Hypertension is recognized as a major risk factor for cognitive impairment and dementia, both dementia on vascular basis and Alzheimer disease (AD). Dementia affects approximately 50 million people worldwide, and its prevalence is expected to continue to grow each year, in part due to a lack of effective therapies. Since mid-life hypertension is associated with late-life dementia, studies elucidating the effects of antihypertensive treatments on cognition are crucial, not only focusing on the effects of blood pressure lowering for the prevention of cognitive decline, but differences between the various drug classes. Prior, although not all, studies have in fact suggested a potential benefit for renin-angiotensin system (RAS) targeting drugs.
A meta-analysis presented by Ho et al.1 in this issue extends these findings and attempts to further discern whether benefits afforded by RAS inhibiting therapies differ based on blood- brain barrier (BBB) crossing potential of RAS targeting compounds (Table 1). Their meta-analysis included 14 studies (n=12,849 participants) from 6 different countries which assessed at least one neuropsychological outcome. Non-dementia participants aged ≥50 and taking an antihypertensive drug affecting the RAS [angiotensin II receptor blockers (ARBs) or angiotensin-converting-enzyme inhibitors (ACEIs)] were selected. Based on previous literature, participants were grouped into taking either BBB-crossing or non-BBB-crossing RAS antihypertensive drugs. Neuropsychological performance on 6 measures were included: mental status, memory, language, executive function, attention, and processing speed. Two outcomes were identified: (1) baseline cognitive performance on these 6 different measures, and (2) cognitive change from baseline in all of these domains. They found that despite no baseline differences in any examined cognitive domain subjects taking BBB-crossing RAS inhibitors had better memory scores (recall) at 3-year follow-up than participants using RAS inhibitors without BBB penetrating capability. Somewhat surprisingly the opposite was true for attention. This last finding may be explained in part by uneven distribution of vascular risk factors between the 2 groups, with subjects taking BBB-crossing drugs having more vascular burden. That possibly could put this group at a disadvantage with respect to cognitive tests (attention) sensitive to cerebrovascular impairment. The fact that a significant difference was detected in memory score change in otherwise non-demented older individuals is worthy of emphasis.
Table 1.
Classification of blood-brain barrier (BBB) crossing potential of angiotensin receptor blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors by Ho et al.1
| Drug Class | Non-BBB-crossing | BBB-crossing |
|---|---|---|
|
| ||
| ARB | Olmesartan Eprosartan Irbesartan Losartan |
Telmisartan Candesartan |
|
| ||
|
ACE
inhibitor |
Benazepril Enalapril Moexipril Quinapril |
Captopril Fosinopril Lisinopril Perindopril Ramipril Trandolapril |
Meta-analysis of available data sets has advantages and disadvantages. Notable strengths of this study are its large participant sample and geographic diversity across 6 countries. The detailed examination of covariates, baseline cardiovascular risk, and patient demographics is also notable. Particularly, this study makes a case that the use of various neuropsychological tests across multiple cognitive domains will provide the field with a more sensitive analysis of cognition rather than only the global mental status measures commonly used. This is especially important given that cognitive decline is a notably slow and gradual process. Disadvantages include the inability to control patient demographics, which in this study resulted in significant differences observed between study groups. Missing information on dose prescribed and drug adherence makes comparisons difficult. Additionally, despite the geographic diversity of the studies, not all studies included participant information on race and ethnicity. This is particularly important given the racial and ethnic disparities observed in dementia diagnosis and care.2 But undoubtably the focus on BBB is the major strength of this report. The notion BBB-permeability enhances RAS drugs effects on cognition seems intuitively true, as it allows the drugs to reach brain targets behind the BBB. And that is what authors were able to confirm with respect to memory. Even more importantly this compelling discovery invites further questions: which target precisely?
Due to a lack of energy reserves, the brain is uniquely dependent on blood flow for a continuous supply of essential nutrients to maintain diverse energetic demands and to clear toxic byproducts. Adequate cerebral perfusion is critical for optimal brain health and cognitive function. Hypertension detrimentally affects both the structure and function of the cerebral vasculature, impairing the homeostatic mechanisms regulating cerebral blood flow, and increasing the brain’s susceptibility to vascular insufficiency. Thus, beneficial actions of RAS inhibitors are, at least in part, related to reduction in blood pressure. Hypertension is also associated with disruption of the BBB, an early biomarker of human cognitive dysfunction.3 However, the prevalence of BBB disruption among patients with hypertension remains unknown. Furthermore, the timing and mechanisms of BBB disruption in patients are not known. Several, but not all, animal models of hypertension present with BBB disruption, and this may be linked with angiotensin type 1 receptor (AT1) activation instead of blood pressure elevation.4 In mice receiving angiotensin II infusion, lowering BP with a combination of hydralazine and hydrochlorothiazide did not prevent the opening of the BBB. It has also been previously shown that elevating BP with phenylephrine does not induce an opening of the BBB. Furthermore, not all models of hypertension have BBB disruption (e.g. deoxycorticosterone acetate [DOCA]-salt). However, in Ang II infused mice, specifically downregulating the AT1 receptor in cerebral endothelial cells does prevent the opening of the BBB.4 These findings make a case that RAS blockade may exert beneficial actions on the brain beyond lowering blood pressure, thus raising the question whether they can be useful for prevention of cognitive decline in patients who are not hypertensive.
Brain specific effects of ARBs and ACEIs indeed suggest benefits beyond blood pressure lowering and systemic vascular benefits. In animal models, ARBs have demonstrated protection against reduced cerebral blood flow, neuroinflammation, and neuronal injury. Thus, BBB-permeable ARBs would potentially be advantageous since they can access the brain parenchyma to exert neuronal effects. Beyond its role in sympathetic activation and hormone production and release, the brain RAS also modulates microglia activation, oxidative stress, learning and memory, and anxiety behavior. Candesartan and telmisartan, the two ARBs grouped as BBB-permeable in this study, are also linked with activation of peroxisome proliferator-activated receptor-γ (PPARg), a transcription factor associated with anti-inflammatory and antioxidant regulation.5 Thus, the mechanisms by which these BBB-permeable ARBs are neuroprotective warrant further investigation.
Many of the neuroprotective and cognition enhancing actions of the RAS system are effected through angiotensin type 2 and 4 receptors (AT2 and AT4) and as discussed by the authors through the axis: angiotensin converting enzyme 2 (ACE2) - Angiotensin (1–7) - Mas receptor.1 Angiotensin II is the agonist (AT2) or substrate for the agonists of these receptors (angiotensin IV and angiotensin 1–7 for AT4 and Mas, respectively) (Figure 1). Conceivably, blocking angiotensin II production by ACEIs could abolish or reduce anti-inflammatory and neuroprotective responses, while selective inhibition of AT1 by ARBs would still leave angiotensin II available to supply the remaining RAS pathways. This is supported by the “angiotensin hypothesis”, suggesting that antihypertensives that increase activity at the AT2 and AT4 receptors provide greater neuroprotection over those that decrease activity at the same receptors.6 In support of this hypothesis, van Dalen et al. reported a reduced dementia risk after a median 7 years of follow-up in community dwelling individuals treated with Ang II-stimulating drugs as compared to Ang II-inhibiting drugs (among others ACEIs).6 However, it is important to note that there is still a paucity of information on the functional role of the AT4 receptor, especially in humans, and thus further research is warranted.
Figure 1.
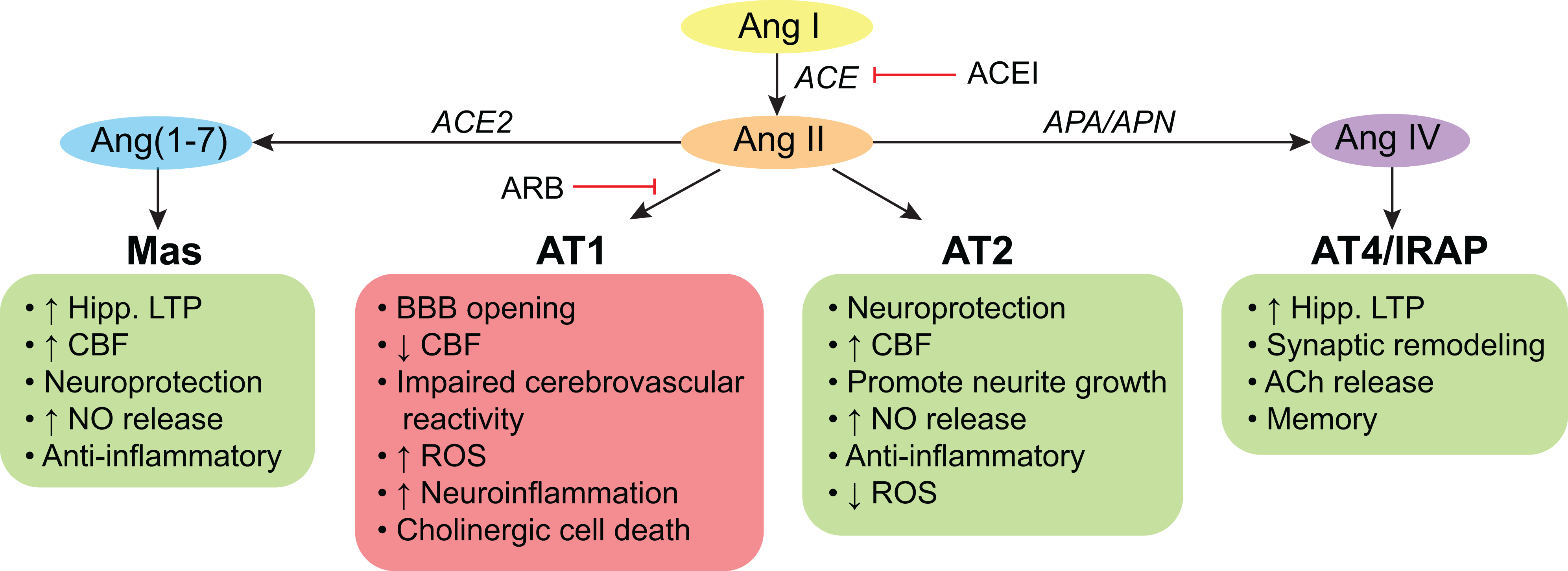
Actions of the brain renin-angiotensin system (RAS) on cognition.
Angiotensin (Ang) II and its derivatives, Ang(1–7) and Ang IV, may act on their receptors to exert effects on cognition. Signaling through the angiotensin type (AT) 1 receptor is deleterious for cognitive health, while signaling through the Mas, AT2, and AT4 receptors is beneficial. Thus, angiotensin type 1 receptor blockers (ARBs) are hypothesized to have greater benefits on cognitive health, because they do not block neuroprotective actions at other receptors, while angiotensin converting enzyme (ACE) inhibitors (ACEI) block all actions by preventing the formation of Ang II. However, not all RAS drugs have access to the brain parenchyma to exert these actions on cognition; therefore blood-brain barrier (BBB) permeable drugs are thus hypothesized to exert greater protection. ACh: acetylcholine, APA: aminopeptidase A, APN: aminopeptidase N, CBF: cerebral blood flow, Hipp: hippocampus, IRAP: insulin-regulated membrane aminopeptidase, LTP: long-term potentiation, NO: nitric oxide, ROS: reactive oxygen species.
A recent meta-analysis of 15 observational studies including over 3 million individuals showed a reduced risk of dementia in subjects taking RAAS inhibiting drugs.7 Interestingly, the beneficial effect was attributable to ARBs rather than ACEIs. ARBs reduced the risk of any dementia by 22% as compared to any antihypertensive medication, and 14% as compared to ACEIs. While their effect on AD was significant (27% reduction in risk), they did not afford the same protection against vascular dementia. These results confirm earlier post-mortem findings of marked reduction in AD pathology in hypertensive subjects treated with ARBs as compared to both any other hypertensive drugs or ACEIs.8 Furthermore, both we9 and others10 have found that in cognitively heathy older subjects ARBs, but not ACEIs use was related to less amyloid deposition as measured with PET tracers. These findings may in part be related to the reported ability of ACE to degrade beta-amyloid, so that inhibiting ACE would facilitate amyloid deposition. Will this be the case also with respect to BBB-crossing RAS inhibitors? Future studies should address whether the observed effect on memory was attributed solely to ARBs, and by doing so further refine the target of “dementia preventing” antihypertensive medication.
Sources of Funding
L.G. is a recipient of an NIH grant NS104364.
M.M. Santisteban is a recipient of the Leon Levy Fellowship in Neuroscience and NIH grant NS123507.
Footnotes
Disclosure
None.
References
- 1.Ho JK, Moriarty F, Manly JJ, et al. Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: a meta-analysis Hypertension. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawas CH, Corrada MM, Whitmer RA. Diversity and Disparities in Dementia Diagnosis and Care: A Challenge for All of Us. JAMA Neurology. 2021. doi: 10.1001/jamaneurol.2021.0285. [DOI] [PubMed] [Google Scholar]
- 3.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santisteban MM, Ahn SJ, Lane D, et al. Endothelium-Macrophage Crosstalk Mediates Blood-Brain Barrier Dysfunction in Hypertension. Hypertension. 2020;76(3):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saavedra JM. Beneficial effects of Angiotensin II receptor blockers in brain disorders. Pharmacological research. 2017;125(Pt A):91–103. [DOI] [PubMed] [Google Scholar]
- 6.van Dalen JW, Marcum ZA, Gray SL, et al. Association of Angiotensin II-Stimulating Antihypertensive Use and Dementia Risk: Post Hoc Analysis of the PreDIVA Trial. Neurology. 2021;96(1):e67–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scotti L, Bassi L, Soranna D, et al. Association between renin-angiotensin-aldosterone system inhibitors and risk of dementia: A meta-analysis. Pharmacological research. 2021;166:105515. [DOI] [PubMed] [Google Scholar]
- 8.Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Archives of neurology. 2012;69(12):1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glodzik L, Rusinek H, Kamer A, et al. Effects of vascular risk factors, statins, and antihypertensive drugs on PiB deposition in cognitively normal subjects. Alzheimer’s & dementia. 2016;2:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouk M, Wu CY, Rabin JS, et al. Associations between brain amyloid accumulation and the use of angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers. Neurobiology of aging. 2021;100:22–31. [DOI] [PubMed] [Google Scholar]


