Abstract
Background: Rapid diagnostic tests (RDTs) for bacteremia allow for early antimicrobial therapy modification based on organism and resistance gene identification. Studies suggest patient outcomes are optimized when infectious disease (ID)-trained antimicrobial stewardship personnel intervene on RDT results. However, data are limited regarding RDT implementation at small community hospitals, which often lack access to on-site ID clinicians. Methods: This study evaluated the impact of RDTs with and without real-time pharmacist intervention (RTPI) at a small community hospital with local pharmacist training and asynchronous support from a remote ID Telehealth pharmacist. Time to targeted therapy (TTT) in patients with bacteremia was compared retrospectively across 3 different time periods: a control without RDT, RDT-only, and RDT with RTPI. Results: Median TTT was significantly faster in both the RDT with RTPI and RDT-only groups compared with the control group (2 vs 25 vs 51 hours respectively; P < .001). TTT was numerically faster for RDT with RTPI compared with RDT-only but did not reach statistical significance (P = .078). Median time to any de-escalation was significantly shorter for RDT with RTPI compared with both RDT-only (14 vs 33 hours; P = .012) and the control group (14 vs 45 hours; P < .001). Median length of stay was also significantly shorter in both RDT groups compared with the control group (4.0 vs 4.1 vs 5.5 hours; P = .013). Conclusion: This study supports RDT use for bacteremia in a small community hospital with ID Telehealth support, suggesting additional benefit with RTPI.
Keywords: rapid diagnostic testing, bacteremia, community hospital, antimicrobial stewardship, infectious diseases, telehealth
Background and Significance
Early appropriate antimicrobial therapy is essential for treatment of bloodstream infections. 1 Traditional organism identification and susceptibility testing can take 48 to 72 hours, which can delay appropriate therapy. However, rapid diagnostic tests (RDTs) such as Verigene® Blood Culture-Gram Negative (BC-GN), Verigene® Blood Culture-Gram Positive (BC-GP), and BioFire FilmArray® Blood Culture Identification (BCID) identify organisms and key resistance genes on average 28 to 29 hours faster than traditional methods, allowing for earlier antimicrobial optimization. 2 RDT implementation decreases time to appropriate therapy, leading to clinical benefits such as decreased mortality and length of stay.3,4
Studies suggest that active antimicrobial stewardship intervention on RDT results is key for optimizing clinical impact. 5 Most published studies were conducted in large academic medical centers or large community hospitals with primarily infectious diseases (ID)-trained pharmacists or ID physicians responding to the RDT results.6,7 However, few studies have examined the logistics and impact of RDT implementation in small community hospitals (SCHs, < 200 beds), which often lack access to on-site ID clinicians. The purpose of this study was to determine the impact of RDT implementation with and without real-time pharmacist intervention (RTPI) on bacteremia outcomes at a SCH without on-site ID personnel.
Setting and Study Design
Logan Regional Hospital is a 146-bed urban community hospital serving northern Utah, southeastern Idaho, and western Wyoming. Pharmacist staffing consisted of 6 on-site daytime pharmacists and 1 overnight pharmacist providing 24-hour coverage. No on-site pharmacists were ID-trained, one was residency-trained, and all rotated through de-centralized clinical responsibilities.
An observational, pre/post implementation study was conducted to investigate the impact of RDT implementation on bacteremia outcomes in 3 time periods: From 5/15/2013 to 5/14/2015, the microbiology lab used traditional methods for workup of positive blood cultures without RDT (control group). Between 5/15/2015 and 1/15/2017, the lab utilized Verigene® BC-GN and BC-GP (RDT-only). The lab switched to BioFire FilmArray® BCID on 1/16/17. Beginning on 3/15/17 through the study end date 3/1/18, a protocol was implemented in which the RDT results were called directly to a staff pharmacist (RDT with RTPI). Positive blood cultures between 1/16/17 and 3/15/17 were excluded because this was a transition period between RDTs and was prior to RTPI implementation. This research was approved by the Intermountain Healthcare Institutional Review Board.
Intermountain’s Electronic Data Warehouse (EDW) was queried to identify adult patients (≥18 years) admitted to Logan Regional Hospital with a positive blood culture from 5/15/13 to 3/1/18. Patients were included with bacteremia due to S. aureus, Coagulase negative Staphylococcus spp. (CoNS), Streptococcus spp., S. pneumoniae, Enterococcus spp., E. coli, K. pneumoniae, K. oxytoca, P. aeruginosa, Enterobacter spp., and Proteus spp. as these organisms are present on both BCID and either BC-GN or BC-GP panels. Data were collected by EDW query regarding demographics, length of stay, and inpatient mortality. Data were collected by chart review for hospital course, antibiotic therapy, severity of illness, and comorbidities.
Patients with polymicrobial bacteremia, those with positive blood cultures at an outside facility prior to admission, and those who died, transferred, or discharged prior to blood culture positivity were excluded. Patients were excluded from the RDT-only and RDT with RTPI groups if no RDT was run and were excluded from the RDT with RTPI group if no pharmacist recommendation was documented in the chart.
Microbiology Workflow, Pharmacy Workflow, and RDT Protocol
For all 3 groups, positive blood cultures were processed real-time 24/7 and were incubated in the BACTEC FX blood culture system. A Gram stain was performed on positive blood cultures, and the results were called by the microbiology lab technician to the patient’s nurse, who communicated results to the clinical team. Identification and susceptibility testing were performed using Microscan Walkaway 96 Plus with ESBL confirmatory testing, and results were entered into the electronic health record (EHR). The control group had only this traditional workflow, and pharmacists did not review blood cultures in a standardized way during this time period. Patients in the RDT-only group had RDT performed following the Gram stain with results reported in the EHR and the same call back process to nursing staff. Pharmacists reviewed alerts for positive blood culture results once per day during daytime hours during this time period (no notification was made for RDT results to pharmacy or nursing staff). Any potential pharmacist recommendations in the control and RDT-only groups were not captured because these were not systematically documented. Patients in the RDT with RTPI group had RDT performed after blood culture positivity prior to the Gram stain. These results were entered into the EHR and called in real-time 24/7 by the microbiology lab technician to an on-site clinical pharmacist (whomever was designated as staff coverage at the time of notification), who contacted the attending physician with an antibiotic recommendation and wrote a note in the EHR.
To guide pharmacist recommendations for RDT with RTPI, an off-site ID Telehealth pharmacist within the Intermountain Healthcare system developed a protocol with preferred and alternative antimicrobials for each organism identified by RDT (see Supplemental Material). The protocol was based on national guidelines, published literature, and the local antibiogram. Beyond antimicrobials, the protocol included considerations for allergies, renal function, severity of illness, possible blood culture contaminants, when to recommend an ID consult (eg, S. aureus bacteremia), and screening for history of resistant organisms (eg, ESBLs). Prior to protocol implementation, the ID Telehealth pharmacist delivered an on-site 1-hour training lecture to local pharmacists and created a competency test for them to pass before receiving RDT notifications from the lab. For ongoing quality assurance, the ID Telehealth pharmacist prospectively monitored positive blood cultures during weekday business hours and provided feedback to the local pharmacists on their notes and recommendations. The ID Telehealth pharmacist was also available for local pharmacist questions regarding blood culture results if needed.
Outcomes
The primary outcome was time to targeted therapy (TTT), measured from index blood culture positivity to ordering of RDT protocol-recommended therapy or stopping therapy in the case of a contaminant (eg, 1 of 4 blood culture bottles positive for CoNS). Targeted therapy was defined as meeting all of the following: (1) Susceptible organism per finalized in vitro susceptibility testing; (2) Intravenous therapy at an appropriate dose for bacteremia and patient’s renal function; (3) The narrowest empiric therapy was chosen based on the local antibiogram and patient culture history. For Gram-negative bacteremia, broader spectrum antibiotics (eg, piperacillin-tazobactam instead of ceftriaxone) were allowed for critically ill patients; (4) Unnecessary agents were discontinued, including stopping therapy for contaminants. Additional agents continued for concomitant infections were allowed; and (5) Therapy was supported by published literature (eg, carbapenems were preferred for bacteremia with ESBL-producing organisms).
Secondary outcomes included inpatient mortality and length of stay. Time to any de-escalation or discontinuation of unnecessary therapy was also analyzed. This differed from the primary outcome in that patients did not have to meet all the criteria for targeted therapy to qualify as de-escalation. For example, in a patient with E.coli bacteremia on empiric vancomycin and meropenem, stopping vancomycin would be de-escalation, but continuing meropenem would not be targeted therapy.
The percent of pharmacist recommendations resulting in a change in therapy (ie, accepted recommendations) was captured for the RDT with RTPI group only. Percent agreement between RDT results and susceptibility testing, number of pharmacist recommendations that deviated from the protocol, and number of patients for whom the protocol would have resulted in inappropriate therapy were captured as safety measures.
Statistical Analysis
TTT and time to de-escalation were compared between the 3 groups with Kaplan-Meier survival analysis. Patients who died or were discharged before receiving targeted or de-escalated therapy were censored at the time of death or discharge. Overall log rank testing was used to evaluate for any time-to-event differences among the groups, and pairwise log rank testing was used to evaluate differences between groups. Kruskal-Wallis analysis was used to compare inpatient length of stay among the 3 groups; significant results were followed by 2-group comparisons using Bonferroni-corrected Wilcoxon testing. All statistical tests were performed by a statistician using SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, N.Y., USA). P-values were 2-sided with a significance level of < 0.05 [except the Bonferroni-corrected Wilcoxon testing for which P < .017 was considered significant (0.017 = 0.05/3 comparisons)].
Results
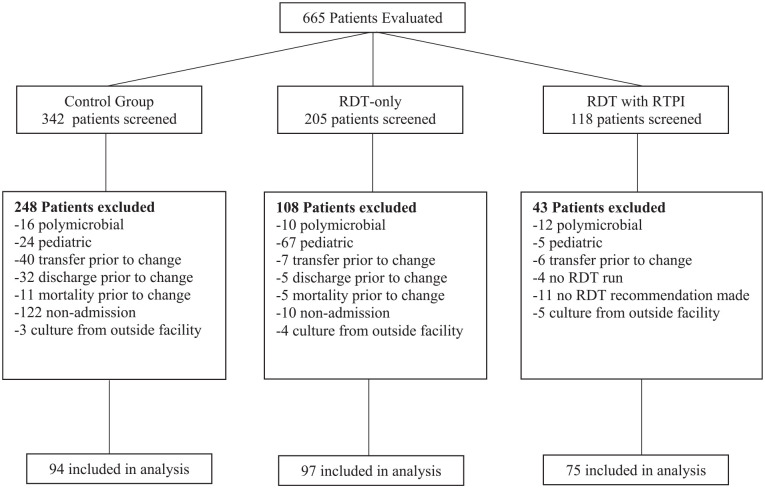
A total of 665 patients were evaluated and 266 met inclusion criteria: 94 in the control group, 97 in the RDT-only group, and 75 in the RDT with RTPI group (Figure 1). Baseline characteristics were well balanced between groups, without significant differences in age, Charlson Comorbidity Index, Pitt Bacteremia Score, source of bacteremia, or proportion of Gram-positive versus Gram-negative isolates (Table 1). More patients were admitted to the intensive care unit (ICU) in the control group than in the other 2 groups. The most common source of bacteremia was urinary followed by skin and soft tissue (SSTI). Almost 14% of positive cultures were determined to be contaminants in all 3 groups (Table 1). Methicillin sensitive S. aureus (MSSA) was the most common Gram-positive pathogen, and E. coli was the most common Gram-negative pathogen (Table 2). Median time to organism identification in the control, RDT-only, and RDT with RTPI groups was 17 versus 3 versus 1 hour, respectively; median time to final susceptibility results was 52 versus 61 versus 61 hours.
Figure 1.
Flowchart of study participants.
Note. RDT = rapid diagnostic test; RTPI = real-time pharmacist intervention.
Table 1.
Baseline Characteristics.
| Characteristic | Control Group (n = 94) | RDT-only (n = 97) | RDT with RTPI (n = 75) |
|---|---|---|---|
| Age (y), median (IQR) | 70 (59-81) | 69 (59-80) | 70 (59-80) |
| Sex, % female | 47.9 | 51.5 | 44.0 |
| Charlson Comorbidity, median (IQR) | 1 (0-2) | 2 (1-3) | 2 (1-3) |
| Pitt Bacteremia, median (IQR) | 1 (0-2) | 1 (0-2) | 1 (0-2) |
| ICU admission, no. (%) | 53 (56.4) | 39 (40.2) | 28 (36.8) |
| Bacteremia Source* | |||
| Endovascular, no. (%) | 5 (5.3) | 5 (5.2) | 1 (1.3) |
| Respiratory, no. (%) | 4 (4.3) | 6 (6.1) | 4 (5.3) |
| Intra-abdominal, no. (%) | 9 (9.6) | 9 (9.2) | 8 (10.7) |
| IV catheter, no. (%) | 5 (5.3) | 2 (2.1) | 4 (5.3) |
| Urinary, no. (%) | 33 (35.1) | 29 (29.9) | 26 (34.7) |
| Bone and joint, no. (%) | 7 (7.4) | 10 (10.3) | 3 (4.0) |
| SSTI, no. (%) | 16 (17.0) | 18 (18.6) | 17 (22.4) |
| Gynecological, no. (%) | 0 (0) | 1 (1.0) | 0 (0) |
| Contamination, no. (%) | 13 (13.8) | 14 (14.4) | 10 (13.3) |
| Unknown, no. (%) | 2 (2.1) | 3 (3.1) | 2 (2.7) |
Note. ICU = intensive care unit; IV = intravenous; SSTI = skin and skin structure infection; RDT = rapid diagnostic test; RTPI = real-time pharmacist intervention.
As determined by treating physician.
Table 2.
Microbiology.
| Organism, # (%) | Control group (n = 94) | RDT-only (n = 97) | RDT with RTPI (n = 75) |
|---|---|---|---|
| Gram positive | 54 (57.4) | 57 (58.8) | 39 (52.0) |
| MSSA | 18 (19.1) | 20 (20.6) | 11 (14.7) |
| MRSA | 3 (3.2) | 2 (2.1) | 1 (1.3) |
| CoNS | 9 (9.6) | 17 (17.5) | 8 (10.7) |
| Beta-hemolytic Streptococci | 8 (8.5) | 12 (12.4) | 9 (12.1) |
| Streptococcus pneumoniae | 3 (3.2) | 1 (1.0) | 3 (4.1) |
| Viridans group Streptococci | 11 (11.7) | 3 (3.1) | 5 (6.9) |
| Enterococci | 2 (2.1) | 2 (2.1) | 2 (2.7) |
| Gram negative | 40 (42.6) | 41 (42.2) | 36 (48.0) |
| E. coli | 29 (30.9) | 33 (34.0) | 25 (33.3) |
| Klebsiella spp. | 7 (7.4) | 3 (3.1) | 4 (5.3) |
| Pseudomonas aeruginosa | 1 (1.1) | 3 (3.1) | 3 (4.1) |
| Enterobacter spp. | 1 (1.1) | 1 (1.0) | 3 (4.1) |
| Proteus spp. | 2 (2.1) | 1 (1.0) | 1 (1.3) |
| ESBL producers | 0 (0) | 0 (0) | 8 (10.7) |
Note. ESBL = extended spectrum beta-lactamase; MSSA = methicillin sensitive S. aureus; MRSA = methicillin resistant S. aureus; CoNS = coagulase negative Staphylococci; RDT = rapid diagnostic test; RTPI = real-time pharmacist intervention.
Primary Outcomes
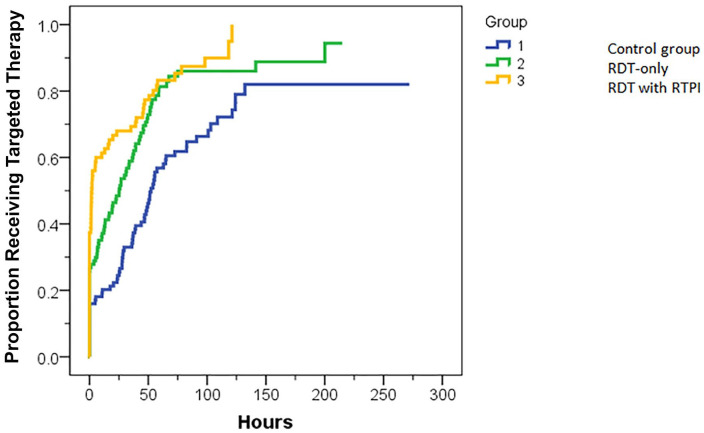
Median TTT was 51 hours in the control group, 25 hours in the RDT-only group, and 2 hours in the RDT with RTPI group. In a Kaplan-Meier analysis (Figure 2), TTT was found to be significantly different among the 3 groups (P < .001). Follow-up pairwise comparisons found TTT to be significantly longer in the control group than for the RDT-only (51 vs 25 hours, P < .001) and RDT with RTPI groups (51 vs 2 hours, P < .001). The difference in TTT between the RDT-only and RDT with RTPI groups did not reach statistical significance (25 vs 2 hours, P = .078).
Figure 2.
Kaplan-Meier curve for time to targeted therapy (TTT). TTT for the control group was significantly longer than for the RDT-only (P < .001), and RDT with RTPI groups (P < .001). TTT for RDT-only was numerically longer than for RDT with RTPI, but not significantly different (P = .078).
Note. RDT = rapid diagnostic test; RTPI = real-time pharmacist intervention.
Secondary Outcomes
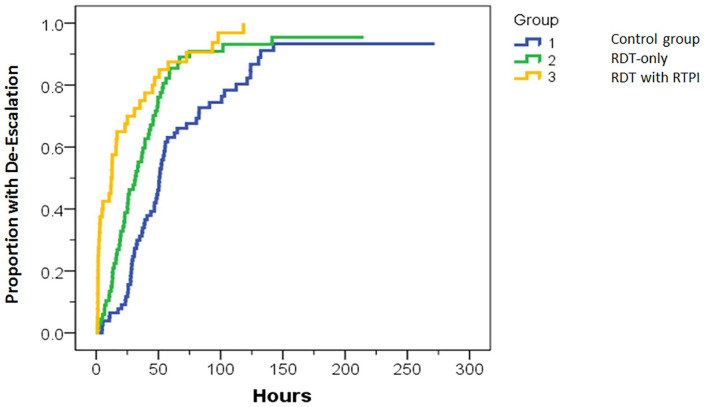
Median time to de-escalation was 45 hours in the control group, 33 hours for RDT-only, and 14 hours for RDT with RTPI. In a Kaplan-Meier analysis (Figure 3), the overall comparison showed a significant difference among the 3 groups (P < .001). Follow-up pairwise comparisons showed time to de-escalation was significantly longer for the control group than for both the RDT-only (45 vs 33 hours, P = .001) and RDT with RTPI groups (45 vs 14 hours, P < .001). Time to de-escalation was significantly longer with RDT-only than for RDT with RTPI (33 vs 14 hours, P = .012).
Figure 3.
Kaplan-Meier curve for time to de-escalation. Time to de-escalation for the control group was significantly longer than for the RDT-only (P = .001) and RDT with RTPI groups (P < .001). RDT-only time to de-escalation was significantly longer than for RDT with RTPI (P = .012).
Note. RDT = rapid diagnostic test; RTPI = real-time pharmacist intervention.
Additional secondary outcomes are displayed in Table 3. Kruskal-Wallis testing found a significant difference in length of stay among the 3 groups (P = .013). Bonferroni-corrected Wilcoxon testing found median length of stay to be significantly longer in the control group versus RDT-only (5.5 vs 4.1 days, P = .011) and RDT with RTPI (5.5 vs 4.0 days, P = .011). Length of stay was not significantly different between the RDT-only and RDT with RTPI groups (4.1 vs 4.0 days, P = .85). The proportion of patients receiving targeted therapy prior to discharge was significantly higher in the RDT with RTPI and RDT-only groups (89.3% and 84.5%, respectively) compared with the control group (70.2%, P =.005 and .03, respectively). No patients (0%) died in the RDT with RTPI group compared to 2 patients (2%) in the control and 3 patients (3%) in the RDT-only group, but statistical comparison was not performed due to low numbers.
Table 3.
Secondary Outcomes.
| Outcome | Control group (n = 94) | RDT-only (n = 97) | RDT with RTPI (n = 75) | P-value |
|---|---|---|---|---|
| LOS (d), median (IQR) | 5.5 (3.6-6.8) | 4.1 (3.0-5.9) | 4.0 (3.0-5.4) | .013* |
| Patients receiving targeted therapy prior to discharge, number (%) | 66 (70.2) | 82 (84.5) | 67 (89.3) | .004* |
| Mortality, number (%) | 2 (2.1) | 3 (3.1) | 0 (0) | N/A |
Note. LOS = inpatient length of stay; RDT = rapid diagnostic test; RTPI = real-time pharmacist intervention.
RDT-only and RDT with RTPI groups significantly different from group 1.
Rapid Diagnostic Test Concordance
The percent agreement between RDT and traditional microbiology testing was 98.9% for the RDT-only group and 94.7% for the RDT with RTPI group. One organism was misidentified as CoNS in the RDT-only group but identified as MSSA by traditional testing. The RDT with RTPI group had 4 patients with organisms identified as MRSA, Klebsiella pneumoniae, MSSA, and Proteus spp. by RDT, which were later identified by traditional testing as MSSA, Enterobacter aerogenes, S. hominis, and E. coli, respectively. It is unknown from retrospective chart review if these represented reporting errors or erroneous testing results. These discrepancies delayed de-escalation in 2 cases but did not result in inappropriate therapy.
Pharmacist Recommendations in the RDT With RTPI Group
Of 75 patients in the RDT with RTPI group, 26 (34.7%) were already on targeted therapy at the time of RDT result. Pharmacist recommendations were made for the remaining 49 patients: 25 (51%) had antibiotics changed to targeted therapy immediately, 16 (14.7%) changed to targeted therapy later, and 8 (10.7%) never received targeted therapy prior to discharge. Of the 25 immediately accepted recommendations, 8 (32%) were in the first 6 months, whereas 17 (68%) were in the following 5 months. Four protocol-based recommendations (5%) would have resulted in inappropriate therapy (all ESBL E. coli with no history of ESBL isolates). Five pharmacist recommendations (7%) diverged from the protocol and prompted feedback from the ID Telehealth pharmacist: 4 recommended vancomycin for CoNS instead of stopping therapy for probable contaminants (vancomycin was stopped after negative repeat cultures) and 1 recommended piperacillin/tazobactam in a patient with a severe penicillin allergy (meropenem was used instead).
Discussion
RDT implementation in a SCH setting with remote ID Telehealth support was associated with shorter TTT, time to de-escalation, and length of stay. Pairing RDT with RTPI was associated with a further decrease in time to de-escalation. These findings are consistent with other studies of RDT implementation, which also showed decreased length of stay and time to targeted therapy. 8 Unlike previous studies, no difference in mortality was observed, which is likely because severely ill patients at Logan Regional Hospital are often transferred to a facility with a higher acuity ICU. This was reflected by the low Pitt Bacteremia Scores and low overall mortality rate (1.9%) in the study population.
Previous studies have shown that RTPI reduces TTT in community hospitals, yet ID-trained personnel were heavily involved in the interventions.6,7 Data from this study suggest that implementation of RDT plus RTPI is both feasible and impactful at SCHs without on-site ID personnel. To facilitate successful implementation, off-site ID resources were leveraged through telehealth. ID pharmacist involvement was essential for protocol development and initial staff training, as well as ongoing surveillance and follow-up education regarding protocol deviations. However, local pharmacists were able to autonomously enact the protocol thereafter without an on-site ID presence.
One of the advantages of this study was the use of 3 cohorts to measure the effects of RDT implementation and RDT with RTPI separately. Previous randomized controlled trials have shown that real-time intervention is essential for optimization.9,10 A prior observational study examined the impact of RDT implementation with and without real-time intervention and did not find a further reduction in time to appropriate therapy with real-time intervention. This study may have had a reduced effect size as their real-time intervention took place only during business hours from Monday to Friday. 11 In the present study, RTPI took place 24/7 leading to a significant difference in time to de-escalation compared to RDT-only. The lack of significant difference in TTT between the RDT-only and RDT with RTPI groups was likely due to small sample size, but RTPI did not appear to impact LOS or mortality compared to RDT-only. While faster time to de-escalation is important for patient safety and healthcare efficiency, additional studies are needed to determine if allocating resources for RTPI improves outcomes for SCH patients.
This study had several limitations. Due to its observational nature, only an association can be established (and not causality) between the interventions and outcomes. The pre-post intervention design did not control for confounding variables (such as more ICU admissions in the control group or pharmacist interventions in the control or RDT-only groups), nor was an interrupted time series analysis conducted to see if the observed changes were already occurring over time. Fifteen patients excluded from the RDT with RTPI group (4 no RDT run, 11 no RDT recommendation made) may have biased the study toward positive findings, although including those patients would not have reflected the true impact of RDT with RTPI. We used multiple statistical tests to identify differences between groups, although the results remained significant using conservative methods (eg, Bonferroni correction) to protect against a Type 1 error. Another limitation was that only 51% of pharmacist recommendations were accepted in the RDT with RTPI group, which likely impacted TTT. Providers appeared hesitant to change therapy in the first 6 months following RTPI implementation with the majority of accepted recommendations coming in the final 5 months of the RTPI period. Obtaining more upfront buy-in from pharmacists and providers or providing additional education when implementing RTPI might lead to increased early acceptance. A final limitation was the switch between RDTs during the study period. Although organisms were identified slightly faster in the RDT with RTPI than RDT-only group, this did not account for the magnitude of difference seen in time to de-escalation or TTT. Therefore, the addition of RTPI was a more likely factor resulting in these differences.
In summary, this study found a significantly shorter TTT and LOS following RDT implementation, and that time to de-escalation was further shortened by adding RTPI. The data suggest that the benefits of RDT can extend to SCHs without on-site ID clinicians. With remote ID Telehealth support, local pharmacists were well-suited to provide the real-time interventions needed for optimization of RDT implementation and bacteremia outcomes.
Supplemental Material
Supplemental material, sj-docx-1-hpx-10.1177_00185787211037554 for No Implementation Without Representation: Real-Time Pharmacist Intervention Optimizes Rapid Diagnostic Tests for Bacteremia at a Small Community Hospital by Brandon J. Tritle, Robert Watteyne, Abby Hickman, Todd J. Vento, Bert K. Lopansri, Dave S. Collingridge and John J. Veillette in Hospital Pharmacy
Acknowledgments
We would like to thank to the front-line pharmacists at Logan Regional Hospital, the microbiology lab technicians, and the microbiology lab supervisor Stephanie Jensen for her help implementing the protocol and educating her lab technicians. Without their work this project would not have been possible.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: John J. Veillette  https://orcid.org/0000-0001-7662-7609
https://orcid.org/0000-0001-7662-7609
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. doi: 10.1097/01.CCM.0000217961.75225.E9 [DOI] [PubMed] [Google Scholar]
- 2. Ward C, Stocker K, Begum J, Wade P, Ebrahimsa U, Goldenberg SD. Performance evaluation of the Verigene® (Nanosphere) and FilmArray® (BioFire®) molecular assays for identification of causative organisms in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis. 2015;34(3):487-496. doi: 10.1007/s10096-014-2252-2 [DOI] [PubMed] [Google Scholar]
- 3. Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis. 2017;64(1):15-23. doi: 10.1093/cid/ciw649 [DOI] [PubMed] [Google Scholar]
- 4. Bauer K, West J, Balada-Llasat J, Pancholi P, Stevenson K, Goff D. An antimicrobial stewardship program’s impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis. 2010;51(9):1074-1080. doi: 10.1086/656623 [DOI] [PubMed] [Google Scholar]
- 5. Beganovic M, McCreary EK, Mahoney MV, Dionne B, Green DA, Timbrook TT. Interplay between rapid diagnostic tests and antimicrobial stewardship programs among patients with bloodstream and other severe infections. J Appl Lab Med. 2019;3(4):601-616. doi: 10.1373/jalm.2018.026450 [DOI] [PubMed] [Google Scholar]
- 6. Box MJ, Sullivan EL, Ortwine KN, et al. Outcomes of rapid identification for Gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy. 2015;35(3):269-276. doi: 10.1002/phar.1557 [DOI] [PubMed] [Google Scholar]
- 7. Porter AM, Bland CM, Young HN, et al. Comparison of pharmacist-directed management of multiplex PCR blood culture results with conventional microbiology methods on effective and optimal therapy within a community hospital. Antimicrob Agents Chemother. 2019;63(1):e01575-18. doi: 10.1128/AAC.01575-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beganovic M, Timbrook TT, Wieczorkiewicz SM. Predictors of time to effective and optimal antimicrobial therapy in patients with positive blood cultures identified via molecular rapid diagnostic testing. Open Forum Infect Dis. 2019;6(1):ofy350. doi: 10.1093/ofid/ofy350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080. doi: 10.1093/cid/civ447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cairns KA, Doyle JS, Trevillyan JM, et al. The impact of a multidisciplinary antimicrobial stewardship team on the timeliness of antimicrobial therapy in patients with positive blood cultures: a randomized controlled trial. J Antimicrob Chemother. 2016;71(11):3276-3283. doi: 10.1093/jac/dkw285 [DOI] [PubMed] [Google Scholar]
- 11. Buss BA, Baures TJ, Yoo M, et al. Impact of a multiplex PCR assay for bloodstream infections with and without antimicrobial stewardship intervention at a cancer hospital. Open Forum Infect Dis. 2018;5(10):ofy258. doi: 10.1093/ofid/ofy258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hpx-10.1177_00185787211037554 for No Implementation Without Representation: Real-Time Pharmacist Intervention Optimizes Rapid Diagnostic Tests for Bacteremia at a Small Community Hospital by Brandon J. Tritle, Robert Watteyne, Abby Hickman, Todd J. Vento, Bert K. Lopansri, Dave S. Collingridge and John J. Veillette in Hospital Pharmacy