Abstract
Objectives
This study aims to determine the role and mechanism of autophagy in endothelial cell dysfunction by glucolipotoxicity.
Methods
Human umbilical vein endothelial cells (HUVECs) were treated with high glucose and high palmitic acid. The number of autophagosomes was evaluated by monodansylcadaverine (MDC) staining and transmission electron microscopy (TEM). The expression of autophagy-related proteins (LC3 and P62) was assessed by Western blotting. Capillary tube-like formation was evaluated on Matrigel. Reactive oxygen species (ROS) production was detected by DCFH-DA. Cell apoptosis was measured by Hoechst 33258 staining and flow cytometry. Phosphorylation of AMPK, mTOR, and ULK1 was also analyzed by Western blotting.
Results
We found that glucolipotoxicity induced autophagy initiation and hindered autophagosomes degradation. Moreover, glucolipotoxicity increased the production of intracellular ROS, decreased the ability of tubular formation, and increased cell apoptosis. However, endothelial cell dysfunction was alleviated by 3-methyladenine, an early-stage autophagy inhibitor. Additionally, glucolipotoxicity promoted the phosphorylation of AMPK and ULK1 and inhibited the phosphorylation of mTOR.
Conclusions
Glucolipotoxicity initiates autophagy through the AMPK/mTOR/ULK1 signaling pathway and inhibits autophagic flow, leading to the accumulation of autophagosomes, thereby inducing apoptosis and impairing endothelial cell function.
Keywords: Glucolipotoxicity, autophagy, endothelial cell dysfunction, diabetes, AMPK/mTOR
Introduction
Diabetes has many complications due to persistent hyperglycemia and long-term insulin resistance, among which chronic vascular complications are the main cause of death and disability in patients with diabetes. 1 As an important barrier to the vascular environment and endocrine organ, vascular endothelium responds to various injuries such as hyperglycemia, dyslipidemia, and hemodynamic changes, and therefore plays a vital role in the occurrence of diabetic vascular complications. 2 In recent years, the role of lipotoxicity and glucolipotoxicity in vascular endothelial dysfunction and diabetic vascular diseases has gradually been recognized. The “lipotoxicity” of diabetes is due to increased triglycerides, decreased high-density lipoproteins, and increased low-density lipoproteins in the blood circulation, leading to insulin resistance and abnormal pancreatic β-cells, thereby impairing glucose homeostasis. The concept of “glucolipotoxicity” was first proposed in diabetic β-cell damage. 3 It is becoming more clearer that lipotoxicity and glucolipotoxicity have synergistic toxic effects in diabetic vascular injury now. 4 This is also consistent with the simultaneous presence of hyperglycemia and hyperlipidemia in patients with diabetes.
Autophagy is a physiological process in which damaged or senescent organelles are degraded in the cell. In the process of autophagy, double-membrane vesicles that wrap the organelles, namely autophagosomes, are formed and transported to lysosomes to degrade the contents. Autophagy ensures the metabolism of intracellular substances and the renewal and recycling of damaged and aging organelles.5,6 Under stress events, such as energy stress, oxidative stress, hypoxia, and nutrient deficiency, cells will accumulate damaged or toxic proteins and organelles, which will drive autophagy to maintain cellular homeostasis. 6 Excessive activation of intracellular autophagy leads to impaired autophagy flux, and induces apoptosis by releasing mitochondrial cytochrome C and activating the caspase system. 7 Autophagy is the pathological basis of many chronic diseases. It is closely related to diabetes, obesity, cardiovascular diseases, tumors, neurodegenerative diseases, autoimmune diseases, infections, and chronic inflammatory diseases.5,8
Autophagy participates in the onset of diabetes and the development of various related vascular complications, but the role of autophagy in regulating endothelial cell function under diabetic conditions is still inconclusive. Studies have found that inhibiting hyperglycemia-induced endothelial cell autophagy has a protective effect on endothelial cell barrier and angiogenesis. 9 However, other studies have shown that under hyperglycemia conditions, autophagy flux of endothelial cells is blocked, so activating autophagy can improve endothelial cell function. 10 At present, most of the studies on glucolipotoxicity have focused on pancreatic β cells. However, the effect of glucolipotoxicity on endothelial cell function and autophagy is rarely studied.
In this study, we used HUVECs treated with glucose and palmitic acid to investigate the effects of glucolipotoxicity on endothelial cell function and the role and mechanism of autophagy in endothelial dysfunction. We found that glucolipotoxicity induces endothelial dysfunction and apoptosis through autophagy.
Materials and methods
Reagents
D-glucose, palmitic acid (P5585), bafilomycin A1 (196000), 3-methyladenine (3-MA, M9281), and rapamycin (V900930) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Bovine serum albumin (BSA, 4240) was purchased from BioFroxx GmbH (Einhausen, Germany). Primary antibodies against β-actin (rabbit polyclonal, 1:1,000, 20536-1-AP), Bcl-2 (rabbit polyclonal, 1:1,000, 12789-1-AP), BAX (rabbit polyclonal, 1:1,000, 50599-2-Ig), P62 (rabbit polyclonal, 1:1,000, 18420-1-AP) and goat anti-rabbit IgG (H+L) (1:10,000, SA00001-2) were from Proteintech (Wuhan, China). Primary antibodies against LC3A/B (rabbit monoclonal, 1:1,000, 12741S), cleaved caspase-3 (rabbit monoclonal, 1:500, 9664S), p-AMPK (rabbit monoclonal, 1:1,000, 2535T), AMPK (rabbit monoclonal, 1:1,000, 5832T), p-mTOR (rabbit monoclonal, 1:1,000, 5536T), mTOR (rabbit monoclonal, 1:1,000, 2983T), ULK1 (rabbit monoclonal, 1:1,000, 8054T), and p-ULK1 (rabbit monoclonal, 1:1,000, 5869T) were purchased from Cell Signaling Technology (Beverly, MA, USA). AMPK specific inhibitor Compound C (S7306) was purchased from Selleck Chemicals (Houston, TX, USA).
Cell culture
HUVECs purchased from American Type Culture Collection (CRL-1730; Manassas, VA, USA) were grown in ECM (cat. no. 1001, Sciencell; San Diego, California, USA) supplemented with 5% fetal bovine serum (FBS, cat. no. 0025, Sciencell; San Diego, California, USA) and 1% endothelial cell growth supplement (ECGS, cat. no. 1052, Sciencell; San Diego, California, USA) at 37°C in a 5% CO2 humidified atmosphere. Cells were treated with 0.5% BSA-ECM as solvent control (CON group), 30 mM glucose plus 100 μM palmitic acid (high glucose and high palmitic acid, GL group), 30 mM glucose, 100 μM palmitic acid plus 5 mM 3-MA (GL+ 3-MA group), or 30 mM glucose, 100 μM palmitic acid plus 5 μM Compound C (GL+ Compound C) for 24 h. Before exposure to glucolipotoxicity, cells were treated with 50 nM bafilomycin A1 (GL+ BafA1 group) or 100 nM rapamycin (GL+ Rapamycin group) for 2 h. In addition, specific configuration steps for 100 μM palmitic acid can be found in Supplementary Material.
Assessment of reactive oxygen species
Intracellular ROS production was measured using a 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay according to the manufacturer’s protocol (S0033S, Beyotime Institute of Biotechnology, Shanghai, China). Cells were washed thrice with serum-free ECM, seeded in 6-well plates at a density of 2 × 105 cells/well, and incubated with 10 μM DCFH-DA for 30 min at 37°C. Subsequently, cells were washed again and observed under an inverted fluorescence microscope (Olympus Corp., Tokyo, Japan). Mean fluorescence intensity were determined using ImageJ software.
Monodansylcadaverine staining
MDC-based autophagy staining kit (KGATG001, KeyGen Biotech, Jiangsu, China) was used to monitor autophagy in living cells. After cultured in a 24-well plate for 24 h under glucolipotoxic conditions, cells were washed, and then stained with MDC in the dark for 15 min at room temperature. Autophagy was observed and captured with a fluorescence microscope with a 355 nm wavelength excitation filter and a 512 nm wavelength blocking filter.
Western blot analysis
Total cellular proteins were extracted with RIPA buffer (KGP702, KeyGen Biotech, Jiangsu, China) and quantified using a BCA kit (KGP902, KeyGen Biotech, Jiangsu, China). Equal amounts of cellular proteins (20 μg) were separated on 12% Tris-glycine gels and transferred to a polyvinylidene membrane. After blocking with 5% non-fat milk at room temperature for 2 h, the membranes were incubated overnight at 4°C with primary antibodies. PVDF membranes were then incubated with the HRP-conjugated secondary antibody (goat anti-rabbit, SA00001-2, 1:10,000, Proteintech) for 2 h at room temperature. The blots were treated using a super sensitive ECL chemiluminescence substrate detection kit (BL523A, Biosharp, Anhui, China). Signals were imaged using the UVP ChemStudio Plus (Analytik Jena, Jena, Germany) and quantified using the Image J software (National Institutes of Health, Bethesda, MD, USA).
Endothelial cell tube formation assay
The formation of capillary-like structures by HUVECs was assessed using a Matrigel basement membrane matrix (354262, Corning Inc., Corning, NY, USA). Briefly, HUVECs were seeded in Matrigel-coated 48-well plates at a density of 2 × 104 cells/cm2. Tube formation was observed under an inverted phase contrast fluorescent microscope (Olympus Corp.), and images were taken with a digital camera. The results were quantified by measuring the total length of the tubular structure in each vision.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 15 min, permeabilized by 0.5% Triton X-100 in phosphate buffered saline (PBS) for 10 min, and blocked in 1% BSA in PBS for 30 min. Then, cells were serially incubated with primary antibodies p62 (1:100) overnight at 4°C and secondary antibodies was goat anti-rabbit IgG H&L (Alexa Fluor® 488) (1:1000, ab150077, Abcam) for 1 h at room temperature. DAPI (1 μg/mL, BS097, Biosharp, Anhui, China) was used for nuclear staining at room temperature for 5 min. Cells were observed with a fluorescence microscope (RX71, Olympus Corp., Japan) and digitized using ImageJ software.
Transmission electron microscopy
After 24 h of treatment, cells were harvested, fixed in 2.5% glutaraldehyde overnight, and incubated with osmium tetroxide at 4°C for 2 h. Subsequently, the epoxy resin embedded specimens were cut into 100 nm and stained with uranyl acetate and lead citrate. Cellular ultrastructure was imaged using a Hitachi HT7700 TEM (Hitachi, Tokyo, Japan).
Hoechst 33258 staining
Hoechst 33258 staining (G3680, Solarbio, Beijing, China) was used to evaluate cell apoptosis. According to the manufacturer’s instructions, HUVECs were washed twice with PBS, stained with Hoechst 33258 (10 μg/mL in PBS) at 37°C in dark for 5 min, and then observed under a fluorescent microscope.
Flow cytometry
Apoptosis was measured after staining with Annexin V-Alexa Fluor 647/propidium iodide (PI) solution (FXP023, 4A Biotech, Beijing, China). Label the cells according to the manufacturer’s protocol. For each sample, at least 1 × 104 cells were assayed by flow cytometry (BD Biosciences, San Jose, CA, USA) and analyzed using the FlowJo software (v.18.0.0.0, Tree Star, Ashland, OR, USA).
Lyso-tracker red staining
HUVECs were stained with lysosome-specific fluorescent dye Lyso-Tracker Red (50 nM, C1046, Beyotime Institute of Biotechnology, Shanghai, China) at 37°C for 15 min and photographed with a fluorescence microscope. The intensity of red fluorescence represents the number of lysosomes.
Statistical analysis
All statistical analyses were performed using the SPSS 22.0 software (SPSS, Chicago, IL, USA). All experiments were repeated at least thrice. Data are expressed as mean ± standard deviation (SD). The comparisons of the means between two groups were assessed by Student t-test, and the comparisons of the means between multiple groups were performed by one-way analysis of variance followed by Tukey’s post hoc test. p < 0.05 was considered a statistically significant difference.
Results
Glucolipotoxicity initiates autophagy and inhibits autophagy flow in human umbilical vein endothelial cells
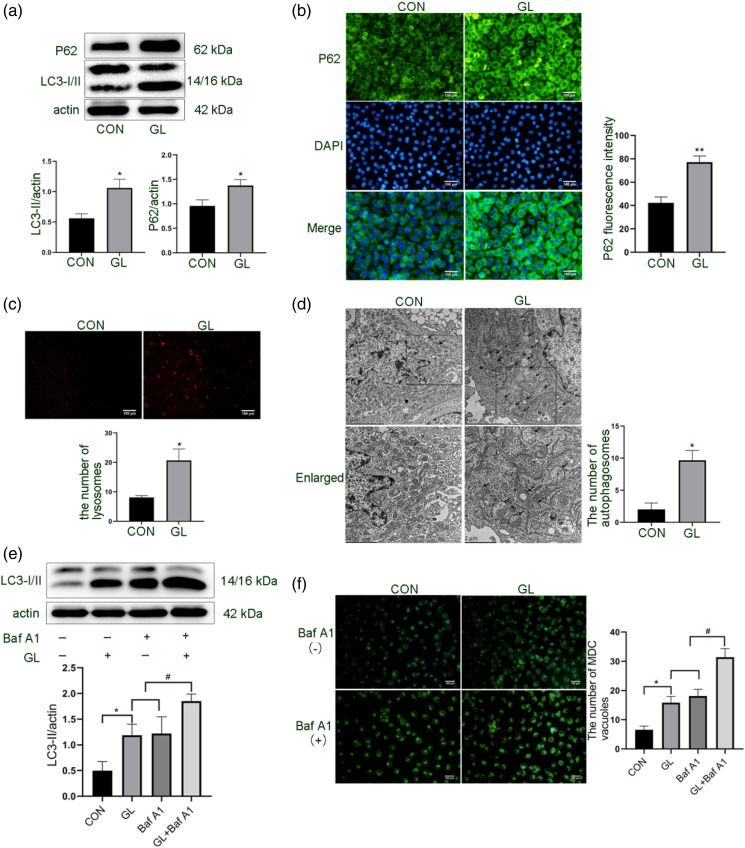
After treating HUVECs with high glucose and high palmitic acid for 24 h, we first detected LC3 conversion, a specific marker for autophagic activation. 11 Although we found that glucolipotoxic treatment increased the turnover of LC3, we also found that the expression of P62 increased (Figure 1(a)). We further confirmed glycolipid-induced P62 expression by immunofluorescence assay (Figure 1(b)). As a substrate of autophagy, P62 is delivered to the lysosome for degradation. The increased expression of P62 after glucolipotoxic exposure may be related to the discontinuous turnover of autophagosomes. 12
Figure 1.
Effect of glucolipotoxicity on autophagic activation and autophagic flow of HUVECs. Cells were treated with 30 mM glucose and 100 μM palmitic acid (GL) for 24 h. A. Expression levels of autophagy proteins LC3 and P62 were detected by Western blot. B. Immunofluorescence detection of P62 expression in cells. Scale bar: 100 μm. C. Quantification of cellular lysosome numbers by Lyso-Tracker Red dye. Scale bar: 100 μm. D. Transmission electron microscopy observation of autophagosomes and autolysosomes in cells. Scale bar: 2/5 μm. E. Western blot analysis of LC3 conversion in cells pretreated with Baf A1 (50 nM) for 2 h and then treated with GL for 24 h. F. MDC staining of autophagic vacuoles. Scale bar: 100 μm. The band densities of LC3-II (A and E) and P62 (A) were quantitated and normalized to those of corresponding loading control Actin. The mean fluorescence intensity of P62 (B), the number of lysosomes (C), the number of autophagosomes (D), and the number of MDC vacuoles (F) were quantitated or counted in the whole field. Representative images are shown or data are expressed as mean ± SD (n = 3) *p < 0.05 vs control group and #p < 0.05 vs GL group or Baf A1 group. CON = control group; GL = glucolipotoxicity group; Baf A1= bafilomycin A1 group; GL+ Baf A1 = glucolipotoxicity + bafilomycin A1 group; MDC= monodansylcadaverine.
It is generally believed that the blockage of autophagic flow is closely related to the change of lysosomal activity. 13 To test this hypothesis, we first observed lysosome numbers with Lyso-Tracker Red dye and found that the red fluorescence of glycolipid-treated cells was enhanced (Figure 1(c)). Further TEM observed an increase in the double membrane structure of autophagosomes and the single membrane structure of autophagolysosomes (autolysosome) in glycolipid-treated cells (Figure 1(d)). The observation of autophagolysosomes (autolysosome) accumulation indicates that the autophagic flow may be inhibited by glucolipotoxicity.
But, the above results cannot confirm that the increase in autophagosome is only due to the blocking of the autophagic flow. This question was clarified by using the autophagosome-lysosome fusion inhibitor bafilomycin A1. 14 Our results showed that bafilomycin A1 further enhanced the effect of glucolipotoxicity on LC3-II expression and the number of autophagic vesicles (Figures 1(e) and (f)), indicating that glucolipotoxicity induces autophagosomes synthesis in HUVECs.
Glucolipotoxicity impairs cell function and increases apoptosis in human umbilical vein endothelial cells
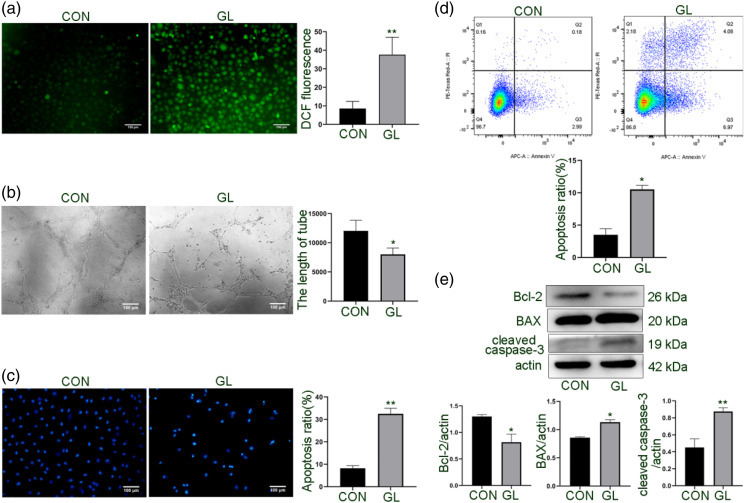
Next, we determined the effect of glucolipotoxicity on the function of HUVECs. As shown in Figure 2(a), HUVECs showed increased ROS production after exposure to glucolipotoxicity. Because angiogenesis is one of the important indicators for evaluating endothelial cell function, 15 we tested the tubule formation ability of HUVECs and found that the tubule formation was significantly reduced in the GL group compared to the untreated control group (Figure 2(b)). Furthermore, both Hoechst 33258 staining and flow cytometry demonstrated that glucolipotoxicity induced cell apoptosis (Figures 2(c) and (d)). In addition, following glucolipotoxic treatment, the expression of anti-apoptotic protein Bcl-2 decreased and the expression of BAX and cleaved caspase-3 increased in HUVECs (Figure 2(e)). These results indicate that glucolipotoxicity damages endothelial cell function and induces apoptosis.
Figure 2.
Effect of glucolipotoxicity on tubular formation and apoptosis of HUVECs. Cells were cultured for 24 h under glucolipotoxic conditions, as described in Figure 1. A. Detection of intracellular ROS production by a fluorescent probe DCFH-DA. The mean fluorescence intensity of ROS was quantitated in the whole field. B. Evaluation of the capillary structure formation of cells on Matrigel and quantification of the tubule length with the ImageJ. Scale bar: 100 μm. C. Quantification of apoptotic cells by Hoechst 33258 staining. The nuclei of apoptotic cells are concentrated, dense, and high blue. Scale bar: 100 μm. D. Flow cytometric analysis of cell apoptosis rate with Annexin V-Alexa Fluor 647/PI staining. E. Western blot analysis of the expression of apoptotic protein BAX, cleaved caspase-3 and anti-apoptotic protein Bcl-2. The band densities were quantitated. Representative images are shown or data are expressed as mean ± SD (n = 3). *p < 0.05 vs control group and **p < 0.01 vs control group. CON = control group; GL= glucolipotoxicity group; PI, propidium iodide; ROS, reactive oxygen species.
Inhibition of autophagy improves cell dysfunction induced by glucolipotoxicity in human umbilical vein endothelial cells
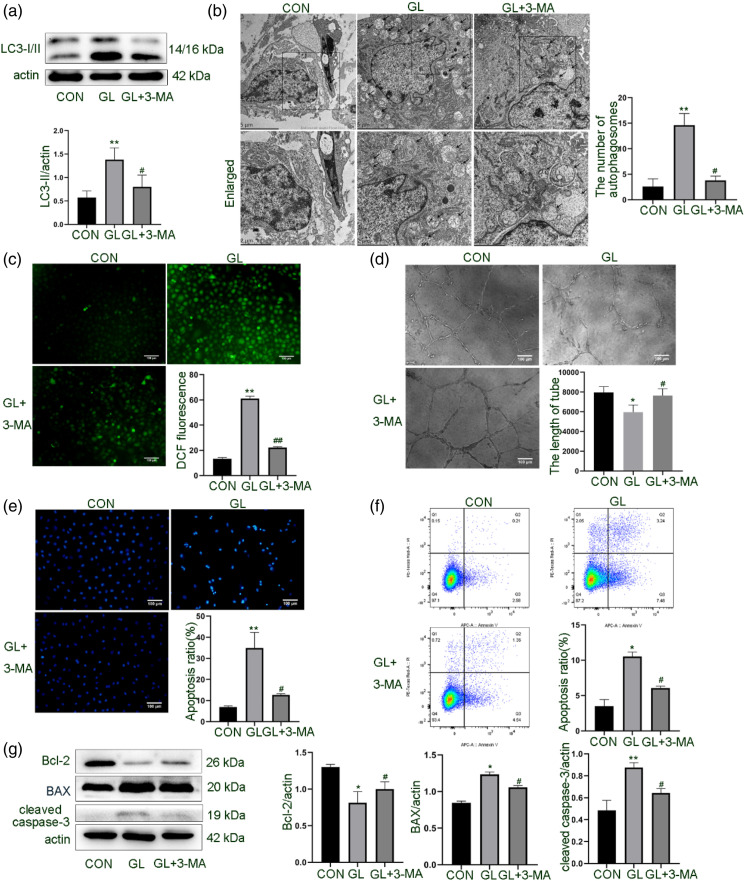
To further understand the role of autophagy in endothelial cell dysfunction induced by glucolipotoxicity and explore the relationship between autophagy and apoptosis, we treated HUVECs with 3-MA to interfere with the formation of autophagosomes. As shown in Figures 3(a) and (b), 3-MA significantly reduced the expression of LC3-II and the number of autophagosomes induced by glucolipotoxicity. After 3-MA inhibited autophagy, the tubular structure formation ability of cells was partially restored, and oxidative stress was also reduced (Figures 3(c) and (d)). Simultaneously, the rate of cell apoptosis measured by different methods was significantly reduced (Figures 3(e) to (g)). These results suggest that autophagy is involved in endothelial cell dysfunction induced by glucolipotoxicity.
Figure 3.
Effect of autophagic inhibition on glucolipotoxicity-induced cell dysfunction in HUVECs. Cells were treated with 3-MA (5 mM), an early-stage autophagy inhibitor, and glucolipotoxicity for 24 h. A. Western blot analysis of LC3 expression. B. Observation of autophagosomes and autolysosomes in HUVECs under TEM. Scale bar: 2/5 μm. C. Detection of intracellular ROS by DCFH-DA. D. Evaluation of tubular formation of cells on Matrigel. E. Quantification of apoptotic cells by Hoechst 33258 staining. Scale bar: 100 μm. F. Flow cytometric analysis of cell apoptosis rate with Annexin V-Alexa Fluor647/PI staining. G. Western blot detection of expression of cleaved caspase-3, BAX and Bcl-2. Representative images are shown or data are expressed as mean ± SD (n = 3). *p < 0.05 vs control group, **p < 0.01 vs control group, and #p < 0.05 vs GL group. CON = control group; GL = glucolipotoxicity group; GL + 3-MA = glucolipotoxicity + 3-MA group; 3-MA, 3-methyladenine; PI, propidium iodide; ROS, reactive oxygen species.
Glucolipotoxicity initiates autophagy through AMPK/mTOR/ULK1 pathway in human umbilical vein endothelial cells
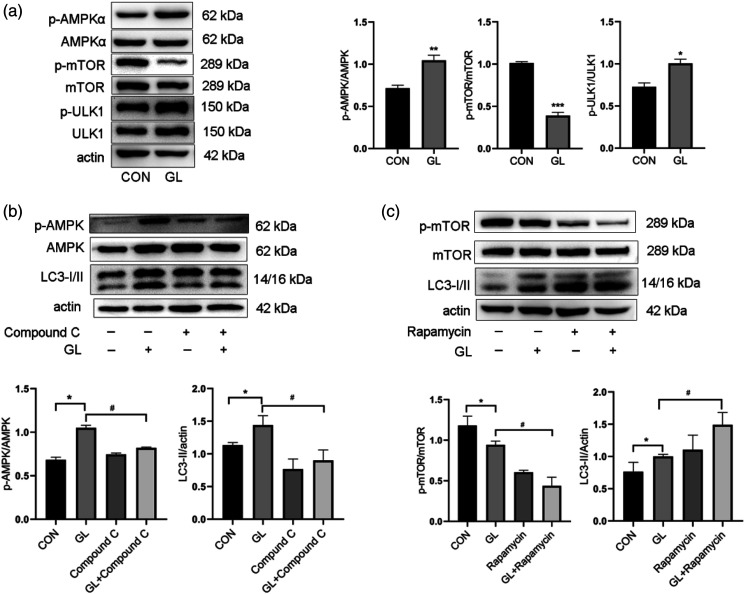
Autophagy is mainly regulated by AMPK and mTOR signaling pathways, which converge on ULK1 and oppositely regulate ULK1 activation. Therefore, we evaluated the role of AMPK and mTOR signaling pathways in autophagy triggered by glucolipotoxicity in HUVECs. As shown in Figures 4(a) and (b), glucolipotoxicity increased the phosphorylation and activation of AMPK and ULK1, and reduced the phosphorylation and activation of mTOR. To further confirm the role of AMPK in glucolipotoxicity-induced autophagy, Compound C, a specific AMPK inhibitor, was used to inactivate AMPK. 16 As expected, Compound C reduced the accumulation of LC3-II induced by glucolipotoxicity (Figure 4(b)). Conversely, rapamycin, a specific inhibitor of mTOR, increased autophagy induced by glucolipotoxicity (Figure 4(c)). These results suggest that glucolipotoxicity initiates autophagy by activating AMPK and inhibiting mTOR.
Figure 4.
The involvement of AMPK/mTOR/ULK1 pathway in autophagy induction by glucolipotoxicity in HUVECs. A. Western blot analysis of AMPK, mTOR, and ULK1 phosphorylation in cells exposed to glucolipotoxicity for 24 h. B. Western blot analysis of AMPK phosphorylation and LC3 expression in cells treated with AMPK specific inhibitor Compound C (5 μM) and glucolipotoxicity for 24 h. C. Western blot analysis of mTOR phosphorylation and LC3 expression in cells pretreated with mTOR inhibitor rapamycin (100 nM) for 2 h and then treated with glucolipotoxicity for 24 h. Representative images are shown or data are expressed as mean ± SD (n = 3). *p < 0.05 vs control group, **p < 0.01 vs control group, ***p < 0.001 vs control group, and #p < 0.05 vs GL group. CON = control group; GL = glucolipotoxicity group; GL+ Compound C = glucolipotoxicity + Compound C group; GL+ Rapamycin = glucolipotoxicity+ rapamycin group.
Discussion
In this study, we demonstrated for the first time that glucolipotoxicity causes endothelial dysfunction by inducing autophagy initiation and inhibiting autophagic flow. Moreover, we found that autophagy initiation in endothelial cell was caused by glucolipotoxicity are through the AMPK/mTOR/ULK1 pathway.
Previous studies have shown that high glucose induces increased autophagy, senescence, and apoptosis via RAS-mediated mitochondrial damage in endothelial cells. 17 Studies have also explored that PA overload of endothelial cells results in the Ca2+-dependent development of autophagy, ultimately leading to programmed necrotic cell death. 18 Besides, there have also been some related studies on the combined effect of glycolipids in recent years. For example, while lipotoxic or glucotoxic insult alone produces a similar increase in ROS during initial exposure, the addition of lipotoxicity to a glucotoxic environment further exacerbates ROS production and accelerates their damaging effect on mitochondrial homeostasis. This study shows that early pharmacological intervention in patients with prediabetes with dyslipidemia can delay the development of retinopathy before hyperglycemia develops. 19 Intriguingly, the active form of vitamin D protects endothelial cells from the harmful effects of glucolipotoxicity by regulating gene expression of related enzymes. 20 Besides, glucolipotoxicity can damage the viability and tube-forming capability of endothelial cells and increase the level of inflammatory factors in the cells. However, co-culture of mesenchymal stem cells and endothelial cells improves the damage of glucolipotoxicity to endothelial cells. 21 In clinical practice, most patients with diabetes with hyperglycemia develop hyperlipidemia, 22 so the role of lipotoxicity in exploring the mechanism of diabetes-related vascular disease cannot be ignored. It is for this reason that we focused on the effects and mechanisms of glycolipid toxicity on endothelial function. In this study, we observed the harmful effects of glucolipotoxicity on endothelial cells, such as impaired tube formation ability, increased ROS production, and increased endothelial cell apoptosis.
Autophagy is a basic catabolic process that wraps excess or impaired cell components to form a double-membrane structure and fuse with lysosomes to degrade the contents. 23 Autophagy seems to play multiple roles in the various cardiovascular complications of diabetes. For example, hyperglycemia can impair endothelial cell function and induce autophagy. However, activation of the Hedgehog pathway and down-regulation of autophagy can improve endothelial dysfunction. 9 In the cardiovascular complications of diabetes, autophagy also reduces cardiac microvascular endothelial cell apoptosis through the mTOR signaling pathway. 24 Diabetes complicated by peripheral arterial disease is still a clinical problem. Studies have shown that endothelial cell-specific knockout of mTORC1 may prevent hindlimb ischemic injury by activating autophagy, reducing oxidative stress, and reducing inflammation.23–25 In diabetic nephropathy, the autophagic activity of endothelial cells and podocytes together protect against glomerular sclerosis caused by diabetes. 26 All these evidence shows that autophagy is a promising new strategy for the treatment of diabetes. The interaction between autophagy and apoptosis is complex and closely related. Also in our study, glucolipotoxicity induced the initiation of autophagy and hindered the unimpeded flux of autophagy, leading to the accumulation of a large number of autophagosomes and then endothelial cell apoptosis. After inhibiting the formation of autophagosomes, endothelial apoptosis was alleviated and endothelial dysfunction was improved.
AMPK and mTOR signaling pathways are considered to be the most important autophagy-regulatory pathways. 27 The ULK1 protein is a bridge connecting upstream nutrient or energy receptors AMPK and mTOR with downstream autophagosomes. 28 AMPK promotes autophagy in two main ways. On the one hand, it directly phosphorylates and activates ULK1 kinase; on the other hand, it indirectly inhibits the phosphorylation of mTOR to alleviate autophagic inhibition. Both ultimately lead to the activation of the Beclin1-VPS34 complex through Beclin1 phosphorylation. 29 In our study, glucolipotoxicity activated the phosphorylation of AMPK and inhibited the phosphorylation of mTOR, thereby synergistically activating the phosphorylation of downstream ULK1 and initiating autophagy. By using the AMPK-specific inhibitor Compound C, we confirmed that the phosphorylation of AMPK was suppressed, resulting in the reduction of LC3-II expression in glucolipotoxicity-treated cells. Moreover, the mTOR inhibitor rapamycin reduced the phosphorylation of mTOR, leading to a further increase in LC3-II expression. All in all, these results suggest that glucolipotoxicity may initiate autophagy by promoting AMPK phosphorylation and inhibiting mTOR phosphorylation.
However, our study has limitations by extrapolating data from in vitro exposure to high glucose and palmitic acid to more complex diabetes. Next, we will design experiments to study endothelial cells from diabetic human donors or diabetic mice to validate the above findings and make them more convincing and meaningful.
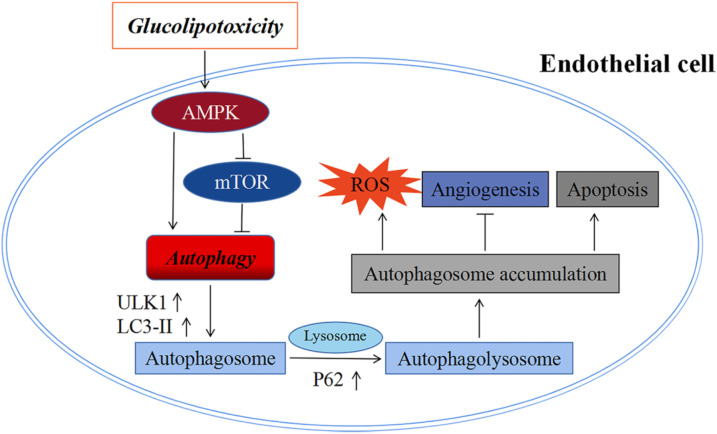
In conclusion, glucolipotoxicity initiates autophagy by activating the AMPK/mTOR/ULK1 signaling pathway and inhibits autophagic flow, leading to a large accumulation of autophagosomes, thereby inducing apoptosis and impairing endothelial cell angiogenesis (Figure 5).
Figure 5.
A flow chart model showing that glucolipotoxicity initiates autophagy through AMPK/mTOR/ULK1 signaling pathway and inhibits autophagic flow, leading to accumulation of autophagosomes, thereby inducing apoptosis and impairing endothelial cell function.
Footnotes
Author contributions: All authors approve the manuscript for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the National Natural Science Foundation of China (grant no. 81870352, 81970252, 82000454), the Key Research and Development Project of Hunan Province (grant no. 2020SK2087, 2019SK2041) and Joint project of Medical Science and Technology Research of Henan (grant no. LHGJ20190092).
ORCID iD
Hongwei Lu https://orcid.org/0000-0001-8005-142X
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018; 14(2): 88–98. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes 2017; 9(5): 434–449. [DOI] [PubMed] [Google Scholar]
- 3.Prentki M, Joly E, El-Assaad W, et al. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes 2002; 51(Suppl 3): S405–S413. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox T, Newman JD, Maldonado TS, et al. Peripheral vascular disease risk in diabetic individuals without coronary heart disease. Atherosclerosis 2018; 275: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morel E, Mehrpour M, Botti J, et al. Autophagy: A druggable process. Annu Rev Pharmacol Toxicol 2017; 57: 375–398. [DOI] [PubMed] [Google Scholar]
- 6.Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cel Biol 2018; 20(5): 521–527. [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay S, Panda PK, Sinha N, et al. Autophagy and apoptosis: where do they meet? Apoptosis 2014; 19(4): 555–566. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med 2020; 383(16): 1564–1576. [DOI] [PubMed] [Google Scholar]
- 9.Niu C, Chen Z, Kim KT, et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy 2019; 15(5): 843–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai X, Yang X, Jia X, et al. CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis. Autophagy 2020; 16(6): 1111–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YK, Jun YW, Choi HE, et al. Development of LC3/GABARAP sensors containing a LIR and a hydrophobic domain to monitor autophagy. EMBO J 2017; 36(8): 1100–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Chen S, Yeo S, et al. Elevated p62/SQSTM1 determines the fate of autophagy-deficient neural stem cells by increasing superoxide. J Cel Biol 2016; 212(5): 545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao XF, Cao J, Xu LM, et al. Perfluorooctane sulfonate blocked autophagy flux and induced lysosome membrane permeabilization in HepG2 cells. Food Chem Toxicol 2014; 67: 96–104. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 2007; 3(6): 542–545. [DOI] [PubMed] [Google Scholar]
- 15.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cel Biol 2011; 12(9): 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen QW, Gerrard DE, Du M. Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem. Meat Sci 2008; 78(3): 323–330. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Chen B, Xiao F, et al. Autophagy protects against senescence and apoptosis via the RAS-mitochondria in high-glucose-induced endothelial cells. Cell Physiol Biochem 2014; 33(4): 1058–1074. [DOI] [PubMed] [Google Scholar]
- 18.Khan M, Rizwan Alam M, Waldeck-Weiermair M, et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells. J Bio Chem 2012; 287(25): 21110–21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 2015; 56(5): 2985–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuricova K, Pleskacova A, Pacal L, et al. 1,25-Dihydroxyvitamin D increases the gene expression of enzymes protecting from glucolipotoxicity in peripheral blood mononuclear cells and human primary endothelial cells. Food Funct 2016; 7(6): 2537–2543. [DOI] [PubMed] [Google Scholar]
- 21.An X, Li L, Chen Y, et al. Mesenchymal stem cells ameliorated glucolipotoxicity in HUVECs through TSG-6. Int J Mol Sci 2016; 17(4): 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzo C, Hartnett S, Hanley A, et al. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clinical Endocrinol Metabol 2013; 98(4): 1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cel Biol 2018; 19(6): 349–364. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhang S, Wang Y, et al. Autophagy inhibits high glucose induced cardiac microvascular endothelial cells apoptosis by mTOR signal pathway. Apoptosis 2017; 22(12): 1510–1523. [DOI] [PubMed] [Google Scholar]
- 25.Fan W, Han D, Sun Z, et al. Endothelial deletion of mTORC1 protects against hindlimb ischemia in diabetic mice via activation of autophagy, attenuation of oxidative stress and alleviation of inflammation. Free Radic Biol Med 2017; 108: 725–740. [DOI] [PubMed] [Google Scholar]
- 26.Lenoir O, Jasiek M, Hénique C, et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 2015; 11(7): 1130–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res 2014; 24(1): 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alers S, Löffler AS, Wesselborg S, et al. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cel Biol 2012; 32(1): 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cel Biol 2011; 13(2): 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]