Learning objectives.
By reading this article, you should be able to:
-
•
Describe the risk factors for abdominal aortic aneurysm (AAA).
-
•
Discuss the assessment and decision-making processes for transfer and initial management of patients with ruptured abdominal aortic aneurysm (rAAA).
-
•
Outline the challenges of anaesthesia for endovascular repair of rAAA, including isolated site working.
-
•
Identify common complications of endovascular rAAA repair.
Key points.
-
•
Mortality from rAAA remains high, at approximately 80%.
-
•
Protocols for the assessment and transfer of patients with rAAA improve outcomes.
-
•
Emergency endovascular repair is becoming more common. Survival outcomes are comparable to those from open repair.
-
•
Challenges of anaesthesia for ruptured endovascular aneurysm repair (REVAR) involve managing an unstable patient in a hybrid theatre.
-
•
Local anaesthesia for REVAR is associated with a better 30-day mortality than GA.
An abdominal aortic aneurysm (AAA) is defined as a widening of the aortic lumen to >1.5 times its normal diameter or to greater than 30 mm in absolute diameter. Ruptured abdominal aortic aneurysm (rAAA) is a surgical emergency and carries a very high mortality. In-hospital mortality is approximately 30–40%, but overall mortality is around 80% when deaths in patients who do not reach hospital are included.1,2 Around 6000 people die each year from rAAA in England and Wales, accounting for approximately 1% of all deaths. Traditionally, rAAA was treated with open repair, which had a 50% in-hospital mortality.3 The widespread introduction of endovascular infrarenal repair has provided an alternative treatment option for some patients with rAAA. Both elective and emergency AAAs can be treated using minimally invasive techniques. The outcomes for endovascular aneurysm repair (EVAR) compared with open repair are comparable both in the emergency and elective settings.4,5 For elective procedures, the early improvement in mortality with endovascular repair has not translated into long-term survival benefit compared with open repair.5 The Immediate Management of Patients with Rupture: Open Versus Endovascular Repair (IMPROVE) trial compared endovascular with open repair in rAAA and demonstrated comparable clinical outcomes both for 30-day mortality and at 3-yr follow-up.6
Risk factors
The risk of AAA is greater in males and increases with age. The incidence is 4% in men older than 65 yrs, which is four times greater than that in women of comparable age. However, the risk of rupture is higher in women (Table 1).7, 8, 9
Table 1.
Risk factors for developing an abdominal aortic aneurysm (AAA) and risk factors for aneurysm rupture (rAAA).
| Risk factors for developing AAA | Risk factors for AAA rupture |
|---|---|
|
|
The screening programme and national vascular services
The goal of a national aneurysm screening programme is to detect asymptomatic disease in order to facilitate patient selection for elective repair before aneurysmal rupture. Population screening for AAA has demonstrated a cost-effective reduction in disease-specific mortality of around 50% and was introduced in the UK in 2009.10 Ultrasonography is used as it is cheap, noninvasive and has a high sensitivity and specificity.10 Males aged >65 yrs are offered screening. If an aneurysm is detected, follow-up scanning is offered annually until the AAA reaches 45 mm when the scan interval decreases to 3 monthly. No further scans are indicated if there is no aneurysmal disease at screening. Males with AAAs exceeding 55 mm detected either at initial screening or at follow-up ultrasound are referred for consideration of surgery. Females are not routinely screened for AAA but, if found incidentally, are referred for consideration of surgery at an AAA diameter of 50 mm. In 2020, only the UK, Sweden, Germany, and the USA had implemented national screening programmes.
National vascular services
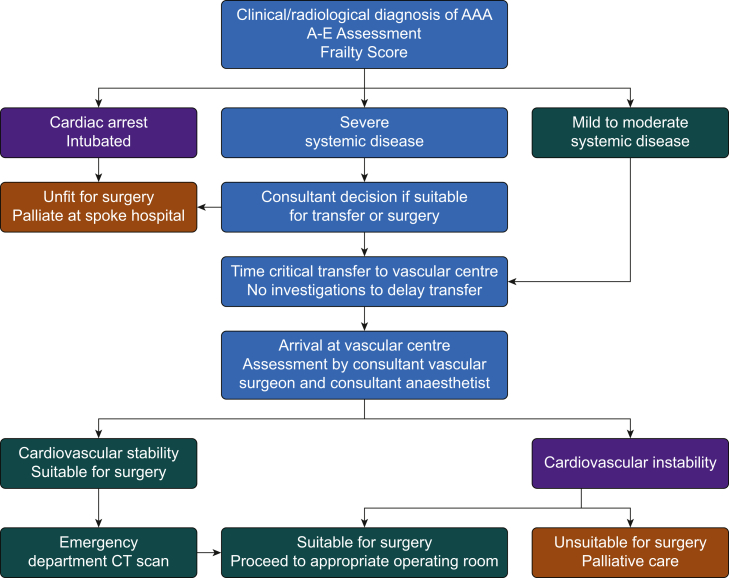
Vascular services in the UK have been centralised since 2009.11 Local vascular networks were established with regional central ‘hub’ sites providing vascular services to local ‘spoke’ hospitals. Locally agreed protocols have streamlined the management and time-critical transfer of patients with ruptured AAA and are recommended by National Institute for Health and Care Excellence (NICE)9 (Fig. 1). The aim of the guidance is to facilitate swift transfer of patients for whom emergency aneurysm repair is likely to be of benefit, whilst avoiding the unnecessary transfer of patients for whom palliative care at their local hospital is more appropriate.
Fig 1.
An example of a decision pathway for referral for ruptured abdominal aortic aneurysm.
‘Turn down’ register
As a result of screening programmes, patients presenting with rupture are often known to have aneurysmal disease. Such patients may have been assessed for elective repair and in some instances a decision made to pursue conservative management because of coexisting morbidities, and predicted poor outcome. It is useful to keep a record of these patients as it can help inform the discussion between clinicians, the patient and their relatives in the event of emergency admission, potentially preventing an inappropriate transfer. Each patient must be assessed at presentation and ‘turn down’ for elective repair should not preclude offering repair in the event of rupture. Many hubs keep such records in the emergency department to render them easily accessible to the relevant clinical staff.
Protocols and guidance
The Society for Vascular Surgery (USA) guidelines recommend that time from first clinical contact to surgical intervention for rAAA should be less than 90 min, broken into a 30:30:30 model. The goal is a decision-to-transfer time of 30 min, maximum 30 min transfer time, and a further 30 min until starting definitive treatment. This guidance is based on the 2004 American College of Cardiology and American Heart Association guidelines for ST-segment elevation myocardial infarction (STEMI).12 The UK College of Emergency Medicine gives similar guidance.13 Good communication between the referring and accepting hospitals is paramount and should include transfer of radiological imaging. The introduction of multidisciplinary protocols has been reported to improve 30-day mortality in Canada and the USA.14,15
Diagnosis of AAA and patient selection for endovascular repair
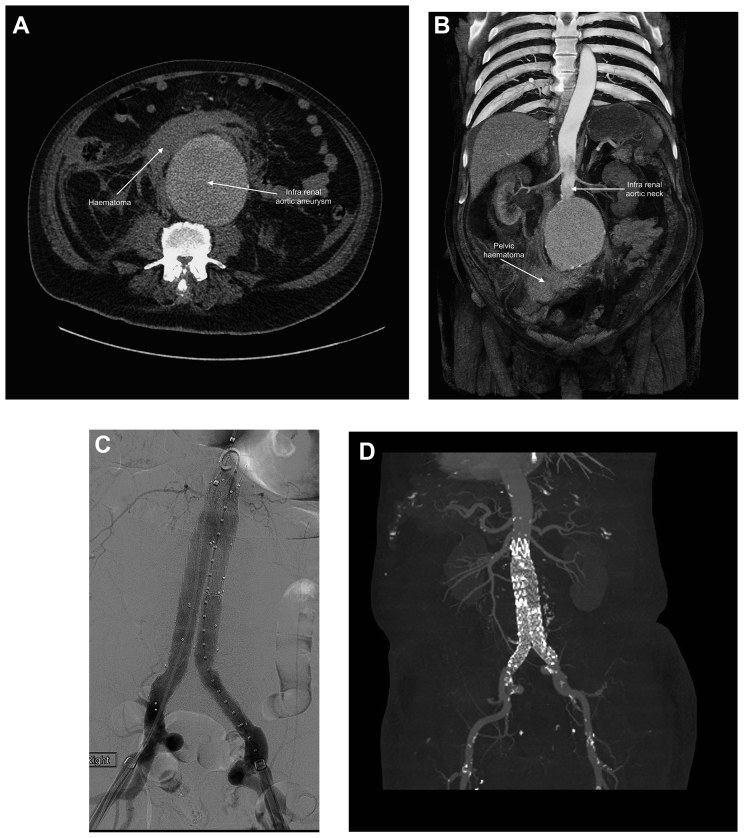
Patients who survive the acute physiological insult of AAA rupture usually present to the emergency department with acute onset of back or abdominal pain, loss of consciousness or cardiovascular collapse. The differential diagnosis includes renal colic, gastrointestinal haemorrhage, acute diverticulitis and pancreatitis. Rupture is more likely in patients older than 60 yrs with an existing diagnosis of AAA, hypertension and a history of smoking tobacco (Table 1). A bedside ultrasound may confirm the presence of AAA, but the sensitivity of detection of free fluid attributable to haemorrhage is low.16 A thin-slice contrast-enhanced arterial-phase CT angiogram is required to confirm diagnosis and assess treatment options, and ideally this should be performed at the vascular hub.16 CT scans from a diagnosis of AAA rupture to successful graft placement are shown in Fig. 2.
Fig 2.
Computed tomography and digital subtraction angiogram views of ruptured abdominal aortic aneurysm (rAAA) and endovascular repair. (A) Axial CT angiogram showing haematoma from the ruptured rAAA. (B) Coronal view showing pelvic haematoma and infrarenal neck suitable for proximal stent deployment. (C) Digital subtraction angiogram showing Medtronic stent placement. (D) Computed tomography after surgery demonstrating acceptable placement of graft with no evidence of endoleak.
The evidence regarding the superiority of either ruptured endovascular aneurysm repair (REVAR) or open repair for rAAA is equivocal. The IMPROVE trial, a large multicentre randomised control trial, was established to compare mortality after open repair with endovascular repair in rAAA. Before CT scanning, patients were randomised to either standard open repair or to a potential endovascular approach with urgent CT assessment of anatomical suitability. Patients who were randomised to the group with unfavourable anatomy on CT for endovascular repair were able to move to the open group and likewise a patient unsuitable for open repair could transfer to the endovascular group if subsequent CT confirmed favourable anatomy. The primary outcome measure was 30-day mortality. Secondary outcomes included 24-h and in-hospital mortality, time to discharge, discharge destination and costs. Results showed comparable 30-day mortality for REVAR (35.4%) and open repair (37.4%). This comparable clinical effectiveness extended to 3-yr follow-up. There was some evidence that women benefitted more than men from endovascular repair.6 Amongst the notable secondary outcomes were quality of life benefits in the REVAR group: shorter duration of hospital stay; increased likelihood of discharge directly to home; and improved quality of life. REVAR was also more cost-effective.17 In contrast, a smaller American study found a statistically significant improvement in 30-day mortality persisting to 5 yrs in patients having endovascular rather than open repair.18 The 2020 National Vascular Registry also reported a 20.2% mortality with REVAR compared with 41.1% for open repair in rAAA.19 These differences compared with the IMPROVE data are thought to be attributable to real-life patient selection, with stable patients who had favourable aortic anatomy more often selected for REVAR. National Institute for Health and Care Excellence has recommended REVAR as first-line management of rAAA in men aged over 70 yrs and for all women with suitable anatomy. Enthusiasm for REVAR is growing in the USA and Europe, but open repair still predominates.20,21
Radiological considerations
The primary goal of REVAR is to ensure rapid control of the aneurysmal rupture and hence survival of the patient.22 Anatomical challenges seen as a barrier to elective EVAR can be considered acceptable in REVAR when palliation would be the only other option. Suitable proximal and distal landing zones must be identified, and angulation or a short tapered infrarenal neck are contraindications. Some clinicians tolerate less-than-ideal anatomy to secure control of haemorrhage, but this may compromise graft durability, giving rise to a need for further interventions during long-term follow-up. A bifurcated graft is preferred to an aorto-uni-iliac (AUI) device because, although time to aneurysm sac exclusion may be longer, the graft can be inserted under local anaesthesia. An AUI followed by open femoral–femoral crossover graft is an alternative where there is uni-iliac occlusion or compromised access. The femoral–femoral crossover extends the duration of the procedure, usually necessitates general anaesthesia and increases the incidence of groin infection after surgery.22,23
Multidisciplinary working
Assessment of a patient's suitability for REVAR should be multidisciplinary, involving senior clinicians at the spoke site and a senior team of vascular surgeon, anaesthetist and radiologist at the hub. A rapid review of the patient's health and functional status should be undertaken, including assessment of common comorbidities such as ischaemic heart disease, cardiac failure, renal disease, respiratory disease and history of smoking. Medications must be reviewed. It is particularly important to ascertain whether the patient is taking anticoagulant drugs, which will potentiate ongoing haemorrhage, and advice should be sought from a haematologist in this instance. Metabolic equivalents of task (MET) and Frailty Index scoring can be used to quantify premorbid functional status, but there is no strong evidence that any individual patient characteristics or scoring systems are useful in deciding whether a patient will survive surgery for rAAA.9 This information should be used in conjunction with clinical assessment of shock and Glasgow Coma Scale (GCS) to determine the suitability of emergency repair for each individual. A full blood count, clotting studies, blood biochemistry, blood gas analysis and an ECG give an indication of any ischaemic insult from hypoperfusion and are also relevant in the assessment of comorbid disease. Urgent group and cross-matching sampling aids provision of group-specific blood in preference to the use of universal donor products. It is recognised that patients who suffer cardiac arrest or who require intubation before transfer have a poor outcome even with surgery, and the local team should consider palliative care in such circumstances.13 Cross-site and interdepartmental communication remains vital throughout, and hub clinicians must ensure that laboratory, operating theatre and radiological staff are informed as soon as is practicable to allow time for preparation of blood products, equipment and suitable staff allocation.
Initial resuscitation
Repeated reassessment of the patient's clinical state is imperative as rapid deterioration may occur rendering surgery futile. Computed tomography images should be reviewed to determine suitability for REVAR or open surgery. Clinical management depends on the degree of shock. Accurate assessment of blood loss is difficult because haemorrhage is concealed, but useful surrogates include cardiovascular stability, level of consciousness, blood haematology, blood biochemistry and acid–base status. Patients who survive to the emergency department are likely to have posterior retroperitoneal tamponade, rather than free intraperitoneal haemorrhage.3 Overzealous therapy with fluids should be avoided to prevent disruption of the clot providing tamponade. Balanced resuscitation includes early use of blood products in a 1:1:1 ratio of red cells, fresh frozen plasma and platelets.24 Excessive degrees of permissive hypotension are contraindicated in these patients who are typically older. In secondary analysis, the IMPROVE trial found a linear correlation between 30-day mortality and the lowest recorded systolic arterial pressure, with average mortality rates of 51% when the recorded systolic pressure was <70 mmHg, and 34.1% when greater. For each 10 mmHg increase the relative survival odds increased by 13%, apparently demonstrating that very low arterial pressure in these patients appeared detrimental.4 It is unknown whether this is a result of undertreatment for hypotension or whether lower systolic pressure is in itself a poor prognostic sign. A permissive hypotension threshold of 70 mmHg is questionable, and arterial pressure targets should be based on clinical review. In the USA 80 mmHg is the accepted lower limit, and the UK Emergency Medicine guidance recommends maintaining systolic arterial pressure between 90 and 120 mmHg with an emphasis on assessment of GCS.13 The use of relative volume restriction should not be confused with a lack of need for wide-bore i.v. access, which must be inserted at the earliest opportunity.
Transfer to a radiology hybrid theatre should be expedited if CT imaging of the patient's aneurysmal morphology demonstrates that endovascular repair is suitable. If REVAR is unsuitable, but open surgery is an option, the patient should proceed directly to the operating theatre. The multidisciplinary care team should carefully consider whether surgery is appropriate in a patient too unstable for radiological assessment. In all cases, consent for surgery and anaesthesia should be succinct, allowing relatives time with the patient but not delaying life-saving intervention.
Massive haemorrhage protocol
Activation of a massive haemorrhage protocol with a named clinician responsible for blood bank communication is recognised as good practice by the Joint Professional Advisory Committee (JPAC) and ensures prioritisation of blood products for the patient.25 The use of cross-matched, group-specific or universal donor blood will depend on the efficiency of sample processing. Early use of tranexamic acid is controversial, with concerns over the high risk of thromboembolic events after rAAA repair.26 There is little published evidence concerning the use of tranexamic acid in rAAA, and NICE recommends it be restricted to enrolment in clinical trials.26
Location of surgery
Hybrid theatre facilities may be situated within the interventional radiology (IR) department or the operating theatre suite. They should be capable of providing high-quality radiological imaging and be able to accommodate conversion to open repair if necessary. If repair is undertaken outside the operating theatre complex, all the caveats of anaesthesia in an isolated site apply. In addition, radiological protection for staff must be considered.
Interventional radiology issues for the anaesthetist
Issues for the anaesthetist may include:
-
•
Communication within and between teams with a large number of multidisciplinary personnel present.
-
•
Limited space.
-
•
An unfamiliar, isolated environment.
-
•
A non-tilting table for induction of anaesthesia.
-
•
The need to transfer anaesthetic equipment from other areas.
-
•
Inaccessibility of the patient because of positioning of imaging equipment.
-
•
Limited ability to warm the patient – warming blankets and mattresses may compromise or obscure radiological imaging.
Anaesthesia for REVAR
Ruptured endovascular aneurysm repair requires an experienced anaesthetic team. Because of its complexity, the numerous practical tasks and the challenge of managing an unstable patient, it is optimum to have at least a second anaesthetist supporting the anaesthetist in charge of the patient's care. Additional support staff experienced in managing of major haemorrhage, cell salvage and endovascular surgery are essential to the team. The IMPROVE trial noted an increased 30-day mortality in patients presenting out of hours, but the relative risk was negligible when adjusted for lowest recorded systolic arterial pressure and fluid volume given, suggesting this was not a significant factor.4
Patient monitoring and resuscitation equipment must be prepared and should include noninvasive and invasive blood pressure monitoring, five-lead ECG, capnography, the option for cardiac output monitoring, pulse oximetry, infusion pumps, a rapid infuser, cell salvage and a comprehensive range of vasoactive, inotropic and other resuscitation drugs. Large-bore i.v. access is necessary to allow adequate resuscitation and the use of vasoactive drugs during induction of anaesthesia. It is desirable to site invasive monitoring cannulae before induction of anaesthesia, but this should not delay the surgical management of haemorrhage. The difficulties of access to the patient should be anticipated and planned for, particularly in relation to insertion of invasive catheters during the procedure. Significant insidious bleeding may occur from groin access sites during a prolonged procedure. The element of blood loss can be quantified by suction collection, cell salvage or swab weighing. Point-of-care testing including haemoglobin, blood gas analysis and blood viscosity assessment can assist in guiding doses of blood product and platelets, but haemoglobin concentration and haematocrit may be misleading and transfusion should be guided by the patient's clinical condition and physiological variables. There are no established transfusion goals specific to rAAA, but management of major haemorrhage guidance published by JPAC provides a working model.25
Anaesthetic technique
Both local (LA) and general anaesthesia (GA) have been used for REVAR. Local anaesthesia infiltration of the groins facilitates device deployment via the femoral arteries and eliminates the cardiovascular depressant effects of general anaesthesia. An IMPROVE subgroup analysis reported a lower 30-day mortality for REVAR under LA (13%) compared with GA (34%).4 This benefit was maintained when adjusted for sex and age. As a result, initial LA infiltration is becoming common practice in the UK and is supported by NICE.8,27 Back and abdominal pain associated with aneurysmal rupture may be intolerable to some patients, who may become agitated and anxious with an extended procedure.12 Some surgical practitioners are reluctant to conduct REVAR under LA because of the theoretical risk of stent malposition and inadvertent renal artery occlusion in an agitated patient.16 The cautious use of sedative and analgesic drugs can help to alleviate this. Low dose target-controlled infusions of short-acting agents such as propofol and remifentanil are ideal and can be titrated to achieve compliance and anxiolysis without compromising the airway or cardiovascular stability.
The decision to use LA or GA will depend on careful anaesthetic assessment and the clinical state of the patient. Airway and starvation status assessment should be undertaken for all rAAA and preparations for GA and intubation made even if LA is preferred. General anaesthesia leads to a reduction in sympathetic tone, which potentiates the hypotension of hypovolaemia in patients experiencing aneurysmal rupture but may still be preferred to facilitate rapid repair in an agitated patient. The initial use of LA to facilitate proximal aneurysm control with later conversion to GA may offer the most pragmatic option in some circumstances. Where GA is the preferred option, i.v. induction agents should be carefully titrated to target effect, and used in combination with fluids and infusion of vasoactive drugs to support the cardiovascular system. Both GA induction or LA infiltration should occur on the operating or radiology table with the patient prepared, surgeons scrubbed and radiologist ready for rapid aortic balloon placement and inflation should this be required.
Resuscitative balloon occlusion of the aorta
The aim of REVAR is rapid control of major haemorrhage which may require resuscitative endovascular balloon occlusion of the aorta (REBOA). A balloon catheter is introduced via the femoral artery using a Seldinger technique and deployed in the supracoeliac aorta with the aim of maintaining cardiac and cerebral perfusion. The balloon inflation results in reduced hepatic, mesenteric and renal blood flow, and so should ideally be inflated for a maximum of 30 min to minimise the risk of end organ damage.24 Aortic rupture caused by repeated balloon inflation or overdistension is potentially fatal. Resuscitative endovascular balloon occlusion of the aorta can also cause large vessel injuries such as aortic dissection, perforation, thrombosis and cholesterol and air embolisation, resulting in distal limb ischaemia. Local injuries at the sheath insertion site include pseudoaneurysm, dissection and arteriovenous fistula formation.28 In view of these significant complications, some operators favour guidewire placement, only inflating the balloon in the event of haemodynamic deterioration.
Perioperative management
Transfusion
Massive transfusion brings the complication of a rapidly evolving coagulopathy. Coagulopathy will be potentiated by hypothermia, so precautions should be taken from the outset to minimise patient exposure. Warmed i.v. fluids should be given and attempts made to maintain body temperature despite the difficulties of using active warming devices. Ongoing requirements for fluids and blood products are guided by laboratory and point-of-care analysis. If available, activated clotting time (ACT) testing may help to guide dosing of heparin.29
Acid–base balance
Ongoing fluid resuscitation is required after surgery to correct the perioperative acidosis resulting from hypovolaemia and end-organ ischaemia. Fluid, vasopressor and inotropic drugs may be needed and should be guided by haemodynamic flow monitoring and regular blood gas analysis with particular attention to lactate and base deficit correction. Massive transfusion of red cells can lead to hyperkalaemia and treatment may be required if concentrations associated with cardiac arrhythmias are reached.
Perioperative complications
Type I endoleak
Type I endoleak occurs when there is inadequate sealing of the proximal endovascular graft within the infrarenal aorta (type Ia endoleak) or distal aspect of the limbs within the iliac vessels (type Ib endoleak). These are high-pressure leaks and result in continuing blood loss into the rupture site. A proximal aortic cuff necessitating ballooning of the graft extension is required for type Ia leak bringing the concomitant risks of aortic rupture. Limb extensions may be necessary for type Ib leaks to ensure graft sealing. There are five types of endoleak, but types II–V do not result in a continued high-pressure leak into the aneurysm sac and are less important in the setting of rupture.
Arterial injury
Arterial injury at the vascular access site may manifest as a retroperitoneal bleed or blood loss at the groin wounds. Blood loss may be covert so a high index of suspicion should be maintained in the event of a rapid arterial pressure decrease during guidewire and device delivery manipulations or REBOA if used. Clinicians should be vigilant into the postoperative period where apparent vasovagal episodes and signs of peritoneal irritation may be caused by insidious bleeding.
Abdominal compartment syndrome
Abdominal compartment syndrome (ACS) is common after both open repair and EVAR. It develops as a result of massive fluid resuscitation, intraperitoneal haemorrhage and splanchnic reperfusion. The increase in intra-abdominal pressure reduces venous return because of compression of the inferior vena cava and can precipitate heart failure, an increase in ventilation pressures, and a reduction in renal and intestinal perfusion causing ischaemic damage. Abdominal compartment syndrome is difficult to diagnose and, left untreated, will lead to multiorgan failure and death. The standard treatment for ACS is decompressive laparotomy.30
Ischaemic colitis
Ischaemic colitis is significantly more common after rAAA than elective repair and occurs more frequently in open repair compared with REVAR (19.3% vs 6.4%).31 Its aetiology is complex: risk factors include rupture, age, renal insufficiency, surgical time and microembolisation. Signs and symptoms include rectal bleeding, diarrhoea, abdominal pain and distension. Prompt endoscopy is required to confirm the diagnosis, but mortality is high (48.2% after open repair and 25.6% after REVAR).31
Acute kidney injury
Acute kidney injury is common after ruptured AAA. The process is multifactorial, and careful monitoring and management of fluid balance is mandatory. These patients frequently present with pre-existing renal impairment with advanced age, hypertension, comorbidities and polypharmacy contributing to their risk. Hypovolaemia, hypotension and the use of nephrotoxic contrast during REVAR exacerbate the renal insult, with embolic phenomena and ACS potentially further compounding the damage.30,32
Lower limb ischaemia
As with open repair, lower limb ischemia and arterial occlusion are recognised complications of REVAR and result from thrombus or cholesterol embolus. It is usually recognised at the end of surgery when distal pulses are checked by Doppler. The treatment of choice is urgent embolectomy, but on occasion limb bypass procedures may be required.
Cholesterol embolisation syndrome
Cholesterol embolisation syndrome is caused by the disruption of atherosclerotic plaque in a large calibre vessel. The cholesterol emboli shower into smaller arteries causing mechanical plugging and stimulating an autoimmune inflammatory process and end organ damage.32 During REVAR, this can be precipitated by the manipulation of guidewires and graft devices including REBOA within the aorta.
Postoperative considerations
Postoperatively, patients should be nursed in a unit capable of level 3 care. Ongoing resuscitation should ensure adequate intravascular volume and correction of abnormal haemoglobin, coagulation and acid–base balance. Nevertheless, the risk of multisystem organ failure remains high with a mortality of around 50–70%.12
Summary
Ruptured endovascular aneurysm repair is an alternative to open surgical repair in selected patients. Network management protocols assist in ensuring timely assessment, transfer and surgical treatment at an appropriately specialised facility. Hybrid operating theatres situated within the main theatre complex offer the most familiar surroundings for anaesthesia, but in some centres REVAR will be performed in the IR suite. Both local and general anaesthetic techniques can be used to facilitate endovascular repair but conversion from local to general anaesthesia should always be anticipated. The concealed nature of haemorrhage makes assessment difficult and is complicated by insidious bleeding from access sites. Clinical assessment and regular point-of-care testing should guide resuscitation management. Appropriate critical care facilities must be available postoperatively.
Acknowledgements
We thank Dr Robert Barker BSc MRCP FRCR, consultant radiologist, Frimley Health NHS Foundation Trust, for help in producing the images for Fig. 2.
Biographies
Kate Berry BSc (Hons) FRCA is a locum consultant at Hampshire Hospitals Foundation Trust. She undertook a vascular fellowship at Frimley Health NHS Foundation.
Judith Gudgeon BSc MRCPFRCA is consultant anaesthetist at Frimley Health. She is lead clinician for cardiopulmonary exercise testing and vascular anaesthesia. She recently completed 9 yrs on the committee of the Vascular Anaesthesia Society of Great Britain and Ireland.
Jeremy Taylor MRCS FRCR is a consultant diagnostic and interventional radiologist at Frimley Health NHS Foundation Trust with a specialist interest in interventional vascular radiology. He is a member of the British Society of Interventional Radiology and the Cardiovascular and Interventional Radiology Society of Europe.
Matrix codes: 1B04, 2A03, 3A05
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Bown M.J., Sutton A.J., Bell P.R.F., Sayers R.D. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. 2002;89:714–730. doi: 10.1046/j.1365-2168.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 2.Ito H. Operative strategy of ruptured abdominal aortic aneurysms and management of postoperative complications. Ann Vasc Dis. 2019;12:323–328. doi: 10.3400/avd.ra.19-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellard L., Djaiani G. Anaesthesia for vascular emergencies. Anaesthesia. 2013;68:72–83. doi: 10.1111/anae.12048. [DOI] [PubMed] [Google Scholar]
- 4.Powell J.T., Hinchliffe R.J., Thmpson M.M., et al. IMPROVE Trial Investigators Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2014;101:216–224. doi: 10.1002/bjs.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R., Powell J.T., Sweeting M.J., Epstein D.M., Barrett J.K., Greenhalgh R.M. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess. 2018;22:1–132. doi: 10.3310/hta22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IMPROVE Trial Investigators Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017;359:j4859. doi: 10.1136/bmj.j4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norman P.E., Powell J.T. Abdominal aortic aneurysm. Circulation. 2007;115:2865–2869. doi: 10.1161/CIRCULATIONAHA.106.671859. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hashimi M., Thompson J. Anaesthesia for elective open abdominal aortic aneurysm repair. Contin Educ Anaesth Crit Care Pain. 2013;13:208–212. [Google Scholar]
- 9.National Institute for Health and Care Excellence Guideline 156. Abdominal aortic aneurysm: diagnosis and management. Available from: https://www.nice.org.uk/guidance/ng156/chapter/recommendations (accessed 8 March 2021). [PubMed]
- 10.Moll F.L., Powell J.T., Fraedrich G., et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41:S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 11.The Vascular Society of Great Britain and Ireland: the provision of services for patients with vascular disease. 2012. https://www.vascularsociety.org.uk/_userfiles/pages/files/Document%20Library/Provision-of-Services-for-Patients-with-Vascular-Disease.pdf Report of a joint working party. Edinburgh Available from: [Google Scholar]
- 12.Chaikof E.L., Dalman R.L., Eskandari M.K., et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e2. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Powell J.T., Ribbons T. 2019. The Royal College of Emergency Medicine best practice guideline: management and transfer of patients with a diagnosis of ruptured abdominal aortic aneurysm to a specialist vascular centre.https://rcem.ac.uk/wp-content/uploads/2021/10/RCEM_BPC_rAAA_220119_FINAL.pdf Available from: [Google Scholar]
- 14.Mehta M., Taggert J., Darling R.C., et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44:1–8. doi: 10.1016/j.jvs.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 15.Moore R., Nutley M., Cina C.S., Motamedi M., Faris P., Abuznadah W. Improved survival after introduction of an emergency endovascular therapy protocol for ruptured abdominal aortic aneurysms. J Vasc Surg. 2007;45:443–450. doi: 10.1016/j.jvs.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 16.European Society for Vascular Surgery (ESVS) Clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;22:123–125. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Braithwaite B., Cheshire N.J., Greenhalgh R.M., et al. Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J. 2015;36:2061–2069. doi: 10.1093/eurheartj/ehv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta M., Byrne J., Darling R.C., et al. Endovascular repair of ruptured infrarenal abdominal aortic aneurysm is associated with lower 30-day mortality and better 5-year survival rates than open surgical repair. J Vasc Surg. 2013;57:368–375. doi: 10.1016/j.jvs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Watson S., Johal A., Birmpili P., et al. The Royal College of Surgeons of England; London: 2020. National vascular Registry: 2020 annual report. [Google Scholar]
- 20.Gupta P.K., Ramanan B., Engelbert T.L., Tefera G., Hoch J.R., Kent K.C. A comparison of open surgery versus endovascular repair of unstable ruptured abdominal aortic aneurysms. J Vasc Surg. 2014;60:1439–1445. doi: 10.1016/j.jvs.2014.06.122. [DOI] [PubMed] [Google Scholar]
- 21.Mani K., Lees T., Beiles B., et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a Vascunet report. Eur J Vasc Endovasc Surg. 2011;42:598–607. doi: 10.1016/j.ejvs.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 22.Bryce Y., Rogoff P., Romanelli D., Reichle R. Endovascular repair of abdominal aortic aneurysms: vascular anatomy, device selection, procedure, and procedure-specific complications. RadioGraphics. 2015;35:593–615. doi: 10.1148/rg.352140045. [DOI] [PubMed] [Google Scholar]
- 23.Resch T., Dias N., Sonesson B. Endovascular repair of ruptured abdominal aortic aneurysms. Techniques and strategies for optimal outcomes. Endovasc Today. 2015;14:52–56. [Google Scholar]
- 24.Luk K.K., Nandate K. Anesthetic considerations for endovascular repair of ruptured abdominal aortic aneurysms. Arch Vasc Med. 2018;2:14–19. [Google Scholar]
- 25.Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee . In: Handbook of transfusion medicine. 5th ed. Norfold D., editor. TSO; Sheffield: 2020. Blood Transfusion and tissue. 7.3: transfusion management of major haemorrhage; pp. 1–16. [Google Scholar]
- 26.NICE Guideline Updates Team . National Institute for Health and Care Excellence (UK); 2020. Use of tranexamic acid during transfer of people with ruptured or symptomatic abdominal aortic aneurysm: abdominal aortic aneurysm: diagnosis and management: evidence review R.http://www.ncbi.nlm.nih.gov/books/NBK556906/ NICE Guideline, No. 156. Available from: [PubMed] [Google Scholar]
- 27.Mouton R., Rogers C.A., Harris R.A., Hinchliffe R.J. Local anaesthesia for endovascular repair of ruptured abdominal aortic aneurysm. Br J Surg. 2019;106:74–81. doi: 10.1002/bjs.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsurukiri J., Akamine I., Sato T., et al. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24:13. doi: 10.1186/s13049-016-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremey B., Szekely B., Schlumberger S., et al. Anticoagulation monitoring during vascular surgery: accuracy of the Hemochron® low range activated clotting time (ACT-LR) Br J Anaesth. 2006;97:453–459. doi: 10.1093/bja/ael194. [DOI] [PubMed] [Google Scholar]
- 30.Rubenstein C., Bietz G., Davenport D.L., Winkler M., Endean E.D. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J Vasc Surg. 2015;61:648–654. doi: 10.1016/j.jvs.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Ultee K.H.J., Zettervall S.L., Soden P.A., et al. Incidence of and risk factors for bowel ischemia after abdominal aortic aneurysm repair. J Vasc Surg. 2016;64:1384–1391. doi: 10.1016/j.jvs.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozkok A. Cholesterol-embolization syndrome: current perspectives. Vasc Health Risk Manag. 2019;15:209–220. doi: 10.2147/VHRM.S175150. [DOI] [PMC free article] [PubMed] [Google Scholar]