Learning objectives.
By reading this article, you should be able to:
-
•
Define major obstetric haemorrhage (MOH) and understand its epidemiology.
-
•
Initiate an appropriate strategy for resuscitation with fluids and blood products.
-
•
Explain the rationale for the medical, pharmacological and surgical treatment options for patients with MOH.
Key points.
-
•
Major obstetric haemorrhage (MOH) is the leading cause of global maternal morbidity and mortality, yet most deaths can be prevented.
-
•
Early recognition is key to the effective management of patients with MOH.
-
•
The choice and dose of uterotonic drug depend on the clinical context.
-
•
Massive transfusion protocols should be adopted locally, as deaths occur from inadequate volume replacement and failure to correct coagulopathy
-
•
Anaesthetists should be familiar with the range of mechanical and surgical options to treat postpartum haemorrhage.
The burden of major obstetric haemorrhage
Major obstetric haemorrhage (MOH) is the leading cause of maternal morbidity and mortality worldwide.1,1, 2 The vast proportion of deaths occur in resource-poor countries.2 However, MOH results in severe morbidity in all healthcare settings, including multiorgan failure, postpartum hysterectomy and long-term psychological trauma, contributing to enormous economic and social costs.3
In the UK and Ireland’s most recent Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries (MBRRACE) report, deaths from haemorrhage were the second most common cause of direct maternal deaths with a rate of 0.64 (0.35–1.08) per 100,000.4 In Wales, the incidence of postpartum haemorrhage (PPH) greater than 1000 ml was reported as 8.6% when measured using a quantitative technique, with 1.3% of women suffering blood loss ≥2000 ml.5 In a large retrospective study of US hospital discharge data, the incidence of PPH was 3.2%, where the method of assessing the quantity of haemorrhage was not reported.6
In resource-rich healthcare settings, an increase in the incidence of PPH has been primarily caused by an increasing incidence of uterine atony.7 Rates of severe PPH requiring interventions, such as blood transfusion and surgery, are also increasing in many countries.8
Definitions of maternal haemorrhage
Definitions of what constitutes maternal haemorrhage are not universally agreed. Antepartum haemorrhage (APH) occurs from 24 weeks’ gestation, and occurs in between 3% and 5% of all pregnancies.9 The Royal College of Obstetricians and Gynaecologists (RCOG) has defined major APH as bleeding of 50-1000mL with no signs of shock, and massive APH as bleeding ≥ 1000 mL and/or bleeding of any volume with clinical signs of shock.10
Primary PPH occurs within the 24 h after delivery and secondary PPH occurs between 24 h and 12 weeks after delivery.11 The WHO defines PPH as ≥500 ml blood loss. However, it is recognised that using a definition with a higher threshold of 1000 ml may be more relevant clinically. In 2014 the American College of Obstetricians and Gynaecologists (ACOG) updated their definitions of PPH to reflect this, defining PPH as being ≥1000 ml within 24 h, with or without associated clinical signs of hypovolaemia, regardless of delivery method.12 In the UK, the RCOG stratifies their definitions of PPH by severity. Major PPH may be defined as moderate (1000–2000 ml) or severe (≥2000 ml).11
Pathophysiology of MOH
The uteroplacental unit functions as a high-flow, low-resistance vascular bed and by term receives 25% of the cardiac output. Consequently, large volumes of blood may be lost rapidly at delivery. During pregnancy, humans have evolved physiologically to anticipate this, consequently moderate blood loss is generally well tolerated.13 Both plasma and red cell mass increase throughout pregnancy, leading to a 50% increase in maternal blood volume. The increase in plasma volume is greater than red cell mass leading to a ‘physiological anaemia of pregnancy’, thereby offering an evolutionary advantage in which less red cell mass is lost per millilitre of blood during haemorrhage. Involution of the uterus and termination of the placental circulation after delivery results in autotransfusion of approximately 500 ml.
Aetiology
Early identification of women who are at risk of haemorrhage allows for safe birth planning and strategic mobilisation of resources; yet, risk stratification for maternal haemorrhage is fraught with difficulty as most women who present with PPH have no discernible risk factors.
Causes of APH include placenta praevia, placental abruption, uterine rupture and bleeding from the vulva, vagina or cervix, although a cause is often not found. The most common cause of PPH is uterine atony, which accounts for approximately 80% of cases of primary PPH. Trauma and injury to the genital tract, retained invasive placenta and coagulopathy are other common causes of PPH. More uncommon causes of primary PPH include uterine inversion and extragenital bleeding. Endometritis or retained products of conception are the most common causes of secondary PPH.
Placenta accreta spectrum (PAS) is a common cause of MOH, and the incidence of this condition is increasing.14 It ranges from placenta that is morbidly adherent to the myometrium (accreta vera), to that which invades into the myometrium (increta) or through the myometrium into surrounding organs (percreta).
Clinical assessment of blood loss
Early recognition of bleeding and prompt intervention reduces the risk of progression to haemorrhagic shock, disseminated intravascular coagulation (DIC) and death. A higher resting heart rate and a lower mean arterial blood pressure compared with the non-pregnant state may contribute to failure to detect signs of haemorrhage.13
Visual estimation of blood loss is variable and often underestimated. Quantitative blood loss measurement offers a more objective and scientific approach to visual assessment and may be measured by gravimetric or photometric means. Quantitative blood loss can be used as part of an alert system with the aim of preventing the progression of minor bleeding to more severe bleeding, and has been successfully incorporated into the Welsh Obstetric Bleeding Strategy.5 Blood loss should be considered in the context of the maternal blood volume, as women who have a smaller blood volume will decompensate more rapidly from a given quantity of bleeding.
Preparation
Major obstetric haemorrhage requires a rapid and highly coordinated response, and therefore systematic preparation is necessary. This includes individual risk stratification, optimisation before delivery, and preparing obstetric units and healthcare staff for massive transfusion events. Bundles of care such as the Safe Motherhood Initiative (SMI) aid in both the planning and execution of safe management by delineating standards and minimising variability in care between obstetric units.15
Early identification of haemorrhage risk in the antenatal period enables the effective mobilisation of healthcare resources. Every woman should be matched to a unit where her clinical needs can be met by the facilities provided.4 Protocols should be in place to ensure rapid transfer of a patient to a consultant-led unit in case of haemorrhage in the community or birthing centres. In those with abnormal placentation, prior structured multidisciplinary planning can reduce blood loss, blood transfusions and emergency Caesarean delivery.16 Women who may refuse blood products in an emergency, including Jehovah’s witnesses, should be identified early in the antenatal period and counselled appropriately.
Screening for anaemia and the optimisation of haemoglobin in the antenatal period is essential to prevent unnecessary blood transfusion in cases of postpartum bleeding, as there are opportunities to maximise maternal red cell mass through improved nutrition, iron supplementation or the use of recombinant erythropoietin.
Maternity units should use best practice resources to plan the organisation and teamwork of crisis situations such as maternal haemorrhage, focussing on the SMI themes of readiness, recognition, response and reporting.15 Massive transfusion algorithms should be developed, adapted and rehearsed locally in each obstetric unit based on best international practice. Women who develop massive haemorrhage should be managed with multidisciplinary involvement including obstetrics, anaesthesia, midwifery, obstetric-trained nurses and transfusion medicine specialists. It is recommended that from the outset a senior clinician should supervise the overall management plan, in order to coordinate all aspects of care and avoid fragmentation of decision making.
Initial clinical management
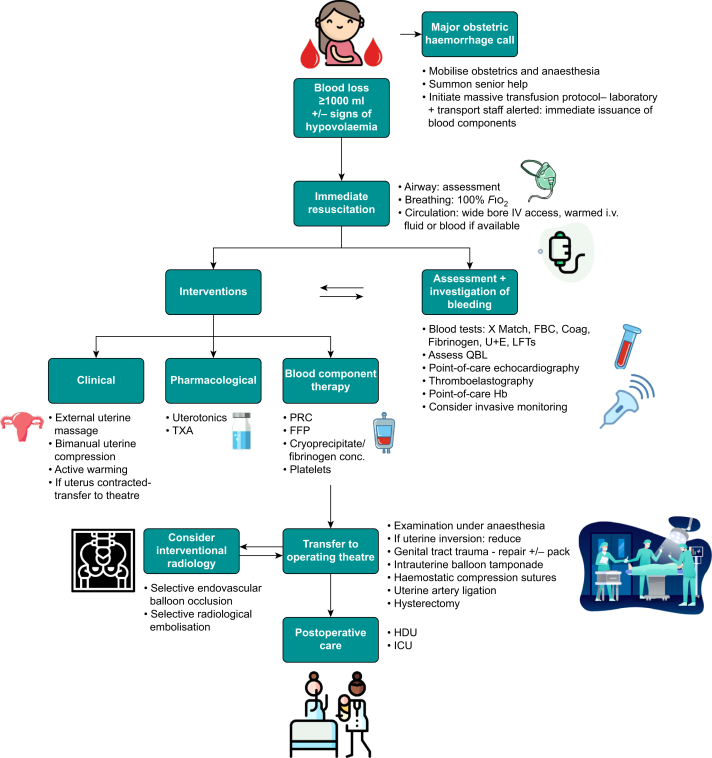
The causes of haemorrhage should be considered and simultaneously investigated while being treated (Fig. 1).
Fig 1.
Flowsheet outlining management of major obstetric haemorrhage in the postpartum setting. Coag, coagulation screen; FBC, full blood count; FFP, fresh frozen plasma; Fio2, fraction of inspired oxygen; Hb, haemoglobin; HDU, high dependency unit; LFTs, liver function tests; PRC, packed red cells; QBL, quantitative blood loss; TXA, tranexamic acid; U+E, urea and electrolytes; X-match, blood cross-match.
The goal during resuscitation is restoration of blood volume and oxygen-carrying capacity. The SMI has categorised obstetric haemorrhage into four stages based on volume of blood loss, the presence of abnormal physiology and the requirement for transfusion.15 Wide bore i.v. access should be established early in at least two sites and a high flow of warmed i.v. crystalloid fluid should be infused until blood products are available. Rapid infusion systems offer the possibility of very high flow rates. Central venous access should be considered if you anticipate that vasopressors might be needed. Left lateral tilt or uterine displacement is required in antepartum cases to optimise preload to the right side of the heart.
Management of the airway is the first priority followed by respiratory support if needed. High-flow oxygen via facemask should be started in all cases of MOH in awake patients. Tracheal intubation may be required to protect the airway in women with a reduced level of consciousness.
Blood should be sent for a full blood count, a coagulation screen and four units of blood should be cross-matched as a minimum.11 Further blood tests should include liver and renal function tests, electrolytes and fibrinogen concentrations. Point-of-care testing for haemoglobin and viscoelastic assays may be useful to detect and monitor ongoing losses. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are point-of-care devices that use whole blood samples to assess coagulation and fibrinolysis providing clinicians with immediate coagulation data and can be used to guide transfusion therapy. There is limited evidence to support the use of these devices from randomised trials.17 It is important to recognise that the initial haemoglobin concentration may not reflect the amount of blood lost and therefore clinical judgement is paramount when initiating and calculating needs for blood transfusion.18 The body temperature should be measured frequently and active warming is mandatory. Extracorporeal membrane oxygenation (ECMO) may offer life-saving support for patients who fail to recover from reversible cardiocirculatory failure.
Control of uterine tone
More than 80% of cases of PPH are attributable to poor uterine tone after delivery. Traditionally, active management of the third stage of labour (AMTSL) is a process in which expulsion of the placenta and membranes is achieved proactively with early cord clamping, controlled cord traction (CCT) and the use of uterotonic drugs. These measures can reduce the incidence of primary PPH by 70% compared with expectant management (spontaneous delivery of the placenta). However, delayed cord clamping has significant benefits for the neonate, and CCT may only be beneficial in the event of a delayed third stage. Uterotonic drug therapy is therefore the primary preventative therapy for PPH; it has excellent clinical efficacy and is universally recommended.2,19,20 Uterotonic drug prophylaxis against PPH is required for all women, as many who suffer PPH have no identifiable risk factors. Uterotonic drugs are also given as therapy where prophylaxis has failed to produce sufficient uterine tone. In 2019 a consensus statement on the use of uterotonic drugs at Caesarean delivery was published in order to address variation in the clinical use of uterotonic drugs and heterogeneity in recommendations from the major international society guidelines.20 Recommended doses of uterotonics are detailed in Table 1.
Table 1.
Comparison of uterotonic drugs used as therapy during major obstetric haemorrhage (MOH). CD, Caesarean delivery; IU, international unit; PPH, postpartum haemorrhage; PR, rectal; PV, vaginal; SL, sublingual.
| Uterotonic drug | Indication | Mechanism of action | Recommended dose during MOH for PPH | Major contraindications |
|---|---|---|---|---|
| Oxytocin | First-line therapy | Via oxytocin receptors on myometrial cell membrane | After vaginal delivery: 5 IU i.v. slowly. Elective Caesarean delivery (CD): bolus 1 IU oxytocin; then infusion at 2.5–7.5 IU h−1. Intrapartum CD: 3 IU oxytocin over ≥30 s; then infusion at 7.5–15 IU h−1. If required after 2 min after initial bolus, give a further dose of 3 IU over ≥30 s. |
Extreme caution in context of haemodynamic instability or cardiovascular disease – deliver drug slowly |
| Carbetocin | First-line therapy | Via oxytocin receptors on myometrial cell membrane | After vaginal delivery: 100 μg over ≥30 s. Elective CD: 100 μg over ≥30 s. Intrapartum CD: 100 μg over ≥30 s. Smaller doses may be sufficient at CD (as low as 20 μg) and may be repeated up to 100 μg. Do not exceed 100 μg in any setting. |
Extreme caution in context of haemodynamic instability or cardiovascular disease – deliver drug slowly |
| Prostaglandins | Second-line therapy: misoprostol may be used as first line where oxytocin/carbetocin unavailable | Via prostaglandin receptors PGE1, PGE2, and PGF2α subtypes | Misoprostol 400–600 μg: sublingual, rectal, vaginal, oral; repeat after 15 min if required, maximum dose 800 μg. Carboprost 250 μg: i.m. or intramyometrial (contraindicated i.v.); up to every 15 min if required, maximum eight doses. Sulprostone 500 μg: i.v. at 100 μg h−1; maximum dose 1500 μg. |
Asthma/obstructive lung disease |
| Ergot alkaloids | Second-line therapy | Via dopamine, α-adrenergic and 5-HT3 receptors | Ergometrine (ergonovine) 200–500 i.m. or slow i.v. in exceptional circumstances; may be repeated after 2 h. | Hypertension Myocardial ischaemia Cardiovascular disease |
Oxytocin
Oxytocin and its analogue carbetocin are recommended as first-line uterotonic prophylaxis and treatment for PPH.2 Oxytocin should be given initially as a small bolus and then as a titrated infusion. There is significant variability in the dose of oxytocin recommended, with most of the major obstetric society guidelines recommending doses of between 5 and 10 IU after both vaginal and Caesarean delivery (5 IU is the maximum allowable i.v. bolus in the UK). However, there is evidence to suggest that these drugs are as effective for prophylaxis in much lower doses.
Women undergoing elective Caesarean delivery (oxytocin naïve) should be considered separately from those undergoing intrapartum Caesarean delivery (prior oxytocin exposure) because of oxytocin receptor desensitisation, whereby prolonged exposure to oxytocin leads to desensitisation of receptors on the myometrium and failure of oxytocin to stimulate effective myometrial contractions. Labouring women with oxytocin-augmentation require much greater doses of oxytocin compared with those at elective Caesarean delivery.
Oxytocin is associated with a range of extrauterine effects, most serious of which are effects on the cardiovascular system and commonly causes tachycardia and hypotension. Chest pain, ECG ST-T segment changes and dysrhythmias may also occur. These effects strongly correlate with both the dose and speed of administration. This is particularly relevant during MOH where oxytocin given in the context of reduced intravascular volume may precipitate rapid decompensation and a number of deaths have occurred in this instance. Oxytocin may be given i.v. or i.m. A reduced dose should be given slowly in those women with cardiac disease. As oxytocin is given via infusion, and has an antidiuretic-like effect in which there is a risk of fluid overload and hyponatraemia in susceptible women (e.g. pre-eclampsia); therefore, the volume of fluids given should be reduced.
Carbetocin
Carbetocin is an oxytocin analogue that is at least as effective as oxytocin, with a favourable adverse effect profile, but its use has been limited by its relatively high cost. Carbetocin acts via the oxytocin receptor system.19 Carbetocin has a duration of action approximately 4–7 times that of oxytocin and so can be given as a slow i.v. bolus without the need for an infusion. It is also available in a heat stable formulation, offering a substantial advantage in certain healthcare settings as cold chain storage is not required. The dose currently recommended is up to 100 μg. Similar to oxytocin, lower doses of carbetocin than those currently recommended are effective at elective Caesarean delivery. The adverse effects and haemodynamic consequences of carbetocin are similar to those described for oxytocin.
Prostaglandins
Prostaglandins act via stimulation of prostaglandin receptors and are unaffected by prior exposure to oxytocin. Prostaglandins are relatively contraindicated in patients with asthma as they may cause bronchospasm. Other adverse effects include hypertension, hypotension, pulmonary oedema, diarrhoea, nausea, vomiting, flushing, pyrexia and myalgia. Misoprostol is a prostaglandin E1 analogue and may be given by oral, sublingual, rectal or vaginal routes. It is recommended as first-line treatment for PPH where oxytocin is not available. Its efficacy is broadly similar to that of oxytocin, but is limited by its unfavourable adverse effects.19 Doses between 400 and 600 μg misoprostol are currently recommended. Carboprost and sulprostone are prostaglandins used to treat PPH where first-line agents have failed, and are not used currently as first-line prophylaxis because of their significant adverse effects. Carboprost is recommended at a dose of 250 μg i.m. every 15 min up to a maximum of eight doses, and i.v. use is contraindicated as it may result in bronchospasm, hypertension or pulmonary oedema.
Ergometrine
Ergometrine is currently recommended as a second-line agent for the treatment of PPH. Ergometrine combined with oxytocin has additional benefit compared with oxytocin alone, but has more adverse effects, especially nausea.19 Ergometrine is contraindicated where hypertensive disorders are present, as α-adrenergic receptor activation may precipitate or exacerbate hypertension. Activity of ergometrine is via α-adrenergic receptors, dopamine receptors and a partial 5-hydroxytryptamine (5-HT) signalling effect. Ergometrine is unaffected by the oxytocin desensitisation phenomenon. Guidelines recommend a dose range of 200–500 μg, and this may be given via either the i.v. (with caution) or i.m. routes.20 The duration of action of ergometrine is from 45 min if given i.v. and may be up to 3 h if given i.m.
Blood transfusion and haemostasis
Delays in accessing blood components for life-threatening haemorrhage leading to unnecessary morbidity and mortality are often reported, including in the 2020 Serious Hazards of Transfusion (SHOT) report.18,21 Transfusion should be protocol-driven with clear local guidelines agreed at each obstetric unit. Blood transfusion may be lifesaving but has risks including transfusion reactions, infection and red cell alloimmunisation. Blood product management is often extrapolated from the trauma literature to the obstetric setting as there is a lack of evidence from specific randomised controlled trials in obstetrics. Fixed transfusion strategies with a high ratio of platelets and fresh frozen plasma (FFP) clotting products to packed red blood cells (e.g. 1:1:1) are often used in massive transfusion protocols. Dilution of fibrinogen is a concern if large volumes of products with a low concentration of fibrinogen are used such as FFP.
Red cell transfusion
When MOH is suspected, the decision to transfuse red cells should be made on clinical grounds, avoiding any delay in waiting to confirm transfusion thresholds on laboratory tests.18 ABO–, rhesus D– (RhD–) and K– (Kell–) compatible red cell units should be transfused. At least four units of O-negative packed red cells should be readily available in each obstetric unit at all times. If blood loss is anticipated during surgery, cell salvage may be useful for autologous transfusion. The use of cell salvage may also be prudent in some cases at risk of major haemorrhage, but no benefit has been demonstrated from its routine use and it may increase the risk of maternal alloimmunisation.22 Cell salvage may be acceptable to some patients who would not otherwise accept blood transfusion, for example Jehovah’s Witnesses. A perceived risk of amniotic fluid embolisation is often cited against the use of cell salvage, yet there is little evidence to support this in practice and safety has been demonstrated robustly. A double suction technique is often used, with the aim of using one suction for amniotic fluid and another for suctioning uncontaminated blood. Cell salvage requires training and experience must be demonstrated in the low risk setting in order to ensure proficiency amongst staff in an emergency.
Plasma and fibrinogen products
Inadequate provision of coagulation products, particularly fibrinogen was highlighted as a concern in recent MBRRACE reports, and many women who died had delayed or inadequate correction of their coagulopathy.4 The risk of coagulopathy increases with bleeding >2000 ml. Coagulopathy varies depending on the aetiology of haemorrhage and may be dilutional, consumptive or attributable to DIC.23 Haemorrhage causes by amniotic fluid embolism, uterine rupture or placental abruption may be associated with early onset DIC.
FFP should be considered early for conditions with a suspected coagulopathy, such as placental abruption or amniotic fluid embolism, or where treatment has been delayed. The coagulation target should be to maintain PT and APTT at less than 1.5× normal. Laboratory processing times may limit the use of these tests in the acute setting.
Pregnancy is a prothrombotic state with higher baseline fibrinogen concentrations of 4–6 g L–1 compared with 2–4 g L–1 in non-pregnant patients, acting as a physiological buffer for haemorrhage. Fibrinogen concentrations decrease more rapidly than other coagulation factors, and this is often a predictor of progression to severe PPH. A target of at least 2 g L–1 fibrinogen is required to maintain haemostasis during haemorrhage. Human fibrinogen concentrate or cryoprecipitate may be used as a fibrinogen source, depending on availability. The concentration of fibrinogen in cryoprecipitate is approximately 15 g L–1, significantly higher than that of FFP (2 g L–1). Fibrinogen concentrate is a fixed dose that may be given in a smaller volume and is viral inactivated, but gram for gram is significantly more expensive than cryoprecipitate.
Platelets
The platelet count, if low, may indicate a consumptive process and be associated with a coagulopathy. A platelet transfusion trigger of 75×109 is recommended as a transfusion trigger during haemorrhage.18
Tranexamic acid
Tranexamic acid (TXA) reduces bleeding by inhibiting the enzymatic breakdown of fibrin, thereby stabilising blood clot architecture and is well established in many non-obstetric situations including trauma and elective surgery. The World Maternal Antifibrinolytic Trial (WOMAN) showed that by giving TXA, deaths from bleeding were reduced by 20% and the greatest benefit was seen when TXA was given within 3 h of childbirth.24 TXA is not associated with an increased risk of thrombosis in healthy women. The dose of TXA recommended by the WHO is a 1 g fixed dose given over 10 min with a second dose if bleeding continues after 30 min.
Mechanical and surgical intervention
Anaesthesia for surgical intervention
Women may need examination under anaesthesia to evaluate the cause of haemorrhage, repair tears to the genital tract, drain a haematoma or other interventions to control bleeding. The choice of anaesthesia will depend on the clinical situation. General anaesthesia may be preferable to regional anaesthesia in cases of haemodynamic instability, compromised consciousness or where the coagulation status of the patient is in question. The choice and dose of i.v. anaesthetic for induction should avoid cardiovascular decompensation, and ‘cardiostable’ induction agents such as ketamine may be preferred. Invasive arterial pressure monitoring will allow detection of rapid shifts in arterial pressure and close titration of vasopressors and fluid balance. Women who suffer MOH should be managed in a high-dependency area with the capacity to deliver high-quality maternal critical care in the presence of the infant, where possible.
Interventions to control haemorrhage
Bimanual uterine compression is recommended as a temporising measure until definitive care is available for the treatment of PPH resulting from uterine atony after vaginal delivery and acts by stimulating contraction of uterine muscle fibres.
Intrauterine balloon catheters such as the Bakri, Rusch and Foley catheters may be used to achieve uterine tamponade, and is effective in 97% of cases of PPH in resource-poor settings.25 The balloon should be deflated preferably during the daytime when there is a full complement of staff available because of the risk of rebleeding.
Haemostatic compression sutures are most commonly used at Caesarean delivery to arrest haemorrhage, particularly in the setting of refractory uterine atony. They are safe and may be fertility preserving. Ligation of the uterine and utero-ovarian arteries can reduce uterine bleeding by obtunding myometrial blood flow. Cross-clamping of the aorta may be used as a temporising measure to control bleeding in the setting of massive haemorrhage.
Interventional radiology offers a minimally invasive, fertility preserving method to treat refractory haemorrhage. Selective arterial occlusion using balloons may be used to stem blood flow to the common internal iliac artery or the aorta to either prevent or treat PPH.26 The use of resuscitative endovascular balloon occlusion of the aorta (REBOA) has resulted in better outcomes than standard therapy without REBOA, but has also been associated with serious complications including ischaemia. Selective radiological embolisation of the uterine artery may be used to treat haemorrhage. Complications including ischaemia, thrombosis and arterial rupture have also been reported.
Peripartum hysterectomy is the definitive last resort to control obstetric haemorrhage and is associated with significant morbidity. The recent MBRRACE report acknowledges that hysterectomy should be resorted to sooner rather than later, especially in cases of accreta or uterine rupture.4
Conclusions
Major obstetric haemorrhage is a significant global burden and a preventable cause of maternal morbidity and mortality. Early identification of haemorrhage, followed by simultaneous investigation and management, is the key to effective treatment. Uterotonic drugs should be chosen based on the clinical context. Intravascular volume resuscitation and blood transfusion should be driven by a protocol with clear local guidelines agreed at each obstetric unit, as many women who have died had delayed or inadequate correction of their coagulopathy. Early senior decision making is critical to coordinate and optimise care. Anaesthetists should familiarise themselves with the range of mechanical and surgical options available to treat haemorrhage. Coordinated preparation, including simulation training, is essential to deliver high-quality care in a crisis.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Thomas Drew FCAI FJFICMI is a consultant anaesthesiologist at the Rotunda and Beaumont Hospitals in Dublin, Ireland.
Jose Carvalho MD PhD is director of obstetric anaesthesia at Mount Sinai Hospital and professor of anaesthesia and obstetrics and gynaecology at the University of Toronto, Toronto, Canada.
Matrix codes: 1A02, 2B05, 3B00
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.GBD 2015 Maternal Mortality Collaborators Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1775–1812. doi: 10.1016/S0140-6736(16)31470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2018. WHO recommendations uterotonics for the prevention of postpartum haemorrhage.https://apps.who.int/iris/bitstream/handle/10665/277276/978 9241550420-eng.pdf?ua=1 Available from. [PubMed] [Google Scholar]
- 3.Sheldon W.R., Blum J., Vogel J.P., et al. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121:5–13. doi: 10.1111/1471-0528.12636. [DOI] [PubMed] [Google Scholar]
- 4.Knight M., Bunch K., Tuffnell D., et al. National Perinatal Epidemiology Unit, University of Oxford; 2021. Saving Lives, Improving Mothers’ Care — lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017–19. [Google Scholar]
- 5.Bell S.F., Watkins A., John M., et al. Incidence of postpartum haemorrhage defined by quantitative blood loss measurement: a national cohort. BMC Pregnancy Childbirth. 2020;20:271. doi: 10.1186/s12884-020-02971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reale S.C., Easter S.R., Xu X., Bateman B.T., Farber M.K. Trends in postpartum hemorrhage in the United States from 2010 to 2014. Anesth Analg. 2020;130:e119–e122. doi: 10.1213/ANE.0000000000004424. [DOI] [PubMed] [Google Scholar]
- 7.Kramer M.S., Berg C., Abenhaim H., et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209:449. doi: 10.1016/j.ajog.2013.07.007. e1–7. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadzia H.K., Grotegut C.A., James A.H. A national update on rates of postpartum haemorrhage and related interventions. Blood Transfus. 2020;18:247–253. doi: 10.2450/2020.0319-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varouxaki N., Gnanasambanthan S., Datta S., Amokrane N. Antepartum haemorrhage. Obstet Gynaecol Reprod Med. 2018;28:237–242. [Google Scholar]
- 10.RCOG . 2011. Antepartum haemorrhage (Green-top Guideline No. 63) [Google Scholar]
- 11.RCOG . 2016. Prevention and Management of Postpartum Haemorrhage (Green-top Guideline No. 52) [DOI] [PubMed] [Google Scholar]
- 12.Menard M.K., Main E.K., Currigan S.M. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150–153. doi: 10.1097/AOG.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 13.Hayes N., Drew T. In: Respiratory Disease in Pregnancy. Lapinsky S., Plante L., editors. Cambridge University Press; Cambridge, UK: 2020. Cardiopulmonary physiological alterations in pregnancy; pp. 25–33. [Google Scholar]
- 14.Bailit J.L., Grobman W.A., Rice M.M., et al. Morbidly adherent placenta treatments and outcomes. Obstet Gynecol. 2015;125:683–689. doi: 10.1097/AOG.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Obstetrics and Gynecology Safe Motherhood Initiative. https://www.acog.org/community/districts-and-sections/district-ii/programs-and-resources/safe-motherhood-initiative/obstetric-hemorrhage Available from:
- 16.Shamshirsaz A.A., Fox K.A., Salmanian B., et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218. doi: 10.1016/j.ajog.2014.08.019. e1–9. [DOI] [PubMed] [Google Scholar]
- 17.Amgalan A., Allen T., Othman M., Ahmadzia H.K. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J Thromb Haemost. 2020;18:1813–1838. doi: 10.1111/jth.14882. [DOI] [PubMed] [Google Scholar]
- 18.RCOG . 2015. Blood transfusions in obstetrics, green-top guideline no. 47. [Google Scholar]
- 19.Gallos I.D., Papadopoulou A., Man R., et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689. doi: 10.1002/14651858.CD011689.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heesen M., Carvalho B., Carvalho J.C.A., et al. International consensus statement on the use of uterotonic agents during caesarean section. Anaesthesia. 2019;74:1305–1319. doi: 10.1111/anae.14757. [DOI] [PubMed] [Google Scholar]
- 21.Narayan S., Poles D., et al. 2021. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2020 Annual SHOT Report. [Google Scholar]
- 22.Khan K.S., Moore P., Wilson M., et al. A randomised controlled trial and economic evaluation of intraoperative cell salvage during caesarean section in women at risk of haemorrhage: the SALVO (cell SALVage in Obstetrics) trial. Health Technol Assess. 2018;22:1–88. doi: 10.3310/hta22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collis R.E., Collins P.W. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015;70(Suppl 1):78–86. doi: 10.1111/anae.12913. e27–e28. [DOI] [PubMed] [Google Scholar]
- 24.Woman Trial Collaborators Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez S., Conde-Agudelo A., Borovac-Pinheiro A., et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293 e1–52. doi: 10.1016/j.ajog.2019.11.1287. [DOI] [PubMed] [Google Scholar]
- 26.Lambrecht S., Van De Velde M. Interventional radiology for the obstetric patient. Curr Opin Anaesthesiol. 2020;33:566–570. doi: 10.1097/ACO.0000000000000884. [DOI] [PubMed] [Google Scholar]