Abstract
Background:
Orphanhood increased dramatically in the 1980s and 1990s in sub-Saharan Africa (SSA) due to HIV mortality. Little is known about the contribution of HIV interventions such as antiretroviral therapy (ART) and male medical circumcision (MMC) to more recent trends in orphanhood.
Methods:
We examined the prevalence of orphanhood among adolescents 15–19 years, before and after roll out of ART in mid 2004 and MMC in 2007, using data from 28 continuously followed communities within the Rakai Community Cohort Study (RCCS). We used multinomial logistic regression (MLR) with clustered standard errors to estimate adjusted relative risk ratios (adj.RRR) for maternal-only, paternal-only, and double orphanhood compared to non-orphanhood over 11 survey rounds between 2001 and 2018. Controlling for community HIV prevalence, household socioeconomic status (SES), and adolescent age, we examined the association between community prevalence of ART use among people living with HIV (PLWHIV) and prevalence of male circumcision (MC) including traditional circumcision.
Findings:
Orphanhood declined from 52% in 2001–2002 to 23% by 2016–2018 (p<0.001), while double orphanhood declined from 20% to 3% (p<0.001). Community prevalence of ART use among PLWHIV rose from 11% in 2005–2006 to 78% in 2016–2018; MC rates rose from 19% to 65%. In the MLR model, a 10% increase in community prevalence of ART use was associated with a decrease in maternal orphanhood (adj.RRR=0.90, 95% CI=0.85–0.95) and double orphanhood (adj.RRR=0.80, 95% CI=0.75–0.85). In the post-ART era, a 10% increase in the community prevalence of MC was associated with a decrease in paternal orphanhood (2005–2018, adj.RRR=0.92, 95% CI=0.86–0.97), and double orphanhood (adj.RRR=0.91, 95% CI=0.85–0.98).
Interpretation:
Widespread availability and uptake of HIV combination prevention was associated with dramatic reductions in orphanhood among adolescents. Reductions in orphanhood promise improved health and social outcomes for young people.
Funding:
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, grants R01HD091003, RO1HD070769, R01HD050180, and P2CHD058486), the National Institute of Allergy and Infectious Diseases (grants R01AI110324, U01AI100031, U01AI075115, R01AI110324, R01AI102939, and K01AI125086-01), the National Institute of Mental Health (grant R01MH107275), and the Division of Intramural Research of the National Institute for Allergy and Infectious Diseases.
Introduction
Orphanhood in SSA increased dramatically in the 1980s and 1990s associated with HIV infection and mortality among parents 1,2. The United Nations Children’s Fund has estimated that between 1990–2015, 10.9 million children and adolescents (age 0–17 years) in SSA had lost one or both parents to HIV infection 3. In Uganda over this same period, 660,000 children and adolescents had lost one or both parents to HIV; this represents about 1/3 of all orphans in the country 3. HIV-related orphanhood among adolescents in sub-Saharan Africa (SSA) has been associated with adverse physical and mental health and social consequences such as mental health distress, truncation of education, child marriage, and behavioral risk for HIV among adolescents 4–10.
Highly active antiretroviral therapy (ART) can substantially reduce HIV-related mortality 11. Since many PLWHIV in SSA are parents, availability of ART should reduce orphanhood among children and adolescents. Limited access to ART in the Rakai region began around 2003, while the President’s Emergency Plan for AIDS Relief (PEPFAR) provided funding for widespread ART availability beginning in June 2004 2. arly reports from Uganda suggested that ART use had reduced orphanhood among children substantially 2,10, 12. In Rakai, Uganda, the prevalence of orphanhood among children age <15 years declined from 17.2% in 2001–2002 to 12.6% in 2006–2009 2. However, less is known about the impact of ART on orphanhood among adolescents across more recent time periods. A 2020 study from rural KwaZulu-Natal found declines in orphanhood among children and adolescents after 2010, coinciding with expanded ART coverage and declines in adult mortality 13. Moreover, ART is both a highly effective treatment and a marker for access to other medical services such as tuberculosis screening and treatment.
Male medical circumcision has been demonstrated to reduce HIV transmission from women to men about 60% 14–17, and thus may prevent HIV-related mortality in men and reduce paternal orphanhood. As circumcised men are less likely to be infected, MMC indirectly reduces transmission from men to women 16. Scale-up of MMC as a routine prevention service was implemented in Rakai immediately after termination of the Rakai clinical trial of MMC in 2007 18. The prevalence of traditional male circumcision in Rakai in 19941998 and prior to availability through the healthcare system was 16.5% among all men in the region, predominantly among Muslim 19. Combination HIV prevention which includes ART and MMC has substantially reduced HIV incidence and prevalence in Rakai within the adult population 18,20.
Other factors can influence HIV epidemic dynamics and therefore influence orphanhood. Higher HIV prevalence within a community increases orphanhood by increasing HIV-related mortality among parents. HIV prevalence in the Rakai District (13% 2001–2002 and 12% in 2016–2018) has been higher than in Uganda as a whole (7% 2001 and 6% in 2018) 21. Socioeconomic status (SES) may influence HIV risk in complex ways as poverty may increase behavioral risk and decrease access to prevention and treatment services; in a recent study from Rakai, Uganda, we found the relationship of SES with HIV incidence changed over time with lower SES increasingly associated with higher HIV incidence 22.
In this paper, we first examined trends over time in maternal-only, paternal-only, and double orphanhood among adolescents (15–19 years) before and after ART and MMC became widely available in the Rakai region of southcentral Uganda. Next, we sought to understand the association between adolescent orphanhood and HIV combination prevention (community-level ART use and prevalence of MMC). Multivariable analyses adjusted for household SES and the prevalence of HIV infection within communities. We hypothesized that increasing combination prevention, including greater use of ART and higher prevalence of MMC, would be associated with lower probability of orphanhood.
Methods
Study design and population
We used data from 28 communities in the Rakai Community Cohort Study (RCCS) in rural southcentral Uganda; these communities were continuously surveyed (i.e., at every survey round) from 1994 to 2018; we used data from 2001–2018 (11 rounds) including the period before and after ART availability beginning in 2004. The RCCS is a population-based, open cohort of consenting individuals 15 to 49 years residing in communities in or near the Rakai District.18 As an open cohort, newly age-eligible 15-year olds and recent inmigrants are enrolled at each round. At each round, community-wide HIV education, individual and couple’s HIV counseling and testing, referral for MMC and, and ART for PLWHIV, are offered free of charge. For unemancipated minors (<18 years), both written minor assent and parental/guardian permission are obtained; 18+ year-olds and emancipated minors provide their own written informed consent. Ethical approvals were obtained from the Research Ethics Committee (REC) of the Uganda Virus Research Institute (UVRI), the Uganda National Council for Science and Technology (UNCST), and Institutional Review Boards at Johns Hopkins and Columbia Universities, and Western IRB, Olympia WA.
Procedures
Each RCCS survey round includes a household census, with data provided by the head of household. All households within RCCS community are included in the census. Household members are enumerated by age, sex, relationship to the head of household, duration of residence in the household, and the status of each parent for all household members (co-resident, alive but not co-resident, or deceased). A household is defined as an individual or group of individuals who eat their primary meals together and live together. While a household usually comprises a family or extended family, it may include non-family members. Households may be composed of multiple separate structures forming a compound. All individuals between the ages of 15 and 49 years within a household are eligible for an interview.
Two to four weeks after the census, consenting residents (including both new enrollees and those followed-up) are interviewed and asked to provide blood for HIV and sexually transmitted infection testing, treatment, and referral. RCCS participation rates are ~95% of those present at time of survey; ~25% of censused residents are absent at each round - for work, school or other travel 18. Acceptance of HIV testing among enrollees is high (>95%). Eligible individuals (age 15–49 years) are interviewed about sexual and reproductive health and HIV risk and offered HIV counseling and testing on the day of interview. HIV test results and post-test counseling are provided - in the past after laboratory testing and currently in the field using a validated rapid test algorithm with enzyme immunoassay confirmation of newly identified HIV-positive persons 18. Data are entered electronically in the field and are immediately reviewed by supervisors/editors to ensure data integrity. Data are not weighted.
The primary outcome was orphanhood among adolescents 15–19 years. Orphanhood in the RCCS is identified using household census data, in which the head of household is asked about the relationships among household members and the status of each person’s mother and father and whether each parent is alive or deceased. Using these data, each adolescent was classified into one of four categories: not an orphan if both parents were alive, a double orphan if both parents were deceased, maternal-only orphan if only the mother was deceased, or a paternal-only orphan if only the father was deceased.
For these analyses, we created two key exposure variables: (1) the community prevalence of ART use among PLWHIV 15–49 years and (2) the community prevalence of MC among men 15–49 years. ART use was based on self-report among PLWHIV (“Have you ever been on the following long term medications?”). Self-reported ART use in the RCCS has high specificity (99%) and moderate sensitivity (77%).23 Prevalence of ART use was calculated as the proportion of PLVHIV 15–49 years in a community who had ever used ART. Access to ART was first measured in the RCCS survey round 11 (January 2005-June 2006). Prevalence of MC was based on the proportion of adolescent and adult men who self-reported that they were circumcised at each round (“Are you circumcised?”).
We also created a variable for community HIV prevalence. HIV community prevalence was calculated based on HIV test results from individuals in each community at each survey round. Although HIV community prevalence among 15–49 years was highly correlated with HIV community prevalence in 30–49 year olds, we used the latter since 30–49 year olds should most closely match the age of parents of the adolescents.
Household SES is an asset-based composite measure of household SES created via principal component analysis and is used in surveys of low and middle income countries 22,24. The household census has collected consistent information on nine household assets related to home construction and household possessions from 1994 to present. These assets were recoded as binary variables: e.g., owning vs not owning a car; household SES scores could vary between 0 and 9. SES scores were divided into quartiles based on the distribution of SES scores over all 18 rounds of RCCS data collection.
RCCS communities are classified as trading centers (villages) on main roads and rural villages on secondary roads. Adolescent age was self-reported and verified by the interviewer using census data.
Statistical Analysis
Statistical software (Stata/SE 16) was used for all analyses. We first examined trends in prevalence of maternal-only, paternal-only, and double orphanhood among adolescents 15–19 years over time between Round 8 (2001–2002) and Round 18 (2016–2018). We also examined trends in community, household, and individual-level independent variables including community-level use of ART among PLWHIV, community prevalence of MC, community HIV prevalence within each community, adolescent age, sex of the adolescent, and household SES. Trends were assessed using linear models using generalized estimating equations (GEE).
Next, to understand the impact of independent variables on orphanhood, we used multinomial logistic regression (MLR) to estimate how each independent variable was associated with maternal-only, paternal-only, and double orphanhood compared with non-orphanhood. In the MLR, beta coefficients were converted to relative risk ratios (RRRs). Adjusted RRRs were adjusted for all other factors within the model; these are age of adolescent, rural vs. trading village, household SES, community HIV prevalence, community ART prevalence among PLHIV, and prevalence of circumcision among men, before and after ART became available. Rural residence was included to account for any potential bias, as rural residence has been associated with lower HIV prevalence and may influence access to HIV-related services.
MLR is used to model a nominal outcome variable with multiple categories that share common risk factors, as is true for maternal, paternal, and double orphanhood. MLR is advantageous compared to several logistic regression models in which a model would be run for each pair of outcomes. MLR allows minimizing Type I Error rate (alpha) for the model at 5%, enabling us to run one single model on the whole sample in contrast to different samples for each logistic regression models.
Statistical clustering of orphanhood was examined on the community level and household levels. There was little evidence of clustering of orphanhood within communities (intraclass correlation coefficient or ICC = 0.005). Orphanhood was strongly correlated within household units (ICC = 0.688). Relative risk ratios (RRR) were estimated with a cluster-robust sandwich estimator of variance to account for within-household correlation.
We initially examined ART use among men and women living with HIV in our MLR model. Similar patterns were found, so we chose to model ART use among all PLWHIV within each community.
We dealt with time in several ways. First, we used time as a continuous variable; time was not significant given strong correlations between time and use of ART and prevalence of MC. Next, we introduced into the model a change point at 2004, when ART became available via the RHSP. Specifically, we allowed the baseline prevalence of orphanhood to differ before and after 2004 and allowed the effects of community-level independent variables (i.e., the prevalence of MC, ART use and HIV) on orphanhood to differ before and after 2004.
Our sample in Table 1 included an average of 1845 observations per round and 20 289 total observations over all 11 rounds. The sample for MLR modeling in Table 2 was somewhat smaller (18 492 observations) given missing data, particularly missing data on SES.
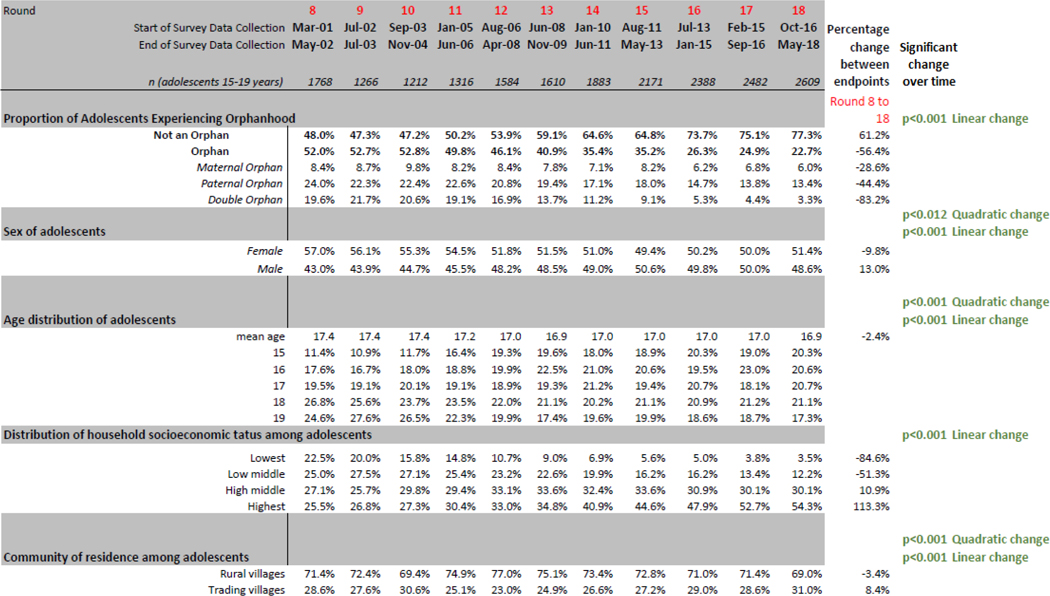
Table 1.
Prevalence of Orphanhood and Risk Factors for Orphanhood among Adolescents 15–19 Years, 28 Communities, Rakai Uganda, Rounds 8–18, 2001–2018
|
Change over time for orphanhood and socioeconomic status were assessed using a multinomial logistic regression model with clustered standard errors. For community of residence, changes over time were assessed using regression models with Generalized Estimating Equation modeling. Percentage change over time compares relative change in endpoints (first and last rounds available).
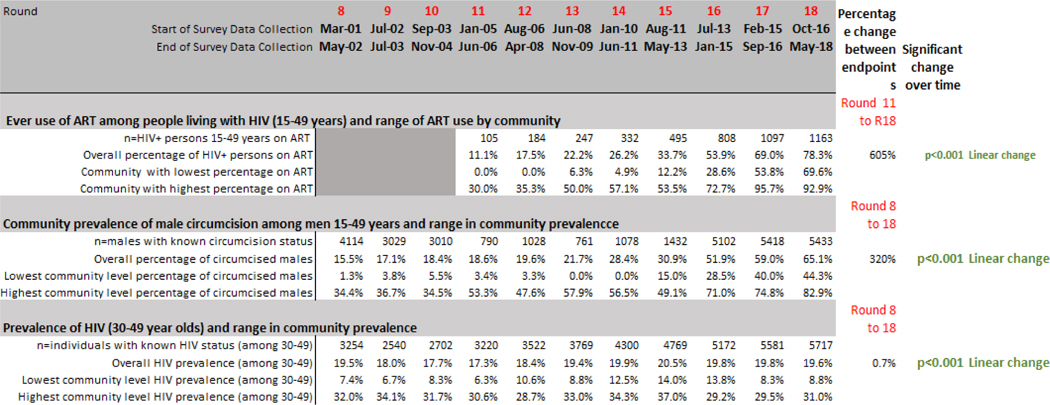
Table 2.
Prevalence of Community-Level Factors among Adolescents 15–19 Years, 28 Communities, Rakai Uganda, Rounds 8–18, 2001–2018
|
Notes: The measurement of household Socioeconomic Status is based house assets and home construction. SES scores were divided into quartiles.
Change over time for community-level factors were assessed using regression models with Generalized Estimating Equation modeling. Percentage change over time compares relative change in endpoints (first and last rounds available).
Role of the Funding Source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
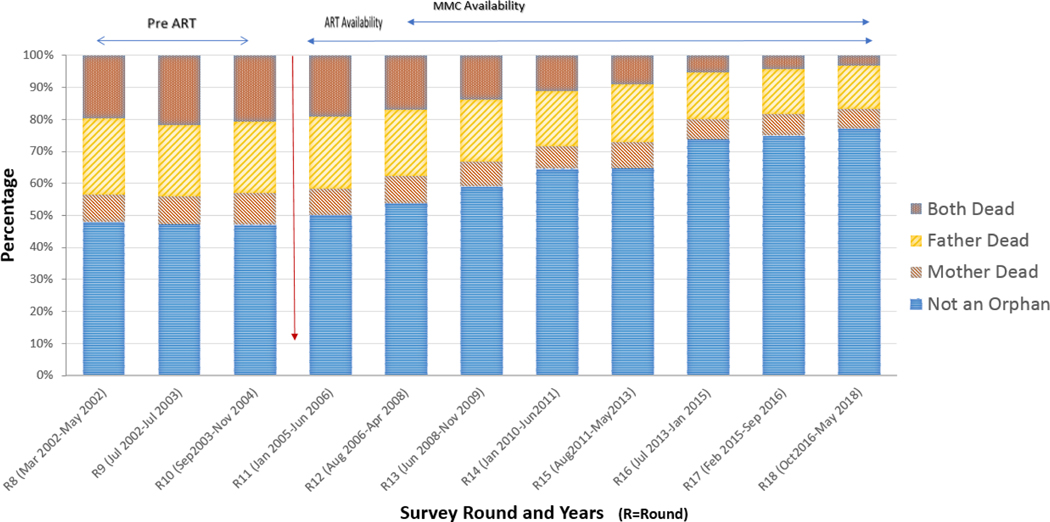
Before widespread availability of ART or MMC, the proportion of adolescents experiencing any orphanhood (maternal-only, paternal-only, or double orphanhood) was 52–53% (survey Rounds 10–12, 2001–2004; Figure 1 and Table 1) and orphanhood declined to 23% by Round 18; this represents a relative decline of 61% from Round 8 to 18 (test for trend p<0.001). Over the same period, double orphanhood declined from 20% (346 out of 1768) to 3% (86 out of 2609), paternal-only orphanhood declined from 24% (425 out of 1768) to 13% (349 out of 2609), and maternal-only orphanhood declined from 8% (149 out of 1768) to 6% (157 out of 2609). The largest decline occurred in double-orphanhood (83%), followed by paternal orphanhood (44%) and maternal orphanhood (29%).
FIGURE 1. ORPHANHOOD AMONG ADOLESCENTS 15–19 YEARS, RAKAI COMMUNITY COHORT STUDY, 2001–2018 (ROUNDS 8–18).
Table 1 also demonstrates change over time in individual, household, and community residence variables. The average age of adolescents decreased from 17.4 to 16.9 over time (linear and quadratic change, both p<.001). Household SES increased steadily between 2001 and 2018.
Table 2 includes our three created community-level variables. Community prevalence of ever use of ART among HIV positive residents rose over time - from 11% at Round 11 to 78% at Round 18. ART use among PLWHIV varied between 0% to 30% across communities in Round 11 compared to 70% to 93% variation in Round 18. The community prevalence of MC among adolescent and adult men (15–49 years) rose from 15% in Round 8 to 65% in Round 18. (The prevalence of male circumcision before the roll out of MMC represents traditional practice among Muslims.) Rates of male circumcision varied considerably among the 28 Rakai communities between 1% and 34% at Round 8 relative to 44% to 83% variation in Round 18. The overall community prevalence of HIV among 30–49 year olds changed little over time, ranging between 17% and 21%. At each round, HIV prevalence was two to five times higher in communities with the highest prevalence, compared to communities with the lowest prevalence.
Table 3 presents the results of the multinominal logistic regression (MLR) model estimating risk of maternal-only, paternal-only, and double orphanhood compared to non-orphans. In the MLR model, older adolescent age (calculated as single year of age) was associated with an increased risk of orphanhood. Compared to rural residence, living in a trading village was associated with an increased risk of paternal orphanhood (adj.RRR=1.44, 95% CI=1.22,1.69). Higher SES were associated with lower risk of orphanhood for paternal and double orphanhood. A 10% increase in the community HIV prevalence was associated with an increase in paternal orphanhood (adj.RRR=1.17, 95% CI=1.03,1.33) and double orphanhood (adj.RRR=1.15, 95% CI=1.00,1.31).
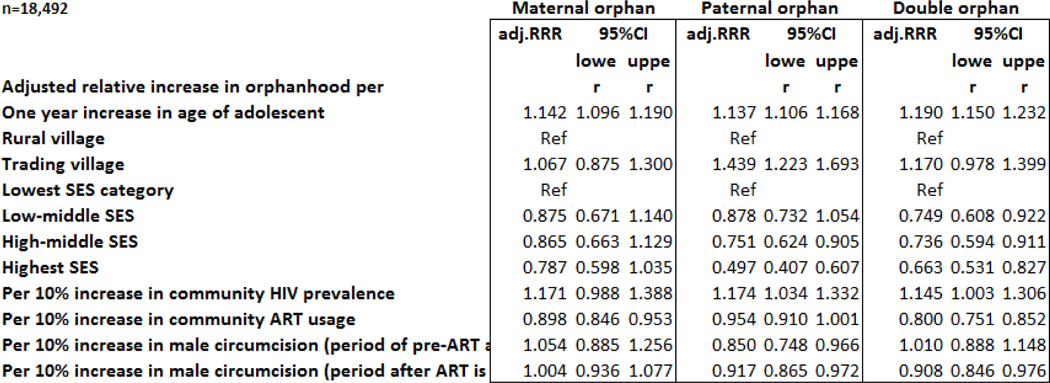
Table 3.
Risk Factors for Maternal, Paternal and Double Orphanhood Among Adolescent 15–19 Years, Using Multmominal Logistic regression, Rakai, Uganda, Rounds 8-R18, 2001–2018
|
Notes: Reference category for multinominal logistic regression is not an orphan. Multinomial Logistic Regression Model accounts for intraclass correlation of household. Adj.RRR-adjusted relative risk ratio. SES is household socioeconomic status. ART is ever use of antiretroviral treatment among people living with HIV (PLHIV). Period of pre-ART availability is rounds ft-10. Period of ART availability is rounds 11–18. Variables in the model are age of adolescent rural vs. trading village, household SES, community HIV prevalence among 30–49 year olds, community ART prevalence among PLHIV, and prevalence of circumcision among men 15–49 years of age, before and after ART became available.
A 10% increase in community-level ART use among PLWHIV was associated with a decrease in maternal orphanhood (adj. RRR=0.90, 95% CI=0.85,0.95) and double orphanhood (adj.RRR=0.80, 95% CI=0.75,0.85). We examined the impact of MC in both the period of ART availability (Round 11–18) and the pre-ART period (Round 8–10) for synergy between these two interventions. In the pre-ART period, MC was associated with lower paternal orphanhood (adj.RRR=0.85, 95% CI=0.75,0.97), but not maternal or double orphanhood. During the ART period, a 10% increase in the community prevalence of MC was associated with a decrease in paternal orphanhood (adj.RRR=0.92, 95% CI=0.87,0.97), and double orphanhood (adj.RRR=0.91, 95% CI=0.85,0.98) but not maternal orphanhood.
In the adjusted model, time period was not statistically significant, after ART and MC were added to the model, suggesting that the declines in orphanhood are explained by changes in ART and MC, not time itself.
Discussion
We found dramatic declines over time in orphanhood, particularly double orphanhood, among adolescents in the Rakai cohort, corresponding with the availability of ART beginning in mid 2004 and of MMC in 2007. Adolescents living in communities with higher ART use among PLWHIV, higher prevalence of MC, and lower HIV prevalence had a lower risk of orphanhood. Higher household SES was also associated with lower prevalence of orphanhood among adolescents, particularly paternal orphanhood. These finding are consistent with – and extend – earlier reports suggesting that parental ART use could reduce orphanhood among younger children in Uganda 2,12. Given the adverse impact of orphanhood, these declines in orphanhood promise improved health and social outcomes for children and adolescents.
Our findings are consistent with prior research on the impact of ART and MMC on HIV infection and mortality due to HIV infection. ART dramatically reduces mortality from HIV infection 11 and – because many PLWHIV in SSA are parents – should reduce orphanhood among children and adolescents. Other analyses have documented positive impacts of access to ART including declines in HIV-related mortality among adults, improved quality of life, HIV viral load suppression, and reduced vertical transmission of HIV infection 25,26. Declines in adult mortality in Rakai coincided with ART availability 11. Likewise, MMC in three clinical trials in SSA has reduced HIV transmission from women to men by 60%14,15,17 and combination prevention (providing both MMC and ART) has reduced HIV incident infection in RCCS communities 18. Thus, our findings are both plausible and consistent with research on population impact of HIV treatment and prevention 18.
The impact of MC on orphanhood was confined to paternal orphanhood and double orphanhood which is consistent with research demonstrating that MMC prevents transmission from HIV positive women to HIV negative men 17 but not from HIV positive men to HIV negative women 28. Reduction of HIV infection among men will indirectly reduce infection among women over time. Likewise, the association of higher community prevalence of HIV with higher rates of maternal and double orphanhood is consistent with current understanding of HIV transmission dynamics; communities with a higher prevalence of HIV would be expected to have higher adult mortality and higher rates of orphanhood.
Orphanhood was also negatively associated with SES which has risen steadily in the RCCS from 1994 to 2018. However, we did not find a sharp inflection point in the mid 2000s, as we found for ART, MC, and orphanhood. Wealthier individuals may be more knowledgeable about HIV risks, better able to use HIV prevention such as condoms, and, if infected, better able to access HIV treatment and thereby prevent HIV-related mortality. In a recent RCCS analysis, we found higher SES was related to lower HIV incidence among 15–49 year olds 22.
Our analyses have a number of strengths and limitations. Variables in our analyses have been consistently measured over time and data collection is closely monitored to ensure completeness and quality. Importantly, orphanhood among adolescents is the cumulative result of mortality of parents over the lifespan of the adolescent thus reflecting both cumulative measures such as community prevalence of MC and HIV and measures of uncertain duration such as duration of ART use. Moreover ART access may be influenced by SES or residence in a trading community which often provide easier access to clinical services. Despite these limitations, we found significant, plausible and independent associations with orphanhood.
HIV-related orphanhood in sub-Saharan Africa (SSA) has been associated with adverse health and social consequences among adolescents such as mental health distress, premature truncation of education, child marriage, and increased behavioral risk for HIV transmission among adolescents 5–9. Reduced mortality among parents should lead to increased family stability and reductions in these adverse social and health outcomes associated with orphanhood. Thus, our findings suggest that access to ART and MC likely had positive impacts on families in Rakai and elsewhere in sub-Saharan Africa. These impacts on reducing orphanhood should be now be considered as one of the triumphs of global efforts to support HIV treatment and prevention services.
Research in Context Panel.
Evidence before this study:
We searched PubMed and Google Scholar for English-language studies published between 1990 and July 1 2020 using the search terms “HIV,” “AIDS,” “Africa,” and “Orphanhood”. We selected articles from eastern and southern Africa that reported on prevalence, risk factors, and trends over time in orphanhood. Multiple studies identified dramatic increases in orphanhood because of HIV infection and the adverse health and social outcomes associated with orphanhood and HIV. Between 1990–2015, an estimated 10.9 million children and adolescents (age 0–17 years) in SSA had lost one or both parents to HIV infection. Two studies reported on declines in orphanhood. An early study in Rakai found a reduction in incidence of orphanhood for children <15 years from 2001 to 2009 – before and after rollout of ART in 2004. A 2020 study from rural KwaZulu-Natal found declines in orphanhood among children and adolescents after 2010, coinciding with expanded ART coverage and declines in adult mortality.
Added value of this study
Prior studies have not examined the impact of male circumcision, community HIV prevalence, and socioeconomic status, in addition to rates of ART, on rates of orphanhood. Orphanhood among adolescents in Rakai District began declining around 2005 - coinciding with the scale-up of ART beginning in 2004 and MMC in 2007. Loss of one or both parents declined from 53% in 2003 to 23% by 2018. Double orphanhood declined from 21% to 3%. Statistical modeling suggests that trends in orphanhood were significantly associated with community prevalence of ART use, MC, and HIV prevalence.
Implications of all the available evidence
Dramatic increases and declines in orphanhood in SSA - before and after 2005 - are closely tied to the HIV pandemic and to HIV combination interventions including ART and MMC. Reductions in orphanhood from HIV prevention and treatment promise improved health and social outcomes among young people.
Acknowledgments
We thank Andrea Deisher and Lee Daniel for their ever helpful assistance with manuscript preparation and revision.
Footnotes
Declaration of Interests
We declare no conflicts of interest related to this research.
Data sharing
A deidentified version of the RCCS data may be provided to interested parties subject to completion of the Rakai Health Sciences Program data request form and signing of a Data Transfer Agreement. Inquiries should be directed to datarequests@rhsp.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John S Santelli, Department of Population and Family Health, Mailman School of Public Health, Columbia University, New York, NY, USA; Department of Pediatrics, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Ivy S Chen, Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, NY, USA.
Dorean Nabukalu, Rakai Health Sciences Program, Kalisizo, Uganda.
Tom Lutalo, Rakai Health Sciences Program, Kalisizo, Uganda.
Esther J Spindler, Department of Population and Family Health, Mailman School of Public Health, Columbia University, New York, NY, USA.
Larry W Chang, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA..
Mary Kate Grabowski, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Pathology, Johns Hopkins School of Medicine, Baltimore, MD, USA..
Stephanie A Grilo, Department of Population and Family Health, Mailman School of Public Health, Columbia University, New York, NY, USA.
Philip Kreniske, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, New York, NY, USA.
Ying Wei, Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, NY, USA.
Fred Nalugoda, Rakai Health Sciences Program, Kalisizo, Uganda.
Susie Hoffman, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, New York, NY, USA; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA.
Mahlet Maru, Department of Sociomedical Sciences, Mailman School of Public Health, Columbia University, New York, NY, USA.
Sofia Chu, Department of Population and Family Health, Mailman School of Public Health, Columbia University, New York, NY, USA.
Fred M Ssewamala, Brown School, Washington University, St. Louis, MO, USA.
William Byansi, Brown School, Washington University, St. Louis, MO, USA.
Joseph Kagaayi, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Epidemiology and Biostatistics, Makerere University School of Public Health, Kampala, Uganda.
Maria J Wawer, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA..
Ronald H Gray, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
David Serwadda, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Disease Control and Environmental Health, Makerere University School of Public Health, Kampala, Uganda.
Fred Makumbi, Rakai Health Sciences Program, Kalisizo, Uganda; Department of Epidemiology and Biostatistics, Makerere University School of Public Health, Kampala, Uganda.
References
- 1.Andrews G, Skinner D, Zuma K. Epidemiology of health and vulnerability among children orphaned and made vulnerable by HIV/AIDS in sub-Saharan Africa. AIDS Care. 2006. Apr;18(3):269–76. [DOI] [PubMed] [Google Scholar]
- 2.Makumbi FE, Nakigozi G, Sekasanvu J, Lukabwe I, Kagaayi J, Lutalo T, et al. Incidence of orphanhood before and after implementation of a HIV care programme in Rakai, Uganda: Alpha Network HIV Supplement. Trop Med Int Health. 2012. Aug;17(8):e94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For Every Child, End AIDS: Seventh Stocktaking Report, 2016. [Internet]. UNICEF. [cited 2020 Oct 12]. Available from: https://www.unicef.org/publications/index_93427.html [Google Scholar]
- 4.Birdthistle IJ, Floyd S, Machingura A, Mudziwapasi N, Gregson S, Glynn JR. From affected to infected? Orphanhood and HIV risk among female adolescents in urban Zimbabwe. AIDS. 2008. Mar 30;22(6):759–66. [DOI] [PubMed] [Google Scholar]
- 5.Chae S. Timing of Orphanhood, Early Sexual Debut, and Early Marriage in Four Sub-Saharan African Countries. Stud Fam Plann. 2013. Jun;44(2):123–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cluver L, Gardner F, Operario D. Psychological distress amongst AIDS-orphaned children in urban South Africa. Journal of Child Psychology and Psychiatry. 2007;48(8):755–63. [DOI] [PubMed] [Google Scholar]
- 7.Foster G, Williamson J. A review of current literature on the impact of HIV/AIDS on children in sub-Saharan Africa. AIDS. 2000;14 Suppl 3:S275–284. [PubMed] [Google Scholar]
- 8.Nyamukapa CA, Gregson S, Lopman B, Saito S, Watts HJ, Monasch R, et al. HIV-associated orphanhood and children’s psychosocial distress: theoretical framework tested with data from Zimbabwe. Am J Public Health. 2008. Jan;98(1):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orkin M, Boyes ME, Cluver LD, Zhang Y. Pathways to poor educational outcomes for HIV/AIDSaffected youth in South Africa. AIDS Care. 2014;26(3):343–50. [DOI] [PubMed] [Google Scholar]
- 10.Makumbi FE, Gray RH, Serwadda D, Nalugoda F, Kiddugavu M, Sewankambo NK, et al. The incidence and prevalence of orphanhood associated with parental HIV infection: a population-based study in Rakai, Uganda. AIDS. 2005. Oct 1;19(15):1669–76. [DOI] [PubMed] [Google Scholar]
- 11.Nabukalu D, Reniers G, Risher KA, Blom S, Slaymaker E, Kabudula C, et al. Population-level adult mortality following the expansion of antiretroviral therapy in Rakai, Uganda. Popul Stud (Camb). 2020;74(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbiize P, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008. Mar 1;371(9614):752–9. [DOI] [PubMed] [Google Scholar]
- 13.Mejia-Pailles G, Berrington A, McGrath N, Hosegood V. Trends in the prevalence and incidence of orphanhood in children and adolescents <20 years in rural KwaZulu-Natal South Africa, 2000–2014. PLOS ONE. 2020. Nov 24;15(11):e0238563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005. Nov;2(11):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007. Feb 24;369(9562):643–56. [DOI] [PubMed] [Google Scholar]
- 16.Gray RH, Li X, Kigozi G, Serwadda D, Nalugoda F, Watya S, et al. The impact of male circumcision on HIV incidence and cost per infection prevented: a stochastic simulation model from Rakai, Uganda: AIDS. 2007. Apr;21(7):845–50. [DOI] [PubMed] [Google Scholar]
- 17.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007. Feb 24;369(9562):657–66. [DOI] [PubMed] [Google Scholar]
- 18.Grabowski MK, Serwadda DM, Gray RH, Nakigozi G, Kigozi G, Kagaayi J, et al. Combination HIV Prevention and HIV Incidence in Uganda. N Engl J Med. 2017. Nov 30;377(22):2154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, Mangen FW, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. AIDS. 2000. Oct 20;14(15):2371–81. [DOI] [PubMed] [Google Scholar]
- 20.Kagaayi J, Chang LW, Ssempijja V, Grabowski MK, Ssekubugu R, Nakigozi G, et al. Impact of combination HIV interventions on HIV incidence in hyperendemic fishing communities in Uganda: a prospective cohort study. Lancet HIV. 2019;6(10):e680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The 2019 HIV Epidemiological Surveillance Report for Uganda - Ministry of Health | Government of Uganda [Internet]. Kampala; 2020. Mar [cited 2021 Jul 15] p. 82. Available from: https://www.health.go.ug/cause/the-2019-hiv-epidemiological-surveillance-report-for-uganda/
- 22.Santelli JS, Chen I, Makumbi F, Wei Y, Nalugoda F, Lutalo T, et al. Household wealth and HIV incidence over time, rural Uganda, 1994–2018. AIDS. 2021. Jun 15; [DOI] [PMC free article] [PubMed]
- 23.Grabowski MK, Reynolds SJ, Kagaayi J, Gray RH, Clarke W, Chang LC, et al. The validity of self-reported antiretroviral use in persons living with HIV: a population-based study. AIDS. 2018. Jan 28;32(3):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006. Nov;21(6):459–68. [DOI] [PubMed] [Google Scholar]
- 25.Frank TD, Carter A, Jahagirdar D, Biehl MH, Douwes-Schultz D, Larson SL, et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. The Lancet HIV. 2019. Dec 1;6(12):e831–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goga A, Slogrove A, Wedderburn CJ, Feucht U, Wessels J, Ramokolo V, et al. The impact of health programmes to prevent vertical transmission of HIV. Advances, emerging health challenges and research priorities for children exposed to or living with HIV: Perspectives from South Africa. S Afr Med J. 2019. Dec 5;109(11b):77–82. [DOI] [PubMed] [Google Scholar]
- 27.Ssewamala FM, Dvalishvili D, Mellins CA, Geng EH, Makumbi F, Neilands TB, et al. The long-term effects of a family based economic empowerment intervention (Suubi+Adherence) on suppression of HIV viral loads among adolescents living with HIV in southern Uganda: Findings from 5-year cluster randomized trial. PLOS ONE. 2020. Feb 10;15(2):e0228370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wawer MJ, Makumbi F, Kigozi G, Serwadda D, Watya S, Nalugoda F, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009. Jul 18;374(9685):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]