Abstract
Background
Dolutegravir monotherapy (DTG-m) results in virological failure (VF) in some people with human immunodeficiency virus (PWH). We sought to identify the independent factors associated with the risk of VF and to explore the effect size heterogeneity between subgroups of PWH enrolled in DTG-m trials.
Methods
We searched for randomized clinical trials (RCTs) evaluating DTG-m versus combined antiretroviral therapy (cART) among PWH virologically controlled for at least 6 months on cART. We performed an individual participant data meta-analysis of VF risk factors and quantified their explained heterogeneity in random-effect models. Definition of VF was a confirmed plasma human immunodeficiency virus (HIV)-1 ribonucleic acid (RNA) >50 copies/mL by week 48.
Results
Among 416 PWH from 4 RCTs, DTG-m significantly increased the risk of VF (16 of 227 [7%] versus 0 of 189 for cART; risk difference 7%; 95% confidence interval [CI], 1%–2%; P = .02; I2 = 51%). Among 272 participants exposed to DTG-m, VF were more likely in participants with the following: first cART initiated ≥90 days from HIV acute infection (adjusted hazard ratio [aHR], 5.16; 95% 95% CI, 1.60–16.65), CD4 T cells nadir <350/mm3 (aHR, 12.10; 95% CI, 3.92–37.40), HIV RNA signal at baseline (aHR, 4.84; 95% CI, 3.68–6.38), and HIV-deoxyribonucleic acid (DNA) copy number at baseline ≥2.7 log/106 peripheral blood mononuclear cells (aHR, 3.81; 95% CI, 1.99–7.30). Among these independent risk factors, the largest effect size heterogeneity was found between HIV DNA subgroups (I2 = 80.2%; P for interaction = .02).
Conclusions
Our study supports the importance of a large viral reservoir size for explaining DTG-m simplification strategy failure. Further studies are needed to link size and genetic diversity of the HIV-1 reservoir.
Keywords: dolutegravir, HIV, individual-participant data meta-analysis, monotherapy, proviral DNA, randomized trial
Among the 4 independent risk factors associated with virological failure under dolutegravir monotherapy (DTG-m), HIV-DNA explained 80% of the effect size heterogeneity in an individual participant data meta-analysis from 4 randomized controlled trials of DTG-m versus cART currently in existence.
Current recommended antiretroviral combinations include triple or dual integrase-strand transfer inhibitor (INSTI)-based regimen [1–3]. The majority of people with human immunodeficiency virus (PWH) are virologically controlled and therefore nontransmitters without acquired immune deficiency syndrome-related complications. However, the antiretroviral treatment (ART) of human immunodeficiency virus (HIV) infection requires life-long therapy, which can be associated with side effects, toxicities, pill burden, drug-drug interactions, and long-term complications particularly for aging PWH [4, 5]. In an effort to improve quality of life and tolerance and decrease healthcare-related costs, several ART simplification maintenance strategies have been investigated [6–16].
Dolutegravir (DTG) is a once-daily, second-generation INSTI with high potency, high genetic barrier to resistance, high forgiveness to missed doses, good safety profile, and few drug-drug interactions [17–23]. These characteristics placed dolutegravir monotherapy (DTG-m) as a good potential candidate for maintenance therapy. Unfortunately, the DTG-m had an unacceptable rate of failure compared with the comparator combined antiretroviral therapy (cART) arm. However, in these 3 randomized controlled trials (RCTs), namely, DOMONO [22], DOLAM [24], and MONCAY [25], which enrolled chronically infected patients, a large fraction of the participants receiving DTG-m was still able to continuously suppress HIV-ribonucleic acid (RNA) replication to undetectable plasma levels. Moreover, in the EARLY-SIMPLIFIED study [26], a fourth RCT enrolling patients who initiated ART during primary HIV infection, DTG-m was noninferior to cART. The risk factors associated with DTG-m virological failure (VL) may well apply to dual therapies including dolutegravir plus a second antiretroviral with low genetic barrier to resistance. This concern of “functional dolutegravir monotherapy” is particularly relevant in the context of previous treatment failure with resistance [1, 13–16, 27]. Finally, potential metabolic problems may arise when DTG is combined with tenofovir alafenamide [28, 29]. However, to identify these factors we require individual-level participants data and large sample size to perform multivariable analysis. One study (DOMONO) found associations between VF on DTG-m and CD4 T-cell nadir, duration between HIV diagnosis and ART initiation, and total HIV deoxyribonucleic acid (DNA) copy numbers, but the sample size precluded complete multivariable analysis [22, 30].
First, we explored the influence of participant-level covariates on the risk of DTG-m virological failure using all available evidence from RCTs. Second, based on the covariates identified to be independently associated with DTG-m virological failure, we explored the heterogeneity of the risk of DTG-m failure compared with cART, through an individual participant data meta-analysis (IPDMA).
METHODS
Study Oversight and Search Strategy
We conducted an IPDMA according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines for the meta-analysis of RCTs [31]. Our protocol was prospectively registered in PROSPERO (number CRD42020221501). We searched Medline, EMBASE, Cochrane Central and Web of Science, using the keywords “dolutegravir monotherapy” and “virological failure”. Furthermore, we searched clinicaltrials.gov for unpublished studies. The last search was conducted on April 21, 2021. We considered all RCTs investigating DTG-m. Participant characteristics of eligible studies included were as follows: aged 18 or above and virologically controlled HIV-1-infected participants (plasma HIV RNA <50 copies/mL) for at least 6 months at the time of screening, and participants randomly assigned to receive DTG-m or cART. No language restrictions were applied. Studies had to report on virological outcomes of participants who switched to DTG-m. Two investigators (A.L.F. and J.-J.P.) independently selected studies based on titles and, in a second step, assessed the eligibility based on the full-text articles. Corresponding authors of eligible studies were asked to participate with a collaborative group to perform an IPDMA by sharing their original study database.
Patient Consent Statement
The patient’s written consent was obtained for participating in trials included in this meta-analysis.
Human Subjects’ Data Protection
The Caen University Hospital signed a data-sharing agreement with all corresponding author institutions. All of the participants provided written informed consent to participate in the RCTs and agreed for further research with anonymized collected data. All of the parent studies received approval from independent ethics committee, as appropriate.
Data Collection and Management
The following data were extracted independently at the study level by 2 reviewers (A.L.F. and J.-J.P.), using a standardized spreadsheet: inclusion and exclusion criteria, definitions of outcomes, and number of participants and their main demographical and clinical characteristics, including immunological status, virological parameters, and HIV integrase mutations.
Regarding individual-level variables, the data manager of each qualified study performed a deidentified individual data extraction including the prespecified following variables: trial arm, date of inclusion, sex, age at inclusion, ethnicity, HIV transmission risk, nadir CD4 T-cell count, date of HIV diagnosis, prior exposure to INSTI, previous genotypic resistance to any ART, previous genotypic resistance to any INSTI, presence of a polymerase chain reaction (PCR) signal below the quantification threshold, CD4 T-cell count, total HIV-1 DNA—at baseline and at week 48—virologic failure, and HIV integrase mutations. A binary variable HIV DNA count at baseline was created with a cutoff at 2.7 log/106 peripheral blood mononuclear cells (PBMCs) according to Trulight study [6]. We built a binary variable nadir of CD4 T-cell count with a cutoff of 350 cells/mm3 and a time before first ART with a cutoff at 90 days according to the medians. After validation by each DM of the parent studies, we merged participants into 1 database formatted with a common naming structure.
For quality control, we compared data collected from the original papers and from the database shared by authors. In cases of discrepancies from the published article or trial protocol, the authors were contacted for clarification. The methodological components of the RCTs such as blinding (participants, personnel and outcome assessor), incomplete outcome data, and other sources of bias were assessed by 2 independent authors (A.L.F. and J.-J.P.) as recommended.
Outcomes
The primary endpoint was virological failure, defined as 2 consecutive viral loads >50 copies/mL during follow-up. Our main outcome was to determine the overall risk difference (RD) of virological failure between the DTG-m arm and cART arm. The secondary outcomes of the study were the safety and the emergent genotypic resistance to the INSTI class in case of virological failure. Only the DOMONO trial used a different definition of virological failure, VL >200 copies/mL, but the 50 copies/mL cutoff was included as secondary endpoint, and thus the data were available to update the outcome variable to fit with our new definition.
Statistical Analysis
Details of each step are provided in Supplemental Appendix. No sample size was estimated a priori and all available data were used. Analyses were conducted on the intention-to-treat dataset for 3 studies [24–26] and on the treatment dataset for 1 study [22]. The statistical analysis plan had 2 objectives: (1) identify risk factors for DTG-m virological failure and (2) explore heterogeneity of the effect size between subgroups stratified by previously identified risk factors.
In brief, we analyzed all patients who received DTG-m during the follow-up into a single-stage meta-analysis [32]. We computed uni- and multivariate Cox models of independent virological failure risk factors. Statistical analyses and data preparation were conducted in STATA version 14.1 (StataCorp, College Station, TX) and SAS version 9.4 (SAS Institute, Cary, NC). Regarding heterogeneity, we also used all participants from the 2 randomized arms (DTG-m and controls). We estimated the incidence of virological failure and then compared time to virological failure between DTG-m arm and cART arm with a Kaplan-Meir method. The effect size of the DTG-m was estimated by the absolute RD and 95% confidence interval (CI) of the cumulative risk of virological failure between DTG-m and cART. To take into account the intrastudy correlation, we performed a 2-stage meta-analysis with random-effect models. Subgroup analyses were defined according to the independent risk factors found in the multivariate analysis, and the degree of heterogeneity was quantified by the I2, as appropriate [33]. Statistical analyses and data preparation were conducted in Revman v5 software.
RESULTS
Twenty-three single studies were identified in the literature search, 4 of which were RCTs and were included in the meta-analysis: EARLY-SIMPLIFIED, Switzerland [26], DOMONO, The Netherlands [22], DOLAM, Spain [24], and MONCAY, France [25] (Supplementary Figure S1). The characteristics of included studies are presented in Supplementary Table S1. The PRISMA quality of studies is shown in Supplementary Figure S2.
Baseline Characteristics
Four hundred sixteen subjects were enrolled in total: the DTG-m arm comprised 227 subjects and the cART arm comprised 189 subjects. Baseline characteristics for the maintenance treatment group and control group were comparable (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Individual Patients’ Data in DTG-m Group and cART Group, in Complete Case Analysis
| Variables | Overall N = 416 | DTG-m N = 227 | cART N = 189 |
|---|---|---|---|
| Male, n (%) | 354 (85.1%) | 197 (86.8%) | 157 (83.1%) |
| Age at baseline, mean (SD) | 46 (12) | 45 (12) | 47 (12) |
| Ethnicity, n (%) | |||
| White | 361 (86.8%) | 199 (87.7%) | 162 (85.7%) |
| Black | 42 (10.1%) | 23 (10.1%) | 19 (10.1%) |
| Other | 13 (3.1%) | 5 (2.2%) | 8 (4.2%) |
| Time before first ART (days)a, median (IQR) | 91 (30.4–759) | 72 (29–547) | 133 (33–863) |
| HIV Transmission Groupa, n (%) | |||
| Men who have sex with men | 288 (69.4%) | 159 (70.0%) | 129 (68.6%) |
| Heterosexual | 98 (23.6%) | 54 (23.8%) | 44 (23.4%) |
| Other | 29 (7.0%) | 14 (6.2%) | 15 (8.0%) |
| Nadir CD4 T-cell count (/mm3), mean (SD) | 362 (178) | 368 (178) | 354 (179) |
| Zenith viral load (log copies/mL)b, mean (SD) | 4.63 (0.85) | 4.69 (0.85) | 4.57 (0.85) |
| BMI (kg/m) at baseline, mean (SD) | 24.8 (3.7) | 24.8 (3.7) | 24.7 (3.8) |
| Duration of cART before inclusionb (years), median (IQR) | 5.9 (2.7–12.9) | 5.0 (2.5–10.5) | 6.9 (3.0–13.9) |
| INI 1st-generation exposure, n (%) | 60 (14.4%) | 27 (11.9%) | 33 (17.5%) |
| Previous Genotypic Resistance to Any Integrase Strand Transfer Inhibitora, n (%) | 5 (2.3%) | 3 (2.6%) | 2 (2.0%) |
| Presence of a PCR signal at baselineb | 72 (17.4%) | 38 (16.7%) | 34 (18.1%) |
| CD4 T-cell count at baseline (/mm3), mean (SD) | 777 (284) | 786 (276) | 767 (294) |
| HIV DNA at baseline (log/106 PBMCs)b, mean (SD) | 2.33 (0.45) | 2.38 (0.43) | 2.25 (0.47) |
Abbreviations: ART, antiretroviral treatment; BMI, body mass index; cART, combined antiretroviral therapy; DNA, deoxyribonucleic acid; DTG-m, dolutegravir monotherapy; HIV, human immunodeficiency virus; INI, integrase inhibitor; IQR, interquartile range; PBMCs, peripheral blood mononuclear cells; PCR, polymerase chain reaction; SD, standard deviation.
Risk Factors of Dolutegravir Monotherapy Failure
We pooled together all the participants on DTG-m in the 4 RCTs, 227 participants of the DTG-m arms plus the 45 participants in the DOMONO trial who switched to DTG-m at week 24. (Supplementary Figure S3). Median follow-up on DTG-m was 48 weeks (interquartile range [IQR], 24–48). Among these 272 participants, 18 virological failures occurred. Incidence of virological failure was 1.69/100 persons-years (95% CI, 1.07–2.69).
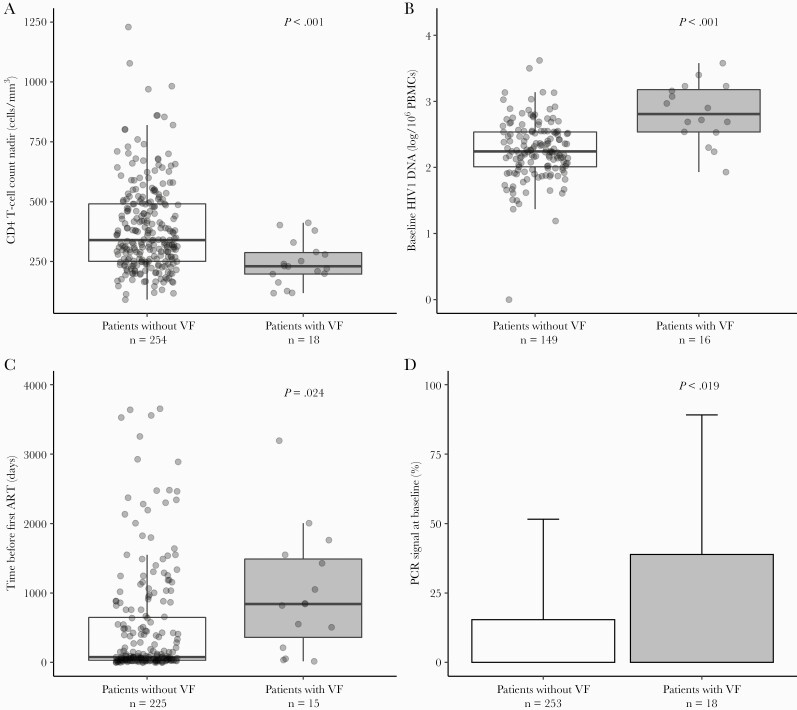
Individuals with virological failure were all male, with a mean age of 50 years (minimum–maximum, 27–68), 83% were Caucasian, and 67% were men who have sex with men. Nadir of CD4 T cells was <350 cells/mm3 for 15 of 18 individuals with virological failure (83%). Zenith of viral load mean was 4.78 log copies/mL (standard deviation = 0.58). Median time between HIV diagnosis and first ART was 2.3 years (IQR, 0.6–4.2). Median time on cART until the switch to DTG-m was 5.4 years (IQR, 4.2–10.5). Time before first ART was ≥90 days in 12 of 15 individuals (80%). A PCR signal at baseline was evidenced in 7 of 18 cases (39%), and HIV DNA at baseline was in ≥2.7/106 PBMCs among 9 of 16 individuals (56%) (Table 2). Univariate and multivariate analysis are shown in Table 2 and Figure 1. Risk factors independently associated with virological failure were a time before first ART ≥90 days (adjusted hazard ratio [aHR], 5.16; 95% CI, 1.60–16.65), a nadir of CD4 T cells lower than 350 (aHR, 12.10; 95% CI, 2.92–37.40), when an RNA plasma PCR signal was detected at baseline (aHR, 4.84; 95% CI, 3.68–6.38), and a HIV DNA level at baseline ≥2.7/106 PBMCs (aHR, 3.81; 95% CI, 1.99–7.30).
Table 2.
Description and Cox Model of Factors Associated With Virological Failure Among Subjects Randomized to Dolutegravir Monotherapy, N = 164
| Variables | No Virological Failure N = 254 | Virological Failure N = 18 | Univariate Analysis Hazard Ratio (95% CI) | P Value | Multivariate Analysis* Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| Gender, n (%) | ||||||
| Male | 221 (87.0%) | 18 (100%) | 1 | .09** | ||
| Female | 33 (13.0%) | 0 (0%) | NA | |||
| Age at baseline, mean (SD) | 45 (12) | 50 (11) | 1.04 (1.01–1.08) | .05 | ||
| Ethnicity, n (%) | ||||||
| White | 219 (86.2%) | 15 (83.3%) | 1 | |||
| Black | 27 (10.6%) | 3 (16.7%) | 1.62 (0.47–5.59) | .58** | ||
| Other | 8 (3.2%) | 0 | NA | |||
| Time before first ART (days)a, n (%) | ||||||
| <90 | 117 (52.0%) | 3 (20.0%) | 1 | 1 | ||
| ≥90 | 108 (48.0%) | 12 (80.0%) | 4.62 (1.30–16.38) | .008 | 5.16 (1.60–16.65) | .006 |
| HIV transmission group, n (%) | ||||||
| Men who have sex with men | 182 (71.7%) | 12 (66.7%) | 1 | .94 | ||
| Heterosexual | 56 (22.0%) | 5 (27.8%) | 1.21 (0.43–3.43) | |||
| Other | 16 (6.3%) | 1 (5.5%) | 0.96 (0.13–7.40) | |||
| Nadir CD4-T cells count (/mm3), n (%) | ||||||
| <350 | 133 (52.4%) | 15 (83.3%) | 4.23 (1.22–14.60) | .009 | 12.10 (3.92–37.40) | <.001 |
| ≥350 | 121 (47.6%) | 3 (16.7%) | 1 | 1 | ||
| Zenith viral load (log copies/mL)***, mean (SD) | 4.62 (0.80) | 4.78 (0.58) | 1.15 (0.62–2.13) | .664 | ||
| BMI (kg/m) at baselinea, mean (SD) | 24.9 (3.8) | 25.5 (3.5) | 1.05 (0.93–1.17) | .47 | ||
| Duration of cART before inclusion*** (days), median (IQR) | 1651 (763–3435) | 1973 (1537–3841) | 1.00 (1.00–1.00) | .845 | ||
| INI 1st-generation exposure, n (%) | ||||||
| Yes | 30 (11.8%) | 1 (5.6%) | 0.46 (0.06–3.44) | .39 | ||
| No | 224 (88.2%) | 17 (94.4%) | 1 | |||
| Presence of a PCR signal at baselinea, n (%) | ||||||
| Yes | 39 (15.4%) | 7 (38.9%) | 3.38 (1.31–8.71) | .02 | 4.84 (3.68–6.38) | <.001 |
| No | 214 (84.6%) | 11 (61.1%) | 1 | 1 | ||
| CD4 T-cell count at baseline (/mm3), mean (SD) | 779.2 (273.9) | 742.2 (233.2) | 0.97 (0.88–1.06)*** | .47 | ||
| HIV DNA at baseline (log/106 PBMCs)b, n(%) | ||||||
| <2.7 | 128 (85.9%) | 7 (43.7%) | 1 | 1 | ||
| ≥2.7 | 21 (14.1%) | 9 (56.3%) | 6.01 (2.24–16.15) | <.001 | 3.81 (1.99–7.30) | <.001 |
Abbreviations: BMI, body mass index; cART, combined antiretroviral therapy; CI, confidence interval; DNA, deoxyribonucleic acid; HIV, human immunodeficiency virus; IQR, interquartile range; NA, nonapplicable; PCR, polymerase chain reaction; PBMCs, peripheral blood mononuclear cells; SD, standard deviation.
Presence of missing data (between <1% and 11%).
Presence of missing data (between 38% and 41%).
*HIV transmission group variable did not meet the proportional risk hypothesis.
**Log-rank test.
***HR represents an increase of 50 cells.
Figure 1.
Distributions of (A) CD4 T-cell nadir, (B) baseline human immunodeficiency virus (HIV)1 deoxyribonucleic acid (DNA) copy number in peripheral blood mononuclear cells (PBMCs), (C) time before first antiretroviral treatment (ART), and (D) presence of polymerase chain reaction signal at baseline in patients with and without virological failure (VF) during dolutegravir monotherapy (DTG-m).
In a sensitivity analysis including the observed median as the cutoff for HIV DNA variable, risk factors associated with virological failure were similar: first cART ≥90 days from HIV diagnosis (aHR, 5.21; 95% CI, 1.40–19.44), CD4 T-cell nadir <350/mm3 (aHR, 13.22; 95% CI, 3.57–48.95), a detected plasma HIV RNA signal at baseline (aHR, 6.24; 95% CI, 4.45–8.74), and HIV DNA copy number at baseline ≥2.3 log/106 PBMCs (aHR, 4.25; 95% CI, 1.52–11.86) (Supplementary Table S2).
The multivariate analyses were performed among 164 of 272 subjects. Comparison of subjects included in the multivariate analyses and others is shown in Supplementary Table S3. No virological failure occurred among the 56 DTG-m participants with a nadir of CD4 T cell above 350/mm³ and an HIV DNA level below 2.7/106 PBMCs.
Effect of Dolutegravir Monotherapy Versus Combined Antiretroviral Therapy
Overall Risk Differences
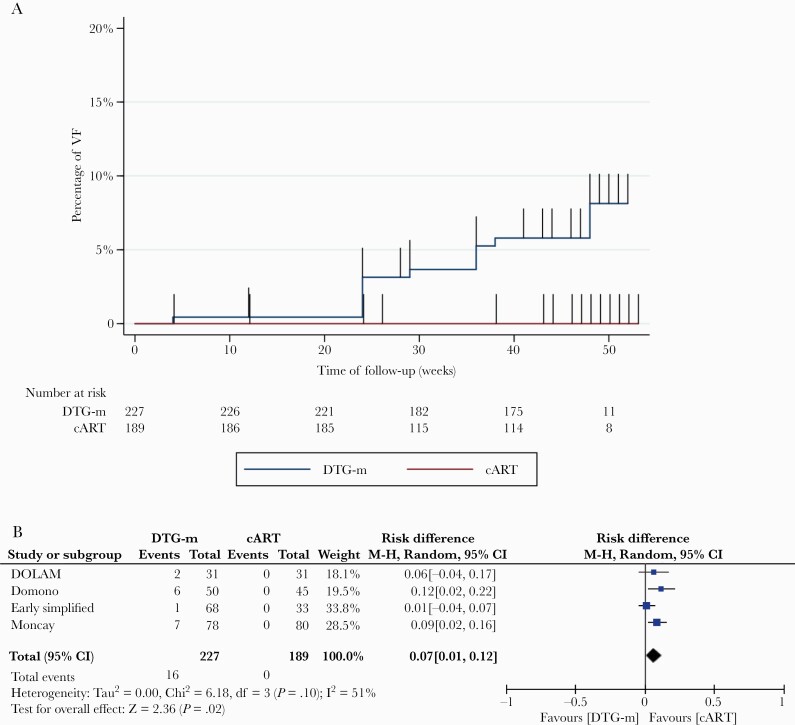
Overall, 16 individuals among 227 (7%) in the DTG-m arm had a virological failure versus none in the cART arm at week 48 of follow-up. Time to virological failure was statistically significantly different between DTG-m and cART groups according to the Kaplan-Meier curve (Figure 2A) (log-rank test, P = .0007). Cumulative RD of virological failure computed by 2-step IPDMA between the 2 arms was significantly different (RD = 0.07; 95% CI, 0.01–0.12; P = .02). Overall, between-study heterogeneity was I2 = 51% (Figure 2B).
Figure 2.
Incidence (A) and risk difference (B) of virological failure (VF) between dolutegravir monotherapy (DTG-m) and combined antiretroviral therapy (cART).
Subgroup Analyses
Analyses of the virological failure differences between individuals in the DTG-m arm versus the cART arm for each group of participants found to be at risk of virological failure in the Cox model are shown in Table 3.
Table 3.
Heterogeneity in Subgroup Analyses of the Virological Failure Difference Between Participants on DTG-m or cART
| Stratification Variables | m-DTG (n/N) | cART Arm (n/N) | D (95% CI) | P Value | Subgroup I2 | P Value for Interaction |
|---|---|---|---|---|---|---|
| Time before first ART ≥90 days | 10/87 | 0/93 | 0.10 (0.01–0.19) | .04 | 47.2% | .17 |
| Time before first ART <90 days | 3/108 | 0/65 | 0.02 (−0.04 to 0.08) | .47 | ||
| Nadir CD4 <350/mm3 | 13/129 | 0/114 | 0.08 (−0.01 to 0.17) | .08 | 29.0% | .24 |
| Nadir CD4 ≥350/mm3 | 3/98 | 0/75 | 0.02 (−0.03 to 0.07) | .52 | ||
| Presence of a PCR signal at baseline | 7/38 | 0/34 | 0.16 (0.03 to 0.30) | .02 | 64.2% | .09 |
| Absence of a PCR signal at baseline | 9/189 | 0/154 | 0.04 (0.01 to 0.08) | .02 | ||
| HIV DNA at baseline ≥2.7 log/106 PBMCs | 9/27 | 0/10 | 0.32 (0.09 to 0.56) | .007 | 80.2% | .02 |
| HIV DNA at baseline <2.7 log/106 PBMCs | 5/106 | 0/70 | 0.04 (−0.03 to 0.11) | .22 |
Abbreviations: ART, antiretroviral treatment; cART, combined antiretroviral therapy; CI, confidence interval; D, risk difference; DNA, deoxyribonucleic acid; DTG-m, dolutegravir monotherapy; HIV, human immunodeficiency virus; n, number of virological failures; N, number of patients; PCR, polymerase chain reaction; PBMCs, peripheral blood mononuclear cells.
NOTE: Data NA: nadir CD4 (n = 0), HIV DNA (n = 203), PCR signal (n = 1), time before ART (n = 63).
A difference of 32% between the 2 arms was found in the subgroup of participants with a baseline HIV DNA ≥2.7 log/106 PBMCs (RD = 0.32; 95% CI, 0.09–0.56) and 4% in the subgroup of participants with a baseline HIV DNA <2.7 log/106 PBMCs (RD = 0.04; 95% CI, −0.03 to 0.11). Heterogeneity was measured I2 = 80.2%, and the RD was significantly higher among the DTG-m arm with a baseline HIV DNA ≥2.7 log/106 PBMCs (P = .007) and the interaction test was significant (P = .02). A difference of 21% between the 2 arms was found in the subgroup of participants with a baseline HIV DNA ≥2.3 log/106 PBMCs (RD = 0.21; 95% CI, −0.03 to 0.45) and 0% in the subgroup of participants with a baseline HIV DNA <2.3 log/106 PBMCs (RD = 0.00; 95% CI, −0.06 to 0.6). Heterogeneity was measured I2 = 63.5% (Supplementary Figures S4–8).
Secondary Outcomes
Amplification for drug resistance testing was successful for 15 of the 18 (83.3%) participants with virological failure. Seven participants with resistance-associated mutations in the integrase gene among the 15 (46.7%) integrase sequencing obtained were found among DOMONO, DOLAM, and MONCAY participant’s plasma at the time of virological failure. The detected mutations were Asn155His found in 4 of 15 (26.7%) cases, Ser147Gly in 2 of 15 (13.3%) cases, Arg263Lys in 2 of 15 (13.3%) cases, Glu138Lys in 1 of 15 (6.7%) cases, and Ser230Arg in 1 of 15 (6.7%) cases (Supplementary Table S4). Among these 7 participants who had emergence of integrase-associated mutations, none were exposed to any integrase inhibitors before DTG-m. Six of these 7 had an integrase sequenced in plasma at baseline, and no resistance-associated mutations in the integrase gene was found.
All DTG plasma concentrations obtained were above the in vitro, protein-adjusted 90% inhibitory concentration of DTG for wild-type virus, for DOLAM, DOMONO, and EARLY-SIMPLIFIED participants with virological failure. Self-reported adherence was >95% among DOMONO, DOLAM, and MONCAY participants with virological failure. DOLAM, EARLY-SIMPLIFIED, and MONCAY participants assigned to DTG-m arm with any adverse event were 146 of 177 (82.5%) versus 99 of 144 (68.7%) among participants in the cART arm. Study drug-related adverse effects were found among 15 of 146 (10.3%) in the DTG-m arm versus 14 of 113 (12.4%) in the cART arm. Neuropsychiatric effects were reported by 14 of 99 (14.1%) among DTG-m participants and 2 of 64 (3.1%) among cART participants. Serious adverse events were reported among 17 of 177 (9.6%) DTG-m participants versus 19 of 144 (13.2%) among participants in the cART arm.
DISCUSSION
In an IPDMA from 4 European RCTs, we found a higher risk of virological failure in participants who received DTG-m as a maintenance therapy compared with cART in PWH. Moreover, in the event of virological failure, DTG-m led to a risk (considered unacceptable in view of current standards) of emerging resistance mutations for the INSTI class. Overall, this result supports current guidelines that recommend avoiding DTG-m [1, 2]. However, based on parameters that are simple to collect, our work also suggests that VF under DTG-m are not occurring at random. The largest effect size was attributed by the difference in total HIV DNA using a cut off of 2.7 log/106 PBMCs.
Dolutegravir monotherapy use was associated with emerging resistance mutations for the INSTI class. It is interesting to note the recently reported NADIA study found that in the context of resistance mutations to both NRTIs of the backbone, DTG-based triple therapy can rarely result in virological failure with INSTI resistance mutations, whereas no mutations to protease inhibitor (PI) were found when the third agent was darunavir [34]. Dual therapy based on DTG is equivalent to triple therapy in terms of efficacy, as a switch [13, 16, 24] as well as initiation of treatment [35]. Several reports, not all, even support the maintenance of high efficacy in the presence of M184V [36–38], without increased risk of integrase mutation in the event of virological failure. Finally, although virological failures with boosted PI monotherapy were more frequent than with triple therapy, they did not increase the risk of resistance [39]. In summary, in an unselected population, DTG-m is clearly a suboptimal treatment compared with the effectiveness of currently recommended antiretroviral regimens [1, 2].
Of all the parameters associated with good virological control under DTG-m, the size of the reservoir (measured here by total HIV DNA) appears to be the most important, as evidenced by the I2 at 80.2% and significant interaction P value (.02), which means that the excess risk of VF with DTG-m differs by HIV DNA strata. A commercial test allows us to measure total HIV DNA in a standardized way (Biocentric, Bandol, France). Human immunodeficiency virus DNA is strongly correlated with replicative forms of the virus, predicts the natural course of HIV disease, as well as the dynamics of HIV infection under treatment [40, 41]. Higher HIV DNA levels have been described as a risk factor for virological failure during boosted PI monotherapy [39, 42, 43]. The threshold of 2.7 log10 per million PBMC corresponds to the median of the HIV DNA measured in PWH with sustained virological suppression [44]. Human immunodeficiency virus DNA below this threshold was associated with a good virological response in a randomized clinical trial with another suboptimal strategy, compared with a triple therapy [6]. Our study supports the concept that in a therapeutic situation of extreme simplification, such as DTG-m, the existence of a small viral reservoir is associated with the maintenance of good virological suppression, whereas in mirror, a large reservoir is associated with a high risk of virological failure. It is reassuring to know that in observational studies, HIV DNA did not increase during the follow-up in participants who maintained viral suppression during various dual- or even mono-therapies [45, 46].
Our study presented several strengths and limitations. An important strength is that we used individual data from each study participant, which is considered the gold standard to explore subgroups. All were randomized, multicentric noninferiority trials. Homogeneity in the definition of the primary efficacy endpoint was allowed by having access to individual dataset of each trial. The compilation of the studies made it possible to obtain the largest number of PWH exposed to DTG-m in existing RCTs, with varied participant profiles and coming from several European countries. The first limitation is the 48-week follow-up. It is not known whether the risk factors identified here protected from VF or simply delayed VF posterior to week 48. All participants were enrolled in trials, meaning selected participants who were probably more adherent to their antiretrovirals than average. Human immunodeficiency virus DNA was unfortunately not available for all participants. Finally, HIV DNA was unfortunately not available for DOLAM patients. A selection bias in the multivariate analysis of virological failure risk factors under DTG-m may exist. However, the only difference found in the comparison between participants included or not included in the analyses was the CD4 T-cell count at baseline higher in the not included group (P = .04) (Supplementary Table S3). In addition, HIV DNA was not measured by the same technique in the 3 RCTs: it is therefore difficult to make a definitive decision on a precise cutoff to predict virologic failure. However, the same technique was used within each trial while effect size was estimated within each trial in a 2-step IPDMA.
CONCLUSIONS
In the context of treatment for several decades (or even “for life”) and more frequent comorbidities in PWH, it is essential to find for each patient the smallest treatment capable of blocking the replication of their virus, without jeopardizing long-term treatment options. Although DTG-m is not likely to serve as an experimental arm for ethical reasons, the quest to simplify therapy is ongoing. Our work has the merit of exploring the drivers of this therapeutic regimen failure, which may have useful pathogenetic implications in future “tailor-made” strategy trials.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all the patients for participating in these 4 randomized clinical trials. We also thank Renaud Verdon, Sylvie Dargére, Julia Dina, Vincent Le Moing, Pierre-Marie Girard, and Cédric Arvieux for fruitful discussions.
Author contributions. J.-J. P. conceived the study idea. A. L. F., L. H., and J.-J. P. developed the study design. A. L. F. and J.-J. P. performed the systematic literature review. All authors collected and prepared the primary study data. A. L. F. and A. R. B. performed the analysis with guidance from J.-J. P. A. L. F., L. H., and J.-J. P. wrote the first draft of the manuscript. All authors critically revised and approved the manuscript.
Financial support. We did not receive external funding for the current study. The DOLAM trial was supported by a research grant from Instituto de Salud Carlos III (PI16/01085) and Red de Investigacio´n en Sida (Grant Numbers RISEST29 and RD12/0017/0001, RD12/0017/0005, RD17/0017/0022, and RD17/0017/0029). The DOMONO trial study was supported by the Erasmus Trustfonds. The MONCAY trial was funded by the Centre Hospitalier Regional d’Orleans–La Source (Orleans, France) with support of the COREVIH Centre-Poitou Charentes (Tours, France). The EARLY-SIMPLIFIED trial was supported by the Swiss National Science Foundation (SNF) (Grant 179571; to H. F. G.) and the University of Zurich’s Clinical Research Priority Program’s (to H. F. G. and D. L. B.) R. D. K. was supported by the SNF (Grant Numbers PZ00P3-142411 and BSSGI0_155851). There was no specific funding for this meta-analysis.
Potential conflicts of interests. L. H. received honoraria from ViiV, Gilead, Mylan, and Merck outside of this study. D. L. B. and J.-J. P. received honoraria from ViiV, Gilead, and Merck outside of this study. E. M. received honoraria from Gilead Sciences, Janssen, MSD, and ViiV Healthcare outside of this study. C. R. received research grants from ZonMW, AIDSfonds, Dutch Federation Medical Specialist, Erasmus MC MRACE, Gilead Sciences, Merck, Janssen-Cilag and ViiV, outside of this study. K. J. M. has received travel grants and honoraria from Gilead Sciences, Roche Diagnostics, GlaxoSmithKline, Merck Sharp & Dohme, Bristol-Myers Squibb, ViiV, and Abbott. The University of Zurich received research grants from Gilead Science, Novartis, Roche, and Merck Sharp & Dohme for studies in which K. J. M. serves as principal investigator, and K. J. M. receives advisory board honoraria from Gilead Sciences. H. F. G., outside of this study, reports grants from the Swiss National Science Foundation, National Institutes of Health (NIH), and the Swiss HIV Cohort Study, unrestricted research grants from Gilead Sciences, Roche, and Yvonne Jacob Foundation, personal fees from consulting or advisory boards or data safety monitoring boards for Merck, Gilead Sciences, ViiV Healthcare, Mepha, and Sandoz. H. F. G.’s institution received money for participation in the following clinical COVID-19 studies: 540-7773/5774 (Gilead), TICO (ACTIV-3, INSIGHT/NIH), and the Morningsky study (Roche).
Contributor Information
Anna L Fournier, INSERM U1311 DYNAMICURE, Université Caen Normandie, Caen, France; Department of Infectious Diseases, Caen University Hospital, Caen, France.
Laurent Hocqueloux, CHR Orleans, Orleans, France.
Dominique L Braun, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Karin J Metzner, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Roger D Kouyos, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
François Raffi, Infectious Diseases Department, Hotel-Dieu Hospital, Nantes, France; INSERM CIC 1413, Nantes University Hospital, Nantes, France.
Anaïs R Briant, Department of Biostatistic and Clinical Research, Caen University Hospital, Caen, France.
Esteban Martinez, University Hospital of Barcelona, Barcelona, Spain.
Elisa De Lazzari, University Hospital of Barcelona, Barcelona, Spain.
Eugenia Negredo, Lluita Contra La Sida Foundation, Germans Trias I Pujol University Hospital, Barcelona, Spain.
Bart Rijnders, Departments of Internal Medicine and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, Netherlands.
Casper Rokx, Departments of Internal Medicine and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, Netherlands.
Huldrych F Günthard, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Jean-Jacques Parienti, INSERM U1311 DYNAMICURE, Université Caen Normandie, Caen, France; Department of Infectious Diseases, Caen University Hospital, Caen, France; Department of Biostatistic and Clinical Research, Caen University Hospital, Caen, France.
References
- 1. Ryom L, Cotter A, De Miguel R, et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med 2020; 21:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryom L, Boesecke C, Gisler V, et al. Essentials from the 2015 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV-positive persons. HIV Med 2016; 17:83–8. [DOI] [PubMed] [Google Scholar]
- 4. Keiser O, Fellay J, Opravil M, et al. Adverse events to antiretrovirals in the Swiss HIV Cohort Study: effect on mortality and treatment modification. Antivir Ther 2007; 12:1157–64. [PubMed] [Google Scholar]
- 5. Demessine L, Peyro-Saint-Paul L, Gardner EM, Ghosn J, Parienti J-J.. Risk and cost associated with drug-drug interactions among aging HIV patients receiving combined antiretroviral therapy in France. Open Forum Infect Dis 2019; 6:ofz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prazuck T, Verdon R, Le Moal G, et al. Tenofovir disoproxil fumarate and emtricitabine maintenance strategy in virologically controlled adults with low HIV-1 DNA: 48 week results from a randomized, open-label, non-inferiority trial. J Antimicrob Chemother 2021. doi: 10.1093/jac/dkab038. [DOI] [PubMed] [Google Scholar]
- 7. Havlir DV, Marschner IC, Hirsch MS, et al. Maintenance antiretroviral therapies in HIV-infected subjects with undetectable plasma HIV RNA after triple-drug therapy. AIDS Clinical Trials Group Study 343 Team. N Engl J Med 1998; 339:1261–8. [DOI] [PubMed] [Google Scholar]
- 8. Pialoux G, Raffi F, Brun-Vezinet F, et al. A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV-1-infected patients. Trilège (Agence Nationale de Recherches sur le SIDA 072) Study Team. N Engl J Med 1998; 339:1269–76. [DOI] [PubMed] [Google Scholar]
- 9. Arribas JR, Girard P-M, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15:785–92. [DOI] [PubMed] [Google Scholar]
- 10. Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15:775–84. [DOI] [PubMed] [Google Scholar]
- 11. Di Giambenedetto S, Fabbiani M, Quiros Roldan E, et al. Treatment simplification to atazanavir/ritonavir + lamivudine versus maintenance of atazanavir/ritonavir + two NRTIs in virologically suppressed HIV-1-infected patients: 48 week results from a randomized trial (ATLAS-M). J Antimicrob Chemother 2017; 72:1163–71. [DOI] [PubMed] [Google Scholar]
- 12. Pulido F, Ribera E, Lagarde M, et al. Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin Infect Dis 2017; 65:2112–8. [DOI] [PubMed] [Google Scholar]
- 13. Llibre JM, Hung C-C, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–49. [DOI] [PubMed] [Google Scholar]
- 14. Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 15. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 16. van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO Study. Clin Infect Dis 2020; 71:1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cottrell ML, Hadzic T, Kashuba ADM.. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013; 52:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 19. Walmsley S, Baumgarten A, Berenguer J, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Molina J-M, Clotet B, van Lunzen J, et al. Once-daily dolutegravir is superior to once-daily darunavir/ritonavir in treatment-naïve HIV-1-positive individuals: 96 week results from FLAMINGO. J Int AIDS Soc 2014; 17:19490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 22. Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV 2017; 4:e547–54. [DOI] [PubMed] [Google Scholar]
- 23. Parienti J-J, Fournier AL, Cotte L, et al. Forgiveness of dolutegravir-based triple therapy compared with older antiretroviral regimens: a prospective multicenter cohort of adherence patterns and HIV-RNA replication. Open Forum Infect Dis 2021; 8:ofab316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. [DOI] [PubMed] [Google Scholar]
- 25. Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic human immunodeficiency virus infection: the randomized noninferiority MONotherapy of TiviCAY trial. Clin Infect Dis 2019; 69:1498–505. [DOI] [PubMed] [Google Scholar]
- 26. Braun DL, Turk T, Tschumi F, et al. Noninferiority of simplified dolutegravir monotherapy compared to continued combination antiretroviral therapy that was initiated during primary human immunodeficiency virus infection: a randomized, controlled, multisite, open-label, noninferiority trial. Clin Infect Dis 2019; 69:1489–97. [DOI] [PubMed] [Google Scholar]
- 27. Rolle C-P, Nguyen V, Hinestrosa F, DeJesus E.. Virologic outcomes of switching to dolutegravir functional mono- or dual therapy with a non-cytosine nucleoside analog: a retrospective study of treatment-experienced, patients living with HIV. AIDS Res Ther 2021; 18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 29. Surial B, Mugglin C, Calmy A, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med 2021; 174:758–67. [DOI] [PubMed] [Google Scholar]
- 30. Wijting I, Rutsaert S, Rokx C, et al. Predictors of virological failure in HIV-1-infected patients switching to dolutegravir maintenance monotherapy. HIV Med 2019; 20:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 2015; 313:1657–65. [DOI] [PubMed] [Google Scholar]
- 32. Smith CT, Williamson PR, Marson AG.. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat Med 2005; 24:1307–19. [DOI] [PubMed] [Google Scholar]
- 33. Hemming K, Hughes JP, McKenzie JE, Forbes AB.. Extending the I-squared statistic to describe treatment effect heterogeneity in cluster, multi-centre randomized trials and individual patient data meta-analysis. Stat Methods Med Res 2021; 30:376–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paton NI, Musaazi J, Kityo C, et al. Dolutegravir or darunavir in combination with zidovudine or tenofovir to treat HIV. N Engl J Med 2021; 385:330–41. [DOI] [PubMed] [Google Scholar]
- 35. Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 36. Charpentier C, Montes B, Perrier M, Meftah N, Reynes J.. HIV-1 DNA ultra-deep sequencing analysis at initiation of the dual therapy dolutegravir + lamivudine in the maintenance DOLULAM pilot study. J Antimicrob Chemother 2017; 72:2831–6. [DOI] [PubMed] [Google Scholar]
- 37. Rial-Crestelo D, de Miguel R, Montejano R, et al. Long-term efficacy of dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: week 96 results of ART-PRO pilot study. J Antimicrob Chemother 2021; 76:738–42. [DOI] [PubMed] [Google Scholar]
- 38. Maggiolo F, Gulminetti R, Pagnucco L, et al. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis 2017; 17:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lambert-Niclot S, Flandre P, Valantin M-A, et al. Factors associated with virological failure in HIV-1-infected patients receiving darunavir/ritonavir monotherapy. J Infect Dis 2011; 204:1211–6. [DOI] [PubMed] [Google Scholar]
- 40. Avettand-Fènoël V, Hocqueloux L, Ghosn J, et al. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin Microbiol Rev 2016; 29:859–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hocqueloux L, Viard J-P.. [Clinical and therapeutic implications of quantifying HIV reservoirs]. Virologie (Montrouge) 2019; 23:241–9. [DOI] [PubMed] [Google Scholar]
- 42. Rutsaert S, De Spiegelaere W, De Clercq L, Vandekerckhove L.. Evaluation of HIV-1 reservoir levels as possible markers for virological failure during boosted darunavir monotherapy. J Antimicrob Chemother 2019; 74:3030–4. [DOI] [PubMed] [Google Scholar]
- 43. Gianotti N, Cozzi-Lepri A, Antinori A, et al. Refining criteria for selecting candidates for a safe lopinavir/ritonavir or darunavir/ritonavir monotherapy in HIV-infected virologically suppressed patients. PLoS One 2017; 12:e0171611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hocqueloux L, Avettand-Fènoël V, Jacquot S, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013; 68:1169–78. [DOI] [PubMed] [Google Scholar]
- 45. Tebano G, Soulié C, Schneider L, et al. Long-term follow-up of HIV-infected patients on dolutegravir monotherapy. J Antimicrob Chemother 2020; 75:675–80. [DOI] [PubMed] [Google Scholar]
- 46. Sculier D, Doco-Lecompte T, Yerly S, Metzner K, Decosterd L, Calmy A.. Stable HIV-1 reservoirs on dolutegravir maintenance monotherapy: the MONODO study. HIV Med 2018; 19:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.