Abstract
Forty-seven Enterococcus faecium strains from different sources were evaluated by restriction endonuclease analysis (REA) of total chromosomal DNA. Strains from chicken, pork, and humans were clearly divided into separate clusters, whereas strains from different countries, strains with different antibiotic resistance profiles, or clinical and healthy-subject strains were not.
Infections by Enterococcus spp. have become increasingly important during the last decade (14, 24), and there has been much interest in Enterococcus faecium since it is prone to take up antibiotic resistance genes (5, 6, 16). Furthermore, enterococci are infamous for their ability to rapidly transfer their resistance genes to other enterococci (14), as well as to bacteria belonging to other genera (12, 17).
It has been proposed that strains are spread from animals to humans (1–3), and since the use of growth promoters and antimicrobial agents in animal husbandry often selects for E. faecium (9, 10, 15), it is important to clarify the role of E. faecium strains of animal origin in human infections.
E. faecium is a homogeneous species (19), and separation of strains belonging to this species requires methods with high discriminatory powers. It has been proposed that restriction endonuclease analysis (REA) is the best method to separate E. faecium strains (25), and this method has been used by other workers (7, 11). In these studies, however, only the ability of REA to separate strains and not the ability to reveal relationships between strains was investigated. In the present study, two separate restriction endonuclease digestions were used, compared to the one digestion used in the previous studies (7, 11). Also, use of a computer-based interpretation system allowed us to analyze gels with higher complexity, which meant that a broader range of DNA fragments could be included in the analysis compared to other studies (7, 11).
The aim of this study was to clarify the genomic relationships among E. faecium strains from different sources and with different levels of antibiotic resistance. REA of total chromosomal DNA with frequently cutting endonucleases was used due to its great capacity to resolve organisms at the strain level (8).
The strains included in this study are listed in Table 1. All of the strains were previously identified as E. faecium strains with methods other than REA (18, 19; data not shown). The test strains were obtained from different specimens and at different times.
TABLE 1.
Clusters of E. faecium strains obtained at the 45% similarity level: sources of strains, identification method(s) used, and antibiotic resistance
| Cluster | Isolate | Sourcea | Identification method(s)b | Antibiotic resistancec |
|---|---|---|---|---|
| I | SE ch 4c | Swedish chicken | phen + RAPD | TC |
| I | SE ch 17d | Swedish chicken | phen + RAPD | TC |
| I | DK ch 5a | Danish chicken | phen + RAPD | EM, VA |
| I | DK ch 6b | Danish chicken | phen + RAPD | EM, PV, VA |
| II | NCC 218d | The Netherlands, human, hosp | RAPD | EM |
| II | SE hum B42d | Sweden, human blood, hosp | phen + RAPD | PV |
| II | DK hum 5/3bd | Denmark, human feces, healthy | RAPD | EM |
| II | DK hum 27bd | Denmark, human feces, healthy | RAPD | NX |
| II | DK hum 24ad | Denmark, human feces, healthy | RAPD | EM, NX |
| II | CCUG 542Te | Type strain, human feces | phen + RAPD | |
| II | SE hum B50d | Sweden, human feces, hosp | phen + RAPD | TC |
| II | G | Danish probiotic yoghurt | phen + RAPD | |
| II | DK hum 19ad | Denmark, human feces, healthy | RAPD | NX, VA |
| III | SE hum B36d | Sweden, human urine, comm | phen + RAPD | AM, EM, NX, PV, TC |
| III | SE hum ET45d | Sweden, human urine, comm | phen + RAPD | AM, EM, NX, PV, TC |
| III | SE hum B49d | Sweden, human wound, comm | phen + RAPD | AM, EM, PV, TC |
| III | SE hum B60d | Sweden, human feces, hosp | phen + RAPD | |
| III | SE hum ET33d | Sweden, human urine, hosp | phen + RAPD | AM, EM, PV |
| III | SE hum ET47d | Sweden, human urine, comm | phen + RAPD | AM, EM, PV, TC |
| III | SE hum multid | Sweden, human blood, hosp | phen + RAPD | AM, CL, EM, PV, PP, TC, TS, VA |
| IV | SE hum ET144d | Sweden, human urine, hosp | phen + RAPD | AM, EM, NX, PV, TC |
| IV | SE hum 19ad | Sweden, human normal flora | phen + RAPD | |
| V | SE ch 14a | Swedish chicken | phen + RAPD | TC |
| V | SE ch 2a | Swedish chicken | phen + RAPD | NX, TC |
| VI | NCC 212d | Probiotic strain | RAPD | CL, EM, PV |
| VI | DK hum 30bd | Denmark, human feces, healthy | RAPD | EM |
| VI | MC74d | Probiotic strain | RAPD | EM |
| VI | SF68d | Probiotic strain | RAPD | EM |
| VI | DK hum 26/2ad | Denmark, human feces, healthy | RAPD | EM |
| VI | NCC 270d | Probiotic strain | RAPD | EM |
| VI | DK hum 23ad | Denmark, human feces, healthy | RAPD | EM, PV |
| VII | DK hum 12ad | Denmark, human feces, healthy | RAPD | EM |
| VII | DK hum 20/2bd | Denmark, human feces, healthy | RAPD | NX |
| VIII | DK hum 1ad | Denmark, human feces, healthy | RAPD | EM |
| VIII | DK hum 17ad | Denmark, human feces, healthy | RAPD | EM |
| IX | DK po 4d | Danish pork | phen + RAPD | |
| IX | DK po 24a | Danish pork | phen + RAPD | EM |
| X | DK hum 22/2ad | Denmark, human feces, healthy | RAPD | CL, EM, TC |
| X | DK hum 11ad | Denmark, human feces, healthy | RAPD | NX |
| XI | DK hum 3bd | Denmark, human feces, healthy | RAPD | TC |
| XI | DK hum 8bd | Denmark, human feces, healthy | RAPD | NX |
| Straggler | DK hum 25ad | Denmark, human feces, healthy | RAPD | EM, PV, TC |
| Straggler | DK ch 6d | Danish chicken | phen + RAPD | EM, PV, VA |
| Straggler | DK ch 7c | Danish chicken | phen + RAPD | EM, VA |
| Straggler | DK po 5d | Danish pork | phen + RAPD | |
| Straggler | SE po 1c | Swedish chicken | phen + RAPD | |
| Straggler | DK po 24b | Danish pork | phen + RAPD | |
| Straggler | DK hum 18bd | Denmark, human feces, healthy | RAPD |
hosp, hospitalized patient; comm, community-based patient; healthy, healthy individual.
Identification procedures were performed by workers in our laboratory. phen, phenotypic identification; RAPD, randomly amplified polymorphic DNA identification.
AM, ampicillin; CL, chloramphenicol; EM, erythromycin; NX, norfloxacin; PP, piperacillin; PV, penicillin V; TS, trimethoprim-sulfamethoxazole; TC, tetracycline; VA, vancomycin.
Received as E. faecium (phenotypically identified).
Identified as E. faecium by workers at the Culture Collection, University of Gothenburg, Gothenburg, Sweden.
The strains were grown overnight in 50 ml of All Purpose Tween broth (Difco Laboratories, Detroit, Mich.). The cells were washed, resuspended in Tris-EDTA buffer (10 mmol of Tris liter−1, 1 mmol of EDTA liter−1; pH 8.3), and stored at −20°C.
The chromosomal DNA was prepared by enzymatic cell lysis, phenol-chloroform extraction, and dye-buoyant density centrifugation gradient, as previously described (23). This method removed most of the plasmid DNA but left the chromosomal DNA intact.
Restriction endonuclease digestion was performed as described previously for Lactobacillus digestion (23), except that two rather than three restriction endonucleases were used to digest the DNA. Two separate digestions were performed, one with EcoRI and one with PvuII (Boeringer-Mannheim Scandinavia, Bromma, Sweden). The DNA fragments were visualized by electrophoresis on a submerged 0.9% agarose gel, stained with ethidium bromide, and photographed as previously described for an analysis of Lactobacillus plantarum (8).
The gel images were scanned into a computer. Combined gel lanes consisting of the EcoRI digestion results and the PvuII digestion results in sequence were constructed, the lanes were compared by using the pattern recognition technique (Pearson coefficient), and dendrograms based on the unpaired group method using arithmetric averages (UPGMA) were constructed. All this was done by using GelCompar 4.0 software (Applied Maths, Kortrijk, Belgium).
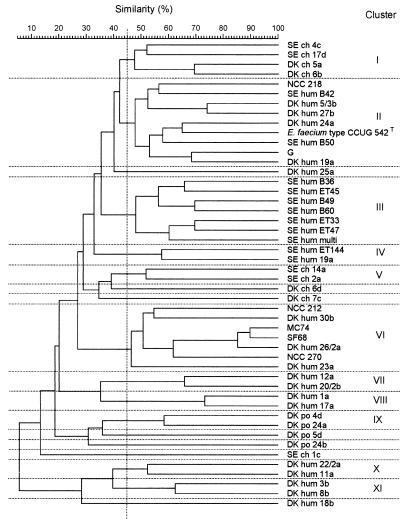
Eleven clusters and seven single strains were identified at a similarity level of 45% in the UPGMA dendrogram based on the combined REA profiles obtained from digestion with EcoRI and digestion with PvuII (Fig. 1). All but two of the strains tested could be clearly separated. The method exhibited good reproducibility (typically, 90 to 95% similarity) (data not shown); thus, the reproducibility was about the same as that previously obtained with lactobacilli (8). The source of isolation (human, chicken, or pig) could be determined in the clusters obtained from the REA profiles, whereas the specific source of isolation (e.g., feces, blood, or urine) could not be determined (Table 1 and Fig. 1). Furthermore, the antibiotic resistance profiles of the strains (Table 1) were not reflected in the dendrogram, and human strains from healthy persons and clinical specimens did not form separate clusters.
FIG. 1.
UPGMA dendrogram based on the REA profiles of E. faecium strains from different sources.
The method used to prepare DNA for the REA included a large number of steps for obtaining pure, unfragmented, essentially plasmid-free DNA. The efficiency of plasmid DNA removal by this DNA preparation method was demonstrated by the fact that the existence of antibiotic resistance genes in many of the strains tested did not affect the clustering (Table 1 and Fig. 1).
The question of whether Enterococcus strains and/or their antibiotic resistance genes are spread between animals and humans has not been clearly answered. Strong evidence that animal enterococci are capable of infecting humans has been presented by Das et al. (3). Several other workers have also suggested that resistant strains are spread from animals to humans (1, 2, 13). Seyed-Akhavani et al. (21) have suggested the possibility that resistance is spread via plasmid transfer from resistant animal strains to previously susceptible human strains. Indeed, the fact that human E. faecium strains are able to receive resistance genes from donor strains from chickens has been demonstrated. For instance, strains DK ch 6d and DK ch 5a, which were used in this investigation, were found to transfer both erythromycin resistance and vancomycin resistance to strain CCUG 542 (18) and to strain G (19a). There are also workers who claim that they have not been able to find any valid evidence for the spread of strains or resistance from animals to humans (4, 20).
The present finding that strains cluster according to their hosts suggests that the strains are host specific. Such specificity has also been observed in Lactobacillus reuteri strains by Ståhl and Molin, who found that strains from humans or pigs could be separated from strains from rats by REA (22). The fact that no strains from animals were found to cluster together with human strains in the present study does not necessarily mean that animal-to-human spread does not occur. In the present study, 48 isolates representing only a minute part of the immense number of strains occurring in nature were investigated.
Acknowledgments
This work was supported by the Swedish Council for Forestry and Agricultural Research and the Swedish National Science Research Council.
REFERENCES
- 1.Bates J, Jordens Z, Selkon J B. Evidence for an animal origin of vancomycin-resistant enterococci. Lancet. 1993;342:490–491. doi: 10.1016/0140-6736(93)91613-q. [DOI] [PubMed] [Google Scholar]
- 2.Bates J, Jordens J Z, Griffiths D T. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antimicrob Chemother. 1994;34:507–516. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 3.Das I, Fraise A, Wise R. Are glycopeptide-resistant enterococci in animals a threat to human beings? Lancet. 1997;349:997–998. doi: 10.1016/S0140-6736(05)62894-2. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly I P, Voss A, Witte W, Murray B E. Does the use in animals of antimicrobial agents, including glycopeptide antibiotics, influence the efficacy of antimicrobial therapy in humans? J Antimicrob Chemother. 1996;37:389–390. doi: 10.1093/jac/37.2.389. . (Reply, P. W. Hayes, R. H. Gustafson, F. K. Lotgering, Jr., and A. J. Mudd, J. Antimicrob. Chemother. 37:390–392, 1996.) [DOI] [PubMed] [Google Scholar]
- 5.Gordon S, Swenson J M, Hill B C, Pigott N E, Facklam R R, Cooksey R, Thornsberry C. Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. J Clin Microbiol. 1992;30:2373–2378. doi: 10.1128/jcm.30.9.2373-2378.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray J, Marsh P J, Stewart D, Pedler S J. Enterococcus bacteraemia: a prospective study of 125 episodes. J Hosp Infect. 1994;27:179–186. doi: 10.1016/0195-6701(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 7.Hall L M C, Duke B, Guiney M, Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992;30:915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson M-L, Quednau M, Ahrné S, Molin G. Classification of Lactobacillus plantarum by restriction endonuclease analysis of total chromosomal DNA, using conventional agarose electrophoresis. Int J Syst Bacteriol. 1995;45:670–675. [Google Scholar]
- 9.Kaukas A, Hinton M, Linton A H. The effect of ampicillin and tylosin on the faecal enterococci of healthy young chickens. J Appl Bacteriol. 1987;62:441–447. doi: 10.1111/j.1365-2672.1987.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaukas A, Hinton M, Linton A H. The effect of growth-promoting antibiotics on the faecal enterococci of healthy young chickens. J Appl Bacteriol. 1988;64:57–64. doi: 10.1111/j.1365-2672.1988.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 11.Lacoux P A, Jordens J Z, Fenton C M, Guiney M, Pennington T H. Characterization of enterococcal isolates by restriction enzyme analysis of genomic DNA. Epidemiol Infect. 1992;109:69–80. [PMC free article] [PubMed] [Google Scholar]
- 12.Leclercq R, Derlot E, Weber M, Duval J, Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989;33:10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy S B. Emergence of antibiotic-resistant bacteria in the intestinal flora of farm inhabitants. J Infect Dis. 1978;157:688–690. [PubMed] [Google Scholar]
- 14.Moellering R C. Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 15.Molitoris E, Krichevsky M I, Fagerberg D J, Quarles C L. Effects of dietary chlortetracycline on the antimicrobial resistance of broiler faecal Streptococcaceae. J Appl Bacteriol. 1986;60:185–193. doi: 10.1111/j.1365-2672.1986.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 16.Montecalvo M A, Horowitz H, Gedris C, Carbonaro C, Tenover F C, Issha A, Cook P, Wormser G P. Outbreak of vancomycin-, ampicillin-, and aminoglycoside-resistant Enterococcus faecium bacteremia in an adult oncology unit. Antimicrob Agents Chemother. 1994;38:1363–1367. doi: 10.1128/aac.38.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble W G, Virani Z, Cree R G A. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;93:195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 18.Quednau M, Ahrné S, Molin G. Antibiotic resistant strains of Enterococcus isolated from Swedish and Danish retailed chicken and pork. J Appl Bacteriol. 1998;84:1163–1170. doi: 10.1046/j.1365-2672.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- 19.Quednau M, Ahrné S, Molin G. Identification of clinically important species of Enterococcus within 1 day using randomly amplified polymorphic DNA (RAPD) Curr Microbiol. 1998;36:332–336. doi: 10.1007/s002849900318. [DOI] [PubMed] [Google Scholar]
- 19a.Quednau, M., S. Ahrné, and G. Molin. Unpublished data.
- 20.Scheidy S F, Pagano J F, McKee J J. The use of drugs in animal feeds. Preceedings of a symposium. National Academy of Sciences National Research Council Publication no. 1679. Washington, D.C: National Academy of Sciences; 1969. Status of research concerning drugs in feeds in relation to drug resistance of microbial populations; pp. 360–367. [Google Scholar]
- 21.Seyed-Akhavani M, Hill R L R, Morrison D, Woodford N, Beighton D, Casewell M W. Program and abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. A molecular comparison of vancomycin-resistant E. faecium (VREF) strains isolated from patients and chickens; p. 42. [Google Scholar]
- 22.Ståhl M, Molin G. Classification of Lactobacillus reuteri by restriction endonuclease analysis of chromosomal DNA. Int J Syst Bacteriol. 1994;44:9–14. [Google Scholar]
- 23.Ståhl M, Molin G, Persson A, Ståhl S. Restriction endonuclease patterns and multivariate analysis as a classification tool for Lactobacillus spp. Int J Syst Bacteriol. 1990;40:189–193. [Google Scholar]
- 24.Tailor S A N, Baily E M, Rybak M J. Enterococcus, an emerging pathogen. Ann Pharmother. 1993;27:1231–1242. doi: 10.1177/106002809302701014. [DOI] [PubMed] [Google Scholar]
- 25.Willey B M, McGeer A J, Ostrowski M A, Kreiswirth B N, Low D E. The use of molecular typing techniques in the epidemiologic investigation of resistant enterococci. Infect Control Hosp Epidemiol. 1994;15:548–556. doi: 10.1086/646976. [DOI] [PubMed] [Google Scholar]