Abstract
Significance:
Mitochondria determine glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells by elevating ATP synthesis. As the metabolic and redox hub, mitochondria provide numerous links to the plasma membrane channels, insulin granule vesicles (IGVs), cell redox, NADH, NADPH, and Ca2+ homeostasis, all affecting insulin secretion.
Recent Advances:
Mitochondrial redox signaling was implicated in several modes of insulin secretion (branched-chain ketoacid [BCKA]-, fatty acid [FA]-stimulated). Mitochondrial Ca2+ influx was found to enhance GSIS, reflecting cytosolic Ca2+ oscillations induced by action potential spikes (intermittent opening of voltage-dependent Ca2+ and K+ channels) or the superimposed Ca2+ release from the endoplasmic reticulum (ER). The ATPase inhibitory factor 1 (IF1) was reported to tune the glucose sensitivity range for GSIS. Mitochondrial protein kinase A was implicated in preventing the IF1-mediated inhibition of the ATP synthase.
Critical Issues:
It is unknown how the redox signal spreads up to the plasma membrane and what its targets are, what the differences in metabolic, redox, NADH/NADPH, and Ca2+ signaling, and homeostasis are between the first and second GSIS phase, and whether mitochondria can replace ER in the amplification of IGV exocytosis.
Future Directions:
Metabolomics studies performed to distinguish between the mitochondrial matrix and cytosolic metabolites will elucidate further details. Identifying the targets of cell signaling into mitochondria and of mitochondrial retrograde metabolic and redox signals to the cell will uncover further molecular mechanisms for insulin secretion stimulated by glucose, BCKAs, and FAs, and the amplification of secretion by glucagon-like peptide (GLP-1) and metabotropic receptors. They will identify the distinction between the hub β-cells and their followers in intact and diabetic states. Antioxid. Redox Signal. 36, 920–952.
Keywords: pancreatic β-cell metabolism, insulin secretion, redox signaling, mitochondrial Ca2+ transport, branched-chain ketoacid oxidation, fatty acid-stimulated insulin secretion, ATP-sensitive K+ channel, TRPM channels, GLP-1
Introduction
Mitochondria as metabolic and redox hub
Mitochondria have been recognized for seven decades as the metabolic and redox hub, not only providing cells with ATP but also with a plethora of metabolites and signaling mechanisms. Mitochondria cannot be ignored in the majority of studies working toward understanding physiological and pathological mechanisms at the subcellular level. For pancreatic β-cells, the ultimate physiological role of mitochondria lies in the notoriously known elevation of ATP synthesis upon glucose-stimulated insulin secretion (GSIS). However, mitochondrial redox signaling is one of its recently discovered roles (104, 194), as well as the transport of Ca2+ across the inner mitochondrial membrane (IMM), synchronized with Ca2+ oscillations evoked by action potential firing, which are caused by the predominantly intermittent opening of voltage-dependent Ca2+ channels (CaV); in rodents, these are mostly L-type channels (CaL) (31, 91, 210, 212, 213).
The additional Ca2+ release is superimposed onto the primary Ca2+ oscillations incoming from the endoplasmic reticulum (ER) (49, 280) and other Ca2+ stores, such as insulin granule vesicles (IGVs) or lysosomes. By stimulating matrix dehydrogenases and adenylyl cyclase, the elevated matrix Ca2+ plays an important amplifying role during the first and second GSIS phase and during amplification mechanisms of insulin secretion, notably in incretin- (glucagon-like peptide [GLP-1]- and gastric inhibitory peptide [GIP]-) and metabotropic receptor signaling (71, 218).
We found that insulin secretion stimulated by branched-chain ketoacids (BCKAs) (194) and partly by fatty acids (FAs) (98, 103) essentially relies on mitochondrial retrograde redox signaling. Due to the relatively low content of cytosolic glutathione (17, 135–137, 270), the redox milieu of pancreatic β-cells promotes the signal spreading from mitochondria up to the targets within the plasma membrane, which can further switch-on CaV opening and action potential firing, followed by IGV exocytosis. It is unknown whether the redox signal spreading is enabled by a H2O2 diffusion or by a redox relay, for example, via peroxiredoxins, thioredoxins, or glutaredoxins, abundant in pancreatic β-cells (97, 204). In any case, β-cells appear to be a perfect redox system, ideally suited for redox signal conduction (105, 274). Nevertheless, the redox state is highly compartmentalized (17, 208).
Also, the specific metabolism of β-cells under fasting and fed conditions contributes via changes and the concomitant effects of NADH/NADPH homeostasis, as well as via the transport of various specific metabolites, for example, coenzyme A-esters (CoA-esters) of FAs, malonyl-CoA, and long-chain acyl-CoA as stimulating insulin secretion (196), formed from the matrix acetoacetate exported to the cytosol (52).
ATP-sensitive K+ channel as prerequisite for triggering of GSIS
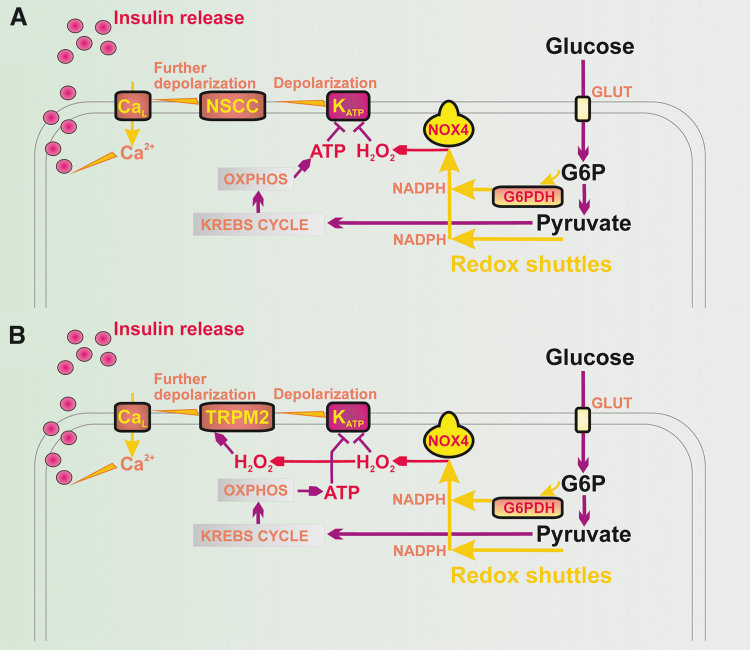
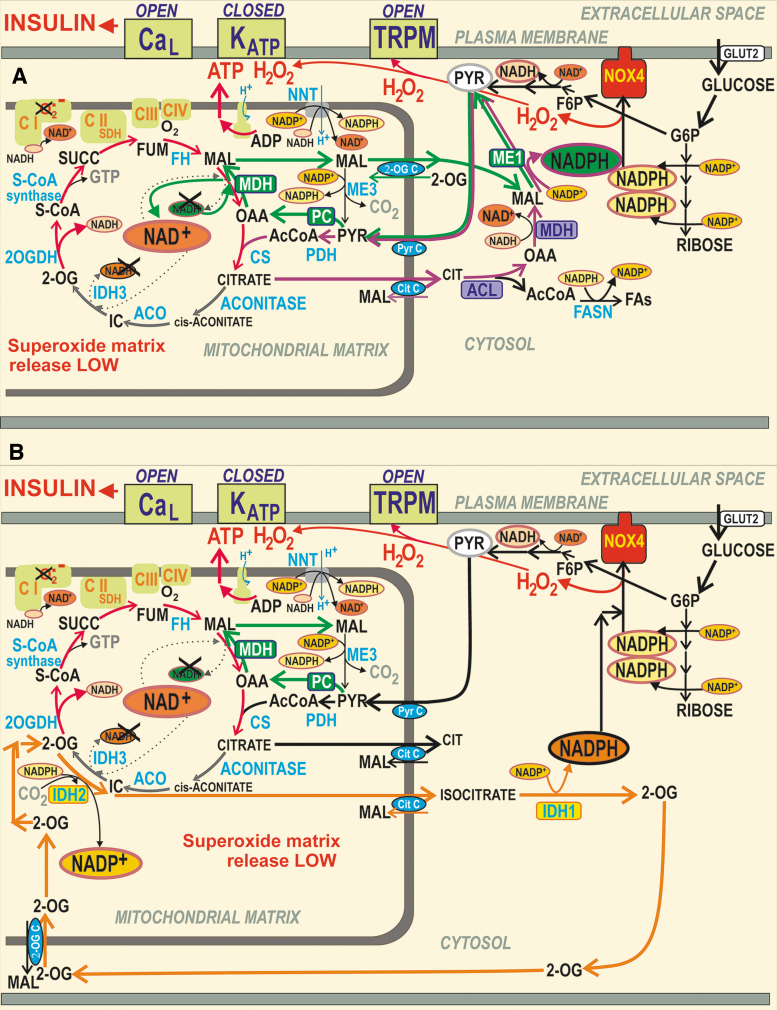
Recently, we reported that GSIS essentially relies on the physiological cytosolic redox signaling provided by H2O2 produced by NADPH oxidase 4 (NOX4) upon glucose intake, followed by the branching of the glucose-6-phosphate (G6P) flux toward the pentose phosphate pathway (PPP) (Fig. 1) (194). The two PPP enzymes produce NADPH, and the elevation of their activities causes an instant elevation of H2O2 formation by NOX4. This new paradigm of the requirement of increased ATP plus increased H2O2 for insulin secretion in response to glucose was concluded from experiments in which NOX4-knockout mice (NOX4KO) or mice with NOX4, ablated specifically in pancreatic β-cells (NOX4βKO mice), exhibited a completely suppressed first GSIS phase, whereas the second phase was only moderately attenuated (194).
FIG. 1.
Redox signaling triggers GSIS in parallel with ATP. (A) Hypothetical model based on Plecita-Hlavata et al. (194), in which only KATP is redox-regulated; KATP closure is only possible when H2O2 and ATP are elevated. (B) Extended hypothetical model, in which TRPM2 is also redox-activated. For an explanation, see the Introduction section and the Plasma Membrane Depolarization in Pancreatic β-Cells section. G6PDH, glucose-6-phosphate dehydrogenase; GSIS, glucose-stimulated insulin secretion; KATP, ATP-sensitive K+ channel; TRPM, transient receptor potential melastin.
The first phase was rescued by NOX4 overexpression in pancreatic islets (PIs) isolated from NOX4βKO mice or by H2O2 addition (194). Moreover, the ATP-sensitive K+ channel (KATP) (8) could not be closed after the glucose addition to the patch-clamped INS-1E cells silenced for NOX4 (194). In contrast, INS-1E cells having vestigial ATP synthase lacking DAPIT and thus having crippled ATP synthesis still maintained GSIS (134).
The textbook paradigm stressed the key role of glucose triggering of the first GSIS phase [reviewed, e.g., in Refs. (104, 210)]. Glycolysis followed by the oxidative phosphorylation (OXPHOS) and elevated synthesis of ATP has been considered to be the only required condition, similar to the exclusive role of KATP. In The Synergy of Membrane Channels section, we will discuss that even 100% closure of the ensemble of KATP is not enough for GSIS triggering. In contrast, certain forms of the maturity-onset diabetes of the young (MODY), that is, of monogenic type of diabetes mellitus, are exemplar cases supporting the important role of KATP.
Thus, homogeneous mutations in Kcnj11 (a gene encoding the KIR6.2 subunit of KATP, when the KIR6.2 tetramer forms the physical channel) and more heterogeneous mutations in the Abcc8 gene (encoding the regulatory subunits sulfonylurea receptor 1 [SUR1]) reduce the ability of ATP to cause channel closure (9). These mutations impair ATP binding at KIR6.2 or how ATP binding translates into the pore closure, respectively (128, 161, 232). They may enhance MgADP activation of SUR1 by increasing the affinity of the nucleotide-binding domains for nucleotides (185). Both mutations can increase the unliganded channel open probability, which leads to a decrease in both ATP and possible sulfonylurea block (11, 199). However, note also that in different MODY types different gene mutations occur (e.g., glucokinase gene GCK or genes encoding transcription factors HNF1α/4α, PDX), all affecting insulin secretion.
In this review, all the above-described aspects of the mitochondrial physiology of pancreatic β-cells will be discussed, including the “logical summation” principle of metabolic plus redox stimulation for the mitochondrial source of H2O2, which besides GSIS plays an essential role in insulin secretion stimulated by BCKAs (194) and partially by FAs (103). Without detailed knowledge of the redox system of pancreatic β-cells and their sensing of glucose or other secretagogues, the health issues that develop due to type 2 diabetes (107, 248) cannot be understood. Hence, we collected up-to-date knowledge on mitochondria as key players in the physiology of pancreatic β-cells and the pathology of diabetes.
Mechanisms of Insulin Secretion
Plasma membrane depolarization in pancreatic β-cells
The synergy of membrane channels
Quite recently, an explanation was suggested as to why a 100% closed KATP population is still insufficient to induce the threshold depolarization (−50 mV) of plasma membrane potential (Vp), required for CaV opening and thus for switching on action potential firing (119, 221). Vp should be shifted far more than enabled by the 100% KATP closure alone. This additional Vp shift can be facilitated by numerous “synergic” channels (210), namely by the opening of nonspecific calcium channels (NSCCs), such as transient receptor potential melastin (TRPM) channel-2 (TRPM2) (79, 123, 210, 285), or by the concerted action of chloride channels (45). Moreover, TRPM2 channels are activated by H2O2 (79, 84, 123, 223), hence they could theoretically also contribute to the “logical sum” of the redox plus metabolic (ATP) signal.
These “synergic” channels provide a small background inward current that cannot depolarize with an open KATP, but it is able to do so with a predominantly closed KATP ensemble since the NSCC conductance is then comparable to the small conductance provided by the remaining open KATP channels (KATP properties, Figs. 1 and 2). Also, the indirect inhibition of KATP by H2O2 was observed in smooth muscle cells (283).
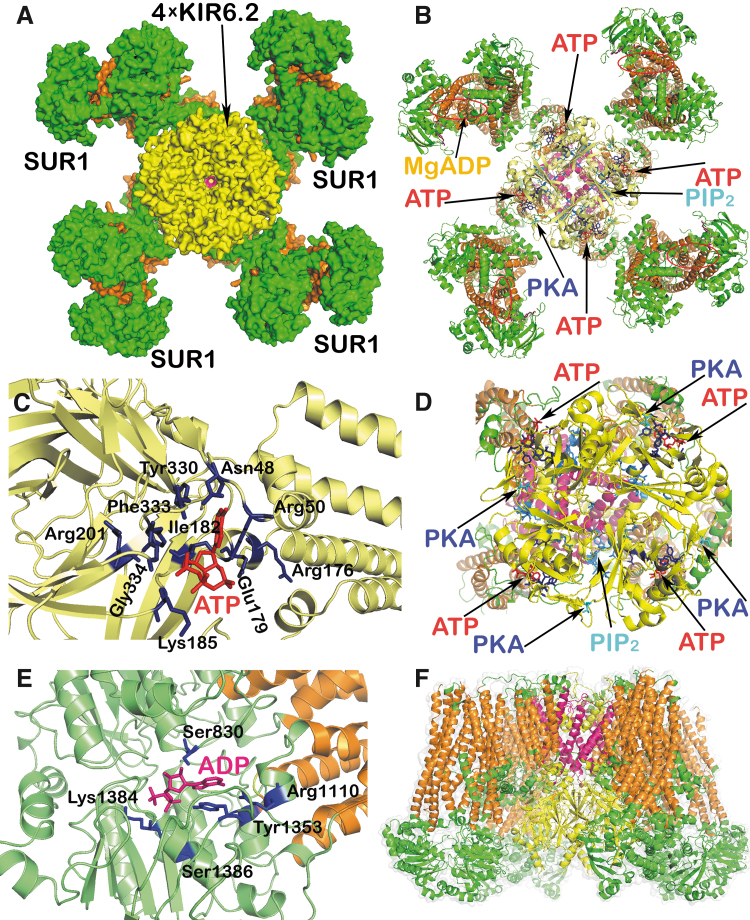
FIG. 2.
KATP channel structure and regulation. Structures of both types of subunits of hetero-octameric KATP have been resolved, that is, the SUR1 (a product of Abcc8 gene) and the pore-forming subunit, a potassium inward rectifier, KIR6.2 (Kcnj11 gene) (101, 142, 159, 210). The displayed model of KATP channel was derived from the cryo-EM structure of the pancreatic ATP-sensitive K+ channel SUR1/Kir6.2 in the presence of ATP and glibenclamide, pdb code 5twv (159), and cryo-EM structure of human KATP bound to ATP and ADP in quatrefoil form, pdb code 6c3o (131). The structure was visualized using the PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC. (A) KATP channel from the intracellular site. (B) Visualization of the ATP and PIP2 binding sites on the Kir6.2 subunits, Mg2+-ADP binding pocket on the SUR1 subunits, and PKA interaction site within the Kir6.2. (C) Detail of the binding domain for ATP (in red) on the Kir6.2 subunit with interacting amino acid residues (in dark blue). (D) Detail of the Kir6.2 with ATP and PIP2 binding domains and PKA interaction site. (E) Detail of the SUR1 Mg2+-ADP binding site (in pink) with interacting amino acid residues (in dark blue). (F) Side view of the KATP channel; color coding: intracellular regions of Kir6.2 subunits in yellow, transmembrane domains in dark pink; intracellular domains of SUR1 subunits in green, transmembrane helices in orange. Four Kir6.2-subunits cluster together, forming the core of the ∼18 × 13 nm entire structure (166). The cytoplasmic Kir6.2 surface contains the ATP-binding site, implicated in the channel closing, exposed 2 nm below the membrane. An overlapping PIP2 binding site stabilizes the open state. Upon PIP2 release, the open probability decreases (14, 166, 234). The channel is closed as soon as the first ATP-binding site is occupied, one of four ATP-binding sites (179). Sensitivity to PIP2 is regulated by the palmitoylation of Cys166 (277). Mg2+-free ATP decreases the duration of channel openings, while periods of closing are longer (36), whereas MgADP acts in the other direction (118). Artificial KATP openers (diazoxide) and KATP blockers, such as sulfonylureas (glibenclamide bound to SUR1), act independent of high ATP (233). Of the eight sites of four SUR1 subunits, each one bears an MgATP- plus MgADP-binding site. At the NBF1 of the former, MgATP is hydrolyzed to MgADP, activating KATP at NBF2 and increasing the ATP-sensitive K+-conductance. This provides lower excitability and sensitivity to ATP inhibition (179). Already 5–15 μM ATP (IC50 10 μM; ∼25 μM with Mg2+) closes the channel in inside–out patches, in which the medium affects the cytosolic side (35, 262). In contrast, in intact resting β-cells, much higher [ATP] is required to close KATP. IC50 of ∼0.6 mM was found for the whole-cell patch-clamp mode (242). This low sensitivity is adjusted by the PKA phosphorylation of Thr224 (144) and Ser372, which increases the KATP open probability (16). Any further closing only occurs at higher [ATP] or even hypothetically requires H2O2. NBF, nucleotide-binding fold; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; SUR1, sulfonylurea receptor 1.
The plasma membrane of β-cells possesses up to 60 channels belonging to 16 ion channel families (210, 280), with a distinct pattern in humans (101). Since the ∼130 mM [K+]in concentration inside the β-cell is much greater than outside ([K+]out ∼5 mM), there would be an equilibrium resting Vpequi of −82 mV, if only there was a K+-channel conductance. The actual VpResting is −75 mV (49); hence, NSCCs and other channels should provide this shift since NSCCs conduct any Na+, Ca2+, and K+. Evidence came from the observed depolarization reversal after the withdrawal of Ca2+ and Na+ at a 10 mM concentration of glucose ([glucose]) in mouse β-cells (213). Without this NSCC conductance, the established Vp would only be equal to Vpequi and the shift to −50 mV, required for CaV opening (212), would not take place, despite the 100% closed KATP ensemble (19, 119, 221, 239, 249, 280).
Besides there being a redox-activated TRPM2 channel (79, 123, 223), there are also the Ca2+- and cAMP-activated TRPM4 and TRPM5 channels in rodent β-cells (119), plus the heat-activated transient receptor potential vanilloid 1 (TRPV1, capsaicin receptor), TRPV2, TRPV4, or transient receptor potential canonical 1 and 3 (TRPC1, TRPC3) channels. TRPC3 provides an additional shift upon G-protein-coupled receptor (GPR) 40 receptor activation by FAs (276). Similarly, Cl− channels (SLC12A, SLC4A, SlC26A, GABAA, GABAB, and glycine receptor Cl−-channel) (45) and others (210) were implicated in Vp shifts, particularly volume-regulated anion channels (VRACs; e.g., the leucine-rich repeat containing 8-isoform A; LRRC8A) (45, 246). TRPM2 is also activated by nicotinic acid dinucleotide phosphate (NAADP) (247), elevated upon GSIS (160, 285). Interestingly, TRPM2 was also reported to interact with peroxiredoxin 2, from which it can receive a redox signal (168, 186).
Action potential firing begins at [glucose] > 6 mM in mouse β-cells (49), stimulated by reaching a depolarization of up to −50 mV. Above −50 mV, CaV opening [predominantly CaL with minor contribution of R-, N-, and P/Q-type Ca2+ channels (228)] is intermittent with the opening of the voltage-dependent K+ channels (KV) in mice (150) or calcium-dependent K+ channels (KCa) in humans (49) since KV (KCa) opening terminates Ca2+ entry, but their time-dependent deactivation allows a new 30–40 ms spike (210). Also, Na+ channels participate in upstrokes in a 30% β-cell population (289). Spikes return to a plateau Vp of −50 to −40 mV, the level of which is also adjusted by the two-pore K+ channels TASK-1 and TALK-1 (50, 264). At 10 mM [glucose], periods of a high and low frequency of action potential spikes exist, including burst and silent interburst phases (210).
The latter is explained by a transient ATP consumption by sarco/ER-Ca2+-ATPase (SERCA) and plasma membrane Ca2+-ATPase (PMCA), that is, ATPases removing Ca2+ (252, 253). At >20 mM glucose, ATP synthesis is thought to overcome its consumption, leading to a permanent action potential firing (49), upon which 100% of KATP channels close (210). The amplitude becomes reduced by 15 mV after ∼3 min.
The resulting pulsatile Ca2+ entry elevates the cytosolic Ca2+ concentration [Ca2+]c. The accumulated Ca2+ pool acts simultaneously on the protein exocytotic machinery and thus stimulates the pulsatile Ca2+-dependent exocytosis of IGVs (213, 214, 260). In human PIs, the threshold is −60 mV, the frequency of action potential spikes is higher, whereas 5 ms spikes are grouped into shorter ∼2 s groups and their termination upon lowering glucose is slow (211). As for the [glucose] dependence in mice, 50% of KATP closing was already reported at 3 mM, keeping Vp constant; while at 5 mM 93% and at 10 mM 97% of KATP channels were closed (251). Thus, Vp depolarization is due to the closure of remaining ∼7% KATP in mouse PIs when [glucose] is increased above 5 mM (235).
In mouse β-cells, CaV isoforms CaV1.2 and CaV1.3 are responsible for 50%, CaV2.1 for 15%, and CaV2.3 for 25% of the whole-cell Ca2+ current, which is activated at −50 mV (228). Interestingly, R-type CaV2.3 channels were reported to open exclusively during the second phase of GSIS (109). The protein kinase A (PKA) phosphorylation of CaV1.2 and CaV1.3 enhances their activity (122). Note that different groups of channels, not only CaV, are involved in action potential spikes in different species, cultured cells or even within individual cells of PIs (210), which is outside the scope of this review.
The deactivation of CaV is switched predominantly by the opening of KV2.1 in rodents (150, 213) or KV2.2 and KCa1.1 channels (BK channels) in humans (49, 101). A delayed rectifier K+ current is induced at positive Vp down to −30 mV (215). The opening of KV2.1 channels repolarizes Vp and thus closes CaV channels. The ablation of KV2.1 reduced Kv currents by ∼80% and prolonged the duration of the action potential, secreting more insulin. Mice with ablated KV2.1 exhibited lower fasting glycemia, but elevated insulin, and improved GSIS (100). Interestingly, glucose, glyceraldehydes, and 2-ketoisocaproate (KIC) were reported to increase Kv currents (284).
Ca2+ oscillations
Besides synergy with other channels, CaV opening intermittent with KV opening leads to Vp oscillations (action potential firing) (91), which induce primary oscillations in [Ca2+]c (210). The latter is further modulated by a Ca2+ efflux from the ER (49, 280), lysosomes, IGVs, or mitochondria (see the Mitochondrial Ca2+ Signaling in Pancreatic β-Cells section). However, the ER Ca2+ efflux cannot be initiated without the preceding primary CaV-mediated Ca2+ influx. The two components are superimposed, that is, fast cytosolic Ca2+ oscillations with 2–60 s periods and slow Ca2+ oscillations with periods reaching up to several minutes (15, 73). The resulting complex Ca2+ oscillations finally induce pulsatile insulin secretion. One can predict more IGVs to be secreted with a higher time-integrated cytosolic Ca2+concentration.
Basic mitochondrial contribution to insulin secretion
ATP supply and its regulation in pancreatic β-cells
Undoubtedly, increasing ATP synthesis by OXPHOS with increasing [glucose] is the first prerequisite for GSIS (152, 156). OXPHOS respiration is determined as the oxygen consumption rate (OCR). The OCR of cultured β-cells or PIs, incubated with low (insulin nonstimulating) [glucose], increases after further glucose elevation (193). Simultaneously, mitochondrial IMM potential ΔΨm also increases, indicating that the OCR increase is not due to uncoupling (protonophoric action), but stems from faster ATP synthesis, while the respiratory proton pumps are fully coupled via the protonmotive force (Δp = ΔΨm + ΔpH) to the H+ backflow through the ATP synthase.
OXPHOS can be semiquantified, when accounting for the ratio (Rr) of OCR to OCROligo. OCROligo values of nonphosphorylating respiration are set by oligomycin, blocking the ATP synthase, hence driven by the H+ leak. For rat INS-1E cells, the ratio Rr exhibits a sharp increase between 3 and 8 mM [glucose] with AC50 at ∼3.5 mM and saturation at >8 mM [glucose] in INS-1E cells (193). This AC50 roughly corresponds to the half-maxima of the surplus in the total cell ATP and in the insulin secretion rate (116). For human healthy and diabetic PIs, AC50 of 4.4 and 5.5 mM were found, respectively (47).
The parameter Ar, where Ar = (OCR − OCROligo)/OCRFCCP, follows a very similar relationship to AC50 (193), reflecting the fraction of the maximum respiration (OCRFCCP) capacity used for ATP synthesis. The extent of this sharp increase in Rr or Ar perfectly correlates with the [glucose] range for which 50%–100% closure of the KATP ensemble proceeds, despite different ranges for rat versus mice versus human PIs (see The Synergy of Membrane Channels section). With oligomycin, the closure of the KATP ensemble in INS-1E cells is incomplete (194).
When a major fraction of ATP synthase molecules are incapable of synthesizing ATP in INS-1E cells, such as upon silencing of the subunit DAPIT, GSIS is virtually unchanged, although elevations of ATP were only ∼10% of those in nontransgenic cells (134). This interpretation stems from the reasoning that the second leg required for GSIS (i.e., redox signaling) was preserved, and the established lower ATP was able, together with H2O2, to close KATP (or simultaneously open TRPM2).
With isolated rat PIs, elevations from resting 2 mM up to insulin-stimulating 4 mM [ATP] (at 10 mM [glucose]) were found (43), while AC50 at ∼3 mM was reported for the total ATP rise and 50% KATP closure in mouse PIs, not correlating with AC50 of ∼12 mM for GSIS (210). Perhaps specific AC50 for the first phase should be considered. In α-toxin-permeabilized PIs, the 84% KATP closure occurred already at 1 mM ATP (251). The perifusion of human PIs with up to 7.5 mM [glucose] leads to ∼30% of maximum GSIS (267), with insulin release observed beginning at 3 mM (89). In humans, blood glycemia of 7.5 mM stimulates a fivefold increase in insulin (266). Note that there is no sudden increase in glucose after a meal in humans, instead glycemia increases over ∼30 min from ∼5 to 8 mM (61).
Description of the diabetic phenotype is out of scope in this review [but cf. Refs. (5, 17, 107, 248)]. Type 2 diabetes etiology originates not only from the impaired molecular mechanisms of insulin secretion but also from low-grade inflammation causing insulin resistance and promoting β-cell oxidative stress, ER stress, and cell death. Pancreatic β-cells first attempt to compensate the glucotoxic metabolic demand by enhancing their mass, which also elevates insulin production. Still, their exhaustion induces further pathogenesis, impaired β-cell biogenesis, leading to dedifferentiation and dysfunction (20). This further deteriorates insulin secretion. During the β-cell mass expansion phase of the type 2 diabetes development, the first GSIS phase is often missing, whereas the second phase is enhanced and prolonged, so higher time-integrated insulin release exists. This was therefore termed hyperinsulinemia (117).
Regulations of ATP synthase by ATPase inhibitory factor 1
Searching for factors that adjust the [glucose] range to the sensing one (3–8 mM in INS-1E cells), we found ATPase inhibitory factor 1 (IF1) to be a key element (115, 116) (Fig. 3). This regulation adds to well-known settings of the sensing [glucose] range due to other factors, including the proper Km of rodent glucose transporter GLUT2/SLC2A2 (human GLUT1/SLC2A1), Km, and the lack of product inhibition for glucokinase, existing smooth fluxes of glycolysis that supply the Krebs cycle, followed by the efficient supply of substrates (NADH, succinate) for the respiratory chain and OXPHOS (227, 290). Note that glucokinase is considered a glucose sensor. This notion is supported by its importance since inactivation of both glucokinase alleles leads to the maturity-onset diabetes of the young type 2 (MODY-2). Nevertheless, this causes defective KATP regulation (205).
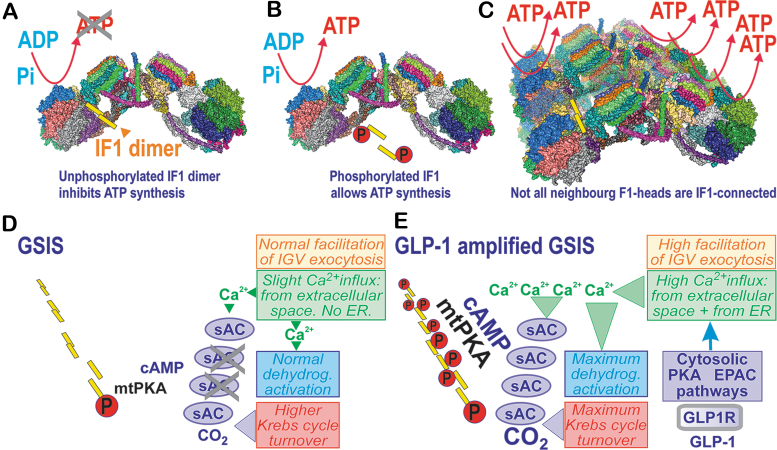
FIG. 3.
IF1 adjusts glucose-sensing concentration range being hypothetically regulated by PKA upon GSIS and its amplification by GLP-1. (A, C) Inhibitory binding of nonphosphorylated IF1 dimer within structure of ATP synthase dimer (A) and four adjacent ATP synthase dimers (C), within model of crista segment. (B) Inability of phosphorylated IF1 to bind and inhibit ATP synthase. (D) Hypothetical phosphorylation of small fraction of IF1 molecules upon GSIS, when mild activation of the soluble adenylyl cyclase within mitochondrial matrix (mt-sAC) produces proper cAMP levels, required to adjust the accurate proper IF1 phosphorylation by the mitochondrial matrix PKA. Mt-sAC is activated by the increased CO2 due to a higher Krebs cycle (glucose metabolism) turnover upon GSIS and also the concomitant cytosolic Ca2+ oscillations relayed to the matrix cause oscillations in [Ca2+]m superimposed onto the steady-state increasing [Ca2+]m levels (cf. the Contribution of Mitochondrial Ca2+ to Insulin Secretion section). (E) Hypothetical higher IF1 phosphorylation state upon GLP-1 amplification of GSIS. Here, in addition to the situation described in (D), the mt-sAC population could be more activated due to higher CO2, resulting from the additional activation of matrix dehydrogenases, which are superactivated (mt-sAC as well) by more integrally intensive [Ca2+]m oscillations superimposed onto the steady-state increasing [Ca2+]m levels. They are determined by cytosolic Ca2+ oscillations with a prolonged duration of bursts onto which the Ca2+ efflux from ER stores is also superimposed (cf. the Mitochondrial Ca2+ Homeostasis upon Receptor-Augmented Insulin Secretion section). As a result, hypothetically, a higher fraction of the matrix IF1 population should be phosphorylated and hence ATP synthesis could be more intensive. ER, endoplasmic reticulum; GLP-1, glucagon-like peptide 1; IF1, ATPase inhibitory factor 1; sAC, soluble adenylyl cyclase.
IF1 was thought to be able to only inhibit the reverse mode of the ATP synthase, in which H+ ions are pumped into the intracristal space (ICS) across the c-subunit-ring of the membrane FO moiety, whereas the energy is supplied by ATP hydrolysis to ADP ongoing at the F1 moiety. This is unlikely in primary cells; in cancer cells, this mode is mixed with the regular ATP synthesis (269).
However, evidence was found for the inhibition of ATP synthesis by IF1 in vivo (115, 116). A mild partial inhibition of a fraction of the ATP synthase (Fig. 3) may just set the proper glucose-sensing range in pancreatic β-cells. With silenced IF1 in INS-1E cells, the insulin secretion dependence on [glucose] was shifted far left with AC50 ∼1 mM (115). A similar shift with increasing [glucose] was observed for the glucose-induced surplus in total cell ATP, which was always higher in IF1-silenced cells (fivefold higher at 1 mM; about twice at 7 mM [glucose]), reflecting a mild inhibition of ATP synthesis in control cells. In contrast, the IF1 overexpression in INS-1E cells inhibited GSIS, so that the maximum saturated insulin release was about half, whereas AC50 was slightly right-shifted to 4.5 mM (116). A more profound shift to ∼7 mM was observed for the [glucose] dependence of cell surplus ATP levels, which were about halved at 1 mM and ∼25% at 7 mM glucose (116). These results reflected the ATP synthase inhibition in vivo by the excessive (overexpressed) IF1.
Structural aspects of IF1 interaction with ATP synthase versus cristae morphology
ATP synthase dimers are organized in arrays or rows along the crista rims (Fig. 3C), while actually determining the morphology of cristae. If we can approximate the two neighboring dimers by the revealed structure of the tetrameric porcine ATP synthase (80), we can also speculate on the actual IF1 localization in vivo.
The IF1 dimers bridge the two F1 moieties, however, not those within a single ATP synthase dimer, but between two neighboring dimers (Fig. 3A–C) (80). These connections (bridges) via the dimeric IF1 are lifted above the membrane of the crista edge. The membrane at this edge is bent into a sharp rim purely due to the single FO-dimer structure. IF1 is attached to the bottom of the interface between the α- and β-subunit, where it meets with the γ-subunit of the F1 moiety (74, 80). Speculatively, one may assume that both the F1 moieties bridged with the IF1 dimer cannot synthesize ATP. In this instance, not all dimers along the ATP synthase row of dimers could be connected by the IF1-IF1 bridges since if this was the case, no ATP synthesis could exist; all F1 moieties would be inhibited.
Moreover, the IF1 dimerization is prevented when IF1 is phosphorylated on Ser39 by PKA (68, 69). Also, fast degradation via the factor IEX1 was reported (230). Therefore, not only the regulation of IF1 expression versus degradation (53) but also PKA signaling provides fine-tuning of ATP synthesis. We hypothesize that the multifaceted natural regulation of IF1 and/or all ATP synthase subunits (including mtDNA-encoded) sets the proper activity within the ensemble of ATP synthases, which provides the properly adjusted rate of ATP synthesis in pancreatic β-cells. This complex regulation predetermines the glucose sensing that starts between 3 and 4 mM (strictly dependent on the elevated NOX4-redox signal) in INS-1E cells or isolated mouse PIs.
When the fraction of phosphorylated IF1 increases within the matrix, an even higher rate of ATP synthesis can be achieved, as simulated by IF1 silencing or additions of dibutyryl-cAMP, which increased cytosolic ATP levels (115). Also, GSIS was upregulated after the dibutyryl-cAMP treatment, but the upregulation ceased in IF1-knockdown cells, indicating that the IF1 phosphorylation enabling higher ATP synthesis was the important component of this mechanism. The dibutyryl-cAMP treatment also compensated the suppressing effect of IF1 on the cytosolic ATP and on the total released insulin amount (116).
Mitochondrial PKA pathways in pancreatic β-cells
PKA either phosphorylates suitable protein residues exposed to the cytosolic face of the outer mitochondrial membrane (OMM; PKAOMM) or even proteins of the mitochondrial matrix (mtPKA) (78, 292). The latter implies the existence of sensors leading to cAMP signaling in the matrix (187, 287). Thus, adenylyl cyclase mt-sAC (soluble adenylyl cyclase), phosphodiesterase mtPDE2A2 (2), and also mtPKA (286), were identified to be localized in the matrix. Indeed, the GPR receptor activator forskolin induced the phosphorylation of matrix proteins, such as IF1 (68, 69). cAMP cannot freely diffuse into the matrix, and no cAMP carrier is known (2); hence, the matrix cAMP pool is independent of the cytosolic one (44, 46). The ICS-localized or peripheral intermembrane space-localized PKAIMS might phosphorylate the Complex IV COXIV-1 subunit, which prevents its inhibition by ATP and hence enhances respiration and OXPHOS (39). For PKAIMS, one could expect the cytosolic cAMP to penetrate at least to the peripheral intermembrane space.
Matrix mt-sACs are hypothetically activated by elevated matrix Ca2+, while experiments reported mt-sAC activation by bicarbonate, which increased matrix cAMP (34, 132). Nevertheless, no mtPKA activation under these conditions was found (132). Since CO2 is increasingly released when the Krebs cycle turnover is elevated, mt-sAC activation could occur upon the metabolic stimulation of insulin secretion. Similarly, increasing responses of matrix [Ca2+]m to cytosolic Ca2+ oscillations and Ca2+ efflux from the ER (Fig. 3D, E) may activate the matrix mtPKA (3), the existence of which was found in Drosophila (286). Thus, OXPHOS is facilitated in the mitochondria of numerous tissues due to the Hsp70-mediated import of the NDUFS4 subunit of Complex I, initiated by phosphorylation, as well as by the phosphorylation of IF1 (69, 115, 116). The observed release of the PKA catalytic subunits by the increased ROS is also noteworthy (216, 243).
Mitochondrial Ca2+ Signaling in Pancreatic β-Cells
Contribution of mitochondrial Ca2+ to insulin secretion
Stimulation of matrix dehydrogenases and OXPHOS machinery by mitochondrial Ca2+
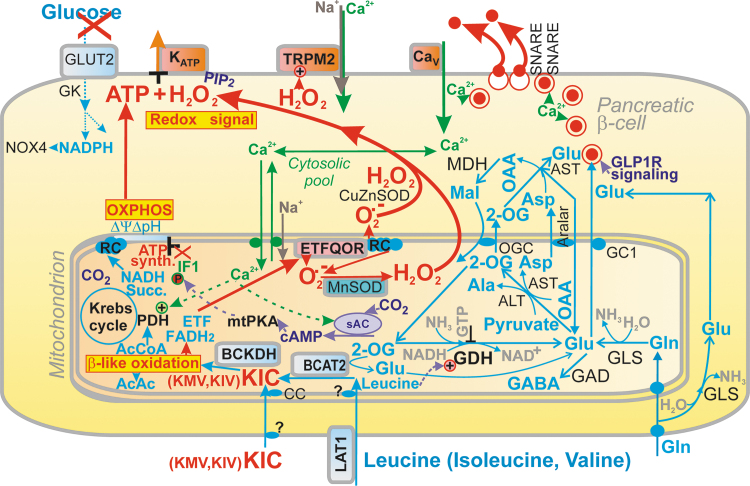
The stimulation of matrix dehydrogenases upon GSIS is one of the most plausible benefits provided by the Ca2+ influx into the matrix via the mitochondrial calcium uniporter (MCU) complex (41, 70, 71) (Fig. 4). The FAD-glycerol-3-phosphate dehydrogenase, localized on the outer IMM surface, is then instead influenced by the cytosolic Ca2+ penetrating into the intramembrane space or ICS (4, 165, 219, 252). Ca2+ activation was also reported for mt-sAC (34, 44, 46), which hypothetically leads to the phosphorylation of IF1 (69, 115, 116) by a putative matrix mtPKA (3, 132). mtPKA releases the IF1-mediated inhibition of the ATP synthase, thus enhancing ATP synthesis (69). A link to Ca2+ was suggested for the observation of 50% GSIS suppression upon ablation of the GTP-providing succinyl-CoA (S-CoA) synthetase, whereas the ablation of its ATP-providing form accelerated GSIS (126). We conclude that the mitochondrial Ca2+ transport represents a key factor of GSIS dependence on mitochondria.
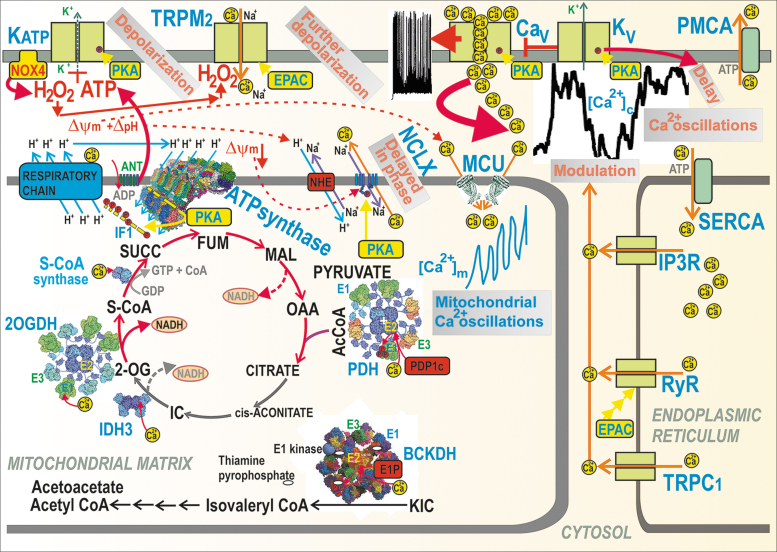
FIG. 4.
Mitochondrial versus cytosolic Ca2+ oscillations and their ability to activate matrix dehydrogenases. Action potential firing is reflected by cytosolic Ca2+ oscillations, which determine the steady-state increase in matrix [Ca2+]m with superimposed [Ca2+]m oscillations and concomitant activation of matrix dehydrogenases. Activation due to the PKA pathway is also indicated, demonstrating phosphorylation (red circles) of (i) IF1 hypothetically forming bridges (yellow) between the neighboring dimers of the ATP synthase within a row of dimers (four dimers are depicted with the indicated H+ backflow that leads to ATP synthesis); (ii) NCLX promoting activation via the ΔΨm decrease; (iii) KATP channels (setting their sensing of ATP to ∼1 mM [ATP]); (iv) CaV channels, thus activating them. Similarly, the EPAC2A pathway (“EPAC”) reportedly activates TRPM2 and RyR. The dashed arrows, pointing to NADH, illustrate the sites where NAD+ is made from NADH due to pyruvate redox shuttles (Fig. 5). CaV, voltage-dependent Ca2+ channels; EPAC, exchange proteins directly activated by cAMP; FUM, fumarate; IC, isocitrate; MAL, malate; NCLX, mitochondrial sodium calcium exchanger; RyR, ryanodine receptor; SUCC, succinate.
Mitochondrial Ca2+ transport upon GSIS
The mitochondrial matrix content of bound Ca2+ and the free Ca2+ concentration [Ca2+]m (124) are finely regulated by the ΔΨm-driven Ca2+ influx via the MCU complex (41), which is balanced by the Ca2+ influx, conducted by the Ca2+/2Na+ antiporter (mitochondrial sodium calcium exchanger [NCLX]) (38). The latter is driven by ΔpH via the Na+/H+ antiporter (plausibly NHE6/SLC9A6). Hypothetically, LETM1 may also ensure Ca2+/2H+ antiport, thus extruding Ca2+ from the matrix (202).
Mitochondrial Ca2+ participates in the first GSIS phase (70) and in GSIS potentiation by GLP-1 (71, 218). A sudden [glucose] elevation in primary β-cells induces the concomitant CaV-dependent [Ca2+]c oscillations, which are relayed to delayed steady-state increases in mitochondrial [Ca2+]m up to saturation (252, 253). The observed [Ca2+]m oscillations, superimposed onto the linearly increased [Ca2+]m, are roughly in phase with [Ca2+]c oscillations. The higher the frequency of the action potential spike within a burst, the higher [Ca2+]m amplitude was reached (252). These changes induced a biphasic increase in the ATP/ADP ratio with its second phase after 5 min (124, 252, 253).
The mechanism behind this is probably enabled by slightly retarded NCLX responses, in which Ca2+ influx exceeds the Ca2+ efflux during these transients and during the entire nearly linear [Ca2+]m increase up to saturation. The major effect of such integrally elevated [Ca2+]m is in the well-known Ca2+ activation of mitochondrial dehydrogenases (4, 164, 165, 219, 252) [doubted in Drews et al. (48)] (Fig. 4).
One cannot identify the above-described second phase in the ATP/ADP increase with the second GSIS phase, nevertheless in MCU-deficient β-cells, such a second-phase-ATP/ADP-increase was missing (252, 253). The [Ca2+]m responses were slightly shifted up upon NCLX silencing (253). Insulin release from primary β-cells, monitored using Zn2+ as a surrogate, was stimulated either by high [glucose] independent of MCU deficiency (which led to delayed responses) or by KATP closing with tolbutamide, which ceased upon MCU deficiency (252). Hence, the activation of dehydrogenases was also delayed and was probably responsible for the observed second-phase ATP/ADP increase.
The overexpression of the Ca2+-binding protein S100G in the matrix of INS-1E cells prevented [Ca2+]m increases responding to [Ca2+]c, blocked the glucose stimulation of respiration and ATP, thus reflecting the prevention of OXPHOS upon impaired [Ca2+]m responses (271). Typical [Ca2+]m elevations up to 880 nM dropped to 530 nM. In primary β-cells, S100G overexpression specifically attenuated the second GSIS phase, while the first phase did not decrease (271). This reflects a delay required for the full-extent activation of matrix dehydrogenases. In a more exaggerated way, this effect is also manifested during GSIS amplification by GLP-1 (90, 258).
Experiments suggested the essential requirement of MCU for GSIS in mice with an ablated MCU-pore, specifically in pancreatic β-cells (70). The insulin release was suppressed the first 5 min following the glucose administration, but after that, the time-integrated insulin release was equal to controls. Thus, in-phase MCU-mediated increases in [Ca2+]m concomitant with [Ca2+]c oscillations upon GSIS or GLP-1 amplification of GSIS (see the Mitochondrial Ca2+ Homeostasis upon Receptor-Augmented Insulin Secretion section) are among the precise mitochondrial machinery, which is required for optimum ATP synthesis.
The MCU complex is composed of the regulatory scaffolds MCU regulator 1 (MCUR1), the essential MCU regulator element (EMRE), and three isoforms of Ca2+-channel/sensors, termed mitochondrial calcium uptake proteins 1, 2, and 3 (MICU1,2,3) (127, 191). Mitochondrial Ca2+ transporters are well known to respond to Ca2+ released from the ER. This is reflected by the silencing of either MCU or MICU1, which reduced [Ca2+]c oscillations and respiration rates and also decreased ATP production and GSIS (4). MCU was found to be activated by kaempferol (22).
A higher ΔΨm allosterically blocks NCLX, hence Ca2+ efflux, and thus increases [Ca2+]m (129). Mechanistically, this requires the interaction of Ser258 with positively charged residues of NCLX, which is disrupted by PKA phosphorylation, hence NCLX becomes insensitive to ΔΨm, and thus active. For pancreatic β-cells, this regulation implies a low NCLX activity at low [glucose] but high activity upon insulin-stimulating [glucose] (129). Speculatively, this allosteric effect may be behind the oscillation of [Ca2+]m since each cycle of MCU-mediated Ca2+ influx may transiently or locally decrease ΔΨm, whereas the concomitant fraction of imported Ca2+ partially upregulates OXPHOS, hence adds to ΔΨm, which in turn would activate NCLX. The regulation of OXPHOS by cytosolic Ca2+ penetrating into the ICS probably occurs via the Ca2+-induced activation of Complex IV subunit Cox4.1, which disrupts its feedback inhibition by ATP (39, 114, 203). The impact on GSIS is yet to be studied.
Synchronization of cytosolic and mitochondrial Ca2+ upon GSIS
Within rodent islets, cooperation between β-cells exists. Synchronization of the electrical activity of the plasma membrane potential within the ensemble of cells in the islet results in synchronization of their cytosolic Ca2+ oscillations and other events (99, 111, 218). A few percent of pacemaker-like β-cells provides such synchronization. These cells were termed hub cells. Since it has been recognized that the second GSIS phase exists in PIs, but not in the β-cells isolated from islets, this cell cooperation was considered to substantiate the second phase. But, the delayed kinetics of the insulin granules (104) plus intercellular synchronization act in parallel.
Of course, major synchronization takes place within the individual β-cells. At first, the initial rise in ATP plus H2O2 upon elevating glucose sets the triggering event for CaV channels by closing KATP, whereas H2O2 can hypothetically also activate TRPM2 channels. With other NSCCs or other synergic channels, depolarization reaches up to the −50 mV threshold of Vp, ultimately activating action potential firing due to the intermittent opening of CaV channels and KV channels.
Second, the pulsatile Ca2+ influx from the exterior causes cytosolic Ca2+ oscillations. Specifically at intermediate [glucose], such as ∼10 mM, Ca2+ oscillations might be terminated because of the transient exhaustion of cytosolic ATP by PMCA and SERCA (252, 253), thus creating silent interburst phases (210) [see the lag between burst [Ca2+]c phases in Fig. 4—part of the records published in Plecita-Hlavata et al. (194)]. Moreover, under the activation of receptors, such as GPR (inositol-1,4,5-triphosphate receptor-diacylglycerol [IP3-DAG] signaling) or GLP-1 receptor (PKA and EPAC2 pathways), an additional amplifying Ca2+ efflux, is induced from the ER, via Ca2+ channels of TRPC1, ryanodine receptor (RyR), or IP3 receptor (IP3R). This ER Ca2+ efflux modulates and superimposes onto the existing cytosolic Ca2+ oscillations.
Third, cytosolic Ca2+ oscillations are relayed to the matrix, causing oscillations in [Ca2+]m superimposed onto the steady-state increasing [Ca2+]m levels (252, 253). This is hypothetically allowed by the in-phase delayed Ca2+ efflux mediated by the NCLX Ca2+/Na+ antiporter, behind the instantly acting MCU. NCLX is inhibited by a higher ΔΨm, but due to higher ATP synthesis and concomitant H+ backflow via the ATP synthase c-ring, ΔΨm is partially diminished, leading to NCLX activation (129). The Ca2+ uniport via MCU is driven by the ΔΨm component of the protonmotive force Δp, whereas the electroneutral Ca2+/2Na+ antiport by NCLX is driven by the Na+-gradient, established by the Na+/H+ antiporter (NHE6), which is then driven by the ΔpH component of Δp.
Fourth, besides the matrix mt-sAC, the resulting [Ca2+]m elevation activates matrix dehydrogenases (42, 164, 218) in the set pace, specifically: (i) the 8-MDa multienzyme complex of pyruvate dehydrogenase (PDH), in which Ca2+ binds to heterodimers of the E2 PDH subunit and the catalytic subunit of pyruvate dehydrogenase phosphatase (PDP1c) within the core of the complex, which leads to the PDP1-mediated dephosphorylation of E1 subunits. The PDH-complex contains a hollow core of numerous dihydrolipoate acetyltransferase subunits (E2), plus 12 E3-binding subunits (E3BP). E3BP attaches subunits of pyruvate decarboxylase (E1) and dihydrolipoate dehydrogenase (E3). Since the phosphorylated E1 causes the inhibition of the overall PDH reaction, the Ca2+-activated dephosphorylation is therefore the key event for the PDH activation (42).
(ii) BCKA-dehydrogenase (BCKDH) complex is activated by mechanism similar to (i), in which Ca2+ activation of the E1P-phosphatase occurs, which dephosphorylates the E1 subunit, which is otherwise inhibited by the phosphorylation enabled by the BCKDH-E1-kinase, and this is in turn inhibited by the cofactor thiaminepyrophosphate. Since the thiaminepyrophosphate-mediated kinase inhibition is strengthened by Ca2+, hence Ca2+ activates BCKDH (180). Moreover, there is a direct Ca2+-induced activation for (iii) Ca2+ binding to the E1 subunit of the 2-oxoglutarate dehydrogenase (2OGDH) multienzyme complex; (iv) Ca2+ binding to βγ-subunit interfaces of the hetero-octameric NAD+-dependent isocitrate dehydrogenase 3 (IDH3) (42), and (v) Ca2+ activates the GTP-producing S-CoA synthase by as yet unknown mechanism (126).
Mitochondrial Ca2+ homeostasis upon receptor-augmented insulin secretion
Signaling by GLP-1 receptor for the amplification of GSIS
Produced in intestinal L-enterocytes, GLP-1 from the bloodstream activates its receptor (GLP1R) on the plasma membrane of pancreatic β-cells (170). GLP1R activation preferentially stimulates G-proteins Gαs, but also Gαq or Gα11 (Fig. 10), and recruits β-arrestin, depending on a biased agonism differently to different agonists, such as exendin-4 and oxyntomodulin (238, 275).
FIG. 10.
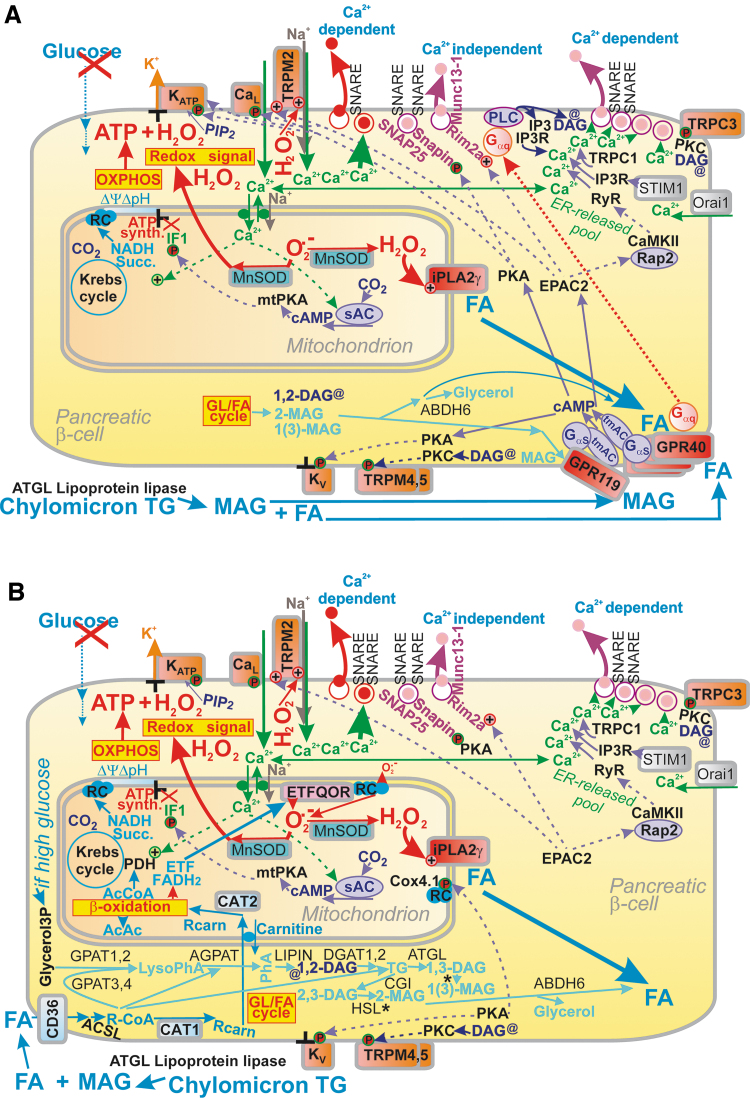
Receptor and metabolic pathways determining FASIS. (A) Receptor component of stimulation is emphasized. (B) Metabolic component is emphasized. When present in islets capillaries, for example, in incoming chylomicrons, FAs are cleaved by lipoprotein lipase (ATGL being the most specific β-cell isoform) and may either (A) act via receptor pathways to stimulate the GPR40 metabotropic receptor (94), in parallel with the second product of the cleavage, MAG, which signals via GPR119. Alternatively, (B) the metabolic pathway begins by FA import into the cell by the CD36 transporter and by ACSL conversion to acyl-CoAs. As for (A), the activation of both receptors leads to CaV-mediated action potential spikes and concomitant pulsatile insulin secretion. GPR40 acts via the Gαq/11, thus activating PLC, which leads to IP3 and DAG release (for DAG downstream pathway, see @). IP3 activates additional Ca2+ efflux from the ER via the IP3R, which is initiated either by the preceding CaV opening (95) or by PLC-TRPC-induced Ca2+ efflux from the ER (276). The most prominent pathway downstream of DAG involves the PKC-mediated phosphorylation of TRPM4 and TRPM5 to activate them. As a result, together with TRPM2, activated by Ca2+ and H2O2, these channels strengthen the necessary shift to −50 mV depolarization at the 100% closed KATP ensemble. The KATP closure is ensured by the metabolic component of FASIS (B). The two components are mutually interrelated since the canonical GPR119 signaling and the biased GPR40 signaling leads to the cAMP-mediated activation of the PKA and EPAC2 pathways (65, 187, 287). PKA phosphorylates the CaVβ2 subunit to activate it, phosphorylates KATP (see legend of Fig. 2) and inhibits Kv channels, which prolongs the already more intensive Ca2+ influx (179). Snapin, which allows IGV docking to the plasma membrane, is also PKA-phosphorylated, enabling initiation of the snapin SNARE complex with a lipid-anchored protein, the SNAP-25 (236). The EPAC2 pathway is based on its guanine nucleotide exchange activity. This induces further TRPM2 activation (285), regulates KATP (121) plus priming of the interaction of Rim2a with Munc13-1, required for the syntaxin 1 interaction of IGV [which activates IGV exocytosis (282)] and, finally, the activation of RyR- mediated Ca2+ efflux from the ER (120). FAs imported by CD36 are converted to acyl-CoAs by AcylCoA-synthetase (ACSL), whereas CAT1 converts acyl-CoAs to acyl-carnitines (207). The carnitine carrier (SLC25A20) imports acylcarnitines into the matrix, exchanging them for carnitine. The matrix CAT2 converts acyl carnitines to acyl-CoAs, which is followed by FA β-oxidation (see also Fig. 8). As described in the Mechanisms of Insulin Secretion section, all the benefits of activation also occur for mitochondrial metabolism, that is, activations upon GSIS and its receptor-mediated amplification (cf. Fig. 4). Also, similar redox signaling due to the increased superoxide formation upon FA β-oxidation occurs during the metabolic branch of FASIS, as with BCKA-stimulated insulin secretion (cf. Fig. 9). Elevated ATP from OXPHOS fortified by FA β-oxidation and elevated cytosolic H2O2 due to the increased H2O2-release from the matrix close the KATP channel (possibly also TRPM2), as they do upon GSIS. Overactivation of GPR40: pathways (A, B) are also interconnected because of the intramitochondrial redox signaling [elevated matrix superoxide/H2O2 due to FA β-oxidation (Fig. 11) directly activates mitochondrial phospholipase iPLA2γ/PNPLA8 (98, 103, 105)]. The phospholipase iPLA2γ cleaves both saturated and unsaturated FAs from the phospholipids of mitochondrial membranes. The cleaved free FAs diffuse up to the plasma membrane, where they activate GPR40 (103). FASIS in iPLA2γ-knockout mice or its isolated islets yields ∼30% insulin in the first fast phase of insulin secretion compared with wt mice (Holendová et al., unpublished data). This supports the existence of such an acute mechanism in vivo. Overactivation of GPR119: FASIS in the presence of high [glucose] (which by itself would stimulate GSIS) also involves the so-called glycerol/FA cycle combining simultaneous lipogenesis and lipolysis, as suggested by Prentki et al. (197). Enzymes involved in this cycle are described in the legend of Figure 8. An important intermediate of the glycerol/FA cycle is 1,2-DAG, which initiates PKC signaling (and TRPM4,5 activation) and activates Munc13-1 to facilitate IGV exocytosis. Moreover, created MAGs (* indicates its diffusion toward GPR119) can diffuse to the plasma membrane and overactivate the GPR119 receptor there. ABDH6, alpha/beta-hydrolase domain containing 6, monoacylglycerol lipase; CaMKII, Ca2+/calmodulin-dependent protein kinase II; FASIS, fatty acid-stimulated insulin secretion; IP3, inositol-1,4,5-triphosphate; IP3R, inositol-1,4,5-triphosphate receptor; iPLA2γ, Ca2+-independent phospholipase A2 isoform γ; Orai1, calcium release-activated calcium modulator 1; PLC, phospholipase C; Rap2, Ras-related protein 2; RC, respiratory chain; SNAP-25, synaptosomal nerve-associated protein 25; SNARE, soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor; tmAC, transmembrane adenylyl cyclase; TRPC, transient receptor potential canonical.
A scaffold protein β-arrestin promotes signaling via Gαs to cAMP, but also to CREB (238), extracellular regulated kinase ERK1,2 (169), and insulin receptor substrate 2 (IRS-2). This activates β-cell growth, differentiation, and β-cell identity maintenance (238). The major Gαs stimulation spreads signals via enhanced cAMP (65, 133, 177) and the initiation of PKA (143), plus the enhanced signaling via exchange proteins directly activated by cAMP 2A-isoform (EPAC2A) pathways (120). However, a putative cAMP-independent pathway may also exist at physiological 1–10 pM [GLP-1] (231). Prolonged cAMP production could even be induced by internalized GLP1R, which partly potentiates GSIS (255). Ex vivo, GLP-1 was found to act at a low range of stimulating [glucose] 6–7.5 mM (5–6 mM in isolated β-cells) (65, 133, 177, 231), which paradoxically is equivalent to fasting glycemia in mice.
The PKA pathway activation leads to a surplus [Ca2+]c above that of the net GSIS (i.e., without any receptor stimulation) (151). This is achieved by the phosphorylation of KV channels, leading to their deactivation. This prolongs the overall Ca2+-stimulation signals and induces somewhat lower frequencies of Ca2+ oscillations, but with each spike lasting longer (231, 265). Also, the second phase of GSIS might be potentiated by such mechanisms. Speculatively, the PKA-pathway evoked [Ca2+]c surplus may activate Cox4.1 in the ICS if the concentration therein would reflect [Ca2+]c. PKA also phosphorylates snapin, a protein of the exocytotic machinery. This promotes soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE)-complex formation by the interaction of synaptosomal nerve-associated protein 25 (SNAP-25) with synaptogamins of IGVs. Thus, exocytosis is facilitated within the first GSIS phase (236, 237).
A parallel EPAC2A pathway activation by the GLP1R signaling stimulates TRPM2 channels (285), providing the essential shift in depolarization in synergy with KATP and thus triggers the action potential firing. EPAC2A also enhances KATP closing (40, 75, 121). The EPAC2A pathway also promotes the docking and priming of IGVs by allowing Rab3A interaction with Rim2α (282); and a hypothetical interaction of EPAC2-Rim2α-Picollo trimers with Rab3A, again facilitating IGV exocytosis (254). Also, Ca2+-release is activated from the ER via the RyR-, which depends on CaV opening (120). A biased GLP1R stimulation via Gαq/11 may also stimulate Ca2+ release from the ER now via IP3R (23) and via TRPM4 and TRPM5 activation due to phosphorylation by protein kinase C (PKC) (231).
Experiments using simultaneous electrophysiological and Ca2+ oscillation monitoring (with Ca2+ fluorescent probes) found that at 2 mM glucose, but with 200 μM tolbutamide blocking KATP (265), the GLP-1 analog liraglutide decreased the frequency of action potential spikes, which became individually wider. This reflects the PKA-mediated inactivation of Kv2.1 channels. With or without liraglutide, each action potential spike matched the triangular peak of the cytosolic Ca2+ rise. Its time-width increased from 2 s to about 5 s with liraglutide (265). The relative duration versus the active phase of Ca2+ spikes was ∼10% at 4 mM, ∼50% at 7 mM, and ∼80% at 9 mM glucose (59). Earlier experiments at 7.7 mM glucose and with GLP-1(7-36)amide (preproglucagon78-107) also reported an increased duration of active and silent electrical activity (58).
Importantly, the observed delayed decay of Ca2+-responses is not only determined by the prolonged action potential spikes but is also affected by Ca2+, released from the intracellular stores, especially the ER, but also from mitochondria. The relay of these complex Ca2+-responses onto the in-phase intermittent responses of proteins of the exocytotic machinery (formation of SNARE complexes) and the resulting pulsatile IGV exocytosis were also monitored by surveying the ATP-activated currents conducted by the artificially overexpressed P2X2 cation channels (145). This was possible due to IGVs containing a high ATP concentration.
Mitochondrial Metabolism of Pancreatic β-Cells
Redox shuttles provided by several mitochondrial anion carriers
Redox shuttles
NADPH has long been considered to be a facilitator of GSIS (107, 110, 112, 183, 193, 209). However, the NADPH increase was not known to activate NOX4 upon GSIS, and PPP was thought to be inactive due to the product-inhibition of G6P-dehydrogenase (227, 290). Later, metabolomics confirmed a significant diversion of G6P flux to PPP upon GSIS (147, 240). Besides the two PPP enzymes, G6P-dehydrogenase and 6-phosphogluconate dehydrogenase, there is a contribution of three metabolic redox shuttles to the increasing [NADPH]c upon GSIS (110), complying with the high pyruvate influx into the matrix (147). We recognize the pyruvate/malate, pyruvate/citrate, and pyruvate/isocitrate shuttle (Fig. 5).
FIG. 5.
Pyruvate-based redox shuttles transfer matrix NADH equivalents to elevate cytosolic NADPH. (A) The pyruvate/malate redox shuttle (green arrows) and pyruvate/citrate shuttle (violet arrows). The pyruvate/malate redox shuttle bypasses PDH and the concomitant entry of the resulting acetyl-CoA into the Krebs cycle via the CS. This bypass exists due to the PC reaction producing OAA. Conditions upon glucose intake into pancreatic β-cells allow the reversed reaction of the matrix MDH2 that produces malate from oxaloacetate at the expense of NADH, which is converted to NAD+. That is why redox equivalents of NADH are transferred into cytosolic NADPH. The transfer is achieved by malate export via the “2-OGC” (SLC25A11) (183), where it is exchanged for 2OG, which is then imported to the matrix. The exported malate increases the cytosolic malate pool, which can be consumed by the ME1 reaction, driven by NADP+ and thus increasing the cytosolic NAPDH pool (82, 195). This reaction direction is driven by an instant return of pyruvate to the mitochondrial matrix ensured by the pyruvate carrier (MPC1 and MPC2, providing pyruvate-H+ symport). In this way, the cycle is achieved. The pyruvate/citrate shuttle is enabled by the citrate export from the matrix after the CS reaction (54). This truncated Krebs cycle has been confirmed using 13C-tracing, demonstrating that high amounts of the cytosolic citrate originate from glucose-derived acetyl-CoA (146). Citrate is exported by the citrate carrier (“Cit C”; SLC25A1), enabling citrate antiport with malate. Together with the pyruvate/malate redox shuttle, malate cycling occurs. The exported citrate is split in the cytosol by the ACL with CoA, yielding oxaloacetate and acetyl-CoA. The cytosolic isoform of MDH1 then converts oxaloacetate into malate, which is again used by ME1 to produce NADPH and pyruvate, which is finally imported back to the matrix. The ACL reaction and hence shuttle operation is minor compared with the acetoacetate pathway operating upon GSIS (52). Under low glucose conditions, levels of short-chain acyl-CoA are preserved. (B) The pyruvate/isocitrate shuttle (orange arrows) exists when the reductive carboxylation reaction of matrix IDH2 takes place. Unlike IDH3, which is the regular Krebs cycle enzyme providing NADH, IDH2 in a “forward” oxidative decarboxylation mode uses NADP+ plus citrate and produces NADPH and 2OG in the matrix. At high [glucose], such conditions are established instead to facilitate the reverse IDH2 reaction, which is the NADPH-driven reductive carboxylation of 2OG in the presence of CO2 (193). This is also facilitated by the Krebs cycle truncation, leaving a slow aconitase reaction and isocitrate formation so that the reverse IDH2 reaction occurs. The citrate carrier finally exports isocitrate to the cytosol, exchanging it for imported malate. The enhanced isocitrate pool in the β-cell cytosol is concomitantly consumed by the cytosolic IDH1, ensuring the NADP+-driven oxidative decarboxylation of isocitrate to 2OG, yielding NADPH (82). As a result, IDH1 within this shuttle contributes to another portion of the cytosolic NADPH increase upon GSIS (209). 2OG, as with the pyruvate/malate shuttle, is imported to the matrix, being exchanged for malate again by the oxoglutarate carrier. 2OG thus contributes to the mitochondrial matrix 2OG pool, consumed massively by the 2OGDH complex within the Krebs cycle. However, a portion of the matrix 2OG pool is used for another cycle of this shuttle, that is, for IDH2-mediated reductive carboxylation. 2OG, 2-oxoglutarate; 2OGC, 2-oxoglutarate carrier, mitochondrial; 2OGDH, 2-oxoglutarate dehydrogenase; ACL, ATP citrate lyase; ACO, aconitase; Cit C, citrate carrier, mitochondrial; CoA, coenzyme-A; CS, citrate synthase; F6P, fructose-6-phosphate; FASN, fatty acid synthase; FH, fumarate hydratase; IDH1, isocitrate dehydrogenase 1, cytosolic NADP+ dependent; IDH2, isocitrate dehydrogenase 2, mitochondrial NADP+ dependent; IDH3, isocitrate dehydrogenase 3, cytosolic NAD+ dependent; MDH, malate dehydrogenase; ME1, malic enzyme 1, cytosolic; OAA, oxaloacetate; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; PyrC, pyruvate carrier, mitochondrial.
Note also that metabolic pathways including metabolic shuttles are plastic, specifically when the key members are altered in their expression, such as identified upon glucotoxicity, lipotoxicity, and glucolipotoxicity in isolated human PIs (108). Therefore, the description below concerns with the most frequent schemes before and after glucose intake into intact pancreatic β-cells.
NADPH and NADH homeostasis in the cytosol and mitochondrial matrix upon GSIS
The redox shuttles, activated upon GSIS, do not allow maximum NADH to be produced in the mitochondrial matrix, but instead more NADPH is produced in the cell cytosol, representing a transfer of redox equivalents from the matrix to the cell cytosol (Fig. 5A, B). The shuttles concomitantly provide an independent, although minor NADPH source, supplying NOX4 to initiate redox signaling that enables insulin secretion upon elevated ATP. Mitochondrial malate dehydrogenase 2 (MDH2) produces less NADH, when compared with the situation of the 100% forward reaction, which proceeds at low [glucose].
Also, less NADH is produced, if isocitrate is not converted by the isocitrate dehydrogenase IDH3 to produce NADH due to the truncated Krebs cycle owing to the citrate export from the matrix, or when the concurrent reaction direction exists so that isocitrate dehydrogenase 2, mitochondrial NADP+ dependent (IDH2) is switched to the inverse (reductive carboxylation) reaction. The exported isocitrate promotes NADPH formation by the cytosolic isocitrate dehydrogenase 1, cytosolic NADP+ dependent (IDH1). Thus again, instead of one NADH molecule produced in the matrix, one NADPH molecule is formed in the cytosol.
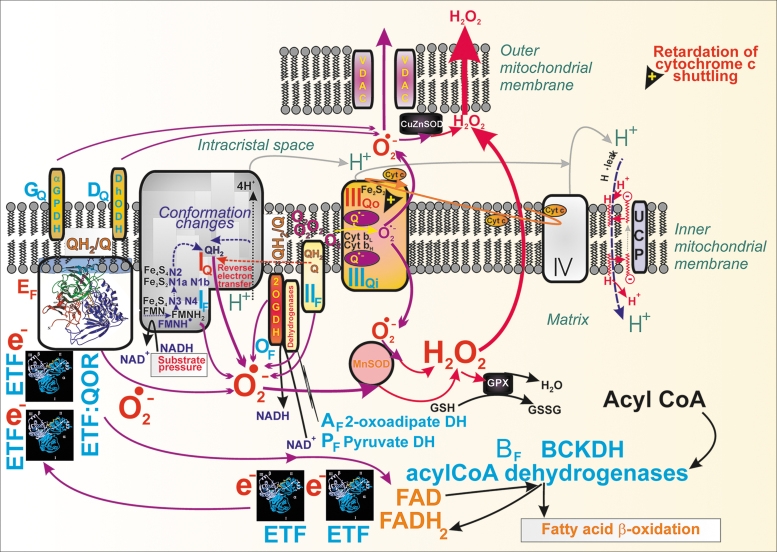
Decreasing matrix NADH ([NADH]m) at high- versus low glucose conditions has one interesting consequence, a diminished matrix NADH/NAD+ ratio, which causes decreased superoxide formation, probably at the IF flavin-site of Complex I (193). As a result, upon GSIS, we do have a dichotomic redox situation in the β-cell cytosol versus mitochondrial matrix. Whereas the cytosolic H2O2 elevation occurs due to NOX4 function, the matrix superoxide formation decreases (likewise H2O2 produced by superoxide dismutase MnSOD). Moreover, typical [NAD+]m, estimated, for example, in HeLa cells, is up to two orders of magnitude higher than [NADH]m. Values of 800 μM [NAD+]m and 5 μM [NADH]m were reported (33). During fast respiration upon GSIS, this difference actually leads to a situation in which each NADH molecule formed by the respective matrix dehydrogenases is instantly consumed by Complex I.
As for the matrix NADPH ([NADPH]m), we found that it decreases with increasing glucose. Specifically, the operation of the pyruvate/isocitrate shuttle and reductive carboxylation by IDH2 consumes NADPH significantly in INS-1E cells (193), and this is not balanced by the increased NADPH formation by the matrix malic enzyme 3 (ME3) nor by the increasing forward (Δp-consuming) mode of nicotinamide nucleotide transhydrogenase (NNT). The matrix ME3 forms pyruvate and NADPH from malate and NADP+ (85). The acute [NADPH]m decrease could lead to a decrease in the reduced glutathione in the matrix, representing a resource sacrificed in exchange for the transfer of redox equivalents, ensuring elevations in cytosolic NADPH.
Other regulators of redox homeostasis
Nicotinamide nucleotide translocase in pancreatic β-cells
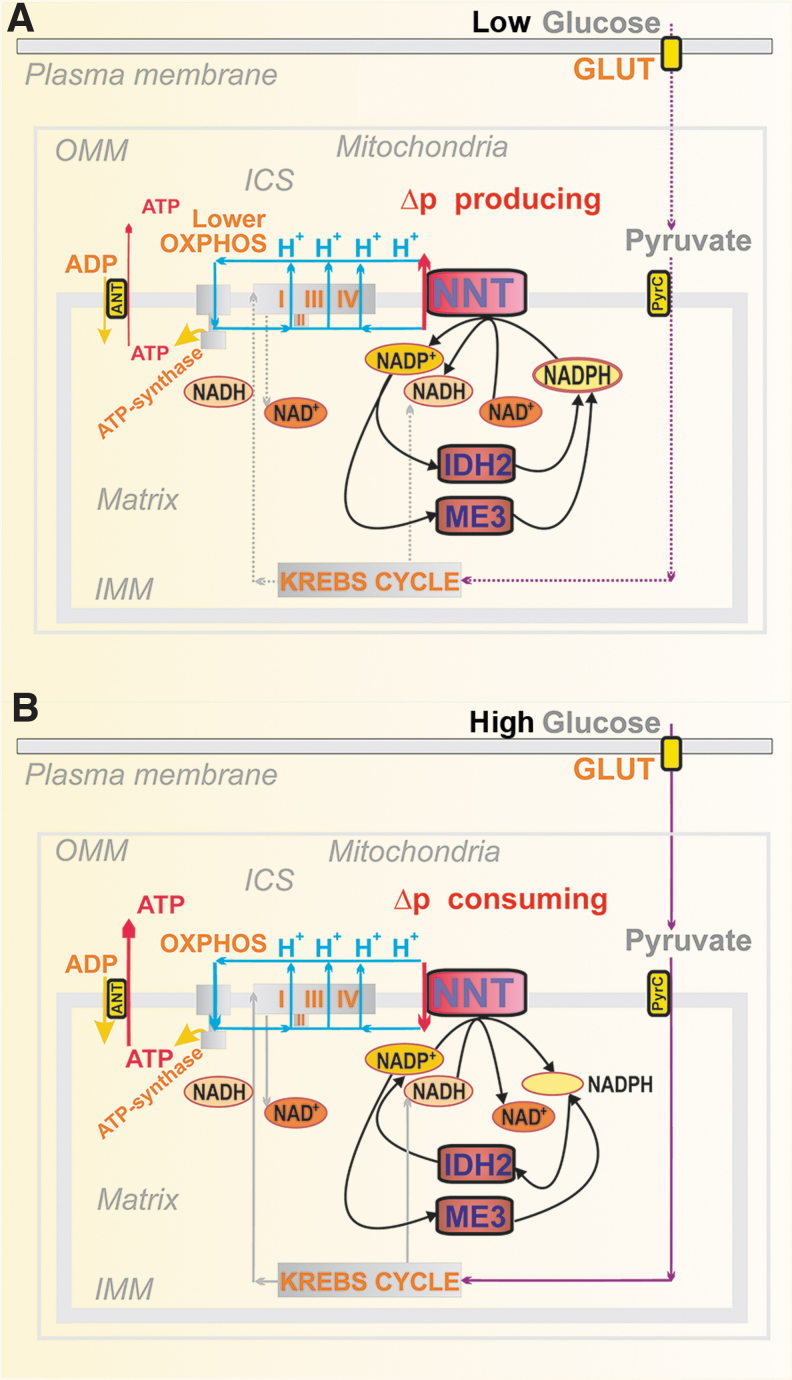
Contradictory findings were reported for the mitochondrial NNT in PIs (Fig. 6). This IMM enzyme exposes its active site to the mitochondrial matrix. In a thermodynamically favored forward mode upon GSIS, NNT consumes Δp (Fig. 6B) by allowing H+ import into the matrix, tightly coupled with the conversion of NADP+ to NADPH and with the simultaneous NADH conversion to NAD+ (220). In this forward mode, NNT contributes to the matrix [NADPH]m pool. Since NNT acts downstream of the redox shuttles, it cannot alter or affect them. Nevertheless, if all these shuttles operate, than IDH2 consumes NADPH and since ME3 cannot balance this consumption, matrix NADPH decreases upon GSIS (193).
FIG. 6.
Hypothetical reverse and forward mode of nicotinamide nucleotide translocase at low and high glucose, respectively. (A) Low glucose conditions: hypothetical reverse NNT mode is depicted, when NNT acts as a proton pump, using the energy of NADPH and NAD+, converting them to NADP+ and NADH, respectively. It would be possible, since under low glucose conditions in pancreatic β-cells, a lower Δp would allow the proton pumping against that Δp. Possible NADPH sources could be the matrix IDH2 providing oxidative decarboxylation and ME3. (B) High glucose conditions: forward NNT mode is possible (193) when NNT uses Δp to translocate H+ into the matrix and drives NADPH formation from NADP+ with the simultaneous conversion of NADH to NAD+. This mode is highly probable upon GSIS since a high Δp is established, thus driving the H+ influx via NNT. Also, since the pyruvate/isocitrate shuttle is activated upon GSIS, it switches the IDH2 reaction direction to reductive carboxylation and NADP+ production. ME3, malic enzyme 3; NNT, nicotinamide nucleotide transhydrogenase.
Despite we could not indicate the reverse mode at low [glucose] (193), NNT was reported to function in the reverse mode (225), in which it pumps protons and thus provides a Δp surplus. This should be coupled with the consumption of NADPH and NAD+, yielding NADP+ and NADH. Such a mode would be possible, since at low [glucose] respiration and ATP synthesis exhibit somewhat slower rates, establishing a lower Δp, than with high [glucose]. The H+ pumping against an intermediate Δp would be possible at rather high NADPH/NADP+ ratio. In contrast, at high [glucose], NNT acts against the higher Δp. However, no direct observation of the actual H+ flux direction was conducted (225). By comparing ΔΨm, monitored using fluorescent probes, we demonstrated ΔΨm-increases in NNT-silenced INS-1E cells, supporting the existence of the forward NNT mode, which produces NADPH (193).
Neither experiments relying on the comparison of C57BL/6J versus C57BL6/N mice were conclusive. They reported that an in-frame five-exon deletion in the Nnt gene spontaneously occurred in C57BL/6J mice, thus removing exons 7–11, causing a complete absence of NNT protein (62, 63, 257). The C57BL6/J mouse strain was claimed to have a highly suppressed GSIS. However, other studies normally used knockouts backcrossed into the C57BL6/J-mice background as controls for GSIS and it exhibited high insulin secretion rates [e.g., Plecita-Hlavata et al. (194) and Wong et al. (273)]. The discrepancy originates from the fact that initially only the quantitative trait loci were identified. Thus, a mere correlation with deletions in the Nnt gene was assumed, and verifications using the artificial Nnt expression can be regarded as inconclusive, since the Nnt expression per se could enhance insulin secretion. This could be subsequently interpreted as an apparent GSIS suppression in C57BL6/J mice (273).
Malate/aspartate shuttle in low and high glucose conditions
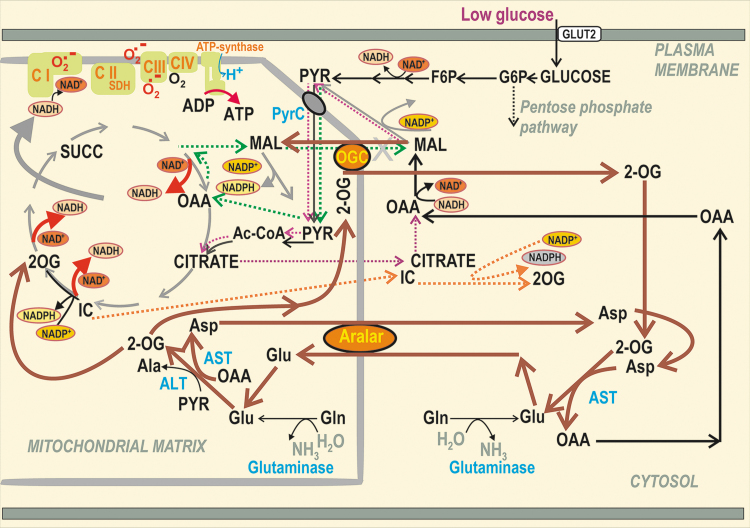
The malate/aspartate shuttle (MAS) was assumed to play a significant role in pancreatic β-cells (147, 240). However, interpretation of the relevant metabolomics data must be provided with caution since they mostly do not resolve metabolites of the mitochondrial matrix versus those from the cytosolic compartment (which is typically greater). One must consider that the metabolite transport direction within the active MAS is the opposite of the pyruvate redox shuttles (193) (Fig. 7). Their existence documented by numerous experiments over the last two decades (107, 110, 112, 183, 193, 209) thus excludes metabolite fluxes required for MAS operation at high [glucose].
FIG. 7.
MAS is plausible when pyruvate-based redox shuttles are not operating. The MAS (brown) could participate in metabolic fluxes in pancreatic β-cells at low nonstimulating [glucose], when the pyruvate-based redox shuttles do not provide the opposite malate fluxes for the 2OGC. Moreover, the MAS transfers redox equivalents of NADH into the mitochondrial matrix; however, matrix NADH was found to decrease upon GSIS and relies on at least one of the two aspartate–glutamate antiporters, that is, SLC25A12/AGC1/aralar (18, 217) and SLC25A13/AGC2 (193) (data not shown). The key enzymes are alanine aminotransferases (cytosolic ALT1 and mitochondrial ALT2; also termed glutamate pyruvate transaminases, GPT1 and GPT2). Within MAS, ALT2 catalyzes the conversion of pyruvate plus l-glutamate to 2OG and l-alanine, whereas ALT1 (omitted for simplicity) would catalyze the reaction in the reverse mode. Analogously, there are aspartate aminotransferases, cytosolic AST1, and mitochondrial AST2 (also termed glutamate oxaloacetate transaminases, GOT1 and GOT2). In MAS, AST2 converts oxaloacetate plus l-glutamate to 2OG and l-aspartate, whereas AST1 should catalyze the opposite reaction to complete the cycle. Due to the reverse character of the aminotransferase reaction, its direction depends on the glutamate metabolism. AGC1, aspartate–glutamate antiporter SLC25A12 (Aralar); ALT, alanine aminotransferase; Aralar, aspartate–glutamate antiporter SLC25A12 (AGC1); AST, aspartate aminotransferase (aka glutamate oxaloacetate transaminase, GOT); GPT, glutamate pyruvate transaminase; MAS, malate/aspartate shuttle.
The 2-oxoglutarate carrier (2OGC) mediates the malate efflux coupled with the 2-oxoglutarate (2OG) uptake to the matrix at high [glucose], whereas the malate import coupled with the 2OG export is required for MAS, if it exists. Unlike with pyruvate-based redox shuttles, at least one of two glutamate-aspartate antiporter isoforms is required for MAS, enabling the glutamate import in exchange for aspartate export from the matrix. In contrast, the aspartate needs to be imported as a part of the pyruvate/malate redox shuttle. However, glutamate formed in the matrix was suggested to be exported to the β-cell cytosol to facilitate IGV maturation and exocytosis (30, 72, 92, 93, 153, 155, 250). This would again require the opposite direction of glutamate flux.
At low [glucose], both aspartate–glutamate antiporters can participate in MAS, that is, SLC25A12/AGC1/aralar (18, 217) and SLC25A13/AGC2 (193). The existence of MAS was derived from the essential requirement of transaminases (aminotransferases) and aspartate–glutamate antiporters for β-cells (18, 217). Metabolomics studies evidenced a decrease in total cell aspartate at the initiation of GSIS, while aconitate, citrate, isocitrate, malate, or fumarate instantly rose, and elevations of 2OG and succinate were delayed until 15 min (241). Elevations in metabolites originate from the disbalance between producing versus consuming reactions, while the latter is slower; whereas for losses of metabolite, the producing reactions are slower. Hence, the observed aspartate losses reflect this disbalance.
Due to providing cytosolic glutamate, MAS was implicated in the GLP-1 amplification of GSIS, but not in GSIS itself (72). The ablation of cytosolic transaminase AST1/GOT1 reversibly transforming 2OG and aspartate to oxaloacetate plus l-glutamate led to the lack of GLP-1 effects. Further experiments are required to evaluate whether the three pyruvate-redox shuttles operate and interfere or not with MAS upon the GLP-1 amplification of GSIS, notably in the sustained second phase.
β-Hydroxybutyrate dehydrogenase and acetoacetate metabolism
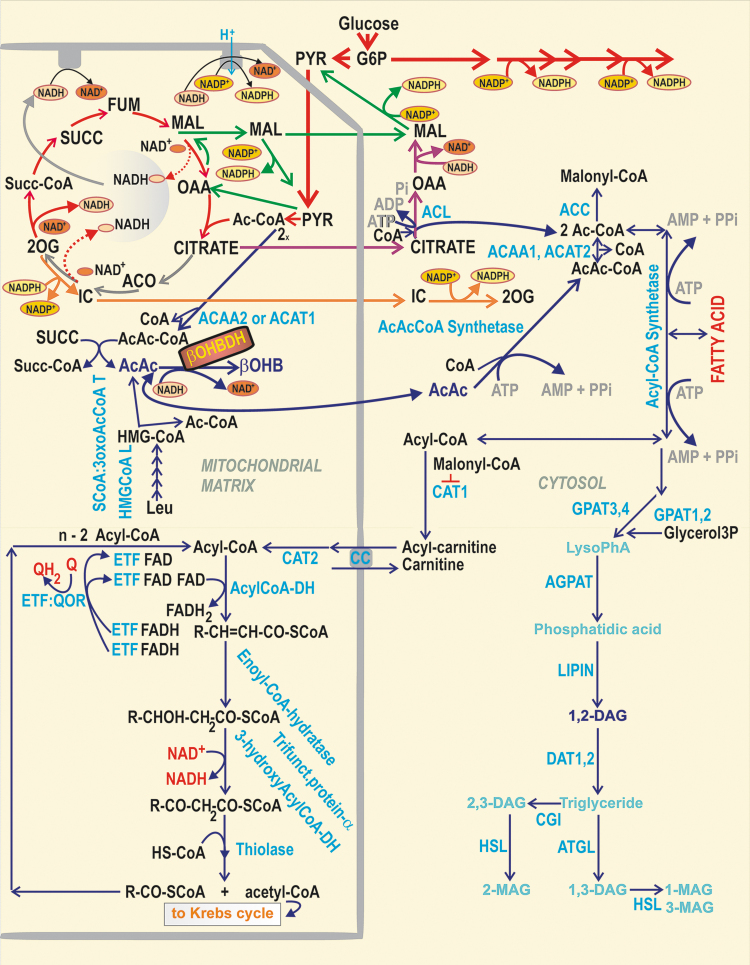
β-Hydroxybutyrate dehydrogenase (β-OHBDH) is exclusive to the matrix in rodent pancreatic β-cells, playing an important role in redox homeostasis (149, 178). In pioneering investigations with hepatocytes, the β-OHBDH reaction was suggested to precisely reflect the matrix NAD+/NADH ratio, which would therefore determine the ratio of (total) β-hydroxybutyrate/acetoacetate concentration (178). However, since the estimated order of magnitude for the matrix NAD+/NADH ratio is >100, such an excess of β-hydroxybutyrate is unlikely. Since we reported the increase in this ratio upon GSIS (193), one could speculate that also matrix β-hydroxybutyrate rises upon GSIS (Fig. 8). However, acetoacetate can also be exported to the cytosol, where it is utilized by other reactions (149, 178). This was thought to facilitate insulin secretion via the formation of various acyl-CoA derivatives (Fig. 8) (149), which could acetylate proteins thus speculatively enhancing GSIS (189, 190). β-Hydroxybutyrate (https://www.brenda-enzymes.org/enzyme.php?ecno=1.1.1.30) can also be formed in the cytosol of human β-cells.
FIG. 8.
β-OHB formation and FA metabolism in pancreatic β-cells. The scheme describes three selected metabolic branches: (i) β-OHB formation and its relationship to leucine metabolism; (ii) FA β-oxidation; and (iii) the cytosolic glycerol/FA cycle. As for (i), at high [glucose], succinate is interconverted with AcAcCoA to S-CoA and acetoacetate by SCoA:3oxoAcCoAT (52). As part of leucine metabolism during a series of oxidative reactions (“β-like oxidation”) resembling FA β-oxidation, HMG-CoA is split by HMGCoAL into acetyl-CoA and acetoacetate. Besides being converted by β-OHBDH, acetoacetate can escape to the cytosol. Distinct enzyme isoforms convert two molecules of acetyl-CoA into CoA and AcAcCoA in the mitochondrial matrix. The latter are ACAT1 and ACAA2, whereas in the cytosol, there are ACAT2 and ACAA1. Cytosolic acetyl-CoA was suggested to facilitate the acetylation of proteins, which might speculatively enhance GSIS (189, 190). (ii) FA β-oxidation: FA is imported via CD36 into β-cells, where AcylCoA-synthetase (ACSL), localized externally to the ER membrane and OMM, converts FAs to acyl-CoAs, whereas the cytosolic CAT1 (synonymous for carnitine palmitoyltransferase, CPT1) converts acyl-CoAs to acylcarnitines (207). The carnitine carrier (SLC25A20) provides the import of acylcarnitines into the matrix, exchanging them for carnitine. The matrix CAT2/CPT2 converts acyl carnitines to acyl-CoAs. The following chain of reactions, termed FA β-oxidation, shortens the FA-acyl chain by two carbons, involving acyl-CoA dehydrogenases, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and β-thiolase. The product of a single cycle is just acyl-CoA shortened by two carbons plus acetyl-CoA. The FA β-oxidation is regulated via the inhibition of CAT1/CPT1 by malonyl-CoA, formed by ACC from acetyl-CoA. (iii) Cytosolic glycerol/FA cycle (197): elevated glucose is converted to glycerol3P, which is esterified by acyl-CoAs by GPATs (bound externally to ER and OMM) to LysoPhA. The latter is further esterified by AGPAT (bound to ER) to PhA. At the ER surface or lipid droplets, lipins transform PhA to 1,2-DAG, initiating PKC signaling and activating Munc13-1. DAG is also acylated there to TG, by diacylglycerol O-acyltransferase-1 and -2 (DGATs). Simultaneously, the lipolytic branch is provided by the cytosolic ATGL, hydrolyzing TG to DAG, upon the facilitation of perilipin (data not shown) and CGI-58 protein (CGI) on the lipid droplet surface. DAG is hydrolyzed to MAG by HSL, again facilitated by perilipin. The created MAGs can overactivate the GPR119 receptor (Fig. 10). The glycerol/FA cycle is completed by the hydrolysis of MAG to glycerol and FAs by the plasma membrane-associated ABHD6 (data not shown), whereas glycerol is exported from β-cells. β-OHB, β-hydroxybutyrate; β-OHBDH, β-hydroxybutyrate dehydrogenase; ABHD6, alpha/beta-hydrolase domain containing 6, monoacylglycerol lipase; ACAA, acetyl-CoA acyltransferase; AcAcCoA, acetoacetyl-CoA; ACAT, acetyl-CoA acetyltransferase; ACC, acetyl-CoA carboxylase; ACSL, long-chain acyl-CoA synthetase; AGPAT, 1-acylglycerol-3-phosphate acyltransferase; ATGL, adipose triglyceride lipase; CAT, carnitine acyltransferase; CGI, comparative gene identification 58, ATGL co-activator (aka ABDH5); CPT, carnitine palmitoyltransferase; DAG, diacylglycerol; DAT, DGAT, diacylglycerol O-acyltransferase; FA, fatty acid; glycerol3P, glycerol-3-phosphate; GPAT1,2, glycerol-3-phosphate acyltransferase 1,2; GPAT3,4, glycerol-3-phosphate acyltransferase 3,4 (1-acylglycerol-3-phosphate O-acyltransferase); GPR, G-protein-coupled receptor; HMG-CoA, hydroxymethyl-glutaryl-CoA; HMGCoAL, hydroxymethyl-glutaryl-CoA lyase; HSL, hormone-sensitive lipase; LysoPhA, lysophosphatidic acid; MAG, monoacylglycerol; OMM, outer mitochondrial membrane; PhA, phosphatidic acid; PKC, protein kinase C; SCoA:3oxoAcCoAT, succinyl-CoA:3-ketoacid-CoA transferase; Succ-CoA, S-CoA, i.e. succinyl-CoA; TG, triglyceride.
Long-chain acyl-CoAs were also reported to bind to the KIR6.2 subunit of KATP (28), which potently activates this channel (27, 77). Since upon GSIS, there is a reduction in total cell acyl-CoAs and malonyl-CoA (146, 198), such a reduction could facilitate KATP closure (146). Alternatively, FA β-oxidation (long-chain acyl-CoA shortening) could also provide the redox signaling toward KATP or TRPM2 (79, 123, 223), as with KIC (194) (see the Mitochondrial Contribution to Insulin Secretion Stimulated by BCKAs and FAs section).
Phosphoenolpyruvate cycle and role of pyruvate kinases
Another cycle, the phosphoenolpyruvate (PEP) cycle was suggested to act in the low glucose conditions. The PEP cycle is cataplerotic, beginning by the mitochondrial PEP-carboxykinase 2 (PEPCK2) conversion of oxaloacetate to PEP, which is exported by the citrate carrier (SLC25A1) from mitochondria. Cytosolic pyruvate kinases (PKs, isoforms constituent M1, recruitable M2 and L), existing in beta cells (167) use then the cytosolic PEP to convert it to pyruvate, which is coupled to ATP formation from ADP. Pyruvate enters mitochondria, where is metabolized either by PDH or by pyruvate carboxylase (PC). The PC flux completes the cycle by pyruvate conversion to oxaloacetate.
Pyruvate kinase isoform recruitable M2 (PKM2) and pyruvate kinase isoform L (PKL), allosterically activated by fructose 1,6-bisphosphate, were recently reported to aid KATP closure, as derived from patch-clamp experiments in excision mode combined with PK activation by a small-molecule activator (141). The authors exemplified PEP cycle switched on/off in the β-cell responses to intermediate 9 mM glucose, when Vp and or [Ca2+]c bursts phases are interchanged with the interburst phases (Fig. 4). The decreased cytosolic ATP/ADP ratio was explained on the basis of the PEP cycle providing ATP synthesis by PK, that is, by “substrate” phosphorylation of ADP, independent of OXPHOS. Naturally low ATP sets KATP-channels open, which occurs before glucose elevation and/or after termination of the burst phase at 9 mM glucose. When OXPHOS continues to elevate ATP further, PEP cycle is less active and more importantly the Krebs cycle control strength overcomes that of PEP cycle. Consequently, the burst phase begins at 9 mM glucose. Also, PKM2 and PKL activator failed to improve GSIS in PEPCK2-knockout mice (1).
Glutamine and glutamate in pancreatic β-cells
Glutamine and glutamate metabolism
The reaction direction of mitochondrial glutamate dehydrogenase (GDH) in pancreatic β-cells was thought to favor the provision of glutamate and NAD+, while consuming 2OG, ammonium, and NADH (30, 92, 153, 155, 250). This would also contribute to decreasing [NADH]m, if acting upon GSIS. During fasting, GDH is activated by ADP and leucine, while at high [glucose], GDH is inhibited by GTP and ATP (67, 278).
Glutamate is exported from the matrix by the glutamate carrier GC1 (SLC25A22) (30, 92, 155). Also, pyruvate utilization by aminotransferases has a certain impact, despite being minor compared with the utilization by the matrix PDH complex and by the oxaloacetate anaplerosis provided by pyruvate carboxylase (6). Thus, cytosolic alanine aminotransferase (ALT1) and mitochondrial ALT2 [also termed glutamate pyruvate transaminases, GPT1 and GPT2 (188)] could catalyze the conversion of pyruvate plus l-glutamate to 2OG and l-alanine (279), which diminishes the matrix glutamate pool. The reverse GPT2 reaction would then produce glutamate and add pyruvate to its fast-consuming pool. Alternatively, AST1/GOT1 and mitochondrial AST2/GOT2 reversibly convert oxaloacetate plus l-glutamate to 2OG and aspartate.
Glutamine is also utilized in PIs, reportedly promoting leucine-stimulated insulin secretion at low [glucose], when phosphate-dependent glutaminase produces glutamate, which is further oxidized by GDH (67).
Glutamate regulation of insulin secretion
Glutamate was first suggested to facilitate GSIS (30, 92, 154, 155, 229), which was questioned by subsequent reports (24, 148). The glutamate effects are instead connected with IGV biology (214, 260). The specific uptake of glutamate into IGVs was found, being driven by ΔpHIGV, established on the IGV membranes by the V-ATPase (10). Anion influx, namely Cl− influx by the ClC3 transporter, also helps to build ΔpHIGV. Additions of membrane-permeant dimethyl glutamate were reported to amplify both phases of GSIS, increasing the frequency of insulin granules merging with the plasma membrane (72).
Sufficient glutamate content inside the IGV lumen results from its uptake mediated by glutamate transporters VGLUT1,2,3 balanced by the EAAT2-mediated glutamate efflux (66). The ablation of VGLUTs reduced the GLP-1-induced amplification of GSIS but not GSIS itself (72). Ablation of the plasma membrane sodium-coupled neutral amino acid transporter 5 (SNAT5) also led to a reduced GLP-1 amplification of GSIS (86).
Mitochondrial Contribution to Insulin Secretion Stimulated by BCKAs and FAs
Insulin secretion by BCKAs involves mitochondrial retrograde redox signaling
Branched-chain amino acids versus BCKAs
A mixed meal leads to elevated levels of amino acids in circulation (244). During fasting or numerous pathologies, branched-chain amino acids (BCAAs) and BCKAs are also elevated in plasma. We will only discuss situations when pancreatic β-cells sense BCAAs and BCKAs in the islet microcirculation, or when these compounds are formed by metabolism. Thus, BCKAs, KIC (leucine metabolite) (7), 2-ketoisovalerate (KIV; valine metabolite), and 2-ketoisomethylvalerate (KMV; isoleucine metabolite) stimulate profound insulin secretion at low glucose (83, 88, 138–140, 163, 189, 190). Leucine can also exert effects by allosterically activating GDH (278).
Retrograde redox signaling (i.e., from mitochondria to cell cytosol, nuclei, or other organelles) is provided when these BCKAs are oxidized in mitochondria (29). This is substantiated by the elevated mitochondrial superoxide formation due to BCKA oxidation, whereas superoxide is transformed to H2O2 either by the matrix MnSOD or by the intermembrane space CuZnSOD (Fig. 9).
FIG. 9.
Mitochondrial retrograde redox signaling determines insulin secretion by BCKAs. KIC, KMV, and KIV may arise from blood capillaries (imported into the matrix via the carnitine carrier, CC) or by being converted from leucine, isoleucine, and valine, respectively, by the branched-chain aminotransferase reaction in the mitochondrial matrix (BCAT2). The BCAA import is ensured by the plasma membrane LAT1 transporter. The BCKDH, which provides electrons to ETF, exists exclusively in β-cell mitochondria and initiates a series of reactions of β-like oxidation. ETF:QOR accepts electrons from two ETFs, when it converts Q to QH2, and as side reactions produces superoxide together with its elevated production at sites IQ and IIIQo (cf. Fig. 11). In the mitochondrial matrix, superoxide is transformed to H2O2 by MnSOD, but by CuZnSOD in the intermembrane space and cytosol. The elevated mitochondrial/cytosolic H2O2 substantiates the retrograde redox signaling that targets KATP to ensure its closure together with ATP (and possibly also TRPM2). As a result, CaV channels are open, providing the Ca2+-signal for IGV exocytosis. The end products of the β-like oxidation of KIC contribute to OXPHOS. Thus, AcCoA enters the Krebs cycle to form citrate, whereas AcAc can be partly exported to the cytosol or converted to β-OHB by a reverse reaction of β-OHBDH at the expense of NADH (Fig. 8). A link to glutamate and glutamine metabolism is also indicated, including the MAS (Fig. 7), which may exist together with BCKA-stimulated insulin secretion at low glucose, since at low glucose, the pyruvate-based redox shuttles are not highly active. AcAc, acetoacetate; AcCoA, acetyl-CoA; BCAA, branched-chain amino acid; BCAT2, branched/chain amino acid transferase 2, mitochondrial; BCKA, branched-chain ketoacid; BCKDH, branched-chain ketoacid dehydrogenase; ETF, electron transfer flavoprotein; ETFQOR or ETF:QOR, electron transfer flavoprotein:quinone oxidoreductase; GAD, glutamate decarboxylase; GC1, glutamate carrier; GLS, glutaminase; IGV, insulin granule vesicle; KIC, 2-ketoisocaproate; KIV, 2-ketoisovalerate; KMV, 2-ketoisomethylvalerate; OXPHOS, oxidative phosphorylation; Q, ubiquinone; QH2, ubiquinol.
Despite there being details missing on how BCAA or BCKA are transported to the mitochondrial matrix, a well-known exclusively matrix BCKDH complex converts CoA plus BCKAs to proper BCKA-CoAs, that is, to isovaleryl-CoA, isobutyryl-CoA, and methyl-isobutyryl-CoA from KIC, KIV, and KMV, respectively. The entire series of reactions, a β-like oxidation (resembling FA β-oxidation) continues, for example, for KIC via methylcrotonyl-CoA carboxylase (MCC), methyl-glutoconyl-CoA hydratase (MGCoAH), and 3-hydroxy-3-methylglutaryl-CoA lyase (HMGCoAL), leading to the end products acetyl-CoA, which drives the Krebs cycle, and acetoacetate.
Mechanism of superoxide formation upon oxidation of BCKAs
The BCKDH reaction includes FAD as an electron acceptor. Subsequently, two electron transfer flavoprotein (ETF) molecules reoxidize the resulting FADH2 since each ETF is only a single-electron carrier. ETFs otherwise transfer electrons from 11 different mitochondrial flavoprotein dehydrogenases to the IMM ubiquinone (Q) pool. During BCKA oxidation, the transfer of electrons proceeds from one electron-reduced ETF one at a time to the lower potential FAD center of the electron transfer flavoprotein:quinone oxidoreductase (ETFQOR) (268). One electron is transferred to the iron cluster. ETF:QOR thus accepts two electrons, and this is coupled with the reduction of ubiquinone to ubiquinol (Q to QH2) with a transient semiubiquinone formation (268). This is a clue to the enhanced superoxide formation upon BCKA oxidation.
Since QH2 binds to the Complex I Q-binding site, the excessive supply of QH2 by ETF:QOR causes a feedback inhibition of the ongoing Q reduction to QH2 in Complex I. As a result, mitochondrial superoxide formation is accelerated at the so-called IQ site of superoxide formation (Fig. 11). The second possible source could be due to the ETF:QOR reaction itself, while electrons are leaking from flavin to oxygen at site EF (Fig. 11), forming initially a radical pair and finally superoxide (25). The third possible source is given by the excessive acetyl-CoA (propionyl-CoA) entry or methylmalonyl and S-CoA entry into the Krebs cycle. Since such enhanced catabolism accelerates respiration, an enhanced superoxide formation can be expected at Complex I site IF and site IQ, and Complex III site IIIQo, when the capacity of electron transfer is exceeded. Also, as discussed above, acetoacetate influences the established redox homeostasis.
FIG. 11.
Superoxide formation due to FA β-oxidation or oxidation of BCKAs. An overview of locations for mitochondrial superoxide sources (blue capitalized fonts), termed according to the nomenclature introduced by Brand (25) is shown with those which increase superoxide formation upon FA β-oxidation or the β-like oxidation of BCKAs emphasized (in red). The electron-transfer flavoprotein:ubiquinone oxidoreductase (ETFQOR) is a common key electron transfer link between the initial dehydrogenases of these reactions and the respiratory chain complexes III and IV (29, 268). ETFQOR accepts electrons sequentially from two ETFs as single-electron carriers, while converting ubiquinone (Q) from its IMM pool to QH2. The electron leak from flavin to the oxygen leads to a radical pair formation and subsequent superoxide formation within the ETF:QOR itself (site EF). Moreover, the requirement of ETF:QOR to react with Q effectively outcompetes Q as the Complex I substrate, resulting in relative electron transfer retardation over the whole respiratory chain, hence superoxide is formed at its sites IQ and IIIQo. Finally, due to the increasing acetyl-CoA entry (propionyl-CoA entry for KIV; through methylmalonyl and succinyl-CoA) and NADH entry into the Krebs cycle, the excessive formation of superoxide at site IF may also contribute. After the conversion of superoxide to H2O2 by the matrix MnSOD and the intermembrane space CuZnSOD, the ongoing H2O2 efflux from mitochondria can be regarded as redox signaling. As in other types of mitochondria, there are in total six sites acting at the ∼280 mV redox potential of the NADH/NAD+ isopotential pool (index F, flavin) and five sites acting at the ∼20 mV redox potential of the QH2/Q isopotential pool (index Q) (25). Of these, only the superoxide sources with more intensive production at higher ΔΨm are attenuated by uncoupling proteins (103). This also involves the reverse electron transfer to the Complex I site IQ. In turn, superoxide formation at site IF increases with the increasing NADH/NAD+ ratio (“substrate pressure”). When cytochrome c shuttling (orange elliptic arrow) is retarded, then the Complex III site IIIQo provides major superoxide formation, which cannot be attenuated by uncoupling. IMM, inner mitochondrial membrane.
Insulin secretion due to BCKAs
Insulin secretion upon elevated BCKA depends on increases in cytosolic ATP plus H2O2, now both supplied by mitochondria (Fig. 9). Indeed, BCKDH silencing blocked KIC-stimulated insulin secretion, as did the mitochondrial-matrix-targeted antioxidant SkQ1 (194). In contrast, the transaminase inhibitor aminooxyacetate had no effect. The blockage correlated with the suppressed matrix superoxide release, which was otherwise elevated by KIC. So, the H2O2 diffusion from the matrix substantiates the retrograde redox signaling from mitochondria. We admit the KIC doses may be supraphysiological in these experiments. A magnitude of a minimum threshold should be further investigated, which still creates sufficient mitochondrial superoxide/H2O2 in vivo to substantiate redox signaling reaching the plasma membrane and concomitant insulin exocytosis.
The most plausible terminal targets are located in the plasma membrane. They could be Cys residues of KATP, or an oxidizable Met residue of TRPM2 (223), or both. Note that TRPM2 or other NSCCs or chloride channels are essentially required to shift the depolarization to the −50 mV threshold required for the opening of CaV, and so for action potential firing (119, 221). This −50 mV threshold cannot be achieved without NSCCs or Cl− channels, even when 100% of KATP channels are closed. The latter is undoubtedly established by a highly elevated cytosolic ATP resulting from β-like oxidation and concomitant OXPHOS.
Redox signaling from mitochondria upon stimulation of insulin secretion by long-chain FAs
Fatty acid-stimulated insulin secretion requires mitochondrial β-oxidation and signaling via the GPR40 receptor
In rodent and human physiology, a mixed or fatty meal leads to the intestinal formation of chylomicrons that are brought by the circulation system to PIs after a few hours in humans, while also lipolysis is inhibited by secreted insulin (61). In contrast, the lipid- or FA-mediated secretion of GLP-1 by intestinal L-enterocytes comes earlier (173). Thus, a fatty meal induces insulin secretion via GLP-1 endocrine effects on PIs, whereas the further delayed insulin secretion due to chylomicrons arises at a time when even the 1-h-long second GSIS phase is terminated. So not only due to molecular mechanistic reasons, but due to physiological timing, it is crucial to study fatty acid-stimulated insulin secretion (FASIS).
In vivo, there is always a concomitant parallel portion of insulin secretion stimulated by 2-monoacylglycerol (MAG), cleaved from postprandial chylomicrons in PI capillaries by lipoprotein lipase (Fig. 10) (37, 158, 182, 272). The resulting MAG and long-chain FAs (37, 158, 182, 272) stimulate their own receptors (171). Adipose triglyceride lipase (ATGL) is the major isoform that cleaves triglycerides in PI capillaries (192), besides secretory phospholipases A2 (64). MAG activates metabotropic receptor GPR119, providing signaling via Gαs and cAMP (76, 87, 94, 96, 157, 171). It has been questioned whether the levels of FAs bound to albumin can initiate FASIS (102, 261), but this could happen with metabolic syndrome and/or obesity and type 2 diabetes since circulating FA levels are then elevated.
The consensus view was that sufficient glucose must always be present for FAs to induce any insulin secretion (51, 76, 87, 94–96, 157, 206, 245, 276). However, FASIS was described to exist at low (insulin nonstimulating) [glucose] (32, 103, 106, 194), but not at zero glucose (32). Human islets perifused without glucose did not increase respiration with long-chain FAs, but released insulin (32), whereas both increasing respiration and insulin release were observed with long-chain FAs plus 5.5 mM glucose. FASIS was evidenced by FA triggering an action potential with a prolonged duration at low (insulin nonstimulating) [glucose] (57) and concomitantly increased Ca2+ influx and hence potentiation of insulin secretion (55, 184). As a result, FASIS should be based on the metabolic components plus metabotropic receptor GPR40 signaling (76, 87, 94, 96, 103, 106, 130, 157, 201, 222, 256, 281, 288). It has to be established whether these two branches are mutually independent.
The major receptor pathway of FASIS (Fig. 10A) includes the metabotropic receptor GPR40, as evidenced by its ablation or its point R258W mutation since both impaired FASIS (222). The major pathway downstream of GPR40 initiates signaling via the Gαq/Gα11 (226), activating phospholipase C-β- (PLC-β-) mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate into DAG and IP3 (23). The major axis involves the phosphorylation of TRPM4 (TRPM5) channels by PKC activated by DAG (231) or TRPC3 activation (276). As with TRPM2, the opening of TRPM4, TRPM5, or TRPC3 determines the necessary depolarization shift, despite the 100% closed KATP ensemble. The KATP closure is provided by the metabolic component of FASIS, that is, by FA β-oxidation providing ATP and H2O2, which may also function under low glucose conditions.
Signaling along the axis of GPR40-Gαq/Gq11-PLC-IP3 promotes additional Ca2+ efflux from the ER (initiated by the CaV opening, Fig. 4) via the IP3R, forming a Ca2+ channel of ER membranes (13, 64). Alternatively, a synergy exists for the plasma membrane channel calcium release-activated calcium modulator 1 (Orai1) with IP3R1 and stromal interaction molecule 1 (STIM1), sensing ER Ca2+ (259). If biased GPR40-Gαs signaling occurs, also the EPAC2-RyR route of Ca2+-release from ER might contribute to Ca2+ oscillations. GPR40 also initiates pathways of protein kinase D (PKD), activated by DAG (56), signal-regulated kinase 1 and 2 (ERK1/2) (201), and p21-activated kinase 4 (PAK4) (21). The latter regulates cytoskeletal dynamics, facilitating IGV exocytosis. Signaling downstream of GPR40 slightly increases respiration during 1-h incubations (130). Thus, PKC (224) and downstream ERK1/2 signaling stimulates OXPHOS, hence mitochondrial ATP synthesis (224).
Long-chain FAs are also imported into pancreatic β-cells by the sirtuin-activated CD36 FA transporter (125) (Fig. 10B). If short-chain FAs are present, they act via the GPR41 metabotropic receptor and contribute to the fine-tuning of insulin secretion in both fed and fasting states (200, 263). Similarly, the metabotropic receptor GPR120, having a different selectivity for agonists, mediates the amplification and/or stimulation of insulin secretion, by, for example, α-linolenic acid and polyunsaturated FAs (12, 172).
FA synthesis versus β-oxidation
During GSIS, due to the operation of pyruvate/isocitrate and pyruvate/citrate shuttles, conditions are set for FA synthesis. Metabolomics studies confirmed this, while observing an increase in free palmitic acid after transitions from low to high [glucose] (240). FA metabolism is even considered to be a prerequisite for GSIS since GSIS attenuation was observed in isolated PIs with inhibited triglyceride lipolysis (162, 176), in mice with deleted lipase specifically in β-cells (60) or in ATGL-knockout mice (192). But the net FASIS was not affected by the ATGL deletion. In contrast, at low [glucose] FA β-oxidation readily proceeds in pancreatic β-cells, supplying OXPHOS (Figs. 8 and 10B). Fifty to seventy percent of FAs generated by long-chain acyl-CoA synthetase (ACSL) are recycled into lipogenesis (197).
Retrograde redox signaling upon FASIS
FA β-oxidation is based on mitochondrial flavoprotein dehydrogenases, such as the short-chain acyl-CoA dehydrogenase (EC 1.3.8.1), medium-chain acyl-CoA dehydrogenase (EC 1.3.8.7), long-chain acyl-CoA dehydrogenase (EC 1.3.8.8), and very long-chain acyl-CoA dehydrogenase (EC 1.3.8.9). All of them donate electrons to ETF:QOR via ETF and therefore contribute to the mitochondrial superoxide formation, which after conversion to H2O2 serves as redox signaling (Fig. 11). Therefore, the mechanism is similar as for the β-like oxidation of BCKAs. Even at low [glucose], the ETF-ETF:QOR redox relay to Complex I and III of the mitochondrial respiratory chain serves as the electron acceptor for dehydrogenases of β-oxidation. Interestingly, GPR40 signaling also activated NOX2 (181).
Redox-sensitive mitochondrial phospholipase iPLA2γ amplifies FASIS
Notably, the first FASIS phase (the second FASIS phase only moderately) was highly amplified by the action of mitochondrial phospholipase iPLA2γ (Ca2+-independent phospholipase A2 isoform γ [PNPLA8]), providing the cleaved mitochondrial FAs to GPR40 in insulinoma INS-1E cells (98, 103) and in mouse islets (Holendová et al., unpublished data). The iPLA2γ is directly activated by H2O2, and it is also activated by the intramitochondrial redox signaling resulting from FA β-oxidation (103). The activated iPLA2γ cleaves free long-chain FAs from mitochondrial phospholipids. The cleaved long-chain FAs diffuse to the plasma membrane and subsequently stimulate GPR40 (Fig. 10A, B).
This was indicated by the direct observation of FA diffusion to the plasma membrane using the FA-sensitive fluorescent protein ADIFAB (103). Thus, the pool of free long-chain FAs generated by the glycerol/FA cycle (Figs. 8 and 10B) and CD36-mediated import is enriched by FAs cleaved from mitochondrial membranes. In this way, FAs self-accelerate the GPR40 signaling and hence insulin secretion. In INS-1E cells, ∼66% of the FASIS first phase was dependent on GPR40, and nearly the same 66% of it was blocked upon the silencing of iPLA2γ (or its ablation in mice, unpublished data). Hypothetically, the remaining part may depend on the elevation of ATP plus redox signaling from β-oxidation.
Mixed GSIS and FASIS
At high [glucose], triglyceride synthesis alternates with triglyceride hydrolysis in pancreatic β-cells (197). Because of ATP consumption, this cycling is futile. Since DAG is one of the intermediates of this cycle, it may provide all the above-specified signaling (Figs. 8 and 10B). Moreover, free FAs released during the cycle enrich the net GSIS with this supplemental FASIS. Indeed, the addition of FAs to β-cells and PIs at insulin-stimulating [glucose] amplifies GSIS, whereas β-cell acyl-CoA levels increase and appear to rapidly esterify glycerol-3-phosphate into lysophosphatidic acid and several different glycerolipids (51). This partly replenishes cytosolic NAD+. Also, glycerol-3-phosphatase produces glycerol and thus regulates glycolysis, the cellular redox state, ATP production, and other important branches of metabolism (175). The largest amount of insulin was secreted by INS-1E cells when palmitic acid plus 25 mM glucose were added, relative to either palmitic acid stimulation alone (∼80% of maximum) and GSIS alone (∼30% of maximum) (103).
Higher glucose decreases acyl-CoA levels in pancreatic β-cells (146). Previously, acyl-CoAs were suggested to activate KATP (26), hence declining acyl-CoAs would ease the KATP closure. Moreover, the metabolism of the remaining long-chain acyl-CoAs leads to superoxide/H2O2 formation, which also aids the opening of CaV. In contrast, incoming higher glucose levels in pancreatic β-cells increase malonyl-CoA (81, 146), which inhibits carnitine palmitoyltransferase 1 (CPT1) and hence FA β-oxidation. This in turn opens the way for FA synthesis stemming from the ATP citrate lyase (ACL) reaction after the citrate efflux from the mitochondria (174). Nevertheless, the silencing of ACL and FA synthase in β-cells did not affect GSIS (113).
Upon increasing [glucose], glycerol-3-phosphate is also esterified, so the abundance of long-chain saturated monoacylglycerols increases (174, 291). These MAGs additionally stimulate insulin secretion via the GPR119 receptor and downstream PKA and EPAC2 pathway; the latter notably facilitates IGV priming by activating the protein Munc13-1 (291) (Figs. 8 and 10A). Note that this is similar to the GPR40 activation by the FAs cleaved in the cell interior, that is, from the mitochondrial membranes by mitochondrial phospholipase iPLA2γ.
Acknowledgment
The authors gratefully acknowledge the precise frequent renumbering of references during the article preparation and molecular modeling for Figure 2 by B.H.
Abbreviations Used
- 2OG
2-oxoglutarate
- 2OGC
2-oxoglutarate carrier, mitochondrial
- 2OGDH
2-oxoglutarate dehydrogenase
- β-OHB
β-hydroxybutyrate
- β-OHBDH
β-hydroxybutyrate dehydrogenase
- ABHD6
alpha/beta-hydrolase domain containing 6, monoacylglycerol lipase
- ACAA
acetyl-CoA acyltransferase
- AcAc
acetoacetate
- AcAcCoA
acetoacetyl-CoA
- ACAT
acetyl-CoA acetyltransferase
- ACC
acetyl-CoA carboxylase
- AcCoA
acetyl-CoA
- ACL
ATP citrate lyase
- ACO
aconitase
- ACSL
long-chain acyl-CoA synthetase
- AGC1
aspartate–glutamate antiporter SLC25A12 (Aralar)
- AGPAT
1-acylglycerol-3-phosphate acyltransferase
- ALT
alanine aminotransferase (aka glutamate pyruvate transaminase, GPT)
- Aralar
aspartate–glutamate antiporter SLC25A12 (AGC1)
- AST
aspartate aminotransferase (aka glutamate oxaloacetate transaminase, GOT)
- ATGL
adipose triglyceride lipase
- BCAA
branched-chain amino acid
- BCAT2
branched-chain amino acid transferase 2, mitochondrial
- BCKA
branched-chain ketoacid
- BCKDH
branched-chain ketoacid dehydrogenase
- CaL
voltage-dependent Ca2+ channels of L-type
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CAT
carnitine acyl transferase
- CaV
voltage-dependent Ca2+ channels
- CGI
comparative gene identification 58, ATGL co-activator (aka ABDH5)
- Cit C
citrate carrier, mitochondrial
- CoA
coenzyme-A
- CS
citrate synthase
- DAG
diacylglycerol
- DAT, DGAT
diacylglycerol O-acyltransferase
- EPAC
exchange proteins directly activated by cAMP
- ER
endoplasmic reticulum
- ERK
extracellular regulated kinase
- ETF
electron transfer flavoprotein
- ETF:QOR
electron transfer flavoprotein:quinone oxidoreductase
- F6P
fructose-6-phosphate
- FA
fatty acid
- FASIS
fatty acid-stimulated insulin secretion
- FASN
fatty acid synthase
- FH
fumarate hydratase
- FUM
fumarate
- G6P
glucose-6-phosphate
- G6PDH
glucose-6-phosphate dehydrogenase
- GAD
glutamate decarboxylase
- GC1
glutamate carrier
- GDH
glutamate dehydrogenase
- GLP-1
glucagon-like peptide 1
- GLP1R
glucagon-like peptide 1 receptor
- GLS
glutaminase
- GLUT
glucose transporter
- glycerol3P
glycerol-3-phosphate
- GPAT1,2
glycerol-3-phosphate acyltransferase 1,2
- GPAT3,4
glycerol-3-phosphate acyltransferase 3,4 (1-acylglycerol-3-phosphate O-acyltransferase)
- GPR
G-protein-coupled receptor
- GPT
glutamate pyruvate transaminase
- GSIS
glucose-stimulated insulin secretion
- HMG-CoA
hydroxymethyl-glutaryl-CoA
- HMGCoAL
hydroxymethyl-glutaryl-CoA lyase
- HSL
hormone-sensitive lipase
- IC
isocitrate
- ICS
intracristal space
- IDH1
isocitrate dehydrogenase 1, cytosolic NADP+ dependent
- IDH2
isocitrate dehydrogenase 2, mitochondrial NADP+ dependent
- IDH3
isocitrate dehydrogenase 3, cytosolic NAD+ dependent
- IF1
ATPase inhibitory factor 1 (i.e., ATP synthase inhibitory factor 1)
- IGV
insulin granule vesicle
- IMM
inner mitochondrial membrane
- IP3
inositol-1,4,5-triphosphate
- IP3R
inositol-1,4,5-triphosphate receptor
- iPLA2γ
Ca2+-independent phospholipase A2 isoform γ (PNPLA8)
- KATP
ATP-sensitive K+ channel
- KIC
2-ketoisocaproate
- KIV
2-ketoisovalerate
- KMV
2-ketoisomethylvalerate
- LysoPhA
lysophosphatidic acid
- MAG
monoacylglycerol
- MAL
malate
- MAS
malate/aspartate shuttle
- MCU
mitochondrial calcium uniporter
- MDH
malate dehydrogenase
- ME1
malic enzyme 1, cytosolic
- ME3
malic enzyme 3, mitochondrial
- MODY
maturity-onset diabetes of the young
- NBF
nucleotide-binding fold
- NCLX
mitochondrial sodium calcium exchanger
- NNT
nicotinamide nucleotide transhydrogenase
- NOX4
NADPH oxidase 4
- NSCC
nonspecific calcium channels
- OAA
oxaloacetate
- OCR
oxygen consumption rate
- OMM
outer mitochondrial membrane
- Orai1
calcium release-activated calcium modulator 1
- OXPHOS
oxidative phosphorylation
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- PEP
phosphoenolpyruvate
- PEPCK2
phosphoenolpyruvate-carboxykinase 2
- PhA
phosphatidic acid
- PI
pancreatic islets
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PK
pyruvate kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PKL
pyruvate kinase isoform L
- PKM2
pyruvate kinase isoform recruitable M2
- PLC
phospholipase C
- PMCA
plasma membrane Ca2+ATPase
- PPP
pentose phosphate pathway
- PyrC
pyruvate carrier, mitochondrial
- Q
ubiquinone
- QH2
ubiquinol
- Rap2
Ras-related protein 2
- RC
respiratory chain
- RyR
ryanodine receptor
- sAC
soluble adenylyl cyclase
- S-CoA
succinyl-CoA
- SCoA:3oxoAcCoAT
succinyl-CoA:3-ketoacid-CoA transferase
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SNAP-25
synaptosomal nerve-associated protein 25
- SNARE
soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor
- SUCC
succinate
- SUR1
sulfonylurea receptor 1
- TG
triglycerides
- tmAC
transmembrane adenylyl cyclase
- TRPC
transient receptor potential canonical
- TRPM
transient receptor potential melastin
- TRPV
transient receptor potential vanilloid
Authors' Contributions
Conceptualization, P.J.; resources, B.H.; writing—original draft preparation, P.J.; writing—review and editing, P.J., B.H., A.D., M.J., and L.P-H.; funding acquisition, P.J.
Author Disclosure Statement
The authors declare no conflicts of interest.
Funding Information
This research was funded by the Grant Agency of the Czech Republic (Grantová Agentura České Republiky, GAČR), grant number 20-00408S.
References
- 1. Abulizi A, Cardone RL, Stark R, Lewandowski SL, Zhao X, Hillion J, Ma L, Sehgal R, Alves TC, Thomas C, Kung C, Wang B, Siebel S, Andrews ZB, Mason GF, Rinehart J, Merrins MJ, and Kibbey RG. Multi-tissue acceleration of the mitochondrial phosphoenolpyruvate cycle improves whole-body metabolic health. Cell Metab 32: 751.e11–766.e11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acin-Perez R, Russwurm M, Günnewig K, Gertz M, Zoidl G, Ramos L, Buck J, Levin LR, Rassow J, Manfredi G, and Steegborn C. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J Biol Chem 286: 30423–30432, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agnes RS, Jernigan F, Shell JR, Sharma V, and Lawrence DS. Suborganelle sensing of mitochondrial cAMP-dependent protein kinase activity. J Am Chem Soc 132: 6075–6080, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, and Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J Biol Chem 287: 34445–34454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, and Bernal-Mizrachi E. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Aspects Med 42: 19–41, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alves TC, Pongratz RL, Zhao X, Yarborough O, Sereda S, Shirihai O, Cline GW, Mason G, and Kibbey RG. Integrated, step-wise, mass-isotopomeric flux analysis of the TCA cycle. Cell Metab 22: 936–947, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashcroft FM, Ashcroft SJ, and Harrison DE. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J Physiol 385: 517–529, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashcroft FM, Harrison DE, and Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312: 446–448, 1984. [DOI] [PubMed] [Google Scholar]
- 9. Ashcroft FM, Puljung MC, and Vedovato N. Neonatal diabetes and the K(ATP) channel: from mutation to therapy. Trends Endocrinol Metab 28: 377–387, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aspinwall CA, Brooks SA, Kennedy RT, and Lakey JR. Effects of intravesicular H+ and extracellular H+ and Zn2+ on insulin secretion in pancreatic beta cells. J Biol Chem 272: 31308–31314, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Babenko AP and Vaxillaire M. Mechanism of KATP hyperactivity and sulfonylurea tolerance due to a diabetogenic mutation in L0 helix of sulfonylurea receptor 1 (ABCC8). FEBS Lett 585: 3555–3559, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai T, Yang H, Wang H, Zhi L, Liu T, Cui L, Liu W, Wang Y, Zhang M, Liu Y, and Zhang Y. Inhibition of voltage-gated K(+) channels mediates docosahexaenoic acid-stimulated insulin secretion in rat pancreatic β-cells. Food Funct 11: 8893–8904, 2020. [DOI] [PubMed] [Google Scholar]
- 13. Barker CJ and Berggren PO. New horizons in cellular regulation by inositol polyphosphates: insights from the pancreatic β-cell. Pharmacol Rev 65: 641–669, 2013. [DOI] [PubMed] [Google Scholar]
- 14. Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, and Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282: 1141–1144, 1998. [DOI] [PubMed] [Google Scholar]
- 15. Beauvois MC, Merezak C, Jonas JC, Ravier MA, Henquin JC, and Gilon P. Glucose-induced mixed [Ca2+]c oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am J Physiol Cell Physiol 290: C1503–C1511, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Béguin P, Nagashima K, Nishimura M, Gonoi T, and Seino S. PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J 18: 4722–4732, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benáková Š, Holendová B, and Plecitá-Hlavatá L. Redox homeostasis in pancreatic β-cells: from development to failure. Antioxidants (Basel) 10: 526, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bender K, Maechler P, McClenaghan NH, Flatt PR, and Newsholme P. Overexpression of the malate-aspartate NADH shuttle member Aralar1 in the clonal beta-cell line BRIN-BD11 enhances amino-acid-stimulated insulin secretion and cell metabolism. Clin Sci (Lond) 117: 321–330, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett K, James C, and Hussain K. Pancreatic β-cell KATP channels: hypoglycaemia and hyperglycaemia. Rev Endocr Metab Disord 11: 157–163, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Bensellam M, Jonas JC, and Laybutt DR. Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol 236: R109–R143, 2018. [DOI] [PubMed] [Google Scholar]
- 21. Bergeron V, Ghislain J, and Poitout V. The P21-activated kinase PAK4 is implicated in fatty-acid potentiation of insulin secretion downstream of free fatty acid receptor 1. Islets 8: 157–164, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bermont F, Hermant A, Benninga R, Chabert C, Jacot G, Santo-Domingo J, Kraus MR, Feige JN, and De Marchi U. Targeting mitochondrial calcium uptake with the natural flavonol kaempferol, to promote metabolism/secretion coupling in pancreatic β-cells. Nutrients 12: 538, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96: 1261–1296, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Bertrand G, Ishiyama N, Nenquin M, Ravier MA, and Henquin JC. The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J Biol Chem 277: 32883–32891, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100: 14–31, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Bränström R, Aspinwall CA, Välimäki S, Ostensson CG, Tibell A, Eckhard M, Brandhorst H, Corkey BE, Berggren PO, and Larsson O. Long-chain CoA esters activate human pancreatic beta-cell KATP channels: potential role in type 2 diabetes. Diabetologia 47: 277–283, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Bränström R, Corkey BE, Berggren PO, and Larsson O. Evidence for a unique long chain acyl-CoA ester binding site on the ATP-regulated potassium channel in mouse pancreatic beta cells. J Biol Chem 272: 17390–17394, 1997. [DOI] [PubMed] [Google Scholar]
- 28. Bränström R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, and Larsson O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6. 2) of the ATP-regulated potassium channel. J Biol Chem 273: 31395–31400, 1998. [DOI] [PubMed] [Google Scholar]
- 29. Bunik VI. Redox-driven signaling: 2-oxo acid dehydrogenase complexes as sensors and transmitters of metabolic imbalance. Antioxid Redox Signal 30: 1911–1947, 2019. [DOI] [PubMed] [Google Scholar]
- 30. Casimir M, Lasorsa FM, Rubi B, Caille D, Palmieri F, Meda P, and Maechler P. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J Biol Chem 284: 25004–25014, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555, 2000. [DOI] [PubMed] [Google Scholar]
- 32. Cen J, Sargsyan E, and Bergsten P. Fatty acids stimulate insulin secretion from human pancreatic islets at fasting glucose concentrations via mitochondria-dependent and -independent mechanisms. Nutr Metab (Lond) 13: 59, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen WW, Freinkman E, Wang T, Birsoy K, and Sabatini DM. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166: 1324.e11–1337.e11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, and Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. [DOI] [PubMed] [Google Scholar]
- 35. Cook DL and Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311: 271–273, 1984. [DOI] [PubMed] [Google Scholar]
- 36. Craig TJ, Ashcroft FM, and Proks P. How ATP inhibits the open K(ATP) channel. J Gen Physiol 132: 131–144, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruz WS, Kwon G, Marshall CA, McDaniel ML, and Semenkovich CF. Glucose and insulin stimulate heparin-releasable lipoprotein lipase activity in mouse islets and INS-1 cells. A potential link between insulin resistance and beta-cell dysfunction. J Biol Chem 276: 12162–12168, 2001. [DOI] [PubMed] [Google Scholar]
- 38. De Marchi U, Galindo AN, Thevenet J, Hermant A, Bermont F, Lassueur S, Domingo JS, Kussmann M, Dayon L, and Wiederkehr A. Mitochondrial lysine deacetylation promotes energy metabolism and calcium signaling in insulin-secreting cells. FASEB J 33: 4660–4674, 2019. [DOI] [PubMed] [Google Scholar]
- 39. De Rasmo D, Micelli L, Santeramo A, Signorile A, Lattanzio P, and Papa S. cAMP regulates the functional activity, coupling efficiency and structural organization of mammalian FOF1 ATP synthase. Biochim Biophys Acta 1857: 350–358, 2016. [DOI] [PubMed] [Google Scholar]
- 40. de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, and Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. [DOI] [PubMed] [Google Scholar]
- 41. De Stefani D, Raffaello A, Teardo E, Szabò I, and Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Detimary P, Dejonghe S, Ling Z, Pipeleers D, Schuit F, and Henquin JC. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. J Biol Chem 273: 33905–33908, 1998. [DOI] [PubMed] [Google Scholar]
- 44. Di Benedetto G, Scalzotto E, Mongillo M, and Pozzan T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab 17: 965–975, 2013. [DOI] [PubMed] [Google Scholar]
- 45. Di Fulvio M and Aguilar-Bryan L.. Chloride transporters and channels in β-cell physiology: revisiting a 40-year-old model. Biochem Soc Trans 47: 1843–1855, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DiPilato LM, Cheng X, and Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A 101: 16513–16518, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doliba NM, Qin W, Najafi H, Liu C, Buettger CW, Sotiris J, Collins HW, Li C, Stanley CA, Wilson DF, Grimsby J, Sarabu R, Naji A, and Matschinsky FM. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab 302: E87–E102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drews G, Bauer C, Edalat A, Düfer M, and Krippeit-Drews P. Evidence against a Ca(2+)-induced potentiation of dehydrogenase activity in pancreatic beta-cells. Pflugers Arch 467: 2389–2397, 2015. [DOI] [PubMed] [Google Scholar]
- 49. Drews G, Krippeit-Drews P, and Düfer M. Electrophysiology of Islet Cells. In: Islam M. (ed). The Islets of Langerhans. Advances in Experimental Medicine and Biology, vol. 654. Springer, Dordrecht. 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- 50. Düfer M, Gier B, Wolpers D, Krippeit-Drews P, Ruth P, and Drews G. Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes 58: 1835–1843, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. El-Azzouny M, Evans CR, Treutelaar MK, Kennedy RT, and Burant CF. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. J Biol Chem 289: 13575–13588, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. El Azzouny M, Longacre MJ, Ansari IH, Kennedy RT, Burant CF, and MacDonald MJ. Knockdown of ATP citrate lyase in pancreatic beta cells does not inhibit insulin secretion or glucose flux and implicates the acetoacetate pathway in insulin secretion. Mol Metab 5: 980–987, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Esparza-Moltó PB and Cuezva JM. Reprogramming oxidative phosphorylation in cancer: a role for RNA-binding proteins. Antioxid Redox Signal 33: 927–945, 2020. [DOI] [PubMed] [Google Scholar]
- 54. Farfari S, Schulz V, Corkey B, and Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49: 718–726, 2000. [DOI] [PubMed] [Google Scholar]
- 55. Feng DD, Luo Z, Roh SG, Hernandez M, Tawadros N, Keating DJ, and Chen C. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology 147: 674–682, 2006. [DOI] [PubMed] [Google Scholar]
- 56. Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, Fielitz J, Olson EN, Prentki M, Biden T, MacDonald PE, and Poitout V. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 55: 2682–2692, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fernandez J and Valdeolmillos M. Increased levels of free fatty acids in fasted mice stimulate in vivo beta-cell electrical activity. Diabetes 47: 1707–1712, 1998. [DOI] [PubMed] [Google Scholar]
- 58. Fernandez J and Valdeolmillos M. Glucose-dependent stimulatory effect of glucagon-like peptide 1(7–36) amide on the electrical activity of pancreatic beta-cells recorded in vivo. Diabetes 48: 754–757, 1999. [DOI] [PubMed] [Google Scholar]
- 59. Fernandez J and Valdeolmillos M. Synchronous glucose-dependent [Ca(2+)](i) oscillations in mouse pancreatic islets of Langerhans recorded in vivo. FEBS Lett 477: 33–36, 2000. [DOI] [PubMed] [Google Scholar]
- 60. Fex M, Haemmerle G, Wierup N, Dekker-Nitert M, Rehn M, Ristow M, Zechner R, Sundler F, Holm C, Eliasson L, and Mulder H. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia 52: 271–280, 2009. [DOI] [PubMed] [Google Scholar]
- 61. Frayn KN. Metabolic Regulation: A Human Perspective. Chichester, UK: A John Wiley & Sons, Ltd., 2010, pp. 53–76; 144–168; 169–212; 306–328. [Google Scholar]
- 62. Freeman H, Shimomura K, Cox RD, and Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a link between insulin secretion, glucose metabolism and oxidative stress. Biochem Soc Trans 34: 806–810, 2006. [DOI] [PubMed] [Google Scholar]
- 63. Freeman HC, Hugill A, Dear NT, Ashcroft FM, and Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55: 2153–2156, 2006. [DOI] [PubMed] [Google Scholar]
- 64. Fujiwara K, Maekawa F, and Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab 289: E670–E677, 2005. [DOI] [PubMed] [Google Scholar]
- 65. Furman B, Ong WK, and Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol 654: 281–304, 2010. [DOI] [PubMed] [Google Scholar]
- 66. Gammelsaeter R, Coppola T, Marcaggi P, Storm-Mathisen J, Chaudhry FA, Attwell D, Regazzi R, and Gundersen V. A role for glutamate transporters in the regulation of insulin secretion. PLoS One 6: e22960, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gao ZY, Li G, Najafi H, Wolf BA, and Matschinsky FM. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes 48: 1535–1542, 1999. [DOI] [PubMed] [Google Scholar]
- 68. García-Aguilar A and Cuezva JM. A review of the inhibition of the mitochondrial ATP synthase by IF1 in vivo: reprogramming energy metabolism and inducing mitohormesis. Front Physiol 9: 1322, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. García-Bermúdez J, Sánchez-Aragó M, Soldevilla B, Del Arco A, Nuevo-Tapioles C, and Cuezva JM. PKA phosphorylates the ATPase inhibitory factor 1 and inactivates its capacity to bind and inhibit the mitochondrial H(+)-ATP synthase. Cell Rep 12: 2143–2155, 2015. [DOI] [PubMed] [Google Scholar]
- 70. Georgiadou E, Haythorne E, Dickerson MT, Lopez-Noriega L, Pullen TJ, da Silva Xavier G, Davis SPX, Martinez-Sanchez A, Semplici F, Rizzuto R, McGinty JA, French PM, Cane MC, Jacobson DA, Leclerc I, and Rutter GA. The pore-forming subunit MCU of the mitochondrial Ca(2+) uniporter is required for normal glucose-stimulated insulin secretion in vitro and in vivo in mice. Diabetologia 63: 1368–1381, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Georgiadou E and Rutter GA. Control by Ca(2+) of mitochondrial structure and function in pancreatic β-cells. Cell Calcium 91: 102282, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gheni G, Ogura M, Iwasaki M, Yokoi N, Minami K, Nakayama Y, Harada K, Hastoy B, Wu X, Takahashi H, Kimura K, Matsubara T, Hoshikawa R, Hatano N, Sugawara K, Shibasaki T, Inagaki N, Bamba T, Mizoguchi A, Fukusaki E, Rorsman P, and Seino S. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep 9: 661–673, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gilon P, Ravier MA, Jonas JC, and Henquin JC. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes 51(Suppl 1): S144–S151, 2002. [DOI] [PubMed] [Google Scholar]
- 74. Gledhill JR, Montgomery MG, Leslie AG, and Walker JE. How the regulatory protein, IF(1), inhibits F(1)-ATPase from bovine mitochondria. Proc Natl Acad Sci U S A 104: 15671–15676, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gloerich M and Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375, 2010. [DOI] [PubMed] [Google Scholar]
- 76. Graciano MF, Valle MM, Curi R, and Carpinelli AR. Evidence for the involvement of GPR40 and NADPH oxidase in palmitic acid-induced superoxide production and insulin secretion. Islets 5: 139–148, 2013. [DOI] [PubMed] [Google Scholar]
- 77. Gribble FM, Proks P, Corkey BE, and Ashcroft FM. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J Biol Chem 273: 26383–26387, 1998. [DOI] [PubMed] [Google Scholar]
- 78. Grimsrud PA, Carson JJ, Hebert AS, Hubler SL, Niemi NM, Bailey DJ, Jochem A, Stapleton DS, Keller MP, Westphall MS, Yandell BS, Attie AD, Coon JJ, and Pagliarini DJ. A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Cell Metab 16: 672–683, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grupe M, Myers G, Penner R, and Fleig A. Activation of store-operated I(CRAC) by hydrogen peroxide. Cell Calcium 48: 1–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gu J, Zhang L, Zong S, Guo R, Liu T, Yi J, Wang P, Zhuo W, and Yang M. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science 364: 1068–1075, 2019. [DOI] [PubMed] [Google Scholar]
- 81. Guay C, Joly E, Pepin E, Barbeau A, Hentsch L, Pineda M, Madiraju SR, Brunengraber H, and Prentki M. A role for cytosolic isocitrate dehydrogenase as a negative regulator of glucose signaling for insulin secretion in pancreatic ß-cells. PLoS One 8: e77097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guay C, Madiraju SR, Aumais A, Joly E, and Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282: 35657–35665, 2007. [DOI] [PubMed] [Google Scholar]
- 83. Gurgul-Convey E, Kaminski MT, and Lenzen S. Physiological characterization of the human EndoC-βH1 β-cell line. Biochem Biophys Res Commun 464: 13–19, 2015. [DOI] [PubMed] [Google Scholar]
- 84. Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, and Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9: 163–173, 2002. [DOI] [PubMed] [Google Scholar]
- 85. Hasan NM, Longacre MJ, Stoker SW, Kendrick MA, and MacDonald MJ. Mitochondrial malic enzyme 3 is important for insulin secretion in pancreatic β-cells. Mol Endocrinol 29: 396–410, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hashim M, Yokoi N, Takahashi H, Gheni G, Okechi OS, Hayami T, Murao N, Hidaka S, Minami K, Mizoguchi A, and Seino S. Inhibition of SNAT5 induces incretin-responsive state from incretin-unresponsive state in pancreatic β-cells: study of β-cell spheroid clusters as a model. Diabetes 67: 1795–1806, 2018. [DOI] [PubMed] [Google Scholar]
- 87. Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, Weinglass AB, Engelstoft MS, Madsen AN, Luckmann M, Miller MW, Trujillo ME, Frimurer TM, Holst B, Howard AD, and Schwartz TW. GPR40 (FFAR1)—combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab 4: 3–14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Heissig H, Urban KA, Hastedt K, Zünkler BJ, and Panten U. Mechanism of the insulin-releasing action of alpha-ketoisocaproate and related alpha-keto acid anions. Mol Pharmacol 68: 1097–1105, 2005. [DOI] [PubMed] [Google Scholar]
- 89. Henquin JC, Dufrane D, and Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55: 3470–3477, 2006. [DOI] [PubMed] [Google Scholar]
- 90. Hodson DJ, Tarasov AI, Gimeno Brias S, Mitchell RK, Johnston NR, Haghollahi S, Cane MC, Bugliani M, Marchetti P, Bosco D, Johnson PR, Hughes SJ, and Rutter GA. Incretin-modulated beta cell energetics in intact islets of Langerhans. Mol Endocrinol 28: 860–871, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, Johnson P, Ashcroft FM, and Rorsman P. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab 10: 455–465, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Høy M, Maechler P, Efanov AM, Wollheim CB, Berggren PO, and Gromada J. Increase in cellular glutamate levels stimulates exocytosis in pancreatic beta-cells. FEBS Lett 531: 199–203, 2002. [DOI] [PubMed] [Google Scholar]
- 93. Hull J, Hindy ME, Kehoe PG, Chalmers K, Love S, and Conway ME. Distribution of the branched chain aminotransferase proteins in the human brain and their role in glutamate regulation. J Neurochem 123: 997–1009, 2012. [DOI] [PubMed] [Google Scholar]
- 94. Husted AS, Trauelsen M, Rudenko O, Hjorth SA, and Schwartz TW. GPCR-mediated signaling of metabolites. Cell Metab 25: 777–796, 2017. [DOI] [PubMed] [Google Scholar]
- 95. Itoh K, Moriguchi R, Yamada Y, Fujita M, Yamato T, Oumi M, Holst JJ, and Seino Y. High saturated fatty acid intake induces insulin secretion by elevating gastric inhibitory polypeptide levels in healthy individuals. Nutr Res 34: 653–660, 2014. [DOI] [PubMed] [Google Scholar]
- 96. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, and Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422: 173–176, 2003. [DOI] [PubMed] [Google Scholar]
- 97. Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in ‘t Veld P, Renström E, and Schuit FC. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54: 2132–2142, 2005. [DOI] [PubMed] [Google Scholar]
- 98. Jabůrek M, Průchová P, Holendová B, Galkin A, and Ježek P. Antioxidant synergy of mitochondrial phospholipase PNPLA8/iPLA2γ with fatty acid–conducting SLC25 gene family transporters. Antioxidants (Basel) 10: 678, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jacob S, Köhler M, Tröster P, Visa M, García-Prieto CF, Alanentalo T, Moede T, Leibiger B, Leibiger IB, and Berggren PO. In vivo Ca(2+) dynamics in single pancreatic β cells. FASEB J 34: 945–959, 2020. [DOI] [PubMed] [Google Scholar]
- 100. Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammälä CE, and Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab 6: 229–235, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jacobson DA and Shyng SL. Ion channels of the islets in type 2 diabetes. J Mol Biol 432: 1326–1346, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jensen MD and Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 56: 68–76, 2007. [DOI] [PubMed] [Google Scholar]
- 103. Ježek J, Dlasková A, Zelenka J, Jabůrek M, and Ježek P. H2O2-activated mitochondrial phospholipase iPLA2γ prevents lipotoxic oxidative stress in synergy with UCP2, amplifies signaling via g-protein–coupled receptor GPR40, and regulates insulin secretion in pancreatic β-cells. Antioxid Redox Signal 23: 958–972, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ježek P, Holendová B, Jabůrek M, Tauber J, Dlasková A, and Plecitá-Hlavatá L. The pancreatic β-cell: the perfect redox system. Antioxidants (Basel) 10: 197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jezek P, Holendova B, and Plecita-Hlavata L. Redox signaling from mitochondria: signal propagation and its targets. Biomolecules 10: 93, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jezek P, Jaburek M, Holendova B, and Plecita-Hlavata L. Fatty acid-stimulated insulin secretion vs. lipotoxicity. Molecules 23: 1483, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ježek P, Jabůrek M, and Plecitá-Hlavatá L. Contribution of oxidative stress and impaired biogenesis of pancreatic β-cells to type 2 diabetes. Antioxid Redox Signal 31: 722–751, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jimenez-Sánchez C, Brun T, and Maechler P. Mitochondrial carriers regulating insulin secretion profiled in human islets upon metabolic stress. Biomolecules 10: 1543, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jing X, Li DQ, Olofsson CS, Salehi A, Surve VV, Caballero J, Ivarsson R, Lundquist I, Pereverzev A, Schneider T, Rorsman P, and Renström E. CaV2.3 calcium channels control second-phase insulin release. J Clin Invest 115: 146–154, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jitrapakdee S, Wutthisathapornchai A, Wallace JC, and MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53: 1019–1032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, Berishvili E, Duncanson P, Watkinson M, Broichhagen J, Trauner D, Rutter GA, and Hodson DJ. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 24: 389–401, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alárcon C, Rhodes CJ, and Newgard CB. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 281: 35624–35632, 2006. [DOI] [PubMed] [Google Scholar]
- 113. Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, and Newgard CB. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282: 31592–31600, 2007. [DOI] [PubMed] [Google Scholar]
- 114. Kadenbach B. Regulation of cytochrome c oxidase contributes to health and optimal life. World J Biol Chem 11: 52–61, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kahancová A, Sklenář F, Ježek P, and Dlasková A. Regulation of glucose-stimulated insulin secretion by ATPase inhibitory factor 1 (IF1). FEBS Lett 592: 999–1009, 2018. [DOI] [PubMed] [Google Scholar]
- 116. Kahancová A, Sklenář F, Ježek P, and Dlasková A. Overexpression of native IF1 downregulates glucose-stimulated insulin secretion by pancreatic INS-1E cells. Sci Rep 10: 1551, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kahn SE, Hull RL, and Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006. [DOI] [PubMed] [Google Scholar]
- 118. Kakei M, Kelly RP, Ashcroft SJ, and Ashcroft FM. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett 208: 63–66, 1986. [DOI] [PubMed] [Google Scholar]
- 119. Kakei M, Yoshida M, Dezaki K, Ito K, Yamada H, Funazaki S, Kawakami M, Sugawara H, and Yada T. Glucose and GTP-binding protein-coupled receptor cooperatively regulate transient receptor potential-channels to stimulate insulin secretion [review]. Endocr J 63: 867–876, 2016. [DOI] [PubMed] [Google Scholar]
- 120. Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, and Holz GG. Epac-selective cAMP analog 8-pCPT-2'-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278: 8279–8285, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kang G, Leech CA, Chepurny OG, Coetzee WA, and Holz GG. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells. J Physiol 586: 1307–1319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kanno T, Suga S, Wu J, Kimura M, and Wakui M. Intracellular cAMP potentiates voltage-dependent activation of L-type Ca2+ channels in rat islet beta-cells. Pflugers Arch 435: 578–580, 1998. [DOI] [PubMed] [Google Scholar]
- 123. Kashio M and Tominaga M. Redox signal-mediated enhancement of the temperature sensitivity of transient receptor potential melastatin 2 (TRPM2) elevates glucose-induced insulin secretion from pancreatic islets. J Biol Chem 290: 12435–12442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kennedy ED, Rizzuto R, Theler JM, Pralong WF, Bastianutto C, Pozzan T, and Wollheim CB. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J Clin Invest 98: 2524–2538, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Khan S and Kowluru A. CD36 mediates lipid accumulation in pancreatic beta cells under the duress of glucolipotoxic conditions: novel roles of lysine deacetylases. Biochem Biophys Res Commun 495: 2221–2226, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, and Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab 5: 253–264, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Klec C, Ziomek G, Pichler M, Malli R, and Graier WF. Calcium signaling in ß-cell physiology and pathology: a revisit. Int J Mol Sci 20: 6110, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, and Barbetti F. The G53D mutation in Kir6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J Clin Endocrinol Metab 93: 1054–1061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kostic M, Katoshevski T, and Sekler I. Allosteric regulation of NCLX by mitochondrial membrane potential links the metabolic state and Ca(2+) signaling in mitochondria. Cell Rep 25: 3465.e4–3475.e4, 2018. [DOI] [PubMed] [Google Scholar]
- 130. Kristinsson H, Bergsten P, and Sargsyan E. Free fatty acid receptor 1 (FFAR1/GPR40) signaling affects insulin secretion by enhancing mitochondrial respiration during palmitate exposure. Biochim Biophys Acta 1853: 3248–3257, 2015. [DOI] [PubMed] [Google Scholar]
- 131. Lee KPK, Chen J, and MacKinnon R. Molecular structure of human KATP in complex with ATP and ADP. Elife 6: e32481, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lefkimmiatis K, Leronni D, and Hofer AM. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J Cell Biol 202: 453–462, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lefkimmiatis K and Zaccolo M. cAMP signaling in subcellular compartments. Pharmacol Ther 143: 295–304, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Leguina-Ruzzi A, Vodičková A, Holendová B, Pavluch V, Tauber J, Engstová H, Dlasková A, and Ježek P. Glucose-induced expression of DAPIT in pancreatic β-cells. Biomolecules 10: 1026, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 36: 343–347, 2008. [DOI] [PubMed] [Google Scholar]
- 136. Lenzen S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim Biophys Acta Gen Subj 1861: 1929–1942, 2017. [DOI] [PubMed] [Google Scholar]
- 137. Lenzen S, Drinkgern J, and Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996. [DOI] [PubMed] [Google Scholar]
- 138. Lenzen S, Formanek H, and Panten U. Signal function of metabolism of neutral amino acids and 2-keto acids for initiation of insulin secretion. J Biol Chem 257: 6631–6633, 1982. [PubMed] [Google Scholar]
- 139. Lenzen S, Schmidt W, and Panten U. Transamination of neutral amino acids and 2-keto acids in pancreatic B-cell mitochondria. J Biol Chem 260: 12629–12634, 1985. [PubMed] [Google Scholar]
- 140. Lenzen S, Schmidt W, Rustenbeck I, and Panten U. 2-ketoglutarate generation in pancreatic B-cell mitochondria regulates insulin secretory action of amino acids and 2-keto acids. Biosci Rep 6: 163–169, 1986. [DOI] [PubMed] [Google Scholar]
- 141. Lewandowski SL, Cardone RL, Foster HR, Ho T, Potapenko E, Poudel C, VanDeusen HR, Sdao SM, Alves TC, Zhao X, Capozzi ME, de Souza AH, Jahan I, Thomas CJ, Nunemaker CS, Davis DB, Campbell JE, Kibbey RG, and Merrins MJ. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab 32: 736.e5–750.e5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Li N, Wu JX, Ding D, Cheng J, Gao N, and Chen L. Structure of a pancreatic ATP-sensitive potassium channel. Cell 168: 101.e10–110.e10, 2017. [DOI] [PubMed] [Google Scholar]
- 143. Light PE, Manning Fox JE, Riedel MJ, and Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol 16: 2135–2144, 2002. [DOI] [PubMed] [Google Scholar]
- 144. Lin YF, Jan YN, and Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J 19: 942–955, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu T, Li H, Gounko NV, Zhou Z, Xu A, Hong W, and Han W. Detection of insulin granule exocytosis by an electrophysiology method with high temporal resolution reveals enlarged insulin granule pool in BIG3-knockout mice. Am J Physiol Endocrinol Metab 307: E611–E618, 2014. [DOI] [PubMed] [Google Scholar]
- 146. Lorenz MA, El Azzouny MA, Kennedy RT, and Burant CF. Metabolome response to glucose in the β-cell line INS-1 832/13. J Biol Chem 288: 10923–10935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, and Sherry AD. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci U S A 99: 2708–2713, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. MacDonald MJ and Fahien LA. Glutamate is not a messenger in insulin secretion. J Biol Chem 275: 34025–34027, 2000. [DOI] [PubMed] [Google Scholar]
- 149. MacDonald MJ, Hasan NM, and Longacre MJ. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta 1780: 966–972, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. MacDonald PE. Signal integration at the level of ion channel and exocytotic function in pancreatic β-cells. Am J Physiol Endocrinol Metab 301: E1065–E1069, 2011. [DOI] [PubMed] [Google Scholar]
- 151. MacDonald PE, Salapatek AM, and Wheeler MB. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K(+) currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes 51(Suppl 3): S443–S447, 2002. [DOI] [PubMed] [Google Scholar]
- 152. Maechler P. Mitochondrial function and insulin secretion. Mol Cell Endocrinol 379: 12–18, 2013. [DOI] [PubMed] [Google Scholar]
- 153. Maechler P. Glutamate pathways of the beta-cell and the control of insulin secretion. Diabetes Res Clin Pract 131: 149–153, 2017. [DOI] [PubMed] [Google Scholar]
- 154. Maechler P, Kennedy ED, Pozzan T, and Wollheim CB. Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic beta-cells. EMBO J 16: 3833–3841, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Maechler P and Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402: 685–689, 1999. [DOI] [PubMed] [Google Scholar]
- 156. Maechler P and Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature 414: 807–812, 2001. [DOI] [PubMed] [Google Scholar]
- 157. Mancini AD, Bertrand G, Vivot K, Carpentier É, Tremblay C, Ghislain J, Bouvier M, and Poitout V. β-Arrestin recruitment and biased agonism at free fatty acid receptor 1. J Biol Chem 290: 21131–21140, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Marshall BA, Tordjman K, Host HH, Ensor NJ, Kwon G, Marshall CA, Coleman T, McDaniel ML, and Semenkovich CF. Relative hypoglycemia and hyperinsulinemia in mice with heterozygous lipoprotein lipase (LPL) deficiency. Islet LPL regulates insulin secretion. J Biol Chem 274: 27426–27432, 1999. [DOI] [PubMed] [Google Scholar]
- 159. Martin GM, Yoshioka C, Rex EA, Fay JF, Xie Q, Whorton MR, Chen JZ, and Shyng SL. Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Elife 6: e24149, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJ, and Galione A. NAADP: a new second messenger for glucose-induced Ca2+ responses in clonal pancreatic beta cells. Curr Biol 13: 247–251, 2003. [DOI] [PubMed] [Google Scholar]
- 161. Masia R, Koster JC, Tumini S, Chiarelli F, Colombo C, Nichols CG, and Barbetti F. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes 56: 328–336, 2007. [DOI] [PubMed] [Google Scholar]
- 162. Masiello P, Novelli M, Bombara M, Fierabracci V, Vittorini S, Prentki M, and Bergamini E. The antilipolytic agent 3,5-dimethylpyrazole inhibits insulin release in response to both nutrient secretagogues and cyclic adenosine monophosphate agonists in isolated rat islets. Metabolism 51: 110–114, 2002. [DOI] [PubMed] [Google Scholar]
- 163. McClenaghan NH and Flatt PR. Glucose and non-glucidic nutrients exert permissive effects on 2-keto acid regulation of pancreatic beta-cell function. Biochim Biophys Acta 1426: 110–118, 1999. [DOI] [PubMed] [Google Scholar]
- 164. McCormack JG, Halestrap AP, and Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990. [DOI] [PubMed] [Google Scholar]
- 165. McKenna JP, Ha J, Merrins MJ, Satin LS, Sherman A, and Bertram R. Ca2+ effects on ATP production and consumption have regulatory roles on oscillatory islet activity. Biophys J 110: 733–742, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, and Ashcroft FM. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J 24: 4166–4175, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mitok KA, Freiberger EC, Schueler KL, Rabaglia ME, Stapleton DS, Kwiecien NW, Malec PA, Hebert AS, Broman AT, Kennedy RT, Keller MP, Coon JJ, and Attie AD. Islet proteomics reveals genetic variation in dopamine production resulting in altered insulin secretion. J Biol Chem 293: 5860–5877, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Miyanohara J, Kakae M, Nagayasu K, Nakagawa T, Mori Y, Arai K, Shirakawa H, and Kaneko S. TRPM2 channel aggravates CNS inflammation and cognitive impairment via activation of microglia in chronic cerebral hypoperfusion. J Neurosci 38: 3520–3533, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, and Bernier M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 140: 1132–1140, 1999. [DOI] [PubMed] [Google Scholar]
- 170. Moon MJ, Park S, Kim DK, Cho EB, Hwang JI, Vaudry H, and Seong JY. Structural and molecular conservation of glucagon-like Peptide-1 and its receptor confers selective ligand-receptor interaction. Front Endocrinol (Lausanne) 3: 141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Moran BM, Abdel-Wahab YH, Flatt PR, and McKillop AM. Activation of GPR119 by fatty acid agonists augments insulin release from clonal β-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol Chem 395: 453–464, 2014. [DOI] [PubMed] [Google Scholar]
- 172. Moran BM, Abdel-Wahab YH, Flatt PR, and McKillop AM. Evaluation of the insulin-releasing and glucose-lowering effects of GPR120 activation in pancreatic β-cells. Diabetes Obes Metab 16: 1128–1139, 2014. [DOI] [PubMed] [Google Scholar]
- 173. Moss CE, Glass LL, Diakogiannaki E, Pais R, Lenaghan C, Smith DM, Wedin M, Bohlooly YM, Gribble FM, and Reimann F. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 77: 16–20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mugabo Y, Zhao S, Lamontagne J, Al-Mass A, Peyot ML, Corkey BE, Joly E, Madiraju SRM, and Prentki M. Metabolic fate of glucose and candidate signaling and excess-fuel detoxification pathways in pancreatic β-cells. J Biol Chem 292: 7407–7422, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mugabo Y, Zhao S, Seifried A, Gezzar S, Al-Mass A, Zhang D, Lamontagne J, Attane C, Poursharifi P, Iglesias J, Joly E, Peyot ML, Gohla A, Madiraju SR, and Prentki M. Identification of a mammalian glycerol-3-phosphate phosphatase: role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc Natl Acad Sci U S A 113: E430–E439, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mulder H, Yang S, Winzell MS, Holm C, and Ahrén B. Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes 53: 122–128, 2004. [DOI] [PubMed] [Google Scholar]
- 177. Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, and Tschöp MH. Glucagon-like peptide 1 (GLP-1). Mol Metab 30: 72–130, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Newman JC and Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr 37: 51–76, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006. [DOI] [PubMed] [Google Scholar]
- 180. Noguchi S, Kondo Y, Ito R, Katayama T, Kazama S, Kadota Y, Kitaura Y, Harris RA, and Shimomura Y. Ca(2+)-dependent inhibition of branched-chain α-ketoacid dehydrogenase kinase by thiamine pyrophosphate. Biochem Biophys Res Commun 504: 916–920, 2018. [DOI] [PubMed] [Google Scholar]
- 181. Nunes Marsiglio-Librais G, Aparecida Vilas-Boas E, Carlein C, Hoffmann MDA, Roma LP, and Carpinelli AR. Evidence for NADPH oxidase activation by GPR40 in pancreatic β-cells. Redox Rep 25: 41–50, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Nyrén R, Chang CL, Lindström P, Barmina A, Vorrsjö E, Ali Y, Juntti-Berggren L, Bensadoun A, Young SG, Olivecrona T, and Olivecrona G. Localization of lipoprotein lipase and GPIHBP1 in mouse pancreas: effects of diet and leptin deficiency. BMC Physiol 12: 14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Odegaard ML, Joseph JW, Jensen MV, Lu D, Ilkayeva O, Ronnebaum SM, Becker TC, and Newgard CB. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem 285: 16530–16537, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Olofsson CS, Salehi A, Holm C, and Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol 557: 935–948, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ortiz D, Voyvodic P, Gossack L, Quast U, and Bryan J. Two neonatal diabetes mutations on transmembrane helix 15 of SUR1 increase affinity for ATP and ADP at nucleotide binding domain 2. J Biol Chem 287: 17985–17995, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ostapchenko VG, Chen M, Guzman MS, Xie YF, Lavine N, Fan J, Beraldo FH, Martyn AC, Belrose JC, Mori Y, MacDonald JF, Prado VF, Prado MA, and Jackson MF. The transient receptor potential melastatin 2 (TRPM2) channel contributes to β-amyloid oligomer-related neurotoxicity and memory impairment. J Neurosci 35: 15157–15169, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ould Amer Y and Hebert-Chatelain E.. Mitochondrial cAMP-PKA signaling: what do we really know? Biochim Biophys Acta Bioenerg 1859: 868–877, 2018. [DOI] [PubMed] [Google Scholar]
- 188. Ouyang Q, Nakayama T, Baytas O, Davidson SM, Yang C, Schmidt M, Lizarraga SB, Mishra S, Ei-Quessny M, Niaz S, Gul Butt M, Imran Murtaza S, Javed A, Chaudhry HR, Vaughan DJ, Hill RS, Partlow JN, Yoo SY, Lam AT, Nasir R, Al-Saffar M, Barkovich AJ, Schwede M, Nagpal S, Rajab A, DeBerardinis RJ, Housman DE, Mochida GH, and Morrow EM. Mutations in mitochondrial enzyme GPT2 cause metabolic dysfunction and neurological disease with developmental and progressive features. Proc Natl Acad Sci U S A 113: E5598–E5607, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Panten U, Früh E, Reckers K, and Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets: role of cytosolic acetyl-CoA. Metabolism 65: 1225–1229, 2016. [DOI] [PubMed] [Google Scholar]
- 190. Panten U, Willenborg M, Schumacher K, Hamada A, Ghaly H, and Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism 62: 1375–1386, 2013. [DOI] [PubMed] [Google Scholar]
- 191. Pathak T and Trebak M. Mitochondrial Ca(2+) signaling. Pharmacol Ther 192: 112–123, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Peyot ML, Guay C, Latour MG, Lamontagne J, Lussier R, Pineda M, Ruderman NB, Haemmerle G, Zechner R, Joly E, Madiraju SR, Poitout V, and Prentki M. Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. J Biol Chem 284: 16848–16859, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Plecitá-Hlavatá L, Engstová H, Holendová B, Tauber J, Špaček T, Petrásková L, Křen V, Špačková J, Gotvaldová K, Ježek J, Dlasková A, Smolková K, and Ježek P. Mitochondrial superoxide production decreases on glucose-stimulated insulin secretion in pancreatic β cells due to decreasing mitochondrial matrix NADH/NAD(+) ratio. Antioxid Redox Signal 33: 789–815, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Plecita-Hlavata L, Jaburek M, Holendova B, Tauber J, Pavluch V, Berkova Z, Cahova M, Schroeder K, Brandes RP, Siemen D, and Jezek P. Glucose-stimulated insulin secretion fundamentally requires H2O2 signaling by NADPH oxidase 4. Diabetes 69: 1341–1354, 2020. [DOI] [PubMed] [Google Scholar]
- 195. Pongratz RL, Kibbey RG, Shulman GI, and Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 282: 200–207, 2007. [DOI] [PubMed] [Google Scholar]
- 196. Prentki M, Joly E, El-Assaad W, and Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes 51(Suppl 3): S405–S413, 2002. [DOI] [PubMed] [Google Scholar]
- 197. Prentki M, Matschinsky Franz M, and Madiraju SRM. Metabolic signaling in fuel-induced insulin secretion. Cell Metab 18: 162–185, 2013. [DOI] [PubMed] [Google Scholar]
- 198. Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, and Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267: 5802–5810, 1992. [PubMed] [Google Scholar]
- 199. Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, and Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci U S A 101: 17539–17544, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Pujol JB, Christinat N, Ratinaud Y, Savoia C, Mitchell SE, and Dioum EHM. Coordination of GPR40 and ketogenesis signaling by medium chain fatty acids regulates beta cell function. Nutrients 10: 473, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Qian J, Gu Y, Wu C, Yu F, Chen Y, Zhu J, Yao X, Bei C, and Zhu Q. Agonist-induced activation of human FFA1 receptor signals to extracellular signal-regulated kinase 1 and 2 through Gq- and Gi-coupled signaling cascades. Cell Mol Biol Lett 22: 13, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Quan X, Nguyen TT, Choi SK, Xu S, Das R, Cha SK, Kim N, Han J, Wiederkehr A, Wollheim CB, and Park KS. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 290: 4086–4096, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ramzan R, Rhiel A, Weber P, Kadenbach B, and Vogt S. Reversible dimerization of cytochrome c oxidase regulates mitochondrial respiration. Mitochondrion 49: 149–155, 2019. [DOI] [PubMed] [Google Scholar]
- 204. Reinbothe TM, Ivarsson R, Li D-Q, Niazi O, Jing X, Zhang E, Stenson L, Bryborn U, and Renström E. Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol Endocrinol 23: 893–900, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Remedi MS, Koster JC, Patton BL, and Nichols CG. ATP-sensitive K+ channel signaling in glucokinase-deficient diabetes. Diabetes 54: 2925–2931, 2005. [DOI] [PubMed] [Google Scholar]
- 206. Remizov O, Jakubov R, Düfer M, Krippeit Drews P, Drews G, Waring M, Brabant G, Wienbergen A, Rustenbeck I, and Schöfl C. Palmitate-induced Ca2+-signaling in pancreatic beta-cells. Mol Cell Endocrinol 212: 1–9, 2003. [DOI] [PubMed] [Google Scholar]
- 207. Ribas GS and Vargas CR. Evidence that oxidative disbalance and mitochondrial dysfunction are involved in the pathophysiology of fatty acid oxidation disorders. Cell Mol Neurobiol 2020, Sep 2. doi: 10.1007/s10571-020-00955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Roma LP and Jonas JC. Nutrient metabolism, subcellular redox state, and oxidative stress in pancreatic islets and beta-cells. J Mol Biol 432: 1461–1493, 2020. [DOI] [PubMed] [Google Scholar]
- 209. Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, and Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281: 30593–30602, 2006. [DOI] [PubMed] [Google Scholar]
- 210. Rorsman P and Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 98: 117–214, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Rorsman P and Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75: 155–179, 2013. [DOI] [PubMed] [Google Scholar]
- 212. Rorsman P, Braun M, and Zhang Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium 51: 300–308, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Rorsman P, Eliasson L, Kanno T, Zhang Q, and Gopel S. Electrophysiology of pancreatic β-cells in intact mouse islets of Langerhans. Prog Biophys Mol Biol 107: 224–235, 2011. [DOI] [PubMed] [Google Scholar]
- 214. Rorsman P and Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 46: 1029–1045, 2003. [DOI] [PubMed] [Google Scholar]
- 215. Rorsman P and Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol 374: 531–550, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Rosca M, Minkler P, and Hoppel CL. Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta 1807: 1373–1382, 2011. [DOI] [PubMed] [Google Scholar]
- 217. Rubi B, del Arco A, Bartley C, Satrustegui J, and Maechler P. The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J Biol Chem 279: 55659–55666, 2004. [DOI] [PubMed] [Google Scholar]
- 218. Rutter GA, Hodson DJ, Chabosseau P, Haythorne E, Pullen TJ, and Leclerc I. Local and regional control of calcium dynamics in the pancreatic islet. Diabetes Obes Metab 19(Suppl 1): 30–41, 2017. [DOI] [PubMed] [Google Scholar]
- 219. Rutter GA, Pralong WF, and Wollheim CB. Regulation of mitochondrial glycerol-phosphate dehydrogenase by Ca2+ within electropermeabilized insulin-secreting cells (INS-1). Biochim Biophys Acta 1175: 107–113, 1992. [DOI] [PubMed] [Google Scholar]
- 220. Rydström J. Mitochondrial NADPH, transhydrogenase and disease. Biochim Biophys Acta 1757: 721–726, 2006. [DOI] [PubMed] [Google Scholar]
- 221. Sabourin J and Allagnat F. Store-operated Ca2+ entry: a key component of the insulin secretion machinery. J Mol Endocrinol 57: F35–F39, 2016. [DOI] [PubMed] [Google Scholar]
- 222. Sabrautzki S, Kaiser G, Przemeck GKH, Gerst F, Lorza-Gil E, Panse M, Sartorius T, Hoene M, Marschall S, Haring HU, Hrabe de Angelis M, and Ullrich S. Point mutation of Ffar1 abrogates fatty acid-dependent insulin secretion, but protects against HFD-induced glucose intolerance. Mol Metab 6: 1304–1312, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sakaguchi R and Mori Y. Transient receptor potential (TRP) channels: biosensors for redox environmental stimuli and cellular status. Free Radic Biol Med 146: 36–44, 2020. [DOI] [PubMed] [Google Scholar]
- 224. Santo-Domingo J, Chareyron I, Dayon L, Núñez Galindo A, Cominetti O, Pilar Giner Giménez M, De Marchi U, Canto C, Kussmann M, and Wiederkehr A. Coordinated activation of mitochondrial respiration and exocytosis mediated by PKC signaling in pancreatic β cells. FASEB J 31: 1028–1045, 2017. [DOI] [PubMed] [Google Scholar]
- 225. Santos LRB, Muller C, de Souza AH, Takahashi HK, Spégel P, Sweet IR, Chae H, Mulder H, and Jonas J-C. NNT reverse mode of operation mediates glucose control of mitochondrial NADPH and glutathione redox state in mouse pancreatic β-cells. Mol Metab 6: 535–547, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sassmann A, Gier B, Gröne HJ, Drews G, Offermanns S, and Wettschureck N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Invest 120: 2184–2193, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, and Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272: 18572–18579, 1997. [DOI] [PubMed] [Google Scholar]
- 228. Schulla V, Renström E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, Obermüller S, Olofsson CS, Salehi A, Wendt A, Klugbauer N, Wollheim CB, Rorsman P, and Hofmann F. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J 22: 3844–3854, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Sener A, Conget I, Rasschaert J, Leclercq-Meyer V, Villanueva-Peñacarrillo ML, Valverde I, and Malaisse WJ. Insulinotropic action of glutamic acid dimethyl ester. Am J Physiol 267: E573–E584, 1994. [DOI] [PubMed] [Google Scholar]
- 230. Shen L, Zhi L, Hu W, and Wu MX. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death Differ 16: 603–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Shigeto M, Ramracheya R, Tarasov AI, Cha CY, Chibalina MV, Hastoy B, Philippaert K, Reinbothe T, Rorsman N, Salehi A, Sones WR, Vergari E, Weston C, Gorelik J, Katsura M, Nikolaev VO, Vennekens R, Zaccolo M, Galione A, Johnson PR, Kaku K, Ladds G, and Rorsman P. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest 125: 4714–4728, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Shimomura K, de Nanclares GP, Foutinou C, Caimari M, Castaño L, and Ashcroft FM. The first clinical case of a mutation at residue K185 of Kir6.2 (KCNJ11): a major ATP-binding residue. Diabet Med 27: 225–229, 2010. [DOI] [PubMed] [Google Scholar]
- 233. Shyng S, Ferrigni T, and Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol 110: 643–654, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Shyng SL and Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282: 1138–1141, 1998. [DOI] [PubMed] [Google Scholar]
- 235. Smith PA, Ashcroft FM, and Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K(+)-currents in isolated mouse pancreatic beta-cells. FEBS Lett 261: 187–190, 1990. [DOI] [PubMed] [Google Scholar]
- 236. Somanath S, Partridge CJ, Marshall C, Rowe T, and Turner MD. Snapin mediates insulin secretory granule docking, but not trans-SNARE complex formation. Biochem Biophys Res Commun 473: 403–407, 2016. [DOI] [PubMed] [Google Scholar]
- 237. Song WJ, Seshadri M, Ashraf U, Mdluli T, Mondal P, Keil M, Azevedo M, Kirschner LS, Stratakis CA, and Hussain MA. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 13: 308–319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, and Olefsky JM. Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc Natl Acad Sci U S A 105: 6614–6619, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Soty M, Visa M, Soriano S, del Carmen Carmona M, Nadal Á, and Novials A. Involvement of ATP-sensitive potassium (K ATP) channels in the loss of beta-cell function induced by human islet amyloid polypeptide. J Biol Chem 286: 40857–40866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Spégel P and Mulder H. Metabolomics analysis of nutrient metabolism in β-cells. J Mol Biol 432: 1429–1445, 2020. [DOI] [PubMed] [Google Scholar]
- 241. Spégel P, Sharoyko VV, Goehring I, Danielsson AP, Malmgren S, Nagorny CL, Andersson LE, Koeck T, Sharp GW, Straub SG, Wollheim CB, and Mulder H. Time-resolved metabolomics analysis of β-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J 450: 595–605, 2013. [DOI] [PubMed] [Google Scholar]
- 242. Speier S, Yang SB, Sroka K, Rose T, and Rupnik M. KATP-channels in beta-cells in tissue slices are directly modulated by millimolar ATP. Mol Cell Endocrinol 230: 51–58, 2005. [DOI] [PubMed] [Google Scholar]
- 243. Srinivasan S, Spear J, Chandran K, Joseph J, Kalyanaraman B, and Avadhani NG. Oxidative stress induced mitochondrial protein kinase A mediates cytochrome c oxidase dysfunction. PLoS One 8: e77129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Stegink LD, Filer LJ Jr., and Baker GL. Plasma amino acid concentrations in normal adults fed meals with added monosodium L-glutamate and aspartame. J Nutr 113: 1851–1860, 1983. [DOI] [PubMed] [Google Scholar]
- 245. Stein DT, Stevenson BE, Chester MW, Basit M, Daniels MB, Turley SD, and McGarry JD. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest 100: 398–403, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Stuhlmann T, Planells-Cases R, and Jentsch TJ. LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion. Nat Commun 9: 1974, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Sumoza-Toledo A and Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol 589: 1515–1525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Swisa A, Glaser B, and Dor Y. Metabolic stress and compromised identity of pancreatic beta cells. Front Genet 8: 21, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Szollosi A, Nenquin M, and Henquin J. Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K+ channels. Br J Pharmacol 159: 669–677, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Takahashi H, Yokoi N, and Seino S. Glutamate as intracellular and extracellular signals in pancreatic islet functions. Proc Jpn Acad Ser B Phys Biol Sci 95: 246–260, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Tarasov AI, Girard CA, and Ashcroft FM. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic beta-cells. Diabetes 55: 2446–2454, 2006. [DOI] [PubMed] [Google Scholar]
- 252. Tarasov AI, Semplici F, Li D, Rizzuto R, Ravier MA, Gilon P, and Rutter GA. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic β cells. Pflugers Arch 465: 543–554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, and Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS One 7: e39722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Teraoku H and Lenzen S. Dynamics of insulin secretion from EndoC-βH1 β-cell pseudoislets in response to glucose and other nutrient and nonnutrient secretagogues. J Diabetes Res 2017: 2309630, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Thompson A and Kanamarlapudi V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gαq pathway. Biochem Pharmacol 93: 72–84, 2015. [DOI] [PubMed] [Google Scholar]
- 256. Tomita T, Hosoda K, Fujikura J, Inagaki N, and Nakao K. The G-protein-coupled long-chain fatty acid receptor GPR40 and glucose metabolism. Front Endocrinol (Lausanne) 5: 152, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, Mijat V, Goldsworthy M, Moir L, Haynes A, Quarterman J, Freeman HC, Ashcroft FM, and Cox RD. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia 48: 675–686, 2005. [DOI] [PubMed] [Google Scholar]
- 258. Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, and Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J 369: 287–299, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Usui R, Yabe D, Fauzi M, Goto H, Botagarova A, Tokumoto S, Tatsuoka H, Tahara Y, Kobayashi S, Manabe T, Baba Y, Kurosaki T, Herrera PL, Ogura M, Nagashima K, and Inagaki N. GPR40 activation initiates store-operated Ca(2+) entry and potentiates insulin secretion via the IP3R1/STIM1/Orai1 pathway in pancreatic β-cells. Sci Rep 9: 15562, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Vakilian M, Tahamtani Y, and Ghaedi K. A review on insulin trafficking and exocytosis. Gene 706: 52–61, 2019. [DOI] [PubMed] [Google Scholar]
- 261. van der Vusse GJ. Albumin as fatty acid transporter. Drug Metab Pharmacokinet 24: 300–307, 2009. [DOI] [PubMed] [Google Scholar]
- 262. Vedovato N, Rorsman O, Hennis K, Ashcroft FM, and Proks P. Role of the C-terminus of SUR in the differential regulation of β-cell and cardiac K(ATP) channels by MgADP and metabolism. J Physiol 596: 6205–6217, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Veprik A, Laufer D, Weiss S, Rubins N, and Walker MD. GPR41 modulates insulin secretion and gene expression in pancreatic β-cells and modifies metabolic homeostasis in fed and fasting states. FASEB J 30: 3860–3869, 2016. [DOI] [PubMed] [Google Scholar]
- 264. Vierra NC, Dadi PK, Jeong I, Dickerson M, Powell DR, and Jacobson DA. Type 2 diabetes-associated K+ channel TALK-1 modulates β-cell electrical excitability, second-phase insulin secretion, and glucose homeostasis. Diabetes 64: 3818–3828, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Vierra NC, Dickerson MT, Philipson LH, and Jacobson DA. Simultaneous real-time measurement of the β-cell membrane potential and Ca(2+) influx to assess the role of potassium channels on β-cell function. Methods Mol Biol 1684: 73–84, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, and Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 57: 678–687, 2008. [DOI] [PubMed] [Google Scholar]
- 267. Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, and Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab 13(Suppl 1): 95–105, 2011. [DOI] [PubMed] [Google Scholar]
- 268. Watmough NJ and Frerman FE. The electron transfer flavoprotein: ubiquinone oxidoreductases. Biochim Biophys Acta 1797: 1910–1916, 2010. [DOI] [PubMed] [Google Scholar]
- 269. Weissert V, Rieger B, Morris S, Arroum T, Psathaki OE, Zobel T, Perkins G, and Busch KB. Inhibition of the mitochondrial ATPase function by IF1 changes the spatiotemporal organization of ATP synthase. Biochim Biophys Acta Bioenerg 1862: 148322, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Welsh N, Margulis B, Borg LA, Wiklund HJ, Saldeen J, Flodström M, Mello MA, Andersson A, Pipeleers DG, and Hellerström C. Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med 1: 806–820, 1995. [PMC free article] [PubMed] [Google Scholar]
- 271. Wiederkehr A, Szanda G, Akhmedov D, Mataki C, Heizmann CW, Schoonjans K, Pozzan T, Spät A, and Wollheim CB. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab 13: 601–611, 2011. [DOI] [PubMed] [Google Scholar]
- 272. Winzell MS, Ström K, Holm C, and Ahrén B. Glucose-stimulated insulin secretion correlates with beta-cell lipolysis. Nutr Metab Cardiovasc Dis 16(Suppl 1): S11–S16, 2006. [DOI] [PubMed] [Google Scholar]
- 273. Wong N, Blair AR, Morahan G, and Andrikopoulos S. The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology 151: 96–102, 2010. [DOI] [PubMed] [Google Scholar]
- 274. Woo HA, Yim SH, Shin DH, Kang D, Yu DY, and Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 140: 517–528, 2010. [DOI] [PubMed] [Google Scholar]
- 275. Wootten D, Reynolds CA, Smith KJ, Mobarec JC, Koole C, Savage EE, Pabreja K, Simms J, Sridhar R, Furness SGB, Liu M, Thompson PE, Miller LJ, Christopoulos A, and Sexton PM. The extracellular surface of the GLP-1 receptor is a molecular trigger for biased agonism. Cell 165: 1632–1643, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Yamada H, Yoshida M, Ito K, Dezaki K, Yada T, Ishikawa SE, and Kakei M. Potentiation of glucose-stimulated insulin secretion by the GPR40-PLC-TRPC pathway in pancreatic β-cells. Sci Rep 6: 25912, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Yang HQ, Martinez-Ortiz W, Hwang J, Fan X, Cardozo TJ, and Coetzee WA. Palmitoylation of the K(ATP) channel Kir6.2 subunit promotes channel opening by regulating PIP(2) sensitivity. Proc Natl Acad Sci U S A 117: 10593–10602, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Yang J, Chi Y, Burkhardt BR, Guan Y, and Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev 68: 270–279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, and Gong DW. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 49: 598–607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Yang SN, Shi Y, Yang G, Li Y, Yu J, and Berggren PO. Ionic mechanisms in pancreatic β cell signaling. Cell Mol Life Sci 71: 4149–4177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Yashiro H, Tsujihata Y, Takeuchi K, Hazama M, Johnson PR, and Rorsman P. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J Pharmacol Exp Ther 340: 483–489, 2012. [DOI] [PubMed] [Google Scholar]
- 282. Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, Numata T, Mori Y, Miyazaki J, Miki T, and Seino S. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab 12: 117–129, 2010. [DOI] [PubMed] [Google Scholar]
- 283. Yasui S, Mawatari K, Morizumi R, Furukawa H, Shimohata T, Harada N, Takahashi A, and Nakaya Y. Hydrogen peroxide inhibits insulin-induced ATP-sensitive potassium channel activation independent of insulin signaling pathway in cultured vascular smooth muscle cells. J Med Invest 59: 36–44, 2012. [DOI] [PubMed] [Google Scholar]
- 284. Yoshida M, Dezaki K, Yamato S, Aoki A, Sugawara H, Toyoshima H, Ishikawa SE, Kawakami M, Nakata M, Yada T, and Kakei M. Regulation of voltage-gated K+ channels by glucose metabolism in pancreatic beta-cells. FEBS Lett 583: 2225–2230, 2009. [DOI] [PubMed] [Google Scholar]
- 285. Yosida M, Dezaki K, Uchida K, Kodera S, Lam NV, Ito K, Rita RS, Yamada H, Shimomura K, Ishikawa SE, Sugawara H, Kawakami M, Tominaga M, Yada T, and Kakei M. Involvement of cAMP/EPAC/TRPM2 activation in glucose- and incretin-induced insulin secretion. Diabetes 63: 3394–3403, 2014. [DOI] [PubMed] [Google Scholar]
- 286. Zhang F, Qi Y, Zhou K, Zhang G, Linask K, and Xu H. The cAMP phosphodiesterase Prune localizes to the mitochondrial matrix and promotes mtDNA replication by stabilizing TFAM. EMBO Rep 16: 520–527, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhang F, Zhang L, Qi Y, and Xu H. Mitochondrial cAMP signaling. Cell Mol Life Sci 73: 4577–4590, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Zhang L, Duan X, Sun W, and Sun H. Perfluorooctane sulfonate acute exposure stimulates insulin secretion via GPR40 pathway. Sci Total Environ 726: 138498, 2020. [DOI] [PubMed] [Google Scholar]
- 289. Zhang Q, Chibalina MV, Bengtsson M, Groschner LN, Ramracheya R, Rorsman NJ, Leiss V, Nassar MA, Welling A, Gribble FM, Reimann F, Hofmann F, Wood JN, Ashcroft FM, and Rorsman P. Na+ current properties in islet α- and β-cells reflect cell-specific Scn3a and Scn9a expression. J Physiol 592: 4677–4696, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, and Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J 24: 1497–1505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Zhao S, Mugabo Y, Iglesias J, Xie L, Delghingaro-Augusto V, Lussier R, Peyot ML, Joly E, Taïb B, Davis MA, Brown JM, Abousalham A, Gaisano H, Madiraju SR, and Prentki M. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab 19: 993–1007, 2014. [DOI] [PubMed] [Google Scholar]
- 292. Zhao X, León IR, Bak S, Mogensen M, Wrzesinski K, Højlund K, and Jensen ON. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol Cell Proteomics 10: M110.000299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]