Abstract
Background
Internationally, diabetes mellitus is recognised as a risk factor for severe COVID-19. The relationship between diabetes mellitus and severe COVID-19 has not been reported in the Australian population.
Objective
The objective of this study was to determine the prevalence of and outcomes for patients with diabetes admitted to Australian intensive care units (ICUs) with COVID-19.
Methods
This is a nested cohort study of four ICUs in Melbourne participating in the Short Period Incidence Study of Severe Acute Respiratory Infection (SPRINT-SARI) Australia project. All adult patients admitted to the ICU with COVID-19 from 20 February 2020 to 27 February 2021 were included. Blood glucose and glycated haemoglobin (HbA1c) data were retrospectively collected. Diabetes was diagnosed from medical history or an HbA1c ≥6.5% (48 mmol/mol). Hospital mortality was assessed using logistic regression.
Results
There were 136 patients with median age 58 years [48–68] and median Acute Physiology and Chronic Health Evaluation II (APACHE II) score of 14 [11–19]. Fifty-eight patients had diabetes (43%), 46 patients had stress-induced hyperglycaemia (34%), and 32 patients had normoglycaemia (23%). Patients with diabetes were older, were with higher APACHE II scores, had greater glycaemic variability than patients with normoglycaemia, and had longer hospital length of stay. Overall hospital mortality was 16% (22/136), including nine patients with diabetes, nine patients with stress-induced hyperglycaemia, and two patients with normoglycaemia.
Conclusion
Diabetes is prevalent in patients admitted to Australian ICUs with severe COVID-19, highlighting the need for prevention strategies in this vulnerable population.
Keywords: COVID-19, Australia, Diabetes, Stress hyperglycaemia
1. Introduction
During previous outbreaks of highly transmissible respiratory viral infections, including severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and the H1N1 influenza virus, a greater risk of infection was observed in people with diabetes.1 This is not the case for coronavirus disease 2019 (COVID-19), with similar rates of diabetes in patients with COVID-19 compared to the general population.2 Despite not increasing the risk of infection, diabetes is strongly associated with progression to severe COVID-19 and death. Worldwide, approximately one-quarter of adults with severe COVID-19 have pre-existing diabetes,3 substantially greater than the global prevalence of <10%.4 Moreover, pre-existing diabetes is associated with nearly double the risk of death from severe COVID-19 in international meta-analyses.5
Hyperglycaemia in severe COVID-19 also occurs frequently in patients with previously normal glucose tolerance—so-called stress-induced hyperglycaemia.1 In addition to the known metabolic effects of critical illness, COVID-19 is thought to be particularly deleterious for glycaemic control, due to direct effects of the virus increasing insulin resistance and impairing insulin secretion.5 , 6 This is further exacerbated by corticosteroid treatment which has become a standard of care for critically ill patients requiring supplemental oxygen and/or mechanical ventilation.7 The distinction between stress-induced hyperglycaemia and the chronic hyperglycaemia attributed to diabetes is likely important, as the presence of chronic hyperglycaemia is known to attenuate the association between acute hyperglycaemia and mortality in a general critically ill population.8
There is a paucity of data assessing the interaction between acute and chronic hyperglycaemia and its impact on mortality in severe COVID-19. Moreover, previous meta-analyses demonstrating the association between diabetes and a worse prognosis have failed to include Australian data.2 , 9 This is important as the 13% mortality rate for severe COVID-19 in Australia10 is substantially lower than that reported globally, with the pooled estimate being 28.1% (95% confidence interval [CI], 23.4–33.0).3
The Short Period Incidence Study of Severe Acute Respiratory Infections (SPRINT-SARI) Australia study10 has been prospectively collecting comprehensive data on critically ill patients with COVID-19 admitted to Australian intensive care units (ICUs) from February 2020. Within the SPRINT-SARI case report form, diabetes status is recorded as well as peak daily blood glucose. We used a nested cohort within the SPRINT-SARI Australia database to determine the clinical characteristics and outcomes for patients with diabetes and stress-induced hyperglycaemia. Our primary hypothesis was that clinical outcomes would be inferior in those patients with diabetes, independent of age and admission illness severity.
2. Materials and methods
We conducted a nested cohort study within a multicentre national registry following the recommendations of the STROBE Statement.11 Ethics approval with full consent waiver was granted under the National Mutual Acceptance scheme by the Alfred Health Human Research Ethics Committee (HREC/16/Alfred/59) or by specific applications at individual sites.
2.1. Study design, setting, and participants
The methodology for the SPRINT-SARI Australia study has been described in detail elsewhere.10 In brief, the SPRINT-SARI Australia study prospectively collected data on all suspected and confirmed COVID-19 admissions to participating ICUs, adult and paediatric. Patients included in this nested cohort study were aged 18 years and older, had a positive polymerase chain reaction (PCR) test for COVID-19, and were admitted to one of four adult mixed medical/surgical ICUs in Melbourne, Australia: Sunshine Hospital, Footscray Hospital, Royal Melbourne Hospital, and The Alfred. Site recruitment occurred through regionally affiliated hospitals in Melbourne, Victoria. Site participation in the nested cohort study was voluntary, and sites were not selected at random. Across these four ICUs, glucose control was achieved with continuous infusions of intravenous insulin titrated according to institutional algorithms and commenced at a threshold of 9 mmol/L (1 site) or 10 mmol/L (3 sites). Patient management decisions were made by treating clinicians.
2.2. Data collection
Data pertaining to baseline demographics, diabetes status (recorded as diabetes mellitus with or without complications), clinical characteristics, treatments, and outcomes were collected prospectively and extracted from the SPRINT-SARI Australia database for patients admitted from 20 February 2020 until 27 February 2021. Across the four sites in the nested cohort, additional data were retrospectively collected on every blood glucose measurement throughout the ICU admission and glycated haemoglobin (HbA1c) if performed within 120 days of the index ICU admission.
2.3. Data definitions
Diabetes mellitus was defined as any patient recorded as having diabetes and/or an HbA1c ≥6.5% (48 mmol/mol).12 Patients with stress-induced hyperglycaemia were patients without diabetes and a random plasma glucose ≥11.1 mmol/L. While the threshold blood glucose to define stress-induced hyperglycaemia remains contentious,13 the American Diabetes Association (ADA) Diabetes in Hospitals Writing Committee Guidelines suggest random plasma glucose ≥11.1 mmol/L is appropriate for use in hospitalised patients.12 Given the majority of critically ill patients receive continuous enteral nutrition,14 we chose to use this threshold prior to reviewing available data. Normoglycaemic patients were those without stress-induced hyperglycaemia or diabetes. Glycaemic variability was analysed as the standard deviation (SD) and the coefficient of variation (SD/mean ×100%) of blood glucose values.15 Moderate hypoglycaemia was defined as any blood glucose ≤4.0 mmol/L and severe hypoglycaemia as a subset with any blood glucose ≤2.2 mmol/L.12
2.4. Statistical analyses
Data are presented as frequencies and proportions for categorical variables and mean (SD) or median [interquartile range] for continuous variables. Proportions were compared using the χ2 or Fisher's exact test. Between-group comparisons were performed by t-test, Wilcoxon rank-sum test, or Kruskal–Wallis test as indicated. Univariate logistic regression was used to assess factors associated with the primary outcome, hospital mortality. The relationship between glycaemia status and hospital outcome was assessed with univariate and multivariable logistic regression, with adjustment for age and severity of illness (as the Acute Physiology and Chronic Health Evaluation II [APACHE II] score) planned a priori. Analysis of glycaemic variability by time period was designated a priori as before and after the online pre-publication of the RECOVERY trial data on the 16th of June 2020.7 Continuous coefficients of glucose variation were analysed using linear regression with the post-RECOVERY time period as a binary indicator variable. Binary glycaemic outcomes were analysed by logistic regression and are presented as the odds ratio, with 95% CI and corresponding P-value. ICU and hospital lengths of stay were analysed by competing risks regression as per Fine and Gray,16 with death as the competing event and estimates presented as the respective sub-hazard ratio (SHR) and corresponding 95% CI. All regression analyses were performed employing robust standard errors to allow for within ICU correlation. Analyses were performed using Stata, version 16.1 (StataCorp, College Station, Texas, United States).
3. Results
Across Australia, there were 503 patients with confirmed COVID-19 admitted to 54 ICUs during the study period, of which 136 (27%) were admitted to one of the four hospitals within the nested cohort; of these, 40% (55/136) were female, with a median [interquartile range] age of 58 [48–68] years. There were 57 patients (42%) who had an available HbA1c. The median APACHE II score was 14 [11–19], and 62% (85/136) of patients required mechanical ventilation for a median of 10 [4–18] days. The median ICU length of stay was 7 [3–16.5] days, and overall hospital mortality was 16% (22/136) with complete data on all patients. The characteristics and daily processes of care are presented in Table 1 .
Table 1.
Patient characteristics and processes of care by glycaemic category.
| Covariate name | Normoglycaemia | Stress-induced hyperglycaemia | Diabetes | P-value |
|---|---|---|---|---|
| Patients, n (%) | 32 (24) | 46 (34) | 58 (43) | – |
| Age (years), median [IQR] | 48 [39.5, 61.5] | 59.5 [49, 67] | 61 [56, 70] | 0.004 |
| Gender (male), n (%) | 17 (53) | 29 (63) | 35 (60) | 0.671 |
| APACHE II score, median [IQR] | 12 [6.5, 15] | 15.5 [11, 20] | 15 [12, 20] | 0.004 |
| Chronic health conditions, n (%) | ||||
| Cardiac disease (exc. hypertension) | 3 (9.4) | 7 (15) | 10 (17) | 0.644 |
| Obesity (per clinical staff) | 8 (25) | 10 (22) | 26 (45) | 0.026 |
| Pulmonary disease (not asthma) | 0 (0) | 4 (8.7) | 4 (6.9) | 0.265 |
| Asthma (physician diagnosed) | 5 (16) | 7 (15) | 4 (6.9) | 0.299 |
| Kidney disease | 0 (0) | 2 (4.3) | 7 (12) | 0.079 |
| Moderate or severe liver disease | 1 (3.1) | 1 (2.2) | 2 (3.4) | 1.000 |
| Malignant neoplasm | 1 (3.1) | 1 (2.2) | 6 (10) | 0.210 |
| Haematologic disease | 2 (6.3) | 3 (6.5) | 2 (3.4) | 0.692 |
| Immunosuppression | 3 (9.4) | 6 (13) | 4 (6.9) | 0.525 |
| Proportion of study days (%), median [IQR] | ||||
| Vasopressors | 0 [0, 15.9] | 80 [45, 95] | 74 [0, 96] | <0.001 |
| Invasive mechanical ventilation | 0 [0, 0] | 53 [25, 75] | 43 [0, 67] | <0.001 |
| Interventions on any study day, n (%) | ||||
| Vasopressors | 6 (19) | 37 (80) | 36 (62) | <0.001 |
| Nasal high flow | 22 (69) | 33 (72) | 39 (67) | 0.884 |
| Noninvasive ventilation | 4 (13) | 12 (26) | 7 (12) | 0.140 |
| Invasive mechanical ventilation | 8 (25) | 40 (87) | 37 (64) | <0.001 |
| Prone ventilation | 3 (9.4) | 17 (37) | 18 (31) | 0.016 |
| Nitric oxide therapy | 1 (3.1) | 6 (13) | 0 (0) | 0.005 |
| ECMO | 0 (0) | 8 (18) | 4 (6.9) | 0.026 |
| Percutaneous tracheostomy | 0 (0) | 9 (20) | 9 (16) | 0.015 |
| Renal replacement therapy | 1 (3.1) | 7 (15) | 9 (15.516) | 0.169 |
| Study days, median [IQR] | 3 [2, 4.5] | 10 [5, 18] | 9 [4, 22] | <0.001 |
| LOS – ICU days, median [IQR] | 2.0 [1.3, 3.7] | 9.1 [3.3, 20.8] | 6.9 [2.8, 20.8] | <0.001 |
| LOS – hospital days, median [IQR] | 7.1 [5.0, 14.1] | 13.7 [7.4, 30.9] | 14.9 [9.0, 25.1] | 0.004 |
| Died in ICU, n (%) | 1 (3.1) | 9 (20) | 9 (16) | 0.092 |
| Died in hospital, n (%) | 2 (6.3) | 10 (22) | 10 (17) | 0.163 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; ECMO: Extra-Corporeal Membrane Oxygenation; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay.
3.1. Glucose control by category of glycaemia
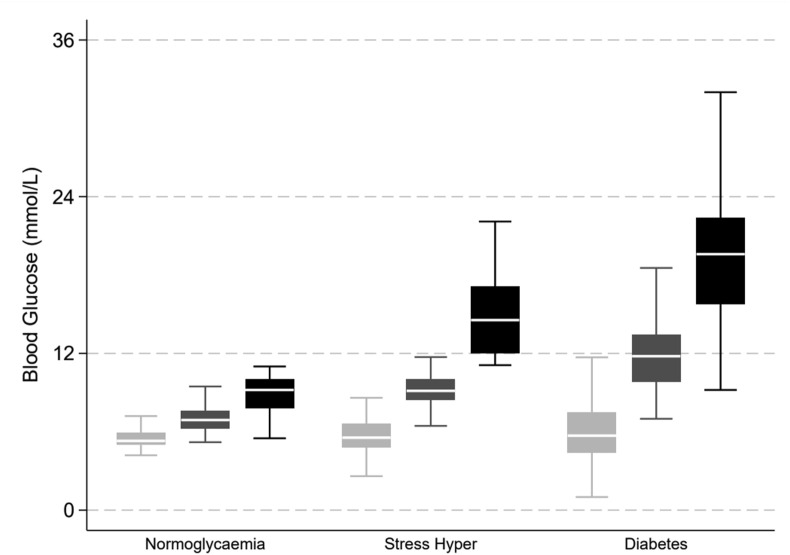
In total, 58 (43%) had diabetes, 46 (34%) had stress-induced hyperglycaemia, and 32 (23%) patients had normoglycaemia. Six (11%) patients were defined as having diabetes by HbA1c criteria alone. There were 11,375 blood glucose measurements recorded with a median of 42 [14–113] measurements per patient. Patients with diabetes had greater mean and maximum glucose concentrations than patients with stress-induced hyperglycaemia or normoglycaemia (Fig. 1 , and Table 2 ). Hypoglycaemia occurred infrequently, with 11 (8.1%) patients recording one or more episodes of moderate hypoglycaemia (≤4.0 mmol/L) and 2 (1.5%) patients with diabetes recording episodes of severe hypoglycaemia (≤2.2 mmol/L) (Table 2). Glycaemic variability was greater among patients with diabetes than among patients with stress-induced hyperglycaemia and normoglycaemia (Table 2).
Figure 1.
Glucose control by category of glycaemia. Light grey = minimum, medium grey = mean, black = maximum blood glucose values. Boxes are median and interquartile range (IQR), with whiskers at 1.5 times the interquartile range; outliers are omitted.
Table 2.
Glucose control by glycaemic category.
| Covariate name | Normoglycaemia | Stress-induced hyperglycaemia | Diabetes | P-value |
|---|---|---|---|---|
| All daily blood glucose measurements, median [IQR] | ||||
| Patients with glucose data | 31 | 46 | 57 | – |
| Tests performed, No. | 12 [2, 21] | 64.5 [28, 146] | 72 [22, 152] | <0.001 |
| Median plasma glucose (mmol/L) | 6.9 [6.3, 7.6] | 9.1 [8.4, 10.0] | 11.8 [9.8, 13.4] | <0.001 |
| Standard deviation | 1.07 [0.85, 1.38] | 1.91 [1.63, 2.39] | 2.77 [2.02, 3.63] | <0.001 |
| Coefficient of variation | 0.15 [0.12, 0.19] | 0.22 [0.18, 0.25] | 0.24 [0.19, 0.30] | <0.001 |
| Minimum | 5.3 [5.0, 5.9] | 5.6 [4.8, 6.6] | 5.7 [4.4, 7.5] | 0.620 |
| Maximum | 9.2 [7.8, 10.0] | 14.6 [12.0, 17.1] | 19.6 [15.8, 22.4] | <0.001 |
| Blood glucose events (number of patients), n (%) | ||||
| >11.0 mmol/L | 0 (0) | 46 (100) | 53 (93) | <0.001 |
| <4.0 mmol/L | 0 (0) | 5 (11) | 6 (11) | 0.347 |
| <2.2 mmol/L | 0 (0) | 0 (0) | 2 (3.6) | 0.691 |
IQR: interquartile range.
3.2. Relationship between glycaemia and outcome
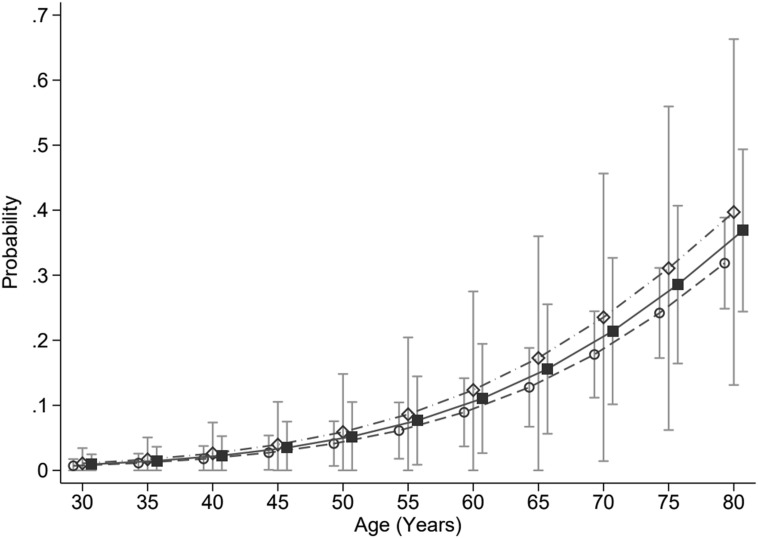
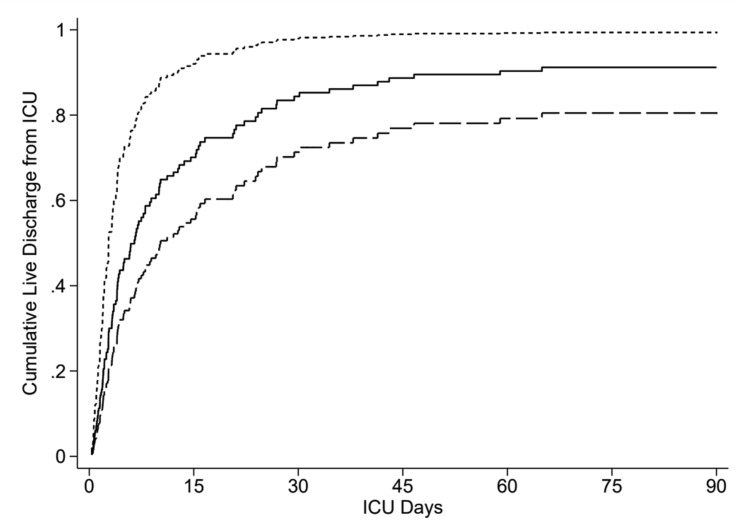
Two of 32 (6.3%) patients with normoglycaemia, 10 of 46 (22%) patients with stress-induced hyperglycaemia, and 10 of 58 (17%) patients with diabetes died in hospital. Normoglycaemic patients were younger and had lower APACHE II scores than patients with stress-induced hyperglycaemia or diabetes (Table 1). After adjusting for the covariates of age and severity of illness (APACHE II), there was no association between acute or chronic hyperglycaemia and hospital mortality, stress-induced hyperglycaemia (OR, 1.61 [95% CI, 0.51–5.13]; P = 0.42), and diabetes (OR, 1.37 [95% CI, 0.52–3.61]; P = 0.53) (Fig. 2 ). ICU length of stay differed markedly across glycaemia groups (Table 2). Under a competing risks regression model, adjusted for age and APACHE2 score, the SHRs for discharge alive from the ICU compared with the normoglycaemia group were: stress induced hyperglycaemia 0.32 (0.20, 0.51), P < 0.001, and diabetes 0.48 (0.36, 0.64), P < 0.001 (Fig. 3 ).
Figure 2.
Adjusted probability of death in hospital by category of glycaemia over age, modelled at the median APACHE II score. Open circles = normoglycaemia, open diamonds = stress-induced hyperglycaemia, solid squares = diabetes mellitus, with error bars the 95% confidence interval for the predicted probability. APACHE: Acute Physiology and Chronic Health Evaluation II.
Figure 3.
Cumulative incidence plots for ICU discharge, plotted by group and at the median values of age and APACHE II score. Normoglycaemia (short dash, uppermost curve), stress-induced hyperglycaemia (long dash, lowermost curve), and diabetes (solid line, middle curve). APACHE: Acute Physiology and Chronic Health Evaluation II; ICU: intensive care unit.
3.3. Effect of routine corticosteroid use on glycaemic control
Prior to 16 June 2020, nine of 19 (47%) patients were administered corticosteroids compared with 107 of 115 (93%) after administration (P < 0.001). Post 16 June 2020, mean blood glucose increased by 1.8 (0.44, 3.19) mmol/L (P = 0.01) and the maximum blood glucose, by 2.8 (0.03, 5.6) mmol/L (P = 0.05); glycaemic variability also increased, with the SD increasing by 0.68 (0.07, 1.29) mmol/L (P = 0.03), albeit without a significant difference in the coefficient of variation (3.05 [−1.29, 7.40], P = 0.17).
4. Discussion
Dysglycaemia was prevalent in patients with severe COVID-19 admitted to Australian adult ICUs. Nearly half of patients had pre-existing diabetes, and another third had stress-induced hyperglycaemia. Routine use of corticosteroids resulted in greater perturbations in glycaemic control with higher mean and peak blood glucose levels and increased glycaemic variability, suggesting that pre-existing insulin protocols were inadequate for these patients. Patients with dysglycaemia had markedly longer ICU and hospital admissions.
The prevalence of diabetes reported in this nested cohort of severe COVID-19 is approximately five times higher than the prevalence in the Australian population (7.4%).17 Moreover, it is nearly double the 22% prevalence of recognised diabetes reported in the Australian critically ill population in the pre-COVID-19 era.8 With this high prevalence of patients with chronic hyperglycaemia, the prevalence of stress-induced hyperglycaemia in our cohort is comparatively lower than the 50% reported in the pre-COVID-19 era.8 The prevalence of hypoglycaemia is similar to historical rates.18 It is likely that there are epidemiological and pathophysiological changes driving worse outcomes for patients with diabetes and COVID-19. It is well recognised that age is strongly associated with worse outcomes in COVID-19 and the prevalence of diabetes increases with age in both the general population and in patients with COVID-19.1 Due to the syndromic nature of the disease, diabetes is associated with hypertension, obesity, and cardiovascular disease. Moreover, older patients with diabetes are more likely to have had a longer exposure to dysglycaemia with a greater prevalence of microvascular and macrovascular complications.19 Several observational studies have reported a greater prevalence of hypertension, cardiovascular disease, cerebrovascular disease, and chronic kidney disease in patients with COVID-19 with diabetes, comorbidities independently associated with worse outcomes.20 In addition to these epidemiological hallmarks of severe disease, it is likely that diabetes is an independent determinant of severe COVID-19. Putative mechanisms include diabetes-induced dysregulated immune responses, altered expression of Renin–Angiotensin–Aldosterone-System effectors, and hyperglycaemia-induced endothelial dysfunction.5
While there is significant geographical heterogeneity in the reported prevalence of diabetes among patients admitted to intensive care with COVID-19, recent large national cohort studies have consistently reported an increased mortality in this population (Italy, n = 3,988, 12.9% with diabetes, adjusted OR, 1.18 [95% CI 1.01, 1.39]; US, n = 2,215, 38.9% with diabetes, adjusted OR, 1.14 [95% CI 0.91–1.43]; UK, n = 19,256, 18.3% with diabetes, adjusted HR, 1.23 [95% CI, 1.14–1.32][21], [22], [23]). For Australian patients with diabetes and severe COVID-19, we report a mortality point estimate consistent with these international data, albeit with wide CIs (adjusted OR, 1.37 [95% CI, 0.52–3.61]). We also report a point estimate favouring increased mortality in patients with COVID-19 with stress-induced hyperglycaemia (adjusted OR, 1.61 [95% CI, 0.51–5.13]). While the 95% CIs are wide, the point estimate is consistent with the recognised association between stress-induced hyperglycaemia and mortality in a non-COVID critically ill population8 and severe COVID-19.24 , 25
In our population, the mortality rate for patients with diabetes was 17% (10/58), markedly lower than reported in Italy, 40% (328/814), the USA, 37% (509/1370), and the UK, 35% (1223/3524).[21], [22], [23] The lower the mortality for patients with diabetes parallels, the higher the survivorship of severe COVID-19 in Australia overall.10 Together with the lower sample size, this contributed to our study being underpowered to detect a mortality difference.
Our results have important implications for the management of patients with diabetes in Australia during the current COVID-19 pandemic. First, since diabetes is overrepresented among patients with severe COVID-19 requiring ICU admission, strategies to prevent COVID-19 in patients with diabetes remain important. Second, even in a nonoverwhelmed critical care setting with the ability to provide vigilant glucose monitoring with high nurse-to-patient ratios, the routine use of dexamethasone worsens glycaemic control and requires vigilant monitoring. Whether this has implications for dexamethasone use in critically ill COVID-19 patients, and/or their prognosis, requires further study.
Strengths of our study include that it was performed using data from a national database in which data collection was performed by experienced research staff using a standardised case report form. The follow-up rate was high with complete data for the primary outcome of hospital mortality. This yielded novel data on metrics of dysglycaemia including glycaemic variability and hypoglycaemia.
There are, however, important limitations. Firstly, as outlined earlier, the small sample size and low mortality rate resulted in the study being an inadequate sample to detect a mortality difference between groups if one truly existed. Secondly, the nested cohort of four hospitals in Melbourne captured 27% of Australian ICU admissions and may not be representative of the national experience with COVID-19. Thirdly, a recent HbA1c was only available in 42% of the cohort, resulting in an additional 11% being classified as having unrecognised diabetes. There may be additional cases with diabetes misclassified as stress-induced hyperglycaemia and the true prevalence of diabetes is likely higher. Observational data in the pre-COVID era would suggest the prevalence of unrecognised diabetes in an adult critically ill population is at least 5%.8 Fourthly, COVID variant was not reliably analysed and provided from local pathology databases; thus, we are unable to comment on variant specific differences. Fifthly, while vaccination status was not reported, by virtue of the timing of the capture period, all of the patients were unvaccinated and further studies are indicated to assess how interactions between diabetes status and outcome are modified in the setting of widespread vaccination. Finally, clinical information on diabetes subtype, duration of diabetes, and the presence of complications was not available and we lacked data to assess the impact of diabetes specific risk factors including premorbid glycaemic control and glucose-lowering medications.26 Given the heterogeneity of diabetes, characterising which risk factors are most useful to identify progression to severe disease is an important area for future research.20 , 23
In summary, in a nested cohort of four adult ICUs in Australia, diabetes complicated nearly half of COVID-19 admissions and was associated with longer ICU and hospital length of stay. The high prevalence of this condition in patients with severe COVID-19 suggests this group is highly vulnerable.
Data sharing statement
Data sharing requests will be considered on an individual basis by the SPRINT-SARI Australia management committee. Requests for de-identified data are to be sent to MNHS-Sprint.Sari@monash.edu. Any such requests will require a formal data sharing agreement, and must be consistent with the Australian and New Zealand Intensive Care – Research Centre (ANZIC-RC) Data Sharing Policy, available at: https://www.monash.edu/__data/assets/pdf_file/0010/2153899/2020-03-19-ANZIC-RC-Terms-of-Reference.pdf.
Funding
SPRINT-SARI Australia is supported by funding from the Australian Department of Health (Standing Deed SON60002733).
CRediT authorship contribution statement
The manuscript was written on behalf of the SPRINT-SARI Australia Management Committee. Roles of listed authors are as follows:
Mark Plummer, Craig French, Tony Trapani, Andrew Udy, and Aidan Burrell were responsible for study conception and design, acquisition of data, and drafting the manuscript. Mark Finnis was responsible for study conception and design, statistical analysis and interpretation, and drafting the manuscript. Adam Deane was responsible was study conception and design and drafting the manuscript. Louise Rait, Tessa Broadley, Samantha Bates, James Douglas, and Mansi Bhurani were responsible for acquisition of data and drafting the manuscript.
Conflict of interest
None.
Acknowledgements
The authors would like to thank the members of the SPRINT-SARI Australia Management Committee: Aidan Burrell, Allen Cheng, Andrew Udy, Annamaria Palermo, Benjamin Reddi, Claire Reynolds, Craig French, D James Cooper, Edward Litton, Husna Begum, Lewis Campbell, Mahesh Ramanan, Mark Plummer, Richard McAllister, Simon Erickson, Tessa Broadley, Tony Trapani, and Winston Cheung. The authors would like to thank the SPRINT-SARI Australia investigators: Adam Visser, Adrian Mattke, Adrian Regli, Alan Rashid, Alexis Tabah, Alison Walker, Allen Cheng, Amanda Corley, Andrew Udy, Anil Ramnani, Anthony Eidan, Bart DeKeulenaer, Benjamin Reddi, Brent Richards, Cameron Knott, Cara Moore, Carmel Delzoppo, Catherine Boschert, Catherine Tacon, Craig French, Danielle Austin, David Brewster, David Cooper, David Crosbie, David Hawkins, Edda Jessen, Eduardo Martinez, Edward Fysh, Edward Litton, Felix Oberender, Forbes McGain, Gavin Salt, Glenn Eastwood, Gopal Taori, Hayden White, Hergen Buscher, Ian Seppelt, Isabel Anne Leditschke, Janelle Young, Jayshree Lavana, Jeremy Cohen, Jessica Lugsdin, John Botha, John Santamaria, Jonathan Barrett, Kasha Singh, Kevin Laupland, Khaled El-Khawas, Kristine Estensen, Kush Deshpande, Kyle White, Leigh Fitzpatrick, Lewis Campbell, Mahesh Ramanan, Manoj Saxena, Marion Kainer, Mark Kol, Mark Page, Mark Plummer, Martin Sterba, Matthew Anstey, Matthew Brain, Matthew Maiden, Myrene Kilminster, Naomi Hammond, Neeraj Bhadange, Nicole Humphreys, Paras Jain, Paul Azzi, Paul Secombe, Paula Lister, Peter Chan, Peter McCanny, Phillip Britton, Pierre Janin, Ravi Krishnamurthy, Ravikiran Sonawane, Ravindranath Tiruvoipati, Richard Totaro, Rinaldo Bellomo, Ritesh Sanghavi, Samantha Bates, Sandra Peake, Shailesh Bihari, Shane George, Simon Erickson, Steve Webb, Subhash Arora, Subodh Ganu, Thomas Rozen, Toni McKenna, Umesh Kadam, Vineet Nayyar, Wei Han Choy, and Wisam Albassam. The authors would also like to thank members performing data entry at the nested cohort sites, Rebecca Morgan, Lalita Prasad, Laloma Carstens, Samantha Bates, Louise Rait, and and Mansi Bhurani. The authors would also like to acknowledge the SPRINT-SARI Australia participating sites: Albury Wodonga Health, Alice Springs Hospital, Angliss Hospital, Austin Hospital, Ballarat Base Hospital, Bankstown-Lidcombe Hospital, Barwon Health, Bendigo Hospital, Box Hill Hospital, Bunbury Hospital, Bundaberg Hospital, Caboolture Hospital, Cabrini Hospital Malvern, Cairns Hospital, Calvary Mater Newcastle, Campbelltown Hospital, Canberra Hospital, Concord Hospital, Dandenong Hospital, Epworth Richmond, Fiona Stanley Hospital, Flinders Medical Centre, Footscray Hospital, Frankston Hospital, Gold Coast University Hospital, Hervey Bay Hospital, Ipswich Hospital, John Hunter Hospital, Joondalup Health Campus, Launceston General Hospital, Lismore Base Hospital, Liverpool Hospital, Logan Hospital, Lyell McEwan Hospital, Maroondah Hospital, Mater Hospital Brisbane, Mildura Base Hospital, Monash Children's Hospital, Monash Medical Centre, Nepean Hospital, Northeast Health Wangaratta, Northern Hospital, Perth Children's Hospital, Port Macquarie Base Hospital, Prince of Wales Hospital, Princess Alexandra Hospital, Queensland Children's Hospital, Redcliffe Hospital, Rockingham Hospital, Royal Adelaide Hospital, Royal Brisbane and Women's Hospital, Royal Children's Hospital, Royal Darwin Hospital, Royal Hobart Hospital, Royal Melbourne Hospital, Royal North Shore Hospital, Royal Perth Hospital, Royal Prince Alfred Hospital, Sir Charles Gairdner Hospital, St George Hospital, St John of God Hospital Midland, St John of God Hospital Murdoch, St Vincent's Hospital Melbourne, St. Vincent's Hospital Sydney, Sunshine Coast University Hospital, Sunshine Hospital, Sydney Children's Hospital Randwick, The Alfred Hospital, The Children's Hospital at Westmead, The Prince Charles Hospital, The Queen Elizabeth Hospital, Toowoomba Hospital, Warrnambool Base Hospital, Werribee Mercy Hospital, Westmead Hospital, Wollongong Hospital, and Women's and Children's Hospital Adelaide.
Contributor Information
SPRINT-SARI Australia Investigators:
Adam Visser, Adrian Mattke, Adrian Regli, Alan Rashid, Alexis Tabah, Alison Walker, Allen Cheng, Amanda Corley, Andrew Udy, Anil Ramnani, Anthony Eidan, Bart DeKeulenaer, Benjamin Reddi, Brent Richards, Cameron Knott, Cara Moore, Carmel Delzoppo, Catherine Boschert, Catherine Tacon, Craig French, Danielle Austin, David Brewster, David Cooper, David Crosbie, David Hawkins, Edda Jessen, Eduardo Martinez, Edward Fysh, Edward Litton, Felix Oberender, Forbes McGain, Gavin Salt, Glenn Eastwood, Gopal Taori, Hayden White, Hergen Buscher, Ian Seppelt, Isabel Anne Leditschke, Janelle Young, Jayshree Lavana, Jeremy Cohen, Jessica Lugsdin, John Botha, John Santamaria, Jonathan Barrett, Kasha Singh, Kevin Laupland, Khaled El-Khawas, Kristine Estensen, Kush Deshpande, Kyle White, Leigh Fitzpatrick, Lewis Campbell, Mahesh Ramanan, Manoj Saxena, Marion Kainer, Mark Kol, Mark Page, Mark Plummer, Martin Sterba, Matthew Anstey, Matthew Brain, Matthew Maiden, Myrene Kilminster, Naomi Hammond, Neeraj Bhadange, Nicole Humphreys, Paras Jain, Paul Azzi, Paul Secombe, Paula Lister, Peter Chan, Peter McCanny, Phillip Britton, Pierre Janin, Ravi Krishnamurthy, Ravikiran Sonawane, Ravindranath Tiruvoipati, Richard Totaro, Rinaldo Bellomo, Ritesh Sanghavi, Samantha Bates, Sandra Peake, Shailesh Bihari, Shane George, Simon Erickson, Steve Webb, Subhash Arora, Subodh Ganu, Thomas Rozen, Toni McKenna, Umesh Kadam, Vineet Nayyar, Wei Han Choy, and Wisam Albassam
References
- 1.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan E., Song J., Deane A.M., Plummer M.P. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest. 2021;159(2):524–536. doi: 10.1016/j.chest.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disease GBD, Injury I, Prevalence C Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts J., Pritchard A.L., Treweeke A.T., Rossi A.G., Brace N., Cahill P., et al. Why is COVID-19 more severe in patients with diabetes? The role of angiotensin-converting enzyme 2, endothelial dysfunction and the immunoinflammatory system. Front Cardiovasc Med. 2020;7:629933. doi: 10.3389/fcvm.2020.629933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C.T., Lidsky P.V., Xiao Y., Lee I.T., Cheng R., Nakayama T., et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 2021;33(8):1565–15676 e5. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer M.P., Bellomo R., Cousins C.E., Annink C.E., Sundararajan K., Reddi B.A., et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014;40(7):973–980. doi: 10.1007/s00134-014-3287-7. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrell A.J., Pellegrini B., Salimi F., Begum H., Broadley T., Campbell L.T., et al. Outcomes for patients with COVID-19 admitted to Australian intensive care units during the first four months of the pandemic. Med J Aust. 2021;214(1):23–30. doi: 10.5694/mja2.50883. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 12.Clement S., Braithwaite S.S., Magee M.F., Ahmann A., Smith E.P., Schafer R.G., et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 13.Finfer S., Wernerman J., Preiser J.C., Cass T., Desaive T., Hovorka R., et al. Clinical review: consensus recommendations on measurement of blood glucose and reporting glycemic control in critically ill adults. Crit Care. 2013;17(3):229. doi: 10.1186/cc12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane A.M., Horowitz M. Dysglycaemia in the critically ill – significance and management. Diabetes Obes Metab. 2013;15(9):792–801. doi: 10.1111/dom.12078. [DOI] [PubMed] [Google Scholar]
- 15.Krinsley J.S. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 16.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Dunstan D.W., Zimmet P.Z., Welborn T.A., De Courten M.P., Cameron A.J., Sicree R.A., et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25(5):829–834. doi: 10.2337/diacare.25.5.829. [DOI] [PubMed] [Google Scholar]
- 18.Krinsley J.S., Egi M., Kiss A., Devendra A.N., Schuetz P., Maurer P.M., et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. 2013;17(2):R37. doi: 10.1186/cc12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 20.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis J.M., Mateen B.A., Sonabend R., Thomas N.J., Patel K.A., Hattersley A.T., et al. Type 2 diabetes and COVID-19-related mortality in the critical care setting: a national cohort study in England, March-July 2020. Diabetes Care. 2021;44(1):50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]