, mammillary body complex; NA, norepinephrine; NAc, nucleus accumbens; OT, oxytocin; OTR, OT receptor; PAG, Periaqueductal gray; PVN, paraventricular nucleus; SEG, Spinal ejaculation generator; SNP, single nucleotide polymorphism; SON, supraoptic nucleus; VMH, Ventromedial hypothalamic nucleus; VP, vasopressin; VTA, ventral tegmental area.
Eating and Drinking
Eating and drinking are important for the growth, energy supply, hydration and biochemical activities for physiological processes, and they are under the regulation of OT.
Eating
Food intake is driven by hunger that is associated with hypothalamic feeding center and vagal activity, which are modulated by OT. A systematic review including 26 studies of 912 participants showed that endogenous OT in nonclinical samples does not change significantly through altered diet or behaviors; in contrast, exogenous OT reduces indices of food intake in clinical and nonclinical samples (Skinner et al., 2018). For instance, IAO can increase proactive control over feeding behavior with overweight or obesity while evoking satiety signals and inhibiting eating in men (Plessow et al., 2021). In chickens, intracerebroventricular injection of OT dose-dependently reduces food intake and increases c-Fos expression in arcuate, dorsomedial nucleus, lateral hypothalamus, PVN, and ventromedial hypothalamus (McConn et al., 2019b). However, endogenous OT may mediate anorexigenic effects of other factors, such as that oleoylethanolamide causes satiety by activation of vagus and OT secretion in humans (Igarashi et al., 2021). Central OT serves as a mediator of other anorexic substances like l-tryptophan as shown in mice (Gartner et al., 2018). In mice, OT delivered into the intestinal tract decreases food intake to those who receive intraperitoneal administration. Oral OT also dramatically increases plasma OT levels and the expression of neural activation marker c-Fos protein in the PVN in mice (Maejima et al., 2020). These facts support the proposal that OT can reduce feeding.
OT neurons are glucose sensors. In rats, insulin activation of phosphatidylinositol 3 kinase increases glucokinase-mediated adenosine triphosphate (ATP) production, which induces closure of ATP-sensitive K+ channels, opens voltage-sensitive Ca2+ channels, and stimulates OT release from the SON (Song et al., 2014). From the PVN, OT can activate brainstem vagal neurons in the dorsal vagal complex (DVC) including the dorsal motor nucleus of the vagus and nucleus tractus solitarius (Althammer & Grinevich, 2017). Activated DVC neurons can increase digestive fluid secretion in response to stomach distention and elevated osmolality, and block consumption of toxic foods in rats (Mitra et al., 2010). OT-decreased food intake driven by energy is also involved in the ventromedial hypothalamic nucleus (Klockars et al., 2017) and the arcuate nucleus (Maejima et al., 2014) as shown in rats. OT directly evokes a substantial excitatory effect on presumptive GABA cells that contain melanin-concentrating hormone (Yao et al., 2012). Thus, OT also influences the secretion of melanin-concentrating hormone that can promote eating (Koop and Oster, 2021). By contrast, OT activation of paraventricular thalamic neurons promotes feeding motivation to attenuate stress-induced hypophagia but does not influence normal feeding in mice (Barrett et al., 2021). In addition, OT effects on appetite may involve CRH signaling in the hypothalamus in chickens (McConn et al., 2019b).
Notably, patients with Prader-Willi syndrome display poor feeding and social skills as infants and fewer hypothalamic OT neurons were documented in adults. IAO significantly increases acylated ghrelin and connectivity of the right superior orbitofrontal network that correlated with changes in sucking and behavior in infants with Prader-Willi syndrome and improves feeding and social skills (Tauber et al., 2017). In patients with anorexia nervosa, plasma OT levels are significantly reduced (Monteleone et al., 2016). IAO can reduce eating concern and cognitive rigidity while lowering salivary cortisol levels and reducing stress responsiveness to food and eating (Russell et al., 2018), thereby promoting nutritional rehabilitation in anorexia nervosa. Although such effects require replication with inclusion of more sensitive subjective measures, as a whole, OT functions as a homeostatic regulator of food consumption, ingestive behavior and energy metabolism.
Drinking
Drinking is essential for water supplement and volemic and hydromineral homeostasis and also important for taking energy drinking and enjoyment. While VP is the major hormones responsible for volemic and osmotic regulation in general (Smith et al., 2015), OT also modulate water and alcohol intake. In a single-case study, IAO for two six-week stages significantly reduced addiction severity index in five domains of medical status, occupational status, alcohol consumption, family status, and mental status (Pirnia et al., 2020). In prairie voles, intraperitoneal injection of OT can reduce 15% ethanol versus water consumption only in the first hour of access after treatment (Stevenson et al., 2017). In rats, intracerebroventricular OT application can completely block alcohol-induced dopamine release within the NAc (Peters et al., 2017). In female mice, disruptions in OT signaling may contribute to increased vulnerability to alcohol-associated addiction (Rodriguez et al., 2020). Moreover, OT may also mediate the regulation of water intake by other hormones, such as endothelin in rats (Samson et al., 1991) and serotonin in water-deprived rats (de Souza Villa et al., 2008). OT administration blocks the increase in voluntary ethanol consumption observed in socially defeated mice (Reguilon et al., 2021). It is likely that OT influence of alcohol consumption is mainly associated with its effect on social cognition and rewarding
Relative to the effect on food intake, endogenous OT inhibition of water intake in humans is weaker. Evidence of OT regulation of water intake is mainly from animal studies. As reported, mesotocin, an OT-like hormone of nonmammalian tetrapods and in most marsupials, decreases food intake and induces water intake; OTR antagonism can prevent mesotocin-mediated changes in food intake but not water intake in chicks (McConn et al., 2019a). Notably, in rats freely accessing to beer and water in their home cages, consumption of beer that has more calories (mainly from carbohydrates and alcohol) but not water was significantly less in the OT pre-treated rats (Bowen et al., 2011). This is in agreement with the finding that OT acting in the ventromedial hypothalamic nucleus of rats decreases food intake driven by energy (e.g. chow) but not by palatability (e.g. energy-dilute saccharin and sucrose solutions) (Klockars et al., 2017). Both beer/alcohol and food are energy-enriched substance while saccharin and sucrose solutions are palatable but energy-diluted. Thus, OT inhibitory effects on feeding and drinking are aimed at reducing the energy/caloric intake.
Reproduction-Associated Activity
OT has extensive regulatory effects on reproduction including sexual behaviors, menstrual cycle, parturition, lactation and aging.
Sexual activity
In both males and females, sexual activity at all stages is closely associated with OT actions. Plasma OT levels increase during sexual arousal and are significantly higher during orgasm/ejaculation than during prior baseline levels in both women and men (Cera et al., 2021; Dekker et al., 2021). In the brain, OT-dopaminergic neural pathways play a role not only in the erectile function and copulation, but also in the motivational and rewarding aspects of the anticipatory phase of sexual behavior. Deficits in OT system or its interactions with brain amine systems can result in loss of libido, impotence and lack of orgasm; conversely, over-activation of these systems may cause abnormal desire and multiple orgasms (Magon & Kalra, 2011). Postpartum women appear to experience a decrease in sexual interest possibly as a feature of a more generalized decrease in amygdala responsiveness to arousing stimuli, which also relates to the actions of OT (Rupp et al., 2013). Thus, appropriate OT levels and actions are essential to maintain the quality of sexual activity. Mechanistic studies in rats reveal that OT in the VTA from the PVN can induce penile erection (Melis et al., 2007) by promoting dopamine and glutamate release into the NAc, mPFC, amygdala, and other forebrain regions (Peris et al., 2017). The coordinated reactions of this OT-PVN-VTA-forebrain/spinal cord pathways make the sexual intercourse accompany with positive motivation and reward of sexual behavior (Magon & Kalra, 2011).
It seems contradictory that over-activation of the OT system causes abnormal desire and multiple orgasms while postpartum women have decreased sexual interest. However, it could be an outcome of reproductive trade-offs whereby mother-infant interactions activate the reward system by breastfeeding and bonding rather than by sexual drive. This notion is supported by the following observations (Gregory et al., 2015). 1) Postpartum women show lower sexual excitation, higher sexual inhibition, and lower sexual desire compared to nulliparous women; 2) Postpartum women show higher reward area activation to infant stimuli and nulliparous women show higher activation to sexual stimuli; and 3) IAO increases VTA but not NAc activation to both crying infant and sexual images for all women. Thus, OT may contribute to the altered reproductive priorities in postpartum women by increasing VTA activation to salient infant stimuli. In addition, the reduced estrogen level in postpartum may also decrease sexual drive, which can mask a transiently increased sexual desire elicited by OT pulse during suckling; however, breastfeeding the baby elicits the reward associated with women's emotional and physical satisfaction. Therefore, decreased sexual interest in breastfeeding mothers occurs, particularly in the first 1–2 months of postpartum period.
Menstruation
Menstrual cycle is a unique physiological phenomenon of women, driven by a series of changes in steroid hormone production and modulated by OT. Plasma OT is different at different stages of menstruation in ovulating women; compared to the luteal phase, OT levels are significantly high during ovulatory phase (Evans et al., 2003). A higher dose of OT could promote the growth of follicles as shown in postpartum buffaloes (Murtaza et al., 2021). In the hypothalamus, OT can stimulate the secretion of gonadotropin-releasing hormone, specifically at pre-ovulation stage as revealed in studies on cycling female rats (Selvage & Johnston, 2004). Consistently, during the proestrus, c-Fos expression in the SON increases significantly in association with facilitatory retraction of astrocytic processes in rats (Liu et al., 2016). Thus, OT secretion is closely associated with the neuroendocrine regulation of menstrual cycle.
Migraine is a major cause of disability worldwide, particularly among women aged 15–49 years. Overall, the incidence of migraine is threefold higher among women than men. In association with menstrual cycle, changes in estrogen and OT secretion can underlie migraines, which can be relieved by OT (Krause et al., 2021). Consistently, intravenous OT application can rapidly and temporally relieve the pain (Phillips et al., 2006). It is believed that cyclically released hypothalamic OT inhibits trigeminal neuronal excitability that can promote migraine pain. Coincident with decreased levels of both estrogen and OT during menstruation, magnesium and cholesterol, which positively modulate the affinity of OT for OTRs, also decrease. Thus, decreased OT alongside the reduction of trigeminal OTR expression and its affinity to OT promotes the activation of meningeal trigeminal nociceptors and increases the likelihood of an menstrual migraine attack (Bharadwaj et al., 2021; Tzabazis et al., 2017).
Pregnancy and parturition
During pregnancy, OT is not only important for the development and protection of fetus, but also critical for the maturation of maternal pulsatile OT secretion machinery during lactation. From the middle stage of gestation, central OTR binding is increased gradually in the SON, PVN, BNST and MPOA (Bealer et al., 2006); its blockade delays OT release and reduces milk yields during suckling in rats (Lipschitz et al., 2003) and in adult marmosets (Taylor & French, 2015). However, brain and blood OT levels do not change significantly during pregnancy until parturition. Functionally, activation of central OTR during gestation can cause morphological changes in magnocellular OT neurons, disinhibition of OT neurons in response to GABA, and adaptations in membrane response characteristics of OT neurons (Bealer et al., 2010). Resultantly, OT neurons become more reactive to neural inputs from the cervix, mammary glands and conditional stimuli from other sources.
When cervix or vaginal walls are stretched, neural signals reach the SON and PVN of the hypothalamus and OT neurons are activated; the resultant release of OT in a bolus initiates self-sustaining cycle of uterine contractions in rats (Higuchi & Okere, 2002). This neural-humoral reflex is called Ferguson reflex. In clinical practice, while using OT to assist parturition remains controversial (Litorp et al., 2021), induction of labor with a single-balloon catheter and OT results in a shorter time from insertion to delivery without increasing the rate of cesarean delivery (Lassey et al., 2021). Thus, how to activate the mechanism underlying Ferguson reflex is worthy of investigation for augmentation of labor.
Lactation
In all mammals, OT is an essential hormone that is used by mothers to provide milk to their offspring, as proved by OT knockout in mice (Winslow & Insel, 2002). Suckling-increased OT secretion is essential for the maintenance of maternal behaviors in the brain and the development of mammary glands in rats (Li et al., 2021e). In response to suckling stimulation, neural signals from the mammary glands reach the hypothalamus, trigger synchronized burst-like activation of OT neurons in the SON and PVN, and result in a pulsatile release of OT from the posterior pituitary and milk ejection (Wakerley et al., 1994). In association with the conventional suckling signals, the hypothalamus also receives signals from other sensory organs, such as the gastrointestinal tract, OBs, vagus and cortex-hypothalamus neural pathway, which allows conditioned OT release available (Uvnas-Moberg et al., 2014). In the activation of OT neurons, many extracellular and intracellular events occur, such as neurochemical environment (Brown et al., 2013), mobilization of OTR-signaling events (Hatton & Wang, 2008), intercellular junctional coupling (Wang et al., 2019a), astrocytic plasticity (Wang & Hatton, 2009), ion channel activity (Li et al., 2020; Li et al., 2021c) identified in rats and others as reviewed (Hou et al., 2016). In addition, synchronization of burst firing of a large pool of OT involving those on bilateral sides of the hypothalamic OT neurons (Wang et al., 1995; Wang et al., 1997) is required to trigger full milk ejections in lactating rats. Such synchronized activity among OT neurons is determined by the activity of the mammillary body complex (Wang et al., 2013). In addition, OT release via activating the Ferguson reflex can increase the strength of the milk-ejection reflex shown in Holstein cattle (Morales et al., 2020). It indicates that different neurohumoral reflexes for OT release can be additive. It remains to clarify the neural circuit that controls the burst synchrony and the bolus of OT secretion.
In clinical trials, IAO has been found to promote breastfeeding in human (Cowley, 2005) and in lactating rats (Li et al., 2021c; Liu et al., 2019). In the neonatal unit setting, IAO can cause initial faster production and then become no difference from placebo treatment (Fewtrell et al., 2006). This finding is consistent with the finding in rat model that acute social stress-reduced OT neuronal activity, maternal depression and hypogalactia can recover spontaneously in chronic social stress (Li et al., 2021d). In cesarean rats that the reduced OT neuronal activity and aberrant maternal behavior at early postpartum stage can recover spontaneously as the elapsing of postpartum time while IAO can reverse depressive-like maternal behavior (Li et al., 2021e). These facts indicate that OT can promote breastfeeding by accelerating maturation of the machinery for the milk-ejection reflex through improving OT neuronal activity and maternal mood at the early postpartum stages.
Anti-Aging
Aging, as a process of negative growth and functional loss, is closely associated with alterations of OT actions. Around 35 years of age, blood OT levels in women decrease gradually (Maestrini et al., 2018). Moreover, there is a positive correlation between serum OT levels and the volume of the left hippocampus and amygdala among people aged 65 years and older (Orihashi et al., 2020). IAO can increase functional connectivity of neural circuits involved in action observation and social perception among old men (Valdes-Hernandeza et al., 2021). Decreased OT levels in elderly people are associated with reduced sex steroid production, socially emotional deficits, symptoms of neurodegenerative diseases, lower sexual ability, reduced vagal nervous tone and low muscle power (Sannino et al., 2017; Zohrabi et al., 2020). As shown in aged male rats, the number of parvocellular OT neurons and their OT release are significantly decreased in the PVN, DVC and substantia gelatinosa of the spinal cord (Calza et al., 1990), which weakens the regulation of visceral activity.
By contrast, OT application can significantly improve erectile dysfunction in aged people with Parkinson's disease (Sakakibara et al., 2010), relieve aging-associated symptoms such as mood disorders in menopausal women (Crowley et al., 2015) and exert many other protective functions, such as reducing blood pressure (Wang et al., 2019b). Notably, chronic IAO has no significant impact on cardiovascular, urine, or serum measures. Relative to placebo, OT does not significantly increase the likelihood of adverse events. Chronic IAO appears safe and well-tolerated in generally healthy older men (Rung et al., 2021). These facts highlight the anti-aging effects and therapeutic potential of OT in aging-associated diseases.
Nociception and Analgesia
In addition to menstrual migraines as stated above, OT extensively suppresses nociception and exerts analgesia effects in humans and animals. Acute dental pain elevates salivary OT levels in pregnant women (Gurler et al., 2021). Women with the minor allele OTR rs53576 report 8.18-fold higher breast and nipple pain severity over time while women with the OTR rs2254298 minor allele also report allodynia (Lucas et al., 2021). This finding suggests that disorders in OT/OTR signaling can cause breast and nipple pain and its associated breastfeeding cessation. By contrast, IAO for 4 weeks can significantly improve chronic pelvic pain in 1/3 women observed (Flynn et al., 2021) and that IAO improves pains in patients with chronic low back pain (Schneider et al., 2020). In rats, neuropathic pain induced by partial sciatic nerve ligation upregulates OT synthesis in the SON and PVN as well as axon terminals of OT neurons in the posterior pituitary and spinal cord (Nishimura et al., 2019). These facts suggest that both magnocellular and parvocellular OT system are activated in response to nociceptive stimulation. Similar effect of OT is also identified in visceral pain in mice (Tsushima et al., 2021). OT analgesia effect can occur at the dorsal root ganglion (Zhu et al., 2021), anterior cingulate (Li et al., 2021 g), medial amygdala, CeA and NAc regions (Saito et al., 2021; Tsushima et al., 2021). In rats, increased release of opiate peptides from the periaqueductal gray area likely mediates OT analgesic effect (Yang et al., 2011). OT analgesic effect on chronic pain is associated with its increases of intrinsic neural activity of the medial frontal, parietal and occipital functional network and the associations between left NAc, left caudate nucleus and right amygdala with pain-specific psychometric scores (Schneider et al., 2020). Moreover, this OT effect involves increased GABAergic transmission at the cortical insular level and activating descending spinal noradrenergic mechanisms (Gamal-Eltrabily et al., 2020). In addition, this OT effect is also related to its suppressing anxiousness as shown in mice (Saito et al., 2021).
Clinical trials also support OT analgesia effects. IAO enhances the pain-relieving effects of social support in romantic couples (Kreuder et al., 2019). IAO enhancement of social proximity may be a mechanism underlying social influences on cardiovascular and mental health; however, IAO effects depend on interpersonal insecurities and may trigger discomfort in avoidantly attached individuals during experimentally-induced pains (Riem et al., 2021). Thus, OT exerts analgesia function for both somatic and visceral pains by activating both endogenous analgesia machinery and anxiolytic physiology.
Sleep
In humans, OT can increase the quality of sleep. Under basal and disease conditions, endogenous OT facilitates sleep in postpartum women (Comasco et al., 2016), among cancer survivors (Lipschitz et al., 2015) and in patients with amyotrophic lateral sclerosis (Gabery et al., 2021). Women with a history of sexual assault have a number of sleep difficulties, increased risk of depression, and overall poorer subjective well-being. Sexual assault survivors who are breastfeeding and have pulsatile OT secretion are at lower risk on all of the sleep and depression parameters than sexual assault survivors who are mixed or formula feeding (Kendall-Tackett et al., 2013). By contrast, sleep deprivation can increase OT in the SON as shown in Syrian Hamsters (Knochel et al., 2021), which may antagonize the effect of awake-evoking factors.
The wakefulness-sleep cycle is related to dynamics of activating and inhibiting forebrain neurotransmitter systems that are modulated by rhythmic release of several hormones such as melatonin, cortisol and orexin (Koop and Oster, 2021). OT improvement of sleep is associated with its inhibition of the HPA axis (Bulbul et al., 2011) and melatonin secretion (Teclemariam-Mesbah et al., 1997). This effect is also associated with OT anxiolytic, anti-depression and sedating effects as well as its increasing emotional stability as shown in postpartum women (Comasco et al., 2016). In addition, OT fibers also innervate the mammillary body complex as shown in mice (Liao et al., 2020) that participates in sleep regulation, memory and many other functions (Benarroch et al., 2015). Thus, OT could modulate sleep by modulating the activity of mammillary body cells.
In clinical trials, a general sleep-promoting effect of OT remains to be established (Raymond et al., 2021). However, in clinical trials of obstructive sleep apnea patients, IAO has been confirmed to increase respiratory rate and reduce obstructive event duration and oxygen desaturation (Jain et al., 2017; Jain et al., 2020). Thus, while sleep-promoting effect of OT remains to be identified, IAO can be used to improve sleep problems in patients of obstructive sleep apnea.
Regulation of Sensorimotor Activity
OTRs distribute in extensive brain regions, which allows OT to modulate sensory and motor activity through acting on OTRs within sensory and motor centers. OT effects on sensorimotor activity involve autonomic neural activity, specific sensation and somatic sensorimotor activities (Figure 4).
Effects on Autonomic Nervous System
OT modulation of visceral activity is closely related to its regulation of autonomic nervous activity, such as cardiac rhythm, gastrointestinal tract contraction, breathing rhythm, and sweating, etc.
Parasympathetic nervous system
Heart rate variability (HRV) refers to variation in the interval between successive heart beats. Low HRV is an indicator of potential autonomic nervous system dysfunction. Listening to a slow-tempo music sequence increases the salivary OT concentration of participants whose high frequency component of the HRV increases while the heart rate decreases (Ooishi et al., 2017). In the postpartum period, multiparous women have higher saliva OT levels, higher parasympathetic nervous activity and lower physical stress index compared with primiparous women. In multiparous perinatal women, OT levels correlate positively with parasympathetic nervous activity, but negatively with physical stress index (Washio et al., 2020). In women at the third trimester of pregnancy and during low-risk labor, application of OT increases vagally mediated activity (Reyes-Lagos et al., 2015). IAO increases high frequency HRV (an index of cardio-parasympathetic activity) in men at clinical high risk for psychosis (Martins et al., 2020) and with 38 men either receiving 24 IU OT intranasally or a placebo spray. While accomplishing an emotion classification task, electrodermal responses are measured as an index of sympathetic activity. Moreover, heart rate changes recorded are additionally mediated by the parasympathetic nervous system. OT enhances differential heart rate responses to facial expressions as a function of the emotional valence, but has no effect on electrodermal activity or tonic measures of physiological arousal. These results indicate that OT specifically modulates phasic activity of the parasympathetic nervous system which potentially reflects an increased motivational value of facial expressions following OT treatment. These findings suggest that anxiolytic effects of OT are not reflected in short-term sympathetic responses and may even be a consequence rather than a prerequisite of improved social information processing (Gamer & Buchel, 2012). In rats of lipopolysaccharide-induced endotoxemia, respiratory rate is elevated and mean R-R interval is reduced, and OT administration significantly attenuates the hyperventilation and restores the values of the mean R-R interval, supporting that OT promotes cardiac cholinergic autonomic coupling (Elorza-Avila et al., 2017). Histologically, OT fibers from the PVN likely innervate the DVC in the brainstem and the intermediolateral and sacral parasympathetic nuclei in rats (Althammer & Grinevich, 2017), which allow OT to regulate visceral activities and maintain homeostasis of the internal environment.
Among these neural connections, the PVN-DVC-vagal-organ pathway is a major approach for OT regulation of visceral activities. As shown in rats, activation of this pathway can increase insulin levels (Bjorkstrand et al., 1996), reduce heart rate and cardiac contractility (Houshmand et al., 2015), suppress food and water intake of the digestive system (Niimi et al., 2001), slow gastric emptying (Tolentino et al., 2021) and regulate hepatic metabolic activity (Dos Santos et al., 2021). Recently, it is also reported that activation of brain OT neurons inhibits colitis-associated cancer progression in association of suppression of sympathetic neuronal activity in the celiac-superior mesenteric ganglion as shown in mice (Pan et al., 2021). Thus, OT can modulate visceral activities by increasing parasympathetic nervous activity although the parasympathetic outflow function of endogenous OT remains to be studied.
Sympathetic nervous system
Sympathetic nerves arise from the intermediolateral nucleus of the lateral grey column from the first thoracic vertebra of the vertebral column to the second or third lumbar vertebra. Their activities are regulated by many sites or centers in the brain, such as the rostral ventrolateral medulla (RVLM), PVN, lateral hypothalamus and limbic system (Kerman et al., 2006). OT fibers are present in these sites and conditionally regulate their activities and sympathetic neural outflows.
On the one hand, the OT system has inhibitory influence on sympathetic activity by decreasing social stress and the HPA axis activity in general (Moberg et al., 2020). In early postpartum mothers, although plasma OT did not increase reliably from pre-stress levels during stressors, greater overall OT level is related to greater vasodilatation and cardiac stroke volume responses to reduction in heart rate and to the cold pressor, as well as to lower plasma norepinephrine and higher prolactin levels (Grewen & Light, 2011). Consistently, in OT knockout mice, there are higher sympathetic tone and higher heart rate (Michelini et al., 2003).
On the other hand, there are also reports supporting OT activation of sympathetic activity. IAO can reduce engagement of the parasympathetic nervous system in patients of chronic neck and shoulder pain and under cognitive stress (Tracy et al., 2018), which are accompanied with relative increase in sympathetic outflows. Histologically, 80% RVLM-projecting PVN cells in rats are OT neurons (Lee et al., 2013) and microinjection of OT into rat RVLM increases sympathetic outflows as indicated by blood pressure increase (Ishizuka et al., 1993). It is also believed that a population of OT neurons in the PVN projects to sympathetic preganglionic neurons in the upper thoracic spinal cord; their activation by OT selectively increases heart rate in rats (Yang et al., 2009). Moreover, OT is a specific component of the sympathetic pathway innervating the spleen in rats (MacNeil et al., 2003). In addition, blocking OTRs attenuates the increase in renal nerve activity induced by intracerebral application of prostaglandin E2 when measured 10 and 30 min post-injection. However, it remains to exclude a pharmacological effect of OT in its activation of sympathetic nervous activity. This is because of the finding that PVN-induced activation of sympathetic activity to the kidney is mainly mediated by glutamate or VP neurons whereas dopamine via Dl receptors may mediate some of the PVN inhibitory effects identified in rats (Yang et al., 2002).
As a whole, OT effects on the autonomic nervous system are not a simply inhibition or excitation, but at maintaining homeostasis of the internal environment. Thus, the PVN-DVC pathway can be prevailing at rest whereas PVN-sympathetic pathway becomes dominant when a stressful environment is present. In addition, this differential function should result from activation of different neural connections between higher brain centers and different sub-population of OT neurons in the PVN. This possibility remains to be validated in experimental studies, particularly involving humans.
Specific Sensation
OT neurons have extensive neural connections with specific sensory organs that also express OTRs. Correspondingly, OT can influence the sense of smell, vision, hearing, taste, and others.
Olfaction
Olfaction deficits are common in schizophrenia and correlate with negative symptoms and low social drive. Individuals with schizophrenia report experiencing more negative emotionality than controls in response to the olfactory stimuli. Lower plasma OT levels are associated with poorer accuracy for pleasant and unpleasant odors and greater severity of asociality among them (Strauss et al., 2015). Olfactory deficits can be improved by IAO in patients with schizophrenia (Woolley et al., 2015). In animal study, intrabulbar infusions of OT induced full maternal behavior in female rats tested within two hours of pup exposure while intrabulbar infusions of OTR antagonist during delivery blocks the occurrence of maternal behavior (Yu et al., 1996a). It has been found that hypothalamic OT could directly modulate main OB cellular activity following release from neural terminals from OT neurons. It has been reported that high-frequency stimulation of the PVN produced inhibitory responses in 80% of mitral cells and excitatory responses in 74% of inhibitory granule cells tested; both responses were blocked by infusion of an OTR antagonist into the OBs and mimicked by intracerebroventricular OT applications (Yu et al., 1996b). OT activation of granule cells is associated with its increasing excitatory postsynaptic currents as shown in granule cell cultures (Osako et al., 2001), suggesting increased glutamatergic synaptic transmission from mitral/tufted cells to granule cells. However, in cultured mitral/tufted cells, OT also reduces the frequency of inhibitory postsynaptic currents (Osako et al., 2000). Consistently, in mouse accessory OBs, OT can modulate glutamatergic NMDA receptor-dependent long-term potentiation at the mitral-to-granule cell synapse (Fang et al., 2008). Thus, OT can function as a gate to control information flows within the local OB neural circuits and from the OBs to the brain regions regulating maternal behavior.
Similarly, intrabulbar OT can act to preserve social recognition responses (Dluzen et al., 1998) by increasing norepinephrine release and activating alpha-adrenoceptors (Dluzen et al., 2000). Interestingly, intrabulbar OT infusion can increase mouse performance in a social interaction task, enhance odor-evoked responses of mitral/tufted cells and neural discrimination of odors but reduce the spontaneous firing rate of mitral/tufted cells. OT also decreases odor-evoked calcium responses specifically in granule cells and modulates ATP-sensitive potassium channels to exert inhibitory effect on mitral cell activity through OTR-Gq-PLC-IP3 signalling pathway (Sun et al., 2021). By contrast, excitatory outputs of olfactory nerves from mitral cells can mediate effects of IAO on the brain activity including OT neurons in the SON in rats (Liu et al., 2017; Meddle et al., 2000; Yeomans et al., 2021). It is likely that OT modulates social interaction including social memory and maternal behavior by increasing the signal-to-noise ratio of odor responses in mitral cells.
Hearing
Auditory signals are important regulator of the OT system as demonstrated in conditioned milk-ejection reflex (McNeilly et al., 1983). OT/OTR signaling also plays an important role in processing auditory information. For example, individuals with the GG genotype of OTRs have less difficulty in hearing and understanding than participants with AA/AG genotypes in noisy environments; older people with reduced OT secretion report more difficulties of hearing and understanding than younger (Tops et al., 2011). Healthy women with the GG genotype of OTRs have more pronounced physiological reactivity to infant crying (Riem et al., 2011). Mother's live voice can significantly decrease premature infant pain profile scores, with a concomitant increase in OT levels over baseline (Filippa et al., 2021). In addition, patients of ASDs have specific impairments in orienting to, and selection of speech sounds from background speech, which can be improved by IAO (Lin et al., 2014; Yoshimura et al., 2018). In mice, that infant cries evoke powerful responses in parents can also be established in pup-naive virgin females after having been co-housed with a mother and litter, which requires cortical activity and the hypothalamic OT system (Schiavo et al., 2020). OT facilitation of auditory fear memory extinction in rats is due to OT activation of the hippocampal-mPFC pathway, and consequently, the release of brain-derived neurotrophic factor (BDNF) in the infralimbic cortex in rats (Bazaz et al., 2022). Thus, OT has both physiological and pharmacological functions in auditory process.
Hearing effects of OT rely on the mediation of OTRs at multiple levels. In bats, OT perikarya are present predominantly in the inferior colliculus, superior olivary complex, and both OT perikarya and fiber terminals exist extensively in the cochlear nucleus (Kanwal & Rao, 2002), which allow OT to exert both neuromodulatory and autoregulatory effects on auditory information processing. In the cerebral cortex, OTRs are mainly present at synapses, axons and glial processes; OT enables long-term synaptic plasticity in the auditory cortex of female mice (Mitre et al., 2016). The intrinsic mechanisms for vocal communication and sociospatial memory are dependent on OT-OTR signaling shown in singing mice (Campbell et al., 2009). OT enables pup retrieval behavior in female mice by enhancing auditory cortical pup call responses through fine-tunes excitatory and inhibitory responses in the left cortex of maternal adults (Marlin et al., 2015). IAO can shorten latency but not amplitude of mismatch negativity, an event-related potential reflecting auditory discrimination in humans (Ochiai et al., 2021), suggesting that OT may promote the comparison-decision stage. Thus, OT can modulate the activity of auditory system at multiple levels, particularly at higher brain centers.
Visualization
Visual signals have important regulatory effects on the OT system (Devarajan et al., 2005; Ciosek & Drobnik, 2012). Visual/light regulates OT neuronal activity via suppressing pineal gland secretion of melatonin, the receptors of which express in the human hypothalamus and pituitary glands (Wu et al., 2006). By contrast, OT critically modulates visual information processing. OT and OTR are expressed in both human and rhesus retina (Halbach et al., 2015; York et al., 2017). OT can regulate epithelial-mesenchymal transition in the retinal pigment epithelium of human retinal pigmental cell line (Tsuji et al., 2020). OT can also cause a sustained increase of intracellular Ca2+ levels in human fetal retina (Halbach et al., 2015) via capacitative Ca2+ entry mechanisms, and the subsequent inhibition of Kir7.1 channel, an important mediator of sub-retinal waste transport and K+ homeostasis in human retinal pigment epithelium (York et al., 2017). Consistently, IAO augments gaze duration toward the eye-region across four different face categories in humans (Marsh et al., 2021). Thus, OT can regulate visual activity at both cerebral and retinal levels.
OT regulates visual activity at multiple levels. Histologically, the same magnocellular neurons can send axon collaterals to the posterior pituitary and retina or autonomic preganglionic centers of the eyes in rats (Csáki et al., 2021). Under pathological conditions, intravitreal application of OT reduces diabetic retinopathy by significantly increasing outer nuclear layer thickness of the retina in streptozotocin-induced diabetic rats (Degirmenci et al., 2019).
Gustation
Taste sensation is critical for feeding, drinking and digestive activity. Human taste cell line termed HTC-8 is sensitive to OT (Hochheimer et al., 2014). IAO can significantly reduce the consumption of sweet foods and salty snacks among young adults (Burmester et al., 2018). IAO can also suppress activation of the hypothalamus and the parabrachial nucleus (PBN) in response to visual food cues, particularly images of high-calorie food in young adults according to the blood oxygen level-dependent response (van der Klaauw et al., 2017). In rodents, suckling in rat pups or re-feeding with dry food can activate neurons in the insular and somatosensory cortices, central amygdaloid nucleus, PVN, SON, lateral PBN, NTS, and area postrema (Barna et al., 2018). However, physiological effect of OT on gustation in humans remains to be established.
OTRs are present in mouse taste buds throughout the oral cavity (Sinclair et al., 2010), neurons in the PBN (Ryan et al., 2017) and many other regions as shown in mustached bat (Kanwal & Rao, 2002). Activation of OTRs in cells of the lateral PBN along the taste neural pathway suppresses food consumption (Barna et al., 2018). By contrast, absence of OT in mice may increase daily intake of solutions of carbohydrate by selectively blunting or masking post-ingestive satiety (Sclafani et al., 2007). Through the nucleus tractus solitarius, the taste signals reach the PBN that may mediate lithium chloride activation of OT cells in the PVN and SON, and the resultant aversive responses in rats (Olszewski et al., 2000).
Somatic Sensorimotor Activity
While OT is considered as a neuroendocrine hormone and major regulator of social cognition and visceral activity, hypothalamic OT neurons also receive somatic sensory inputs and modulate skeletal muscle activity through modulating motor neuron activity (Figure 4).
Histological basis
It is known that central projections of OT neurons extend to portions of the limbic system, mesencephalon, brain stem and spinal cord, which are associated with the control of behaviors and central stress response as well as motor and vegetative functions (Jirikowski, 2019). For example, spinal cord neurons mainly in the dorsal horn are sensitive to OT originating from parvocellular OT neurons in rat PVN (Condes-Lara et al., 2007). OT presynaptically inhibits the nociceptive input from the peripheral primary afferent fibers by activating γ-Aminobutyric acid (GABA) interneuron and modulating glial cells in rats (Martinez-Lorenzana et al., 2021). In addition, OT fibers are observed in the substantia nigra compact part that modulates fine motor activity and the Barrington's nucleus that controls micturition in mice (Liao et al., 2020). Moreover, OT fibers also innervate the caudate putamen to regulate locomotion and social exploration in rats (Zhang et al., 2021). These structural connections allow OT neurons to receive sensory information and regulate some somatic activities.
OT and neurohumoral reflexes
OT can influence some somatic activity through neural approach. For instance, in both men and women, there are very high positive correlations between OT and electromyography intensity prior to and during orgasm, which is associated with OT excitation of the spinal ejaculation generator in the central gray (lamina X) of the lumbar spinal cord in rats (Uta et al., 2021). The motor centers which contain cerebral peduncles and the cerebellum have lower levels of OTR expression in the human brain (Quintana et al., 2019). The antipsychotic-like effects of OT on prepulse inhibition of the startle response, a measure of sensorimotor gating, are related to basal differences in OT-mediated mechanisms in the mPFC in rats (Tapias-Espinosa et al., 2021). OT modulation of body activity is mainly through neurohumoral reflexes in addition to the milk-ejection reflex and Ferguson reflex. For example, frequent physical touch or hug increases OT levels and in turn causes lower blood pressure, better sleep, and a lower likelihood of inflammation in older adults (Thomas & Kim, 2021). These effects are related to increased OT neuronal activity in the PVN and the inhibition of septohypothalamic nucleus in female rats (Okabe et al., 2021). OT also participates in the regulation of respiratory and cardiovascular activities, partially via projections to the RVLM and phrenic nuclei (Mack et al., 2002). Thus, OT can extensively influence somatosensory activity by acting on both central and peripheral sites in association with neurohumoral reflexes (Table 3).
Table 3.
OT Effect on Sensorimotor Activity and the Underlying Mechanisms.
| Category | Physiologic effects | Neural mechanisms | Pharmacological effects | References | |
|---|---|---|---|---|---|
| Visceral activity (determined by the autonomic nervous activity) |
Maintain homeostasis of the internal environment by selectively activating or inhibiting parasympathetic and sympathetic nervous system. | OT fibers innervate the DVC in the brainstem and the intermediolateral and sacral parasympathetic nuclei and 80% RVLM-projecting PVN cells in rats are OT neurons. OT can increase parasympathetic nervous activity through the PVN-DVC-vagal-organ pathway and increase sympathetic outflows by RVLM way. In addition, there is a population of OT neurons projecting to sympathetic preganglionic neurons in the upper thoracic spinal cord to increase sympathetic outflows directly. | Anxiolysis, reduce heart rate and cardiac contractility, attenuate hyperventilation, suppress food and water intake of the digestive system, slow gastric emptying and regulate hepatic metabolic activity. | (Althammer & Grinevich, 2017; Dos Santos et al., 2021; Elorza-Avila et al., 2017; Gamer & Buchel, 2012; Houshmand et al., 2015; Niimi et al., 2001; Tolentino et al., 2021) | |
| Specific sensation | Olfaction | Modulate main OB cellular activity and enhance neural discrimination of odors. | Increase glutamatergic synaptic transmission from mitral/tufted cells to granule cells and control information flows within the local OB neural circuits and from the OBs to the brain. | Improve olfactory deficits in schizophrenia. | (Osako et al., 2001; Sun et al., 2021; Woolley et al., 2015; Yu et al., 1996b) |
| Hearing | OT may promote the comparison-decision stage and modulate the activity of auditory system at multiple levels. | OT perikarya and fiber terminals exist extensively in the cochlear nucleus and OT enables long-term synaptic plasticity in the auditory cortex. OT facilitation of auditory fear memory extinction through the activation of the hippocampal-mPFC pathway. | Facilitate the auditory fear memory extinction. Improve impairments in selection of speech sounds from background speech in ASDs patients. |
(Bazaz et al., 2022; Kanwal and Rao, 2002; Lin et al., 2014; Yoshimura et al., 2018) | |
| Visualization | Modulate visual information processing and visual activity at both brain levels and at the retina. | OT neurons send axon collaterals to retina and OTR are expressed in retina. | Reduce diabetic retinopathy | (Csáki et al., 2021; Degirmenci et al., 2019; Halbach et al., 2015; Marsh et al., 2021; York et al., 2017) | |
| Gustation | Increase satiety and decrease daily intake of carbohydrate. | OTRs are present in taste buds throughout the oral cavity and neurons in PBN. Activation of OTRs in cells of the lateral PBN along the taste neural pathway suppresses food consumption. | Reduce the intake of sweet foods in diabetes. | (Barna et al., 2018; Burmester et al., 2018; Ryan et al., 2017; Sclafani et al., 2007; Sinclair et al., 2010) | |
| Somatic sensorimotor activity | Receive sensory information and regulate some somatic activities. | OT neurons project to portions of the limbic system, mesencephalon, brain stem, substantia nigra compact part, caudate putamen and spinal cord, which are associated with motor. OT presynaptically inhibits the nociceptive input from the peripheral primary afferent fibers by activating GABA interneuron. OT modulates somatosensory activity mainly through neurohumoral reflexes. | .- | (Jirikowski, 2019; Liao et al., 2020; Martinez-Lorenzana et al., 2021; Zhang et al., 2021) | |
Abbreviations: RVLM, the rostral ventrolateral medulla; HTC-8, Human taste cell line; PBN, the parabrachial nucleus.
Perspectives
OT participates in not only the milk-ejection reflex and parturition, but also a variety of body functions throughout the development, reproduction and aging. A dramatic OT function is its regulation of both the higher brain functions and instinctive behaviors by releasing OT into different brain and spinal cord regions from the SON and PVN as well as extrahypothalamic OT cells. OT also regulates somatic, specialized senses, visceral and motor activities by interactions with the sensorimotor brain and the autonomic nervous system. Notably, many OT effects are achieved through activation of GABAergic interneurons, such as those in the mPFC, amygdala and hippocampus, and spinal cord, which coexists with direct OT activation of some other targets, thereby making OT regulation of mental and physical activities more coordinately. These functions indicate an essential role of OT in neuromodulation and form the foundation for its clinical usage.
Registered Trials
The extensive peripheral effects (Figure 1) and broad neural functions (Figure 2 and Figure 3) of OT have triggered the enthusiasm of researchers and clinicians for clinical trials. According to the https://clinicaltrials.gov on March 16, 2022, there have been 1013 items under OT clinical trials of which 264 associated with varieties of diseases. In 584 completed trials, there are 92 about social behavior, 52 about analgesia, 46 about social cognition, 42 about depression, 37 items about ASDs, 25 about memory, 20 about the autonomic nervous system, 16 about feeding, 13 about addiction,10 about sleep, and 10 about sensation, andamong many others ,e.g., 5 about neurodegenerative diseases and 4 about anorexia. In these studies, OT is mainly applied through intranasal approach (155/584) while intravenous (92/584) and oral (61/584) OT applications are also tested. A large portion of these trials have achieved predicted results; however, clinical usages of OT are largely limited to improve social behaviors and parturition. This situation is largely attributable to the complex mechanisms of OT/OTR signaling processes, suboptimal approaches and doses of OT application, and sexual dimorphisms of OT functions.
Key Consideration of OT Clinical Trials
OT effects are determined by OT levels and OTR expressions. For instance, ASDs are usually associated with low OT levels. However, in ASD patients with OTR SNPs, OT levels can be relatively high in children and adolescents (Yang et al., 2017) and increased OT supplement can only worsen the disorders in OT/OTR signaling but have no improving effect. Moreover, basal OT levels have significant individual variations, most dramatically for women at different stages of reproduction and menstruation. This requires individualized OT doses to achieve ideal efficiency. In case of OT hyposecretion-associated diseases, the dose- and time-dependence of OT actions should also be considered carefully. At first, OT activation of OTR relies on a relative increase but not an absolute amount of OT applied. Administrating OT in higher doses briefly or low doses for a longer time can cause the reversal of excitatory to inhibitory effects as shown in rats (Wang et al., 2006). This effect is associated with OTR internalization and desensitization in uterine muscle strips isolated from wild-type mice (Grotegut et al., 2011) and reduction of OTR binding within the septum, basolateral and medial amygdala, and median raphe nucleus in mice (Peters et al., 2014). Thus, it is important to adjust dose, time, duration and pattern before OT application.
Sexual Dimorphism
Different sexes have dramatic differences in OTR expressions and OT effects. In studies on OT functions, significant difference between males and females emerges gradually. Serum OT levels are significantly higher among women than men (Kunitake et al., 2021), which reflects higher basal OT neuronal activity in women. Salivary OT level in male infants has decreased post massage, while OT level in female infants has increased post massage in mothers with normal bonding in humans (Moussa et al., 2021). These findings suggest inherent difference in the OT system between them. Consistently, left hippocampal volumes in boys are significantly larger than girls in GG-homozygotes of OTR gene rs53576 compared to A-allele carriers (Acosta et al., 2021). Correspondingly, ASDs have disproportionately low probability in the female who has higher levels of OT than boys (Miller et al., 2013). The sex dimorphism in the expressions of OT and OTRs underlies sex-specific functions and behaviors as well as different strategies of treatments.
Approaches of OT Application
OT actions are also dependent on the approaches of application. To achieve optimal neural effects, intranasal, intravenous or intrathecal OT application is available. Intrathecal route is directly accessible to periventricular neural structures; however, it is not commonly acceptable due to its more invasive nature. Intravenous infusion is commonly used in obstetrics and gynecology, and circulating OT may be transported into the brain via peptide transporters on the capillary endothelial cells of the neurovascular unit (Lee & Jayant, 2019). However, the short half-life and relatively low efficiency of this route make it improper to be used for curing neural diseases. IAO has been accepted extensively as an optimal route of OT usages in treating brain diseases. IAO can reach brain by diffusing along nerves, through the cerebrospinal fluid or by neural conduction and via the blood supply (Quintana et al., 2021). Thus, it seems a preferred approach of intranasal administration OT or OTR agonist to treat neural diseases involving OT hyposecretion-associated neural disorders.
Future Research
Accumulative studies have greatly enriched our knowledge about the OT system and it neural functions. However, many questions remain to be addressed in future studies. First, the afferent monosynaptic inputs and their specific influence on OT neurons are largely unclear, particularly under pathological conditions. Second, functional differentiation of the parvocellular OT neurons in the PVN and their upstream neural connections are not identified, specifically for those innervating DVC neurons versus thoracic sympathetic preganglionic neurons. Third, integrative functions of OT/OTR signaling should be evaluated comprehensively, considering not only OT levels but OTR genomic modulation and expression status; the integrative efficiency of OT/OTR signaling may explain many previously controversial findings in ages, sex, and disease-associated OT effects. Fourth, interactive effects of OT with other neuropeptides on neural activities at cellular and molecular levels are largely unclear. Fifth, it is necessary to validate findings based on invasive non-human study results with modern non-invasive imaging techniques in humans while identifying mechanisms underlying human diseases through animal studies. Lastly, it is important to validate therapeutic effects of OT identified in animals and in vitro studies in well-designed clinical trials. Through these studies, controversial effects derived from different neural connections, OT receptor gene dimorphism and methylation, complex interactions with other hormones should be clarified. Importantly, long expected clinical usages of OT and OTR-regulating compounds will likely contribute to modern therapeutic library and really benefit human health.
Acknowledgments
This work was supported by the Fund of the Ministry of Science and Technology of China (grant No. G2021011014L), and the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971 to VP); VP is an Honorary Professor at University of Rijeka, Croatia.
Footnotes
Author Contribution: PW, SCW, XYL, and SWJ wrote the first draft; XRW, TL and JWY drew the figures and discussed the contents critically; SCW, VP and YFW conceived the study and edited the text.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fund of the Ministry of Science and Technology of China, National Institute of General Medical Sciences of the National Institutes of Health, (grant number G2021011014L, R01GM123971).
ORCID iD: Yu-Feng Wang https://orcid.org/0000-0001-8543-8906
References
- Acosta H., Tuulari J. J., Kantojarvi K., Lewis J. D., Hashempour N., Scheinin N. M., Lehtola S. J., Fonov V. S., Collins D. L., Evans A., Parkkola R., Lahdesmaki T., Saunavaara J., Merisaari H., Karlsson L., Paunio T., Karlsson H. (2021). A variation in the infant oxytocin receptor gene modulates infant hippocampal volumes in association with sex and prenatal maternal anxiety. Psychiatry Research Neuroimaging, 307, 111207. 10.1016/j.pscychresns.2020.111207 [DOI] [PubMed] [Google Scholar]
- Althammer F., Grinevich V. (2017). Diversity of oxytocin neurones: Beyond magno- and parvocellular cell types? Journal of Neuroendocrinology, 30, e12549. 10.1111/jne.12549 [DOI] [PubMed] [Google Scholar]
- Andari E., Nishitani S., Kaundinya G., Caceres G. A., Morrier M. J., Ousley O., Smith A. K., Cubells J. F., Young L. J. (2020). Epigenetic modification of the oxytocin receptor gene: Implications for autism symptom severity and brain functional connectivity. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 45(7), 1150–1158. 10.1038/s41386-020-0610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assinder S. J. (2022). The importance of experimental investigation of the peripheral oxytocin system. Methods in Molecular Biology, 2384, 1–27. 10.1007/978-1-0716-1759-5_1 [DOI] [PubMed] [Google Scholar]
- Barna J., Renner E., Arszovszki A., Cservenak M., Kovacs Z., Palkovits M., Dobolyi A. (2018). Suckling induced activation pattern in the brain of rat pups. Nutritional Neuroscience, 21(5), 317–327. 10.1080/1028415X.2017.1286446 [DOI] [PubMed] [Google Scholar]
- Barrett L. R., Nunez J., Zhang X. (2021). Oxytocin activation of paraventricular thalamic neurons promotes feeding motivation to attenuate stress-induced hypophagia. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 46(5), 1045–1056. 10.1038/s41386-021-00961-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaz A., Ghanbari A., Vafaei A. A., Khaleghian A., Rashidy-Pour A. (2022). Oxytocin in dorsal hippocampus facilitates auditory fear memory extinction in rats. Neuropharmacology, 202(1), 108844. 10.1016/j.neuropharm.2021.108844 [DOI] [PubMed] [Google Scholar]
- Bealer S. L., Armstrong W. E., Crowley W. R. (2010). Oxytocin release in magnocellular nuclei: Neurochemical mediators and functional significance during gestation. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 299(2), R452–R458. 10.1152/ajpregu.00217.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer S. L., Lipschitz D. L., Ramoz G., Crowley W. R. (2006). Oxytocin receptor binding in the hypothalamus during gestation in rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 291(1), R53–R58. 10.1152/ajpregu.00766.2005 [DOI] [PubMed] [Google Scholar]
- Benarroch E. E., Schmeichel A. M., Parisi J. E., Low P. A. (2015). Histaminergic tuberomammillary neuron loss in multiple system atrophy and dementia with Lewy bodies. Movement Disorders : Official Journal of the Movement Disorder Society, 30(8), 1133–1139. 10.1002/mds.26287 [DOI] [PubMed] [Google Scholar]
- Bertoni A., Schaller F., Tyzio R., Gaillard S., Santini F., Xolin M., Diabira D., Vaidyanathan R., Matarazzo V., Medina I., Hammock E., Zhang J., Chini B., Gaiarsa J. L., Muscatelli F. (2021). Oxytocin administration in neonates shapes hippocampal circuitry and restores social behavior in a mouse model of autism. Molecular Psychiatry, 26(12), 1–14. https://doi:10.1038/s41380-021-01227-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem R. A. I., Lombardo M. V., Lai M. C., Auyeung B., Crockford S. K., Deakin J., Soubramanian S., Sule A., Kundu P., Voon V., Baron-Cohen S. (2017). Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Translational Psychiatry, 7(4), e1099. 10.1038/tp.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj V. N., Porreca F., Cowan R. P., Kori S., Silberstein S. D., Yeomans D. C. (2021). A new hypothesis linking oxytocin to menstrual migraine. Headache, 61(7), 1051–1059. 10.1111/head.14152, [DOI] [PubMed] [Google Scholar]
- Bjorkstrand E., Eriksson M., Uvnas-Moberg K. (1996). Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology, 63(4), 377–383. 10.1159/000126978 [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O., Ambrogini P., Chruscicka B., Lindskog M., Crespo-Ramirez M., Hernandez-Mondragon J. C., Perez de la Mora M., Schellekens H., Fuxe K. (2021). The role of central serotonin neurons and 5-HT heteroreceptor complexes in the pathophysiology of depression: A historical perspective and future prospects. International Journal of Molecular Sciences, 22(4), 1927. 10.3390/ijms22041927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M. T., Carson D. S., Spiro A., Arnold J. C., McGregor I. S. (2011). Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PloS one, 6(11), e27237. 10.1371/journal.pone.0027237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M. T., Neumann I. D. (2017). Rebalancing the addicted brain: oxytocin interference with the neural substrates of addiction. Trends in Neurosciences, 40(12), 691–708. 10.1016/j.tins.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Brackley A. D., Toney G. M. (2021). Oxytocin receptor activation rescues opioid-induced respiratory depression by systemic fentanyl in the rat. Journal of Pharmacology and Experimental Therapeutics, JPET-AR-2021-000535. https://doi.org/10.1124/jpet.121.000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla M., Manenti R., de Girolamo G., Adenzato M., Bocchio-Chiavetto L., Cotelli M. (2016). Effects of intranasal oxytocin on long-term memory in healthy humans: A systematic review. Drug Development Research, 77(8), 479–488. 10.1002/ddr.21343 [DOI] [PubMed] [Google Scholar]
- Bridges R. S. (2015). Neuroendocrine regulation of maternal behavior. Frontiers in Neuroendocrinology, 36, 178–196. 10.1016/j.yfrne.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. H., Bains J. S., Ludwig M., Stern J. E. (2013). Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. Journal of Neuroendocrinology, 25(8), 678–710. 10.1111/jne.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbul M., Babygirija R., Cerjak D., Yoshimoto S., Ludwig K., Takahashi T. (2011). Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain Research, 1387, 39–45. 10.1016/j.brainres.2011.02.091 [DOI] [PubMed] [Google Scholar]
- Burmester V., Higgs S., Terry P. (2018). Rapid-onset anorectic effects of intranasal oxytocin in young men. Appetite, 130, 104–109. 10.1016/j.appet.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Calza L., Giardino L., Velardo A., Battistini N., Marrama P. (1990). Influence of aging on the neurochemical organization of the rat paraventricular nucleus. Journal of Chemical Neuroanatomy, 3(3), 215–231. [PubMed] [Google Scholar]
- Campbell P., Ophir A. G., Phelps S. M. (2009). Central vasopressin and oxytocin receptor distributions in two species of singing mice. The Journal of Comparative Neurology, 516(4), 321–333. 10.1002/cne.22116 [DOI] [PubMed] [Google Scholar]
- Cera N., Vargas-Caceres S., Oliveira C., Monteiro J., Branco D., Pignatelli D., Rebelo S. (2021). How relevant is the systemic oxytocin concentration for human sexual behavior? A systematic review. Sexual Medicine, 9(4), 100370. 10.1016/j.esxm.2021.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li Q., Zhang Q., Kou J., Zhang Y., Cui H., Wernicke J., Montag C., Becker B., Kendrick K. M., Yao S. (2020). The effects of intranasal oxytocin on neural and behavioral responses to social touch in the form of massage. Frontiers in Neuroscience, 14, 589878. 10.3389/fnins.2020.589878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosek J., Drobnik J. (2012). Function of the hypothalamo-neurohypophysial system in rats with myocardial infarction is modified by melatonin. Pharmacological Reports : PR, 64(6), 1442–1454. 10.1016/S1734-1140(12)70942-8 [DOI] [PubMed] [Google Scholar]
- Comasco E.,, Gulinello M., Hellgren C., Skalkidou A., Sylven S., Sundstrom-Poromaa I. (2016). Sleep duration, depression, and oxytocinergic genotype influence prepulse inhibition of the startle reflex in postpartum women. European Neuropsychopharmacology : the Journal of the European College of Neuropsychopharmacology, 26(4), 767–776. 10.1016/j.euroneuro.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Condes-Lara M., Martinez-Lorenzana G., Rojas-Piloni G., Rodriguez-Jimenez J. (2007). Branched oxytocinergic innervations from the paraventricular hypothalamic nuclei to superficial layers in the spinal cord. Brain Research, 1160, 20–29. 10.1016/j.brainres.2007.05.031 [DOI] [PubMed] [Google Scholar]
- Courtiol E.,, Menezes E. C., Teixeira C. M. (2021). Serotonergic regulation of the dopaminergic system: implications for reward-related functions. Neuroscience and Biobehavioral Reviews, 128, 282–293. 10.1016/j.neubiorev.2021.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley K. C. (2005). Psychogenic and pharmacologic induction of the let-down reflex can facilitate breastfeeding by tetraplegic women: A report of 3 cases. Archives of Physical Medicine and Rehabilitation, 86(6), 1261–1264. 10.1016/j.apmr.2004.10.039 [DOI] [PubMed] [Google Scholar]
- Cox B. M., Bentzley B. S., Regen-Tuero H., See R. E., Reichel C. M., Aston-Jones G. (2017). Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biological Psychiatry, 81(11), 949–958. 10.1016/j.biopsych.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley S. K., Pedersen C. A., Leserman J., Girdler S. S. (2015). The influence of early life sexual abuse on oxytocin concentrations and premenstrual symptomatology in women with a menstrually related mood disorder. Biological Psychology, 109, 1–9. 10.1016/j.biopsycho.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csáki A., Katalin Köves K., Zsolt Boldogkői Z., Dóra Tombácz D., Tóth Z. E. (2021). The same magnocellular neurons send axon collaterals to the posterior pituitary and retina or to the posterior pituitary and autonomic preganglionic centers of the eye in rats. NeuroSci, 2(1), 27–44. 10.3390/neurosci2010002 [DOI] [Google Scholar]
- Cuneo M. G.,, Szeto A., Schrepf A., Thaker P. H., Goodheart M., Cole S. W., Sood A. K., McCabe P. M., Mendez A. J., Lutgendorf S. K. (2021). Positive psychosocial factors and oxytocin in the ovarian tumor microenvironment. Psychosomatic Medicine, 83(5), 417–422. 10.1097/PSY.0000000000000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu C. K., Greer L. L., Van Kleef G. A., Shalvi S., Handgraaf M. J. (2011). Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America, 108(4), 1262–1266. 10.1073/pnas.1015316108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirmenci C., Afrashi F., Erbas O., Aktug H., Taskiran D. (2019). The preventive effect of oxytocin on retinopathy in streptozotocin-induced diabetic rats. Turkish Journal of Ophthalmology, 49(2), 68–72. 10.4274/tjo.galenos.2018.47897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong T. R., Neumann I. D. (2018). Oxytocin and aggression. Current Topics in Behavioral Neurosciences, 35, 175–192. 10.1007/7854_2017_13 [DOI] [PubMed] [Google Scholar]
- Dekker A., Wenzlaff F., Biedermann S. V., Briken P., Fuss J. (2021). VR Porn as “empathy machine"? perception of self and others in virtual reality pornography. Journal of Sex Research, 58(3), 273–278. 10.1080/00224499.2020.1856316 [DOI] [PubMed] [Google Scholar]
- de Souza Villa P., Menani J. V., de Arruda Camargo G. M., de Arruda Camargo L. A., Saad W. A. (2008). Activation of the serotonergic 5-HT1A receptor in the paraventricular nucleus of the hypothalamus inhibits water intake and increases urinary excretion in water-deprived rats. Regulatory Peptides, 150(1-3), 14–20. 10.1016/j.regpep.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Devarajan K., Marchant E. G., Rusak B. (2005). Circadian and light regulation of oxytocin and parvalbumin protein levels in the ciliated ependymal layer of the third ventricle in the C57 mouse. Neuroscience, 134(2), 539–547. 10.1016/j.neuroscience.2005.04.034 [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Muraoka S., Engelmann M., Ebner K., Landgraf R. (2000). Oxytocin induces preservation of social recognition in male rats by activating alpha-adrenoceptors of the olfactory bulb. The European Journal of Neuroscience, 12(2), 760–766. 10.1046/j.1460-9568.2000.00952.x [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Muraoka S., Engelmann M., Landgraf R. (1998). The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides, 19(6), 999–1005. 10.1016/S0196-9781(98)00047-3 [DOI] [PubMed] [Google Scholar]
- Dolen G., Darvishzadeh A., Huang K. W., Malenka R. C. (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos K. M., Moraes D. J. A., da Silva M. P., Antunes V. R. (2021). Exercise training rescues the electrical activity of liver-projecting DMNV neurones in response to oxytocin in spontaneously hypertensive rats. Journal of Neuroendocrinology, 33(5), e12977. [DOI] [PubMed] [Google Scholar]
- Eliava, M., Melchior, M., Sophie Knobloch-Bollmann, H., Wahis, J., da Silva Gouveia, M., Tang, Y., Ciobanu, A. C., del Rio, R. T., Roth, L. C., Althammer, F., Chavant, V., Goumon, Y., Gruber, T., Petit-Demoulière, N., Busnelli, M., Chini, B., Tan, L. L., Mitre, M., Froemke, R. C., … Grinevich, V. (2016). A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron, 89(6), 1291–1304. 10.1016/j.neuron.2016.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B. J., Horn A. J., Carter C. S., van I. M. H., Bakermans-Kranenburg M. J. (2021). Developmental programming of oxytocin through variation in early-life stress: four meta-analyses and a theoretical reinterpretation. Clinical Psychology Review, 86, 101985. 10.1016/j.cpr.2021.101985 [DOI] [PubMed] [Google Scholar]
- Elorza-Avila A. R., Reyes-Lagos J. J., Hadamitzky M., Pena-Castillo M. A., Echeverria J. C., Ortiz-Pedroza M. D., Luckemann L., Schedlowski M., Pacheco-Lopez G. (2017). Oxytocin’s role on the cardiorespiratory activity of endotoxemic rats. Respiratory Physiology & Neurobiology, 236, 19–22. 10.1016/j.resp.2016.10.008 [DOI] [PubMed] [Google Scholar]
- Evans J. J., Reid R. A., Wakeman S. A., Croft L. B., Benny P. S. (2003). Evidence that oxytocin is a physiological component of LH regulation in non-pregnant women. Human Reproduction, 18(7), 1428–1431. 10.1093/humrep/deg291 [DOI] [PubMed] [Google Scholar]
- Fang L. Y., Quan R. D., Kaba H. (2008). Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neuroscience Letters, 438(2), 133–137. 10.1016/j.neulet.2007.12.070 [DOI] [PubMed] [Google Scholar]
- Feifel D., Macdonald K., Cobb P., Minassian A. (2012). Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophrenia Research, 139(1-3), 207–210. 10.1016/j.schres.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Ferrer-Perez C., Castro-Zavala A., Lujan M. A., Filarowska J., Ballestin R., Minarro J., Valverde O., Rodriguez-Arias M. (2019). Oxytocin prevents the increase of cocaine-related responses produced by social defeat. Neuropharmacology, 146, 50–64. 10.1016/j.neuropharm.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Fewtrell M. S., Loh K. L., Blake A., Ridout D. A., Hawdon J. (2006). Randomised, double blind trial of oxytocin nasal spray in mothers expressing breast milk for preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition, 91(3), F169–F174. 10.1136/adc.2005.081265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippa M., Monaci M. G., Spagnuolo C., Serravalle P., Daniele R., Grandjean D. (2021). Maternal speech decreases pain scores and increases oxytocin levels in preterm infants during painful procedures. Scientific Reports, 11, 17301. 10.1038/s41598-021-96840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E. R., Rocha N. P., Morales-Scheihing D. A., Venna V. R., Furr-Stimming E. E., Teixeira A. L., Rossetti M. A. (2021). The relationship between plasma oxytocin and executive functioning in huntington’s disease: A pilot study. Journal of Huntington’s Disease, 10(3), 349–354. 10.3233/JHD-210467 [DOI] [PubMed] [Google Scholar]
- Flynn M. J., Campbell T. S., Robert M., Nasr-Esfahani M., Rash J. A. (2021). Intranasal oxytocin as a treatment for chronic pelvic pain: A randomized controlled feasibility study. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics, 152(3), 425–432. 10.1002/ijgo.13441 [DOI] [PubMed] [Google Scholar]
- Francesconi W.,, Berton F., Olivera-Pasilio V., Dabrowska J. (2021). Oxytocin excites BNST interneurons and inhibits BNST output neurons to the central amygdala. Neuropharmacology, 192, 108601. 10.1016/j.neuropharm.2021.108601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabery S., Ahmed R. M., Caga J., Kiernan M. C., Halliday G. M., Petersen A. (2021). Loss of the metabolism and sleep regulating neuronal populations expressing orexin and oxytocin in the hypothalamus in amyotrophic lateral sclerosis. Neuropathology and Applied Neurobiology, 47(7), 979–989. 10.1111/nan.12709 [DOI] [PubMed] [Google Scholar]
- Galbally M., Lewis A. J., Ijzendoorn M., Permezel M. (2011). The role of oxytocin in mother-infant relations: A systematic review of human studies. Harvard Review of Psychiatry, 19(1), 1–14. 10.3109/10673229.2011.549771 [DOI] [PubMed] [Google Scholar]
- Gamal-Eltrabily M., Espinosa de Los Monteros-Zuniga A., Manzano-Garcia A., Martinez-Lorenzana G., Condes-Lara M., Gonzalez-Hernandez A. (2020). The rostral agranular insular cortex, a new site of oxytocin to induce antinociception. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 40(29), 5669–5680. 10.1523/JNEUROSCI.0962-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Buchel C. (2012). Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology, 37(1), 87–93. 10.1016/j.psyneuen.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Gardner E. L. (2011). Addiction and brain reward and antireward pathways. Advances in Psychosomatic Medicine, 30, 22–60. 10.1159/000324065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S. N., Aidney F., Klockars A., Prosser C., Carpenter E. A., Isgrove K., Levine A. S., Olszewski P. K. (2018). Intragastric preloads of l-tryptophan reduce ingestive behavior via oxytocinergic neural mechanisms in male mice. Appetite, 125, 278–286. 10.1016/j.appet.2018.02.015 [DOI] [PubMed] [Google Scholar]
- Georgiou P., Zanos P., Hourani S., Kitchen I., Bailey A. (2016). Cocaine abstinence induces emotional impairment and brain region-specific upregulation of the oxytocin receptor binding. The European Journal of Neuroscience, 44(7), 2446–2454. 10.1111/ejn.13348 [DOI] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. (2001). The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews, 81(2), 629–683. 10.1152/physrev.2001.81.2.629 [DOI] [PubMed] [Google Scholar]
- Gregory R., Cheng H., Rupp H. A., Sengelaub D. R., Heiman J. R. (2015). Oxytocin increases VTA activation to infant and sexual stimuli in nulliparous and postpartum women. Hormones and Behavior, 69, 82–88. 10.1016/j.yhbeh.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen K. M., Light K. C. (2011). Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biological Psychology, 87(3), 340–349. 10.1016/j.biopsycho.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotegut C. A., Feng L., Mao L., Heine R. P., Murtha A. P., Rockman H. A. (2011). beta-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. American Journal of Physiology Endocrinology and Metabolism, 300(3), E468–E477. 10.1152/ajpendo.00390.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A. J., Mitchell P. B., Mathews F. (2008). Oxytocin enhances the encoding of positive social memories in humans. Biological Psychiatry, 64(3), 256–258. 10.1016/j.biopsych.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Guoynes C. D., Marler C. A. (2021). An acute dose of intranasal oxytocin rapidly increases maternal communication and maintains maternal care in primiparous postpartum california mice. PloS one, 16(4), e0244033. 10.1371/journal.pone.0244033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurler E. B., Iriboz E., Kaya O. T. C., Turkaydin D., Ovecoglu H. S. (2021). Acute dental pain elevates salivary oxytocin in women: A risk factor during pregnancy. General Dentistry, 69(3), 73–77. [PubMed] [Google Scholar]
- Halbach P., Pillers D. A., York N., Asuma M. P., Chiu M. A., Luo W., Tokarz S., Bird I. M., Pattnaik B. R. (2015). Oxytocin expression and function in the posterior retina: A novel signaling pathway. Investigative Ophthalmology & Visual Science, 56(2), 751–760. 10.1167/iovs.14-15646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton G. I., Wang Y. F. (2008). Neural mechanisms underlying the milk ejection burst and reflex. Progress in Brain Research, 170, 155–166. 10.1016/S0079-6123(08)00414-7 [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz Y., Gonzalez-Castro T. B., Tovilla-Zarate C. A., Lopez-Narvaez M. L., Genis-Mendoza A. D., Castillo-Avila R. G., Ramos-Mendez M. A., Juarez-Rojop I. E. (2021). Oxytocin levels in individuals with schizophrenia are high in cerebrospinal fluid but low in serum: A systematic review and meta-analysis : oxytocin and schizophrenia. Metabolic Brain Disease, 36(8), 2415–2424. 10.1007/s11011-021-00836-y [DOI] [PubMed] [Google Scholar]
- Higuchi T., Okere C. O. (2002). Role of the supraoptic nucleus in regulation of parturition and milk ejection revisited. Microscopy Research and Technique, 56(2), 113–121. 10.1002/jemt.10016 [DOI] [PubMed] [Google Scholar]
- Hochheimer A., Krohn M., Rudert K., Riedel K., Becker S., Thirion C., Zinke H. (2014). Endogenous gustatory responses and gene expression profile of stably proliferating human taste cells isolated from fungiform papillae. Chemical Senses, 39(4), 359–377. 10.1093/chemse/bju009 [DOI] [PubMed] [Google Scholar]
- Horrell N. D., Hickmott P. W., Saltzman W. (2019). Neural regulation of paternal behavior in mammals: sensory, neuroendocrine, and experiential influences on the paternal brain. Current Topics in Behavioral Neurosciences, 43, 111–160. 10.1007/7854_2018_55 [DOI] [PubMed] [Google Scholar]
- Hou D., Jin F., Li J., Lian J., Liu M., Liu X., Xu Y., Zhang C., Zhao C., Jia S., Jiao R., Liu X. Y., Wang X., Zhang Y., Wang Y.-F. (2016). Model roles of the hypothalamo-neurohypophysial system in neuroscience study. Biochem Pharmacol (Los Angel), 5, 3. https://doi.org/10.4172/2167-0501.1000211 [Google Scholar]
- Houghton B., Kouimtsidis C., Duka T., Paloyelis Y., Bailey A. (2021). Can intranasal oxytocin reduce craving in automated addictive behaviours? A systematic review. British Journal of Pharmacology, 178(21), 4316–4334. https://doi.org/10.1111/bph.15617. [DOI] [PubMed] [Google Scholar]
- Houshmand F., Faghihi M., Zahediasl S. (2015). Role of atrial natriuretic peptide in oxytocin induced cardioprotection. Heart, Lung & Circulation, 24(1), 86–93. 10.1016/j.hlc.2014.05.023 [DOI] [PubMed] [Google Scholar]
- Hu J., Qi S., Becker B., Luo L., Gao S., Gong Q., Hurlemann R., Kendrick K. M. (2015). Oxytocin selectively facilitates learning with social feedback and increases activity and functional connectivity in emotional memory and reward processing regions. Human Brain Mapping, 36(6), 2132–2146. 10.1002/hbm.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Huang X., Ebstein R. P., Yu R. (2021). Intranasal oxytocin in the treatment of autism spectrum disorders: A multilevel meta-analysis. Neuroscience and Biobehavioral Reviews, 122, 18–27. 10.1016/j.neubiorev.2020.12.028 [DOI] [PubMed] [Google Scholar]
- Hung L. W., Neuner S., Polepalli J. S., Beier K. T., Wright M., Walsh J. J., Lewis E. M., Luo L., Deisseroth K., Dolen G., Malenka R. C. (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science (New York, N.Y.), 357(6358), 1406–1411. 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M., Iwasa K., Yoshikawa K. (2021). Feeding regulation by oleoylethanolamide synthesized from dietary oleic acid. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 165, 102228. 10.1016/j.plefa.2020.102228 [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Wei X., Kubo T. (1993). Cardiovascular effects of microinjections of thyrotropin-releasing hormone, oxytocin and other neuropeptides into the rostral ventrolateral medulla of the rat. Archives Internationales de Pharmacodynamie et de Therapie, 322(3), 35–44. [PubMed] [Google Scholar]
- Jahangard L., Shayganfard M., Ghiasi F., Salehi I., Haghighi M., Ahmadpanah M., Sadeghi Bahmani D., Brand S. (2020). Serum oxytocin concentrations in current and recent suicide survivors are lower than in healthy controls. Journal of Psychiatric Research, 128, 75–82. 10.1016/j.jpsychires.2020.05.014 [DOI] [PubMed] [Google Scholar]
- Jain V., Kimbro S., Kowalik G., Milojevic I., Maritza Dowling N., Hunley A. L., Hauser K., Andrade D. C., Del Rio R., Kay M. W., Mendelowitz D. (2020). Intranasal oxytocin increases respiratory rate and reduces obstructive event duration and oxygen desaturation in obstructive sleep apnea patients: A randomized double blinded placebo controlled study. Sleep Medicine, 74, 242–247. 10.1016/j.sleep.2020.05.034 [DOI] [PubMed] [Google Scholar]
- Jain V., Marbach J., Kimbro S., Andrade D. C., Jain A., Capozzi E., Mele K., Del Rio R., Kay M. W., Mendelowitz D. (2017). Benefits of oxytocin administration in obstructive sleep apnea. American Journal of Physiology Lung Cellular and Molecular Physiology, 313(5), L825–L833. 10.1152/ajplung.00206.2017 [DOI] [PubMed] [Google Scholar]
- Janecek M., Dabrowska J. (2019). Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell and Tissue Research, 375(1), 143–172. 10.1007/s00441-018-2889-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A., Young L. J., Triana-Del Rio R., LaPrairie J. L., Gonzalez-Mariscal G. (2015). Neuroanatomical distribution of oxytocin receptor binding in the female rabbit forebrain: variations across the reproductive cycle. Brain Research, 1629, 329–339. 10.1016/j.brainres.2015.10.043 [DOI] [PubMed] [Google Scholar]
- Jirikowski G. F. (2019). Diversity of central oxytocinergic projections. Cell and Tissue Research, 375(1), 41–48. 10.1007/s00441-018-2960-5 [DOI] [PubMed] [Google Scholar]
- John S.,, Jaeggi A. V. (2021). Oxytocin levels tend to be lower in autistic children: A meta-analysis of 31 studies. Autism : the International Journal of Research and Practice, 25(8), 2152–2161. https://doi.org/10.1177/13623613211034375. [DOI] [PubMed] [Google Scholar]
- Johns J. M., Caldwell J. D., Pedersen C. A. (1993). Acute cocaine treatment decreases oxytocin levels in the rat hippocampus. Neuropeptides, 24(3), 165-169. 10.1016/0143-4179(93)90081-K [DOI] [PubMed] [Google Scholar]
- Josef L., Goldstein P., Mayseless N., Ayalon L., Shamay-Tsoory S. G. (2019). The oxytocinergic system mediates synchronized interpersonal movement during dance. Scientific Reports, 9, 1894. 10.1038/s41598-018-37141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joushi S., Esmaeilpour K., Masoumi-Ardakani Y., Esmaeili-Mahani S., Sheibani V. (2021). Intranasal oxytocin administration facilitates the induction of long-term potentiation and promotes cognitive performance of maternally separated rats. Psychoneuroendocrinology, 123, 105044. 10.1016/j.psyneuen.2020.105044 [DOI] [PubMed] [Google Scholar]
- Jung T., Jang M., Noh J. (2021). Role of medial prefrontal cortical neurons and oxytocin modulation in the establishment of social buffering. Experimental Neurobiology, 30(1), 48–58. 10.5607/en20038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B., Neumann I. D. (2018). The oxytocin receptor: from intracellular signaling to behavior. Physiological Reviews, 98(3), 1805–1908. 10.1152/physrev.00031.2017 [DOI] [PubMed] [Google Scholar]
- Kanwal J. S., Rao P. D. (2002). Oxytocin within auditory nuclei: A neuromodulatory function in sensory processing? Neuroreport, 13(17), 2193–2197. 10.1097/00001756-200212030-00006 [DOI] [PubMed] [Google Scholar]
- Kapetaniou G. E., Reinhard M. A., Christian P., Jobst A., Tobler P. N., Padberg F., Soutschek A. (2021). The role of oxytocin in delay of gratification and flexibility in non-social decision making. eLife, 10. 10.7554/eLife.61844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett K., Cong Z., Hale T. W. (2013). Depression, sleep quality, and maternal well-being in postpartum women with a history of sexual assault: A comparison of breastfeeding, mixed-feeding, and formula-feeding mothers. Breastfeeding Medicine : the Official Journal of the Academy of Breastfeeding Medicine, 8(1), 16–22. 10.1089/bfm.2012.0024 [DOI] [PubMed] [Google Scholar]
- Kerman I. A., Akil H., Watson S. J. (2006). Rostral elements of sympatho-motor circuitry: A virally mediated transsynaptic tracing study. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 26(13), 3423–3433. 10.1523/JNEUROSCI.5283-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury M. A., Bilbo S. D. (2019). The inflammatory event of birth: how oxytocin signaling may guide the development of the brain and gastrointestinal system. Frontiers in Neuroendocrinology, 55, 100794. 10.1016/j.yfrne.2019.100794 [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Matsumura K., Baba M., Kondo M., Takemoto T., Nagayasu K., Ago Y., Seiriki K., Hayata-Takano A., Kasai A., Takuma K., Hashimoto R., Hashimoto H., Nakazawa T. (2021). Intranasal oxytocin administration ameliorates social behavioral deficits in a POGZ(WT/Q1038R) mouse model of autism spectrum disorder. Molecular Brain, 14(1), 56. 10.1186/s13041-021-00769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockars O. A., Waas J. R., Klockars A., Levine A. S., Olszewski P. K. (2017). Neural basis of ventromedial hypothalamic oxytocin-driven decrease in appetite. Neuroscience, 366, 54–61. 10.1016/j.neuroscience.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Knobloch H. S., Grinevich V. (2014). Evolution of oxytocin pathways in the brain of vertebrates. Frontiers in Behavioral Neuroscience, 8, 31. 10.3389/fnbeh.2014.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochel C., Frickmann H., Nurnberger F. (2021). Effects of sleep deprivation by olfactorily induced sexual arousal compared to immobilization stress and manual sleep deprivation on neuromessengers and time keeping genes in the suprachiasmatic nuclei and other cerebral entities of Syrian hamsters-an immunohistochemical study. International Journal of Environmental Research and Public Health, 18(17), 9169. 10.3390/ijerph18179169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Volkow N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet. Psychiatry, 3(8), 760–773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop S., Oster H. (2021). Eat, sleep, repeat - endocrine regulation of behavioural circadian rhythms. The FEBS Journal, 15, 183–191. https://doi.org/10.1016/j.cophys.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Kovatsi L., Nikolaou K. (2019). Opioids and the hormone oxytocin. Vitamins and Hormones, 111, 195–225. 10.1016/bs.vh.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Krause D. N., Warfvinge K., Haanes K. A., Edvinsson L. (2021). Hormonal influences in migraine - interactions of oestrogen, oxytocin and CGRP. Nature Reviews Neurology, 17(10), 621–633. 10.1038/s41582-021-00544-2 [DOI] [PubMed] [Google Scholar]
- Kreuder A. K., Wassermann L., Wollseifer M., Ditzen B., Eckstein M., Stoffel-Wagner B., Hennig J., Hurlemann R., Scheele D. (2019). Oxytocin enhances the pain-relieving effects of social support in romantic couples. Human Brain Mapping, 40(1), 242–251. 10.1002/hbm.24368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann J. C., Fendt M. (2021). Intranasal oxytocin compensates for estrus cycle-specific reduction of conditioned safety memory in rats: implications for psychiatric disorders. Neurobiology of Stress, 14, 100313. 10.1016/j.ynstr.2021.100313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitake Y., Imamura Y., Mizoguchi Y., Matsushima J., Tateishi H., Murakawa-Hirachi T., Nabeta H., Kawashima T., Kojima N., Yamada S., Monji A. (2021). Serum oxytocin levels and logical memory in older people in rural Japan. Journal of Geriatric Psychiatry and Neurology, 34(2), 156–161. 10.1177/0891988720915526 [DOI] [PubMed] [Google Scholar]
- La Fratta I., Franceschelli S., Speranza L., Patruno A., Michetti C., D’Ercole P., Ballerini P., Grilli A., Pesce M. (2021). Salivary oxytocin, cognitive anxiety and self-confidence in pre-competition athletes. Scientific Reports, 11(1), 16877. 10.1038/s41598-021-96392-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrazolo-Lopez A., Kendrick K. M., Aburto-Arciniega M., Arriaga-Avila V., Morimoto S., Frias M., Guevara-Guzman R. (2008). Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience, 152(3), 585–593. 10.1016/j.neuroscience.2008.01.024 [DOI] [PubMed] [Google Scholar]
- Lassey S. C., Haber H. R., Kanbergs A., Robinson J. N., Little S. E. (2021). Six versus twelve hours of single-balloon catheter placement with oxytocin administration for labor induction: A randomized controlled trial. American Journal of Obstetrics and Gynecology, 224(6), 611. e1–611. e8. https://doi.org/10.1016/j.ajog.2021.03.021. [DOI] [PubMed] [Google Scholar]
- Latt H. M., Matsushita H., Morino M., Koga Y., Michiue H., Nishiki T., Tomizawa K., Matsui H. (2018). Oxytocin inhibits corticosterone-induced apoptosis in primary hippocampal neurons. Neuroscience, 379, 383–389. 10.1016/j.neuroscience.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Lee G., Yoon M. (2021). Association of plasma concentrations of oxytocin, vasopressin, and serotonin with docility and friendliness of horses. Domestic Animal Endocrinology, 74, 106482. 10.1016/j.domaniend.2020.106482 [DOI] [PubMed] [Google Scholar]
- Lee M. R., Jayant R. D. (2019). Penetration of the blood-brain barrier by peripheral neuropeptides: New approaches to enhancing transport and endogenous expression. Cell and Tissue Research, 375(1), 287–293. 10.1007/s00441-018-2959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Ryu P. D., Lee S. Y. (2013). Differential distributions of neuropeptides in hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla in the rat. Neuroscience Letters, 556, 160–165. 10.1016/j.neulet.2013.09.070 [DOI] [PubMed] [Google Scholar]
- Li D., Li T., Yu J., Liu X., Jia S., Wang X., Wang P., Wang Y. F. (2021b). Astrocytic modulation of supraoptic oxytocin neuronal activity in rat dams with pup-deprivation at different stages of lactation. Neurochemical Research, 46(10), 2601–2611. 10.1007/s11064-020-03129-5 [DOI] [PubMed] [Google Scholar]
- Li D., Liu H., Liu X., Wang H., Li T., Wang X., Jia S., Wang P., Wang Y. F. (2020). Involvement of hyperpolarization-activated cyclic nucleotide-gated channel 3 in oxytocin neuronal activity in lactating rats with pup deprivation. ASN neuro, 12, 1759091420944658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu H., Wang H., Jia S., Wang X., Ling S., Chen G., Liu X., Wang Y. F. (2021d). Astrocytic hydrogen sulfide regulates supraoptic cellular activity in the adaptive response of lactating rats to chronic social stress. ASN neuro, 13, 17590914211043087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu X., Li T., Wang X., Jia S., Wang P., Wang Y. F. (2021a). Involvement of protein kinase A in oxytocin neuronal activity in rat dams with pup deprivation. Neurochemical Research, 46(4), 980–991. 10.1007/s11064-020-03218-5 [DOI] [PubMed] [Google Scholar]
- Li D., Liu X., Liu H., Li T., Jia S., Wang X., Wang P., Qin D., Wang Y. F. (2021c). Key roles of cyclooxygenase 2-protein kinase A-hyperpolarization-activated cyclic nucleotide-gated channel 3 pathway in the regulation of oxytocin neuronal activity in lactating rats with intermittent pup-deprivation. Neuroscience, 452, 13–25. 10.1016/j.neuroscience.2020.10.016 [DOI] [PubMed] [Google Scholar]
- Li T., Jia S. W., Hou D., Liu X., Li D., Liu Y., Cui D., Wang X., Hou C., Brown C. H., Wang Y. F. (2021e). Intranasal oxytocin restores maternal behavior and oxytocin neuronal activity in the supraoptic nucleus in rat dams with cesarean delivery. Neuroscience, 468, 235–246. 10.1016/j.neuroscience.2021.06.020 [DOI] [PubMed] [Google Scholar]
- Li T., Jia S. W., Hou D., Wang X., Li D., Liu Y., Cui D., Liu X., Hou C. M., Wang P., Brown C. H., Wang Y. F. (2021f). Oxytocin modulation of maternal behavior and its association with immunological activity in rats with cesarean delivery. ASN neuro, 13, 17590914211014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Matsuura T., Xue M., Chen Q. Y., Liu R. H., Lu J. S., Shi W., Fan K., Zhou Z., Miao Z., Yang J., Wei S., Wei F., Chen T., Zhuo M. (2021). G) oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Reports, 36(3), 109411. 10.1016/j.celrep.2021.109411 [DOI] [PubMed] [Google Scholar]
- Liao P. Y., Chiu Y. M., Yu J. H., Chen S. K. (2020). Mapping central projection of oxytocin neurons in unmated mice using cre and alkaline phosphatase reporter. Frontiers in Neuroanatomy, 14, 559402. 10.3389/fnana.2020.559402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin I. F., Kashino M., Ohta H., Yamada T., Tani M., Watanabe H., Kanai C., Ohno T., Takayama Y., Iwanami A., Kato N. (2014). The effect of intranasal oxytocin versus placebo treatment on the autonomic responses to human sounds in autism: A single-blind, randomized, placebo-controlled, crossover design study. Molecular Autism, 5(1), 20. 10.1186/2040-2392-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley Baron-Cohen K., Feldman R., Fearon P., Fonagy P. (2021). Intranasal oxytocin administration improves mood in new mothers with moderate low mood but not in mothers with elevated symptoms of postnatal depression: A randomised controlled trial. Journal of Affective Disorders, 300, 358–365. 10.1016/j.jad.2021.11.062 [DOI] [PubMed] [Google Scholar]
- Lipschitz D. L., Crowley W. R., Bealer S. L. (2003). Central blockade of oxytocin receptors during late gestation disrupts systemic release of oxytocin during suckling in rats. Journal of Neuroendocrinology, 15(8), 743–748. 10.1046/j.1365-2826.2003.01052.x [DOI] [PubMed] [Google Scholar]
- Lipschitz D. L., Kuhn R., Kinney A. Y., Grewen K., Donaldson G. W., Nakamura Y. (2015). An exploratory study of the effects of mind-body interventions targeting sleep on salivary oxytocin levels in cancer survivors. Integrative Cancer Therapies, 14(4), 366–380. 10.1177/1534735415580675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litorp H., Sunny A. K., Kc A. (2021). Augmentation of labor with oxytocin and its association with delivery outcomes: A large-scale cohort study in 12 public hospitals in Nepal. Acta Obstetricia et Gynecologica Scandinavica, 100(4), 684–693. 10.1111/aogs.13919 [DOI] [PubMed] [Google Scholar]
- Liu C., Lan C., Li K., Zhou F., Yao S., Xu L., Yang N., Zhou X., Yang J., Yong X., Ma Y., Scheele D., Kendrick K. M., Becker B. (2021a). Oxytocinergic modulation of threat-specific amygdala sensitization in humans is critically mediated by serotonergic mechanisms. Biological Psychiatry Cognitive Neuroscience and Neuroimaging, 6(11), 1081–1089. https://doi.org/10.1016/j.bpsc.2021.04.009 [DOI] [PubMed] [Google Scholar]
- Liu N., Yang H., Han L., Ma M. (2022). Oxytocin in women’s health and disease. Frontiers in Endocrinology, 13, 786271. 10.3389/fendo.2022.786271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y., Cui D., Li D., Jiao R., Wang X., Jia S., Hou D., Li T., Liu H., Wang P., Wang Y. F. (2017). Oxytocin removes estrous female vs. Male preference of virgin male rats: mediation of the supraoptic nucleus via olfactory bulbs. Frontiers in Cellular Neuroscience, 11, 327. 10.3389/fncel.2017.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y., Hou D., Wang J., Lv C., Jia S., Zhang Y., Wang R., Jin H., Zhu H., Wang Y. F. (2016). Expression of glial fibrillary acidic protein in astrocytes of rat supraoptic nucleus throughout estrous cycle. Neuro Endocrinology Letters, 37(1), 41–45. [PubMed] [Google Scholar]
- Liu X. Y., Li D., Li T., Liu H., Cui D., Liu Y., Jia S., Wang X., Jiao R., Zhu H., Zhang F., Qin D., Wang Y. F. (2019). Effects of intranasal oxytocin on pup deprivation-evoked aberrant maternal behavior and hypogalactia in rat dams and the underlying mechanisms. Frontiers in Neuroscience, 13, 122. 10.3389/fnins.2019.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang D., Li H. (2021b). Oxytocin modulates neural individuation/categorization processing of faces in early face-selective areas. Cerebral Cortex, 32(6), 1159-1169. https://doi.org/10.1093/cercor/bhab277. [DOI] [PubMed] [Google Scholar]
- Logan C. N., Rojas G., Wilkinson C. S., Polo Escorcia A. K., Reichel C. M., Peris J., Knackstedt L. A. (2021). Systemic oxytocin increases glutamate efflux in the nucleus accumbens core of cocaine-experienced male and female rats but only increases dopamine efflux in males. Behavioural Brain Research, 417, 113590. 10.1016/j.bbr.2021.113590 [DOI] [PubMed] [Google Scholar]
- Lopatina O. L., Panina Y. A., Malinovskaya N. A., Salmina A. B. (2021). Early life stress and brain plasticity: From molecular alterations to aberrant memory and behavior. Reviews in the Neurosciences, 32(2), 131–142. 10.1515/revneuro-2020-0077 [DOI] [PubMed] [Google Scholar]
- Lucas R., Zhang Y., Walsh S. J., Starkweather A., Young E. (2021). OXTR Rs53576 variation with breast and nipple pain in breastfeeding women. Pain Management Nursing : Official Journal of the American Society of Pain Management Nurses, 22(3), 369–376. 10.1016/j.pmn.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Sabatier N., Bull P. M., Landgraf R., Dayanithi G., Leng G. (2002). Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature, 418(6893), 85–89. 10.1038/nature00822 [DOI] [PubMed] [Google Scholar]
- Mack S. O., Kc P., Wu M., Coleman B. R., Tolentino-Silva F. P., Haxhiu M. A. (2002). Paraventricular oxytocin neurons are involved in neural modulation of breathing. Journal of Applied Physiology, 92(2), 826–834. 10.1152/japplphysiol.00839.2001 [DOI] [PubMed] [Google Scholar]
- MacNeil B. J., Jansen A. H., Greenberg A. H., Nance D. M. (2003). Neuropeptide specificity of prostaglandin E2-induced activation of splenic and renal sympathetic nerves in the rat. Brain, Behavior, and Immunity, 17(6), 442–452. 10.1016/S0889-1591(03)00050-3 [DOI] [PubMed] [Google Scholar]
- Maejima Y., Horita S., Otsuka A., Hidema S., Nishimori K., Shimomura K. (2020). Oral oxytocin delivery with proton pump inhibitor pretreatment decreases food intake. Peptides, 128, 170312. 10.1016/j.peptides.2020.170312 [DOI] [PubMed] [Google Scholar]
- Maejima Y., Sakuma K., Santoso P., Gantulga D., Katsurada K., Ueta Y., Hiraoka Y., Nishimori K., Tanaka S., Shimomura K., Yada T. (2014). Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS letters, 588(23), 4404–4412. 10.1016/j.febslet.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Maestrini S., Mele C., Mai S., Vietti R., Di Blasio A., Castello L., Surico D., Aimaretti G., Scacchi M., Marzullo P. (2018). Plasma oxytocin concentration in pre- and postmenopausal women: its relationship with obesity, body composition and metabolic variables. Obesity Facts, 11(5), 429–439. 10.1159/000492001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magon N., Kalra S. (2011). The orgasmic history of oxytocin: love, lust, and labor. Indian Journal of Endocrinology and Metabolism, 15(Suppl 3), S156–S161. 10.4103/2230-8210.84851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A., Scheele D., Spengler F. B., Menba T., Mohr F., Gunturkun O., Stoffel-Wagner B., Kinfe T. M., Maier W., Khalsa S. S., Hurlemann R. (2019). Oxytocin reduces a chemosensory-induced stress bias in social perception. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 44(2), 281–288. 10.1038/s41386-018-0063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin B. J., Mitre M., D’Amour J A., Chao M. V., Froemke R. C. (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature, 520(7548), 499–504. 10.1038/nature14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N., Scheele D., Postin D., Onken M., Hurlemann R. (2021). Eye-Tracking reveals a role of oxytocin in attention allocation towards familiar faces. Frontiers in Endocrinology, 12, 629760. 10.3389/fendo.2021.629760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lorenzana G.,, Palma-Tirado L., Cifuentes-Diaz C., Gonzalez-Hernandez A., Condes-Lara M. (2021). Ultrastructural evidence for oxytocin and oxytocin receptor at the spinal dorsal horn: mechanism of nociception modulation. Neuroscience, 475, 117–126. 10.1016/j.neuroscience.2021.09.004 [DOI] [PubMed] [Google Scholar]
- Martins D., Davies C., De Micheli A., Oliver D., Krawczun-Rygmaczewska A., Fusar-Poli P., Paloyelis Y. (2020). Intranasal oxytocin increases heart-rate variability in men at clinical high risk for psychosis: A proof-of-concept study. Translational Psychiatry, 10(1), 227. 10.1038/s41398-020-00890-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H., Latt H. M., Koga Y., Nishiki T., Matsui H. (2019). Oxytocin and stress: neural mechanisms, stress-related disorders, and therapeutic approaches. Neuroscience, 417, 1–10. 10.1016/j.neuroscience.2019.07.046 [DOI] [PubMed] [Google Scholar]
- McConn B. R., Koskinen A., Denbow D. M., Gilbert E. R., Siegel P. B., Cline M. A. (2019b). Central injection of oxytocin reduces food intake and affects hypothalamic and adipose tissue gene expression in chickens. Domestic Animal Endocrinology, 67, 11–20. 10.1016/j.domaniend.2018.10.005 [DOI] [PubMed] [Google Scholar]
- McConn B. R., Siegel P. B., Cline M. A., Gilbert E. R. (2019a). Anorexigenic effects of mesotocin in chicks are genetic background-dependent and are associated with changes in the paraventricular nucleus and lateral hypothalamus. Comparative biochemistry and physiology part A. Molecular & Integrative Physiology, 232, 79–90. 10.1016/j.cbpa.2019.03.009 [DOI] [PubMed] [Google Scholar]
- McNeilly A. S., Robinson I. C., Houston M. J., Howie P. W. (1983). Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed), 286(6361), 257–259. 10.1136/bmj.286.6361.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle S. L., Leng G., Selvarajah J. R., Bicknell R. J., Russell J. A. (2000). Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience, 101, 1013–1021. 10.1016/S0306-4522(00)00300-6 [DOI] [PubMed] [Google Scholar]
- Meixner F., Montag C., Herbert C. (2019). Affective language, interpretation bias and its molecular genetic variations: exploring the relationship between genetic variations of the OXTR gene (rs53576 and rs2268498) and the emotional evaluation of words related to the self or the other. Frontiers in Psychology, 10, 68. 10.3389/fpsyg.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M. R., Melis T., Cocco C., Succu S., Sanna F., Pillolla G., Boi A., Ferri G. L., Argiolas A. (2007). Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. The European Journal of Neuroscience, 26(4), 1026–1035. 10.1111/j.1460-9568.2007.05721.x [DOI] [PubMed] [Google Scholar]
- Michelini L. C., Marcelo M. C., Amico J., Morris M. (2003). Oxytocinergic regulation of cardiovascular function: Studies in oxytocin-deficient mice. American Journal of Physiology Heart and Circulatory Physiology, 284(6), H2269–H2276. 10.1152/ajpheart.00774.2002 [DOI] [PubMed] [Google Scholar]
- Miller M., Bales K. L., Taylor S. L., Yoon J., Hostetler C. M., Carter C. S., Solomon M. (2013). Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Research : Official Journal of the International Society for Autism Research, 6(2), 91–102. 10.1002/aur.1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Gosnell B. A., Schioth H. B., Grace M. K., Klockars A., Olszewski P. K., Levine A. S. (2010). Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides, 31(7), 1346–1352. 10.1016/j.peptides.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre M., Marlin B. J., Schiavo J. K., Morina E., Norden S. E., Hackett T. A., Aoki C. J., Chao M. V., Froemke R. C. (2016). A distributed network for social cognition enriched for oxytocin receptors. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 36(8), 2517–2535. 10.1523/JNEUROSCI.2409-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg K. U., Handlin L., Petersson M. (2020). Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact - with a particular focus on the oxytocinergic system. Infant Behavior & Development, 61, 101482. 10.1016/j.infbeh.2020.101482 [DOI] [PubMed] [Google Scholar]
- Monteleone A. M., Scognamiglio P., Volpe U., Di Maso V., Monteleone P. (2016). Investigation of oxytocin secretion in anorexia nervosa and bulimia nervosa: relationships to temperament personality dimensions. European Eating Disorders Review : the Journal of the Eating Disorders Association, 24(1), 52–56. 10.1002/erv.2391 [DOI] [PubMed] [Google Scholar]
- Morales R., Criollo M. A., Gonzalez M., Medina G., Manriquez O. M., Gonzalez V. M., Villa-Angulo C. (2020). Benefit of oxytocin released by cervix stimulation in Mexican Holstein cattle. Journal of Advanced Veterinary and Animal Research, 7(4), 608–613. 10.5455/javar.2020.g458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa S., Fawaz L., Ibrahim W., Fathelbab Elsayed M., Mostafa Ahmed M. (2021). Effect of infant massage on salivary oxytocin level of mothers and infants with normal and disordered bonding. Journal of Primary Care & Community Health, 12, 21501327211012942. 10.1177/21501327211012942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue S. I., Liang M., Harashima A., Zhong J., Furuhara K., Boitsova E. B., Cherepanov S. M., Gerasimenko M., Yuhi T., Yamamoto Y., Higashida H. (2021). Transport of oxytocin to the brain after peripheral administration by membrane-bound or soluble forms of receptors for advanced glycation end-products. Journal of Neuroendocrinology, 33(3), e12963. 10.1111/jne.12963 [DOI] [PubMed] [Google Scholar]
- Munetomo A., Ishii H., Miyamoto T., Sakuma Y., Kondo Y. (2016). Puerperal and parental experiences alter rat preferences for pup odors via changes in the oxytocin system. The Journal of Reproduction and Development, 62(1), 17–27. 10.1262/jrd.2015-046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaza S., Sattar A., Ahmad N., Jamil Ahmad M., Akhtar S., Ahmad E., Ahmad T., Omer T. (2021). Effect of exogenous administration of oxytocin on postpartum follicular dynamics, oestrous rate and ovulation in nili-ravi buffaloes. Reproduction in Domestic Animals = Zuchthygiene, 56(11), 1369-1376. https://doi.org/ 10.1111/rda.14001 [DOI] [PubMed] [Google Scholar]
- Niimi M., Murao K., Taminato T. (2001). Central administration of neuromedin U activates neurons in ventrobasal hypothalamus and brainstem. Endocrine, 16(3), 201–206. 10.1385/ENDO:16:3:201 [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kawasaki M., Suzuki H., Matsuura T., Motojima Y., Ohnishi H., Yamanaka Y., Yoshimura M., Maruyama T., Saito R., Ueno H., Sonoda S., Nishimura K., Onaka T., Ueta Y., Sakai A. (2019). Neuropathic pain up-regulates hypothalamo-neurohypophysial and hypothalamo-spinal oxytocinergic pathways in oxytocin-monomeric red fluorescent protein 1 transgenic rat. Neuroscience, 406, 50–61. 10.1016/j.neuroscience.2019.02.027 [DOI] [PubMed] [Google Scholar]
- Ocakoglu F. T., Kose S., Ozbaran B., Onay H. (2018). The oxytocin receptor gene polymorphism -rs237902- is associated with the severity of autism spectrum disorder: A pilot study. Asian Journal of Psychiatry, 31, 142–149. 10.1016/j.ajp.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Ochiai H., Shiga T., Hoshino H., Horikoshi S., Kanno K., Wada T., Osakabe Y., Miura I., Yabe H. (2021). Effect of oxytocin nasal spray on auditory automatic discrimination measured by mismatch negativity. Psychopharmacology, 238(7), 1781–1789. 10.1007/s00213-021-05807-w [DOI] [PubMed] [Google Scholar]
- Okabe S., Takayanagi Y., Yoshida M., Onaka T. (2021). Post-weaning stroking stimuli induce affiliative behavior toward humans and influence brain activity in female rats. Scientific Reports, 11(1), 3805. 10.1038/s41598-021-83314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski P. K., Shi Q., Billington C. J., Levine A. S. (2000). Opioids affect acquisition of LiCl-induced conditioned taste aversion: Involvement of OT and VP systems. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 279(4), R1504–R1511. 10.1152/ajpregu.2000.279.4.R1504 [DOI] [PubMed] [Google Scholar]
- Ooishi Y., Mukai H., Watanabe K., Kawato S., Kashino M. (2017). Increase in salivary oxytocin and decrease in salivary cortisol after listening to relaxing slow-tempo and exciting fast-tempo music. PloS one, 12(12), e0189075. 10.1371/journal.pone.0189075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihashi R., Mizoguchi Y., Imamura Y., Yamada S., Ueno T., Monji A. (2020). Oxytocin and elderly MRI-based hippocampus and amygdala volume: A 7-year follow-up study. Brain Communications, 2(2), fcaa081. 10.1093/braincomms/fcaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osako Y.,, Otsuka T., Taniguchi M., Oka T., Kaba H. (2000). Oxytocin depresses spontaneous gamma-aminobutyric acid-ergic inhibitory postsynaptic currents in cultured mitral cells of the rat olfactory bulb by a presynaptic mechanism. Neuroscience Letters, 289(1), 25–28. 10.1016/S0304-3940(00)01235-0 [DOI] [PubMed] [Google Scholar]
- Osako Y., Otsuka T., Taniguchi M., Oka T., Kaba H. (2001). Oxytocin enhances presynaptic and postsynaptic glutamatergic transmission between rat olfactory bulb neurones in culture. Neuroscience Letters, 299(1-2), 65–68. 10.1016/S0304-3940(00)01779-1 [DOI] [PubMed] [Google Scholar]
- Pan S., Yin K., Tang Z., Wang S., Chen Z., Wang Y., Zhu H., Han Y., Liu M., Jiang M., Xu N., Zhang G. (2021). Stimulation of hypothalamic oxytocin neurons suppresses colorectal cancer progression in mice. eLife, 10, e67535. https://doi.org/10.7554/eLife.67535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C. A. (2017). Oxytocin, tolerance, and the dark side of addiction. International Review of Neurobiology, 136, 239–274. 10.1016/bs.irn.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Pedretti G., Wirobski G., Range F., Marshall-Pescini S. (2021). Artificially elevated oxytocin concentrations in pet dogs are associated with higher proximity-maintenance and gazing towards the owners. Physiology & Behavior, 237, 113451. 10.1016/j.physbeh.2021.113451 [DOI] [PubMed] [Google Scholar]
- Peris J., MacFadyen K., Smith J. A., de Kloet A. D., Wang L., Krause E. G. (2017). Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. The Journal of Comparative Neurology, 525(5), 1094–1108. 10.1002/cne.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Slattery D. A., Uschold-Schmidt N., Reber S. O., Neumann I. D. (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology, 42, 225–236. 10.1016/j.psyneuen.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Peters S. T., Bowen M. T., Bohrer K., McGregor I. S., Neumann I. D. (2017). Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction Biology, 22(3), 702–711. 10.1111/adb.12362 [DOI] [PubMed] [Google Scholar]
- Pfundmair M., Echterhoff G. (2021). Does oxytocin shield against negative effects of ostracism? A replication and extension. Biological Psychology, 163, 108128. 10.1016/j.biopsycho.2021.108128 [DOI] [PubMed] [Google Scholar]
- Phillips W. J., Ostrovsky O., Galli R. L., Dickey S. (2006). Relief of acute migraine headache with intravenous oxytocin: Report of two cases. Journal of Pain & Palliative Care Pharmacotherapy, 20(3), 25–28. 10.1080/J354v20n03_05 [DOI] [PubMed] [Google Scholar]
- Pirnia B., Hamdieh M., Kazemi Ashtiani M., Malekanmehr P., Pirnia K., Zahiroddin A., Sadeghi P. (2020). The effectiveness of intranasal oxytocin on addiction severity index and anhedonia symptoms in an alcoholic case with oropharyngeal cancer, a protocol for a single-case experimental design pilot study. Iranian Journal of Pharmaceutical Research : IJPR, 19(3), 18–23. https://doi.org/10.22037/ijpr.2020.14338.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia G., Luedicke J. M., Nazarloo H. P., Carter C. S., Ebner N. C. (2019). Plasma oxytocin and vasopressin levels in young and older men and women: functional relationships with attachment and cognition. Psychoneuroendocrinology, 110, 104419. 10.1016/j.psyneuen.2019.104419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessow F., Marengi D. A., Perry S. K., Lawson E. A. (2021). Oxytocin administration increases proactive control in men with overweight or obesity: A randomized, double-blind. Placebo-Controlled Crossover Study. Obesity, 29(1), 56–61. https://doi.org/10.1002/oby.23010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D. S., Lischke A., Grace S., Scheele D., Ma Y., Becker B. (2021). Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Molecular Psychiatry, 26(1), 80–91. 10.1038/s41380-020-00864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D. S., Rokicki J., van der Meer D., Alnaes D., Kaufmann T., Cordova-Palomera A., Dieset I., Andreassen O. A., Westlye L. T. (2019). Oxytocin pathway gene networks in the human brain. Nature Communications, 10(1), 668. 10.1038/s41467-019-08503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. S., Rehn S., Hoyos C. M., Bowen M. T. (2021). The influence of oxytocin-based interventions on sleep-wake and sleep-related behaviour and neurobiology: A systematic review of preclinical and clinical studies. Neuroscience and Biobehavioral Reviews, 131, 1005–1026. 10.1016/j.neubiorev.2021.10.016 [DOI] [PubMed] [Google Scholar]
- Reguilon M. D., Ferrer-Perez C., Minarro J., Rodriguez-Arias M. (2021). Oxytocin reverses ethanol consumption and neuroinflammation induced by social defeat in male mice. Hormones and Behavior, 127, 104875. 10.1016/j.yhbeh.2020.104875 [DOI] [PubMed] [Google Scholar]
- Reichova A., Schaller F., Bukatova S., Bacova Z., Muscatelli F., Bakos J. (2021). The impact of oxytocin on neurite outgrowth and synaptic proteins in Magel2-deficient mice. Developmental Neurobiology, 81(4), 366–388. 10.1002/dneu.22815 [DOI] [PubMed] [Google Scholar]
- Reyes-Lagos J. J., Echeverria-Arjonilla J. C., Pena-Castillo M. A., Garcia-Gonzalez M. T., Ortiz-Pedroza Mdel R., Pacheco-Lopez G., Vargas-Garcia C., Camal-Ugarte S., Gonzalez-Camarena R. (2015). A comparison of heart rate variability in women at the third trimester of pregnancy and during low-risk labour. Physiology & Behavior, 149, 255–261. 10.1016/j.physbeh.2015.05.041 [DOI] [PubMed] [Google Scholar]
- Riem M. M., Pieper S., Out D., Bakermans-Kranenburg M. J., van Ijzendoorn M. H. (2011). Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Social Cognitive and Affective Neuroscience, 6(3), 294–300. 10.1093/scan/nsq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem M. M. E., Kunst L. E., Kop W. J. (2021). Intranasal oxytocin and the stress-buffering effects of social support during experimentally induced pain: the role of attachment security. Journal of Affective Disorders, 278, 149–156. 10.1016/j.jad.2020.09.057 [DOI] [PubMed] [Google Scholar]
- Roberto M.,, Kirson D., Khom S. (2021). The role of the central amygdala in alcohol dependence. Cold Spring Harbor Perspectives in Medicine, 11(2). 10.1101/cshperspect.a039339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Santos C., Braga D. C., Ceroni A., Michelini L. C. (2020). Activity-Dependent neuroplastic changes in autonomic circuitry modulating cardiovascular control: the essential role of baroreceptors and chemoreceptors signaling. Frontiers in Physiology, 11, 309. 10.3389/fphys.2020.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez K. M., Smith B. L., Caldwell H. K. (2020). Voluntary alcohol consumption is increased in female, but not male, oxytocin receptor knockout mice. Brain and Behavior, 10(9), e01749. 10.1002/brb3.1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rung J. M., Horta M., Tammi E. M., Perez E., Ojeda M. C., Lin T., Harris G., Somerville J., Salmeron D., Beltz S. E., Sandesara B., Feifel D., Ebner N. C. (2021). Safety and tolerability of chronic intranasal oxytocin in older men: Results from a randomized controlled trial. Psychopharmacology, 238(9), 2405–2418. 10.1007/s00213-021-05862-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan A., Lengel D., Huh J. W., Barson J. R., Raghupathi R. (2021). Intranasal administration of oxytocin attenuates social recognition deficits and increases prefrontal cortex inhibitory postsynaptic currents following traumatic brain injury. eNeuro, 8(3), ENEURO.0061-21.2021. 10.1523/ENEURO.0061-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp H. A., James T. W., Ketterson E. D., Sengelaub D. R., Ditzen B., Heiman J. R. (2013). Lower sexual interest in postpartum women: Relationship to amygdala activation and intranasal oxytocin. Hormones and Behavior, 63(1), 114–121. 10.1016/j.yhbeh.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Maguire S., Hunt G. E., Kesby A., Suraev A., Stuart J., Booth J., McGregor I. S. (2018). Intranasal oxytocin in the treatment of anorexia nervosa: randomized controlled trial during re-feeding. Psychoneuroendocrinology, 87, 83–92. 10.1016/j.psyneuen.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Ruthschilling C. A., Albiero G., Lazzari V. M., Becker R. O., de Moura A. C., Lucion A. B., Almeida S., Veiga A. B., Giovenardi M. (2012). Analysis of transcriptional levels of the oxytocin receptor in different areas of the central nervous system and behaviors in high and low licking rats. Behavioural Brain Research, 228(1), 176–184. 10.1016/j.bbr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Ryan P. J., Ross S. I., Campos C. A., Derkach V. A., Palmiter R. D. (2017). Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nature Neuroscience, 20(12), 1722–1733. 10.1038/s41593-017-0014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe M., Zhao N., Crippa A., Strauss G. P., Kaiser S. (2021). Intranasal oxytocin for negative symptoms of schizophrenia: Systematic review, meta-analysis and dose-response meta-analysis of randomized controlled trials. The International Journal of Neuropsychopharmacology, 24(8), 601–614. https://doi.org/10.1093/ijnp/pyab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Hidema S., Otsuka A., Suzuki J., Kumagai M., Kanaya A., Murakami T., Takei Y., Saito K., Sugino S., Toyama H., Saito R., Tominaga T., Nishimori K., Yamauchi M. (2021). Effects of oxytocin on responses to nociceptive and non-nociceptive stimulation in the upper central nervous system. Biochemical and Biophysical Research Communications, 574, 8–13. 10.1016/j.bbrc.2021.08.042 [DOI] [PubMed] [Google Scholar]
- Sakakibara R., Uchiyama T., Yamanishi T., Kishi M. (2010). Genitourinary dysfunction in Parkinson’s disease. Movement Disorders : Official Journal of the Movement Disorder Society, 25(1), 2–12. 10.1002/mds.22519 [DOI] [PubMed] [Google Scholar]
- Samson W. K., Skala K. D., Alexander B. D., Huang F. L. (1991). Hypothalamic endothelin: Presence and effects related to fluid and electrolyte homeostasis. Journal of Cardiovascular Pharmacology, 17(Suppl 7), S346–S349. 10.1097/00005344-199100177-00099 [DOI] [PubMed] [Google Scholar]
- Samuni L., Preis A., Mundry R., Deschner T., Crockford C., Wittig R. M. (2017). Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proceedings of the National Academy of Sciences of the United States of America, 114(2), 268–273. 10.1073/pnas.1616812114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino S., Chini B., Grinevich V. (2017). Lifespan oxytocin signaling: maturation, flexibility, and stability in newborn, adolescent, and aged brain. Developmental Neurobiology, 77(2), 158–168. 10.1002/dneu.22450 [DOI] [PubMed] [Google Scholar]
- Schiavo J. K., Valtcheva S., Bair-Marshall C. J., Song S. C., Martin K. A., Froemke R. C. (2020). Innate and plastic mechanisms for maternal behaviour in auditory cortex. Nature, 587(7834), 426–431. 10.1038/s41586-020-2807-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I., Schmitgen M. M., Boll S., Roth C., Nees F., Usai K., Herpertz S. C., Wolf R. C. (2020). Oxytocin modulates intrinsic neural activity in patients with chronic low back pain. European Journal of Pain, 24(5), 945–955. 10.1002/ejp.1543 [DOI] [PubMed] [Google Scholar]
- Schulze L., Lischke A., Greif J., Herpertz S. C., Heinrichs M., Domes G. (2011). Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology, 36(9), 1378–1382. 10.1016/j.psyneuen.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Sclafani A., Rinaman L., Vollmer R. R., Amico J. A. (2007). Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 292(5), R1828–R1833. 10.1152/ajpregu.00826.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvage D. J., Johnston C. A. (2004). Interaction between norepinephrine, oxytocin, and nitric oxide in the stimulation of gonadotropin-releasing hormone release from proestrous rat basal hypothalamus explants. Journal of Neuroendocrinology, 16(10), 819–824. 10.1111/j.1365-2826.2004.01235.x [DOI] [PubMed] [Google Scholar]
- Shabalova A. A., Liang M., Zhong J., Huang Z., Tsuji C., Shnayder N. A., Lopatina O., Salmina A. B., Okamoto H., Yamamoto Y., Zhong Z. G., Yokoyama S., Higashida H. (2020). Oxytocin and CD38 in the paraventricular nucleus play a critical role in paternal aggression in mice. Hormones and Behavior, 120, 104695. 10.1016/j.yhbeh.2020.104695 [DOI] [PubMed] [Google Scholar]
- Shishido E., Shuo T., Shinohara K., Horiuchi S. (2021). Effects of epidural anesthesia on postpartum maternity blues and fatigue and its relation to changes in oxytocin. Japan Journal of Nursing Science : JJNS:e, 12406. https://doi.org/10.111/jjns.12406 [DOI] [PubMed] [Google Scholar]
- Sinclair M. S., Perea-Martinez I., Dvoryanchikov G., Yoshida M., Nishimori K., Roper S. D., Chaudhari N. (2010). Oxytocin signaling in mouse taste buds. PloS one, 5(8), e11980. 10.1371/journal.pone.0011980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M. T., Goodman S. J., Yellan I., Butcher D. T., Jangjoo M., Grafodatskaya D., Rajendram R., Lou Y., Zhang R., Zhao C., Nicolson R., Georgiades S., Szatmari P., Scherer S. W., Roberts W., Anagnostou E., Weksberg R. (2021). DNA Methylation of the oxytocin receptor across neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 51(10), 3610-3623. https://doi.org/10.1007/s10803-020-04792-x [DOI] [PubMed] [Google Scholar]
- Skinner J. A., Garg M. L., Dayas C. V., Fenton S., Burrows T. L. (2018). Relationship between dietary intake and behaviors with oxytocin: A systematic review of studies in adults. Nutrition Reviews, 76(5), 303–331. 10.1093/nutrit/nux078 [DOI] [PubMed] [Google Scholar]
- Smith J. A., Pati D., Wang L., de Kloet A. D., Frazier C. J., Krause E. G. (2015). Hydration and beyond: Neuropeptides as mediators of hydromineral balance, anxiety and stress-responsiveness. Frontiers in Systems Neuroscience, 9, 46. https://doi.org/10.3389/fnsys.2015.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Levin B. E., Stevens W., Sladek C. D. (2014). Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 306(7), R447–R456. 10.1152/ajpregu.00520.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman M. Q., Duque-Wilckens N., Trainor B. C. (2019). Complementary neural circuits for divergent effects of oxytocin: social approach versus social anxiety. Biological Psychiatry, 85(10), 792–801. 10.1016/j.biopsych.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J. R., Wenner S. M., Freestone D. M., Romaine C. C., Parian M. C., Christian S. M., Bohidar A. E., Ndem J. R., Vogel I. R., O’Kane C. M. (2017). Oxytocin reduces alcohol consumption in prairie voles. Physiology & Behavior, 179, 411–421. 10.1016/j.physbeh.2017.07.021 [DOI] [PubMed] [Google Scholar]
- Strathearn L., Mertens C. E., Mayes L., Rutherford H., Rajhans P., Xu G., Potenza M. N., Kim S. (2019). Pathways relating the neurobiology of attachment to drug addiction. Frontiers in Psychiatry, 10, 737. 10.3389/fpsyt.2019.00737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G. P., Keller W. R., Koenig J. I., Gold J. M., Ossenfort K. L., Buchanan R. W. (2015). Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophrenia Research, 162(1-3), 57–61. 10.1016/j.schres.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Yin Z., Li B. Z., Du H., Tang K., Liu P., Hang Pun S., Lei T. C., Li A. (2021). Oxytocin modulates neural processing of mitral/tufted cells in the olfactory bulb. Acta Physiologica, 231(4), e13626. https://doi.org/10.1111/apha.13626 [DOI] [PubMed] [Google Scholar]
- Sundar M., Patel D., Young Z., Leong K. C. (2021). Oxytocin and addiction: potential glutamatergic mechanisms. International Journal of Molecular Sciences, 22(5), 2405. 10.3390/ijms22052405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J., Yamada D., Ueta Y., Iwai T., Koga E., Tanabe M., Oka J. I., Saitoh A. (2020). Oxytocin reverses abeta-induced impairment of hippocampal synaptic plasticity in mice. Biochemical and Biophysical Research Communications, 528(1), 174–178. 10.1016/j.bbrc.2020.04.046 [DOI] [PubMed] [Google Scholar]
- Tapias-Espinosa C., Canete T., Sampedro-Viana D., Brudek T., Kaihoj A., Oliveras I., Tobena A., Aznar S., Fernandez-Teruel A. (2021). Oxytocin attenuates schizophrenia-like reduced sensorimotor gating in outbred and inbred rats in line with strain differences in CD38 gene expression. Physiology & Behavior, 240, 113547. 10.1016/j.physbeh.2021.113547 [DOI] [PubMed] [Google Scholar]
- Tauber M., Boulanouar, K., Diene, G., Cabal-Berthoumieu, S., Ehlinger,V., Fichaux-Bourin, P., Molinas, C., Faye, S., Valette, M., Pourrinet, J., Cessans, C., Viaux-Sauvelon, S., Bascoul, C., Guedeney, A., Delhanty, P., Geenen, V., Martens, H., Muscatelli, F., Cohen, D., Consoli, A., Payoux, P., Arnaud,C., Salles, J.P. (2017). The use of oxytocin to improve feeding and social skills in infants with Prader-Willi syndrome. Pediatrics, 139(2), e201629776. 10.1542/peds.2016-2976 [DOI] [PubMed] [Google Scholar]
- Taylor J. H., French J. A. (2015). Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Hormones and Behavior, 75, 154–159. 10.1016/j.yhbeh.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R., Kalsbeek A., Buijs R. M., Pevet P. (1997). Oxytocin innervation of spinal preganglionic neurons projecting to the superior cervical ganglion in the rat. Cell and Tissue Research, 287(3), 481–486. 10.1007/s004410050772 [DOI] [PubMed] [Google Scholar]
- Thomas P. A., Kim S. (2021). Lost touch? Implications of physical touch for physical health. The journals of gerontology series B. Psychological Sciences and Social Sciences, 76, e111–e115. 10.1093/geronb/gbaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelman E., Warmink-Perdijk W., Henrichs J., Peters L., Schellevis F. G., Berger M. Y., Burger H. (2021). Intrapartum synthetic oxytocin, behavioral and emotional problems in children, and the role of postnatal depressive symptoms, postnatal anxiety and mother-to-infant bonding: A Dutch prospective cohort study. Midwifery, 100, 103045. 10.1016/j.midw.2021.103045 [DOI] [PubMed] [Google Scholar]
- Tolentino M., Palheta-Junior R. C., Silva C. M. S., Cavalcante A. K. M., Quetz J. D. S., Havt A., de Lima J. B. M., Mecawi A. S., de Castro M., Antunes-Rodrigues J., de Oliveira R. B., Magalhaes P. J. C., Dos Santos A A. (2021). Role of cholecystokinin and oxytocin in slower gastric emptying induced by physical exercise in rats. Physiology & Behavior, 233, 113355. 10.1016/j.physbeh.2021.113355 [DOI] [PubMed] [Google Scholar]
- Tops M., Koole SL H. I. J., Buisman-Pijlman F. T. (2014). Why social attachment and oxytocin protect against addiction and stress: insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry, and Behavior, 119, 39–48. 10.1016/j.pbb.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Tops M., van Ijzendoorn M. H., Riem M. M., Boksem M. A., Bakermans-Kranenburg M. J. (2011). Oxytocin receptor gene associated with the efficiency of social auditory processing. Frontiers in Psychiatry, 2, 60. 10.3389/fpsyt.2011.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres N., Martins D., Monteiro L., Santos A. J., Vaughn B. E., Verissimo M. (2022). Salivary oxytocin after play with parents predicts behavioural problems in preschool children. Psychoneuroendocrinology, 136, 105609. 10.1016/j.psyneuen.2021.105609 [DOI] [PubMed] [Google Scholar]
- Tracy L. M., Gibson S. J., Labuschagne I., Georgiou-Karistianis N., Giummarra M. J. (2018). Intranasal oxytocin reduces heart rate variability during a mental arithmetic task: A randomised, double-blind, placebo-controlled cross-over study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 81, 408–415. 10.1016/j.pnpbp.2017.08.016 [DOI] [PubMed] [Google Scholar]
- Tse W. S., Siu A. F. Y., Zhang Q., Chan H. Y. E. (2018). Maternal oxytocin responsiveness improves specificity of positive social memory recall. Psychoneuroendocrinology, 98, 148–152. 10.1016/j.psyneuen.2018.08.026 [DOI] [PubMed] [Google Scholar]
- Tsuji T., Inatani M., Tsuji C., Cheranov S. M., Kadonosono K. (2020). Oxytocin induced epithelium-mesenchimal transition through rho-ROCK pathway in ARPE-19 cells, a human retinal pigmental cell line. Tissue & Cell, 64, 101328. 10.1016/j.tice.2019.101328 [DOI] [PubMed] [Google Scholar]
- Tsushima H., Zhang Y., Muratsubaki T., Kanazawa M., Fukudo S. (2021). Oxytocin antagonist induced visceral pain and corticotropin-releasing hormone neuronal activation in the central nucleus of the amygdala during colorectal distention in mice. Neuroscience Research, 168, 41–53. 10.1016/j.neures.2021.04.011 [DOI] [PubMed] [Google Scholar]
- Tyzio R., Nardou R., Ferrari D. C., Tsintsadze T., Shahrokhi A., Eftekhari S., Khalilov I., Tsintsadze V., Brouchoud C., Chazal G., Lemonnier E., Lozovaya N., Burnashev N., Ben-Ari Y. (2014). Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science (New York, N.Y.), 343(6171), 675–679. 10.1126/science.1247190 [DOI] [PubMed] [Google Scholar]
- Tzabazis A., Kori S., Mechanic J., Miller J., Pascual C., Manering N., Carson D., Klukinov M., Spierings E., Jacobs D., Cuellar J., Frey W. H., 2nd, Hanson L., Angst M., Yeomans D. C. (2017). Oxytocin and migraine headache. Headache, 57(Suppl 2), 64–75. 10.1111/head.13082 [DOI] [PubMed] [Google Scholar]
- Uta D., Oti T., Sakamoto T., Sakamoto H. (2021). In vivo electrophysiology of peptidergic neurons in deep layers of the lumbar spinal cord after optogenetic stimulation of hypothalamic paraventricular oxytocin neurons in rats. International Journal of Molecular Sciences, 22(7), 3400. https://doi.org/10.3390/ijms22073400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas-Moberg K., Handlin L., Petersson M. (2014). Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Frontiers in Psychology, 5, 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzefovsky F., Bethlehem R. A. I., Shamay-Tsoory S., Ruigrok A., Holt R., Spencer M., Chura L., Warrier V., Chakrabarti B., Bullmore E., Suckling J., Floris D., Baron-Cohen S. (2019). The oxytocin receptor gene predicts brain activity during an emotion recognition task in autism. Molecular Autism, 10, 12. 10.1186/s13229-019-0258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Hernandeza P. A.,, Polk R., Horta M., Frazier I., Perez E., Ojeda M., Porges E., Cruz-Almeida Y., Feifel D., Ebner N. C. (2021). Chronic oxytocin administration in older men modulates functional connectivity during animacy perception. Aging Brain, 1, 100023. 10.1016/j.nbas.2021.100023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Klaauw A. A., Ziauddeen H., Keogh J. M., Henning E., Dachi S., Fletcher P. C., Farooqi I. S. (2017). Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Scientific Reports, 7(1), 4266. 10.1038/s41598-017-04600-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hedger K., Bershad A. K., Lee R., de Wit H. (2020). Effects of intranasal oxytocin on stress-induced cigarette craving in daily smokers. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco, 22(1), 89–95. 10.1093/ntr/nty159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakerley J. B., Clarke G., Summerlee A. J. (1994). Milk ejection and its control. In Knobil E., Neill J. D. (Eds.), The physiology of reproduction (pp. 1131–1177). Raven Press. [Google Scholar]
- Wang P., Wang S. C., Li D., Li T., Yang H. P., Wang L., Wang Y. F., Parpura V. (2019a). Role of connexin 36 in autoregulation of oxytocin neuronal activity in rat supraoptic nucleus. ASN neuro, 11, 1759091419843762. https://doi.org/10.1177/1759091419843762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang S. C., Yang H., Lv C., Jia S., Liu X., Wang X., Meng D., Qin D., Zhu H., Wang Y. F. (2019b). Therapeutic potential of oxytocin in atherosclerotic cardiovascular disease: mechanisms and signaling pathways. Frontiers in Neuroscience, 13, 454. 10.3389/fnins.2019.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yang H. P., Tian S., Wang L., Wang S. C., Zhang F., Wang Y. F. (2015). Oxytocin-secreting system: A major part of the neuroendocrine center regulating immunologic activity. Journal of Neuroimmunology, 289, 152–161. 10.1016/j.jneuroim.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Wang S. C., Parpura V., Wang Y. F. (2021a). Astroglial regulation of magnocellular neuroendocrine cell activities in the supraoptic nucleus. Neurochemical Research, 46(10), 2586–2600. 10.1007/s11064-020-03172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. C., Zhang F., Zhu Z., Yang H.-P., Liu Y., Wang P., Parpura V., Wang Y.-F. (2022). Potential of endogenous oxytocin in endocrine treatment and prevention of COVID-19. Front Endocrinol, 13, 799521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Escobar J. B., Mendelowitz D. (2021b). Sex differences in the hypothalamic oxytocin pathway to locus coeruleus and augmented attention with chemogenetic activation of hypothalamic oxytocin neurons. International Journal of Molecular Sciences, 22(16), 8510. https://doi.org/10.3390/ijms22168510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2009). Astrocytic plasticity and patterned oxytocin neuronal activity: Dynamic interactions. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 29(6), 1743–1754. 10.1523/JNEUROSCI.4669-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Negoro H., Higuchi T. (2013). Lesions of hypothalamic mammillary body desynchronise milk-ejection bursts of rat bilateral supraoptic oxytocin neurones. Journal of Neuroendocrinology, 25(1), 67–75. 10.1111/j.1365-2826.2012.02368.x [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Negoro H., Honda K. (1995). Effects of hemitransection of the midbrain on milk-ejection burst of oxytocin neurones in lactating rat. The Journal of Endocrinology, 144(3), 463–470. 10.1677/joe.0.1440463( [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Negoro H., Honda K. (1997). Effect of interhemispheric sections of the hypothalamus on milk-ejection bursts of supraoptic oxytocin neurones during bilateral and unilateral suckling in the rat. Neuroscience Letters, 227(1), 17–20. 10.1016/S0304-3940(97)00300-5 [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Ponzio T. A., Hatton G. I. (2006). Autofeedback effects of progressively rising oxytocin concentrations on supraoptic oxytocin neuronal activity in slices from lactating rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 290(5), R1191–R1198. 10.1152/ajpregu.00725.2005 [DOI] [PubMed] [Google Scholar]
- Warfvinge K., Krause D., Edvinsson L. (2020). The distribution of oxytocin and the oxytocin receptor in rat brain: Relation to regions active in migraine. The Journal of Headache and Pain, 21(1), 10. 10.1186/s10194-020-1079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrener C. D., Valentin E. M., Gallin C., Richey L., Ross D. B., Hood C. J., Lori A., Cubells J., Rauch S. A. M., Rilling J. K. (2021). The role of oxytocin signaling in depression and suicidality in returning war veterans. Psychoneuroendocrinology, 126, 105085. 10.1016/j.psyneuen.2020.105085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio H., Takeshita D., Sakata S. (2020). Parasympathetic nervous activity is associated with oxytocin in multiparous, but not primiparous, women during the perinatal period. Clinical and Experimental Pharmacology & Physiology, 47(6), 955–965. 10.1111/1440-1681.13267 [DOI] [PubMed] [Google Scholar]
- Wigton R., Tracy D. K., Verneuil T. M., Johns M., White T., Michalopoulou P. G., Averbeck B., Shergill S. (2022). The importance of pro-social processing, and ameliorating dysfunction in schizophrenia. An FMRI study of oxytocin. Schizophrenia Research. Cognition, 27, 100221. 10.1016/j.scog.2021.100221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow J. T., Insel T. R. (2002). The social deficits of the oxytocin knockout mouse. Neuropeptides, 36(2-3), 221–229. 10.1054/npep.2002.0909 [DOI] [PubMed] [Google Scholar]
- Wong S. F., Cardoso C., Orlando M. A., Brown C. A., Ellenbogen M. A. (2021a). Depressive symptoms and social context modulate oxytocin’s effect on negative memory recall. Social Cognitive and Affective Neuroscience, 16(12), 1234–1243. 10.1093/scan/nsab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. F., Cardoso C., Orlando M. A., Brown C. A., Ellenbogen M. A. (2021b). Depressive symptoms and social context modulate oxytocin’s effect on negative memory recall. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. F., Vaillancourt S., Grossman S., Kelly-Turner K., Blackwell S. E., Ellenbogen M. A. (2021c). Intranasal oxytocin increases state anhedonia following imagery training of positive social outcomes in individuals lower in extraversion, trust-altruism, and openness to experience. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 165, 8–17. 10.1016/j.ijpsycho.2021.03.013 [DOI] [PubMed] [Google Scholar]
- Woolley J. D., Lam O., Chuang B., Ford J. M., Mathalon D. H., Vinogradov S. (2015). Oxytocin administration selectively improves olfactory detection thresholds for lyral in patients with schizophrenia. Psychoneuroendocrinology, 53, 217–222. 10.1016/j.psyneuen.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. H., Zhou J. N., Balesar R., Unmehopa U., Bao A., Jockers R., Van Heerikhuize J., Swaab D. F. (2006). Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. The Journal of Comparative Neurology, 499(6), 897–910. 10.1002/cne.21152 [DOI] [PubMed] [Google Scholar]
- Xie S., Hu Y., Fang L., Chen S., Botchway B. O. A., Tan X., Fang M., Hu Z. (2022). The association of oxytocin with major depressive disorder: Role of confounding effects of antidepressants. Reviews in the Neurosciences, 33(1), 59–77. 10.1515/revneuro-2020-0128 [DOI] [PubMed] [Google Scholar]
- Yang H. P., Wang L., Han L., Wang S. C. (2013). Nonsocial functions of hypothalamic oxytocin. ISRN neuroscience, 20123, 179272. https://doi.org/10.1155/2013/179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liang J. Y., Li P., Pan Y. J., Qiu P. Y., Zhang J., Hao F., Wang D. X. (2011). Oxytocin in the periaqueductal gray participates in pain modulation in the rat by influencing endogenous opiate peptides. Peptides, 32(6), 1255–1261. 10.1016/j.peptides.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Yang S., Dong X., Guo X., Han Y., Song H., Gao L., Dai W., Su Y., Zhang X. (2017). Serum oxytocin levels and an oxytocin receptor gene polymorphism (rs2254298) indicate social deficits in children and adolescents with autism spectrum disorders. Frontiers in Neuroscience, 11, 221. 10.3389/fnins.2017.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang W., Wang X. T., Wang Y. W. (2021). A meta-analysis of hormone administration effects on cooperative behaviours: oxytocin, vasopressin, and testosterone. Neuroscience and Biobehavioral Reviews, 126, 430–443. 10.1016/j.neubiorev.2021.03.033 [DOI] [PubMed] [Google Scholar]
- Yang Z., Han D., Coote J. H. (2009). Cardiac sympatho-excitatory action of PVN-spinal oxytocin neurones. Autonomic Neuroscience : Basic & Clinical, 147(1-2), 80–85. 10.1016/j.autneu.2009.01.013 [DOI] [PubMed] [Google Scholar]
- Yang Z., Wheatley M., Coote J. H. (2002). Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Experimental Physiology, 87, 663–674. 10.1113/eph8702439 [DOI] [PubMed] [Google Scholar]
- Yao Y., Fu L. Y., Zhang X., van den Pol A. N. (2012). Vasopressin and oxytocin excite MCH neurons, but not other lateral hypothalamic GABA neurons. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 302, R815–824. 10.1152/ajpregu.00452.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans D. C., Hanson L. R., Carson D. S., Tunstall B. J., Lee M. R., Tzabazis A. Z., Jacobs D., Frey W. H., 2nd (2021). Nasal oxytocin for the treatment of psychiatric disorders and pain: Achieving meaningful brain concentrations. Translational Psychiatry, 11(1), 388. 10.1038/s41398-021-01511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Kim Y. K. (2020). The role of the oxytocin system in anxiety disorders. Advances in Experimental Medicine and Biology, 1191, 103–120. 10.1007/978-981-32-9705-0_7 [DOI] [PubMed] [Google Scholar]
- York N., Halbach P., Chiu M. A., Bird I. M., Pillers D. M., Pattnaik B. R. (2017). Oxytocin (OXT)-stimulated inhibition of Kir7.1 activity is through PIP2-dependent ca(2 + ) response of the oxytocin receptor in the retinal pigment epithelium in vitro. Cellular Signalling, 37, 93–102. 10.1016/j.cellsig.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Takayanagi Y., Inoue K., Kimura T., Young L. J., Onaka T., Nishimori K. (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 29(7), 2259–2271. 10.1523/JNEUROSCI.5593-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara C.,, Numan M., Kuroda K. O. (2018). Oxytocin and parental behaviors. Current Topics in Behavioral Neurosciences, 35, 119–153. 10.1007/7854_2017_11 [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Kikuchi M., Hiraishi H., Hasegawa C., Hirosawa T., Takahashi T., Munesue T., Kosaka H., Hiagashida H., Minabe Y. (2018). Longitudinal changes in the mismatch field evoked by an empathic voice reflect changes in the empathy quotient in autism spectrum disorder. Psychiatry Research Neuroimaging, 281, 117–122. 10.1016/j.pscychresns.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Yu G. Z., Kaba H., Okutani F., Takahashi S., Higuchi T. (1996a). The olfactory bulb: A critical site of action for oxytocin in the induction of maternal behaviour in the rat. Neuroscience, 72(4), 1083–1088. 10.1016/0306-4522(95)00600-1 [DOI] [PubMed] [Google Scholar]
- Yu G. Z., Kaba H., Okutani F., Takahashi S., Higuchi T., Seto K. (1996b). The action of oxytocin originating in the hypothalamic paraventricular nucleus on mitral and granule cells in the rat main olfactory bulb. Neuroscience, 72(4), 1073–1082. 10.1016/0306-4522(95)00599-4 [DOI] [PubMed] [Google Scholar]
- Yuan W., He Z., Hou W., Wang L., Li L., Zhang J., Yang Y., Jia R., Qiao H., Tai F. (2019). Role of oxytocin in the medial preoptic area (MPOA) in the modulation of paternal behavior in mandarin voles. Hormones and Behavior, 110, 46–55. 10.1016/j.yhbeh.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Zhang B., Qiu L., Xiao W., Ni H., Chen L., Wang F., Mai W., Wu J., Bao A., Hu H., Gong H., Duan S., Li A., Gao Z. (2021). Reconstruction of the hypothalamo-neurohypophysial system and functional dissection of magnocellular oxytocin neurons in the brain. Neuron, 109(2), 331–346 e337. 10.1016/j.neuron.2020.10.032 [DOI] [PubMed] [Google Scholar]
- Zheng S., Punia D., Wu H., Liu Q. (2021a). Graph theoretic analysis reveals intranasal oxytocin induced network changes over frontal regions. Neuroscience, 459, 153–165. 10.1016/j.neuroscience.2021.01.018 [DOI] [PubMed] [Google Scholar]
- Zheng X., Xu X., Xu L., Kou J., Luo L., Ma X., Kendrick K. M. (2021b). Intranasal oxytocin may help maintain romantic bonds by decreasing jealousy evoked by either imagined or real partner infidelity. Journal of Psychopharmacology, 35(6), 668–680. 10.1177/0269881121991576 [DOI] [PubMed] [Google Scholar]
- Zhu J., Li Y., Liang J., Li J., Huang K., Li J., Liu C. (2021). The neuroprotective effect of oxytocin on vincristine-induced neurotoxicity in mice. Toxicology Letters, 340, 67–76. 10.1016/j.toxlet.2021.01.008 [DOI] [PubMed] [Google Scholar]
- Zohrabi I., Abedi P., Ansari S., Maraghi E., Shakiba Maram N., Houshmand G. (2020). The effect of oxytocin vaginal gel on vaginal atrophy in postmenopausal women: A randomized controlled trial. BMC women’s Health, 20(1), 108. 10.1186/s12905-020-00935-5 [DOI] [PMC free article] [PubMed] [Google Scholar]