Abstract
Previous systematic reviews have confirmed that carbohydrate (CHO) mouth rinse may boost physical exercise performance, despite some methodological aspects likely affecting its ergogenic effect. In this review, we discussed if the exercise mode, pre-exercise fasting status, CHO solutions concentration, CHO solutions temperature, mouth rinse duration, and CHO placebo effects may potentially reduce the CHO mouth rinse ergogenic effect, suggesting possible solutions to manage these potential confounders. The effectiveness of CHO mouth rinse as a performance booster is apparently related to the origin of the exercise-induced neuromuscular fatigue, as CHO mouth rinse unequivocally potentiates endurance rather than sprint and strength exercises performance. Furthermore, ergogenic effects have been greater in fasting than fed state, somehow explaining the varied magnitude of the CHO mouth rinse effects in exercise performance. In this regard, the CHO solution concentration and temperature, as well as the mouth rinse duration, may have increased the variability observed in CHO mouth rinse effects in fasting and fed state. Finally, placebo effects have challenged the potential of the CHO mouth rinse as an ergogenic aid. Therefore, we suggest that future studies should consider methodological controls such as sample size and sample homogeneity, proper familiarization with experimental procedures, and the use of alternative placebo designs to provide unbiased evidence regarding the potential of the CHO mouth rinse as an ergogenic aid.
Keywords: Central fatigue, Peripheral fatigue, Nutritional supplementation, Placebo, Physical tests
Introduction
Carbohydrate (CHO) ingestion improves endurance exercise performance1,2 as this strategy maintains the blood glucose concentrations within adequate levels, thus sparing muscle and liver glycogen contents during exercise.3–6 Given the key role of muscle glycogen as a fuel during exercise, 7 CHO ingestion has been also shown to improve exercise performance either during prolonged (i.e., > 1 h) 8 or short duration exercises (i.e.,< 1 h),9–11 even though neither muscle glycogen stores nor hypoglycemia has been considered as a limiting factor of exercises lasting shorter than 1 h. 12 This intriguing aspect led researchers to suggest a centrally mediated action of the CHO supplementation ergogenic effects, as isolated peripheral mechanisms involving muscle glycogen stores and blood glucose levels could not explain the CHO effects on exercises shorter than 1 h. 13
A seminal study composed of two experiments provided support to this hypothesis. Using magnetic resonance imaging technique in the first experiment, 14 Chambers et al. observed a greater activation in reward-system-related brain areas such as the insula and anterior cingulate cortex, when participants rinsed their mouth with solutions containing glucose or maltodextrin. In the second experiment, the authors observed that participants improved the 1 h cycling time trial performance when they rinsed their mouth with CHO solutions. Together, these results indicated that improvements in cycling time trial performance were likely associated with a CHO mouth rinse-induced activation in cerebral areas, as no CHO was ingested by participants. Indeed, original15–17and review18,19 studies have supported the use of CHO mouth rinse to boost exercise performance. For example, Peart 18 detected a small but significant improvement in performance with CHO mouth rinse during exercise lasting ⩾ 25 min (effect size, ES = 0.25) and ~1 h time trials (ES = 0.31), in contrast to a trivial effect in resistance exercise (ES =-0.09) or exercise protocols lasting ⩽ 3 min (ES = 0.06). 18 These results confirmed the effectiveness of the CHO mouth rinse to improve exercise performance. However, studies have also reported a varied magnitude of the CHO mouth rinse effects, so that methodological aspects likely playing as confounding factors should be discussed.
Studies designed to investigate the CHO mouth rinse effects on exercise performance have shown a variety of methodological aspects with potential confounding factors. For example, the type of exercise used to assess performance likely influences the potential of the CHO mouth rinse as an ergogenic aid, as the central–peripheral fatigue interplay is related to the exercise type-induced neuromuscular fatigue. 20 Moreover, given the oropharyngeal receptors-mediated CHO mouth rinse effects, one may hypothesize that mouth rinse duration and CHO solution concentration may likely affect the potential of this strategy as ergogenic aid. 21 As a result, the pre-exercise feeding state (i.e., fasting versus fed state) may constitute a further additional confounding factor, as the CHO solution-derived energetic content signalized to brain areas may vary between postprandial states.22,23 Finally, the use of different placebos may be an important source of bias in CHO mouth rinse literature, as limitations to produce comparable placebo and CHO compounds (i.e., taste, smell, and color) may have led to an ineffective blinding procedure in some studies, thereby increasing the chances of placebo-induced effect on exercise performance. 24 Unfortunately, however, neither systematic nor narrative reviews have addressed these methodological features comprehensively (Figure 1), proposing possible directions to improve the methodological control in future CHO mouth rinse studies.
Figure 1.
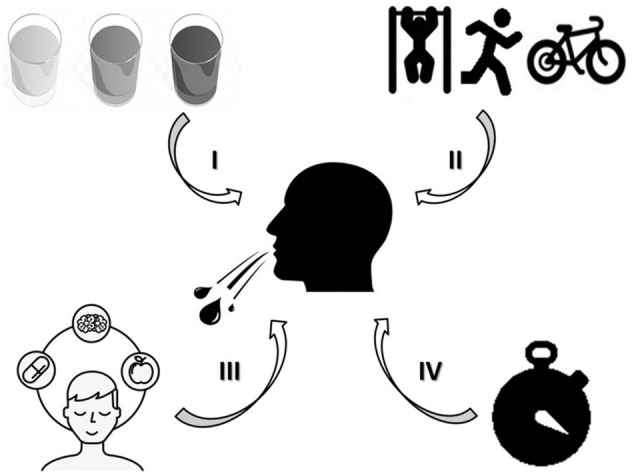
Potential manipulating determinants of the CHO mouth rinse ergogenic effects, with regard to the CHO solution concentration (Panel I), the type of exercise protocol (Panel II), the placebo effect (Panel III), and the mouth rinse duration (Panel IV).
Therefore, the aim of this narrative review was to discuss potential confounding factors present in the CHO mouth rinse literature, providing methodological insights and directions for future studies.
Type of Exercise Protocol
Most studies investigating the potential ergogenic effects of CHO mouth rinse were designed to assess cycling and running performance in endurance exercise protocols lasting longer than 30 min, as shown by meta-analytical reviews.18,19 Overall, it has been reported improvements from ~1.5% to 11.5% in endurance performance,18,19,25 but effects in performance of single- and repeated-sprint exercises or strength exercises have been controversial. While some have shown a beneficial CHO mouth rinse effect on single-sprint performance,26,27 others have challenged this nutritional ergogenic strategy in this type of exercise.28–30 Likewise, some studies have shown improvements in repeated-sprint performance ability,31,32 while others have failed to show beneficial effects.33–35 A recent systematic review and meta-analysis found trivial CHO mouth rinse ergogenic effects in single- as well as repeated-sprint performance, 36 thus suggesting that this strategy may be ineffective to improve performance in sprint exercises. Accordingly, studies assessing CHO mouth rinse effects on strength exercise have also shown controversial findings37–40 on muscular strength and/or muscle endurance performance outcomes.17,29,41–46 Somehow, this controversy can be associated with methodological aspects of previous studies.
Issues regarding the methodological control of short and very high-intensity exercise protocols may be partially related to some controversial results. First, most CHO mouth rinse studies using short and very high-intensity exercises have failed to provide well-controlled familiarization sessions. The use of familiarization sessions is a key methodological aspect to reduce learning-derived systematic errors before assessing performance outcomes in single and multiple experimental trials. 47 For example, three or more trials may be necessary to familiarize participants with testing procedures until producing stable and reliable performance measures, mainly in novice participants. Second, studies have recruited either recreationally active participants or trained individuals from a broad range of training status and experience level, thereby increasing the difficulty of providing reliable performance assessment. For example, previous investigations showed that trained individuals exhibited a greater consistency in motor performance than their untrained counterparts during physical tasks requiring high levels of muscle force, 48 likely due to the greater motor unit synchronization and improved electrical properties of motor units49,50 in trained rather than untrained participants. Therefore, using participants from varied training experiences without preliminary trials to adequately familiarize them with testing procedures likely contributed to increase the variability in performance outcomes of high-intensity exercise tests. Considering that significant CHO mouth rinse beneficial effects on performance may be of small magnitude,18,19 the lack of procedures to minimize the variability in high-intensity exercise test-derived performance outcomes may have reduced the sensibility in assessing true CHO mouth rinse effects in previous studies. Hence, studies aiming at CHO mouth rinse ergogenic effects on sprint and strength exercise performance should make efforts to minimize possible sources of variability in exercise protocols, recruiting trained and experienced individuals and performing adequate familiarization sessions with experimental procedures before the experimental trials. In order to provide unbiased evidence with a higher level of reliability on CHO mouth rinse results, future studies are recommended to use familiarization trials until assessing performance outcomes within acceptable variation or conduct preliminary studies to calculate the typical error of measurement51,52 before the CHO mouth rinse intervention.
Regarding endurance rather than strength or sprints exercise performance, the consistency of the CHO mouth rinse as ergogenic aid is apparently associated with the nature of neuromuscular fatigue development. The mechanisms underlying neuromuscular fatigue are multifactorial and vary according to intensity and duration of the exercise task. 53 Supporting this notion, Thomas et al. 20 found that different exercise durations elicited different magnitudes of central and peripheral fatigue as assessed through electrical twitch interpolation technique, as there was a shift in the central–peripheral fatigue interplay toward a central limitation as the cycling time trial duration increased from 4 km to 20 km and 40 km. A greater peripheral fatigue was detected as the shorter cycling time trial progressed (i.e., 4 km cycling time trial), in contrast to the greater central fatigue found after longer cycling time trials such as 20 km and 40 km. 20 The hypothesis was that participants completing the 4 km cycling trial faced higher levels of muscle metabolites-induced neuromuscular fatigue (47) such as hydrogen and potassium accumulation,54,55 thus contrasting to centrally mediated reductions in motor command and voluntary activation in 40 km cycling time trial. 56 Somehow, this different exercise intensity-neuromuscular fatigue interplay may provide rationale to the controversial results involving endurance exercise protocols, as addressed below.
As suggested earlier by Chambers et al., 14 CHO mouth rinse effects are centrally mediated through activation of reward-system cerebral areas associated with motivation and perceived exertion.14,57 Recently, studies also have found that CHO mouth rinse increased cortical activity in areas involved in motor planning and motor command.14,58,59 Consequently, beneficial CHO mouth rinse effects on performance are potentially higher in exercises eliciting central more than peripheral fatigue, as the CHO centrally mediated effects could counteract fatigue-induced reductions in motor command and voluntary activation. Indeed, in a recent study, we observed that CHO mouth rinse mitigated central fatigue during a 4 km cycling time trial, although being ineffective to enhance performance (likely due to a peripheral fatigue nature of this trial). 60 Accordingly, Bastos-Silva et al. 61 verified beneficial CHO mouth rinse effects on a cycling-to-exhaustion test performance in intensity showing central rather than peripheral fatigue, that is low-to-moderate (80% of the respiratory compensation point) rather than high-intensity exercise (110% of the peak power output). Together, these studies61,62 provided some support to the idea that CHO mouth rinse may be beneficial to improve performance mainly in exercise protocols that induce a central–peripheral fatigue interplay toward central limitations (i.e., moderate intensity, long duration). Accordingly, one may argue that CHO mouth rinse may also have different beneficial effects in upper versus lower limbs strength exercises, given the different fatigue development between them.63,64 However, the relationship between exercise type (intensity and duration) and lower versus upper limbs, and CHO mouth rinse ergogenic effects remains poorly understood so that mechanistic and applied studies are necessary to reveal the true CHO mouth rinse ergogenic effects on endurance and strength exercises having different neuromuscular fatigue etiology.
Pre-exercise Nutritional Status
The pre-exercise nutritional status may be another methodological factor to influence the CHO mouth rinse effects on exercise performance. Beneficial CHO mount rinse effects have been reported mainly by studies assessing performance in post-absorptive state longer than 4 h or following an overnight fasting,14,65–67 However, controversial results including ineffective60,68–70 and effective CHO mouth rinse effects 71 have been found in exercise performance assessed with varied postprandial conditions.
An earlier study by Fares and Kayser 72 showed that CHO mouth rinse improved exercise performance in a cycling-to-exhaustion exercise at 60% of maximum power output irrespective of the participants’ nutritional state, thereby reinforcing the notion of a beneficial CHO mouth rinse effect even after a CHO-enriched breakfast. However, in addition to those methodological issues addressed previously such as testing protocols (high variability), participants (trained versus novice), and familiarization trials (inadequate reliability), other two aspects may have contributed to increase the concern over these results. First, information regarding the total energy density and CHO amount in pre-exercise meals was insufficient to properly evaluate the participants’ nutritional state. Second, instead of a solution with comparable taste, color, and smell to CHO solution, the authors used water as a placebo, thus introducing a serious concern on the effectiveness in blinding participants from the true CHO content. In a well-designed and controlled study, Lane et al. 73 detected that CHO mouth rinse was capable of improving the mean power output during a ~1 h cycling time trial after both an overnight fasting or a high-CHO meal, although the magnitude of the performance enhancement has been higher in fasted (3.4%) than fed state (1.8%). Results of this later study suggest that CHO mouth rinse ergogenic effects may partially rely on the participants’ prandial state.
Despite poorly understood, insights about the mechanisms behind the reduced CHO mouth rinse effects in fed state may have been found by an earlier study. An fMRI study comparing cortical responses to oral sucrose-induced taste stimuli (219 g·L−1; 22% CHO) either after an overnight fast (12 h) or immediately after the ingestion of a 700-kcal liquid meal 74 observed that cerebral regions such as insula showed a greater activation in fasting than postprandial state. Somehow, it is possible that variations in the food-derived metabolic signals such as plasma ghrelin and leptin75,76 played a role in the CHO mouth rinse-induced modulation in cerebral activity, inducing greater responses mainly after long periods of food abstinence (> 4 h).
The hypothesis of a higher CHO mouth rinse ergogenic aid in fasting than fed state does not necessarily discourage the use of this nutritional strategy in practical settings, as Lane et al. 73 showed that the best performance increment was achieved by combining both CHO-rich pre-exercise meal with CHO month rinse throughout a 1 h simulated cycling time trial. In addition, CHO mouth rinse could be an interesting strategy to athletes engaged in restrictive diets such as intermittent fasting 77 or those suffering from gastrointestinal discomfort, who are likely to abstain from food intake before a training session or a competitive challenge. Nevertheless, studies are required to better explore the fasting versus fed state effects on CHO mouth rinse ergogenic aid.
Mouth Rinse Duration
Perhaps, the CHO mouth rinse duration has been one of the first methodological aspects highlighted as a confounding factor in the literature. However, only a few studies26,46,78,79 have attempted to verify the influence of longer mouth rinses with CHO solution on exercise performance.
Most studies have used mouth rinses lasting a short 5s period,18,19,80 thus the question that remains to be answered is that exposing oral receptors to CHO solution during longer periods would induce a higher stimulation of reward-system cerebral regions involved in behavioral and autonomic responses, 14 thereby facilitating motor command to peripheral muscles as a result. Using transcranial magnetic stimulation of the primary motor cortex to assess corticomotor excitability and voluntary force production, Gant et al. 59 reported significant increases in motor output and force production when the oral cavity was exposed to CHO content for approximately 60 s. Study by Sinclair et al. 79 was likely the first to compare the effect of different CHO mouth rinse durations (i.e., 5 versus 10 s) on the total distance covered during a 30 min cycling time trial. When compared with the placebo-controlled condition, results showed that participants covered longer total distances only if rinsing their mouth with CHO during 10s, although increases in performance have also been found in ten out of the 11 participants with 5s mouth rinse. Importantly, when comparing 10s to 5s, eight out of the 11 participants cycled longer when they rinsed their mouth during 10s. Despite these results may suggest a duration–ergogenic effect relationship of the CHO mouth rinse, 79 methodological limitations such as the absence of familiarization sessions and the lack of a double-blind, randomized, and counterbalanced design limited these results to infer to a higher-level inference.
Accordingly, Tomko et al. 78 verified if serial CHO mouth rinses produced higher improvements in sprint performance than single, isolated rinses. Participants performed three sets of repeated anaerobic sprint tests (6 x 35 m sprints interspersed by 7 min between them) either rinsing their mouth with a single rinse for 5s before the sprint test or rinsing their mouth with multiple 5, 10, and 15s rinses (before the first, second, and third sets of the sprint test, respectively). Overall, serial rinses resulted in changes that were trivial on peak and average sprint time compared to single rinses. However, methodological aspects such as the absence of familiarization sessions may have increased the heterogeneity in sprint test performance outcomes. Moreover, the use of an exercise protocol that elicited peripheral rather than central fatigue may also have surrendered an additional difficulty to reveal the true CHO mouth rinse effects. 78 Indeed, a recent study by Nehme et al. 81 found no benefits in performance measured in the Yo-Yo Intermittent Recovery Test Level 1 with serial CHO mouth rinses. Interestingly, neither serial nor single CHO mouth rinses exhibited superior beneficial effects than placebo mouth rinses. Hence, future studies using straightforward methodologies (i.e., including central fatigue-limited exercise tests; adequate familiarization trials; appropriate randomized, double-blind controlled designs; trained participants) should investigate if CHO mouth rinses with varied durations affect its ergogenic effects.
CHO Solution Concentration
Furthermore, the concentration of the CHO content seems to play a role in the effectiveness of the CHO mouth wash ergogenic effects. Possibly, the oral receptors-mediated reward-system cerebral areas are sensitive to the energetic value of the CHO content.21,82 The fact that a previous study 21 found that cerebral areas bordering the reward-system such as hypothalamic areas were progressively activated when participants ingested CHO content with 8.3% and 25% concentrations, respectively, offers some support to this notion. Since the CHO-triggered effects were observed immediately after the ingestion, before any absorption-related effect, this result suggested that comparable effects in reward-system areas could be found if rinsing the mouth with CHO (i.e., free from ingestion-derived “metabolic” effects), as observed elsewhere. 14 Consequently, one may argue that CHO mouth rinse activates the reward-system cerebral areas in a dose-dependent manner, so that the higher the CHO solution concentration, the higher the cerebral activation is. Consequently, a greater exercise performance improvement may also be expected with higher CHO solution concentrations, assuming a cerebral activation–performance improvement relationship.
Studies have tried to clarify a potential CHO dose–response relationship.70,83–88 The study by Wright and Davison 83 was probably the first to investigate this hypothesis. They observed that solutions with 6% and 12% CHO content similarly enhanced exercise performance as measured as the total distance covered in a 90 min running test. However, the limited sample size and the lack of familiarization sessions were important concerns of this study. 83 Accordingly, later studies also provided biased evidence regarding a potential CHO dose–response relationship. For example, Kulaksiz et al. 84 and Clarke et al. 86 found no additive effects of CHO mouth rinses in cycling and running time trial performance with concentrations progressively higher such as 3%, 6%, and 12% CHO content, respectively. Despite no dose–response relationship has been detected, the inclusion of untrained and inexperienced individuals in a small and heterogeneous sample size, together with inadequate familiarization sessions, and the use of a single- rather than double-blind design may have reduced the power to detect a true CHO dose–response relationship.84,86 The fact neither CHO mouth rinse ergogenic effects were detected by these studies suggests that bias may have threatened the conclusions regarding a potential CHO dose–response relationship in endurance exercise performance.
Regarding the effects of different CHO concentrations on strength performance, study by Karayigit et al. 88 compared the effect of 6%, 12%, and 18% CHO concentrations mouth rinses on maximum strength and strength endurance of resistance-trained young women. The authors detected no differences in strength performance among CHO concentrations, given that no CHO mouth rinse ergogenic effect was observed. Again, the inappropriate familiarization sessions and control of potential confounding factors such as menstrual cycle variations may have threatened conclusions in the study.
Overall, studies assessing performance in ~1 h simulated cycling time trial have found controversial results as well. For example, Isopoglou et al. 70 found no CHO dose–response relationship effects in exercise performance investigating 4%, 6%, and 8% CHO content. Accordingly, Devenney et al. 87 showed that CHO mouth rinse increased performance regardless of concentrations of 6% and 12%. Recently, James et al. 85 also found that cyclists rinsing their mouth with 7% and 14% CHO solution enhanced the ~1 h cycling time trial performance by ~3.5% irrespective of the different CHO concentrations. As previously pointed out to other studies, the inclusion of untrained and inexperienced cyclists together with inadequate familiarization sessions may have biased some of these results. Hence, future studies with straightforward design and methodology are still necessary to unravel this issue.
Temperature of the CHO Solution
Other potential confounding factor includes the temperature of the CHO solution, as this may affect performance mainly if the exercise is performed in hot and humid environments. Theoretically, CHO mouth rinsing solutions at low temperatures might confer a competitive advantage by providing a pleasant sensation, decreasing the perception of effort, and increasing the chances of maintaining adequate activation of the neural drive to the muscles. 89 In fact, studies investigating the effects of the temperature of sports drinks support the notion of a core temperature modulation and endurance performance improvement after the ingestion of sports drinks at low temperatures during prolonged exercises in hot environments.90,91 Therefore, one may hypothesize that the CHO solutions offered in lower temperatures could provide eventual beneficial CHO mouth rinse effects in performance, mainly if the exercise is performed in environments with challenging temperatures. However, this hypothesis has not been explored yet, and future studies are required to clarify this.
Potential Placebo Effects on the CHO Mouth Rinse Ergogenic Aid
The placebo effect has been neglected as a possible confounding factor of studies pertaining the CHO mouth rinse ergogenic effects. Placebo effect is a complex phenomenon triggered by psychosocial factors related to inert substance-derived positive outcomes due to the expectation of beneficial effects, thereby possibly altering motor performance and associated responses. 92 Although the mechanisms underlying the ergogenic placebo effect are not fully elucidated, it has been suggested that they involve endogenous opioids and reward-related dopaminergic system that result in decreased pain and augmented corticospinal facilitation.93,94 Given the fact that placebo-induced cerebral changes share similar routes of CHO mouth rinse-induced cerebral changes14,58 such as the reward-system circuitry activation,14,95 one may argue that potential placebo effects can have contributed to controversial results in the CHO mouth rinse literature.
Concerns involving potential placebo effects have been highlighted in sports nutrition fields.96,97 Most CHO mouth rinse studies have used either single- or double-blind designs controlled by a placebo. 98 However, the use of a single placebo as control without including baseline trials has been challenged,99,100 as the expectation of a beneficial effect when ingesting a placebo may change the participants’ motivation and potentiate performance.97,101 Hence, CHO mouth rinse studies using single- or double-blinded designs may have observed an additive placebo effect to the CHO mouth rinse effect on exercise performance outcomes. We argue that the use of a double-control design including a baseline trial together with a placebo trial58,102 is necessary to determine the magnitude of a potential CHO mouth rinse placebo effect, thus accurately assessing the true CHO mouth rinse effects in performance. Discussions in sports nutrition fields including caffeine supplementation have suggested similar approach, 24 so we suggest that it is time to do the same in the CHO mouth rinse literature.
Furthermore, the substance used to mimic the CHO solution has challenged the adequate control by placebo. Studies have employed artificial sweeteners or water as a placebo of the CHO solution, 19 so that the effectiveness in blinding participants from the true CHO solution is a challenge. It is important to point out that an unsuccessful blinding procedure may lead participants to correctly guess the true allocation of CHO and placebo substances, adding bias in experimental procedures and influencing the beneficial CHO mouth rinse effects in exercise performance. Likewise, the unsuccess to adequately blind participants from true placebo trials may negatively influence the exercise performance (i.e., nocebo effect) and add bias. Hence, future studies should improve the experimental procedures to obtain placebos that adequately mimic the true CHO solutions, thus reducing the risk of bias in CHO mouth rinse investigations. First, the application of specific surveys103,104 to check the blinding efficacy may help researchers to monitor the placebo-blinding efficacy. However, given that the taste itself can be ergogenic and elicit a placebo effect, 105 CHO mouth rinse studies should also make efforts to design a straightforward methodology to separate CHO mouth rinse effects from placebo effects. For example, in contrast to traditional randomized clinical trials, the use of CHO-perceived placebo designs may be an alternative to investigate CHO mouth rinse effects on exercise performance while minimizing potential placebo effects, given that comparable cortical responses between CHO and placebo solutions have been found regardless of performance responses. 58 This design makes possible to control for the individuals’ expectancies regarding the CHO solution, as the participants’ expectation in placebo trial is driven toward the CHO solution106,107 so that eventual differences between CHO and placebo mouth rinse can be attributed to the true active substance.15,108
Summary of Concerns and Future Directions
Despite several studies supporting the notion that CHO mouth rinse is a strategy capable of improving exercise performance, confounding factors may have introduced bias in the CHO mouth rinse literature. Future studies aiming at CHO mouth rinse effects in exercise performance are suggested to design straightforward methodologies to manage factors such as sample size and sample homogeneity, adequate familiarization trials, and the use of alternative placebo designs to provide unbiased evidence regarding the role of the exercise mode, feeding state, CHO solution concentration, mouth rinse duration, and temperature of the CHO solution as confounding factors. The use of CHO mouth rinses instead of CHO ingestion as a strategy to improve exercise performance is promising, as it has the advantage of inducing no gastrointestinal discomfort. Hence, CHO mouth rinse may be considered as a useful and applicable ergogenic strategy for athletes and exercise practitioners in sports scenarios. However, given that most evidence has been obtained in laboratory performance tests, future studies are also required to expand these results to real-world settings.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Vitor S Painelli is grateful to the Individual Research Program of the Paulista University for his scholarship of productivity in research (#7-02-1166/2021). Flávio O Pires is grateful to the National Council for Scientific and Technological Development (CNPq—Brazil) for his scholarship of productivity in research (#310355/2019-2). Cayque Brietzke is grateful to São Paulo Research Foundation (FAPESP) for his scholarship (#2020/04827-0).
ORCID iDs: Cayque Brietzke  https://orcid.org/0000-0002-7557-3140
https://orcid.org/0000-0002-7557-3140
Flávio Oliveira Pires  https://orcid.org/0000-0003-4538-1751
https://orcid.org/0000-0003-4538-1751
References
- 1. Casazza GA, Tovar AP, Richardson CE, et al. Energy availability, macronutrient intake, and nutritional supplementation for improving exercise performance in endurance athletes. Curr Sports Med Rep 2018; 17(6): 215–223. [DOI] [PubMed] [Google Scholar]
- 2. Tiller NB, Roberts JD, Beasley L, et al. International society of sports nutrition position stand: nutritional considerations for single-stage ultra-marathon training and racing. J Int Soc Sports Nutr 2019; 16: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coyle EF, Coggan AR, Hemmert MK, et al. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol 1986; 61(1): 165–172. [DOI] [PubMed] [Google Scholar]
- 4. Jeukendrup AE, Raben A, Gijsen A, et al. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol 1999; 515(2): 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuipers H, Costill DL, Porter DA, et al. Glucose feeding and exercise in trained rats: mechanisms for glycogen sparing. J Appl Physiol 1986; 61(3): 859–863. [DOI] [PubMed] [Google Scholar]
- 6. Howlett K, Angus D, Proietto J, et al. Effect of increased blood glucose availability on glucose kinetics during exercise. J Appl Physiol 1998; 84(4): 1413–1417. [DOI] [PubMed] [Google Scholar]
- 7. Bergström J, Hermansen L, Hultman E, et al. Diet, muscle glycogen and physical performance. Acta Physiol 1967; 71: 140–150. [DOI] [PubMed] [Google Scholar]
- 8. Temesi J, Johnson NA, Raymond J, et al. Carbohydrate ingestion during endurance exercise improves performance in adults. J Nutr 2011; 141(5): 890–897. [DOI] [PubMed] [Google Scholar]
- 9. Jeukendrup A, Brouns F, Wagenmakers AJ, et al. Carbohydrate-electrolyte feedings improve 1 h time trial cycling performance. Int J Sports Med 1997; 18(2): 125–129. [DOI] [PubMed] [Google Scholar]
- 10. Carter J, Jeukendrup AE, Mundel T, et al. Carbohydrate supplementation improves moderate and high-intensity exercise in the heat. Pflugers Arch 2003; 446(2): 211–219. [DOI] [PubMed] [Google Scholar]
- 11. Desbrow B, Anderson S, Barrett J, et al. Carbohydrate-electrolyte feedings and 1h time trial cycling performance. Int J Sport Nutr Exerc Metab 2004; 14: 541–549. [DOI] [PubMed] [Google Scholar]
- 12. Carter JM, Jeukendrup AE, Mann CH, et al. The effect of glucose infusion on glucose kinetics during a 1-h time trial. Med Sci Sports Exerc 2004; 36(9): 1543–1550. [DOI] [PubMed] [Google Scholar]
- 13. Jeukendrup AE, Chambers ES. Oral carbohydrate sensing and exercise performance. Curr Opin Clin Nutr Metab Care 2010; 13(4): 447–451. [DOI] [PubMed] [Google Scholar]
- 14. Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol 2009; 587: 1779–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brietzke C, Franco-Alvarenga PE, Canestri R, et al. Carbohydrate mouth rinse mitigates mental fatigue effects on maximal incremental test performance, but not in cortical alterations. Brain Sci 2020; 10(8): 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraga C, Velasques B, Koch AJ, et al. Carbohydrate mouth rinse enhances time to exhaustion during treadmill exercise. Clin Physiol Funct Imaging 2017; 37(1): 17–22. [DOI] [PubMed] [Google Scholar]
- 17. Clarke ND, Hammond S, Kornilios E, et al. Carbohydrate mouth rinse improves morning high-intensity exercise performance. Eur J Sport Sci 2017; 17(8): 955–963. [DOI] [PubMed] [Google Scholar]
- 18. Peart DJ. Quantifying the effect of carbohydrate mouth rinsing on exercise performance. J Strength Cond Res 2017; 31(6): 1737–1743. [DOI] [PubMed] [Google Scholar]
- 19. Brietzke C, Franco-Alvarenga PE, Coelho-Júnior HJ, et al. Effects of carbohydrate mouth rinse on cycling time trial performance: a systematic review and meta-analysis. Sports Med 2019; 49(1): 57–66. [DOI] [PubMed] [Google Scholar]
- 20. Thomas K, Goodall S, Stone M, et al. Central and peripheral fatigue in male cyclists after 4-, 20-, and 40-km time trials. Med Sci Sports Exerc 2015; 47(3): 537–546. [DOI] [PubMed] [Google Scholar]
- 21. Smeets PAM, De Graaf C, Stafleu A, et al. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 2005; 24: 363–368. [DOI] [PubMed] [Google Scholar]
- 22. Cummings TA, Powell J, Kinnamon SC. Sweet taste transduction in hamster taste cells: evidence for the role of cyclic nucleotides. J Neurophysiol 1993; 70(6): 2326–2336. [DOI] [PubMed] [Google Scholar]
- 23. Varkevisser B, Kinnamon SC. Sweet taste transduction in hamster: role of protein kinases. J Neurophysiol 2000; 83(5): 2526–2532. [DOI] [PubMed] [Google Scholar]
- 24. Painelli V, de S, Brietzke C, et al. Comment on: “Caffeine and exercise: what next?” Sports Med 2020; 50: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 25. Ataide-Silva T, de Souza MECA, Amorin JF, et al. Can carbohydrate mouth rinse improve performance during exercise? A systematic review. Nutrients 2013; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips SM, Findlay S, Kavaliauskas M, et al. The influence of serial carbohydrate mouth rinsing on power output during a cycle sprint. J Sports Sci Med 2014; 13(2): 252–258. [PMC free article] [PubMed] [Google Scholar]
- 27. Chong E, Guelfi KJ, Fournier PA. Combined glucose ingestion and mouth rinsing improves sprint cycling performance. Int J Sport Nutr Exerc Metab 2014; 24(6): 605–612. [DOI] [PubMed] [Google Scholar]
- 28. Gam S, Tan M, Guelfi KJ, et al. Mouth rinsing with a bitter solution without ingestion does not improve sprint cycling performance. Eur J App Physiol 2015; 115: 129–138. [DOI] [PubMed] [Google Scholar]
- 29. Pribyslavska V, Scudamore EM, Johnson SL, et al. Influence of carbohydrate mouth rinsing on running and jumping performance during early morning soccer scrimmaging. Eur J Sport Sci 2016; 16(4): 441–447. [DOI] [PubMed] [Google Scholar]
- 30. Chong E, Guelfi KJ, Fournier PA. Effect of a carbohydrate mouth rinse on maximal sprint performance in competitive male cyclists. J Sci Med Sport 2011; 14(2): 162–167. [DOI] [PubMed] [Google Scholar]
- 31. Beaven CM, Maulder P, Pooley A, et al. Effects of caffeine and carbohydrate mouth rinses on repeated sprint performance. Appl Physiol Nutr Metab 2013; 38(6): 633–637. [DOI] [PubMed] [Google Scholar]
- 32. Simpson GW, Pritchett R, O’Neal E, et al. Carbohydrate mouth rinse improves relative mean power during multiple sprint performance. Int J Exerc Sci 2018; 11(6): 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dorling JL, Earnest CP. Effect of carbohydrate mouth rinsing on multiple sprint performance. J Int Soc Sports Nutr 2013; 10(1): 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dolan P, Witherbee KE, Peterson KM, et al. Effect of carbohydrate, caffeine, and carbohydrate + caffeine mouth rinsing on intermittent running performance in collegiate male lacrosse athletes. J Strength Cond Res 2017; 31(9): 2473–2479. [DOI] [PubMed] [Google Scholar]
- 35. Cherif A, Meeusen R, Ryu J, et al. Repeated-sprints exercise in daylight fasting: carbohydrate mouth rinsing does not affect sprint and reaction time performance. Biol Sport 2018; 35: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. José Jonas Crisp AH, de O, Barbosa CGR, et al. Influence of carbohydrate mouth rinse on sprint performance: a systematic review and meta-analysis. J Ex Physiol 2017; 20: 88–99. [Google Scholar]
- 37. Bazzucchi I, Patrizio F, Felici F, et al. CHO mouth rinsing improves neuromuscular performance during isokinetic fatiguing exercise. Int J Sports Physiol Perform 2017; 12(8): 1031–1038. [DOI] [PubMed] [Google Scholar]
- 38. Bastos-Silva VJ, Prestes J, Geraldes AAR. Effect of carbohydrate mouth rinse on training load volume in resistance exercises. J Strength Cond Res 2019; 33(6): 1653–1657. [DOI] [PubMed] [Google Scholar]
- 39. Decimoni LS, Curty VM, Almeida L, et al. Carbohydrate mouth rinsing improves resistance training session performance. Int J Sports Sci Coach 2018; 13(5): 804–809. [Google Scholar]
- 40. Durkin M, Akeroyd H, Holliday A. Carbohydrate mouth rinse improves resistance exercise capacity in the glycogen-lowered state. Appl Physiol Nutr Metab 2021; 46(2): 126–132. [DOI] [PubMed] [Google Scholar]
- 41. Dunkin JE, Phillips SM. The effect of a carbohydrate mouth rinse on upper-body muscular strength and endurance. J Strength Cond Res 2017; 31(7): 1948–1953. [DOI] [PubMed] [Google Scholar]
- 42. Jensen M, Stellingwerff T, Klimstra M. Carbohydrate mouth rinse counters fatigue related strength reduction. Int J Sport Nutr Exerc Metab 2015; 25(3): 252–261. [DOI] [PubMed] [Google Scholar]
- 43. Painelli VS, Roschel H, Gualano B, et al. The effect of carbohydrate mouth rinse on maximal strength and strength endurance. Eur J Appl Physiol 2011; 111(9): 2381–2386. [DOI] [PubMed] [Google Scholar]
- 44. Clarke ND, Kornilios E, Richardson DL. Carbohydrate and caffeine mouth rinses do not affect maximum strength and muscular endurance performance. J Strength Cond Res 2015; 29(10): 2926–2931. [DOI] [PubMed] [Google Scholar]
- 45. Black CD, Schubert DJ, Szczyglowski MK, et al. Carbohydrate mouth rinsing does not prevent the decline in maximal strength after fatiguing exercise. J Strength Cond Res 2018; 32(9): 2466–2473. [DOI] [PubMed] [Google Scholar]
- 46. Karayigit R, Ali A, Rezaei S, et al. Effects of carbohydrate and caffeine mouth rinsing on strength, muscular endurance and cognitive performance. J Int Soc Sports Nutr 2021; 2618(1): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hibbert AW, Billaut F, Varley MC, et al. Familiarization protocol influences reproducibility of 20-km cycling time-trial performance in novice participants. Front Physiol 2017; 8: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grgic J, Lazinica B, Schoenfeld BJ, et al. Test-retest reliability of the one-repetition maximum (1RM) strength assessment: a systematic review. Sport Med Open 2020; 176(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duez L, Qerama E, Fuglsang-Frederiksen A, et al. Electrophysiological characteristics of motor units and muscle fibers in trained and untrained young male subjects. Muscle Nerve 2010; 42(2): 177–183. [DOI] [PubMed] [Google Scholar]
- 50. Semmler JG, Nordstrom MA. Motor unit discharge and force tremor in skill- and strength-trained individuals. Exp Brain Res 1998; 119(1): 27–38. [DOI] [PubMed] [Google Scholar]
- 51. Canestri R, Franco-Alvarenga PE, Brietzke C, et al. Effects of experimentally induced muscle pain on endurance performance: a proof-of-concept study assessing neurophysiological and perceptual responses. Psychophysiology 2021; 58(6): e13810. [DOI] [PubMed] [Google Scholar]
- 52. Brietzke C, Vinícius Í, Franco-Alvarenga PE, et al. Proof-of-concept and test-retest reliability study of psychological and physiological variables of the mental fatigue paradigm. Int J Environ Res Public Health 2021; 1018(18): 9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 1994; 74(1): 49–94. [DOI] [PubMed] [Google Scholar]
- 54. Abbiss CR, Laursen PB. Models to explain fatigue during prolonged endurance cycling. Sports Med 2005; 35(10): 865–898. [DOI] [PubMed] [Google Scholar]
- 55. Lima F, de R, de Barreto CB, et al. Traditional models of fatigue and physical performance. J Phys Ed 2017; 29: e2915. [Google Scholar]
- 56. Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 2008; 104(2): 542–550. [DOI] [PubMed] [Google Scholar]
- 57. Turner CE, Byblow WD, Stinear CM, et al. Carbohydrate in the mouth enhances activation of brain circuitry involved in motor performance and sensory perception. Appetite 2014; 80: 212–219. [DOI] [PubMed] [Google Scholar]
- 58. Brietzke C, Franco-Alvarenga PE, Canestri R, et al. Carbohydrate mouth rinse mitigates mental fatigue effects on maximal incremental test performance, but not in cortical alterations. Brain Sci 2020; 10(8): 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gant N, Stinear CM, Byblow WD. Carbohydrate in the mouth immediately facilitates motor output. Brain Res 2010; 21350: 151–158. [DOI] [PubMed] [Google Scholar]
- 60. Pires FO, Brietzke C, Pinheiro FA, et al. Carbohydrate mouth rinse fails to improve four-kilometer cycling time trial performance. Nutrients 2018; 1210(3): 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bastos-Silva VJ, Melo A de A, Lima-Silva AE, et al. Carbohydrate mouth rinse maintains muscle electromyographic activity and increases time to exhaustion during moderate but not high-intensity cycling exercise. Nutrients 2016; 98(3): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pires FO, Brietzke C, Pinheiro FA, et al. Carbohydrate mouth rinse fails to improve four-kilometer cycling time trial performance. Nutrients 2018; 1210(3): 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol 1990; 76(6): 509–519. [DOI] [PubMed] [Google Scholar]
- 64. Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol 1992; 72(5): 1631–1648. [DOI] [PubMed] [Google Scholar]
- 65. Carter JM, Jeukendrup AE, Jones DA. The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Med Sci Sports Exerc 2004; 36(12): 2107–2111. [DOI] [PubMed] [Google Scholar]
- 66. Rollo I, Williams C, Gant N, et al. The influence of carbohydrate mouth rinse on self-selected speeds during a 30-min treadmill run. Int J Sport Nutr Exerc Metab 2008; 18(6): 585–600. [DOI] [PubMed] [Google Scholar]
- 67. Rollo I, Cole M, Miller R, et al. Influence of mouth rinsing a carbohydrate solution on 1-h running performance. Med Sci Sports Exerc 2010; 42(4): 798–804. [DOI] [PubMed] [Google Scholar]
- 68. Whitham M, McKinney J. Effect of a carbohydrate mouthwash on running time-trial performance. J Sports Sci 2007; 25(12): 1385–1392. [DOI] [PubMed] [Google Scholar]
- 69. Beelen M, Berghuis J, Bonaparte B, et al. Carbohydrate mouth rinsing in the fed state: lack of enhancement of time-trial performance. Int J Sport Nutr Exerc Metab 2009; 19(4): 400–409. [DOI] [PubMed] [Google Scholar]
- 70. Ispoglou T, O’Kelly D, Angelopoulou A, et al. Mouth rinsing with carbohydrate solutions at the postprandial state fail to improve performance during simulated cycling time trials. J Strength Cond Res 2015; 29(8): 2316–2325. [DOI] [PubMed] [Google Scholar]
- 71. Pottier A, Bouckaert J, Gilis W, et al. Mouth rinse but not ingestion of a carbohydrate solution improves 1-h cycle time trial performance. Scand J Med Sci Sports 2010; 20(1): 105–111. [DOI] [PubMed] [Google Scholar]
- 72. Fares EJ, Kayser B. Carbohydrate mouth rinse effects on exercise capacity in pre- and postprandial States. J Nutr Metab 2011; 2011: 385962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lane SC, Bird SR, Burke LM, et al. Effect of a carbohydrate mouth rinse on simulated cycling time-trial performance commenced in a fed or fasted state 2013; 38(2): 134–139. [DOI] [PubMed] [Google Scholar]
- 74. Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 2009; 44(3): 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sadaf Farooqi I, Bullmore E, Keogh J, et al. Leptin regulates striatal regions and human eating behavior. Science 2007; 317(5843): 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Malik S, McGlone F, Bedrossian D, et al. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008; 7(5): 400–409. [DOI] [PubMed] [Google Scholar]
- 77. Bataineh MF, Al-Nawaiseh AM, Abu Altaieb MH, et al. Impact of carbohydrate mouth rinsing on time to exhaustion during Ramadan: a randomized controlled trial in Jordanian men. Eur J Sport Sci 2018; 18(3): 357–366. [DOI] [PubMed] [Google Scholar]
- 78. Tomko PM, Laurent CM, Fullenkamp AM, et al. Mouth rinsing carbohydrates serially does not improve repeated sprint time. J Hum Kinet 2019; 67: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sinclair J, Bottoms L, Flynn C, et al. The effect of different durations of carbohydrate mouth rinse on cycling performance. Eur J Sport Sci 2014; 14(3): 259–264. [DOI] [PubMed] [Google Scholar]
- 80. de Salles Painelli V, Nicastro H, Lancha AHJ. Carbohydrate mouth rinse: does it improve endurance exercise performance? Nutr J 2010; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nehme R, de Branco FMS, Vieira PF, et al. Single and serial carbohydrate mouth rinsing do not improve Yo-Yo intermittent recovery test performance in soccer players. Int J Sport Nutr Exerc Metab 2022; 132(1): 22–29. [DOI] [PubMed] [Google Scholar]
- 82. van Rijn I, Griffioen-Roose S, de Graaf C, et al. Neural processing of calories in brain reward areas can be modulated by reward sensitivity. Front Behav Neurosci 2016; 149: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wright BF, Davison G. Carbohydrate mouth rinse improves 1.5 h run performance: is there a dose-effect. Int J Exerc Sci 2013; 6(4): 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kulaksız TN, Koşar ŞN, Bulut S, et al. Mouth rinsing with maltodextrin solutions fails to improve time trial endurance cycling performance in recreational athletes. Nutrients 2016; 8(5): 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. James RM, Ritchie S, Rollo I, et al. No dose response effect of carbohydrate mouth rinse on cycling time trial performance. Int J Sport Nutr Exerc Metab 2017; 27(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 86. Clarke ND, Thomas JR, Kagka M, et al. No dose-response effect of carbohydrate mouth rinse concentration on 5-km running performance in recreational athletes. J Strength Cond Res 2017; 31(3): 715–720. [DOI] [PubMed] [Google Scholar]
- 87. Devenney S, Collins K, Shortall M. Effects of various concentrations of carbohydrate mouth rinse on cycling performance in a fed state. Eur J Sport Sci 2016; 16(8): 1073–1078. [DOI] [PubMed] [Google Scholar]
- 88. Karayigit R, Forbes SC, Naderi A, et al. Different doses of carbohydrate mouth rinse have no effect on exercise performance in resistance trained women. Int J Environ Res Public Health 2021; 18(7): 3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guest S, Grabenhorst F, Essick G, et al. Human cortical representation of oral temperature. Physiol Behav 2007; 592(5): 975–984. [DOI] [PubMed] [Google Scholar]
- 90. de Salles Painelli V, da Eira Silva V, Guilherme GA, et al. A temperatura dos repositores hídricos pode influenciar a capacidade aeróbia? Rev Bras Ciênc Mov 2017; 25(2): 205. [Google Scholar]
- 91. Tan PM, Lee JK. The role of fluid temperature and form on endurance performance in the heat. Scand J Med Sci Sports 2015; 25(Suppl. 1): 39–51. [DOI] [PubMed] [Google Scholar]
- 92. Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain 2005; 114(1–2): 3–6. [DOI] [PubMed] [Google Scholar]
- 93. Finniss DG, Kaptchuk TJ, Miller F, et al. Placebo effects: biological, clinical and ethical advances. Lancet 2010; 375(9715): 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pollo A, Carlino E, Benedetti F. The top-down influence of ergogenic placebos on muscle work and fatigue. Eur J Neurosci 2008; 28(2): 379–388. [DOI] [PubMed] [Google Scholar]
- 95. de la Fuente-Fernández R. The placebo-reward hypothesis: dopamine and the placebo effect. Parkinsonism Relat Disord 2009; 15(Suppl. 3): S72–S74. [DOI] [PubMed] [Google Scholar]
- 96. Beedie C, Benedetti F, Barbiani D, et al. Consensus statement on placebo effects in sports and exercise: the need for conceptual clarity, methodological rigour, and the elucidation of neurobiological mechanisms. Eur J Sport Sci 2018; 18(10): 1383–1389. [DOI] [PubMed] [Google Scholar]
- 97. Marticorena FM, Carvalho ADE, Oliveira LF, et al. Nonplacebo controls to determine the magnitude of ergogenic interventions: a systematic review and meta-analysis. Med Sci Sports Exerc 2021; 53(8): 1766–1777. [DOI] [PubMed] [Google Scholar]
- 98. Day SJ, Altman DG. Blinding in clinical trials and other studies. BMJ 2000; 321(7259): 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Beedie CJ, Foad AJ. The placebo effect in sports performance: a brief review. Sports Med 2009; 39(4): 313–329. [DOI] [PubMed] [Google Scholar]
- 100. Kaptchuk TJ. The double-blind, randomized, placebo-controlled trial: Gold standard or golden calf? J Clin Epidemiol 2001; 54(6): 541–549. [DOI] [PubMed] [Google Scholar]
- 101. Hurst P, Foad A, Coleman D, et al. Athletes intending to use sports supplements are more likely to respond to a placebo. Med Sci Sports Exerc 2017; 49(9): 1877–1883. [DOI] [PubMed] [Google Scholar]
- 102. Gam S, Guelfi KJ, Fournier PA. Opposition of carbohydrate in a mouth-rinse solution to the detrimental effect of mouth rinsing during cycling time trials. Int J Sport Nutr Exerc Metab 2013; 23(1): 48–56. [DOI] [PubMed] [Google Scholar]
- 103. Bang H, Flaherty SP, Kolahi J, et al. Blinding assessment in clinical trials: a review of statistical methods and a proposal of blinding assessment protocol. Clin Res Reg Affairs 2010; 27(2): 42–51. [Google Scholar]
- 104. Kolahi J, Bang H, Park J. Towards a proposal for assessment of blinding success in clinical trials: up-to-date review. Community Dent Oral Epidemiol 2009; 37(6): 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Best R, McDonald K, Hurst P, et al. Can taste be ergogenic. Eur J Nutr 2021; 60(1): 45–54. [DOI] [PubMed] [Google Scholar]
- 106. Foad AJ, Beedie CJ, Coleman DA. Pharmacological and psychological effects of caffeine ingestion in 40-km cycling performance. Med Sci Sports Exerc 2008; 40(1): 158–165. [DOI] [PubMed] [Google Scholar]
- 107. Brietzke C, Asano RY, de Russi de Lima F, et al. Caffeine effects on VO 2 max test outcomes investigated by a placebo perceived-as-caffeine design. Nutr Health 2017; 23(4): 231–238. [DOI] [PubMed] [Google Scholar]
- 108. Pires FO, Dos Anjos CAS, Covolan RJM, et al. Caffeine and placebo improved maximal exercise performance despite unchanged motor cortex activation and greater prefrontal cortex deoxygenation. Front Physiol 2018; 9: 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]


