Abstract
Introduction
The ProximaTM point of care (POC) device enables arterial blood gas (ABG) samples to be analysed without the nurse leaving the patient. The benefits of this for work efficiency have not been evaluated.
Methods
We compared the time taken to obtain an ABG result using ProximaTM versus a standard ABG sampling system. Twenty patients were randomized to ABG sampling using ProximaTM, or a standard ABG system. Nurses were observed performing all ABG sampling episodes for a minimum of 24 hours and no more than 72 hours.
Results
The mean time taken to obtain a result using ProximaTM was 4:56 (SD = 1:40) minutes compared to 6:31 (SD = 1:53) minutes for the standard ABG technique (p < 0.001). Mean time away from the patient's bedside was 3.07 (SD = 1:17) minutes using the standard system and 0 minutes using ProximaTM (p < 0.001).
Conclusions
Reduced time for blood gas sampling and avoidance of time away from patients may have significant patient safety and resource management implications, but the clinical and financial significance were not evaluated.
Keywords: Blood specimen collection, time and motion studies, point of care technology, critical care
Background
Arterial blood gas (ABG) sampling and analysis is carried out routinely in intensive care units (ICU). 1 Typically, a nurse at the bedside aspirates blood from the arterial line. The blood is injected into an ABG machine located within or close to the ICU. In order to ensure safety whilst the nurse leaves the patient to perform this analysis, another staff member is required to observe the patient. Sampling frequency is increased in patients with more severe illness, 2 requiring more frequent absences from the patient’s bedside.
Each ABG sample is obtained using a heparinised syringe. The syringe is capped off and carried to the ABG machine for analysis. The ProximaTM point of care (POC) device enables ABG samples to be analysed at the bedside and offers several theoretical advantages over conventional ABG analysis. 3 The nurse does not need to leave the patient to perform ABG analysis,3,4 so there is no requirement for a colleague to look after the patient whilst analysis takes place. The integrity of the arterial line sampling system is not broken during sampling, decreasing the risk of splash injury and blood-borne infection, and there is no requirement to carry blood around the unit in an unsealed container.
We aimed to evaluate the impact of one of these theoretical advantages in routine clinical practice in a UK intensive care unit. Specifically, we set out to evaluate whether the time taken to obtain a result using the ProximaTM system was less than the time taken to obtain a result using the standard ABG sampling system. Furthermore, we sought to quantify how long the nurse was absent from the patient’s bedside whilst performing ABG analysis using the standard system.
Methods
Overview
Twenty patients admitted to a cardiac ICU, following elective cardiac surgery, were randomized to ABG sampling using the ProximaTM POC ABG device or the standard ABG system. Nurses were observed performing all ABG sampling on each recruited participant for a minimum of 24 hours and for no more than 72 hours post operation. Healthcare professionals carried out the observations, following a time and motion study methodology. 5
Research governance
The study was conducted in compliance with the Research Governance Framework for Health and Social Care and Good Clinical Practice (GCP). All investigators were ICH-GCP trained. National Research Ethics Service approval was obtained from London – Queen Square Research Ethics Committee (15/LO/1726) before the trial commenced. The trial was registered with the University Hospital Southampton (UHS) Research and Development department for the purposes of data protection compliance. Research and financial sponsorship were provided by Sphere Medical Limited, the manufacturer of the ProximaTM machine.
Setting and participants
All adults (aged 18 years or above) scheduled to undergo non-complex elective cardiac surgery at UHS were screened for eligibility, excluding Grown Up Congenital Heart surgery. Eligible patients were approached before surgery if they were anticipated to require the insertion of an arterial line for clinical management after their operation. Exclusion criteria were: not anticipated to require an arterial line; anticipated to require one for less than 24 hours; less than 18 years of age; pre-existing raised serum phosphate or low or raised serum calcium levels prior to surgery; or scheduled to be admitted to the cardiac high dependency unit immediately post operation.
The cardiac ICU currently utilizes 15 beds. There are two ABG analysers on the unit, and a third available in a laboratory in the vicinity. Each unit based analyser is positioned at the centre of a line of eight beds. The bed furthest from an analyser is at a distance of 29 metres, and the nearest is at a distance of 6 metres.
Recruitment and randomisation
Participants were asked to provide written consent preoperatively. All participants were free to withdraw at any time from the study without giving reasons and without prejudicing further treatment. A research administrator, not involved in running the study, generated an allocation sequence using block randomization, and held it in a password protected electronic folder. The block randomization created five blocks of four allocations. Block randomization was used to reduce the risk of bias that can occur from using simple randomization in small studies, as it balances the allocation of participants to each arm of the study, preventing a run of participants being allocated to one arm, and then the other.
On the day of surgery, the research nurse clarified the planned post-operative destination for the patient – either cardiac ICU, or cardiac high dependency. If the patient’s destination was to be the cardiac ICU, the patient’s anaesthetist was contacted once the patient was in theatre and on cardiac bypass. The anaesthetist was asked to confirm that there were no clinical contraindications to including the patient in the trial. Once this was confirmed the research nurse contacted the research administrator to obtain the allocation group; either ABG sampling using the ProximaTM machine (Proxima group) or the standard ABG sampling system (Standard group).
Data and study management
Trained healthcare practitioners using case report forms (CRF) developed specifically for this study carried out data collection. A data collector remained in the vicinity of the participant for the duration of the data collection period, 24 hours a day. The moment the nurse looking after the patient indicated they were intending to take a blood gas sample the data collector started a stop watch and observed the sampling episode whilst completing a CRF. The CRF consisted of one A4 sheet of paper attached to a clipboard. The data collector was required to record timings related to data collection. sample volume, and tick boxes related to equipment used to take each sample (eg gloves), missing data, and causes of interruptions to the whole process. One CRF was used for each sample episode. The CRF used was specific to each arm of the study. Participants were allocated a unique identifying study number to ensure that it was not possible to identify them from the data. Data from each CRF was entered onto password protected Trust computers and only accessed by investigators from the ICU research team. The manual files were stored in secure offices within the Trust. Euroscore II values were calculated for each participant. Euroscore II, a European system for cardiac operative risk evaluation, is a cumulative score of risks derived from patient, cardiac and operation related factors. 6
Data obtained from the two sampling methods were not identical. Each method provided data on time, pH, PaO2, PaCO2, HCO3, HCT, K+, and BE. In addition, the standard method provided Anion gap, Na+, Ca++, Cl–, Glucose, Lactate, and MetHb. Therefore, the data collectors took an additional ABG sample from participants in the Proxima arm and processed it using the standard ABG method to ensure clinical care was not different between the two groups. This was done immediately after data collection for the sample episode had been completed.
Training
Training in the use of the ProximaTM POC machine was provided by Sphere Medical Limited for ProximaTM at Level 2 to data collectors required to collect data related to the ProximaTM sample episodes. Level 2 training covered advanced training in all aspects of using the Proxima system including assembly, calibration, quality control, sampling, disposal and troubleshooting. Thus, these data collectors were assessed as competent by the company to use the device, and to train other staff in its use for sample analysis only.
Outcome measures
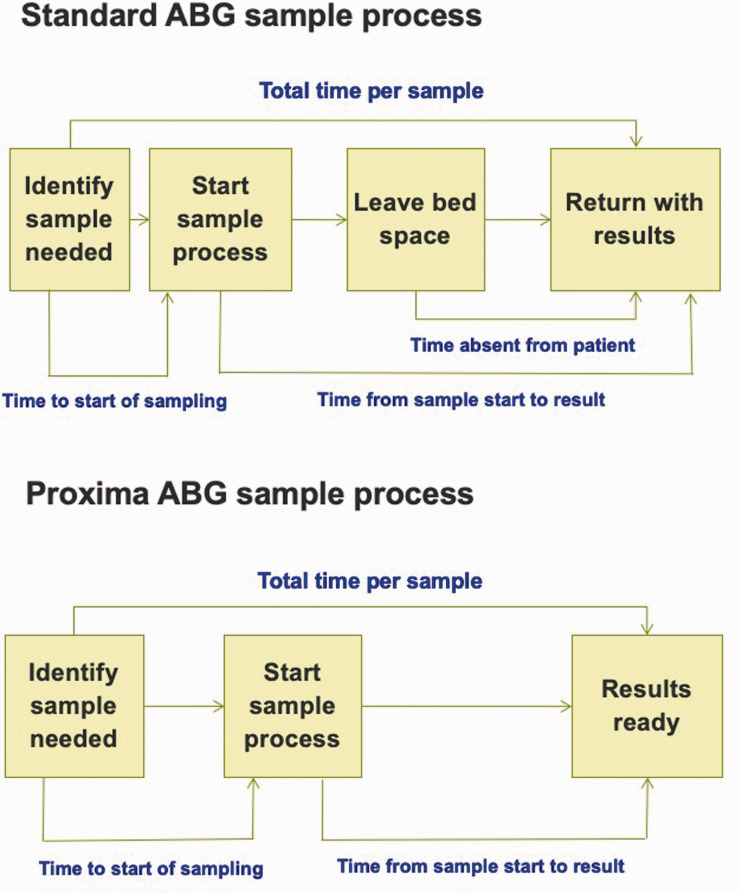
The primary outcome measure was the average (mean) time taken to obtain an ABG sample result using the ProximaTM POC device compared with using the standard ABG sampling system. Sample time was defined as the time period from the point when a decision to take a sample was made through to the point when the sample results were available at the patient's bedside, see Figure 1.
Figure 1.
Various time points of sampling process for ProximaTM and Standard arterial blood gas sampling.
Secondary outcome measures included the average (mean) time per group for components of each sample episode, the average (mean) time the nurse was absent from the patient's bedside whilst carrying out the processing of the sample, and the reasons for delay to the sampling process (descriptive outcome only).
Statistical analysis and sample size calculation
A sample size calculation was performed to establish the number of sampling episodes required to demonstrate a difference in sampling duration between the two groups. A duration difference of greater than two minutes between the ProximaTM machine and the standard ABG sampling method was deemed clinically significant.
A sample size of 166 sampling episodes was estimated to be needed to demonstrate a duration difference between the two groups of greater than two minutes, giving a power level of 95% and a significance level of 5%. A dropout rate of 25% of recruited patients was assumed, where each patient would undergo at least eleven sample episodes in the 24-hour period of observation, requiring 10 patients in each group. All times were reported as minutes and seconds (minutes: seconds)
We performed all statistical analyses on the basis of a pre-specified statistical analysis plan. The main comparison was total sample episode time between groups. A p value of <0.05 was deemed to indicate a statistically significant result. Statistical analysis was carried out using the statistical package SPSS, (IBM SPSS Statistics version 24). Continuous variables are reported as means and standard deviations.
We used an independent t-test to compare between-group differences in the primary outcome. A Mann Whitney U test was also performed. An independent t-test was used to compare between-group differences in the quantitative secondary outcome measures. As a post-hoc analysis, the relationships between a severity illness score (Euroscore II) and total number of samples taken per patient, and also total time absent from patients in the Standard group were explored using a Pearson correlation. Tests were two-tailed. Descriptive outcomes are divided into those directly attributable and those not directly attributable to the technology used.
Results
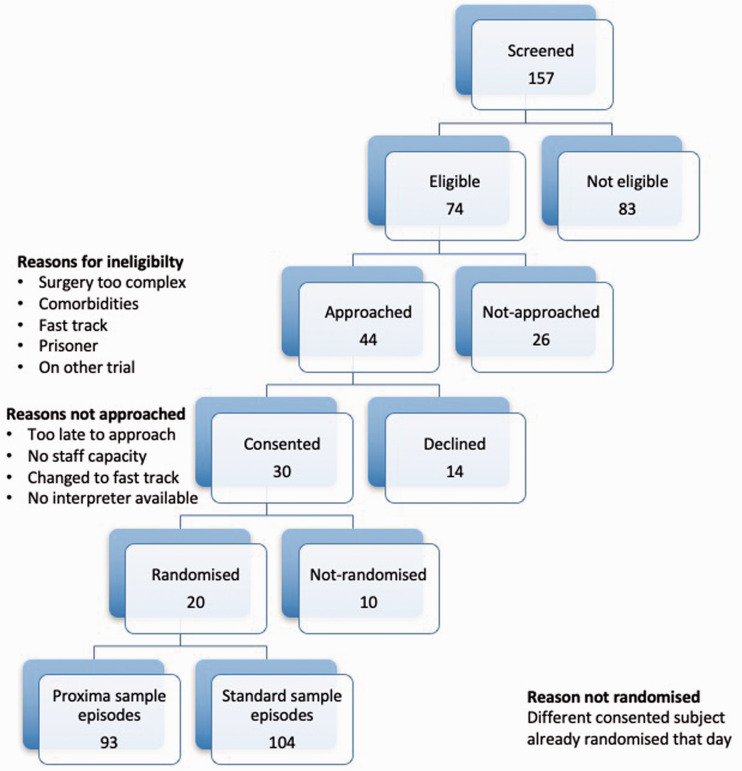
Twenty patients (13 male) were recruited and randomized and 197 sampling episodes were observed between April and May 2016. See Consort 7 diagram (Figure 2) for eligibility and recruitment figures.
Figure 2.
Consort diagram.
No patient chose to withdraw once they had agreed to participate. The mean number of sampling episodes per patient was 9.85 (SD = 2.43) and there was no difference in the number of sampling episodes between groups (Proxima – mean = 9.3, SD = 3.02; Standard – mean = 10.4, SD = 1.65; p = 0.32).
Four sample episodes were removed from the final analysis. Two sampling episodes from the same participant were removed from the Proxima group due to problems aspirating the arterial line (two occasions) resulting in the machine timing out on the sample process so that total time per sample could not be recorded. One sampling episode was removed from the Standard group because the data collector failed to record the sample episode details. Another sampling episode was removed from the Standard group because the data collector failed to record the time the sample collector left the bed space. Therefore, although total sample time could be determined, time of absence from the bed space could not.
One patient’s participation in the study (Proxima group) was discontinued early because the patient was bleeding and a plan was made to return to theatre (unrelated to trial conduct). This patient’s data were included in the final analysis (4 sampling episodes). Use of the ProximaTM in the operating theatre was not included within the study ethical approval so the machine was removed from the patient, but in the event the patient’s condition settled and a return to theatre did not take place. The one reported adverse event (pacing problem) was deemed by independent clinicians to be unrelated to the study conduct but was none-the-less reported to both the Research and Development department and the trial sponsor.
Primary outcome
The mean time taken to obtain a result using the ProximaTM machine was 4:56 (SD = 1:40) minutes in comparison with 6:31 (SD = 1:53) minutes for the standard blood gas technique (p < 0.001). This result was robust to analysis using alternative (non-parametric) analysis (Proxima group median = 4:33 (IQR = 4:11-5:01); Standard group median = 6:10 (IQR = 5:23-7:22); p < 0.001). The mean times using Proxima were also shorter than using the standard blood gas technique for each portion of the process (Table 1).
Table 1.
Time and motion data for 193 sampling episodes after removing 4 samples from final analysis.
| Proxima | Standard | ||
|---|---|---|---|
| Participants | 13 male | 10 | 10 |
| Total episodes (postCut) | N = 193, Mean = 9.65, SD = 2.18 | ||
| Sample episodes (post cut) | N (Mean, SD) | 91 (9.1, 2.64) | 102 (10.2, 1.55) |
| p-value | p = 0.27 | ||
|
Time period |
Statistical test |
Proxima |
Standard |
| Time to start of sampling | Mean (SD) | 0:39 (1:04) | 1:28 (1:11) |
| Sig. (2-tailed) | p < 0.001 | ||
| Time sample start to result | Mean (SD) | 4:17 (1:13) | 5:02 (1:30) |
| Sig. (2-tailed) | p < 0.001 | ||
| Time absent from patient | Mean (SD) | 0:00 (0:00) | 3:07 (1:17) |
| Sig. (2-tailed) | p < 0.001 | ||
| Total time per sample | Mean (SD) | 4:56 (1:40) | 6:31 (1:53) |
| Sig. (2-tailed) | p < 0.001 | ||
| Median (IQR) | 4:33 (4:11-5:01) | 6:10 (5:23-7:22) | |
Secondary outcomes
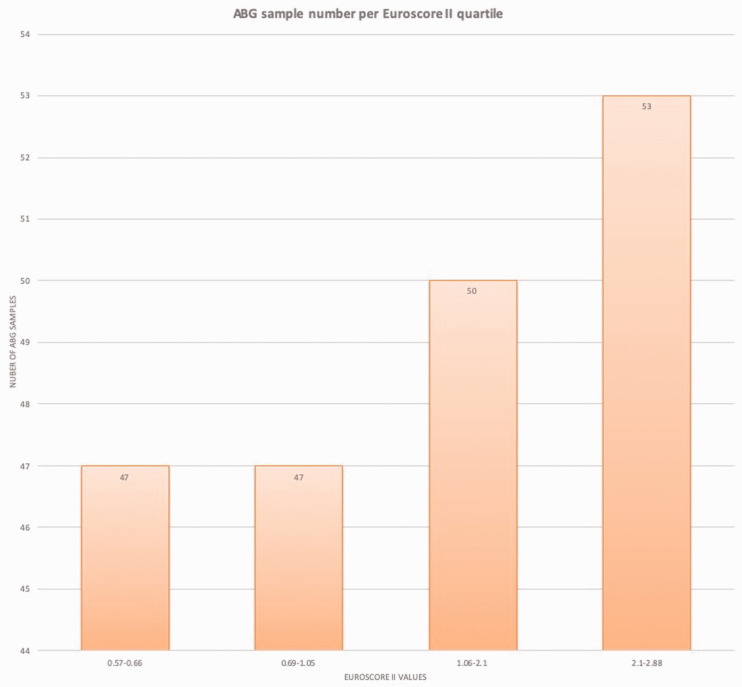
The mean time away from the patient's bedside was 3.07 (SD = 1:17) minutes using the standard system and 0 minutes using the ProximaTM system (p < 0.001). There was no correlation between a measure of severity of illness (Euroscore II) and number of samples per patient (r = 0.21, p = 0.36), even when analysed by group (see Table 2). There was a trend towards a significant correlation between Euroscore II and total time away from patient’s bedside (mean = 31:49, SD = 5:44, r = 0.62, p = 0.054) (Standard group) (see Table 3). Figure 3 shows a histogram of the number of samples within each quartile of the range of Euroscore II values of participants in this study. It should be noted that all the Euroscore II values were less than 3 so considered low risk.
Table 2.
Correlation of Euroscore against number of sample episodes per patient.
| Item | Statistical test | Proxima | Standard |
|---|---|---|---|
| Euroscore II overall | r Sig. (2-tailed) | 0.21 (p = 0.36) | |
| Euroscore II | Mean (SD) | 1.34 (0.90) | 1.43 (0.64) |
| Median (IQR) | 0.8 (0.65/2.23) | 1.2 (0.90/2.15) | |
| Sig. (2-tailed) | t(18) = –2.6, p = 0.79 | ||
| # samples per patient | Mean (SD) | 9.1 (2.64) | 10.2 (1.55) |
| Median (IQR) | 9.5 (7.5/10.5) | 10 (9.75/11) | |
| Sig. (2-tailed) | t(18) = −1.13, p = 0.27 | ||
| Pearson correlation | r, Sig. (2-tailed) | 0.15 (p = 0.67) | 0.33 (p = 0.35) |
Table 3.
Correlation of Euroscore against total time absent per patient in Standard group.
| Item | Statistical test | Standard |
|---|---|---|
| Euroscore II | Mean (SD) | 1.43 (0.64) |
| Median (IQR) | 1.2 (0.90/2.15) | |
| Total time absent from patient | Mean (SD) | 31:49 (5:44) |
| Median (IQR) | 31:33 (30:17/36:06) | |
| Pearson correlation | r, Sig. (2-tailed) | 0.62 (p = 0.054) |
Figure 3.
ABG sample number per Euroscore II quartile.
Discussion
Statement of principal findings
Less time was taken to obtain a sample result using ProximaTM than the standard ABG sampling system. This result was statistically significant (p < 0.001), but the clinical significance is uncertain.
Strengths
This was a prospective study, carried out using an a priori analysis plan. The study used a continuous time and motion study methodology. 8 A data collector was placed within a workplace and captured everything that happened relating to the person or process they were interested in. Data capture started at the time the person or process arrived in the workplace, and continued until the person or process completes. This method is considered the gold standard of time and motion study methodologies, but is not always used as it is associated with a high cost in terms of time and resource. 9
Limitations
The study was single centre, using a homogeneous patient group, and observing staff highly experienced in taking arterial blood gases using the standard arterial blood gas system. The study was carried out on non-complex elective patients undergoing cardiac surgery, so it is unclear whether there would be greater benefit in using the Proxima TM in a 24-hour period, sampling in patients with more severe illness. In our study, there was no significant correlation between Euroscore II and the number of sampling episodes the participant was exposed to. However, there was a trend towards a significant correlation between Euroscore II and total time away from the patient’s bedside. But, the study may have been underpowered in relation to these outcomes as Euroscore II was a post-hoc analysis and was not taken into account when performing the sample size calculation.
The nurses taking arterial blood gas samples using the Proxima system were only trained in using the Proxima system at the beginning of their shift. Therefore, sample episode time may be reduced further in practice when the ProximaTM machine is operated by staff experienced in its use.
Secondary outcome
The mean time the nurse was away from the bed-space to analyse a sample using the standard system was 3:07 minutes. This is sufficient time for a patient to self-extubate or develop an acute physiological disturbance (e.g. arrhythmia). ProximaTM sample analysis does not require the nurse to leave the patient’s bedside. The more samples required in a 24-hour period the more time the nurse would be absent from the bed space if using a standard ABG sampling system, suggesting a greater level of risk in sicker patients, and on units where patient: staff ratios are under pressure. Our data showed a trend towards a significant correlation between total time absent from the patient and a measure of severity of illness.
Once a decision to take a sample was made, sampling was commenced faster in the ProximaTM group than the Standard group (p < 0.001). This may have been related to the nurse needing to ensure they would be able arrange cover to leave the bed space to process the sample once they had taken it in the Standard group.
There were a number of causes of delay to the sampling process using the standard ABG sampling system, (see Table 4). Some of these delays were related to the requirement of the nurse to leave the bed space specifically to perform sample analysis (e.g. no one to watch the patient, calibration and queues at the ABG machine). In units that have a reduced nurse to patient ratio, or a lower machine to bed ratio, these delays would be expected to increase in number. Other delays were due to causes that were independent from the sampling process (e.g. collecting kit, discussing the result with another member of staff, or being called to help elsewhere on the unit). The actions they represent would have had to take place regardless of the sampling process. Of the 86 counts of reasons for delay to the sample process in the Standard group, more than half (60%) could be more frequent occurrences in sicker patients (collecting kit, discussing the result with another member of staff, taking laboratory samples). It is not possible to take additional laboratory blood samples as part of the arterial blood gas sampling procedure using the ProximaTM machine. Laboratory samples can be taken via the ProximaTM system, but not as part of the sampling procedure.
Table 4.
Count of descriptive reasons recorded for prolonging sample episode duration related to sampling method.
| Count of reasons recorded for prolonged sample time (related to sampling method) | ||
|---|---|---|
| ABG machine calibrating | 7 | Standard |
| Queue at ABG machine | 12 | Standard |
| No one to watch patient | 7 | Standard |
| Possible blood on sensor warning | 3 | Proxima |
| Difficulty aspirating/returning/flushing line | 12 | Proxima |
| Count of reasons recorded for prolonged absence from bed space | ||
| Take lab samples in addition to ABGa | 10 | Standard |
| Discussion of result with other staffa | 17 | Standard |
| Collect kit from unit storesa | 25 | Standard |
| Call for help from staff at another bed space | 8 | Standard |
aLikely to be increased in sicker patients.
Compare with previous studies
Previous studies of arterial blood gas analysis have focussed on the accuracy of different measurement techniques.10,11 We have not found any reports of studies measuring the time taken to obtain a result and its impact on adult ICU nurses’ work processes. Paediatric studies are reported only in abstract form. 12
Meaning of the study (implications for clinicians)
The user can obtain an ABG result faster using the ProximaTM ABG machine than a standard ABG sampling system. This absolute difference, whilst statistically significant, is unlikely to be clinically significant in isolation. However, the cumulative reduction in time required to obtain ABG sample results may be significant in sicker patients requiring more frequent sampling. Currently, ABG processing is likely to require the nurse to leave the bed space to process the sample, requiring another member of staff to be available to observe the patient. In units struggling to maintain staff: patient ratios this may prolong sample processing time further.
Unanswered questions and future research
It may be useful to examine Adverse Event Reports related to nurse absence from the bed space, and blood splash incidents. It may also be useful to explore haemodilution risk in relation to ABG sampling frequency. Finally, it may be useful to carry out time and motion studies in other healthcare settings where ABG machines are used such at operating theatres or general intensive care units
Conclusion
The duration of arterial blood gas sampling using the ProximaTM machine was shorter (1 minute 35 seconds) than using the standard blood gas technique. Each step of the blood gas sampling process (time to start sampling, time to obtain result, time away from patient) was shorter using the ProximaTM machine: most notably time away from the patient's bedside was >3 minutes using the standard system and 0 minutes using the ProximaTM system. There was a trend towards a correlation between severity of illness and total time away from the patient’s bedside. Reduced total time for blood gas sampling and avoidance of time away from patients may have significant patient safety and resource management implications but the clinical and financial significance of these findings was not evaluated.
Supplemental Material
Supplemental material, sj-pdf-1-inc-10.1177_1751143720973847 for Comparison of time taken to obtain an arterial blood gas result at the bedside using the ProximaTM point of care machine vs. a standard remote arterial blood gas analyser: A randomized controlled trial by Kay Mitchell, Karen E Salmon, David Egbosimba, Gavin Troughton and Mike PW Grocott in Journal of the Intensive Care Society
Acknowledgements
We would like to express our deepest gratitude to the following: Dr Mike Herbertson and Matron Rachel Spreadborough for giving their support for the research to take place on the Cardiac ICU, the Critical Care Research Area team for their invaluable assistance with recruitment and data collection, the Cardiac ICU clinical team for performing blood gas analysis, and especially the research participants.
Authors' contributions: KM, KES and MPWG designed the study. KM, KES, and DE were responsible for data collection and data handling. KM and MPWG analysed the data. KM performed the statistical analysis. KM and MPWG drafted the manuscript. DE, KES and GT contributed with critical revisions to the manuscript. All authors have read and approved of the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MPWG serves on the Medical Advisory Board of Sphere Medical and the study was conducted in collaboration with the company.
GT and DE were both employees of Sphere Medical Ltd at the time the study was conducted.
The study was funded by an unrestricted grant from the company. KM’s time was in part paid for from this study grant.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research and financial sponsorship were provided by Sphere Medical Limited, the manufacturer of the ProximaTM machine.
ORCID iD: Kay Mitchell https://orcid.org/0000-0001-6393-8475
Ethics approval and consent to participate
Informed consent was obtained directly from the patients. Information was provided both in writing and orally, underscoring that the patient could withdraw from the study at any time. National Research Ethics Service approval was obtained from London – Queen Square Research Ethics Committee (15/LO/1726) before the trial commenced.
Trial registration: UK Clinical Trials gateway, CSP Ref 171084. Registered 17 August 2016 – Retrospectively registered, https://www.ukctg.nihr.ac.uk
References
- 1.Andrews T andWaterman H.. What factors influence arterial blood gas sampling patterns? Nurs Crit Care 2008; 13: 132–137. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman JE, Seneff MG, Sun X, et al. Evaluating laboratory usage in the intensive care unit: patient and institutional characteristics that influence frequency of blood sampling. Crit Care Med 1997; 25: 737–748. [DOI] [PubMed] [Google Scholar]
- 3.Fox J andClutton-Brock T.. Evaluation of a patient-dedicated blood gas analyser. Crit Care 2015; 19: P261. [Google Scholar]
- 4.Richardson A. Frontline leadership, innovation and best practice: 10 hot topics every critical care nurse should be aware of. Nurs Crit Care 2015; 20: 3–4. [DOI] [PubMed] [Google Scholar]
- 5.Zheng K Guo MH andHanauer DA.. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc 2011; 18: 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nashef SA, Roques F, Sharples LD, Nilsson J, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744. discussion 744–735. [DOI] [PubMed] [Google Scholar]
- 7.Schulz KF, Altman DG, Moher D, et al.; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopetegui M, Yen PY, Lai A, et al. Time motion studies in healthcare: what are we talking about? J Biomed Inform 2014; 49: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke TA, McKee JR, Wilson HC, et al. A comparison of time-and-motion and self-reporting methods of work measurement. J Nurs Adm 2000; 30: 118–125. [DOI] [PubMed] [Google Scholar]
- 10.Gelsomino S, Lorusso R, Livi U, et al. Assessment of a continuous blood gas monitoring system in animals during circulatory stress. BMC Anesthesiol 2011; 11: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahutte CK, Holody M, Maxwell TP, et al. Development of a patient-dedicated, on-demand, blood gas monitor. Am J Respir Crit Care Med 1994; 149: 852–859. [DOI] [PubMed] [Google Scholar]
- 12.The introduction and potential development in practice of an inline blood gas analyser within adult and paediatric critical care, http://careconvention.co.uk/wp-content/uploads/2017/05/Collated-Abstract-Document-1.pdf (accessed 3 November 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-inc-10.1177_1751143720973847 for Comparison of time taken to obtain an arterial blood gas result at the bedside using the ProximaTM point of care machine vs. a standard remote arterial blood gas analyser: A randomized controlled trial by Kay Mitchell, Karen E Salmon, David Egbosimba, Gavin Troughton and Mike PW Grocott in Journal of the Intensive Care Society