Abstract
Background:
Neisseria gonorrhoeae culture is required for antimicrobial susceptibility testing, but recovering isolates from clinical specimens is challenging. Although many variables influence culture recovery, studies evaluating the impact of culture specimen collection timing and patient symptomstatus are limited. This study analyzed urogenital and extragenital culture recovery data from Centers for Disease Control and Prevention’s Strengthening the US Response to Resistant Gonorrhea (SURRG) program, a multisite project, which enhances local N. gonorrhoeae culture and antimicrobial susceptibility testing capacity.
Methods:
Eight SURRG jurisdictions collected gonococcal cultures from patients with N. gonorrhoeae–positive nucleic acid amplification test (NAAT) results attending sexually transmitted disease and community clinics. Matched NAAT and culture specimens from the same anatomic site were collected, and culture recovery was assessed. Time between NAAT and culture specimen collection was categorized as same day, 1 to 7 days, 8 to 14 days, or ≥15 days, and patient symptoms were matched to the anatomic site where culture specimens were collected.
Results:
From 2018 to 2019, among persons with N. gonorrhoeae–positive NAAT, urethral infections resulted in the highest culture recovery (5927 of 6515 [91.0%]), followed by endocervical (222 of 363 [61.2%]), vaginal (63 of 133 [47.4%]), rectal (1117 of 2805 [39.8%]), and pharyngeal (1019 of 3678 [27.7%]) infections. Culture recovery was highest when specimens were collected on the same day as NAAT specimens and significantly decreased after 7 days. Symptoms were significantly associated with culture recovery at urethral (P = <0.0001) and rectal (P = <0.0001) sites of infection but not endocervical, vaginal, or pharyngeal sites.
Conclusions:
Culture specimen collection timing and patient symptomatic status can impact culture recovery. These findings can guide decisions about culture collection protocols to maximize culture recovery and strengthen detection of antimicrobial-resistant infections.
Neisseria gonorrhoeae, the causative agent of gonorrhea, has progressively acquired resistance to each class of antibiotics used for treatment over the last 80+ years.1 Currently, ceftriaxone, an injectable extended-spectrum cephalosporin, is the only antibiotic recommended for treatment of uncomplicated gonorrhea in the United States.2 However, susceptibility to ceftriaxone is decreasing in some regions of the world,3,4 and the threat of untreatable gonorrhea is a major public health concern.5
Over the past decade, nucleic acid amplification tests (NAATs) have become the primary method for diagnosing gonorrhea owing to their high sensitivity and specificity.6 The increased use of commercial NAATs has resulted in a decline of N. gonorrhoeae culture collection by clinicians and reduced laboratory capacity to isolate N. gonorrhoeae. However, culture is still required to assess antimicrobial resistance (AMR) in gonorrhea treatment failure cases and AMR surveillance programs such as the Centers for Disease Control and Prevention’s (CDC) Gonococcal Isolate Surveillance Project7 and Strengthening the US Response to Resistant Gonorrhea (SURRG).8
For clinics and laboratories that continue to culture N. gonorrhoeae, many variables influence culture success. From the clinical perspective, the anatomic site of infection impacts N. gonorrhoeae culture recovery, with reduced culture sensitivity from endocervical9,10 and extragenital infections.11,12 In addition, limited data suggest that symptomatic urogenital infections result in more positive culture specimens compared with asymptomatic infections in men and women.9,11 Unfortunately, the relationship between symptomatic extragenital infections and N. gonorrhoeae culture recovery has not been resolved.11,13 A less studied variable is the time between NAAT and culture specimen collection. Culture specimens may not be collected at the initial clinic visit because of a number of reasons (e.g., no patient symptoms, limited resources, clinic workflow). In a recent analysis, collecting culture 7 days after NAAT specimens was shown to negatively impact N. gonorrhoeae culture recovery,14 but this analysis was not stratified by anatomic site of infection.
Antimicrobial susceptibility testing of N. gonorrhoeae isolates is critical for patient care and public health surveillance and requires clinics and laboratories to maintain culture capacity. However, the effects of delayed culture collection and patient symptom status on culture recovery are unclear. Better understanding the influence of these variables on culture recovery can help inform clinical decision on resource allocation, time management, and culture specimen collection protocols. Therefore, the objective of this study was to examine the impacts of anatomic site, time between NAAT and culture specimen collection, and symptom status on N. gonorrhoeae culture recovery.
METHODS
For this analysis, we used data from 2018 to 2019, collected from 8 grantees of the CDC’s SURRG program.8 Grantees included California (San Francisco County), Colorado (Denver County/Denver), Indiana (Marion County/Indianapolis), Hawaii (Honolulu County/Honolulu), New York City, North Carolina (Guilford County/Greensboro), Washington (King County/Seattle), and Wisconsin (Milwaukee). As part of the SURRG program, health departments partnered with sexually transmitted disease (STD) clinics and other community healthcare facilities that frequently diagnose gonorrhea (e.g., emergency departments, HIV care providers, Planned Parenthood health centers) to collect culture specimens from patients of all genders and perform antibiotic susceptibility testing on isolates from any positive cultures.
Specimen Collection
Gonorrhea diagnosis via NAAT testing was performed with Aptima Combo 2, Roche cobas 4800 CT/NG, and Abbott m2000 RealTime CT/NG assays. Nucleic acid amplification test specimens included urine, urethral, vaginal, endocervical, rectal, and pharyngeal swabs. For antimicrobial susceptibility testing, N. gonorrhoeae culture was collected from urethral, vaginal, endocervical, rectal, and pharyngeal anatomic sites. In addition, one grantee (Washington) collected 19 urine specimens for culture. Culture specimen collection methods varied by clinic, although most grantees used fiberwrapped swabs and nutritive transport systems or media developed for N. gonorrhoeae isolation.8 In addition, the culture specimen collection techniques and guidelines were locally developed at each participating clinic. N. gonorrhoeae culture confirmation methods also varied, but biochemical assays, such as API NH and Vitek 2 (bioMérieux, La Balme-les-Grottes, France), were most often used. Culture specimen collection criteria varied across jurisdiction. Each grantee developed criteria to maximize specimen collection–based local gonorrhea morbidity while also managing resources efficiently.
Data Collection
Patients were considered symptomatic during the initial clinic visit if they self-reported symptoms that matched the anatomic site of specimen collection, including dysuria, discharge, abdominal pain, rectal pain, and throat pain. Nucleic acid amplification test and culture specimens were sent to the local public health laboratory for processing and testing. Laboratory data, including specimen type (i.e., NAAT or culture specimen), specimen anatomic source, specimen collection date, and laboratory results, and clinical data, including symptomatic status and patient gender, from each jurisdiction were submitted to the CDC as standardized deidentified line-listed data.8
Our analytic sample included N. gonorrhoeae–positive NAAT specimens with an accompanying specimen collected for culture. Additional data included associated clinical and laboratory data for each specimen, such as the anatomic site of collection, N. gonorrhoeae culture results, time (in days) between NAAT and culture specimen collection, and reported symptoms at the site of collection. Culture recovery at each anatomic site was defined as the percent of N. gonorrhoeae–positive NAAT specimens with a corresponding N. gonorrhoeae–positive culture specimen.
Statistical Analysis
For analyses, the time between NAATand culture specimen collection was categorized as same day, 1 to 7 days, 8 to 14 days, or ≥15 days. Fisher exact or χ2 test with Yates correction were used to determine the association between culture recovery and categorical time differences between NAAT and culture specimen collection and between patient symptomatic status and culture recovery. Significant differences were defined at P < 0.05. All statistical analyses were performed using Prism Software (GraphPad, San Diego, CA).
Human Subjects Protection
The CDC’s institutional review board reviewed the SURRG protocol and determined the project to be a public health activity and not human subject research.
RESULTS
From 2018 to 2019, 13,626 specimens were collected for N. gonorrhoeae culture from 10,412 NAAT-positive patients attending clinics participating in SURRG. One hundred thirty-two (1.0%) NAAT and culture specimen pairs were excluded because of errors in culture processing, indeterminate culture identification results, or isolation of other Neisseria species. N. gonorrhoeae–positive NAAT specimens with corresponding culture specimens were most commonly collected for urethral infections (48.3%), followed by pharyngeal (27.2%), rectal (20.8%), endocervical (2.7%), and vaginal (0.99%) infections. Of these 13,494 specimen pairs, urogenital infections resulted in the highest culture recovery (urethral, 91.0%; endocervical, 61.2%; vaginal, 47.4%), whereas extragenital sites had lower culture recovery (rectal, 39.8%; pharyngeal, 27.7%) (Table 1).
TABLE 1.
Culture Recovery by Anatomic Site
| Anatomic Site | Culture Specimens from Patients with N. gonorrhoeae-positive NAAT | N. gonorrhoeae-Positive Culture Specimen(s) | N. gonorrhoeae Culture Recovery |
|---|---|---|---|
| Urethrala | 6515 | 5927b | 91.0% |
| Endocervical | 363 | 222c | 61.2% |
| Vaginal | 133 | 63d | 47.4% |
| Rectal | 2805 | 1117 | 39.8% |
| Pharyngeal | 3678 | 1019 | 27.7% |
Urethral culture specimens include 19 urine specimens, of which 5 were N. gonorrhoeae-positive.
Two hundred twenty nine N. gonorrhoeae-positive extragenital culture specimens were collected from patients with N. gonorrhoeae-positive urethral culture specimens (i.e., 132 urethral/pharyngeal, 71 urethral/rectal, and 13 urethral/pharyngeal/rectal N. gonorrhoeae-positive paired culture specimens).
Thirty four N. gonorrhoeae-positive extragenital culture specimens were collected from patients with N. gonorrhoeae-positive endocervical culture specimens (i.e., 28 endocervical/pharyngeal, 4 endocervical/rectal, and 1 endocervical/pharyngeal/rectal N. gonorrhoeae-positive paired culture specimens).
Fifteen N. gonorrhoeae-positive extragenital culture specimens were collected from patients with N. gonorrhoeae-positive vaginal culture specimens (i.e., 7 vaginal/pharyngeal, 2 vaginal/rectal, and 3 vaginal/pharyngeal/rectal N. gonorrhoeae-positive paired culture specimens).
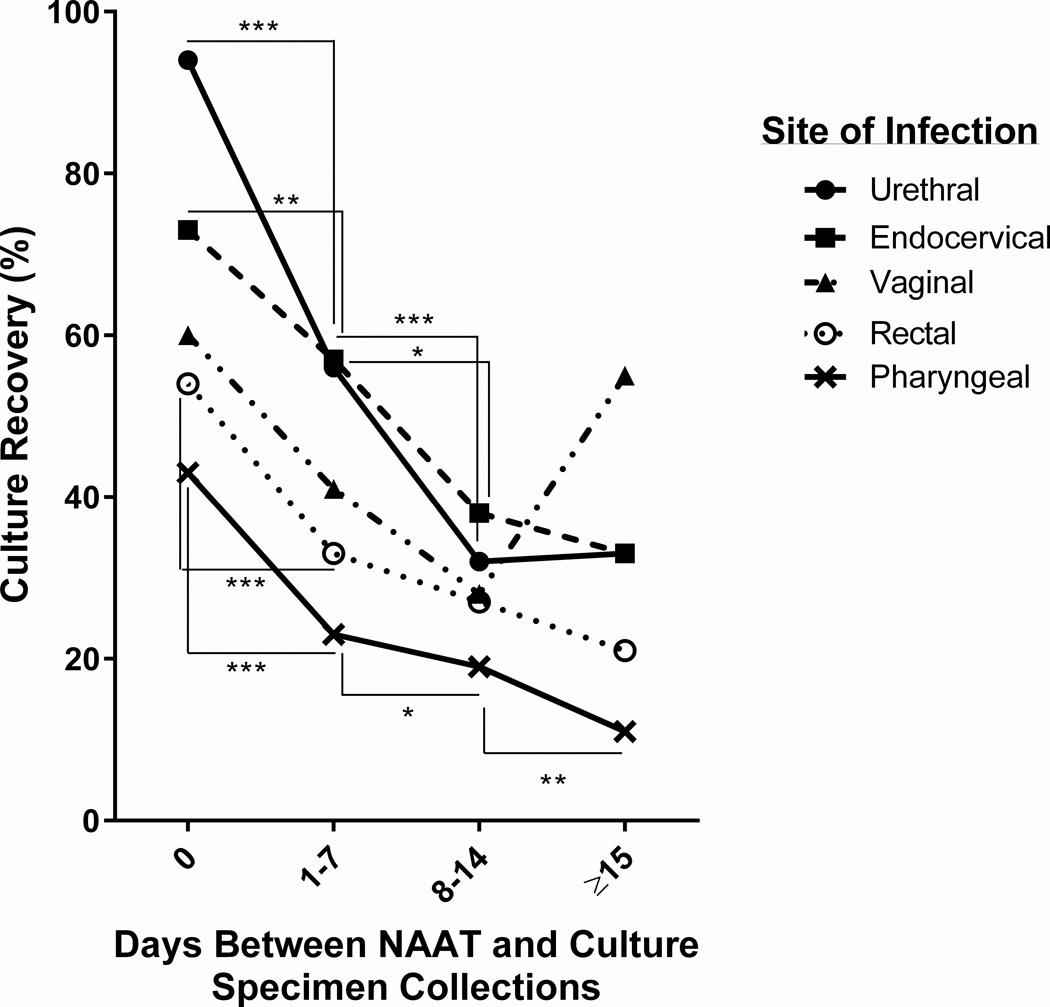
We further stratified the data by the time of culture collection. Based on local culture specimen collection criteria, a patient may be swabbed for N. gonorrhoeae culture on the same day as NAAT specimen collection, or a culture specimen may be collected at a return clinic appointment after a confirmed gonorrhea diagnosis based on NAAT results. Median time between specimen collection for NAAT and culture was 0 days (i.e., same-day collection), with an interquartile range of 0 to 5 days. Urogenital culture specimens were most frequently collected on the same day (urethral, n = 6042 [92.7%]; endocervical, n = 193 [53.2%]; vaginal, n = 53 [40.0%]), whereas the majority of extragenital culture specimens were collected between 1 and 7 days after NAAT collection (rectal, n = 1155 [41.2%]; pharyngeal, n = 1754 [47.7%]) (Table 2). Culture recovery was highest for all anatomic sites when collected on the same day as NAAT (Fig. 1). By 1 to 7 days after NAAT collection, culture recovery was significantly reduced for urethral, endocervical, rectal, and pharyngeal culture specimens compared with same-day collection. Vaginal culture recovery decreased during this time, but not significantly (P = 0.07). After 2 weeks, culture recovery was reduced by approximately 50% (range, 47.7%–65.7% by anatomic site) from same-day specimen collection across all anatomic sites.
TABLE 2.
N. gonorrhoeae Culture Recovery by Time of Culture Collection
| Time from NAAT Specimen collection to Culture Specimen Collection | Anatomic Site | N. gonorrhoeae Culture Specimen(s) Collected | N. gonorrhoeae-Positive Culture Specimen(s) | N. gonorrhoeae Culture Recovery (%) | p-value a |
|---|---|---|---|---|---|
| Same Day vs 1–7 Days | |||||
| Same Day | Urethral | 6042 | 5691 | 94.2% | <0.0001 |
| Endocervical | 193 | 141 | 73.1% | 0.009 | |
| Vaginal | 53 | 32 | 60.4% | 0.07, nsb | |
| Rectal | 1068 | 586 | 54.9% | <0.0001 | |
| Pharyngeal | 1039 | 453 | 43.6% | <0.0001 | |
| 1–7 Days vs 8–14 Days | |||||
| 1–7 Days | Urethral | 338 | 192 | 56.8% | <0.0001 |
| Endocervical | 91 | 52 | 57.1% | 0.04 | |
| Vaginal | 46 | 19 | 41.3% | 0.31, ns | |
| Rectal | 1155 | 381 | 33.0% | 0.06, ns | |
| Pharyngeal | 1754 | 410 | 23.4% | 0.05 | |
| 8–14 Days vs ≥ 15 Days | |||||
| 8–14 Days | Urethral | 96 | 31 | 32.3% | 0.88, ns |
| Endocervical | 55 | 21 | 38.2% | 0.80, ns | |
| Vaginal | 25 | 7 | 28.0% | 0.22, ns | |
| Rectal | 401 | 112 | 27.9% | 0.06, ns | |
| Pharyngeal | 659 | 129 | 19.6% | 0.009 | |
| ≥15 Days | Urethral | 39 | 13 | 33.3% | |
| Endocervical | 24 | 8 | 33.3% | ||
| Vaginal | 9 | 5 | 55.6% | ||
| Rectal | 181 | 38 | 21.0% | ||
| Pharyngeal | 226 | 27 | 11.9% |
Culture recovery data from consecutive time categories was analyzed with Fisher’s exact test or Chi Square test with Yate’s correction where applicable.
not significant
Figure 1.
N. gonorrhoeae culture recovery decreases overtime. Culture recovery was calculated for urethral, endocervical, vaginal, rectal, and pharyngeal culture specimens collected on the same day (0), 1 to 7 days, 8 to 14 days, or ≥15 days after the NAAT specimen. Statistical differences between time points were determined by Fisher exact test or Chi-square test with Yates correction where applicable. ***P < 0.0001; **P < 0.01; *P ≤ 0.05.
Symptoms were reported by patients at the time of NAAT specimen collection. For each culture specimen collected, the associated symptomatic status at the cultured anatomic site was analyzed. In total, 7300 (54.1%) N. gonorrhoeae–positive NAAT specimens were collected from symptomatic infections (Table 3), and 83.3% of these infections had a N. gonorrhoeae–positive culture specimen. Alternatively, culture recovery from specimens collected at asymptomatic sites was reduced (36.7%), despite similar numbers of asymptomatic infections (n = 6169). The majority of urethral (91.1%), endocervical (62.8%), and vaginal (51.9%) infections were symptomatic, whereas 76.9% of rectal and 88.5% of pharyngeal infections were asymptomatic. χ2 Tests show that culture recovery from culture specimens collected from symptomatic urethral and rectal infections was significantly higher ( P = <0.0001) than culture recovery from asymptomatic urethral and rectal infections. However, the presence of symptoms did not influence culture recovery among endocervical, vaginal, or pharyngeal infections. When symptomatic status was analyzed by anatomic site and time of culture collection, we found that 81.3% of all symptomatic infections were urethral infections and 92.7% of urethral culture specimens were collected on the same day as paired NAAT specimens. Thus, among symptomatic infections, 76.9% were urethral infections collected on the same day as the paired NAAT.
TABLE 3.
Association Between Symptoms and N. gonorrhoeae-Positive Culture Specimens
| Anatomic Site | Symptomatic Infections, n(%) | N. gonorrhoeae-Positive Culture Specimens and Culture Recovery from Symptomatic Infections, n(CRa) | Asymptomatic Infections, n(%) | N. gonorrhoeae-Positive Culture Specimens and Culture Recovery from Asymptomatic Infections, n(CRa) | χ2, p-valueb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Sites of Infection | 7300 | (54.1%) | 6078 | (83.3%) | 6194 | (45.9%) | 2270 | (36.7%) | 3084 | <0.0001 |
| Urethral | 5933 | (91.1%) | 5469 | (92.2%) | 582 | (8.9%) | 458 | (78.7%) | 115.7 | <0.0001 |
| Endocervical | 228 | (62.8%) | 137 | (61.0%) | 135 | (37.2%) | 85 | (63.0%) | 0.19 | 0.67, nsc |
| Vaginal | 69 | (51.9%) | 38 | (55.1%) | 64 | (48.1%) | 25 | (39.1%) | 2.8 | 0.09, ns |
| Rectal | 648 | (23.1%) | 307 | (47.4%) | 2157 | (76.9%) | 810 | (37.6%) | 19.7 | <0.0001 |
| Pharyngeal | 422 | (11.5%) | 127 | (30.1%) | 3256 | (88.5%) | 892 | (27.4%) | 1.23 | 0.27, ns |
Culture Recovery
Chi-squared test of association with Yates’ correction for culture specimen positivity and symptomatic infection
not significant
DISCUSSION
Culture-independent diagnostic methods for gonorrhea diagnosis, such as NAATs, continue to predominate clinics and laboratories, diminishing N. gonorrhoeae culture capacity. When culture is performed, culture recovery is often low, with culture sensitivity ranging from 85% to 95% for symptomatic infection and only 50% for asymptomatic cases.15,16 Our limited ability to culture N. gonorrhoeae necessitates a better understanding of clinical variables that foster gonococcal culture. This study reinforces the difficulties in culturing N. gonorrhoeae, as overall culture recovery was only 83.3% in symptomatic infections and 36.7% in asymptomatic infections. By quantifying the impact of culture specimen collection timing on culture recovery, this study demonstrated that culture recovery is highest for all anatomic sites when NAAT and culture specimens are collected on the same day, and recovery decreases as time between NAAT and culture specimen collections increase.
Analysis of NAAT and culture data from the SURRG program demonstrated a high culture recovery from urogenital infections, consistent with published data.17,18 Specifically, urethral infections had the highest success for positive culture at 91.0%. Many factors contribute to this outcome. First urethral infections are often symptomatic.19 Our data confirmed this, as 91.1% of all urethral infections were self-reported as symptomatic, with patients experiencing dysuria and urethral discharge. In settings such as STD clinics where Gram stain is available, symptomatic urethral infections often trigger clinicians to perform Gram stain to quickly identify gram-negative diplococci in urethral discharge. Because this diagnostic step is performed during the initial visit, NAAT and culture specimens can be collected at the same visit. We found same-day culture collection significantly impacted culture recovery; thus, symptoms and the ability to quickly confirm gonorrhea with Gram stain (when available) influence urethral culture recovery.
Overall, symptomatic infections were significantly associated with culture-positive specimens. This association was further demonstrated in urethral and rectal infections, whereas symptoms did not seem to influence culture positivity in endocervical, vaginal, and pharyngeal infections. The lack of association between culture-positive specimens and symptomatic women may reflect the fact that symptoms in women are not specific for N. gonorrhoeae infection. Sexually transmitted infections, such as chlamydia, trichomoniasis, and bacterial vaginosis, can present with similar symptoms as gonorrhea in women.20 Although our study did not evaluate coinfections on N. gonorrhoeae culture recovery or symptomatic status, other studies support our findings and demonstrate that the presence of N. gonorrhoeae, confirmed by culture and NAAT, was equivalent in asymptomatic and symptomatic women.21 Furthermore, successful culturing of N. gonorrhoeae is not impacted by chlamydia coinfection or symptoms in women.14
More than one-third of all asymptomatic infections were successfully cultured in this study, suggesting that other variables influence culture recovery.14 One such variable demonstrated by our study was the time of culture specimen collection. Culture recovery from almost all anatomic sites was significantly reduced when culture collection was delayed by 1 to 7 days after NAAT specimen collection. Because of the well-documented difficulties of isolating N. gonorrhoeae from extragenital culture specimens,13,22 rectal and pharyngeal infections were only cultured in approximately 50% of confirmed gonorrhea infections when NAAT and culture specimens were collected on the same day. Delayed culture collection by 1 to 7 days reduced culture recovery to 33% and 23.4% in rectal and pharyngeal infections, respectively. However, culturing extragenital infections for AMR testing remains worthwhile as these sites can harbor antimicrobial-resistant N. gonorrhoeae strains.3,23 Thus, clinics should consider their local gonorrhea morbidity and high-risk populations when developing culture policies.
Vaginal culture specimens were the only specimen type to demonstrate a nonsignificant ( P = 0.07) decrease in culture recovery between same-day and 1- to 7-day collection time categories. Our data and others24,25 demonstrate that endocervical culture specimens are more sensitive than vaginal specimens, which could explain why these specimen types do not have the same culture recovery results over time. However, the percent change in culture recovery from same-day to 1- to 7-day time categories is actually greater for vaginal culture specimens (vaginal, 31.6% decrease; endocervical, 21.1% decrease). With the small sample size of vaginal culture specimens (<1% of all culture specimens), it is likely the statistical analysis could change if more vaginal culture specimens were available. In addition, when endocervical and vaginal culture specimens are combined, the culture recovery from same-day to 1- to 7-day time categories is significantly different ( P = 0.0004).
Spontaneous clearance of gonorrhea (i.e., without antibiotic treatment) is reported in 6% to 33% of cases and can occur in all genders and anatomic sites of infection.26 This phenomenon may impact culture recovery, as a delay in culture collection allows additional time for the bacteria to clear. The median time between NAAT testing and bacterial clearance was 10 days, and the time interval (in days) between initial gonorrhea diagnosis and follow-up testing was equivalent between spontaneous clearance and sustained infection.26 We observe a decline in culture recovery as early as 1 to 7 days from NAAT specimen collection, suggesting that factors other than spontaneous clearance influence culture recovery at early time points. Most symptoms (e.g., discharge, bleeding, rectal and throat pain) were not associated with spontaneous clearance, and dysuria was reported more often in patients with a sustained infection. This suggests that culture recovery from symptomatic patients may not be impacted by spontaneous clearance.
This study has several limitations related to nonstandardized methods of sample collection. First, the NAAT platform used to diagnose gonorrhea was not consistent between all jurisdictions. The Aptima Combo 2 assay was used in 7 of 8 laboratories participating in SURRG, whereas Marion County Public Health Laboratory (Indiana) performed NAAT testing on the Roche cobas 4800 and Abbott m2000 platforms. The Roche cobas 4800 platform has similar performance characteristics to Aptima Combo 2 assay,27 whereas the Abbott m2000 platform has demonstrated lower positive predictive agreement and similar negative predicative agreement compared with Aptima Combo 2 assay.28 This suggests that some patients with gonorrhea could have been excluded from this analysis because of false-negative NAAT results. However, Indiana contributed the median number of culture specimens (n = 1522 [11.3%]) to this study, and the impact of these missed specimens should be minimal because more than 13,000 paired NAAT and culture specimens were analyzed.
A second limitation of the study pertains to culture collection methods. Clinics participating in SURRG were not directed to use specific swabs or culture transport systems for culture collection. Fiber-wrapped swabs made from cotton, polyester, calcium alginate, or rayon were commonly used, but some clinics used flocked swabs for culture collection. Within a jurisdiction, swab type could vary by clinic or by site of infection, that is, urethral versus extragenital infections. The CDC recommends dacron- or calcium alginate–tipped swabs, as cotton tips might inhibit N. gonorrhoeae.29 However, it is unclear the extent to which different fibers interfere with growth. In addition, various culture transport systems were used to maintain N. gonorrhoeae viability after collection. Most clinics used nutritive transport systems such as InTray (Biomed Diagnostics, White City, OR) or MTM-JEMBEC (BD, Franklin Lakes, NJ), but nonnutritive swab preservatives such as CultureSwab Plus (BD) were also common. Studies have demonstrated higher N. gonorrhoeae culture recovery from nutritive transport systems,30 but these require more technical skill to properly inoculate the culture plate, which could impact culture recovery if performed incorrectly. Additional studies are needed to determine which N. gonorrhoeae transport system best supports clinical culture specimens.
A third limitation is the use of nonstandardized, locally developed culture criteria based on the high-risk patient population and screening practices specific to each clinic. Nonstandardized specimen collection across clinics may have influenced the culture recovery we observed if a disproportionately high number of NAAT and culture specimen pairs were collected from one clinic. For example, the STD clinic in New York City collected 62.2% of all urethral NAAT and culture specimen pairs. This clinic uses fairly restrictive culture criteria for symptomatic patients, as only the anatomic site(s) at which patients are symptomatic are cultured. Because we determined that symptomatic urethral infections are significantly associated with culture positivity, the overall culture recovery for urethral infections may have been driven by the culture protocol at New York City clinics.
In conclusion, this study shows that the time of culture collection significantly impacts the success of N. gonorrhoeae culture recovery. Clinicians are encouraged to collect culture as close as possible to NAAT specimen collection, as resources and clinical operations allow. Whether this involves the expansion of culture collection criteria at a patient’s initial visit or persuading patients with gonorrhea to return for culture collection and treatment as soon as possible will depend on the clinic’s time and resources. Efforts to maximize culture recovery will strengthen surveillance for gonococcal susceptibility and detection of antimicrobial-resistant infections.
Acknowledgments:
SURRG Working Group: Michael M. Denny, Lance Chinna, Joey Dewater, Alberto Clemente, Karen Gieseker, Karen Wendel, Elisabeth Phillips, Rose Finney, JanetArno, ChristineHeumann, Stephanie Cohen, Robert Kohn, Trang Nguyen, Madeline Sankaran, Lindley Barbee, Roxanne Kerani, Rushlenne Pascual, Olusegun O. Soge, Sopheay Hun, Noah Leigh, Ruthie Burich-Weatherly, Wendy Wittman, Victoria Mobley, Brandy Sessoms, Cindy Toler, Candice McNeil, Elizabeth Palavecino, Kimberly Johnson, Preeti Pathela, Zachary Perry, Erica Terrell, Tamara Baldwin, Chun Wang, Rebecca Abelman, Ruchi Pandey, Kyle Bernstein, Kim M. Gernert, Alesia Harvey, Ellen Kersh, RobertD.Kirkcaldy, Jennifer Ludovic, Jennifer Reimche, Brad Roland, Matthew Schmerer, Samera Sharpe, Lizzi Torrone, Katy Town.
Conflict of Interest and Sources of Funding:
The authors have no conflicts of interest to disclose. Strengthening the US Response to Resistant Gonorrhea activities described in this article were supported by federal Antibiotic Resistance Initiative funding and administered through the US Centers for Disease Control and Prevention’s Epidemiology and Laboratory Capacity for the Prevention and Control of Infectious Diseases Cooperative Agreement (CK19-1904).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012; 7: 1401–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyre DW, Sanderson ND, Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23:1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Golparian D, Nicholas R, et al. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Shafer WM. Antibiotic resistance in Neisseria gonorrhoeae: Origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 2011; 1230:E19–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low N, Unemo M, Skov Jensen J, et al. Molecular diagnostics for gonorrhoea: Implications for antimicrobial resistance and the threat of untreatable gonorrhoea. PLoS Med 2014; 11:e1001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkcaldy RD, Harvey A, Papp JR, et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance—the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65:1–19. [DOI] [PubMed] [Google Scholar]
- 8.Schlanger K, Learner ER, Pham CD, et al. Strengthening the US Response to Resistant Gonorrhea: An overview of a multisite program to enhance local response capacity for antibiotic-resistant Neisseria gonorrhoeae. Sex Transm Dis 2021; 48(12S):S97–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Der Pol B, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol 2001; 39: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll KC, Aldeen WE, Morrison M, et al. Evaluation of the ligase chain reaction assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine and genital swab specimens from a sexually transmitted disease clinic population. J Clin Microbiol 1998; 36:1630–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect 2009; 85:182–186. [DOI] [PubMed] [Google Scholar]
- 12.Stary A, Ching SF, Teodorowicz L, et al. Comparison of ligase chain reaction and culture for detection of Neisseria gonorrhoeae in genital and extragenital specimens. J Clin Microbiol 1997; 35:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35:637–642. [DOI] [PubMed] [Google Scholar]
- 14.Vyth R, Leval A, Eriksson B, et al. Gonorrhoea diagnostic and treatment uncertainties: Risk factors for culture negative confirmation after positive nucleic acid amplification tests. PLoS One 2016; 11:e0155017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Preventive Services Task Force. Screening for gonorrhea: Recommendation statement. Ann Fam Med 2005; 3:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra-Pladevall J, Caballero E, Roig G, et al. Comparison between conventional culture and NAATs for the microbiological diagnosis in gonococcal infection. Diagn Microbiol Infect Dis 2015; 83:341–343. [DOI] [PubMed] [Google Scholar]
- 17.Bromhead C, Miller A, Jones M, et al. Comparison of the cobas 4800 CT/NG test with culture for detecting Neisseria gonorrhoeae in genital and nongenital specimens in a low-prevalence population in New Zealand. J Clin Microbiol 2013; 51:1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wind CM, de Vries HJ, Schim van der Loeff MF, et al. Successful combination of nucleic acid amplification test diagnostics and targeted deferred Neisseria gonorrhoeae culture. J Clin Microbiol 2015; 53: 1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong JJ, Fethers K, Howden BP, et al. Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service. Clin Microbiol Infect 2017; 23:555–559. [DOI] [PubMed] [Google Scholar]
- 20.Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: A cross-sectional study. Sex Transm Infect 2014; 90:580–587. [DOI] [PubMed] [Google Scholar]
- 21.Martin DH, Cammarata C, Van Der Pol B, et al. Multicenter evaluation of AMPLICOR and automated COBAS AMPLICOR CT/NG tests for Neisseria gonorrhoeae. J Clin Microbiol 2000; 38:3544–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J Clin Microbiol 2009; 47:902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill 2011; 16:19792. [PubMed] [Google Scholar]
- 24.Bhattacharyya MN, Jephcott AE, Morton RS. Diagnosis of gonorrhoea in women: Comparison of sampling sites. Br Med J 1973; 2:748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmale JD, Martin JE Jr., Domescik G. Observations on the culture diagnosis of gonorrhea in women. JAMA 1969; 210:312–314. [PubMed] [Google Scholar]
- 26.Mensforth S, Ayinde OC, Ross J. Spontaneous clearance of genital and extragenital Neisseria gonorrhoeae: Data from GToG. Sex Transm Infect 2020; 96:556–561. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Pol B, Liesenfeld O, Williams JA, et al. Performance of thecobas CT/NG test compared to the Aptima AC2 and Viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2012; 50:2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doernberg SB, Komarow L, Tran TTT, et al. Simultaneous evaluation of diagnostic assays for pharyngeal and rectal Neisseria gonorrhoeae and Chlamydia trachomatis using a master protocol. Clin Infect Dis 2020; 71:2314–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Recommendations for thelaboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 30.Papp JR, Henning T, Khubbar M, et al. Recovery of Neisseria gonorrhoeae from 4 commercially available transport systems. Diagn Microbiol Infect Dis 2016; 86:144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]