Abstract
Background
COVID-19 and lysosomal storage disorders (LSDs) share a common immunological pathway as they cause the release of cytokines in a similar pattern. We aimed to evaluate the immunity status and reveal the course of COVID-19 in patients with LSDs.
Results
The median age of 110 patients with LSDs was 129 months (range: 21–655), and all but one patient with mucopolysaccharidosis (MPS) type III were regularly receiving enzyme replacement therapy (ERT). In 53.6% (n = 56) of the patients (23 patients with Gaucher disease [10 type III, 13 type I], 26 patients with MPS [8 type VI, 11 type IVA, 1 type III, 3 type II, and 3 type I], and 7 patients with Pompe disease), an abnormality in at least one of the autoimmunity or immunodeficiency parameters was reported. Furthermore, 12 (57%) of 21 Gaucher cases (7 type III, 5 type I), 18 (40.9%) of 44 MPS cases (9 type IVA, 5 type VI, 1 type I, 2 type II, and 1 type III), and six (66%) of nine Pompe cases were reported to involve abnormalities in at least one of the parameters related to immunodeficiency. Immunoglobulin (Ig) M and IgA levels were reported to be lower, and there were abnormalities in the lymphocyte counts and subgroups in the MPS group. ANA was reported to be positive in one patient with Gaucher type III, anti-DNA in two patients with Gaucher type I and one patient with MPS type VI, antithyroglobulin in two patients with Gaucher type I, anti-TPO in one patient with Gaucher type I, TRAB in one patient with Gaucher type I, antiphospholipid IgM in three patients with Gaucher type III and one patient with Gaucher type I, anticardiolipin IgM in one patient with Gaucher type I, one patient with Gaucher type III, and one patient with MPS type II. However, no clinical presentation was consistent with the laboratory results except for one patient with Gaucher type I disease with Hashimoto thyroiditis. Two of the four patients who survived the COVID-19 infection with mild symptoms had a diagnosis of Gaucher type I, and no abnormality was detected in their laboratory tests. The other two patients had a diagnosis of MPS types VI and II. Immune dysfunction was detected in the patient with a diagnosis of MPS type II. Four of our patients were discharged without any sequelae.
Conclusion
Problems with immunity did not cause any noticeable clinical results. Being well protected by reducing social contact might have played a role. However, we believe that it should be borne in mind that cardiac and pulmonary involvement, as well as immune dysfunction in LSDs, may cause an increased need for intensive care because of secondary bacterial infections.
Keywords: Immunodeficiency, Autoimmunity, COVID-19, MPS, Gaucher, Pompe
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the COVID-19 pandemic, was first detected in Wuhan, China, on December 31, 2019 [1]. Since it emerged, the disease has led to high mortality and morbidity in certain groups of people, while causing milder symptoms in others, a matter that has not been clarified yet. While respiratory viral infections are usually more common and severe in children, it has been surprisingly reported that COVID-19 has a course with milder symptoms and a low mortality rate in children. The incidence of asymptomatic infections is higher in children [2]. It is well known that viral infections cause severe presentations and are associated with a worse prognosis in immunocompromised patients; however, surprisingly, SARS-CoV-2 has not led to severe symptoms in immunocompromised children [3]. The explanations for this phenomenon include age-specific mechanisms such as higher counts of regulatory B and T cells in children and their involvement in the immune response, resulting in a lower inflammatory immune response, higher ACE-2 receptor expression in type 2 lung pneumocytes, and plasmablasts generated in response to SARS-CoV-2; furthermore, not smoking and a lower prevalence of comorbid diseases also play a role [3]. The initial course of COVID-19 includes symptoms of fever and cough, similar to SARS 2003; however, the immune system-induced cytokine storm that comes into play in the subsequent phase results in a rapid deterioration. It has been reported that increased cytokines in patients are directed to the lungs, and the increased cytokine attack because of the inflammatory response cannot be stopped. Inflammation is triggered to restore postinfection homeostasis in severe COVID-19 cases. Lymphopenia develops as a result of the sequestration of the hyperactivated T cells in the lung. Activation of immunity can be considered a double-edged sword [4], and thus lymphocytopenia is associated with a poor prognosis in COVID-19 infection [5]. Moreover, susceptibility to COVID-19 infection develops in the presence of cardiovascular disease, which worsens the clinical presentation [6].
SARS-CoV-2 uses the lysosomal endosomal system to infect the cells [7]. Healthy lysosomes play a fundamental role in the normal host response, maintenance of normal inflammatory response, autophagy, and regulation of sphingolipid metabolism [8]. In lysosomal storage disorders (LSDs), dysfunctional lysosomes and substrate accumulation are responsible for activating the inflammatory pathway lead to impaired autophagy, hyper-inflammation, and decreased infection control. LSDs are a heterogeneous group of diseases with immune hyperactivity because of secondary immune dysfunction. Mutations in different genes affect the lysosomal pathway creating the pathophysiology of LSD [9]. It is not clear whether the patients in this group are at extra risk during COVID-19 infection. It is shown that an increase in cellular cholesterol blocks SARS-CoV-2. The plasma entry and cholesterol biosynthesis pathway and identifying host genes guide comprehending the landscape and course of SARS-CoV-2 pathogenicity need to be elucidated [10]. Lysosomes are essential factors in maintaining inflammation and immunity. Autophagy is a crucial key factor for normal homeostasis and is triggered by stress, especially when exposed to toxic molecules [9,11]. Defective autophagy occurs in LSDs, particularly in sphingolipid metabolism. Therefore, autophagy and mitochondrial metabolism are important factors to understand the pathophysiology of LSDs. Impaired autophagy is often associated with the primary alteration of lysosomal function in LSDs. Lysosomes are essential for maintaining neuronal life.
Gaucher disease is an LSD with an autosomal recessive (AR) inheritance pattern and three subtypes that occur due to beta-glucosidase enzyme deficiency. There are insufficient data to clarify whether patients with Gaucher disease are at risk for infection. Analyses of macrophages from patients with Gaucher disease demonstrated an increased secretion of inflammatory cytokines, especially interleukin (IL)−1β, which activates inflammasomes and results in impaired autophagy [12,13]. Mucopolysaccharidosis (MPS) is an LSD comprising subtypes I, II, III, IV, V, VI, VII, and IX and is characterized by glycosaminoglycan accumulation. Abnormal pathological substrate accumulation in MPS causes autophagy, cell dysfunction, and lysosomal membrane stabilization disorder, thus triggering the innate immune response and increasing inflammatory cytokines [14]. Pompe disease is an AR-LSD known as “glycogen storage disease type II,” which is caused by a deficiency of acid alpha-glucosidase (GAA). GAA degrades glycogen, and thus its mutations cause glycogen accumulation followed by accumulation of autophagosomes. Autophagy substrates were reported in the myotubes of these patients and in the primary myoblasts of GAA-deficient mice. Dysfunctional autophagy contributes to the pathogenesis of Pompe disease [15]. This study aimed to reveal whether the COVID-19 pandemic creates additional risk in this patient group compared with the healthy population. Although the potentially increased risk is emphasized in the literature for infections via physiopathology, no detailed study has been reported demonstrating this with clinical data.
2. Materials and methods
Approval dated 06.10.2020 and numbered 105–5 was obtained from the ethics committee of the Çukurova University Faculty of Medicine for the study. All patients with LSDs admitted to the hospital between March 2020 and January 2021 and followed up for more than 1 year were included in the study. Written consent was requested from adult patients themselves and from the parents of patients younger than 18 years. This study aimed to investigate the prevalence of autoimmunity and immunodeficiency in patients with LSDs through clinical findings, enzyme analysis, and genetic studies who were followed up at the Çukurova University, Division of Pediatric Metabolism and Nutrition. The following parameters were recorded: complete blood count; absolute neutrophil, lymphocyte, eosinophil counts; eosinophil percentage; blood type, HBsAg; anti-HBs; IgG, IgA, IgM, and IgE levels; CD16+ CD56+, CD3+ CD4+, CD3+ CD8+, CD19+, CD45+; and human leukocyte antigen (HLA)-DR In terms of autoimmunity, the following were measured: antinuclear antibodies (ANA), anti-DNA, antithyroglobulin, anti-thyroid peroxidase (TPO), anti-gliadin IgG and IgA, antiphospholipid IgG and IgM, anticardiolipin IgG and IgM, tissue transglutaminase antibody, thyroid function tests, aspartate transaminase (AST), and alanine transaminase (ALT) levels, blood glucose, and HbA1c levels. If blood glucose was elevated, measurement of anti-islet cell antibodies (ICA), and if there was an elevation in AST/ALT, measurement of anti-liver-kidney antibodies were requested. Not all tests planned could be performed for every patient because of the pandemic conditions. Data were analyzed using the software SPSS for Windows 22. Descriptive statistics are expressed as mean ± standard deviation or median (minimum–maximum) for discontinuous numeric variables, and categorical variables are expressed as numbers and percentages. Means of groups were compared using the Mann−Whitney test. For quantitative data, comparisons of three and more groups, ANOVA was used if the data were normally distributed, and the Kruskal−Wallis test was used if the data were not normally distributed. For qualitative data, the chi-square test was used if there were more than three groups, and Fischer's exact test was used if the chi-square test condition was not met. A value of p<0.05 was considered statistically significant.
3. Results
3.1. Age, type of diagnosis, time of ERT onset, and duration
Of the 216 patients with MPS followed up in our clinic, 27 (12.5%) had a diagnosis of type I, 23 (10.6%) type II, 51 (23.6%) type III, 50 (23.1%) type IVA, and 65 (30.1%) type VI. Of the patients followed up with a diagnosis of Gaucher disease, 51 (50%) were type I, five (4.9%) were type II, and 46 (45%) were type III. All patients with Gaucher type II had died before the onset of the study. There were 41 patients with Pompe followed up in the LSD group. The median age of 110 patients who were followed up with Gaucher, MPS, and Pompe diagnoses and who could be included in the study for evaluation of immunity was 129 months (range: 21–655), and the gender distribution was 63 (57.2%) female and 47 (42.8%) male patients. Of the patients, 43 were diagnosed with Gaucher, 55 with MPS, and 12 with Pompe disease. There were 30 patients over the age of 18, 11 of whom had Gaucher type I, 9 had Gaucher type III, 7 had MPS type IVA, 1 had MPS type III, 1 had MPS type I, and 1 patient had Pompe disease. Seven (23%) of these patients were male, and 23 (77%) were female. The median duration of receiving enzyme replacement therapy (ERT) for patients included in the study was as follows: 33 months (range: 8–100) for MPS type I, 53 months (range: 19–143) for MPS type II, 31 months (range: 6–98) for MPS type IVA, 44.5 months (range: 19–120) for MPS type VI, 72 months (range: 22–173) for Gaucher type I, 80 months (range: 11–200) for Gaucher type III, and 80 months (range: 20–130) for Pompe disease.
3.2. Immunodeficiency parameters
Of 74 patients whose immunodeficiency parameters could be assessed, 21 had a diagnosis of Gaucher disease, 44 of MPS, and nine of Pompe disease. Their median age was 123.5 months (range: 21–655) and the median duration of ERT was 56.5 months (range: 6–200). All tests were performed outside of the infection period. The serum immunoglobulin levels of the patients were evaluated by considering the ranges based on their ages [16]. Except for two patients, all patients had normal white blood cell (WBC) and absolute neutrophil count (ANC) values. The lymphocyte count was below 1500/mm3 in one patient with Gaucher type III and one patient with Pompe disease. When the three LSD groups were compared in terms of T and B lymphocyte counts, no significant difference was found between them (Table 1 ). Eosinophilia (>5%) was detected in 7 patients (3 Pompe, 2 Gaucher, and 2 MPS cases). When the Gaucher, MPS, and Pompe groups were compared in pairs in terms of the prevalence of eosinophilia, the percentage of eosinophilia was higher in the Gaucher group than in the MPS group, and it was significantly higher in the Pompe group than in the MPS group (p<0.05, p<0.05, respectively). These patients had no known history of allergies, parasitosis, or reactions to ERT. IgG levels could be measured for 76 patients (Table 1). Four of these patients had IgG levels below the normal range for age. There were a total of five patients with IgM levels below the normal range for age (Table 2 ). When patients with a diagnosis of LSDs were compared in terms of Ig levels, IgM and IgA levels were reported to be significantly lower in the MPS group than in the Gaucher and Pompe groups (p<0.05, p<0.05, respectively) (Table 1). IgM and IgA levels were reported to be elevated in only one patient with Gaucher disease (C12) (Table 3 ). Because this patient had a severe accident, no further examination was performed during the study regarding gammopathy. Although there was no statistically significant difference between the groups, the IgG, IgM, and IgA levels in the Gaucher group were reported to be closer to the upper limit for age but within the normal ranges. There was no significant difference between Gaucher type I and type III subgroups in terms of IgG, IgA (p = 0.40, p = 0.37, respectively), and IgM (p = 0.97) levels. CD16+ CD56+(NK) levels were reported to be low in 14 patients (Tables 2 and 3). Six of them were followed up with a diagnosis of Gaucher (28.5% of Gaucher patients), six with MPS (16.6% of MPS patients), and two with Pompe disease (25% of patients with Pompe). Of the patients with low T CD8 (CD3+ CD8+) levels, one had been diagnosed with MPS type II and two with Pompe disease. One patient with CD3+CD4+ deficiency had been diagnosed with MPS type I, and one patient with CD3+deficiency with MPS type II. Of the four patients with low B cell (CD19) counts, one was followed up for Gaucher type III, one for MPS type I, one for MPS type II, and one for MPS type VI (Table 3). The HLA-DR levels of one patient with MPS type IVA and one patient with Pompe disease were below the normal range for age. When the group with abnormal immune deficiency parameters and the group with normal results were compared, there was no significant difference between the two groups in terms of ERT duration and current age (p = 0.75, p = 0.3, respectively) (Table 2). Overall, 12 (57%) of 21 patients with Gaucher disease (7 type III, 5 type I), 18 (40.9%) of 44 patients with MPS (9 type IVA, 5 type VI, 1 type I, 2 type II, and 1 Type III), and six (66%) of nine patients with Pompe disease were reported to have abnormalities in at least one of the parameters related to immunodeficiency. Abnormality was detected in the immunodeficiency parameters in a total of 11 (30%) patients, including in one MPS type I, one MPS type III, three MPS type IVA, four Gaucher type III, and two Gaucher type I patients who were over 18 years of age. The mutations detected in patients with abnormal immunodeficiency parameters were not similar, and the same mutations were reported in patients with normal test results (Table 2).
Table 1.
Count blood cell and immunoglobulin levels of patients with Gaucher, MPS and Pompe diseases.
| Gaucher disease (N = 21) | MPS disease (N = 46) | Pompe disease (N = 9) | p | p* | p** | p# | ||
|---|---|---|---|---|---|---|---|---|
| White blood cell count (× 109/L) | Median(range) | 7.4(4.6–12.6) | 8.7(5.3–17) | 8.2(5.9–12.1) | 0.1 | |||
| Neutrophil (× 109/L) | Median(range) | 3.6(1.5–8.6) | 3.45(1.5–9.3) | 3.6(2.4–6.5) | 0.95 | |||
| Eosinophils(× 103/ml) | Median(range) | 0.2(0–0.9) | 0.1(0–1.1) | 0.2(0,1–0,8) | 0.230 | |||
| Eosinophils (%) | Median(range) | 2,4(0.8–7) | 1,6(0–8.8) | 2,6(1.2–8.2) | 0.0 | 0.01 | 0.01 | 0.31 |
| Immunoglobulin G, mg/dL | Median(range) | 1055(629–1999) | 975(410–1480) | 960(581–1240) | 0.07 | |||
| Immunoglobulin M, mg/dL | Median(range) | 152(73–1680) | 119.5(16–278) | 146(42–161) | 0.01 | 0.02 | 0.28 | 0.42 |
| Immunoglobulin A, mg/dL | Median(range) | 163,5(32–650) | 101(10–230) | 110(44–330) | 0.03 | 0.004 | 0.59 | 0.15 |
| Total immunoglobulin E, IU/ml | Median(range) | 80.9(23.4–1180) | 35.9(5–1680) | 23(5–336) | 0.852 | |||
MPS: mucopolysaccharidosis; statistically significant at p < 0.05; p: comparison of laboratory findings for Gaucher, MPS, and Pompe disease, p*:comparison of laboratory findings for Gaucher and MPS disease; p**: comparison of laboratory findings for MPS and Pompe disease; p#: comparison of laboratory findings for Gaucher and Pompe disease.
Table 2.
Lymphocyte levels of patients with Gaucher, MPS and Pompe diseases.
| Lymphocytes | Gaucher disease N = 21 | MPS N = 36 | Pompe disease N = 8 | p | |
|---|---|---|---|---|---|
| Total lymphocytes × 109/L | Median (range) | 2700(1300–6800) | 3900(1600–7700) | 4100(1400–6900) | 0.156 |
| T cells (CD3+) cells/μL | Median(range) | 67.4(60.1–83.6) | 70.9(55.8–92) | 73.7(62.6–78.4) | 0.173 |
| T CD4+ (CD3+CD4+) cells/μL | Median(range) | 40(27.2–54.8) | 38.4(11.9–53.8) | 43.3(33.5–55.7) | 0.946 |
| T CD8+ (CD3+CD8+) cells/μL | Median(range) | 25,1(20–42.8) | 24.8(7.1–52) | 22.8(5.9–39) | 0.426 |
| B cells (CD19+) cells/μL | Median(range) | 17.5(8.5–27.8) | 17.5(8.5–27.8) | 18.5(10.1–29.4) | 0.319 |
| NK cells (CD3+/CD16+/CD56+) cells/μL | Median(range) | 10.5(2.7–24) | 8.5(2.8–30.7) | 8.45(5.7–13) | 0.238 |
| HLA-DR cells/μL | Median(range) | 25.2(12.2–45.9) | 25.3(10–55) | 25.1(14.8–32.8) | 0.553 |
MPS: mucopolysaccharidosis; NK: natural killer; HLA-DR: human leukocyte antigen–DR isotype; statistically significant at p < 0.05.
Table 3.
Detailed data of patients with positivity in immune deficiency parameters.
| Case number | Diagnosis | Age (years) | Gender | Gene | Mutation | Duration of ERT (months) | Blood group | WBC (× 109/L) | ANS (× 109/L) | HBsAg (mIU) | Anti-HBs (U/L) | Ig G(mg/dL) | Ig M (mg/dL) | Ig A (mg/dL) | Total Ig E (IU/ml) | NK (CD3+/CD16+/CD56+) (cells/μL) | T CD8 (CD3+ CD8+) (cells/μL) | T CD4(CD3+CD4+) (cells/μL) | T cells CD3+ (cells/μL) | B cells CD19+(cells/μL) | HLA DR (cells/μL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | Gaucher Type III | 28.1 | F | GBA | p.D448H/p.D448H | 51 | 0+ | 5700 | 2100 | 0.57 | 467.9 | 1010 | 100 | 150 | 335 | 6.8 | 35.5 | 44.5 | 81.9 | 8.50 | 12.2 |
| C2 | Gaucher Type III | 11 | M | p.D448H/p.D448H | 125 | 0+ | 5800 | 2700 | 0.6 | 2 | 1020 | 137 | 210 | 23.4 | 16 | 26 | 41 | 70 | 25 | 26 | |
| C3 | Gaucher Type III | 8.6 | F | p.L483P/p.L483P | 80 | A+ | 7400 | 4300 | 0.5 | 27.35 | 1630 | 130 | 170 | 53.9 | 2.7 | 23.6 | 54.8 | 83.6 | 13.00 | 16.4 | |
| C4 | Gaucher Type III | 18.3 | F | p.L444P/p.L444P | 99 | 0- | 6000 | 3600 | 0.4 | 20 | 950 | 123 | 259 | 35 | 6.1 | 42.8 | 34.9 | 80.3 | 12.70 | 20.5 | |
| C5 | Gaucher Type III | 20.7 | F | p.L335V/p.L335V | 11 | 0+ | 6100 | 3700 | 0.4 | 1000 | 890 | 167 | 167 | 42 | 6.8 | 25.1 | 41.2 | 67.1 | 25.90 | 32 | |
| C6 | Gaucher Type III | 22.9 | F | p.L335V/p.L335V | 35 | A+ | 8100 | 6200 | 0.4 | 2 | 1040 | 186 | 170 | 304 | 15 | 25 | 40 | 68 | 23 | 25 | |
| C7 | Gaucher Type III | 9.2 | F | p.L483P/p.L483P | 92 | A- | 7300 | 3400 | 0.63 | 5.4 | 1310 | 113 | 150 | 110 | 9.8 | 24.4 | 38.2 | 67.4 | 21.7 | 24.2 | |
| C8 | Gaucher Type 1 | 8.3 | M | p.D448H/p.D448H | 40 | 0+ | 7700 | 4300 | 0.4 | 26.3 | 940 | 64 | 120 | 54 | 4.4 | 20 | 51.1 | 74.7 | 19.40 | 25.2 | |
| C9 | Gaucher Type 1 | 13 | M | p.D448H/p.L335V | 32 | A+ | 5700 | 2900 | 0.3 | 25 | 875 | 132 | 150 | 32 | 5.8 | 36.6 | 33.9 | 79.6 | 13.00 | 14.7 | |
| C10 | Gaucher Type 1 | 18 | F | p.N370S/p.N370S | 144 | 0+ | 6000 | 2730 | 0.6 | 9.7 | 1360 | 190 | 170 | 353 | 17.6 | 24 | 41 | 66.4 | 13 | 28.7 | |
| C11 | Gaucher Type 1 | 16.6 | M | p.L335V/p.L335V | 135 | 0+ | 5760 | 1790 | 0.7 | 2 | 1290 | 80 | 32 | 137 | 16.7 | 24.5 | 32.5 | 62.3 | 15.7 | 24.4 | |
| C12 | Gaucher Type 1 | 33.4 | M | IVS2+1G>A/IVS2+1G>A | 58 | A- | 70,000 | 4200 | 0.3 | 2 | 1990 | 1680 | 650 | 356 | 14.3 | 24.7 | 44.6 | 72.1 | 19 | 17.4 | |
| C13 | MPS Type VI | 7 | M | ARSB | p.L321P/p.L321P | 19 | A+ | 10,400 | 2900 | 0.5 | 32 | 745 | 145 | 124 | 25 | 3.20 | 40.4 | 32 | 75.5 | 16 | 21.00 |
| C14 | MPS Type VI | 12.67 | M | p.E346Sfs*13/p.E346Sfs*13 | 101 | B- | 6400 | 5600 | 0.4 | 345 | 1130 | 49 | 30 | 15.4 | 13.4 | 30.9 | 33.2 | 70 | 11.50 | 20.7 | |
| C15 | MPS Type VI | 10.8 | F | p.E346Sfs*13/p.E346Sfs*13 | 120 | B+ | 7500 | 4300 | 0.5 | 2 | 980 | 123 | 100 | 43 | 3.3 | 27.9 | 53.4 | 88.1 | 5.6 | 10.80 | |
| C16 | MPS Type VI | 3.1 | F | p.L321P/p.L321P | 30 | A+ | 17,000 | 8500 | 0.8 | 2 | 590 | 109 | 40 | 8.87 | 6.4 | 14.6 | 43.5 | 59.6 | 31.2 | 36.2 | |
| C17 | MPS Type VI | 6.75 | F | c.1143_1_1143delGTinsAC/ c.1143_1_1143delGTinsAC | 60 | A+ | 9100 | 4100 | 0.5 | 2 | 990 | 222 | 150 | 1680 | 6.3 | 30.6 | 38.3 | 77.3 | 13.8 | 20.6 | |
| C18 | MPS Type IV | 23.1 | F | GALNS | p.W141R/p.W141R | 62 | 0- | 9400 | 6300 | 0.6 | 2 | 1140 | 114 | 200 | 11.6 | 8.6 | 33.8 | 39 | 75.3 | 13.1 | 26.2 |
| C19 | MPS Type IV | 7.1 | F | p.R90W/p.R90W | 12,00 | A+ | 8400 | 3900 | 0.5 | 30 | 570 | 65 | 70 | 17.5 | 8.4 | 19.6 | 36 | 61.4 | 29.80 | 34.9 | |
| C20 | MPS Type IV | 8.5 | M | p.R219*/p.R219* | 36 | B+ | 12,700 | 8010 | 0.3 | 20 | 456 | 137 | 89 | 96.9 | 9 | 20 | 35 | 62 | 32.00 | 35 | |
| C21 | MPS Type IV | 20 | F | p.W141R/p.W141R | 9 | 0+ | 8100 | 5300 | 0.47 | 10 | 990 | 134 | 150 | 30.9 | 20.8 | 22.9 | 42.7 | 66 | 10.5 | 14.40 | |
| C22 | MPS Type IV | 20.8 | F | p.C308R/p.C308R | 60 | A+ | 8300 | 3900 | 0.52 | 21 | 970 | 159 | 140 | 24.3 | 6.6 | 34.5 | 36.6 | 73.6 | 24.4 | 21.4 | |
| C23 | MPS Type IV | 12 | F | p.W141R/p.W141R | 18 | 0- | 8450 | 2680 | 0.8 | 31.3 | 1480 | 147 | 170 | 146 | 5.6 | 26.2 | 47.7 | 77.6 | 14.6 | 21.2 | |
| C24 | MPS Type IV | 4.5 | M | p.P179S/p.P179S | 34 | A+ | 6700 | 1300 | 0.7 | 17.7 | 470 | 134 | 90 | 52 | 7.3 | 18.7 | 42 | 71.9 | 20.7 | 25.3 | |
| C25 | MPS Type IV | 5.8 | F | p.L36P/p.L36P | 14 | B+ | 12,800 | 9400 | 0.4 | 6 | 1050 | 167 | 100 | 25 | 9.2 | 34 | 41 | 75 | 14 | 27.00 | |
| C26 | MPS Type IV | 8 | F | p.C308R/p.C308R | 10 | A+ | 7100 | 1800 | 0.8 | 6 | 1140 | 110 | 100 | 5 | 9.3 | 27.6 | 31.6 | 68.4 | 19.9 | 28.70 | |
| C27 | MPS Type III | 22.1 | F | NAGLU (Alpha-Nacetylglucosaminidase: 0,15 (10–45) umol/1/h) | -- | — | A+ | 6700 | 3100 | 0.57 | 2 | 1341 | 154 | 218 | 188 | 2.8 | 42.8 | 44.9 | 91.1 | 4.50 | 15.8 |
| C28 | MPS Type II | 13.9 | M | IDS | p.A85T/p.A85T | 137 | B+ | 9300 | 3500 | 0.5 | 30 | 800 | 200 | 150 | 14 | 11.4 | 26.6 | 37.3 | 67.6 | 17.7 | 21.6 |
| C29 | MPS Type II | 1.8 | M | p.R468Q/p.R468Q | 19 | A- | 5900 | 1100 | 0.8 | 423.8 | 410 | 40 | 40 | 5 | 12.8 | 7.1 | 50.2 | 62 | 22.9 | 25.7 | |
| C30 | MPS Type I | 23.3 | M | IDUA | p.S633L/p.S633L | 100 | A+ | 6300 | 3300 | 0.43 | 410 | 1260 | 30 | 23 | 288 | 12 | 35 | 34 | 80 | 15.00 | 30 |
| C31 | MPS Type I | 4.2 | M | p.Y202*/ p.Y202* | 21 | A+ | 12,100 | 6000 | 0.7 | 230 | 460 | 48 | 30 | 5 | 3.3 | 15.4 | 35.8 | 55.8 | 39.90 | 43.2 | |
| C32 | MPS Type I | 1.7 | M | Homozygous deletion of exon 2 | 17 | B+ | 7000 | 3800 | 0.3 | 638 | 630 | 16 | 10 | 5.4 | 30.7 | 48 | 11.9 | 62.5 | 13.00 | 55 | |
| C33 | Pompe | 9.9 | F | GAA | p.R660C/p.R660C | 114 | AB- | 8200 | 3600 | 0.3 | 2 | 900 | 145 | 110 | 8.3 | 7.00 | 26.1 | 46.3 | 77.9 | 14.3 | 20.90 |
| C34 | Pompe | 6.9 | F | IVS10+1G>T/IVS10+1G>T | 78 | B+ | 6900 | 3300 | 0.4 | 15 | 1124 | 151 | 101 | 108 | 12 | 23.6 | 44.9 | 74.4 | 21.4 | 30.40 | |
| C35 | Pompe | 2.1 | M | p.L299P/p.L299P | 20 | A+ | 7400 | 1500 | 0.47 | 1000 | 810 | 42 | 60 | 18.8 | 5.7 | 5.9 | 55.7 | 63.3 | 29.40 | 32.8 | |
| C36 | Pompe | 7.3 | F | p.E888*/p.E888* | 86 | A+ | 6500 | 3100 | 0.8 | 10.8 | 950 | 152 | 97 | 45 | 7.9 | 15.9 | 41.8 | 65.9 | 24.3 | 29.6 | |
| C37 | Pompe | 10.8 | F | p.Q914Pfs*30/p.Q914Pfs*30 | 130 | 0+ | 8100 | 4400 | 0.3 | 2.72 | 960 | 118 | 140 | 5.58 | 10.1 | 22.1 | 37.6 | 62.6 | 25.4 | 29.4 | |
| C38 | Pompe | 8.6 | M | p.E888*/p.E888* | 101 | 0+ | 12,100 | 4700 | 0.5 | 2 | 1210 | 146 | 260 | 23 | 13 | 33.2 | 33.60 | 73.1 | 13.7 | 20.2 |
F:female; M: male; ERT: enzyme replacement therapy; WBC: white blood cell; ANC: absolute neutrophil count; HBsAg: hepatitis B surface antigen; Anti-HBs: hepatitis B surface antibody; Ig: immunoglobulin; NK: natural killer; HLA DR: human leukocyte antigen–DR isotype; CD8: cluster of differentiation 8; CD4: cluster of differentiation 4.
The hepatitis B surface antigen (HBsAg) and anti-HBs levels of 57 patients could be measured. All these patients were vaccinated on time within the framework of the national vaccination program. HBsAg was normal in all patients, while the anti-HBs levels -were above 10 U/L in 40 patients and below 10 U/L in 17 patients. Of the patients with an anti-HBs level below 10 U/L (Table 2), the median HBsAg level was 0.5 mIU (range: 0.1–0.9) and the median anti-HBs level was 146.9 U/L (range: 2–1000).
3.3. Autoimmunity parameters
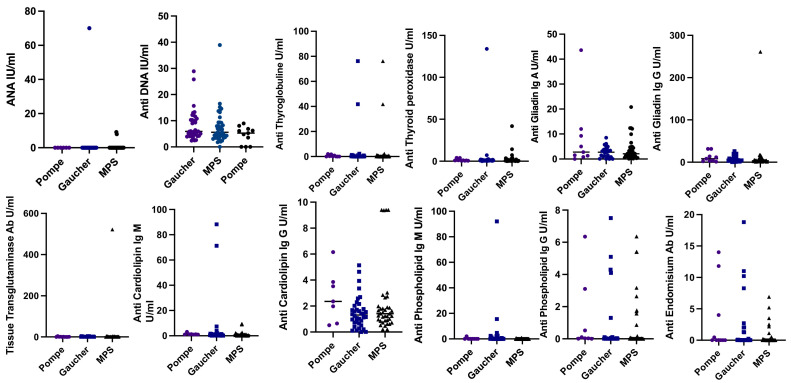
The median age of 85 patients (40 MPS, 37 Gaucher, and 8 Pompe) who had been diagnosed with Gaucher, MPS, and Pompe disease and whose autoimmunity parameters could be analyzed was 129 months (range: 8–655) and the median duration of receiving ERT was 56 months (range: 6–200). Of these 85 patients, one antibody was positive in 23 (27%), two antibodies were positive in four, and three antibodies were positive in one patient. Among patients with antibody positivity (13 patients), 46.4% were older than 18 years, including 10 patients with Gaucher disease (4 type III), one with MPS type III, one with MPS type IVA, and one with MPS type VI. Antibody positivity was reported in nine (22.5%) of 40 patients with MPS included in the study (1 antibody in 7 patients, 2 antibodies in 2 patients). Of the eight patients with Pompe disease, three patients (37.5%) had positivity to one antibody, and one patient had positivity to two antibodies (Table 4 ). Antibody positivity was detected in 15 (40.5%) of 37 Gaucher patients. One antibody was positive in 13 patients, two antibodies were positive in one, and three antibodies were positive in one patient. When autoantibody levels were compared, no significant difference was reported between the three groups (Fig. 1 ). When Gaucher type I and III subgroups were compared separately in terms of autoantibody levels and prevalence of autoantibody positivity, only the anti-endomysial antibody level was significantly higher in the Gaucher type I group than in the Gaucher type III group (p = 0.04). However, there was no difference between the groups in terms of positivity rates.
Table 4.
Detailed data of patients with positivity in autoimmune parameters.
| Case number | Diagnosis | Current age (years) | Gender | Gene | Mutation | Duration of ERT (months) | Autoantibody positivity (Antiphospholipid IgM (>10 U/ml)), Anti-gliadin IgA (> 10 U/ml) , Anti-gliadin IgG (>12 U/ml), ANA (>60), Anti-DNA (>20 IU/ml), Anticardiolipin IgM (>20 U/ml), Anti Thyroglobulin (>4 U/ml)), Anti TPO (>9 U/ml)) |
|---|---|---|---|---|---|---|---|
| C39 | Gaucher disease Type III | 40.5 | M | GBA | p.L335V/p.L335V | 62 | Antiphospholipid IgM: 92 |
| C2 | Gaucher disease Type III | 11 | M | p.D448H/p.D448H | 125 | Anti-gliadin IgG: 14 | |
| C40 | Gaucher disease Type III | 19.5 | F | p.D448H/p.D448H | 78 | ANA: 70 | |
| C41 | Gaucher disease Type III | 27.5 | F | p.N370S/p.N370S | 20 | Antiphospholipid IgM: 14 | |
| C5 | Gaucher disease Type III | 20.7 | F | p.L335V/p.L335V | 11 | Anticardiolipin IgM;71.3 | |
| C6 | Gaucher disease Type I | 22.9 | F | p.L335V/p.L335V | 35 | Anticardiolipin IgM: 88.3 | |
| C42 | Gaucher disease Type I | 2.5 | M | p.L335V/p.L335V | 22 | Anti-gliadin IgG; 12.6 | |
| C43 | Gaucher disease Type 1 | 23 | F | p.N370S/pR502H | 57 | Anti-thyroglobulin:76.2 | |
| C44 | Gaucher disease Type 1 | 2.7 | F | p.L483P/p.L483P | 30 | ICA positive | |
| C45 | Gaucher disease Type 1 | 54.6 | F | p.N370S/p.N370S | 139 | TRAb positive | |
| C46 | Gaucher disease Type 1 | 11.4 | M | ARSB | p.S366T/p.S366T | 86 | ICA Positive |
| C47 | Gaucher disease Type 1 | 30.4 | M | p.N370S/p.N370S | 30 | Anti-DNA: 25.8 | |
| C9 | Gaucher disease Type 1 | 13 | M | p.D448H/p.L335V | 32 | Anti-gliadin IgG: 19.8 | |
| C48 | Gaucher disease Type 1 | 26.5 | F | p.N370S/p.N370S | 127 | Anti-DNA: 28.9, anti-gliadin IgG: 26.1 | |
| C49 | Gaucher disease Type 1 | 24.8 | F | IVS2+1G>A/IVS2+1G>A | 173 | Anti-thyroglobulin: 41.8, anti-TPO: 133, anti-Gliadin IgG: 18.7 | |
| C50 | MPS Type VI | 12.6 | M | p.E346Sfs*13/p.E346Sfs*13 | 101 | Anti-DNA: 38.9 | |
| C51 | MPS Type VI | 5.2 | M | pp.L321P/p.L321P | 44 | Anti-gliadin IgG: 13.9 | |
| C52 | MPS Type VI | 20.5 | M | p.L321P/p.L321P | 62 | Anti-tissue transglutaminase Ab: 522 | |
| C18 | MPS Type IV | 23.1 | F | GALNS | p.W141R/p.W141R | 62 | Anti-gliadin IgG: 15.7 |
| C53 | MPS Type IV | 4.5 | M | p.P179S/p.P179S | 34 | Anti-gliadin Ig A: 12.1 | |
| C54 | MPS Type IV | 13.9 | F | Homozygous deletion of exon 14 | 8 | Anti-gliadin Ig A: 12.4, anti-gliadin IgG: 17 | |
| C27 | MPS Type III | 22.1 | F | NAGLU Alpha-N-acetylglucosaminidase: 0,15 (10–45) umol/1/h | -- | - | Anti-gliadin Ig A: 20.8 anti-gliadin IgG: 261 |
| C55 | MPS Type II | 13.9 | M | IDS | p.S87R/p.S87R | 137 | Anticardiolipin Ig M: 25.1 |
| C31 | MPS Type I | 4.2 | M | IDUA | p.Y202*/ p.Y202* | 21 | ICA positive |
| C36 | Pompe disease | 7.3 | F | GAA | p.E888*/p.E888* | 86 | ICA positive |
| C38 | Pompe disease | 8.6 | M | p.E888*/p.E888* | 101 | Anti-gliadin Ig A: 43.6 | |
| C35 | Pompe disease | 2.1 | M | p.L299P/p.L299P | 20 | Anti-gliadin Ig G: 31,3 | |
| C56 | Pompe disease | 4.3 | F | p.L299P/p.L299P | 50 | Anti-gliadin Ig A: 12, anti-Gliadin IgG: 31.4 |
ERT: enzyme replacement therapy; Ig: immunoglobulin, ANA: antinuclear antibody; TPO: thyroid peroxidase; ICA: islet cell antibodies; TRAb: thyrotropin receptor autoantibodies.
Fig. 1.
Distribution of autoantibodies in Pompe, Gaucher, and MPS groups.
Scatter plots showing the distribution ANA (p>0.05), anti-DNA (p>0.05), anti-thyroglobulin (p>0.05), anti-thyroid peroxidase (p>0.05), anti-gliadin IgA (p>0.05), anti-gliadin IgG (p>0.05), tissue transglutaminase Ab (p>0.05), anti-cardiolipin IgM (p>0.05), anti-cardiolipin IgG (p>0.05), anti-phospholipid IgM (p = 0.05) and anti-phospholipid IgG in Pompe (n = 8), Gaucher (n = 37), and MPS (n = 40) patients. Means of groups were compared using the Mann−Whitney test. For quantitative data, compared to three and more groups, ANOVA was used if the data were normally distributed, and Kruskal−Wallis was used if the data were not normally distributed. ANA: antinuclear antibody; Ig: immunoglobulin; MPS: mucopolysaccharidoses.
ANA was reported to be positive in one patient with Gaucher type III, anti-DNA was positive in two patients with Gaucher type I and in one patients with MPS type VI, antithyroglobulin was positive in two patients with Gaucher type I, anti-TPO was positive in one patient with Gaucher type I, TRAB was positive in one patient with Gaucher type I, antiphospholipid IgM was positive in three patients with Gaucher type III and in one patient with Gaucher type 1, anticardiolipin IgM was positive in one patient with Gaucher type I, one patient with Gaucher type III, and one patient with MPS type II. None of the patients had positive anti-endomysial antibody, anticardiolipin IgG, and antiphospholipid IgG levels (Table 4). When the groups with and without antibody positivity were compared in terms of ERT duration and age, no significant difference was found between them (p = 0.67, p = 0.3, respectively).
The anti-gliadin IgG level was positive for 10 patients, including one patient with MPS type IVA, one patient with MPS type VI, one patient with MPS type III, two patients with Pompe disease, four patients with Gaucher disease type I, and one patient with Gaucher disease type III. Anti-gliadin IgA positivity was detected in one patient with MPS type III, two patients with MPS type IVA, and two patients with Pompe disease. Tissue transglutaminase antibody was positive in only one patient with MPS type VI. Patients with positive antibodies did not have weight loss, malnutrition, or iron, folate, and vitamin B12 deficiencies. ICA positivity was detected in two patients with Gaucher disease type I, one patient with Pompe disease, and one patient with MPS type I; however, the Hba1c and insulin levels of these patients were within the normal range.
In a 54-year-old patient (C45) followed up for the diagnosis of Gaucher type I, the following findings were reported on the 10th year of imiglucerase treatment at 30 IU/kg every 2 weeks: TSH: 0.01 (0.38–5.33) mIU/mL; fT4: 1.63 (0.61–1.12) ng/dL; and fT3: 6.87 (2.3–4.2) pg/mL. Although TRAB was reported to be positive in the first analysis, TRAB measurements and thyroid function tests were in the normal range 4 months later. The patient was diagnosed with subacute thyroiditis. The antithyroglobulin and anti-TPO levels were elevated in a 25-year-old patient (C49) who was followed up for the diagnosis of Gaucher type I. The following were reported: TSH: 2.28 (0.38–5.33) mIU/mL and fT4: 0.8 (0.61–1.12) ng/dL. The ultrasonographic findings were consistent with the diagnosis and the patient was monitored without medication for Hashimoto's thyroiditis. His-brother, aged 33 with the same mutation (C12), who was followed up for the diagnosis of Gaucher type I, had normal autoantibody levels. Other patients with autoantibody positivity had no clinical or laboratory findings related to dermatological, rheumatological, endocrinological, and gastrointestinal systems. The scheduled endoscopy and colonoscopy could not be performed in the patient who was reported to have tissue transglutaminase antibody positivity because of the pandemic.
In total, 10 of 110 patients—including two patients with Gaucher type III, two patients with Gaucher type I, one patient with MPS type III, one patient with MPS type IVA, one patient with MPS type I, and three patients with Pompe diseases—were reported to have abnormalities in both immunodeficiency and autoimmunity parameters (C2, C5, C6, C9, C18, C27, C31, C35, C36, C38) (Tables 3 and 4 ). In 56 (53.6%) of 110 patients included in the study, 23 patients with Gaucher disease (10 type III, 13 type I), 26 patients with MPS (8 type VI, 11 type IVA, 1 type III, 3 type II, and 3 type I), and seven patients with Pompe disease were reported to have an abnormality in at least one of the parameters related to autoimmunity or immunodeficiency.
There were four patients with a diagnosis of LSD who had a COVID-19 infection. One of them was a 17-year-old girl with Gaucher type I who was included in the study. She had been receiving ERT (30 IU/kg 2 weeks) for 11 years. The patient had weakness and headache but did not report a fever, cough, or sore throat. No abnormality was found in her biochemical tests and acute phase reactants. Following favipiravir treatment for 5 days, the result of her COVID-19 PCR test performed on the ninth day was reported to be negative, and thus she was discharged. No abnormality had been reported in her autoimmunity and immunodeficiency parameters before the disease. The other patient had a diagnosis of MPS type II, was 14 years old, and had been receiving ERT for 11 years. The anticardiolipin IgM level was elevated, and among the immunodeficiency parameters, CD3 T cell count was low in this patient. The patient, who was hospitalized and received favipiravir treatment for COVID-19-related pneumonia, was discharged without any sequelae.
A late-diagnosed patient with the severe form of MPS type VI who had not been analyzed for immunity parameters was hospitalized and treated in an external center with a diagnosis of COVID-19; this patient was not intubated and was discharged upon recovery. A COVID-19 PCR test result was positive in an adult patient with Gaucher type I who had not been analyzed for immunity parameters; however, the disease was mildly symptomatic. During this period, two patients with a diagnosis of LSD died. A patient with MPS type III (C27) with interstitial lung disease died because of COVID-19-negative atypical pneumonia. This patient had a low count of natural killer (NK) cells. Another patient with Pompe disease died because of COVID-19-negative bacterial pneumonia and sudden arrhythmia with cardiac arrest. Immunity parameters were not analyzed in this patient. Several COVID-19 PCR tests were performed for these two patients, which always yielded negative results.
4. Discussion
COVID-19 infection and LSDs, where we observe various degrees of hyperimmune response, share a similar immune activation pathway; however, COVID-19 is known to induce a much more severe hyperimmune response. In view of this hyperimmune response, LSDs and conditions that may lead to immunodeficiency represent an essential risk factor for COVID-19. On the other hand, accumulated glycolipids in patients with LSD might spark immune tolerance rather than enhance inflammation; a case in point is Niemann–Pick disease type C, which is found to be potentially resistant to COVID-19. LSDs interestingly seem to offer protection against virus-related complications [17]. We wanted to highlight the importance of the immunity status and the predisposition to infections in our patients. Therefore, our study on immunity in LSDs investigated whether there was an additional predisposition to COVID-19 infection in LSDs. As far as we know, this is the first large study with real-life data on the immune status of patients with LSDs in relation to COVID-19, which was conducted between March 2020 and January 2021. Overall, 269 of 360 patients with Gaucher, MPS, and Pompe disease who were being followed up regularly were reached to obtain information and it was found out that only four patients had a history of non-severe COVID-19 infection. Since PCR tests were only performed in mildly symptomatic cases in our study, the incidence of COVID-19 in asymptomatic carriers is unknown. Seropositivity of COVID-19 was found in two out of 97 patients with Gaucher disease in the present study. With time, a few articles have been published on Gaucher disease that suggested the disease was not involved in the incidence of COVID-19 and related complications [17], [18], [19]. The incidence of COVID-19 in patients with Gaucher disease from Spain was 8 (7.2%), six of whom had contact with an infected person. It is significant that in patients with comorbidities such as diabetes mellitus and hypertension, COVID-19 progressed much more severely [18].
Disturbances in the transport of lipids and their metabolites lead to progressive accumulation of non-degradable and partially degradable proteins in the lysosomes. It is interesting that in recent articles, patients with LSDs are hypothesized to have a reduced risk for COVID-19 infection, whereby it is suggested that NPC1 inhibitor drugs could present as anti-SARS-CoV-2 agents. Some clinically approved drugs inhibit NPC1 and disturb viral SARS-CoV-2 infectivity through three stages—viral entry, replication, and exit—subsequently producing lipid accumulation and disturbing the optimal environment for viral infectivity [20]. These hypotheses were later experimentally tested in other publications that investigated host genetics. Some authors recently used pharmacological treatments for COVID-19 and identified that the cholesterol biosynthesis pathway that is induced results in viral inhibition. The initial impression from the studies was that there could be a considerable complexity between the genetic structure of viruses and humans [10]. There is undoubtedly a great deal of evidence to support the effectiveness of perturbing cholesterol trafficking mechanisms with the use of molecules targeting the host NPC1 receptor in COVID-19 patients [21]. Other essential host factors were determined in another study, such as phosphatidylinositol phosphate biosynthesis, cholesterol regulators, the lysosomal protein TMEM106B, which is unique to SARS-CoV-2 infection, ACE2, and heparan sulfate. Pharmacological inhibition of phosphatidylinositol kinases decreases virus infectivity [22]. Understanding the landscape of LSDs might help elucidate the treatment mechanisms of coronaviruses.
It is the cytokine storm that is responsible for tissue damage, i.e., the hyperimmune response, and not the deficiency in the immune system [3]. Recent publications have shown that COVID-19 can occur in children with severe hyperinflammatory syndrome, albeit rarely [1,23]. Of the patients included in the study, 70% were under the age of 18. However, this rate alone does not seem sufficient to explain the low incidence of COVID-19 infection. Since cardiac and chronic pulmonary diseases are an unfavorable prognostic factor for COVID-19 infection, patients with LSDs are at greater risk because of heart valve problems and interstitial lung disease [24]. Gaucher type III patients with parenchymal lung involvement and valvular and aortic calcification may be more exposed to the severe effects of COVID-19. However, there were no COVID-19 infection cases in the Gaucher type III group during this period. It is considered that the risk is higher in Pompe patients compared with their healthy counterparts due to skeletal muscle involvement and hypotonia as well as cardiac involvement. COVID-19 infection did not develop in any of the patients in our study who were followed up for Pompe disease. Only one patient died because of COVID-19-negative bacterial pneumonia. PCR tests were performed several times for this patient, and the results were consistently negative.
It is known that valve involvement, obstruction caused by accumulations in the respiratory tract, and chest deformities in patients with MPS are important adverse risk factors for a severe course of COVID-19.
Except for a patient with severe and early-onset MPS type VI who had aortic and mitral insufficiency, none of our patients with MPS had a COVID-19 infection. Although it is still not known precisely how the immunosuppressive condition affects the response to COVID-19, preliminary data suggest that immunosuppression in both children and adults is a good prognostic factor in the course of the disease compared with the general population [25]. Considering the mortality and morbidity data from SARS-CoV, it is understood that the immunosuppressive condition alone is not an indicator of poor prognosis [26,27]. On the contrary, it has been reported that it has a protective effect and prevents tissue damage. Immunosuppressed patients are not immune to COVID-19. When infected, the vast majority of cases show mild symptoms of the disease and, since they are undiagnosed, they serve as a source for viral spread [2]. Although we had a bedridden patient with MPS type III and low Ig levels who died because of severe bacterial pneumonia during this period, we are more concerned that especially patients with low immunodeficiency parameters may be asymptomatic carriers based on the study results.
A study conducted with an MPS type II family found that all family members had a low number of NK cells, and five family members had low B cells counts, but IgG, IgM, and IgA levels were reported to be normal [28]. In the present study, low Ig levels and NK cell counts were not found in patients with MPS type II. When examined in terms of immunodeficiency, it was observed that there were patients with low Ig levels and low levels of T cells, B cells, and NK cells in all three patient groups.
In a total of 12 of 20 (57%) patients with Gaucher, 18 of 44 (40.9%) patients with MPS, and six of nine (66%) patients with Pompe disease, at least one of the parameters related to immunodeficiency was found to be abnormal. Decreased levels of HLA-DR pose a risk for secondary and severe bacterial infections in COVID-19 patients [29]. It has been reported that there are patients who are hospitalized in intensive care and who develop secondary bacterial infection and end-organ damage [6]. In one patient with MPS type IVA and one patient with Pompe disease in our study, the HLA-DR level was below the normal range for age. The patient diagnosed with Pompe disease, who had a low HLA-DR level, had been previously hospitalized twice because of bacterial pneumonia. We believe that more attention should be paid to the LSD patient group in terms of secondary bacterial infections if they have a COVID-19 infection. While patients diagnosed with MPS and Pompe had been more frequently hospitalized due to bacterial pneumonia before the pandemic, only two patients with a diagnosis of Pompe (not included in the study) were hospitalized in external centers due to non-COVID-19 pneumonia during the period of COVID-19, which demonstrated once again the fact that more protection from community-acquired pneumonia requiring hospitalization was possible through compliance with hygiene rules and decreased contact, which has often been emphasized. However, a patient with Pompe disease who died in an external center because of arrhythmia shows that a subgroup of patients might not be taken to hospital until their complaints reach the final stage due to the fear of coronavirus infection.
Mechanisms leading to disease progression were examined. It was seen that autoimmunity-mediated cellular dysfunction associated with accumulated metabolites and the immune response to specific self-antigens may cause chronic inflammation and neuroinflammation, leading to cell death in LSDs [30]. Therefore, anti-inflammatory treatments have been used in diseases such as Niemann–Pick disease type C, Sandhoff, and MPS, and it has been shown that there is an increase in adaptive immune response and autoantibody production in some LSDs [31,32]. In COVID-19 patients, increased cytokines in the circulation are indicative of hypercytokinemia. The hyperinflammatory responses rarely occur when bioactive lipids accumulate and trigger inflammation in congenital metabolic diseases, and the hyperinflammatory responses described in SARS-CoV-2 patients are similar [7]. Specific biochemical abnormality and B cell proliferation contribute to alternative macrophage activation induced by cytokines. IgG accumulation has been shown in the brain biopsy of a patient with GM2 gangliosidosis [33]. Accumulation is likely to occur in other patients with LSD. It is known that immunological disorders that can occur in sphingolipidoses may be in the form of increased polyclonal Ig in patients diagnosed with Gaucher disease. Studies demonstrated that patients with Gaucher disease have abnormal Ig levels, monoclonal gammopathy, and other disorders with immune phenotyping [34]. In these patients, an association has been shown between oligoclonal gammopathy that develops on the basis of hypergammaglobulinemia and benign monoclonal gammopathy and multiple myeloma [35] . In only one patient with Gaucher type I (C12) was the IgG level close to the upper limit for age, while the IgM and IgA levels were above the normal range. Moreover, Ig levels were reported to be very close to the upper limits for age in patients diagnosed with Gaucher disease. There was no difference between the Ig levels in Gaucher disease type I and III. One study reported that patients with the folding homozygous mutation in N370S in Gaucher disease were more prone to monoclonal gammopathy [36], In the present study, in two of four patients with Gaucher type I (54 and 26 years old) with the same mutation, TRAB, anti-gliadin IgG and anti-DNA antibodies were positive. The 56-year-old patient with temporary TRAB positivity had the N370S/V499M mutation. In patients with critical SARS-CoV-2 pneumonia in China, the presence of autoimmune disease-associated SSA/Ro antibody and antinuclear antibody at a rate of 20%–50% was demonstrated [37]. The prevalence of autoantibodies in LSD patients was reported to be 32.9% in this study. However, we do not yet know how COVID-19 infection would affect the immune response triggered in patients. Increased cytokines cause a similar inflammation in Gaucher disease and COVID-19 infection [7]. It should be noted that additional studies are needed to determine whether SARS-CoV-2 has similarities with GD in terms of hypercytokinemia [38]. In this study, positivity was found in autoimmunity markers examined during an infection-free period in nine of 40 patients with MPS patients, four of eight patients with Pompe disease, and 15 of 37 patients with Gaucher disease. All these patients were receiving ERT regularly. It was a remarkable finding that 15 of 37 patients with Gaucher disease (40.5%) had autoantibody positivity, and four of them had both autoantibody positivity and low levels of immunodeficiency parameters.
In a study of Gaucher disease conducted on mice, there was an increase in anti-DNA; however, this was not related to SLE, and it was concluded that autoantibodies were natural antibodies, i.e., they were not pathogenic. Natural antibodies develop in healthy people, do not cause tissue damage, and are different from autoantibodies produced in patients with autoimmune diseases that cause tissue damage. Natural antibodies are involved in normal homeostasis and are essential for removing harmful foreign bodies and creating a network in controlling the immune response [39]. There is chronic stimulation of the humoral immune system in Gaucher disease [15]. In this study, autoantibody positivity did not cause any clinical results or biochemical abnormalities in any patient except one patient with Hashimoto disease. One of the patients who survived the COVID-19 infection with mild symptoms had Gaucher disease type I and the other had MPS type II. While no abnormality was found in the study parameters of the patient with Gaucher disease, the patient with MPS type II had immune dysfunction. Autoantibody positivity was reported in the elder sister of the patient with Gaucher type I, who was followed up for the same diagnosis, but she did not have COVID-19 infection. One study reported that 17 patients who were receiving ERT at a higher dose of 47.5 IU/kg had a lower cytokine response compared with 14 patients who were receiving a dose of 23.57 IU/kg [40]. It is possible that because all of our Gaucher patients were regularly receiving ERT during the COVID-19 pandemic, 60% of them were found to have a normal autoantibody response. Therefore, further studies are warranted on the relationship between inflammatory response and COVID-19 in patients with Gaucher disease who are and are not regularly receiving ERT.
Hypercytokinemia is observed in patients with Pompe disease. Antibody response, cytokine release, and CD4+ T cell response can develop [41]. It was observed that four of eight patients with Pompe disease included in the study had at least one autoantibody positivity. Unlike those in the literature, these patients were receiving ERT at a high dose of 20 U/kg weekly, none of them developed an enzyme-related reaction, and none of them had COVID-19.
5. Conclusion
We aimed to evaluate whether the hypothesis that patients with LSDs are susceptible to COVID-19 infection and have a severe infection is a myth, which has been theoretically suggested but lacked sufficient patient data. In our study, 56 of 110 patients had an abnormality in at least one of the autoimmunity or immunodeficiency parameters. However, no clinical presentation that matched with the laboratory findings was observed in any patient, except in one patient with Hashimoto disease. Except for two patients with Gaucher type I disease, one patient with MPS type II, and one patient with MPS type IV, no patient had a COVID-19 infection.
The evidence supports the view that cell entry is one of the main routes for the virus to infect the host along with immunity, including receptor binding after membrane fusion, which is activated by lysosomal proteases. This view is also supported by the fact that the deposition of macromolecules by macrophages triggers inflammation and causes disease progression in LSDs via varying degrees of damage to lysosomal proteases. Additionally, this brings about low infection rates among patients with LSDs. The other factor leading to this result might be these patients protect themselves much more by reducing contact because of the fear of being infected. On the other hand, several theories indicate that immune activity against metabolites in LSDs might induce a different immunological pathway, including NK and B cells. Therefore, it is remarkable that patients with LSD did not have a much more serious risk of contracting COVID-19 infection than the healthy population. Further investigations of lysosome biology in hosts will help establish the patterns of LSDs and vulnerability to COVID-19. This may feasibly help create a cure for humans. All but one patient in our study were receiving ERT, and the frequency of COVID-19 was estimated to be low. In previous studies, ERT was shown to reduce the number of upper airway infections [42]. Additionally, in one study, ERT was demonstrated to correct neutrophil chemotaxis. It is undeniable that this is only a hypothesis; however, it is worth investigating to what degree ERT ameliorates these immunological pathways and assessing the difference between ERT and non-ERT-receiving genetic models in the vulnerability to COVID-19 severity.
Financial disclosure
The authors declare that this study received no financial support.
Research funding
None declared.
Declaration of Competing Interest
No conflict of interest was declared by the authors.
References
- 1.Leung D.T., Tam F.C., Ma C.H., et al. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J Infect Dis. 2004;190:379–386. doi: 10.1086/422040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu X., Zhang L., Du H., et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsetti R., Quintarelli C., Quinti I., et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc Health. 2020;4:414–416. doi: 10.1016/S2352-4642(20)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry B.M., Lippi G., Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;58:1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mistry P., Balwani M., Barbouth D., et al. Gaucher disease and SARS-CoV-2 infection: emerging management challenges. Mol Genet Metab. 2020;130:164–169. doi: 10.1016/j.ymgme.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt F.M., d'Azzo A., Davidson B.L., Neufeld E.F., Tifft C.J. Lysosomal storage diseases. Nat Rev Dis Primers. 2018;4:27. doi: 10.1038/s41572-018-0025-4. [DOI] [PubMed] [Google Scholar]
- 9.Ivanova M.M., Changsila E., Iaonou C., Goker-Alpan O. Impaired autophagic and mitochondrial functions are partially restored by ERT in Gaucher and Fabry diseases. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniloski Z., Jordan T.X., Wessels H.H., et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2021;184 doi: 10.1016/j.cell.2020.10.030. 92–105 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magalhaes J., Gegg M.E., Migdalska-Richards A., Doherty M.K., Whitfield P.D., Schapira A.H. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet. 2016;25:3432–3445. doi: 10.1093/hmg/ddw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman A.P., Puertollano R., Raben N., Slaugenhaupt S., Walkley S.U., Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012;8:719–730. doi: 10.4161/auto.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aflaki E., Moaven N., Borger D.K., et al. Lysosomal storage and impaired autophagy lead to inflammasome activation in Gaucher macrophages. Aging Cell. 2016;15:77–88. doi: 10.1111/acel.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker H., Bigger B.W. The role of innate immunity in mucopolysaccharide diseases. J Neurochem. 2019;148:639–651. doi: 10.1111/jnc.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascimbeni A.C., Fanin M., Tasca E., Angelini C., Sandri M. Impaired autophagy affects acid alpha-glucosidase processing and enzyme replacement therapy efficacy in late-onset glycogen storage disease type II. Neuropathol Appl Neurobiol. 2015;41:672–675. doi: 10.1111/nan.12214. [DOI] [PubMed] [Google Scholar]
- 16.Bayram R.O., Ozdemir H., Emsen A., Turk Dagi H., Artac H. Reference ranges for serum immunoglobulin (IgG, IgA, and IgM) and IgG subclass levels in healthy children. Turk J Med Sci. 2019;49:497–505. doi: 10.3906/sag-1807-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimran A., Szer J., Revel-Vilk S. Impact of Gaucher disease on COVID-19. Intern Med J. 2020;50(7):894–895. doi: 10.1111/imj.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade-Campos M., Escuder-Azuara B., de Frutos L.L., et al. Direct and indirect effects of the SARS-CoV-2 pandemic on Gaucher Disease patients in Spain: time to reconsider home-based therapies? Blood Cells Mol Dis. 2020;85 doi: 10.1016/j.bcmd.2020.102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fierro L., Nesheiwat N., Naik H., Narayanan P., Mistry P.K., Balwani M. Gaucher disease and SARS-CoV-2 infection: experience from 181 patients in New York. Mol Genet Metab. 2021;132:44–48. doi: 10.1016/j.ymgme.2020.12.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturley S.L., Rajakumar T., Hammond N., et al. Potential COVID-19 therapeutics from a rare disease: weaponizing lipid dysregulation to combat viral infectivity. J Lipid Res. 2020;61:972–982. doi: 10.1194/jlr.R120000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y., Feng F., Hu G., et al. A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nat Commun. 2021;12:961. doi: 10.1038/s41467-021-21213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R., Simoneau C.R., Kulsuptrakul J., et al. Genetic screens identify host factors for SARS-CoV-2 and common cold coronaviruses. Cell. 2021;184 doi: 10.1016/j.cell.2020.12.004. 106–19 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahamov A., Elstein D., Gross-Tsur V., et al. Gaucher's disease variant characterised by progressive calcification of heart valves and unique genotype. Lancet. 1995;346:1000–1003. doi: 10.1016/s0140-6736(95)91688-1. [DOI] [PubMed] [Google Scholar]
- 25.Minotti C., Tirelli F., Barbieri E., Giaquinto C., Dona D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81 doi: 10.1016/j.jinf.2020.04.026. e61-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui D.S., Azhar E.I., Kim Y.J., Memish Z.A., Oh M.D., Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18 doi: 10.1016/S1473-3099(18)30127-0. e217-e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 28.Torres L.C., Soares D.C., Kulikowski L.D., Franco J.F., Kim C.A. NK and B cell deficiency in a MPS type II family with novel mutation in the IDS gene. Clin Immunol. 2014;154:100–104. doi: 10.1016/j.clim.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang Y., Peng H., Chen Y., Zhou S., Chen Y. Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Front Biosci (Landmark Ed) 2017;22:1344–1354. doi: 10.2741/4547. [DOI] [PubMed] [Google Scholar]
- 30.Vitner E.B., Platt F.M., Futerman A.H. Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem. 2010;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith D., Wallom K.L., Williams I.M., Jeyakumar M., Platt F.M. Beneficial effects of anti-inflammatory therapy in a mouse model of Niemann-Pick disease type C1. Neurobiol Dis. 2009;36:242–251. doi: 10.1016/j.nbd.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Jeyakumar M., Smith D.A., Williams I.M., et al. NSAIDs increase survival in the Sandhoff disease mouse: synergy with N-butyldeoxynojirimycin. Ann Neurol. 2004;56:642–649. doi: 10.1002/ana.20242. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi A., Katsuyama K., Nagahama K., Takai T., Aoki I., Yamanaka S. Possible role of autoantibodies in the pathophysiology of GM2 gangliosidoses. J Clin Invest. 2004;113:200–208. doi: 10.1172/JCI19639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox T.M., Rosenbloom B.E., Barker R.A. Gaucher disease and comorbidities: B-cell malignancy and parkinsonism. Am J Hematol. 2015;90(Suppl 1):S25–S28. doi: 10.1002/ajh.24057. [DOI] [PubMed] [Google Scholar]
- 35.Marti G.E., Ryan E.T., Papadopoulos N.M., et al. Polyclonal B-cell lymphocytosis and hypergammaglobulinemia in patients with Gaucher disease. Am J Hematol. 1988;29:189–194. doi: 10.1002/ajh.2830290403. [DOI] [PubMed] [Google Scholar]
- 36.Beutler E. Gaucher disease as a paradigm of current issues regarding single gene mutations of humans. Proc Natl Acad Sci U S A. 1993;90:5384–5390. doi: 10.1073/pnas.90.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y., Han T., Chen J., et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13(6):1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tufan A., Avanoglu Guler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoenfeld Y., Beresovski A., Zharhary D., et al. Natural autoantibodies in sera of patients with Gaucher's disease. J Clin Immunol. 1995;15:363–372. doi: 10.1007/BF01541326. [DOI] [PubMed] [Google Scholar]
- 40.Matta M.C., Vairo F., Torres L.C., Schwartz I. Could enzyme replacement therapy promote immune tolerance in Gaucher disease type 1? Blood Cells Mol Dis. 2018;68:200–202. doi: 10.1016/j.bcmd.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Doerfler P.A., Nayak S., Corti M., Morel L., Herzog R.W., Byrne B.J. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol Ther Methods Clin Dev. 2016;3:15053. doi: 10.1038/mtm.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Concolino D., Deodato F., Parini R. Enzyme replacement therapy: efficacy and limitations. Ital J Pediatr. 2018;44:120. doi: 10.1186/s13052-018-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]