Abstract
We describe the case of a patient who developed resistant hypertension due to a giant atheroma with acquired physiologic mimic of coarctation of the aorta. This presentation illustrates an extremely rare etiology to consider in adults in whom aortic isthmus stenosis remains often of congenital origin. (Level of Difficulty: Intermediate.)
Key Words: aorta, aortic coarctation, atherosclerosis, hypertension, thrombosis
Abbreviations and Acronyms: BP, blood pressure; COA, coarctation of the aorta; CT, computed tomography; LV, left ventricle; RH, resistant hypertension; TA, Takayasu’s arteritis
Central Illustration
History of Presentation
A 48-year-old woman presented with rapidly severe resistant hypertension (RH) despite a quintuple antihypertensive medication, associated with mild symptoms of dyspnea, weakness, and reduced arterial pulses of both lower limbs.
Learning Objectives
-
•
To identify acquired obstruction of the aortic isthmus.
-
•
To learn about localized giant atheroma.
-
•
To make differential diagnosis of RH in young adults.
-
•
To hierarchize investigations in the decision-making process.
Medical History
There was a history of postpartum high blood pressure (BP), complicated by immunoglobulin M glomerular nephropathy (at age 24 years), which was treated with cortisone for 2 years. There were 2 more uneventful pregnancies, 3 and 7 years later. Some difficulties to control hypertension with a single medication since her first pregnancy was reported. However, it was only a few weeks before hospital admission that her BP was destabilized despite an increase in treatments by her physician. She was also diagnosed with essential hyperlipidemia (no familial hypercholesterolemia) approximately 4 months before the clinical worsening. The low-density lipoprotein cholesterol was controlled at 70 mg/dL on a moderate dose of atorvastatin. She has a body mass index of 32 kg/m2 with a past 20-pack-year tobacco use. On admission, her left brachial BP was 150/61 mm Hg, under quintuple antihypertensive medication (nicardipine, urapidil, bisoprolol, irbesartan, and hydrochlorothiazide).
Differential Diagnosis
The differential diagnosis included severe hypertension due to RH, congenital undiagnosed coarctation of the aorta (COA), thrombotic pathology of the aorta possibly aneurysmal, progressive glomerulopathy, or renal insufficiency.
Investigations
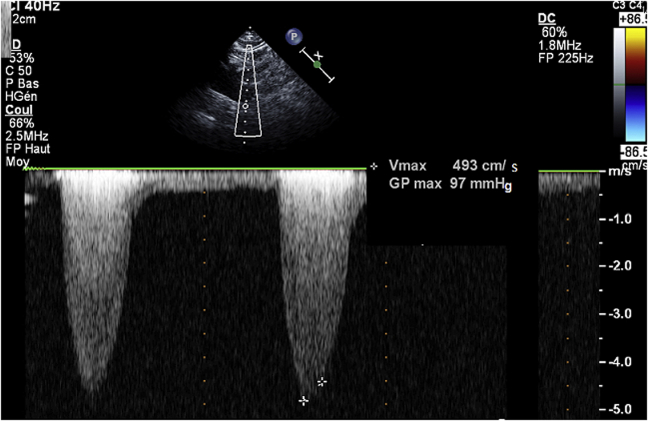
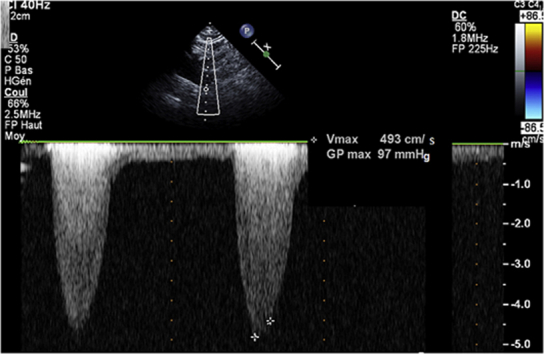
On examination, there was a 3/6 systolic murmur over the left parasternal region and lower extremity pulses were diminished. Electrocardiogram was normal. Transthoracic echocardiography demonstrated turbulence flow at the origin of the descending aorta with a continuous Doppler pressure gradient of 97 mm Hg, without diastolic tail (Figure 1). The left ventricle (LV) cavity was not dilated, with normal ejection fraction and wall motion. The LV mass was 97.2 g/m2. There was LV impaired relaxation. The right ventricle was normal, as well as the pulmonary artery pressure. There was no congenital abnormality.
Figure 1.
Echocardiogram
Continuous Doppler in the descending aorta showing a high systolic peak pressure gradient without diastolic tail.
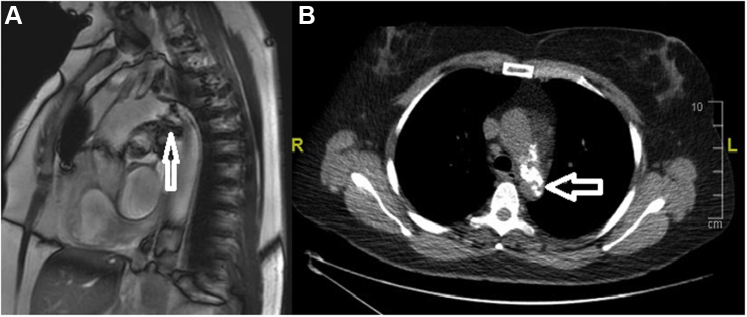
The glomerular filtration ratio and all inflammatory markers were normal. The diagnosis was made on cardiac magnetic resonance imaging and cardiothoracic computed tomography (CT) scan, which demonstrated a severe critical calcified stenosis at the level of the isthmus of the aorta (Figures 2 and 3). Mesenteric artery and celiac truncus were spared. There was no peri-aortic inflammatory change or collateral circulation. Abdominal ultrasonography showed normal kidney morphology without renal artery stenosis. Finally, a radial coronary angiography did not demonstrate significant coronary lesion.
Figure 2.
Magnetic Resonance Imaging and Computed Tomography Scan
Endovascular localized calcified subocclusion (arrows) of the aortic isthmus using magnetic resonance imaging (A) and computed tomography scan (B).
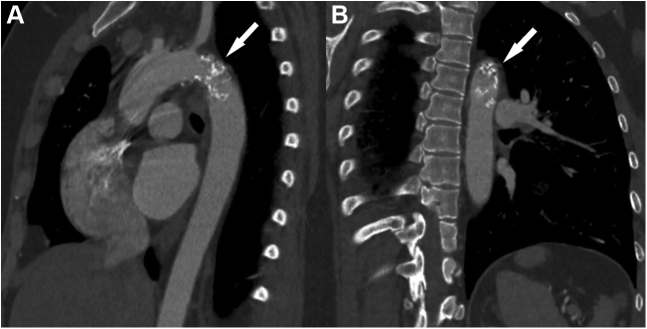
Figure 3.
3-Dimensional Computed Tomography Scan
Severe occlusion (arrows) of the aortic isthmus without coarctation on lateral (A) and posterior oblique (B) views.
Management
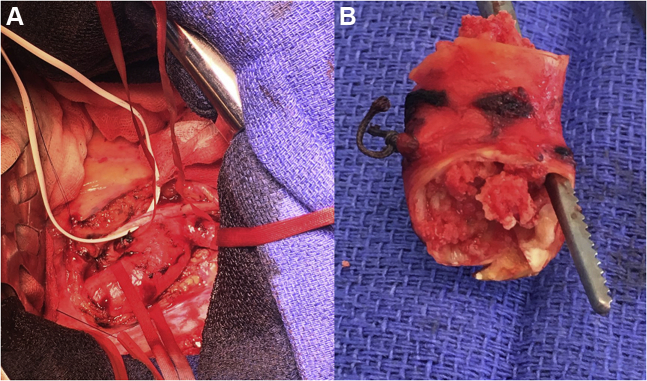
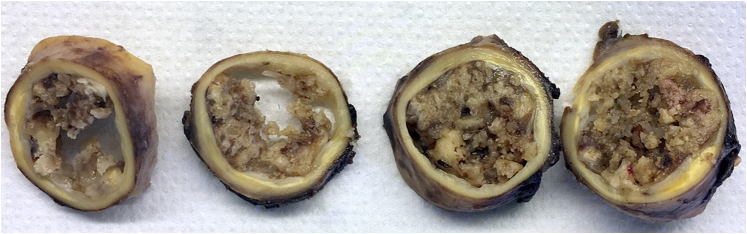
The heart team agreed for an urgent surgery, through a left lateral thoracotomy. An arterial cannula was inserted well below the isthmus of the aorta, while a venous cannula was inserted into the left femoral vein. A partial cardiopulmonary bypass was established at 3 L/min (duration 80 minutes, minimal temperature 34°C, 60 minutes of aortic clamping upstream the left subclavian artery) with a mean lower limb perfusion pressure of 60 mm Hg. The aorta was opened widely to remove ulcerated plaques and multiple friable debris, as a mixture of calcified thrombus and atheroma was causing a severe obstruction. The calcified thrombus was removed with the underlying narrowed segment of the aorta (Figure 4), which was replaced with a 23-mm tube. Weaning from the cardiopulmonary bypass, the patient was hemodynamically stable. The postoperative course was uneventful.
Figure 4.
Operating Room
(A) View of the aortic arch, left lateral thoracotomy. (B) Section of the occluded segment with residual lumen illustrated by the clamp.
Histological examination showed localized giant atheromatous lesions at the wall of the aorta, causing intima-media wall thickness (Figure 5), without lesions suggestive of a congenital origin. There was evidence of cholesterol crystals, associated with histiocytes, macrophages, and rare inflammatory elements (lymphocytes, polynuclear neutrophils), without giant cells. The aortic lumen was obstructed by calcifications.
Figure 5.
Histologic Examination
Giant atheromatous lesions at the wall of the aorta with intima-media wall thickness.
Discussion
Our patient presented with a rapidly evolving RH. Characteristics such as mild obesity, high baseline BP, and female sex might places her at increased risk of RH, which is often multifactorial.1,2 However, the differential diagnosis of RH usually requires further investigation.2 The patient was previously diagnosed with nephropathy; however, no specific investigation had ever been carried out to eliminate congenital COA, yet often associated with RH in young adults.2 In this observation, COA was suspicious from the clinical examination, whereas CT, which is one of the imaging modalities used for the treatment planning, demonstrated a critical localized stenosis. Localized obstruction in a suprarenal aorta of normal diameter caused by an eccentric calcified lesion is exceptional in the literature.3 Rare cases of acquired narrowing of the descending aorta due to coral reefs, which is a different entity, have also been mentioned with multiple calcifications. Based on rare reports, clinical presentation might then include lower limb claudication, signs of visceral ischemia, LV hypertrophy, or heart failure.3,4
Any patient who would justify rapid increase in antihypertensive medication should always have calculation of a differential BP between upper and lower extremities or an ankle-brachial index, as well as a palpation of lower limb pulses.
In case of fixed aortic obstruction, like in our patient, 2 compensatory mechanisms can counteract for the increased LV afterload: LV hypertrophy (with anatomic and electrical remodeling) or aortic collateralization. Interestingly, both mechanisms were absent, and this might point to an acute process. Thus, our report highlights the absence of LV hypertrophy, which in adults with RH, should never exclude an acquired physiologic mimic of COA. The absence of either collateralization or congenital aortic wall lesion on histological examination appear also discordant with a congenital etiology.
Concentric vessel aortic wall thickening with fibrosis and thrombus formation may be due to an atheromatous process or to a vasculitis. Giant cell arteritis and polyarteritis nodosa share similar pathogenesis and imaging features. Giant cell arteritis has a predilection for the carotid arteries, whereas polyarteritis nodosa frequently involves gastrointestinal, renal, and mesenteric vessels. Takayasu’s arteritis (TA) is a chronic granulomatous inflammation that affects large vessels, mainly the aorta and its branches, causing occlusive or ectatic vessel changes that should enter in the differential diagnosis.5 Acquired obstruction of the aortic isthmus can be associated with specific aortitis, like TA, nonspecific aortitis, fibromuscular dysplasia, mycotic aneurysms, or severe atherosclerosis. Both atherosclerosis and TA can lead to calcified atheroma of a large vessel. However intimal changes are more pronounced in atherosclerosis, in comparison with TA, in which inflammatory changes are more pronounced in the adventitia and media.6 Moreover, the patient’s history was significant for hypertension, hyperlipidemia, and smoking, which are all associated with atherosclerosis.
The patient’s final diagnosis is an acquired obstruction of atherosclerotic etiology. Such extremely rare case presenting with a rapidly evolving RH due to acquired atheroma, advocate for a systematic CT scan in any case of abnormal findings on echocardiography.
Follow-Up
At the 1-year follow-up visit, our patient’s BP is well controlled with 2 antihypertensive drugs. Echocardiogram demonstrated no residual gradient.
Conclusions
The diagnosis of acquired obstruction of the aortic isthmus should always be considered, especially in all young adults with RH not explained by other etiologies. Early management is crucial to prevent complications. Estimating routinely in all hypertensive patients the BP in upper and lower extremities is recommended. In any suspicious case, CT scan of the aorta should be considered.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors acknowledge Ms Mélanne Ghahraman and Mrs Maria Minassian for their assistance with manuscript proofreading.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Muxfeldt E.S., Chedier B., Rodrigues C.I.S., Muxfeldt E.S., Chedier B., Rodrigues C.I.S. Resistant and refractory hypertension: two sides of the same disease? J Bras Nefrol. 2019;41(2):266–274. doi: 10.1590/2175-8239-jbn-2018-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey R.M., Calhoun D.A., Bakris G.L., et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53–e90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakattu S., Murtaza G., Dinesh S., Sivagnanam K., Schoondyke J., Paul T. Supersized atheroma causing acquired coarctation of aorta leading to heart failure. J Investig Med High Impact Case Rep. 2017;5(1) doi: 10.1177/2324709616689477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palcau L., Gouicem D., Cameliere L., Berger L. Calcified obstructive disease of the aortic arch. Interact Cardiovasc Thorac Surg. 2014;18(5):683–684. doi: 10.1093/icvts/ivt503. [DOI] [PubMed] [Google Scholar]
- 5.Espinoza J.L., Ai S., Matsumura I. New insights on the pathogenesis of Takayasu arteritis: revisiting the microbial theory. Pathogens. 2018;7(3):73. doi: 10.3390/pathogens7030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma B.K., Jain S., Suri S., Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54(Suppl):S141–S147. doi: 10.1016/s0167-5273(96)88783-3. [DOI] [PubMed] [Google Scholar]