Abstract
L-Arginine (L-Arg), the precursor of nitric oxide (NO), plays an important role in muscle function. Fast-twitch glycolytic fibres are more susceptible to age-related atrophy than slow-twitch oxidative fibres. The effect of L-Arg/NO on protein metabolism of fast- and slow-twitch muscle fibres was evaluated in chickens. In Exp. 1, 48 chicks at 1 day old were divided into 4 groups of 12 birds and subjected to 4 treatments: basal diet without supplementation or supplemented with 1% L-Arg, and water supplemented with or without L-nitro-arginine methyl ester (L-NAME, 18.5 mM). In Exp. 2, 48 chicks were divided into 4 groups of 12 birds fed with the basal diet and subjected to the following treatments: tap water (control), tap water supplemented with L-NAME (18.5 mM), or molsidomine (MS, 0.1 mM), or 18.5 mM L-NAME + 0.1 mM MS (NAMS). The regulatory effect of L-Arg/NO was further investigated in vitro with myoblasts obtained from chicken embryo pectoralis major (PM) and biceps femoris (BF). In vivo, dietary L-Arg supplementation increased breast (+14.94%, P < 0.05) and thigh muscle mass (+23.40%, P < 0.05); whereas, MS treatment had no detectable influence. However, L-NAME treatment blocked the beneficial influence of L-Arg on muscle development. L-Arg decreased (P < 0.05) protein synthesis rate, phosphorylated mTOR and ribosomal protein S6 kinase beta-1 (p70S6K) levels in breast muscle, which was recovered by L-NAME treatment. In vitro, L-Arg or sodium nitroprusside (SNP) reduced protein synthesis rate, suppressed phosphorylated mTOR/p70S6K and decreased atrogin-1 and muscle RING finger 1 (MuRF1) in myoblasts from PM muscle (P < 0.05). L-NAME abolished the inhibitory effect of L-Arg on protein synthesis and the mTOR/p70S6K pathway. However, myoblasts from BF muscle showed the weak influence. Moreover, blocking the mTOR/p70S6K pathway with rapamycin suppressed protein synthesis of the 2 types of myoblasts; whereas, the protein expression of atrogin-1 and MuRF1 levels were restricted only in myoblasts from PM muscle. In conclusion, L-Arg/NO/mTOR/p70S6K pathway enhances protein accumulation and muscle development in fast-twitch glycolytic muscle in chickens. L-Arg/NO regulates protein turnover in a muscle fibre specific way, which highlights the potential clinical application in fast-twitch glycolytic muscle fibres.
Keywords: Muscle fibre, L-Arginine, Nitric oxide/mTOR/p70S6K, Atrogin-1, Muscle RING finger 1, Chicken
1. Introduction
L-Arginine (L-Arg) is an essential amino acid for young mammals (Wu et al., 2000) and chickens (Yu et al., 2018). L-Arg has beneficial effects on the regulation of nutrient metabolism, enhancing lean tissue deposition in humans (McNeal et al., 2016). L-Arg also enhances protein synthesis in the skeletal muscle of piglets (Frank et al., 2007; Yao et al., 2008) and ameliorates muscle dysfunction in mdx mice (Barton et al.,2010). It is well-known that part of the advantageous effect of L-Arg is related to enhanced blood circulation in skeletal muscles (Kalliokoski et al., 2006).
Recently, it is suggested that L-Arg is associated with the regulation of muscle development by directly stimulating muscle protein synthesis (Wang et al., 2018). L-Arg is associated with the regulation of muscle development via the mechanistic target of rapamycin (mTOR) pathway in C2C12 cells (Wang et al., 2018). As a serine/threonine protein kinase, mTOR couples energy and nutrient abundance to the execution of cell growth and division. The activation of mTOR complex 1 (mTORC1) is responsible for the increased protein synthesis and skeletal muscle growth (Baar and Esser, 1999; Rommel et al., 2001; Hornberger and Chien, 2010; Shimizu et al., 2011). L-Arg protects muscle cells from wasting in vitro in an mTORC1-dependent manner (Ham et al., 2014). Nitric oxide (NO) is produced from L-Arg by nitric oxide synthase (NOS), and NOS has 3 isoforms: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). In the skeletal muscle of mammals, nNOS is the major NOS isoform (Brenman et al., 1995). However, in chickens, iNOS is the primary NOS isoform (Lee et al., 1994; Lin et al., 1996; Kim et al., 1999). NO is an important gasotransmitter that participates in specific signal transduction pathways in cell communication (Moncada et al., 1991; Nathan, 1992; Hemish et al., 2003). In skeletal muscle, NO is crucial for skeletal muscle regrowth after an immobilization period, potentially via the mTOR signaling pathway (Aguiar et al., 2017). In L6 myocytes, L-nitro-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, reduces the phosphorylated Akt and p70S6K levels, suggesting that NO is involved in the activation of PI3K/Akt/mTOR signaling pathway (Miniaci et al., 2015). In C2C12 cells, it is observed that L-Arg activates mTOR (Thr 2446)/p70S6K signaling pathway in NO-dependent manner (Wang et al., 2018).
Muscle RING finger 1 (MuRF1) and muscle atrophy F-box (MAFbx)/atrogin-1 are 2 muscle-specific E3 ubiquitin ligases that involving in skeletal muscle atrophy (Bodine and Baehr, 2015). L-Arg is involved in the regulation of protein hydrolysis by suppressing atrogin-1 and MuRF1 mRNA levels in C2C12 cells (Herningtyas et al., 2008). In vivo, L-Arg reverses the resistance exercise induced upregulation of atrogin-1 and MuRF-1 mRNA levels (Morais et al., 2018). NO is suggested to be involved in the regulation of L-Arg on protein degradation, and the NO donor treatment attenuates the suspension induced disuse muscle atrophy and decreases atrogin-1 protein level in hind limbs of mice (Anderson et al., 2017). However, during catabolic conditions, L-Arg protects myocytes from wasting in a NO-independent manner (Ham et al., 2014). Thus, the effect of L-Arg/NO on protein catabolism remains to be fully elucidated.
Fast-twitch glycolytic fibres are more susceptible to age-related atrophy than slow-twitch oxidative fibres (Larsson et al., 2010; Braga et al., 2016). Broiler chickens are genetically selected to possess a fast growth rate and muscle development. In chickens, the pectoralis major (PM) muscle primarily comprises fast-twitch glycolytic fibres, but the biceps femoris (BF) muscle mainly contains slow-twitch oxidative fibers, making the chicken an interesting animal model. In broilers, L-Arg is suggested to be necessary for improved muscle development (Fernandes et al., 2009) and leads to an overall body growth and increased lean (Castro et al., 2019). In ovo feeding of L-Arg improves breast muscle growth, which may be associated with enhanced protein deposition (Yu et al., 2018). NO is proved to be a critical determinant of myogenesis in the early phase of embryonic development of chickens (Cazzato et al., 2014). Hence, we hypothesized that L-Arg/NO regulated muscle protein metabolism in a different way within fast growing muscles.
Therefore, the aim of the present study was to evaluate the L-Arg/NO pathway on the protein metabolism of skeletal muscle within an in vivo chicken model and an in vitro cultured chicken embryo myoblast model. Protein synthesis rate, the activation of mTOR/p70S6K pathway, and the expression of MuRF1 and atrogin-1 were measured in 2 muscle fibres: the fast-twitch glycolytic fibres and slow-twitch oxidative fibres.
2. Materials and methods
2.1. Animal experiments
A total of 96 male broilers (Arbor Acres) at 1 day old were obtained from a local breeder farm (Dabao hatchery, Tai'an, China). The brooding temperature was maintained at 35 °C (65% relative humidity) for the first 2 d and then gradually reduced to 30 °C by d 8 (Zhao et al., 2012). The nutrient contents of the feed ingredients and nutrient levels of the basal diet are provided in Table 1. All study procedures were approved by the Animal Care and Use Committee of Shandong Agricultural University and were in accordance with the Guidelines for Experimental Animals, established by the Ministry of Science and Technology (Beijing, China).
Table 1.
Ingredients and nutrition levels of basal diet (DM basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 47.36 |
| Brown rice | 8.00 |
| Wheat flour | 10.00 |
| Corn gluten meal (60% CP) | 4.00 |
| Soybean meal (45% CP) | 12.00 |
| Peanut meal (46% CP) | 7.00 |
| Fermented cotton seed meal | 2.00 |
| Oil | 1.50 |
| Meat and bone meal | 5.50 |
| Limestone | 0.30 |
| Sodium chloride (98.5%) | 0.25 |
| Choline chloride (60%) | 0.05 |
| L-Lysine sulfate (70%) | 0.90 |
| Methionine hydroxyl analogue (liquid, 88%) | 0.35 |
| L-Threonine (98.5%) | 0.23 |
| Valine | 0.10 |
| Sodium humate | 0.15 |
| Complex enzymes | 0.10 |
| Xylanase | 0.05 |
| Phytase | 0.03 |
| Mineral and vitamin premix1 | 0.13 |
| Total | 100 |
| Nutrition levels2 | |
| ME, kcal/kg | 2,728 |
| CP | 21.67 |
| EE | 4.60 |
| DM | 87.86 |
| Ash | 5.49 |
| Ca | 0.80 |
| TP | 0.53 |
| Asp | 1.13 |
| Thr | 0.58 |
| Ser | 0.70 |
| Glu | 2.60 |
| Gly | 0.52 |
| Ala | 0.66 |
| Cys | 0.07 |
| Val | 0.52 |
| Met | 0.22 |
| Ile | 0.47 |
| Leu | 1.13 |
| Tyr | 0.37 |
| Phe | 0.71 |
| Lys | 1.01 |
| His | 0.36 |
| Arg | 0.80 |
EE = ethanol extract; TP = total phosphorus.
The mineral and vitamin premix provide the follow quantities per kilogram of diet: Fe, 100 mg; Cu, 8 mg; Mn, 120 mg; Zn, 100 mg; I, 0.7 mg; Se, 0.3 mg; vitamin A, 8,000 IU; vitamin D3, 1,000 IU; vitamin E, 20 IU; vitamin K, 0.5 mg; vitamin B1, 2 mg; vitamin B12, 0.01 mg; iboflavin, 8 mg; pantothenic acid, 10 mg; niacin, 35 mg; pyridoxine, 3.5 mg; biotin, 0.18 mg; folic acid, 0.55 mg.
ME is calculated value and the others are measured values.
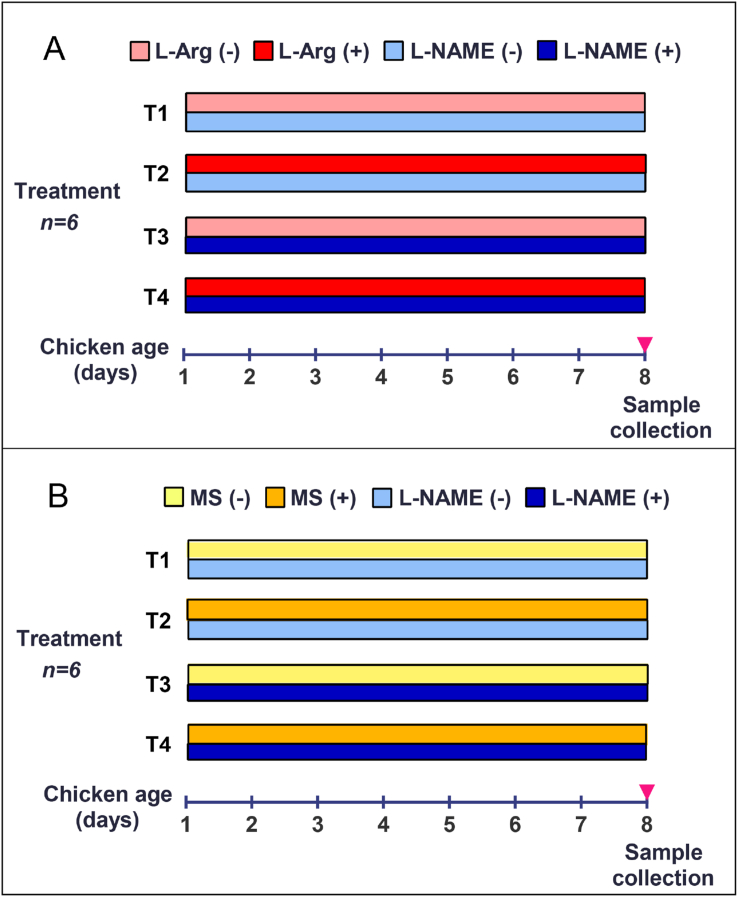
Two experiments were conducted from d 1 to 8 post–hatch (Fig. 1). In Exp. 1, 48 chicks were divided into 4 groups of 12 birds and subjected to 4 treatments: basal diet and water without supplementation (control), basal diet supplemented with 1% L-Arg and water, basal diet and water supplemented with L-nitro-arginine methyl ester (L-NAME, an inhibitor of NOS; Sigma, Saint Louis, USA) at 18.5 mM, and basal diet supplemented with 1% L-Arg and water supplemented with L-NAME at 18.5 mM. In Exp. 2, 48 chicks were divided into 4 groups of 12 birds. The experimental chicks were fed with the basal diet and subjected to the following treatments: tap water (control), or tap water supplemented with 0.1 mM molsidomine (MS, a donor of NO; Sigma, Saint Louis, USA), or 18.5 mM L-NAME, or 0.1 mM MS + 18.5 mM L-NAME (NAMS). At the end of the experiment, 6 birds from each group were randomly selected for sampling.
Fig. 1.
The Gantt chart of animal experiment. (A) In Exp. 1, chicks from d 1 to 8 post–hatch were subjected to 1 of 4 treatments: T1, basal diet + tap water; T2, basal diet supplemented with 1% L-Arg + tap water; T3, basal diet + tap water supplemented with L-NAME (an inhibitor of nitric oxide synthase) at 18.5 mM; and T4, basal diet supplemented with 1% L-Arg + tap water supplemented with L-NAME at 18.5 mM. (B) In Exp. 2, to further investigate the role of NO in regulating protein metabolism, molsidomine (MS, a donor of NO) was used, and chicks from d 1 to 8 post–hatch were fed with the basal diet and subjected to 1 of the 4 treatments: T1, tap water (control), tap water supplemented with MS at 0.1 mM (T2), tap water supplemented with L-NAME at 18.5 mM (T3), and tap water supplemented with MS at 0.1 mM and L-NAME at 18.5 mM (T4). At 8 d, 6 birds from each group were randomly selected for sampling. L-Arg = L-arginine; L-NAME = L-nitro-arginine methyl ester; NO = nitric oxide.
2.2. Primary cell culture
The specific pathogen free (SPF) chicken embryos were obtained (Jinan SAIS Poultry CO., Ltd, Jinan, China). The method to culture cells was performed as previously described (Wang et al., 2019). Briefly, myoblasts were obtained respectively from the PM and BF muscles of d 14 and 12 embryo, seeded in 6 well plates at a density of 1 × 106 cells/mL and cultured in SILAC DMEM Flex Media (DMEM without D-glucose, L-glutamine, L-arginine and L-lysine; Gibco, Grand Island, USA) supplemented with D-glucose (4.5 g/L, Sigma, Saint Louis, USA), L-glutamine (4 mM, Sigma, Saint Louis, USA), L-arginine (397.6 μM, Sigma, Saint Louis, USA), L-lysine (799.3 μM, Sigma, Saint Louis, USA), 15% foetal bovine serum and 1% penicillin-streptomycin (Solarbio, Beijing, China) in a humidified atmosphere at 37 °C with 5% CO2. Cells were then subjected to the treatments specified in the protocols for the experiments described below.
2.3. Cell viability assay
Cell viability was detected by CCK-8 Cell Counting Kit (Vazyme, Nanjing, China). Briefly, cells were cultured in 96-well plate in a humidified incubator (37 °C, 5% CO2). After L-Arg and L-NAME treatments, 90 μL DMEM with 10 μL CCK-8 solution was added to each well of the plate for 2 h. Absorbance was measured at 450 nm with a microplate reader (Elx808, Bio-Tek, Winooski, VT).
2.4. L-Arg, L-NAME, sodium nitroprusside (SNP), and rapamycin treatments
2.4.1. L-Arg treatment
Myoblasts cultured were treated in SILAC DMEM Flex Media (Gibco, Grand Island, USA) supplemented with D-glucose (4.5 g/L), L-glutamine (4 mM), L-arginine (397.6 μM) and L-lysine (799.3 μM). Extra L-arginine supplementation at a dose of 400 μM was used to treat myoblasts for 12 h according to the results of cell viability and protein synthesis rate (Fig. S1; Fig. S2).
2.4.2. L-NAME treatment
Myoblasts were treated with 400 μM L-Arg, 1 mM L-NAME (Sigma, Saint Louis, USA), and L-Arg supplementation (400 μM) plus L-NAME (1 mM) according to the pre-trial result (Fig. S1).
2.4.3. SNP treatment
Myoblasts were treated with SNP (sodium nitroprusside, 10 μM; a NO donor; Sigma, Saint Louis, USA) for 12 h. The doses of L-Arg, L-NAME, and SNP were selected based on previous studies (Long et al., 2006; Wang et al., 2018).
2.4.4. Rapamycin treatment
Myoblasts were treated with rapamycin (Solarbio, Beijing, China), an inhibitor of mTOR, which was dissolved in dimethyl sulfoxide (DMSO) at 25 mg/mL. The 0.5 and 1 μM concentrations of rapamycin were used for 6 h. After treatment, cells were collected and used for protein expression measurement.
2.5. Protein synthesis rate measurement
Protein synthesis rates were measured with a nonradioactive method. In animal experiments, puromycin (10 mg/kg body mass, Solarbio, Beijing, China) was administered by intraperitoneal injection to measure protein-synthesis rate in the skeletal muscle of chickens for 1 h before sample collected. For the in vitro treatments, puromycin (10 μM; Sigma, Saint Louis, USA) was added to cell culture media for 30 min, after which the total protein was extracted and used to measure protein synthesis rates. Newly synthesized polypeptides were labelled with puromycin at low concentrations and then detected with an anti-puromycin antibody to reflect the rate of protein synthesis (Schmidt et al., 2009; Goodman et al., 2010; Wang et al., 2019).
2.6. NO2− concentration and NOS activity assays
NO2− concentrations in cells and the culture media were measured with a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). NO is very chemically active and easily converted into NO2− and then NO3−. In this reaction system, the concentration of NO2− was measured after conversion of NO3− into NO2− by nitrate reductase. The absorbance of the supernatant was determined at 550 nm using a spectrophotometer (Beijing Pgeneral, Beijing, China).
Intracellular NOS activities, including those of total NOS enzymes (TNOS) and iNOS, were determined using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. In the reaction system, NOS catalyzes L-arginine to produce NO, which reacts with nucleophilic substances to form nonferrous compounds. The experiment was also performed in the absence of calcium and the presence of a calcium chelator to determine the calcium-independent NOS activity, which was assumed to represent iNOS activity. The absorbance at 530 nm was determined using a UV-2450 spectrophotometer.
2.7. Measurement of cGMP
Chicken cyclic guanosine monophosphate (cGMP) in myoblasts from chicken embryo PM and BF muscle was detected via an ELISA Kit (Enzyme-linked Biotechnology Co., Ltd, Shanghai, China), and measured at 450 nm using a spectrophotometer (Elx808, Bio-Tek, Winooski, VT).
2.8. Protein preparation and Western blotting
Cells were washed with PBS and then lysed with the lysis buffer. The supernatants were obtained and used for immunoblot analysis. The protein concentration was detected using a BCA Protein Assay Kit (Beyotime, Nanjing, China). Total protein (18 μg) was separated by SDS-PAGE and was transferred to PVDF membranes (Millipore, Billerica, Germany). The membrane was then blocked with blocking buffer (Beyotime, Nanjing, China) at room temperature for 1 h before being incubated with the following primary antibodies, anti-phospho-mTOR (Ser-2448) (#2971, anti-rabbit, Cell Signaling Technology, Boston, USA), anti-mTOR (#2983, anti-rabbit, Cell Signaling Technology, Boston, USA), anti-phospho-p70S6K (Thr-389) (#9234, anti-rabbit, Cell Signaling Technology, Boston, USA), anti-p70S6K (#2708, anti-rabbit, Cell Signaling Technology, Boston, USA), anti-puromycin (EQ0001, anti-mouse, Kerafast, Boston, USA), and anti-tubulin (AT819, anti-mouse, Beyotime, Nanjing, China) overnight at 4 °C. After being washed, the proteins were probed with horseradish peroxidase-linked anti-rabbit or anti-mouse secondary antibodies (Beyotime, Nanjing, China). The membranes were subsequently exposed to enhanced chemiluminescence plus Western blot detection reagents (Beyotime, Nanjing, China). The films were then scanned, and the intensities of specific bands were quantified using ImageJ 1.43 software (National Institutes of Health, Bethesda, USA) and normalized to those of tubulin in the same sample (Wang et al., 2017, 2019).
2.9. Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA of myoblasts from chicken embryo PM and BF muscle after treatments was isolated with Trizol (Invitrogen, Carlsbad, USA). The transcriptor first-strand cDNA synthesis kit (Roche, Basel, Switzerland) was used for cDNA synthesis of mRNA, followed by the amplification by qPCR with FastStart Universal SYBR Green Master (Rox) (Roche, Basel, Switzerland). A standard curve was plotted to calculate the efficiency of the real-time PCR primers. The mRNA level of β-actin was measured as an internal control, and the relative expression of target genes was analyzed by the 2−ΔΔCT method. Primers used for qRT-PCR were designed by the NCBI Primer BLAST program and DNAMAN software. The primer sequences were listed in Table 2.
Table 2.
The primer sequences used for quantitative reverse transcription polymerase chain reaction (qRT-PCR).
| Gene | Accession no. | Primer sequence (5′ to 3′) |
|---|---|---|
| iNOS | NM_204961 | F: GTGGTATGCTCTGCCTGCTGTTG |
| R: GTCTCGCACTCCAATCTCTGTTCC | ||
| nNOS | XM_004934480.1 | F: CTCGGATGCACGGAAGTCCT |
| R: CGTGAACCCAGCCCAAACAC | ||
| eNOS | JQ434761.1 | F: GGATGTGCTGCACGGTCTGC |
| R: AGGACGTGCTGCGGACACAG | ||
| β-actin | NM_205518 | F: TGCGTGACATCAAGGAGAAG |
| R: TGCCAGGGTACATTGTGGTA |
iNOS = inducible nitric oxide synthase; nNOS = neuronal nitric oxide synthase; eNOS = endothelial nitric oxide synthase.
2.10. Immunofluorescence staining and muscle fiber diameter measurement
Cells were cultured on coverslips under identical conditions and treatments as described above. The cells were fixed with 4% (wt/vol) paraformaldehyde for 30 min at room temperature, and permeabilized with cold methanol for 20 min. After washed with wash buffer (Beyotime, Nanjing, China) for 3 times, cells were blocked for 1 h at room temperature, and then incubated with primary antibodies anti-Dystrophin (1:500, Abcam, Cambridge, UK) at 4 °C overnight. Secondary antibody with Alexa Fluor 555 (1:500, Beyotime, Nanjing, China) was incubated for 3 h at 4 °C after washed for 3 times. Cell nucleuses were stained with DAPI solution (Beyotime, Nanjing, China). Images were taken using a confocal laser scanning microscope TCS SPE (Leica, Weztlar, Germany). Cells were also observed in bright field at a low magnification (10×, 20×) with a light microscope (NIKON, Tokyo Metropolis, Japan), and measured the diameter of muscle fiber with the software NIS-elements D.
2.11. Statistical analysis
The data were analysed with a one-way ANOVA model to estimate the main effects of treatment (SAS version 8.1; SAS Institute Inc., NC, USA). When the main effect of the treatment was significant, the differences between means were compared using Tukey's multiple comparisons test. P < 0.05 was considered statistically significant.
3. Results
3.1. Effects of L-Arg and L-NAME on muscle development, NO2− levels and NOS activity
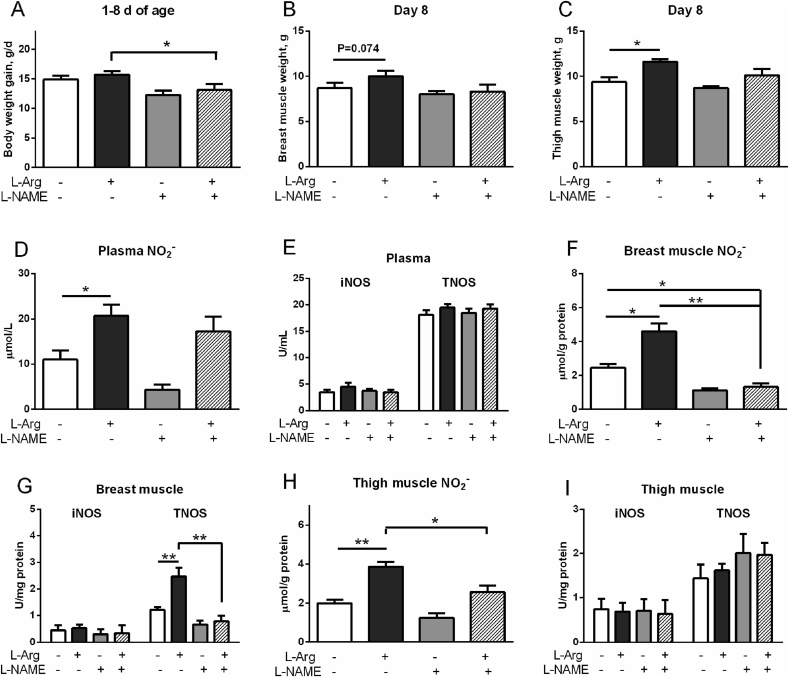
Compared to the control, dietary L-Arg supplementation had no significant influence (P > 0.05) on BW gain and breast muscle weight, whereas increased the thigh muscle mass (P < 0.05, Fig. 2A to C). L-NAME treatment had no detectable influence (P > 0.05) on BW gain and breast and thigh muscle masses. Compared to L-Arg treatment, L-NAME suppressed BW gain and breast and thigh muscle masses (P < 0.05).
Fig. 2.
Effects of L-arginine (L-Arg, 1%) and L-nitro-arginine methyl ester (L-NAME, 18.5 mM) treatments on the muscle development, nitrite (NO2−) concentration, activities of inducible nitric oxide synthase (iNOS), and total NOS (TNOS) in plasma and breast and thigh muscles of broilers. (A) Body weight gain; (B) breast muscle weight; (C) thigh muscle weight; (D) plasma NO2−; (E) plasma iNOS and TNOS activities; (F) NO2− concentration in breast muscle; (G) iNOS and TNOS activities in breast muscle; (H) NO2− concentration in thigh muscle; (I) iNOS and TNOS activities in thigh muscle. The data were presented as the mean ± SD (n = 6); ∗P < 0.05, ∗∗P < 0.01.
Compared with control birds, L-Arg increased but L-NAME decreased plasma NO2− concentration (P < 0.05, Fig. 2D). The iNOS and TNOS activities were not changed by any treatments (P > 0.05, Fig. 2E). In breast muscle, L-Arg increased whereas L-NAME suppressed NO2− levels (P < 0.05, Fig. 2F), compared with control. The iNOS activity was not changed by any treatments whereas TNOS was elevated by L-Arg treatment compared to control and L-NAME treatments (P < 0.01, Fig. 2G). In thigh muscle, NO2− level was higher in L-Arg group than that in control (P < 0.01), L-Arg + L-NAME (P < 0.05), and L-NAME treatments (P < 0.01, Fig. 2H). The iNOS and TNOS activities were not influenced by any treatments (P > 0.05, Fig. 2I).
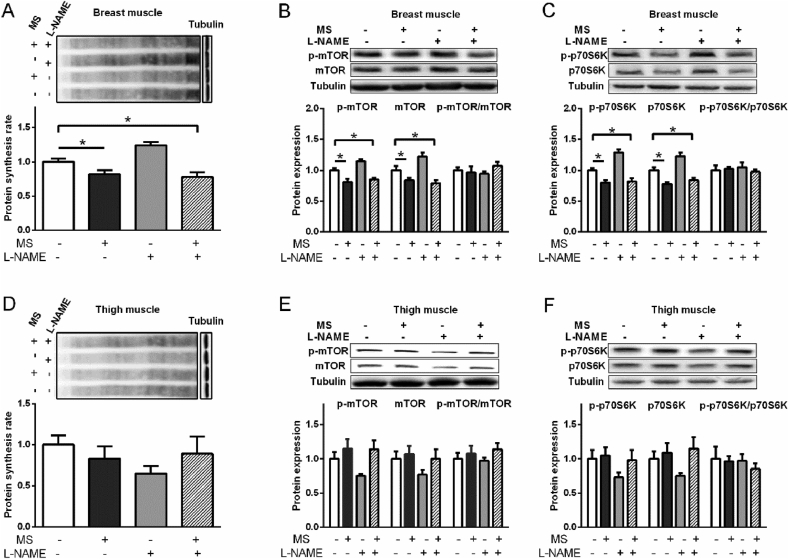
3.2. Effects of L-Arg and L-NAME on protein synthesis and mTOR/p70S6K pathway
In breast muscle, compared with control, the protein synthesis rate was decreased by L-Arg (P < 0.05) but increased by L-NAME treatment (P < 0.01, Fig. 3A). In the present of L-NAME, the inhibitory effect of L-Arg on protein synthesis rate was eliminated and increased to a higher level than that of control (P < 0.01, Fig. 3A). L-Arg decreased phosphorylated mTOR (p-mTOR), total mTOR and phosphorylated p70S6K (p-p70S6K), but did not change total p70S6K, p-mTOR/mTOR ratio, and p-p70S6K/p70S6K ratio compared to the control (P < 0.05, Fig. 3B, C). L-NAME increased p-mTOR (P < 0.05), mTOR (P < 0.05), p-p70S6K (P < 0.01), and reversed the inhibitory effect of L-Arg on p-mTOR (P < 0.05), mTOR (P < 0.05), and p-p70S6K (P < 0.01, Fig. 3B, C).
Fig. 3.
Effects of L-arginine (L-Arg, 1%) and L-nitro-arginine methyl ester (L-NAME, 18.5 mM) treatments on protein synthesis rate and phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K) in the breast and thigh muscles of broilers. (A) The protein synthesis rate in breast muscle; (B) the protein levels of total and phosphorylated mTOR in breast muscle; (C) the total and phosphorylated p70S6K in breast muscle; (D) the protein synthesis rate in thigh muscle; (E) the protein levels of total and phosphorylated mTOR in thigh muscle; (F) the total and phosphorylated p70S6K in thigh muscle. The data were presented as the mean ± SD (n = 6); ∗P < 0.05, ∗∗P < 0.01.
In thigh muscle, the protein synthesis rate was not changed by L-NAME or L-Arg treatment (P > 0.05, Fig. 3D). The total mTOR protein level was reduced by L-NAME treatment (P < 0.05, Fig. 3E), whereas the protein synthesis rate, p-mTOR, p-p70S6K, p70S6K and ratios of p-mTOR/mTOR and p-p70S6K/p70S6K were not changed by either L-Arg or L-NAME treatments (P > 0.05, Fig. 3D to F).
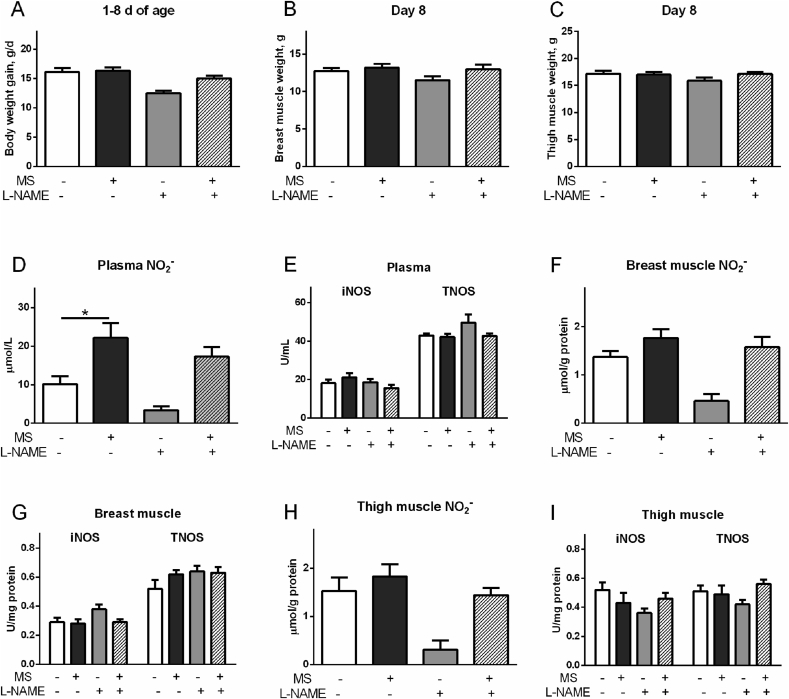
3.3. Effects of L-NAME and MS on muscle development, NO2− levels, and NOS activity
Compared to control, L-NAME decreased BW gain (P < 0.05, Fig. 4A), whereas MS had no significant influence on BW gain (P > 0.05). In contrast, the reduced BW gain by L-NAME was recovered in the presence of MS (P < 0.05, Fig. 4A). Compared to control, neither L-NAME nor MS treatment had an influence on breast and thigh muscle mass (P > 0.05, Fig. 4B, C). However, MS chicks had higher breast muscle mass than that of L-NAME chicks (P < 0.05, Fig. 4B).
Fig. 4.
Effects of molsidomine (MS, 0.1 mM) or L-nitro-arginine methyl ester (L-NAME, 18.5 mM) on the muscle development, nitrite (NO2−) concentration, and activities of inducible nitric oxide synthase (iNOS) and total NOS (TNOS) in plasma and breast and thigh muscles of broilers. (A) Body weight gain; (B) breast muscle weight; (C) thigh muscle weight; (D) plasma NO2− concentrations; (E) plasma activities of iNOS and TNOS; (F) NO2− concentrations in breast muscle; (G) activities of iNOS and TNOS in breast muscle; (H) NO2− concentrations in thigh muscle; (I) activities of iNOS and TNOS in thigh muscle. The data were presented as the mean ± SD (n = 6); ∗P < 0.05.
Plasma concentration of NO2− was increased by MS (P < 0.01) but decreased by L-NAME (P < 0.05), compared to control (Fig. 4D). Plasma activities of iNOS and TNOS were not changed by either L-NAME or MS treatment (P > 0.05, Fig. 4E). In breast and thigh muscle tissues, compared to control, the NO2− level was not changed by MS treatment (P > 0.05), but was decreased by L-NAME treatment (P < 0.01, Fig. 4F, H). In breast muscle, iNOS activity was increased by L-NAME (P < 0.05), whereas TNOS was not influenced (P > 0.05) by any treatments (Fig. 4G). In contrast, iNOS activity was decreased by L-NAME in thigh muscle compared to control (P < 0.05, Fig. 4I).
3.4. Effects of L-NAME and MS on protein synthesis and mTOR/p70S6K pathway
In breast muscle, L-NAME treatment increased but MS and NAMS treatments suppressed the protein synthesis rate (P < 0.05), compared to control (Fig. 5A). The levels of p-mTOR, mTOR, p-p70S6K, and p70S6K were increased by L-NAME (P < 0.05) and decreased by MS or NAMS treatment, compared to control (P < 0.05, Fig. 5B, C). The p-mTOR/mTOR ratio and p-p70S6K/p70S6K ratio were not significantly changed by all the treatments (P > 0.05, Fig. 5B, C). In thigh muscle, however, the protein synthesis rate, p-mTOR, mTOR, p-p70S6K, and p70S6K were decreased by L-NAME (P < 0.05) but were not influenced by other treatments, compared to the control (P > 0.05, Fig. 5D to F). Compared with L-NAME treatment, MS increased (P < 0.05) p-mTOR, mTOR, p-p70S6K, and p70S6K levels, whereas NAMS elevated p-mTOR and p70S6K levels (P < 0.05, Fig. 5E, F). The p-mTOR/mTOR ratio and p-p70S6K/p70S6K ratio were not influenced by all treatments (P > 0.05, Fig. 5E, F).
Fig. 5.
Effects of molsidomine (MS, 0.1 mM) and L-nitro-arginine methyl ester (L-NAME, 18.5 mM) treatments on protein synthesis rate and phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K) in breast and thigh muscles of broilers. (A) Protein synthesis rate in breast muscle; (B) total and phosphorylated mTOR in breast muscle; (C) total and phosphorylated p70S6K in breast muscle; (D) protein synthesis rate in thigh muscle; (E) total and phosphorylated mTOR in thigh muscle; (F) total and phosphorylated p70S6K in thigh muscle. The data were presented as the mean ± SD (n = 6); ∗P < 0.05, ∗∗P < 0.01.
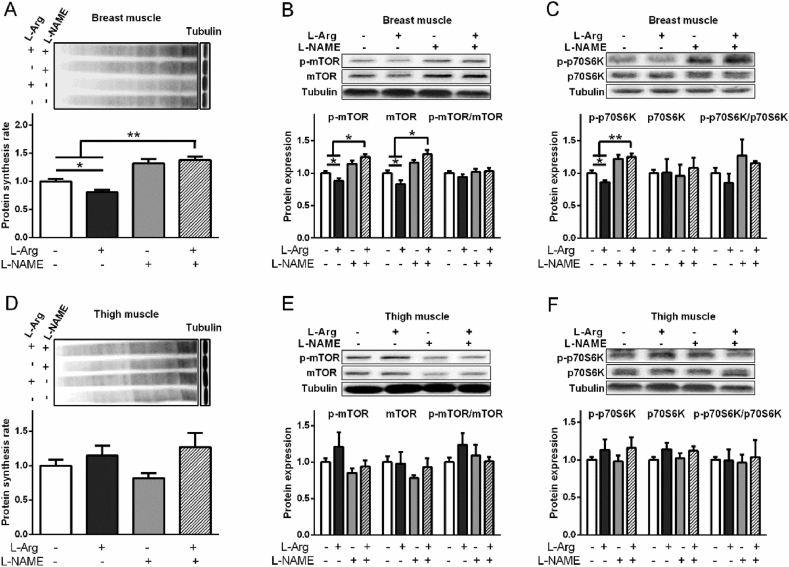
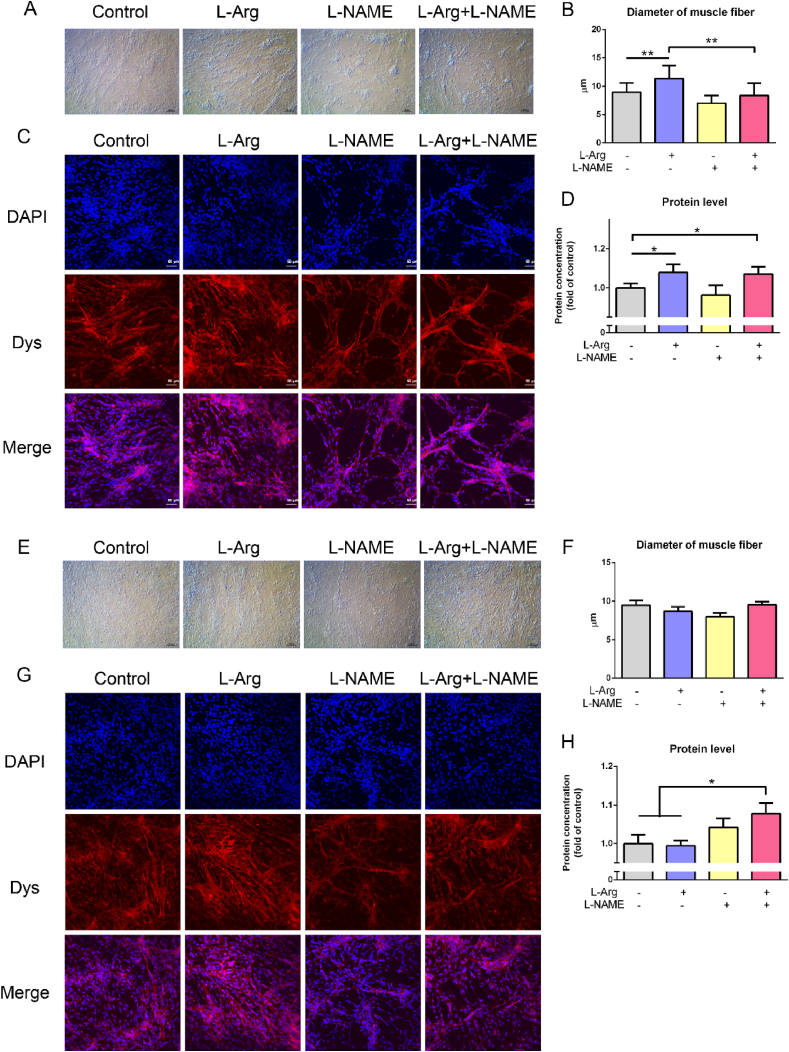
3.5. Effects of L-Arg and L-NAME on myoblast diameter, and protein content
In myoblasts from chicken embryo PM muscle, L-Arg increased (P < 0.05), whereas L-NAME decreased (P < 0.05) the diameter of muscle fibres (Fig. 6A to C). In contrast, L-Arg treatment increased (P < 0.05) the protein content of myoblasts, whereas L-NAME had no detectable influence (P > 0.05, Fig. 6D). In myoblasts from chicken embryo BF muscle, the diameter of myoblasts was not influenced by L-Arg (P > 0.05). Adversely, the diameter of myoblasts was decreased by L-NAME (P < 0.05), which was restored in the presence of L-Arg (P < 0.05, Fig. 6E to G). L-Arg had no significant effect on protein content (P > 0.05), whereas L-NAME treatment significantly increased the protein content (P < 0.05, Fig. 6H).
Fig. 6.
Effects of L-arginine (L-Arg, 400 μM) and L-nitro-arginine methyl ester (L-NAME, 1 mM) treatments on protein content and fiber diameter of myoblasts from chicken embryo pectoralis major (PM) and biceps femoris (BF) muscles. Myoblasts from PM: (A) Bright field of muscle fiber; (B) muscle fiber diameter (n = 20); (C) Dys immunofluorescence (10×, bar = 50 μm); (D) protein content (n = 6). Myoblasts from BF: (E) bright field of muscle fiber; (F) muscle fiber diameter (n = 20); (G) Dys immunofluorescence (10× magnification, bar = 50 μm); (H) protein content (n = 6). The data were presented as the mean ± SD; ∗P < 0.05; ∗∗P < 0.01.
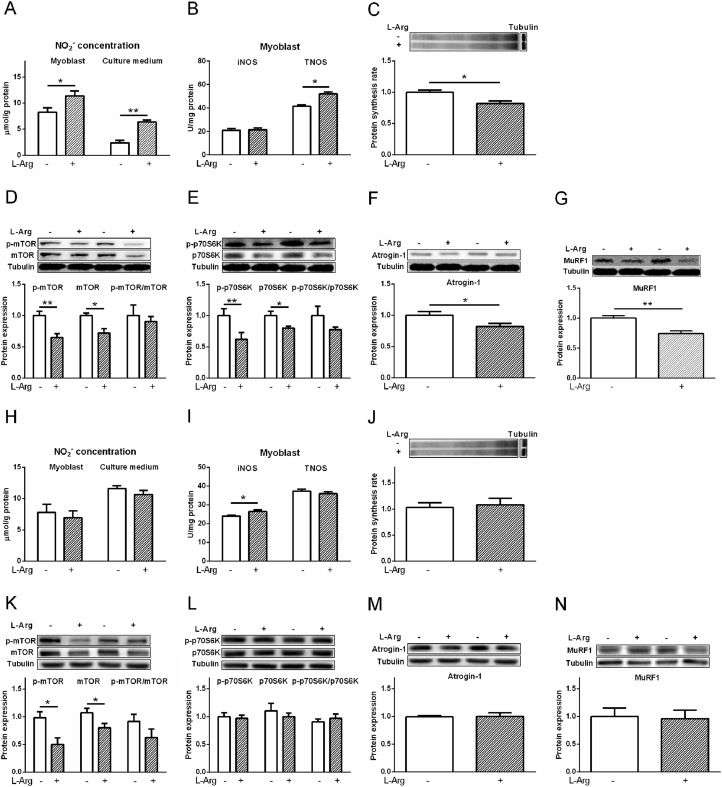
3.6. Effects of L-Arg, L-NAME, and SNP treatments on myoblasts from pectoralis major muscle
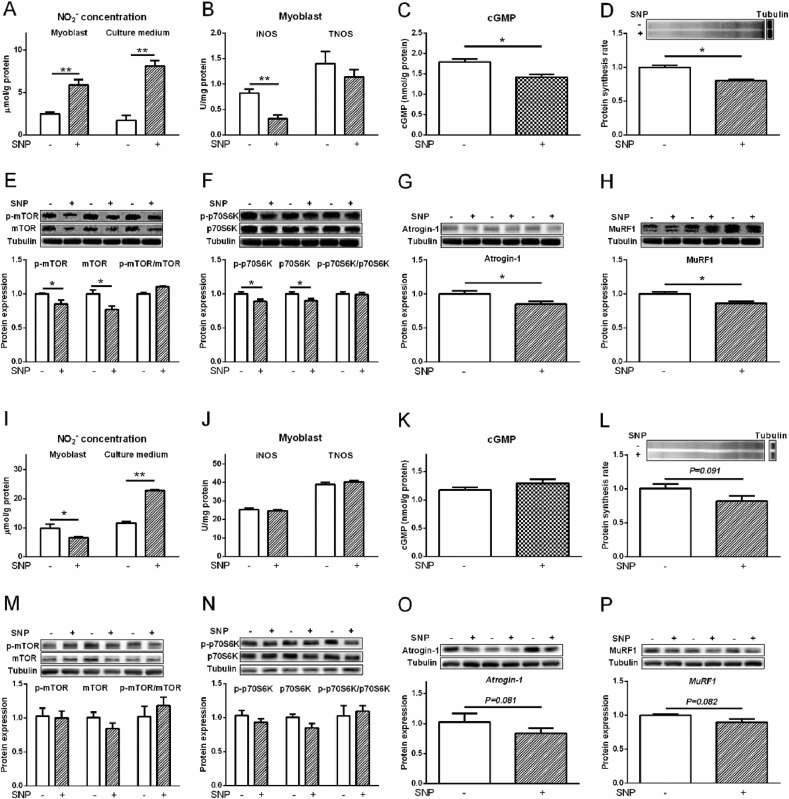
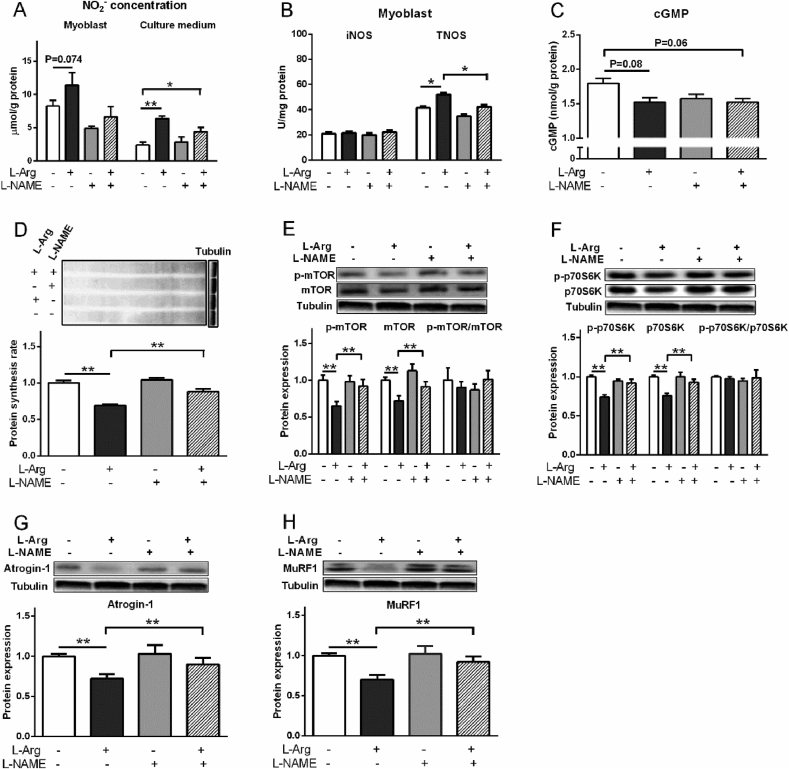
Compared with the control, L-Arg (400 μM) increased NO2− level in myoblasts from chicken embryo PM muscle and cell culture medium, and the activity of TNOS (P < 0.05, Fig. 7A, B). L-Arg decreased protein synthesis rate, p-mTOR, mTOR, p-p70S6K, p70S6K, atrogin-1, and MuRF1 levels compared to control (P < 0.05, Fig. 7C to G). SNP treatment significantly increased the NO2− concentration in myoblasts and cultural media (P < 0.01, Fig. 8A). The activity of iNOS and cGMP levels were decreased (P < 0.05) but TNOS activity was not influenced (P > 0.05) by SNP treatment (Fig. 8B, C). Compared to control, the protein synthesis rate, protein expression levels of p-mTOR, mTOR, p-p70S6K, p70S6K were all decreased by SNP treatment (P < 0.05, Fig. 8D to F). The p-mTOR/mTOR ratio and p-p70S6K/p70S6K ratio were not influenced by SNP treatment (P > 0.05, Fig. 8E, F). The protein levels of atrogin-1 and MuRF1 were suppressed by SNP treatment as well (P < 0.05, Fig. 8G, H).
Fig. 7.
Effects of L-arginine (L-Arg, 400 μM) on protein synthesis rate, phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K), and the protein levels of atrogin-1 and muscle RING finger 1 (MuRF1) in myoblasts from chicken embryo pectoralis major (PM) and biceps femoris (BF) muscles. Myoblasts from PM: (A) nitrite (NO2−) concentrations; (B) inducible nitric oxide synthase (iNOS) and total nitric oxide synthase (TNOS) activities; (C) protein synthesis rate; (D) total and phosphorylated mTOR; (E) total and phosphorylated p70S6K; (F) atrogin-1; (G) MuRF1. Myoblasts from BF: (H) NO2− concentrations; (I) iNOS and TNOS activities; (J) protein synthesis rate; (K) total and phosphorylated mTOR; (L) total and phosphorylated p70S6K; (M) atrogin-1; (N) MuRF1. The data were presented as the mean ± SD (n = 6); ∗P < 0.05; ∗∗P < 0.01.
Fig. 8.
Effects of sodium nitroprusside (SNP, 10 μM) treatment on protein synthesis rate, phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K), and the protein levels of atrogin-1 and muscle RING finger 1 (MuRF1) in myoblasts from chicken embryo pectoralis major (PM) and biceps femoris (BF) muscles. Myoblasts from PM: (A) nitrite (NO2−) concentrations; (B) inducible nitric oxide synthase (iNOS) and total nitric oxide synthase (TNOS) activities; (C) cyclic guanosine monophosphate (cGMP) level; (D) protein synthesis rate; (E) total and phosphorylated mTOR; (F) total and phosphorylated p70S6K; (G) atrogin-1; (H) MuRF1. Myoblasts from BF: (I) NO2− concentrations; (J) iNOS and TNOS activities; (K) cGMP level; (L) protein synthesis rate; (M) total and phosphorylated mTOR; (N) total and phosphorylated p70S6K; (O) atrogin-1; (P) MuRF1. The data were presented as the mean ± SD (n = 6); ∗P < 0.05; ∗∗P < 0.01.
L-NAME, however, decreased (P < 0.01) NO2− level in myoblasts which was increased (P = 0.074) by L-Arg, but not in the culture medium (P > 0.05, Fig. 9A). The iNOS activity was not changed by all the treatments (P > 0.05), whereas TNOS activity was increased (P < 0.05) by L-Arg but decreased (P < 0.05) by L-NAME treatment (Fig. 9B). Compared to control, L-Arg and L-Arg + L-NAME tended to decrease the cGMP level (Fig. 9C). The protein synthesis rate, p-mTOR, mTOR, p-p70S6K and p70S6K levels were suppressed by L-Arg treatment (P < 0.01, Fig. 9D to F) when compared to the control, and were not influenced by L-NAME or L-Arg + L-NAME treatment (P > 0.05). The p-mTOR/mTOR ratio and p-p70S6K/p70S6K ratio were not influenced by all the treatments (P > 0.05, Fig. 9E, F). Similarly, compared to control, the protein levels of atrogin-1 and MuRF1 were suppressed by L-Arg (P < 0.01), whereas were not influenced by L-NAME or L-Arg + L-NAME treatment (P > 0.05, Fig. 9G, H).
Fig. 9.
Effects of L-arginine (L-Arg, 400 μM) and L-nitro-arginine methyl ester (L-NAME, 1 mM) treatments on nitrite (NO2−) concentration, activities of inducible nitric oxide synthase (iNOS) and total nitric oxide synthase (TNOS), protein synthesis rate, phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K), and protein levels of atrogin-1 and muscle RING finger 1 (MuRF1) in myoblasts from chicken embryo pectoralis major muscle. (A) NO2− concentrations; (B) iNOS and TNOS activities; (C) cyclic guanosine monophosphate (cGMP) level; (D) protein synthesis rate; (E) total and phosphorylated mTOR; (F) total and phosphorylated p70S6K; (G) atrogin-1; (H) MuRF1. The data were presented as the mean ± SD (n = 6); ∗P < 0.05, ∗∗P < 0.01.
3.7. Effects of L-Arg, L-NAME, and SNP treatments on myoblasts from biceps femoris muscle
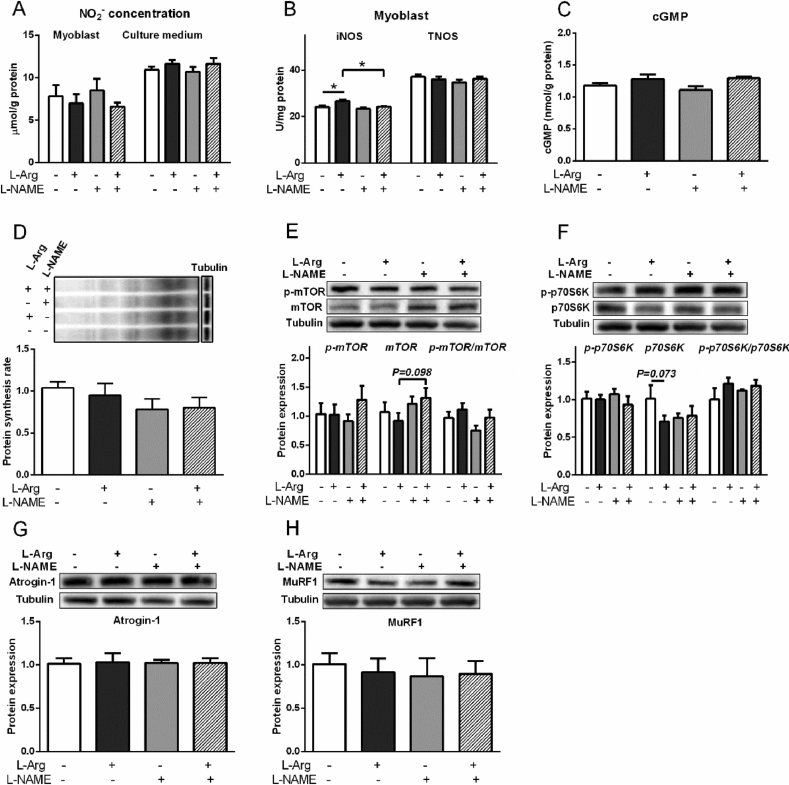
Compared with myoblasts from PM muscle, the NO2− level and the activity of TNOS were lower (P < 0.05) in myoblasts from BF muscle (Fig. S3). The mRNA expression of iNOS was higher than that of nNOS and eNOS (P < 0.05, Fig. S4) in myoblasts from PM and BF muscle. In the myoblast from BF muscle, L-Arg (400 μM) increased the activity of iNOS (P < 0.05, Fig. 7I), whereas the NO2− concentration in myoblasts and cultural media, and TNOS activity were not influenced (P > 0.05, Fig. 7H, I). The phosphorylated and total mTOR levels were decreased (P < 0.05, Fig. 7K), whereas, the protein synthesis rate, p-p70S6K, p70S6K, atrogin-1, MuRF1 levels, and p-mTOR/mTOR ratio and p-p70S6K/p70S6K ratio were not influenced by L-Arg (P > 0.05, Fig. 7J, L to N).
The iNOS activity was increased (P < 0.05) by L-Arg, whereas decreased (P < 0.05) by L-NAME treatment (Fig. 10B). The NO2− level in myoblasts and cell culture medium, and the TNOS activity were not changed by all the treatments (P > 0.05, Fig. 10A, B). Compared to control, the cGMP level was not altered by all treatments (Fig. 10C). L-Arg showed the tendency to decrease p70S6K level (P = 0.073, Fig. 10F), and L-NAME tended (P = 0.095) to decrease protein synthesis rate (Fig. 10D).
Fig. 10.
Effects of L-arginine (L-Arg, 400 μM) and L-nitro-arginine methyl ester (L-NAME, 1 mM) treatments on nitrite (NO2−) concentration, activities of inducible nitric oxide synthase (iNOS) and total nitric oxide synthase (TNOS), protein synthesis rate, phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K), and protein levels of atrogin-1 and muscle RING finger 1 (MuRF1) in myoblasts from chicken embryo biceps femoris muscle. (A) NO2− concentrations; (B) iNOS and TNOS activities; (C) cyclic guanosine monophosphate (cGMP) level; (D) protein synthesis rate; (E) total and phosphorylated mTOR; (F) total and phosphorylated p70S6K; (G) atrogin-1; (H) MuRF1. The data were presented as the mean ± SD (n = 6); ∗P < 0.05.
SNP treatment increased the NO2− concentration in cell culture medium (P < 0.01) whereas decreased in myoblasts (P < 0.05, Fig. 8I). The activities of iNOS and TNOS, and cGMP concentration were not influenced by SNP treatment (P > 0.05, Fig. 8J, K). SNP treatment showed a trend to decrease (P = 0.091) the protein synthesis rate of myoblasts (Fig. 8L). The protein expression levels of p-mTOR, mTOR, p-p70S6K, p70S6K, and the ratios of p-mTOR/mTOR, and p-p70S6K/p70S6K were not changed by SNP treatment (P > 0.05, Fig. 8M, N), whereas atrogin-1 (P = 0.081, Fig. 8O) and MuRF1 (P = 0.082, Fig. 8P) levels tended to be decreased by SNP.
3.8. Effects of rapamycin on the expression of atrogin-1 and MuRF1 in myoblasts
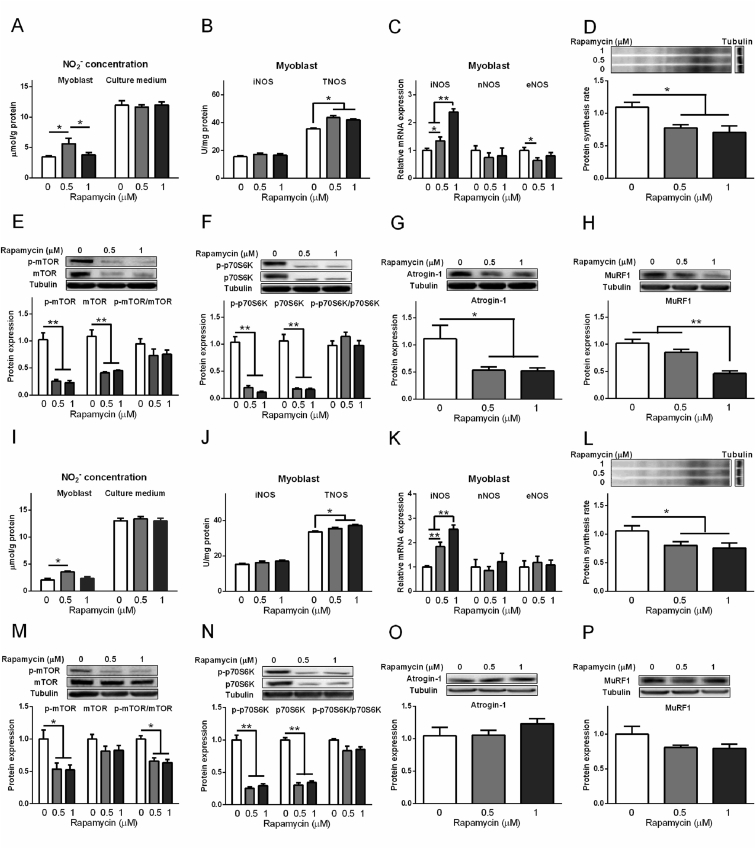
In the myoblast from chicken embryo PM muscle, compared to control, rapamycin increased the NO2− concentration in myoblasts at 0.5 μM (P < 0.05, Fig. 11A), and increased the activity of TNOS (P < 0.05, Fig. 11B). Rapamycin increased the mRNA expression of iNOS of myoblasts from PM and BF muscle in a dose-dependent way (P < 0.05, Fig. 11C, K); whereas, it had no detectable influence on the expression of nNOS. In contrast, eNOS expression was decreased by 0.5 μM rapamycin in myoblasts from PM muscle (P < 0.05, Fig. 11C). Rapamycin decreased the protein synthesis rate (P < 0.05, Fig. 11D), reduced p-mTOR, mTOR, p-p70S6K, and p70S6K levels (P < 0.01, Fig. 11E, F); however, the p-mTOR/mTOR ratio and p-p70S6K/p70S6K ratio were not changed by rapamycin treatment. Compared to control, atrogin-1 was decreased by rapamycin (P < 0.05, Fig. 11G), whereas MuRF1 level was reduced by rapamycin only at 1 μM (P < 0.05, Fig. 11H).
Fig. 11.
Effects of rapamycin treatment on protein synthesis rate, phosphorylation of mTOR and protein S6 kinase beta-1 (p70S6K), protein levels of atrogin-1 and muscle RING finger 1 (MuRF1) in myoblasts from chicken embryo pectoralis major (PM) and biceps femoris (BF) muscle. Myoblasts from PM: (A) nitrite (NO2−) concentrations; (B) inducible nitric oxide synthase (iNOS) and total nitric oxide synthase (TNOS) activities in myoblasts; (C) the relative mRNA expression of iNOS, nNOS, and eNOS; (D) the protein synthesis rate; (E) the total and phosphorylated mTOR; (F) the total and phosphorylated p70S6K; (G) atrogin-1; (H) MuRF1. Myoblasts from BF: (I) NO2− concentrations; (J) iNOS and TNOS activities in myoblasts; (K) the relative mRNA expression of iNOS, nNOS, and eNOS; (L) the protein synthesis rate; (M) the total and phosphorylated mTOR; (N) the total and phosphorylated p70S6K; (O) atrogin-1; (P) MuRF1. The data were presented as the mean ± SD (n = 6); ∗P < 0.05; ∗∗P < 0.01.
In the myoblast from chicken embryo BF muscle, the NO2− concentration in myoblasts was increased at 0.5 μM (P < 0.05, Fig. 11I) by rapamycin treatment. The activity of TNOS was also increased (P < 0.05, Fig. 11J). The protein synthesis rate, protein expression levels of p-mTOR, p-mTOR/mTOR, p-p70S6K, and p70S6K were reduced (P < 0.05, Fig. 11L to N), whereas mTOR, p-p70S6K/p70S6K, atrogin-1 and MuRF-1 levels were not changed by rapamycin (P > 0.05, Fig. 11M to P).
4. Discussion
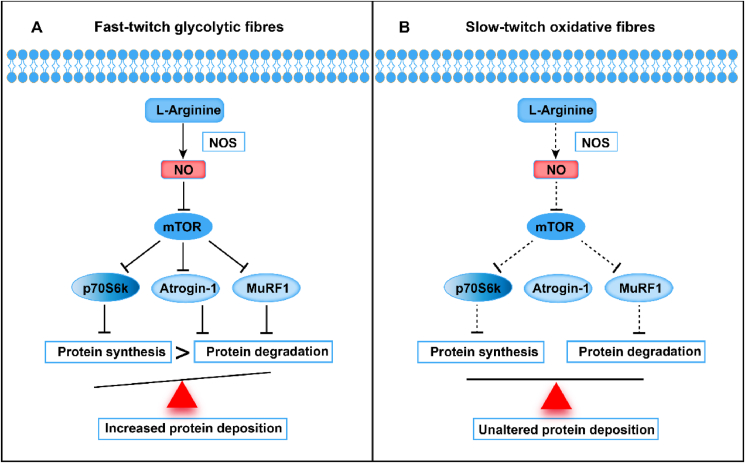
The present study indicated that L-Arg has a beneficial effect on the development of skeletal muscle. In myoblasts from chicken embryo PM muscle, L-Arg/NO and SNP inhibited protein synthesis rates simultaneously with the suppression of protein hydrolysis by hindering the mTOR/p70S6K and atrogin-1/MuRF-1 pathways, resulting in enhanced muscle fibre development. In contrast, myoblasts from BF muscle were less sensitive to the L-Arg/NO pathway. The result suggests that L-Arg/NO promotes skeletal muscle development by suppressing protein hydrolysis via mTOR/p70S6K pathway in a tissue dependent way (Fig. 12).
Fig. 12.
Schematic illustration of regulatory effect of L-Arg/NO on protein turnover. L-Arg/NO suppresses protein synthesis via mTOR/p70S6K pathway in a muscle fibre-dependent manner in chickens. (A) In myoblasts from chicken embryo pectoralis major muscle (fast-twitch glycolytic fibres), L-Arg/NO increases protein deposition by inhibiting protein synthesis via mTOR/p70S6K and suppressing protein hydrolysis via atrogin-1/MuRF-1 pathways. (B) In myoblasts from chicken embryo biceps femoris muscle (slow-twitch oxidative fibers), the effect of L-Arg/NO shows a weak effect. Solid arrowhead: strong positive effect; dotted arrowhead: weak positive effect; solid T-line, strong negative effect; dotted T-line, weak negative effect. L-arginine = L-Arg; NO = nitric oxide; NOS = nitric oxide synthase; p70S6K = protein S6 kinase beta-1; MuRF1 = muscle RING finger 1.
L-Arg tended to increase BW gain (+5.3%) and breast muscle mass (+14.9%), whereas significantly increased thigh muscle mass (+22.9%) compared to the control, indicating that L-Arg supplementation is beneficial for the development of skeletal muscles. This result is in agreement with the previous finding that dietary supplementation with L-Arg leads to an overall body growth and increased growth of breast muscle in broiler chickens (Yu et al., 2018; Castro et al., 2019; Subramaniyan et al., 2019). Similarly, L-arginine supplementation increased the gastrocnemius to body mass ratio of rats (Stefani et al., 2018) and enhanced swine lean-tissue growth (Tan et al., 2009). Hence, the result indicates that L-Arg has a beneficial effect on skeletal muscle development in both mammals and chickens. As there are interactions between L-Arg and other amino acids such as lysine and methionine (Chamruspollert et al., 2002; Zampiga et al., 2018), and L-Arg may act as a donor of NO, the in vivo effect of L-Arg on muscle protein deposition needs to be investigated further.
It is well known that L-Arg is the precursor of NO, which is an important gasotransmitter that participates in cell communication (Moncada et al., 1991; Nathan, 1992; Bredt, 1999; Hemish et al., 2003) and skeletal muscle function (Aguiar et al., 2017). Hence, we investigated the involvement of NO in the regulating effect of L-Arg. L-NAME supplementation abolished the favourable effect of L-Arg on BW gain and skeletal muscle mass, suggesting NO is associated with the regulating effect of L-Arg. This result was in accordance with the reduced NO2− concentration in plasma and skeletal muscle tissue and suppressed TNOS activity in breast muscle in chickens under L-NAME treatment. We further tested the hypothesis via the dietary supplementation of MS, a NO donor that has been used in humans (Chander and Chopra, 2005). MS treatment, however, had no influence on the NO level and activities of iNOS and TNOS in both breast and thigh muscles. Though, it elevated the plasma NO concentration. This result was consistent with the unobvious effect of MS on body weight gain and the breast and thigh muscle weights. As the NO levels in breast and thigh muscles were not altered by the MS treatment, the effect of NO on muscle development cannot be excluded. Adversely, the arrested BW gain by L-NAME was partially recovered and meanwhile the decreased NO2− concentrations in plasma, breast, and thigh muscle tissue by L-NAME were restored. In the in vivo model of the present study, however, the involvement of NO was not evaluated by using NO scavenger such as cPTIO, which will be further studied in the future. Collectively, the result suggests that NO contributes at least partially to the beneficial effect of L-Arg on skeletal muscle development.
Skeletal muscles are composed of several types of muscle fibres, exhibiting oxidative or glycolytic metabolism. It is more susceptible to age-related atrophy of fast-twitch glycolytic fibres when compared with slow-twitch oxidative fibres in mammals (Larsson et al., 2010; Braga et al., 2016). In chickens, the PM muscle primarily comprises of fast-twitch glycolytic fibers, whereas the BF muscle mainly contains slow-twitch oxidative fibers (Barbut and Shai, 2002). Hence, we further investigated the regulation of L-Arg on protein synthesis of the PM and BF muscles. By using a nonradioactive method, the protein synthesis rate was detected via measuring newly synthesized proteins labelled by puromycin (Schmidt et al., 2009; Goodman et al., 2010; Wang et al., 2018). The decreased protein synthesis rate by L-Arg in breast muscle indicated the negative regulation of L-Arg on in vivo protein synthesis. This speculation was supported by the result that L-NAME rescued the restrained protein synthesis rate by L-Arg. This observation was contrary to the reports in mammals. L-Arg enhances protein synthesis in skeletal muscle of piglets (Frank et al., 2007; Yao et al., 2008). However, this result was further solidified by the observation that MS treatment suppressed the protein synthesis rate in breast muscle. Adversely, L-NAME cannot restore the suppressive effect of MS, when the NO2− concentration in breast muscle was not significantly changed by L-NAME. Furthermore, the inhibited protein synthesis rate by L-Arg or MS observed in the breast muscle was not detected in the thigh muscle, indicating that the regulating effect of L-Arg/NO is different in the breast from that in thigh muscle. Collectively, the result suggested that L-Arg suppressed protein synthesis of skeletal muscle in a NO-dependent and tissue specific way.
In mammals, the activation of mTORC1 is responsible for the increased protein synthesis and skeletal muscle growth (Baar and Esser, 1999; Rommel et al., 2001; Hornberger and Chien, 2010; Shimizu et al., 2011). In chickens, mTOR is associated with appetite control in hypothalamus (Liu et al., 2015), and the protein synthesis in intestinal function (Liu et al., 2016, 2018) and skeletal muscle (Wang et al., 2019). Hence, we further measured the activation of mTOR/p70S6K pathway. The suppressed phosphorylation levels of mTOR and p70S6K were observed in the breast muscle of chicks with L-Arg treatment, suggesting the arrested mTOR pathway. In the presence of L-NAME, the suppressed mTOR/p70S6K was totally abolished. In contrast, MS decreased the levels of phosphorylated mTOR and p70S6K in breast muscle, and the suppression effect was not altered by the presence of L-NAME. Hence, the result suggested that NO should be responsible for the block of mTOR/p70S6K pathway in breast muscle.
In order to test the hypothesis, we further verified the observation in cultured myoblasts in vitro. In myoblasts obtained from PM muscle, L-Arg treatment increased intracellular NO2− level simultaneously with the suppressed protein synthesis rate and phosphorylated mTOR and p70S6K, indicating that L-Arg restrained protein synthesis with the involvement of mTOR/p70S6K pathway. In the presence of L-NAME, however, the reduced intra- and inter-cellular NO2− concentrations and TNOS activity indicated that L-NAME decreased the production of NO. This result was consistent with the in vivo experiments, where TNOS but not iNOS activity changed consistently with NO2−, indicating that TNOS is responsible for the formation of NO in breast muscle. The suppressed protein synthesis rate and phosphorylated-mTOR and p70S6K levels by L-Arg treatment were reversed by L-NAME, indicating that NO hinders the protein synthesis with the involvement of mTOR/p70S6K pathway. SNP, a NO donor, increased NO2− level but suppressed the activity of iNOS, suggesting the feedback effect of NO on iNOS activity. The decreased protein synthesis rate and reduced phosphorylated mTOR and p70S6K levels by SNP indicated that NO should be responsible for the restrained protein synthesis and hindered the mTOR/p70S6K pathway. The interaction of NO with heme-containing proteins is exemplified in the binding to guanylate cyclase, which activates the enzyme and thereby raises cGMP levels (Stamler and Meissner, 2001; Godfrey and Schwarte, 2010). In this study, cGMP level was reduced in the presence of L-Arg and SNP, indicating the suppressed cascade of NO/cGMP pathway. The underlying mechanism remains to be further elucidated. In the presence of rapamycin, the suppressed protein synthesis and blocked mTOR/p70S6K activation also indicated that the mTOR/p70S6K pathway is involved in the regulation of L-Arg/NO system on the regulation of protein synthesis of fast-twitch glycolytic fibres.
In thigh muscle, the NO level was increased by L-Arg but not MS treatment, and the iNOS activity was not influenced by either L-Arg or MS. However, in the in vitro cultured myoblasts from BF muscle, L-Arg increased iNOS activity and showed no detectable effect on NO2− levels. Whereas, SNP reduced the intracellular NO but did not alter iNOS and TNOS activities. Moreover, SNP increased the NO level in the culture medium but differently changed the NO level in myoblasts from PM and BF. The result indicated the discrepancies between the in vivo and in vitro systems and between different muscle types. In contrast to the breast muscle, in vivo L-Arg or MS treatments had no obvious influence on the protein synthesis rate of thigh muscle, indicating that thigh muscle is less sensitive to L-Arg/NO treatment compared to breast muscle. Moreover, L-NAME treatment suppressed the protein synthesis rate in thigh muscle, which was restored by the presence of MS or L-Arg. In the in vitro cultured myoblasts from BF muscle, L-Arg had no detectable influence but SNP tended to suppress the protein synthesis rate. This observation, however, disagreed with the result in C2C12 cells, in which L-Arg promotes protein synthesis in a NO-dependent manner (Wang et al., 2019). The activation of mTOR/p70S6K pathway was further determined and the result indicated that L-Arg and MS had no significant influence on the phosphorylation of mTOR and p70S6K in the thigh muscle of broilers, whereas L-NAME decreased the phosphorylation of mTOR. The result indicated that L-Arg/NO cannot activate mTOR/p70S6K pathway in thigh muscle. In the in vitro cultured myoblasts obtained from embryonic BF muscle, the phosphorylated mTOR level was differently influenced by L-Arg and SNP; whereas, the phosphorylation level of p70S6K was not altered by either L-Arg or SNP, suggesting that the mTOR/p70S6K pathway is not activated. Collectively, the result implies that the L-Arg/NO system in BF is not involved in the regulation of protein synthesis via mTOR/p70S6K pathway. NO and related molecules are associated with the regulation of skeletal muscle functions such as force production, blood flow, and myocyte differentiation, respiration, and glucose homeostasis (Stamler and Meissner, 2001). In mammals, nNOS-1/NO system of skeletal muscles exerts its biological role especially in fast-oxidative myofibers, because these myofibers express more NOS-1 than fast-glycolytic or slow-oxidative ones (Planitzer et al., 2001). In this study, the result showed the difference in the fast-twitch glycolytic fibres (PM) and slow-twitch oxidative fibers (BF). Hence, the role of NOS in the breast and thigh muscle needs to be investigated further. Collectively, the present result suggests that L-Arg/NO regulates protein synthesis in a tissue-specific way, playing a role on the protein synthesis of fast-twitch glycolytic fibres and having little influence on the protein synthesis in the slow-twitch oxidative fibres.
As the in vivo experiment indicated the beneficial effect of L-Arg/NO on muscle development, we further investigated the effect of L-Arg on proteolysis in myoblasts. Atrogin-1 and MuRF1, as 2 critical ubiquitin-protein ligases (E3s), are critical regulators of proteolysis, leading to muscle atrophy (Bodine et al., 2001). The myogenic transcription factors such as elongation initiation factor 3 (eIF3-f), myogenic differentiation antigen (MyoD), and myogenin are targets of atrogin-1 (Foletta et al., 2011). In C2C12 cells, the upregulated expression of MuRF1 is associated with the glucocorticoid-induced suppression of protein synthesis (Wang et al., 2016). In myoblasts from PM muscle, atrogin-1 and MuRF1 were downregulated by L-Arg or SNP treatments, and the repressing effect of L-Arg, but not SNP, was abolished by L-NAME. The result indicates that L-Arg inhibits protein degradation via arresting MuRF1 and atrogin-1 expression in a NO-dependent way. In myoblasts from BF muscle, however, high levels of L-Arg and SNP tended to inhibit the protein levels of atrogin-1 and MuRF1, suggesting that L-Arg/NO is a minor regulator in the protein degradation of myoblasts from thigh muscle. This result was in line with the observation that L-Arg increased the protein content and diameter of myotubes from myoblasts of PM. It was previously reported that the branched-chain amino acids and arginine could suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway (Herningtyas et al., 2008). Our results are in agreement with the previous study, which demonstrated that blocking the mTOR pathway with rapamycin resulted in restrained atrogin-1 and MuRF1 protein levels. It is interesting to note that rapamycin increased NO level at a dose of 0.5 μM in the 2 types of myoblasts, in line with the increased iNOS expression level. As a key signalling molecule, NO plays an important role in the maintenance of both skeletal muscle integrity and proper signalling mechanisms during adaptation to mechanical and metabolic stimulation (Kobayashi et al., 2019). Hence, if NO is involved in rapamycin-induced inactivation of mTOR/p70S6K pathway remains to be elucidated further. Collectively, the result indicates that L-Arg/NO induces the arrested protein synthesis simultaneously with the suppressed proteolysis to more serious extents, resulting in reduced protein turnover and increased protein accumulation. As this effect was not detected in the slow-twitch oxidative fibres, this suggests the beneficial effect of L-Arg/NO is muscle-fibre specific.
5. Conclusion
The result demonstrates that L-Arg/NO enhances protein accumulation and muscle development by suppressing protein turnover in a muscle fibre specific way, suppressing protein synthesis simultaneously with proteolysis to a more serious extent in the fast-twitch glycolytic fibres but not in the slow-twitch oxidative fibres. The result suggests that the L-Arg/NO/mTOR/p70S6K pathway is involved in the protein metabolism of fast-twitch glycolytic muscle. The result highlights the potential clinical application of L-Arg or NO in the treatment of muscle wasting in fast-twitch glycolytic muscle fibres.
Author contributions
Ruxia Wang: conceptualization, data curation, writing - original draft; Kelin Li: investigation, data curation, writing - original draft; Li Sun: investigation, project administration; Hongchao Jiao: methodology, resources; Jingpeng Zhao: methodology, software; Yunlei Zhou: validation, visualization; Haifang Li: supervision, validation; Xiaojuan Wang: supervision, project administration; Hai Lin: conceptualization, project administration, writing - review & editing, funding acquisition.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by the Key Technologies Research and Development Program of China (2021YFD1300405), the Earmarked Fund for China Agriculture Research System (CARS-40-K09), National Natural Science Foundation of China (31772619) and Key Technology Research and Development Program of Shandong Province (2019JZZY020602).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2022.04.010.
Contributor Information
Jingpeng Zhao, Email: zjp@163.com.
Hai Lin, Email: hailin@sdau.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Aguiar A.F., Vechetti-Júnior I.J., Souza R.W., Piedade W.P., Pacagnelli F.L., Leopoldo A.S., et al. Nitric oxide synthase inhibition impairs muscle regrowth following immobilization. Nitric Oxide. 2017;69:22–27. doi: 10.1016/j.niox.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Anderson J.E., Zhu A., Mizuno T.M. Nitric oxide treatment attenuates muscle atrophy during hind limb suspension in mice. Free Radic Biol Med. 2017;115:458–470. doi: 10.1016/j.freeradbiomed.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Baar K., Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Barbut S., Shai D. CRC Press; Boca Raton: 2002. Poultry products processing: an industry guide. [Google Scholar]
- Barton E.R., Morris L., Kawana M., Bish L.T., Toursel T. Systemic administration of L-arginine benefits mdx skeletal muscle function. Muscle Nerve. 2010;32:751–760. doi: 10.1002/mus.20425. [DOI] [PubMed] [Google Scholar]
- Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bodine S.C., Baehr L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2015;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga A.S., Padilha F.G.F., Ferreira A.M.R. Evaluation of muscle fiber types in German shepherd dogs of different ages. Anat Rec (Hoboken) 2016;299:1540–1547. doi: 10.1002/ar.23464. [DOI] [PubMed] [Google Scholar]
- Bredt D.S. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- Brenman J.E., Chao D., Xia S.H., Aldape K., Bredt D. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Cazzato D., Assi E., Moscheni C., Brunelli S., De Palma C., Cervia D., et al. Nitric oxide drives embryonic myogenesis in chicken through the upregulation of myogenic differentiation factors. Exp Cell Res. 2014;320:269–280. doi: 10.1016/j.yexcr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Castro F.L.S., Su S., Choi H., Koo E., Kim W.K. L-Arginine supplementation enhances growth performance, lean muscle, and bone density but not fat in broiler chickens. Poultr Sci. 2019;98:1716–1722. doi: 10.3382/ps/pey504. [DOI] [PubMed] [Google Scholar]
- Chamruspollert M., Pesti G.M., Bakalli R.I. Dietary interrelationships among arginine, methionine, and lysine in young broiler chicks. Br J Nutr. 2002;88:655–660. doi: 10.1079/BJN2002732. [DOI] [PubMed] [Google Scholar]
- Chander V., Chopra K. Renal protective effect of molsidomine and L-arginine in ischemia-reperfusion induced injury in rats. J Surg Res. 2005;128:132–139. doi: 10.1016/j.jss.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Fernandes J.I., Murakami A.E., Martins E.N., Sakamoto M.I., Garcia E.R. Effect of arginine on the development of the pectoralis muscle and the diameter and the protein: deoxyribonucleic acid rate of its skeletal myofibers in broilers. Poultr Sci. 2009;88:1399–1406. doi: 10.3382/ps.2008-00214. [DOI] [PubMed] [Google Scholar]
- Foletta V.C., White L.J., Larsen A.E., Léger B., Russell A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflügers Archiv. 2011;461:325–335. doi: 10.1007/s00424-010-0919-9. [DOI] [PubMed] [Google Scholar]
- Frank J.W., Escobar J., Nguyen H.V., Jobgen S.C., Jobgen W.S., Davis T.A., et al. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr. 2007;137:315–319. doi: 10.1093/jn/137.2.315. [DOI] [PubMed] [Google Scholar]
- Godfrey E.W., Schwarte R.C. Nitric oxide and cyclic GMP regulate early events in agrin signaling in skeletal muscle cells. Exp Cell Res. 2010;316:1935–1945. doi: 10.1016/j.yexcr.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Goodman C.A., Mabrey D.M., Frey J.W., Miu M.H., Schmidt E.K., Pierre P., et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2010;25:1028–1039. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham D.J., Caldow M.K., Lynch G.S., Koopman R. Arginine protects muscle cells from wasting in vitro in an mTORC1-dependent and NO-independent manner. Amino Acids. 2014;46:2643–2652. doi: 10.1007/s00726-014-1815-y. [DOI] [PubMed] [Google Scholar]
- Hemish J., Nakaya N., Mittal V., Enikolopov G. Nitric oxide activates diverse signaling pathways to regulate gene expression. J Biol Chem. 2003;278:42321–42329. doi: 10.1074/jbc.M308192200. [DOI] [PubMed] [Google Scholar]
- Herningtyas E.H., Okimura Y., Handayaningsih A.E., Yamamoto D., Maki T., Iida K., et al. Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochim Biophys Acta. 2008;1780:1115–1120. doi: 10.1016/j.bbagen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Hornberger T.A., Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2010;97:1207–1216. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]
- Kalliokoski K.K., Langberg H., Ryberg A.K., Scheede-Bergdahl C., Doessing S., Kjaer A., et al. Nitric oxide and prostaglandins influence local skeletal muscle blood flow during exercise in humans: coupling between local substrate uptake and blood flow. Am J Physiol Regul Integr Comp Physiol. 2006;291:R803–R809. doi: 10.1152/ajpregu.00808.2005. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Uchida H., Kofuji A., Ito J., Shimizu M., Kim H., et al. Molecular regulation of skeletal muscle mass and the contribution of nitric oxide: a review. FASEB Bioadv. 2019;1:364–374. doi: 10.1096/fba.2018-00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.K., Hong E.K., Lee K.H., Kim J.I., Song W.K. Molecular cloning andexpression of nitric oxide synthase gene in chick embryonic muscle cells. Cell Biochem Funct. 1999;17:261–270. doi: 10.1002/(SICI)1099-0844(199912)17:4<261::AID-CBF838>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Larsson L., Biral D., Campione M., Schiaffino S. An age-related type IIB to IIX myosin heavy chain switching in rat skeletal muscle. Acta Physiol Scand. 2010;147:227–234. doi: 10.1111/j.1748-1716.1993.tb09493.x. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Baek M.Y., Moon K.Y., Song W.K., Chung C.H., Ha D.B., et al. Nitric oxide as a messenger molecule for myoblast fusion. J Biol Chem. 1994;269:14371–14374. [PubMed] [Google Scholar]
- Lin A.W., Chang C.C., McCormick C.C. Molecular cloning and expression of an avian macrophage nitric-oxide synthase cDNA and the analysis of the genomic 5'-flanking region. J Biol Chem. 1996;271:11911–11919. doi: 10.1074/jbc.271.20.11911. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang X., Jiao H., Zhao J., Lin H. Glucocorticoids inhibited hypothalamic target of rapamycin in high fat diet-fed chicks. Poultry Sci. 2015;94:2221–2227. doi: 10.3382/ps/pev168. [DOI] [PubMed] [Google Scholar]
- Liu S.Q., Zhao J.P., Fan X.X., Liu G.H., Jiao H.C., Wang X.J., et al. Rapamycin, a specific inhibitor of the target of rapamycin complex 1, disrupts intestinal barrier integrity in broiler chicks. J Anim Physiol Anim Nutr (Berl) 2016;100:323–330. doi: 10.1111/jpn.12375. [DOI] [PubMed] [Google Scholar]
- Liu S.Q., Wang L.Y., Liu G.H., Tang D.Z., Fan X.X., Zhao J.P., et al. Leucine alters immunoglobulin a secretion and inflammatory cytokine expression induced by lipopolysaccharide via the nuclear factor-κB pathway in intestine of chicken embryos. Animal. 2018;12:1903–1911. doi: 10.1017/S1751731117003342. [DOI] [PubMed] [Google Scholar]
- Long J.H., Lira V.A., Soltow Q.A., Betters J.L., Sellman J.E., Criswell D.S. Arginine supplementation induces myoblast fusion via augmentation of nitric oxide production. J Muscle Res Cell Motil. 2006;27:577–584. doi: 10.1007/s10974-006-9078-1. [DOI] [PubMed] [Google Scholar]
- McNeal C.J., Meininger C.J., Reddy D., Wilborn C.D., Wu G. Safety and effectiveness of arginine in adults. J Nutr. 2016;146 doi: 10.3945/jn.116.234740. 2587S–93S. [DOI] [PubMed] [Google Scholar]
- Miniaci M.C., Dattolo M.G., Irace C., Capuozzo A., Santamaria R., Scotto P. Glucose deprivation promotes activation of mTOR signaling pathway and protein synthesis in rat skeletal muscle cells. Pflügers Archiv. 2015;467:1357–1366. doi: 10.1007/s00424-014-1583-2. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Morais S.R.L., Brito V.G.B., Mello W.G., Oliveira S.H.P. L-arginine modulates inflammation and muscle regulatory genes after a single session of resistance exercise in rats. Scand J Med Sci Sports. 2018;28:425–435. doi: 10.1111/sms.12935. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- Planitzer G., Miethke A., Baum O. Nitric oxide synthase-1 is enriched in fast-twitch oxidative myofibers. Cell Tissue Res. 2001;306:325–333. doi: 10.1007/s004410100449. [DOI] [PubMed] [Google Scholar]
- Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Schmidt E.K., Clavarino G., Ceppi M., SUnSET Pierre P. A nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Yoshikawa N., Ito N., Maruyama T., Suzuki Y., Takeda S., et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metabol. 2011;13:170–182. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Stamler J.S., Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Stefani G.P., Marmett B., Alves J.P., Möller G.B., Heck T.G., Frizzo M.N., et al. Resistance training and L-arginine supplementation are determinant in genomic stability, cardiac contractility and muscle mass development in rats. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan S.A., Kang D.R., Park J.R., Siddiqui S.H., Ravichandiran P., Yoo D.J., et al. Effect of in ovo injection of L-arginine in different chicken embryonic development stages on post-hatchability, immune response, and myo-D and myogenin proteins. Animals. 2019;9:357. doi: 10.3390/ani9060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., Yin Y., Liu Z., Li X., Xu H., Kong X., et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- Wang R., Jiao H., Zhao J., Wang X., Lin H. Glucocorticoids Enhance muscle proteolysis through a myostatin-dependent pathway at the early stage. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Jiao H., Zhao J., Wang X., Sun S., Lin H. Allicin alleviates reticuloendotheliosis virus-induced immunosuppression via erk/mitogen-activated protein kinase pathway in specific pathogen-free chickens. Front Immunol. 2017;8:1856. doi: 10.3389/fimmu.2017.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Jiao H., Zhao J., Wang X., Lin H. L-Arginine enhances protein synthesis by phosphorylating mTOR (Thr 2446) in a nitric oxide-dependent manner in C2C12 cells. Oxid Med Cell Longev. 2018;2018:1–13. doi: 10.1155/2018/7569127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Li K., Wang H., Jiao H., Wang X., Zhao J., et al. Endogenous CSE/hydrogen sulfide system regulates the effects of glucocorticoids and insulin on muscle protein synthesis. Oxid Med Cell Longev. 2019;2019:1–15. doi: 10.1155/2019/9752698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Meininger C., Knabe D.A., Bazer F.W., Rhoads J.M. Arginine nutrition in development, health and disease. Curr Opin Clin Nutr Metab Care. 2000;3:59–66. doi: 10.1097/00075197-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Yao K., Yin Y.L., Chu W., Liu Z., Deng D., Li T., et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008;138:867–872. doi: 10.1093/jn/138.5.867. [DOI] [PubMed] [Google Scholar]
- Yu L., Gao T., Zhao M., Lv P., Zhang L., Li J., et al. Effects of in ovo feeding of l-arginine on breast muscle growth and protein deposition in post-hatch broilers. Animal. 2018;12:2256–2263. doi: 10.1017/S1751731118000241. [DOI] [PubMed] [Google Scholar]
- Zhao J., Jiao H., Jiang Y., Song Z., Wang X., Lin H. Cool perch availability improves the performance and welfare status of broiler chickens in hot weather. Poultr Sci. 2012;91:1775–1784. doi: 10.3382/ps.2011-02058. [DOI] [PubMed] [Google Scholar]
- Zampiga M., Laghi L., Petracci M., Zhu C., Meluzzi A., Dridi S., et al. Effect of dietary arginine to lysine ratios on productive performance, meat quality, plasma and muscle metabolomics profile in fast-growing broiler chickens. J Anim Sci Biotechnol. 2018;9:79. doi: 10.1186/s40104-018-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.