Abstract
Background
There are few reports of mechanical circulatory support (MCS) in patients with cardiogenic shock (CS) due to end-stage heart failure (ESHF). We evaluated our institutional MCS strategy and compared the outcomes of INTERMACS 1 and 2 patients with CS due to ESHF.
Methods
Retrospective analysis of prospectively collected data (November 2014 to July 2019) from a single centre. ESHF was defined by a diagnosis of HF prior to presentation with CS. Other causes of CS (eg: acute myocardial infarction) were excluded. We compared the clinical course, complications and 90-day survival of patients with CS due to ESHF in INTERMACS profile 1 and 2.
Results
We included 60 consecutive patients with CS due to ESHF Differences in baseline characteristics were consistent with the INTERMACS profiles. The duration of MCS was similar between INTERMACS 1 and 2 patients (14 (10–33) vs 15 (7–23) days, p = 0.439). There was no significant difference in the number of patients with complications that required intervention. Compared to INTERMACS 2, INTERMACS 1 patients had more organ dysfunction on support and significant lower 90-day survival (66% vs 34%, p = 0.016).
Conclusion
Our temporary MCS strategy, including earlier intervention in patients with CS due to ESHF at INTERMACS 2 was associated with less organ dysfunction and better 90-day survival compared to INTERMACS 1 patients.
Keywords: Cardiogenic shock
Background
‘Pump failure’ and cardiogenic shock (CS) is a major mode of death in patients with end-stage heart failure (ESHF) due to ischemic or non-ischemic cardiomyopathies. Durable left-ventricular assist devices (LVAD) and orthotopic heart transplantation (OHT) are established therapeutic options in patients with ESHF, but outcomes in patients with severe CS (ie: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile 1) are significantly worse compared to patients with other INTERMACS profiles1,2 (description of INTERMACS profiles in Supplementary material). As a result, temporary mechanical circulatory support (MCS) is increasingly deployed in patients with CS to reverse organ dysfunction and bridge patients with ESHF to durable LVAD or OHT. The different modalities of temporary MCS has been extensively reviewed. 3
However, the optimal temporary MCS strategy in patients with CS due to ESHF is uncertain. A number of studies have described clinical outcomes based on a specific MCS device or modality,4–6 thus directly or indirectly advocating a ‘device-centric’ or ‘device-directed’ approach. The temporary MCS bridging strategy at our centre is ‘patient-directed’, tailoring MCS modality based on a number of factors, including the clinical profile of the patient. 7 We undertook this study to evaluate our temporary MCS bridging strategy in a cohort of patients with CS due to ESHF (other causes of CS such as myocardial infarction or myocarditis were excluded). We hypothesized that our temporary MCS strategy in patients with CS due to ESHF, including earlier intervention with MCS in patients who are deteriorating on inotropes (INTERMACS profile 2) would reduce organ dysfunction and improve 90-day survival compared to INTERMACS profile 1.
Methods
Patient selection
Consecutive patients treated at our institution from November 2014–July 2019 with temporary MCS (intra-aortic balloon pumps were not considered temporary MCS for the purpose of this study) for CS associated with prior diagnosis of HF were included in this retrospective analysis. Prior diagnosis of HF was defined as a diagnosis of HF due to an underlying ischemic or non-ischemic cardiomyopathy with objective evidence of cardiac dysfunction and treated with conventional medical therapy before the admission or transfer to our institution for MCS. Patients who presented acutely de novo with cardiogenic shock at the index admission or complicating acute cardiac disease (eg: myocardial infarction, myocarditis, takotsubo cardiomyopathy and pulmonary embolism) were excluded. Other indications for temporary MCS such as post-cardiotomy shock and post-transplant graft dysfunction were excluded. Vasoactive-inotrope score (VIS) was calculated as follows based on Davidson et al.: 8 VIS = Dopamine (in mcg/kg/min) + dobutamine (in mcg/kg/min) + 100 ×adrenaline (in mcg/kg/min) + 100 × noradrenaline (in mcg/kg/min) + 10 × milrinone (in mcg/kg/min) + 10,000 ×vasopressin (in U/kg/min). The sequential (or sepsis-related) organ failure assessment (SOFA) score was calculated at 48 hours post-MCS as previously described. 9
In our institution, MCS is undertaken only with consensus from the multi-disciplinary team, typically in patients with evidence of low cardiac output, persistent lactatemia and organ dysfunction despite inotropic support. Patients with contraindications to OHT were not supported, as our MCS program, like others in the UK is funded with the intention to bridge to heart transplantation. Contraindications include severe underlying disease (cancer, hematologic malignancy, and chronic respiratory disease), severe and irreversible neurologic disease and significant psychosocial concerns.
The Birmingham MCS strategy
There are a number of temporary MCS modalities and our strategy is not centered on a single modality for the wide range of patients that we encounter in practice. We developed our temporary MCS strategy to take into consideration the clinical presentation (underlying aetiology, cardiac arrest and INTERMACS profile), right and left heart function and technical considerations (body habitus, anatomy and previous cardiac surgery): 7
Venoarterial extracorporeal membrane oxygenation (VA ECMO) is used as the primary MCS modality in patients with INTERMACS 1 cardiogenic shock, particularly in the presence of ventricular arrhythmias, cardiac arrest and/or poor right heart function.
Impella CP is used as the modality of choice for left ventricular unloading in patients on peripheral VA ECMO.
Temporary Centrimag (Abbott, USA) biventricular assist device (BIVAD) is used in patients with INTERMACS 2 cardiogenic shock, particularly in the presence of ventricular arrhythmias and/or poor right heart function (based on clinical signs of right heart failure, high central venous pressure relative to pulmonary artery occlusion pressure in mmHg (>0.63) 10 and pulmonary artery pulse pressure, and/or large right relative to left ventricular dimension (>0.75) 11 ).
Patients initially supported by VA ECMO are converted to Centrimag BIVAD to bridge to OHT, particularly if unsuitable for durable LVAD. We avoid bridging patients directly from VA ECMO to OHT due to poor outcomes with this direct VA ECMO bridging strategy.
Statistical analysis
Categorical data are presented as percentages and continuous data as means ± standard deviation or medians with inter-quartile range (IQR) where appropriate. Pearson’s chi-squared test was used to compute the significance of the difference between groups for categorical variables. Normality of continuous variables was tested using the Shapiro-Wilk test. The Student’s t–test and Mann-Whitney U test were used, where appropriate, to compare groups for continuous variables. For time-to-event analyses, Kaplan–Meier estimates of survival were created for patients with INTERMACS profile 1 and 2, and the log-rank test was used to compare survivor functions. All statistical analyses were performed using R (version 3.1.1) and a two sided p-value of <0.05 was considered statistically significant.
Results
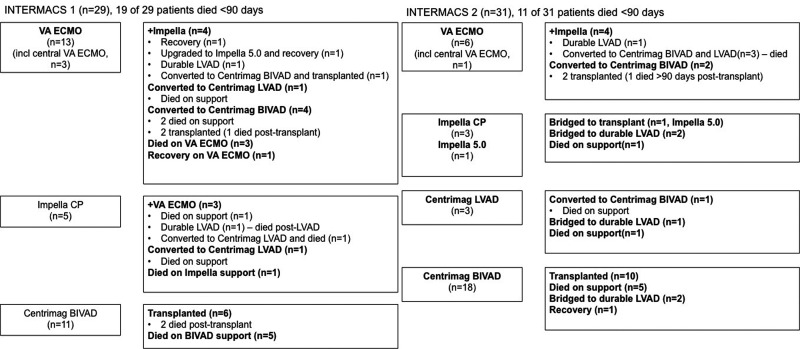
We included 60 consecutive patients with CS due to ESHF into this study. The majority of patients had a diagnosis of HF of over a year. There were numerically more patients with restrictive cardiomyopathy in the INTERMACS 1 group, but the underlying aetiology was not a contraindication to transplantation. Of the 60 patients, 29 and 31 patients were INTERMACS profile 1 and 2 respectively, at the time of MCS. A higher proportion of patients in INTERMACS profile 1 were mechanically ventilated, on multiple vasoactive drugs and had suffered prior cardiac arrest and significantly higher lactate levels, compared to INTERMACS 2 patients, which is consistent with more severe circulatory shock (Table 1). In accordance with our MCS strategy, the majority of patients in INTERMACS 1 were supported with VA ECMO and/or Impella CP, in contrast to patients in INTERMACS 2, who were more likely to undergo Centrimag BIVAD support (Figure 1).
Table 1.
Baseline characteristics (n = 60).
| INTERMACS 1 (n = 29) | INTERMACS 2 (n = 31) | p | |
|---|---|---|---|
| Age (years) | 41 ± 6 | 45 ± 5 | 0.217 |
| Males (n, %) | 19 (66) | 22 (71) | 0.650 |
| BMI (kg/m2) | 25.6 ± 2.1 | 27.3 ± 2.3 | 0.293 |
| Aetiology | 0.507 | ||
| Ischemic | 4 (14) | 5 (16) | |
| Non-ischemic DCM | 21 (72) | 25 (81) | |
| Restrictive | 4 (14) | 1 (3) | |
| HF > 1 year (n, %) | 17 (59) | 21 (68) | 0.697 |
| Ventilated (n, %) | 13 (45) | 2 (10) | 0.002 |
| Pre-MCS cardiac arrest (n, %) | 8 (28) | 1 (3) | 0.008 |
| 1 inotrope (n, %) | 9 (31) | 16 (55) | 0.040 |
| 2 inotropes (n, %) | 11 (38) | 11 (38) | |
| 3 or 4 inotropes (n, %) | 9 (31) | 2 (7) | |
| VIS | 18.3 (6.6–23.6) | 4.7 (3.6–11.7) | <0.001 |
| Pre-MCS IABP (n, %) | 8 (28) | 2 (7) | 0.028 |
| LVEF (%) | 10 (10–12) | 10 (8–16) | 0.825 |
| Severe MR (n, %) | 5 (21) | 8 (36) | 0.243 |
| TAPSE (mm) | 11 (9–13) | 11 (9–12) | 0.506 |
| Severe TR (n, %) | 5 (21) | 8 (36) | 0.243 |
| Pre-MCS CVP (mmHg) | 19 (14–22) | 17 (14–22) | 0.479 |
| Pre-MCS mean PAP (mmHg)* | 38 ± 3 | 37 ± 3 | 0.614 |
| Pre-MCS CI (L/min/m2)* | 1.72 (1.47–1.89) | 1.43 (1.19–1.74) | 0.160 |
| Pre-MCS CPOi* | 0.10 (0.06–0.14) | 0.08 (0.05–0.15) | 0.342 |
| Baseline Na (mmol/L) | 132 ± 2 | 132 ± 2 | 0.897 |
| Baseline creatinine (umol/L) | 135 (107–180) | 121 (99–150) | 0.410 |
| Baseline bilirubin (umol/L) | 42 (28–62) | 30 (19-50) | 0.107 |
| Base excess (mmol/L) | –9.5 (−14.0–2.7) | 2.9 (–1.3–4.3) | <0.001 |
| Lactate | 11.0 (4.8–16.3) | 2.8 (2.6–3.8) | <0.001 |
BMI: body mass index; CI: cardiac index; CPOi: cardiac power output index (=(mean arterial pressure-CVP) x CI/451); CVP: central venous pressure; DCM: dilated cardiomyopathy; HF: heart failure; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; IABP: intra-aortic balloon pump; LVEF: left ventricular ejection fraction; MCS: mechanical circulatory support; MR: mitral regurgitation; Na: sodium; PAP:pulmonary artery pressure; VIS: vasoactive inotrope score; TAPSE: tricuspid annular plane systolic excursion, TR: tricuspid regurgitation.
*No data in 3 patients.
Figure 1.
Mechanical circulatory support modalities and clinical outcomes. VA ECMO: venoarterial extracorporeal membrane oxygenation; BIVAD: biventricular assist device; LVAD: left ventricular assist device.
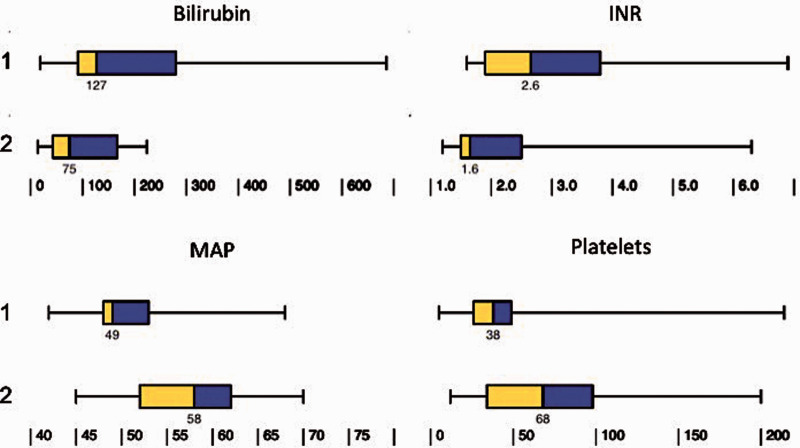
The median duration of MCS was comparable between INTERMACS 1 and 2 patients (14 (10–33) vs 15 (7-23) days, p = 0.439). The post-support clinical course was more complicated in INTERMACS 1 patients, with higher peak bilirubin, international normalized ratio, lower trough platelet count and mean arterial blood pressure (Figure 2). A higher proportion of INTERMACS 1 patients required renal support (15/29 (52%) vs 3/31 (10%), p < 0.001), indicating greater degree of multi-organ dysfunction. INTERMACS 1 patients were ventilated for significantly longer duration compared to INTERMACS 2 patients (10 (5–17) vs 5 (3–7) days, p = 0.021). The SOFA score at 48 hours post-MCS was also significantly higher in INTERMACS 1 compared to INTERMACS 2 patients (14 (13–16) vs 11 (8–12), p < 0.001). There was no statistically significant difference in the number of complications that were directly attributable to the MCS that required intervention between INTERMACS 1 and 2 patients (13/29 (45%) vs 10/31 (32%), p = 0.317). Bleeding requiring intervention is the most common complication (Table 2). Stroke or intracranial bleed were fatal in all 4 patients.
Figure 2.
Clinical course following MCS in INTERMACS 1 (top row) and 2 (bottom row) patients. INR: international normalised ratio; MAP: mean arterial blood pressure; bilirubin and platelets refer to the highest bilirubin level and lowest platelet count during support.
Table 2.
Number of patients with MCS-related complications requiring intervention.
| INTERMACS 1 (n = 29) | INTERMACS 2 (n = 31) | Total (n = 60) | |
|---|---|---|---|
| Bleeding* | 6 (21%) | 6 (19%) | 12 (20%) |
| Stroke or intracranial bleed | 3 (10%) | 1 (3%) | 4 (7%) |
| Hemolysis | 1 (3%) | 1 (3%) | 2 (3%) |
| Device thrombosis | 1 (3%) | 0 | 1 (2%) |
| Cannula positioning | 2 (6%) | 2 (6%) | 4 (7%) |
*Bleeding requiring surgical exploration in all cases except 1 gastrointestinal bleed in an INTERMACS 2 patient.
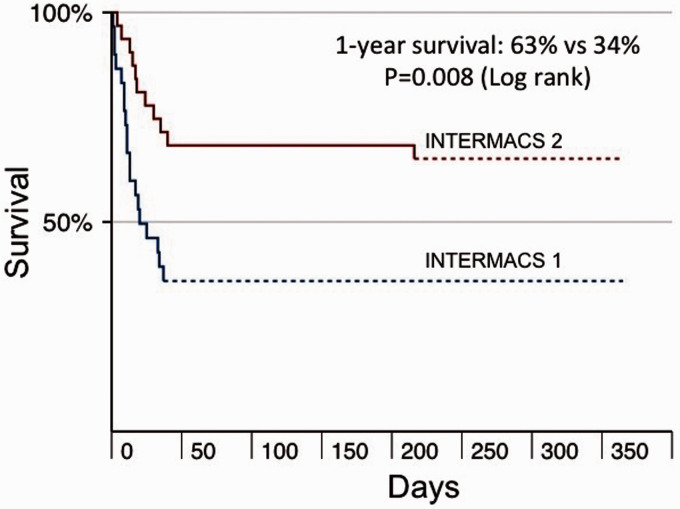
Overall, 50% of our patients were bridged to OHT or durable LVAD. The median length of stay on the intensive care unit post-OHT or LVAD was 13 (6–19) days, and the length of stay on the ward was 21 (14–28) days. A greater proportion of INTERMACS 2 patients were successfully bridged to OHT or durable LVAD compared to INTERMACS 1. All the INTERMACS 2 patients who were bridged to OHT or durable LVAD survived at least 90 days compared to only 64% of patients in INTERMACS 1. The 90-day survival of patients bridged to OHT or durable LVAD in the whole cohort was 87% (Table 3). Overall, 50% of patients died at 90 days of follow-up. Survival at 12 months was significantly higher in INTERMACS 2 compared to INTERMACS 1 patients (63% vs 34%, Log rank test, p = 0.008) (Figure 3). Six of the 9 patients with prior cardiac arrest died <12 months.
Table 3.
Outcomes of bridging to heart transplantation and durable LVAD.
| INTERMACS 1(n = 29) | INTERMACS 2 (n = 31) | Total (n = 60) | |
|---|---|---|---|
| Bridged to transplant | 9 (31%) | 13 (42%) | 22 (37%) |
| Bridged to durable LVAD | 2 (7%) | 6 (19%) | 8 (13%) |
| Number (%) survived at 90 days post-transplant or durable LVAD | 7 (63%) | 19 (100%)* | 26/30 (87%) |
*1 patient died >90 days post-heart transplant.
Figure 3.
Kaplan-Meier survival curves based on INTERMACS profiles.
Discussion
Successful outcome from MCS in CS is dependent on the ‘right’ timing for the ‘right’ patient with the ‘right’ modality of support. 12 Therefore, in this study, we defined the timing (INTERMACS class), the patient population (CS due to ESHF) and the MCS strategy. Our results indicate a more favorable clinical course and 90-day survival with institution of MCS in patients who are beginning to ‘slide’ on inotropes (INTERMACS profile 2) compared to INTERMACS 1 patients in critical cardiogenic shock.
Cardiogenic shock due to ESHF represents a distinct group of patients characterized by the chronicity of illness, concomitant medical therapy and the underlying cardiomyopathic aetiology. However, the majority of published studies on temporary MCS have centred on other causes of CS, particularly acute myocardial infarction (AMI) or myocarditis.13,14 These data derived from other patient populations cannot be simply extrapolated to patients with ESHF, as (i) there are major differences in the hemodynamic and metabolic phenotype between patients with CS due to AMI and ESHF 15 ; and (ii) recovery may be more likely in some patients with CS due to AMI or acute myocarditis, which would obviate the need for prolonged bridging with multiple MCS modalities to LVAD or OHT.
There are few studies that have focused on patients with CS due to ESHF, despite rapidly expanding use of MCS in patients with ESHF. 16 For example, Takayama and colleagues described their experience with different MCS modalities to bridge patients with CS to recovery, OHT or durable LVAD; but their study included patients with CS due a range of aetiologies, including AMI, myocarditis and decompensated HF. 17 Bermudez and colleagues noted significantly worse survival in a small series of patients with CS due to ESHF compared to AMI, using a MCS strategy that was largely limited to VA ECMO. 18
The largest cohort study of temporary MCS in patients with CS due to ESHF was reported by Dangers et al. 5 Overall 1-year survival in the whole cohort of 105 patients was 38%. Survivors in their study had blood lactate levels at the time of MCS (3 (2-5)mmol/L) that were similar to our INTERMACS profile 2 patients, and supports our strategy of earlier MCS in these patients. However, unlike our strategy, Dangers et al used a primary VA ECMO strategy in all their patients and the majority of the patients were maintained on VA ECMO. 5 Large registries have shown that the outcomes of VA ECMO-bridging to OHT are poor. 19 Similarly, Garan et al reported a mortality of 44% in patients who were bridged directly from VA ECMO to durable LVAD. In contrast, all the patients who were bridged via Centrimag VAD to durable LVAD survived. 20 The overall survival to discharge in their cohort of 52 patients was 46%.
Few studies have reported on the clinical course and major complication rates. We found comparable complication rates between INTERMACS 1 and 2 patients in our study. However, INTERMACS profile 1 patients had more severe organ dysfunction, as evidenced by the higher peak bilirubin, lower trough platelet count and greater use of hemofiltration – all of which have been linked with poorer survival in shocked states.21–23 Reduced platelet count is common in critically ill patients supported with VA ECMO and probably reflects more severe critical illness. 24
In the face of a patient with CS due to ESHF who is deteriorating on inotropes (INTERMACS 2), clinicians must weigh up the options of (i) increasing inotropic support, which may be sufficient to stabilize the patient; or (ii) deploy temporary MCS, which may be more effective in preventing or reversing organ dysfunction associated with CS. However, the former risks further deterioration into multi-organ failure, while the latter will expose the patient to potentially life-threatening MCS-related complications. Our results suggest that the benefit of early institution of effective biventricular support (Centrimag BIVAD) in limiting or preventing multi-organ dysfunction in CS may outweigh the risks of MCS. Minimizing MCS-related complications with alternative techniques (eg: minimally invasive Centrimag BIVAD 25 or percutaneous biventricular support) may lead to further improvement in outcomes.
Our approach to cardiogenic shock can be divided into 4 therapeutic phases: “recognition and rescue”, “optimization”, “stabilization” and “exit therapy or de-escalation” (acronym ROSE). In brief, during the “recognition and rescue” phase, patients at risk (eg: acute myocardial infarction or myocarditis) are identified and closely monitored for evolving cardiogenic shock (eg: arterial and central venous blood gases and invasive haemodynamic monitoring); co-morbidities and candidacy for intensive care and MCS are assessed; patients with cardiogenic shock discussed early with the regional cardiogenic shock centre regarding indications, candidacy and possible transfer for MCS; inotropes and vasopressors are initiated to halt or reverse haemodynamic deterioration, and institute MCS in patients with critical cardiogenic shock.
During the “optimization” phase, complications of MCS are addressed; fluid status, inotropes and vasopressors titrated; coagulopathy, gas exchange and metabolic derangements and infective complications are treated. The “stabilization” phase is characterized by liberation from mechanical ventilation, control of coagulopathy, metabolic derangements and infection. De-escalation with weaning of MCS in the event of recovery or exit therapy (heart transplantation, LVAD or palliation) is considered following stabilization.
Study limitations
There are a number of limitations. Firstly, this is a single centre study has the same inherent limitations with biases and external validity. Secondly, there was clear patient selection bias based on candidacy for heart transplantation. Different institutions with different patient selection criteria may have different outcomes. Thirdly, the modality of MCS was decided by the clinical team and not randomized. Hence, we cannot determine the efficacy of different MCS modalities. Indeed, it was our aim was to evaluate our temporary MCS strategy, not to compare the efficacy of different MCS modalities.
In conclusion, our data suggest that our temporary MCS strategy, which included earlier institution of MCS in INTERMACS profile 2 patients with CS due to ESHF may be associated with less organ dysfunction and better 90-day survival, compared to INTERMACS profile 1. A prospective randomized study of CS due to ESHF is warranted to further define the appropriate timing and strategy for intervention in this patient population.
Supplemental Material
Supplemental material, sj-pdf-1-inc-10.1177_1751143720988706 for Outcomes of temporary mechanical circulatory support in cardiogenic shock due to end-stage heart failure by Hoong Sern Lim, Aaron Ranasinghe, David Quinn, Colin Chue and Jorge Mascaro in Journal of the Intensive Care Society
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hoong Sern Lim https://orcid.org/0000-0002-6569-1805
David Quinn https://orcid.org/0000-0003-2465-305X
Supplemental material: Supplemental material for this article is available online.
References
- 1.Eduardo BC, Javier SC, Luis AB, et al. Preoperative INTERMACS profiles determine postoperative outcomes in critically ill patients undergoing emergency heart transplantation. Circ Hear Fail 2013; 6: 763–772. [DOI] [PubMed] [Google Scholar]
- 2.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant 2011; 30: 402–407. [DOI] [PubMed] [Google Scholar]
- 3.Combes A, Price S, Slutsky AS, et al. Temporary circulatory support for cardiogenic shock. Lancet 2020; 396: 199–212. [DOI] [PubMed] [Google Scholar]
- 4.Cheng R, Tank R, Ramzy D, et al. Clinical outcomes of impella microaxial devices used to salvage cardiogenic shock as a bridge to durable circulatory support or cardiac transplantation. Asaio J 2019; 65: 642–648. [DOI] [PubMed] [Google Scholar]
- 5.Dangers L, Bréchot N, Schmidt M, et al. Extracorporeal membrane oxygenation for acute decompensated heart failure. Crit Care Med 2017; 45: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 6.Badu B, Cain MT, Durham 3rd LA, et al. A Dual-Lumen percutaneous cannula for managing refractory right ventricular failure. Asaio J 2020; 66: 915--921. [DOI] [PubMed] [Google Scholar]
- 7.Musa TA, Chue CD, Lim HS. Mechanical circulatory support for decompensated heart failure. Curr Heart Fail Rep 2017; 14: 365–375. [DOI] [PubMed] [Google Scholar]
- 8.Davidson J, Tong S, Hancock H, et al. Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med 2012; 38: 1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 10.Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010; 139: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 11.Vivo RP, Cordero-Reyes AM, Qamar U, et al. Increased right-to-left ventricle diameter ratio is a strong predictor of right ventricular failure after left ventricular assist device. J Heart Lung Transplant 2013; 32: 792–799. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS. The “right” patients for temporary mechanical circulatory support. J Heart Lung Transplant 2018; 37: 936. [DOI] [PubMed] [Google Scholar]
- 13.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016; 42: 370–378. [DOI] [PubMed] [Google Scholar]
- 14.Truby L, Naka Y, Kalesan B, et al. Important role of mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock. Eur J Cardiothorac Surg 2015; 48: 322–328. [DOI] [PubMed] [Google Scholar]
- 15.Lim HS, Howell N. Cardiogenic shock due to end-stage heart failure and acute myocardial infarction: characteristics and outcome of temporary mechanical circulatory support. Shock 2018; 50: 167–172. [DOI] [PubMed] [Google Scholar]
- 16.Stretch R, Sauer CM, Yuh DD, et al. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014; 64: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 17.Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant 2013; 32: 106–111. [DOI] [PubMed] [Google Scholar]
- 18.Bermudez CA, Rocha RV, Toyoda Y, et al. Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg 2011; 92: 2125–2131. [DOI] [PubMed] [Google Scholar]
- 19.Lund LH, Edwards LB, Dipchand AI, et al. The registry of the international society for heart and lung transplantation: thirty-third adult heart transplantation report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016; 35: 1158–1169. [DOI] [PubMed] [Google Scholar]
- 20.Garan AR, Malick WA, Habal M, et al. Predictors of survival for patients with acute decompensated heart failure requiring extra-corporeal membrane oxygenation therapy. ASAIO J 2019; 65: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freundt M, Lunz D, Philipp A, et al. Impact of dynamic changes of elevated bilirubin on survival in patients on venoarterial extracorporeal life support for acute circulatory failure. PLoS One 2017; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opfermann P, Bevilacqua M, Felli A, et al. Prognostic impact of persistent thrombocytopenia during extracorporeal membrane oxygenation: a retrospective analysis of prospectively collected data from a cohort of patients with left ventricular dysfunction after cardiac surgery. Crit Care Med 2016; 44: e1208–18–e1218. [DOI] [PubMed] [Google Scholar]
- 23.Tarvasmäki T, Haapio M, Mebazaa A, et al. Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. Eur J Heart Fail 2018; 20: 572–581. [DOI] [PubMed] [Google Scholar]
- 24.Abrams D, Baldwin MR, Champion M, et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med 2016; 42: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Garan AR, Ando M, et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg 2017; 52: 1055–1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-inc-10.1177_1751143720988706 for Outcomes of temporary mechanical circulatory support in cardiogenic shock due to end-stage heart failure by Hoong Sern Lim, Aaron Ranasinghe, David Quinn, Colin Chue and Jorge Mascaro in Journal of the Intensive Care Society