Highlights
-
•
Magnetoencephalographic effects of fear conditioning predict exposure outcomes.
-
•
No associations between fear ratings of conditioned stimuli and exposure outcomes.
-
•
Prefrontal correlates of safety processing and/or fear inhibition are treatment-relevant.
-
•
Individual neural differences might be a promising predictor of exposure success.
Keywords: Exposure outcome, Anxiety disorders, Fear conditioning, MEG/EEG, Specific phobia, Virtual reality exposure therapy
Abstract
Background
Models of anxiety disorders and the rationale of exposure therapy (ET) are grounded on classical fear conditioning. Yet, it is unclear whether lower fear ratings of conditioned safety versus threat cues and corresponding neural markers of safety-learning and/or fear inhibition assessed before treatment would predict better outcomes of behavioral exposure.
Methods
Sixty-six patients with spider phobia completed pre-treatment clinical and experimental fear conditioning assessments, one session of virtual reality ET, a post-treatment clinical assessment, and a 6-month follow-up assessment. Tilted Gabor gratings served as conditioned stimuli (CS) that were either paired (CS+) or remained unpaired (CS-) with an aversive phobia-related and phobia-unrelated unconditioned stimulus (UCS). CS+/CS- differences in fear ratings and magnetoencephalographic event-related fields (ERFs) were related to percentual symptom reductions from pre- to post-treatment, as assessed via spider phobia questionnaire (SPQ), behavioral avoidance test (BAT), and remission status at 6-month follow-up.
Results
We observed no associations between pre-treatment CS+/CS- differences in fear ratings and any treatment outcome. CS+/CS- differences in source estimations of ERFs revealed that higher CS- activity in bilateral dorsolateral prefrontal cortex (dlPFC) was related with SPQ- and BAT-reductions. Associations between CS+/CS- differences and treatment outcomes were also observed in left ventromedial prefrontal cortex (vmPFC) regions, which additionally revealed associations with the follow-up remission status.
Conclusions
Results provide initial evidence that neural pre-treatment CS+/CS- differences may hold predictive information regarding outcomes of behavioral exposure. Our findings highlight a key role of neural responses to safety cues with potentially inhibitory effects on affect-generating structures during fear conditioning.
1. Introduction
Exposure-based cognitive-behavioral therapy is the first-line treatment for anxiety disorders, including specific phobia (SP) (Bandelow et al., 2021). Although this therapy has medium to large effect sizes (Bandelow et al., 2015, Carpenter et al., 2018), non-response rates reach up to 50% (Loerinc et al., 2015, Taylor et al., 2012), demonstrating the need to identify mechanisms that may explain this substantial variance in treatment effects. In the attempt to identify treatment response-relevant factors for patients with SP mixed findings have been revealed (Böhnlein et al., 2020). Yet, identified factors were predominantly related to learning processes (Böhnlein et al., 2020, Lueken et al., 2016), which also play a key role in etiological models of anxiety disorders (Mineka and Oehlberg, 2008, Mowrer, 1947) and in the rationale of exposure therapy (ET) (Craske et al., 2008, Craske et al., 2018, Craske et al., 2012, Foa and Kozak, 1986).
Based on findings from classical fear conditioning and extinction studies (Bouton and Moody, 2004, Lonsdorf and Merz, 2017, Myers and Davis, 2007), contemporary ET models (Craske et al., 2008, Craske et al., 2018, Craske et al., 2012) posit that ET produces new “safety memory traces” associating phobic stimuli with the absence of the expected aversive outcome (unconditioned stimuli, UCS). These safety memory traces are thought to compete with fear-generating “threat memory traces” that link the phobic stimulus with the presence of aversive outcomes. Consequently, if patients learn to successfully discriminate between safety and threat signals and inhibit fear responses in the presence of safety cues, reductions of experienced fear levels are expected (Jovanovic et al., 2012). In line with this, a study employing functional Near Infrared Spectroscopy during virtual reality exposure therapy (VRET) in acrophobia showed stronger prefrontal activations with more exposures, which might underpin an improved inhibition of fear-associated memories (Landowska et al., 2018).
Although the rationale of ET is mainly grounded on the theory of fear conditioning, this rationale is primarily validated by symptom reductions and other clinical indices. Associations between individual learning functions indexing the discrimination of conditioned stimuli (CS) signaling safety (CS-) versus threat (CS+) in differential classical fear conditioning experiments and treatment outcomes may shed light on relevant neurocognitive mechanisms and support the identification of potential predictors for treatment response.
Fear conditioning studies in anxiety patients compared to healthy controls have revealed robustly increased fear responses to CS- (Duits et al., 2015, Lissek et al., 2005). Several processes might be involved in this effect: First, it has been argued that non-reinforcement of specific CS in experimental paradigms conveying threat (i.e. the CS- in the fear acquisition phase), induces an inhibitory component to these CS (e.g. Haaker et al., 2015). In support of this assumption, regulatory (inhibitory?) dorsolateral prefrontal brain-responses have been observed in response to any (non-threat) stimuli presented in threat-associated contexts – particularly if the expected threat was unpredictable (Klinkenberg et al., 2016). Convergently, fear conditioning research linking inhibitory processing of CS- with trait anxiety (e.g. Haaker et al., 2015), has typically employed paradigms with relatively low contingency rates between the CS+ und the UCS, thus conveying rather high levels of uncertainty (for review see Lonsdorf & Merz, 2017). Following this work, and in support of the inhibitory learning framework of ET, increased fear responses to the CS- in anxiety patients may therefore “represent an impaired ability to inhibit fear in the presence of safety cues (CS-)” (Duits et al., 2015). Second, this effect might also be rooted in a generalization of learned fear responses to the CS- (Duits et al. 2015) and/or – third – in dysfunctions in safety processing (Fullana et al., 2016).
In anxiety patients with specific phobias (SP), aberrant fear conditioning indicated by overestimations of CS+/UCS contingencies and altered evaluations of the CS (e.g. fear ratings) seems to be more pronounced when phobia-relevant stimuli are employed as CS (Wiemer et al., 2014) or UCS (Schweckendiek et al., 2011), respectively. Such “domain-specific” biases in SP are underpinned by brain activations in the dorsolateral prefrontal cortex (DLPFC) (Wiemer et al., 2014) and the amygdala (Schweckendiek et al., 2011). Electro- and magnetoencephalography (EEG, MEG) data from healthy participants suggest that sensory (Miskovic and Keil, 2012, Steinberg et al., 2012) as well as ventral (Rehbein et al., 2014) and dorsolateral (Rehbein et al., 2015, Roesmann et al., 2020) prefrontal regions support the discrimination of CS+ and CS- during fear conditioning. Interestingly, frontal effects were not only revealed for late stages of stimulus processing (>300 ms), known to be influenced by strategic emotion-regulation (Hajcak & Nieuwenhuis, 2006), but occurred already at very early (<100 ms), early (100–200 ms) and mid-latency (200–300 ms) processing stages (Rehbein et al., 2015, Roesmann et al., 2020).
In an attempt to link laboratory research on classical fear conditioning with treatment outcomes, studies yielded evidence for associations between behavioral and neural pre-treatment correlates of extinction (Ball et al., 2017, Forcadell et al., 2017, Hahn et al., 2015, Lange et al., 2020, Lueken et al., 2013, Raeder et al., 2020, Waters and Pine, 2016) or fear generalization (Roesmann et al., 2022) and treatment outcomes. Both approaches have linked this relationship with the ventromedial prefrontal cortex (vmPFC) (Lange et al., 2020, Roesmann et al., 2022b), a core hub for fear inhibition (Milad & Quirk, 2012) as well as fear and safety learning (Battaglia et al., 2020, Fullana et al., 2016; for a recent discussion on the contribution of subregions, see Battaglia et al., 2021). Additionally, lateral prefrontal regions were shown to underpin the link between fear generalization and treatment outcome (Roesmann et al., 2022).
Yet, the few studies investigating associations between treatment outcomes and indices of pre-treatment fear conditioning effects, i.e. CS+/CS- differences, have revealed mixed results (Lueken et al., 2013, Waters and Pine, 2016). Data on the spatiotemporal mechanisms involved in these associations are missing.
Tackling the theoretical link between fear conditioning and responses to exposure therapy, the current study investigated associations of behavioral and neural pre-treatment correlates of fear conditioning and treatment success. First, we predicted that higher CS+/CS- differences in fear ratings – particularly due to lower fear ratings of the CS- – would be associated with better treatment outcomes. Second, we predicted that dorsolateral and ventromedial prefrontal structures involved in safety processing and/or the inhibition of fear during fear conditioning (Battaglia et al., 2021, Fullana et al., 2016, Rehbein et al., 2015, Roesmann et al., 2022b) and during exposure therapy (Landowska et al., 2018) would show higher activations to CS- in treatment responders.
2. Methods and materials
2.1. Participants
66 patients with spider phobia (79% female) entered the analyses presented here. This sample had a mean age of 28.15 years (SD = 6.88 years), 14.71 years of education (SD = 2.82 years), high scores in the Spider Phobia Questionnaires (SPQ, Klorman et al., 1974, German version Rinck et al., 2002, M = 22.68, SD = 2.11) and low depression scores on the Beck Depression Inventory II (BDI-2, Hautzinger et al., 2006, M = 3.97, SD = 4.82). Out of these 66 patients, 59 patients additionally completed the 6- months follow-up (retention rate from post to follow-up = 89.39%), in which 26 patients (44.07%) were remitted according to the structured clinical interview for DSM-IV (SCID, Wittchen et al., 1997).
Being part of the Transregional Collaborative Research Center (CRC-TRR58) “Fear, Anxiety, Anxiety Disorders” funded by the German Research Foundation this study was embedded in a prospective longitudinal project. Details on the recruitment pathway (shared by three CRC-TRR58 sub-projects C07, C08, C09) have been published previously (Schwarzmeier et al., 2019).
Inclusion criteria comprised the diagnosis of a specific phobia (animal subtype: spider phobia) as assessed via a structured clinical interview for DSM-IV (SCID, (Wittchen et al., 1997)), SPQ scores above the clinical cut-off score, i.e. >19 (Hamm, 2006), an age between 18 and 65 years, right-handedness, fluent German language and the willingness to participate in one session of VRET. Conversely, patients with current pharmacological or psychotherapeutic treatment, as well as those already previously treated with exposure-based CBT, were excluded. Other exclusion criteria were pregnancy or lactation, MRI- related exclusion criteria and another primary anxiety disorder (panic disorder, agoraphobia, social phobia, generalized anxiety disorder), acute suicidality, psychotic, bipolar I, obsessive–compulsive disorder, posttraumatic stress disorder, severe major depression, borderline personality disorder or substance dependency (except nicotine).
As described in detail elsewhere (Roesmann et al., 2022), 89 out of 100 eligible patients completed all relevant assessments for this study: (1) a clinical pre-treatment assessment, (2) a behavioral and MEG pre-treatment assessment comprising the fear conditioning paradigm, (3) the VRET, and (4) a clinical post-treatment assessment.
Out of these 89 patients, 13 showed pre-processing artefacts and 10 patients met outlier criteria in the MEG fear conditioning data and were thus excluded from the analyses reported here.
The Ethics Committee of the Medical Faculty of the University of Münster approved this study.
2.2. Procedure and experimental design
Relevant assessments for this study took place on four separate days (for details, see Roesmann et al., 2022b). After a (1) clinical pre-treatment assessment, in which primary and secondary outcome measures and clinical and demographic characteristics were obtained, patients completed (2) a behavioral and a MEG pre-treatment assessment including the fear conditioning paradigm. In this paradigm, audiovisual stimuli depicting spiders and fearful faces were used as phobia-relevant and irrelevant UCS, respectively. Tilted Gabor gratings, which were either paired or left unpaired with the UCS, became CS+ or CS- respectively.
Hereafter, a (3) one-session VRET was conducted by board-certified psychotherapists (for details, see also Roesmann et al., 2022b, Schwarzmeier et al., 2019). During VRET, patients were exposed to varying numbers of spiders in up to five virtual reality scenarios. Within each scenario, we defined specific anchor points that should be achieved by each patient. The time to reach the anchor points varied across patients. During approach of and when reaching the anchor points, fear ratings were obtained: Upon prompts by the therapist, patients verbally rated their individual levels of fear on a scale from 0 = “no fear at all” to 100 = “extremely strong fear” before (i.e. anticipatory anxiety) and during each scenario. If fear ratings dropped below 20 or stagnated three times in succession, the responsible therapist proceeded with the next scenario. The preparation of patients and the subsequent exposure lasted for a maximum of 2.5 h (Mean duration of the exposure in the current sample: M = 77.69 min, SD = 22.78, Range: 25 to 135). The VRET environment was delivered by means of the VR-software (VT+ research systems, VTplus GmbH, Würzburg) and was displayed via an Oculus Rift DK2 head-mounted display.
The VRET was followed by a (4) clinical post-treatment assessment, in which outcome criteria were assessed again.
More detailed information on the content, sequence and duration of assessments and the VRET, as well as information on clinical effects of the VRET, can be found in accompanying publications with different research foci (Leehr et al., 2021, Roesmann et al., 2022b, Schwarzmeier et al., 2019).
Here, to investigate the predicted associations between behavioral and magnetoencephalographic pre-treatment CS+/CS- differences and treatment outcomes to VRET, we employed a 2x2 factorial fear conditioning paradigm with the within-subject factors CS-TYPE (CS+, CS-) and UCS-TYPE (phobia-related, phobia-unrelated). We tested UCS-independent and UCS-dependent associations of CS+/CS- differences and the primary and secondary clinical outcome measures.
2.3. Clinical outcome measures
The primary outcome was defined as symptom reduction in the German translation of the Spider Phobia Questionnaire (SPQ) (Klorman et al., 1974, Rinck et al., 2002) from the clinical pre- to post-treatment assessment in percent (see preregistration at ClinicalTrials.gov: NCT03208400).
The secondary outcome was the percentual reduction of behavioral avoidance indexed by the distance (in cm) between a spider (Grammostola rosea) and the patient from pre- to post-treatment assessment, as assessed by an in vivo behavioral avoidance test (BAT, see supplementary materials, SM1.1) (Schwarzmeier et al., 2019, Shiban et al., 2015).
Finally, remission status was assessed at a 6-months follow-up via the structured clinical interview for DSM-IV (Wittchen et al., 1997). Patients, who did no longer fulfil the clinical DSM-IV criteria for spider phobia were classified as remitted.
To facilitate readability and comprehensiveness of figures, patients with a reduction in the SPQ-score by more than 30% or in the BAT distance by more than 50% will be labelled “responders” according to the primary or secondary outcome, respectively. These values were defined as clinically significant in our methods paper (Schwarzmeier et al., 2019).
2.4. Behavioral and MEG pre-treatment assessment
2.4.1. Conditioned stimuli (CS)
We used four pairs of differently tilted sinus-shaped Gabor patches as CS+ and CS- respectively. To prevent pop-out effects, CS+/CS- pairs had no prominent orientations (0°, 45°, 90°, 135°) but orientations of 11°/35°, 101°/125°, 56°/80°, and 146°/170°. The assignment of stimuli as CS+ and CS- was counterbalanced across patients. Different pairs were employed in different blocks.
2.4.2. Unconditioned stimuli (UCS)
The phobia-related audiovisual UCS depicted a spider and the phobia-unrelated audiovisual UCS a fearful female face with a screaming open mouth (Tottenham et al., 2009). To intensify the UCS and to adhere to previous studies employing mainly auditory or audiovisual UCS (e.g. Onat and Büchel, 2015, Schiele et al., 2016, Roesmann et al., 2022a), pictures were presented together with an aversive scream, taken from the ‘IADS‘ (Bradley & Lang, 1999) or a white noise with matched acoustic characteristics and stimulus duration (1200 ms). Both sounds were presented 60 dB above the patients individual hearing threshold (see SM1.2) and were combined with both pictures in a counterbalanced manner.
2.4.3. Fear conditioning paradigm
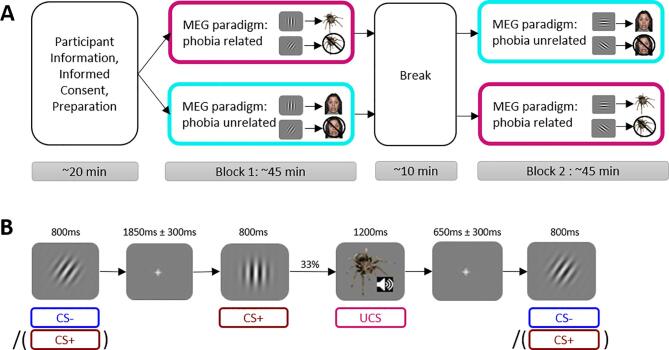
The fear conditioning paradigm consisted of a block with the phobia-related UCS and a block with the phobia-unrelated UCS (Fig. 1A). The order of blocks was counterbalanced across patients. Each block comprised behavioral fear ratings and an MEG-assessment.
Fig. 1.
Experimental procedure and stimulus presentation for the behavioral and MEG assessment on fear conditioning. A) Overview of the procedure, including a pre-experimental preparation block, two experimental blocks and a break in between. The two experimental blocks were similar but used different CS+/CS- pairs of Gabor patches and different UCS- TYPES (phobia-related (magenta) vs. phobia-unrelated (cyan)). The order of blocks was counterbalanced across all patients. B) Stimulus sequence during the fear conditioning phase in a phobia-related block. The stimuli were presented repeatedly. While the CS- (blue) served as a safety- signal and never predicted the UCS, the CS+ (red) predicted the UCS in 33% of the cases. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In each block, patients were instructed that one of two differently tilted grating stimuli would regularly be followed the phobia-related (or phobia-unrelated) UCS (CS+), while the other would remain unpaired (CS-). CS + and CS- stimuli were each presented 60 times in each block in pseudorandom order. Each CS was presented for 800 ms and was followed by a white fixation cross (1850 ± 300 ms) or – in one third of all CS+ presentations (20 times) – by the respective UCS (SOA = 1200 ms) and a fixation cross (SOA = 650 ± 300 ms) (Fig. 1B). The CS- was never followed by the UCS. By employing a rather low contingency rate, we intended to enhance uncertainty, prolong the fear acquisition phase and avoid ceiling effects. We thereby adhere to previous studies focusing on the fear inhibitory component of the CS- during the acquisition phase (for review, see Lonsdorf and Merz, 2017). Phobia related and unrelated blocks were equivalent except for the UCS, the employed CS set, and the exact order of stimulus presentation. Note that the pre-treatment MEG session – in addition to the fear conditioning phase – included a perceptual mid-point task and a generalization phase that have been reported elsewhere (Roesmann et al., 2022).
2.4.3.1. Fear ratings
After the conditioning phase, patients were asked to rate the fear levels elicited by CS+, CS- and the UCS on numeric rating scales ranging from 1=“no fear” to 10=“extreme fear” via a push-button on the right-hand side.
CS fear ratings were analyzed using repeated-measures ANOVAS as implemented in R, Version 3.6.2 (2019) with the factors CS-TYPE (CS+, CS-) and UCS TYPE (phobia-related, phobia-unrelated). To test our prediction that higher CS+/CS- differences in fear ratings would be associated with better treatment outcomes, effects of dimensional treatment outcomes (SPQ-reduction, BAT-reduction) or remission status on main effects of CS-TYPE and CS-TYPE by UCS-TYPE interactions were addressed via separate repeated-measures ANCOVAs and ANOVAs, respectively. Equivalent analyses on UCS fear ratings are presented in SM2. All statistical analyses were based on a significance level of α = 0.05.
2.4.3.2. MEG
During the conditioning phase, continuous MEG signals were recorded by a 275-sensor whole-head MEG sensor system (Omega 275, CTF, VSM MedTech Ltd., Coquitlam, Canada) with first- order axial SQUID gradiometers (frequency range: 0–150 Hz, sampling rate: 600 Hz, for details, see Roesmann et al., 2022). Three landmark coils (two auditory channels and the nasion) were digitized using a 3D tracking device (Polhemus, Colchester, VT, USA), to determine the patients’ head positions in the MEG scanner. MEG data were filtered offline using a 48 Hz low-pass and a 0.1 Hz high-pass filter and sampled down to 300 Hz. Epochs from 200 ms before to 600 ms after CS onset were extracted and baseline-adjusted using the −150 ms to 0 ms baseline interval. Single trials were edited and artefacts were corrected following the method for statistical control of artefacts in high-density EEG/MEG data (Junghöfer et al., 2000). Patients were rejected from further analysis if more than 30% of the trials in any block did not meet an a-priori defined quality criterion (N = 13/89). Data were averaged individually for each participant as a function of CS-TYPE and UCS-TYPE. To avoid statistical artifacts due to outliers in specific experimental conditions, patients were excluded if the mean of the standard deviation across time between conditions or the mean number of residual trials across conditions differed from the sample median by more than four standard deviations (N = 199/89).
The neural generators of the averaged event-related magnetic fields were estimated using the L2-MNE (Hämäläinen & Ilmoniemi, 1994). A spherical shell with 350 evenly distributed dipole pairs (azimuthal and polar direction) with a source shell radius approximately corresponding to the grey matter depth (i.e. 87% of the individually fitted head) served as source model. The Tikhonov regularization parameter Lambda was set to 0.1. Topographies of source-direction-independent neural activities – the vector length of the estimated source activities at each position – were calculated for each individual participant, condition and time point. These data were used to compute CS+/CS- differences of estimated neural source activities.
2.4.3.2.1. Correlation analyses on primary and secondary outcomes
To assess the predicted associations of CS+/CS- differences with treatment outcomes, we computed pointwise correlations between the neural CS+/CS- differences and percentual SPQ- and BAT-reductions for each of the 350 estimated dipoles, i.e., for each estimated source. First, to investigate UCS-TYPE independent associations, CS+/CS- differences (averaged across UCS-TYPES) entered the analysis. Second, to investigate UCS-TYPE dependent associations, i.e. differences in correlations between blocks with phobia-related and phobia-unrelated UCS, we in parallel computed positive/negative point-wise correlations of the neural CS+/CS- difference activities during the phobia-related/ phobia-unrelated blocks with the individual percentage of SPQ- and BAT-reductions.
In all analyses, we controlled for multiple testing via a cluster-permutation analysis (Maris & Oostenveld, 2007) of point-wise Pearson correlation coefficients. In this procedure, the Pearson correlation coefficients per time point and estimated dipole, entered so-called spatio-temporal cluster masses, if they exceeded a critical alpha level of p = .05 (sensor-level criterion) in at least five adjoining sensors and five following time points. Then significant cluster masses were tested against 1,000 random cluster-permutations of the data generated by Monte-Carlo-Simulation. If the calculated cluster mass was greater than 950 of the biggest cluster masses of each of the 1,000 permutations, it surpassed the critical cluster level and was considered significant (cluster level criterion; p-cluster < 0.05). Tests were performed within the predefined time windows of interest, i.e. separately for 0–100 ms (very early effects), 100–200 ms (early effects), 200–300 ms (mid-latency effects) and 300–600 ms (late effects). All analyses concerned the whole brain, no regions of interest were defined. If a resulting cluster reached the border of a pre-defined time interval, the interval was extended by steps of 50 ms and the analysis was repeated, so that the actual start and end points of the resulting cluster could be estimated. If additional clusters emerged in such extended time intervals, they were disregarded. For visualization purposes, the significant clusters were projected on a standard 3D brain model.
The topography of clusters, and the direction of correlations within significant clusters were evaluated with reference to the prediction that that dorsolateral and ventromedial prefrontal structures would show relatively higher activations to CS- in treatment responders, i.e. negative correlations between SPQ/BAT reductions and CS+/CS- differences. In clusters revealing UCS-TYPE dependent differences in correlations, separate correlations were computed for the phobia-related and the phobia-unrelated condition with Bonferroni-corrected significance levels (p = .05/2).
2.4.3.2.2. Follow-up remission analysis
To address the clinical significance of the observed clusters regarding later remission status, CS+/CS- differences within significant clusters were submitted to repeated measures ANOVAs assessing REMISSION-STATUS × UCS-TYPE interaction effects. MEG data were pre-processed and analyzed with the MATLAB-based EMEGS (Version 3.1, Peyk et al., 2011). Continuative analyses were conducted using R.
3. Results
3.1. Clinical measures
Average effect sizes of d = 2.635 (SPQ) and d = 1.220 (BAT) from pre- to post-treatment, and of d = 3.253 (SPQ) and d = 1.496 (BAT) from pre to follow-up in BAT and SPQ rendered the intervention highly effective (for details on the larger sample, see Leehr et al., 2021). While SPQ-REDUCTION and BAT-REDUCTION were significantly correlated (R(64) = 0.32, p = .007), no correlations were observed between REMISSION-STATUS and SPQ-REDUCTION (R(57) = 0.022, p = .871) or BAT-REDUCTION (R(57) = 0.203, p = .123).
3.2. Fear ratings
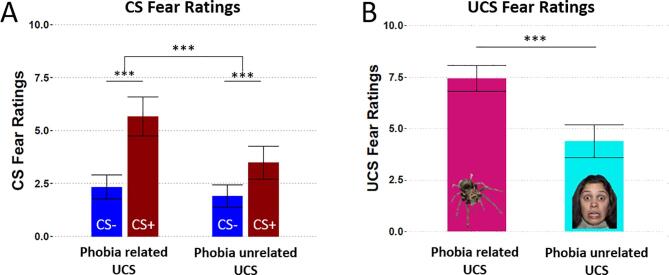
Fear ratings of the CS revealed a main effect of CS-TYPE (F(1,65) = 124.944, p < .001, η2 = 0.658) with stronger fear ratings of CS+ compared to CS- (t(65) = 11.178, p < .001) and a main effect of UCS-TYPE (F(1,65) = 35.114, p < .001, η2 = 0.351) with higher fear ratings in the phobia-related compared to the phobia-unrelated UCS block (t(65) = 5.926, p < .001). Further, an interaction of UCS-TYPE × CS-TYPE (F(1,87) = 28.9, p < .001, η2 = 0.249) indicated a stronger CS+/CS- differentiation in the phobia-related (t(65) = 9.777, p < .001) compared to the phobia-unrelated (t(65) = 5.663, p < .001) block (see Fig. 2A). Fear ratings of the UCS were higher for phobia-related compared to unrelated UCS (see Fig. 2B and SM2).
Fig. 2.
Results of the CS- and UCS- fear ratings. A) CS fear ratings revealed higher ratings for the CS+ (red) than the CS- (blue) in both blocks. The CS+ elicited more fear in the phobia-related block than in the phobia-unrelated block. B) UCS fear ratings confirmed that phobia-related UCS (magenta) elicited more fear than phobia-unrelated UCS (cyan). Error bars denote 95% confidence intervals. *0.05, **0.01, ***0.001 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Percentual reductions in the primary (SPQ) or the secondary outcome (BAT) neither modulated main effects of CS-TYPE (SPQ: F(1,65) = 0.284, p = .596 , η2 = 0.004; BAT: F(1,65) = 0.005, p = .943, η2 < 0.001) or UCS-TYPE (SPQ: F(1,65) = 0.281, p = .598, η2 = 0.004; BAT: F(1,65) = 0.071, p = .791, η2 = 0.001), nor their interaction (SPQ: F(1,64) = 0.006, p = .939, η2 < 0.001; BAT: F(1,64) = 0.440, p = .509, η2 = 0.007). ANOVAs employing the between subject factor REMISSION-STATUS yielded no evidence for associations between remission status and CS-TYPE (F(1,57) = 0.160, p = .691, η2 = 0.003), UCS-TYPE (F(1,57) = 0.131, p = .719, η2 = 0.002), or their interaction (F(1,57) = 1.315, p = .256, η2 = 0.023) in CS fear ratings. Thus, against our hypotheses, we found no support for the assumption of higher CS+/CS- differences in fear ratings in treatment responders.
3.3. MEG results
3.3.1. UCS-TYPE independent effects
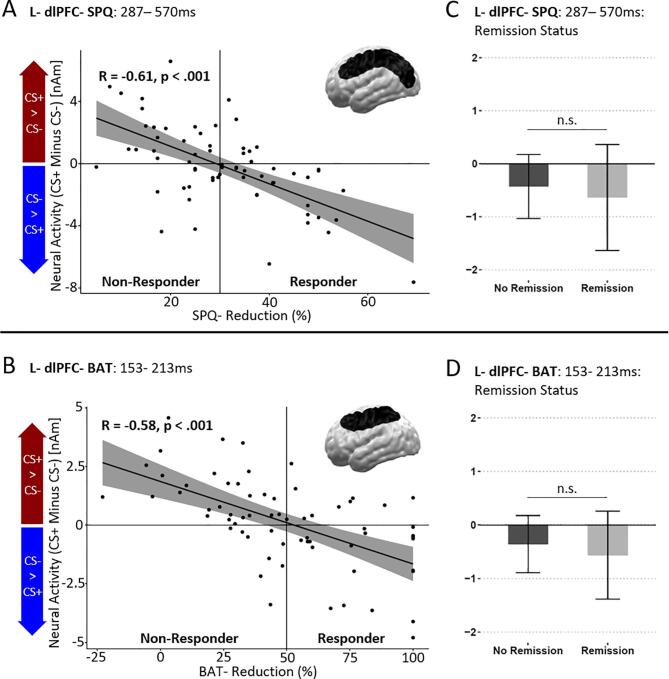
3.3.1.1. SPQ-reduction (left dorsolateral prefrontal cortex)
Correlation analysis including SPQ-reductions revealed a trend-wise significant cluster spanning the left dorsolateral prefrontal and occipito-parietal regions between 287 ms and 570 ms after stimulus onset (L-dlPFC-SPQ, p-cluster = 0.067; see Fig. 3A). In line with the hypothesis that dorsolateral prefrontal structures would show higher activations to CS- in treatment responders., neural CS+/CS- differences within this cluster negatively correlated with the percentual SPQ-reduction (R = -0.61, p < .001). Specifically, responders compared to non-responders revealed a relatively higher activity to the safety-signaling CS- compared to the CS+ .
Fig. 3.
Significant spatiotemporal clusters revealing UCS-independent associations between neural CS+/CS- difference activations and SPQ-reduction (top row) as well as BAT-reduction (bottom row). A and B) Left dorsolateral prefrontal cortex (dlPFC)- SPQ (A) and left dlPFC- BAT (B) clusters revealed significant negative correlations between the neural CS+/CS- difference and the percentual SPQ and BAT reduction of each patient. Both clusters revealed a negative correlation indicating associations of relatively higher ratings for the CS- and better treatment outcomes. The L-dlPFC-SPQ cluster (top row) which extended to occipito-parietal regions appeared at a rather late latency (287–570 ms) while the L-dlPFC- BAT- cluster (bottom row) appeared at early to mid-latencies (153–213 ms). C and D) There were no significant associations of the remission status and neural CS+/CS- differences in the L-dlPFC-SPQ (C) or L- dlPFC-BAT (D) cluster. Error bars and fields around the correlation line display 95% confidence intervals.
3.3.1.2. BAT-reduction (left dorsolateral prefrontal cortex)
Correlation analysis including BAT reductions also revealed a single left dorsolateral prefrontal cluster extending to occipito-parietal regions in a preceding early to mid-latency time interval (L-dlPFC-BAT, p-cluster = 0.048, 153 ms-213 ms; see Fig. 3B). The neural CS+/CS- difference of this cluster also negatively correlated with the percentual BAT reduction (R = −0.58, p < .001). Again, and as predicted, responders compared to non-responders revealed a relatively higher activity to the safety-signaling CS- compared to the CS+.
3.3.1.3. Remission status at follow up assessment
Within these clusters, CS+/CS- differences were not associated with REMISSION-STATUS (L-dlPFC-SPQ: t(57) = -0.601, p = .550; L-dlPFC-BAT: t(57) = 0.810, p = .421; see Fig. 3C + D).
3.3.2. UCS-TYPE dependent effects
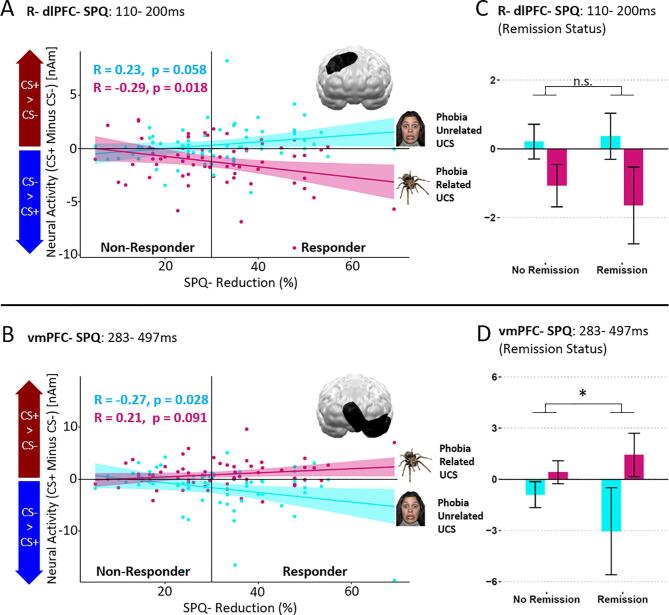
3.3.2.1. SPQ and BAT reductions (right dorsolateral prefrontal cortex)
Correlation analyses testing for UCS-TYPE dependent differences in correlations of CS+/CS- difference activities and treatment outcomes yielded significant clusters at almost symmetric locations in the right dlPFC at early latencies (110 ms–200 ms) for both the SPQ and BAT (R-dlPFC-SPQ; R-dlPFC-BAT). Due to the strong similarities with respect to timing, location and correlations of both clusters, R-dlPFC-SPQ represents both clusters in Fig. 4 (top row; for the highly comparable R-dlPFC-BAT cluster, see SM3).
Fig. 4.
Significant spatiotemporal clusters that depended on the UCS-TYPE. A and B) Right dorsolateral prefrontal cortex (dlPFC)-SPQ (A) and ventromedial prefrontal cortex (vmPFC)-SPQ (B) clusters revealed significant correlations between the neural CS+/CS- difference and the percentual SPQ reduction that differed between UCS-TYPEs. Both scatterplots depict correlations in the phobia-related (magenta) and the phobia-unrelated block (cyan). The right dlPFC-SPQ cluster (top row) appeared at an early to mid-latency (110–200 ms) time interval and was located at the right dorsolateral cortex. While the correlation in the phobia-unrelated block (cyan) was non-significant, the phobia-related block revealed a significant negative correlation (magenta). Higher neural activity evoked by the CS- was associated with better treatment outcomes in the phobia-related block. In contrast, the vmPFC-SPQ cluster (bottom row) occurred at rather late latencies (283–497 ms). Correlations in the phobia-related and phobia-unrelated block were reversed in comparison to the R- dlPFC-SPQ cluster. In the vmPFC-SPQ cluster, correlations were non-significant after Bonferroni correction. C and D) Associations of the remission status and the neural CS+/CS- difference. Remission status was not associated with the neural CS+/CS- difference in the R-dlPFC-SPQ cluster (C). However, the vmPFC- SPQ cluster (D) revealed a significant association between neural CS+/CS- differences and the remission status. Remitted patients showed an increased neural CS+/CS- difference in the phobia-related block (magenta) and reduced CS+/CS- difference in the phobia-related block (cyan). Yet, post-hoc tests were non-significant following Bonferroni correction. The y- axis is the same as used in A and B. Error bars and fields around the correlation line display 95% confidence intervals. *p < .05, ** p < .01, *** p < .001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
R-dlPFC-SPQ (p-cluster = 0.034) showed a significant negative correlation during the phobia-related block (R = -0.29, p = .018), but no or even a trend-wise positive correlation in the phobia-unrelated block (R = 0.23, p = .058). Thus, responders compared to non-responders revealed the predicted higher neural activity to the safety-signalling CS- compared to the CS+ in the phobia-related block only.
Within the cluster R-dlPFC-SPQ, effects of UCS-TYPE on CS+/CS- differences were not modulated by REMISSION-STATUS (F(1,57) = 0.997, p = .332, η2 = 0.017 (see Fig. 4C).
3.3.2.2. SPQ and BAT reductions (ventromedial prefrontal cortex)
Correlation analyses testing UCS-TYPE dependent differences in correlations of CS+/CS- difference activities and treatment outcome additionally yielded significant clusters spanning the vmPFC and anterior temporal regions for both SPQ (vmPFC-SPQ, see Fig. 4 bottom row) and BAT (vmPFC-BAT, see SM3) reductions. While vmPFC-SPQ was located at the left vmPFC and left anterior temporal pole and occurred between 283 ms and 497 ms (p-cluster = 0.02), vmPFC-BAT spanned over the vmPFC of both hemispheres and the left anterior temporal pole (p-cluster = 0.018) but occurred in an almost identical time interval (273 ms-497 ms). As above, due to the strong similarities with respect to timing, location and correlations of both clusters, vmPFC-SPQ is presented in the bottom row of Fig. 4 on behalf of both clusters (for vmPFC-BAT, see SM3).
In contrast to the R-dlPFC cluster, in the vmPFC-SPQ cluster, correlations between the CS+/CS- difference and the percentual reductions in SPQ in the phobia-unrelated block (R = -0.27, p = .028) and the phobia-related block (R = 0.21, p = .091) did not reach significance following Bonferroni-correction (p = .05/2).
However, within this cluster, effects of UCS-TYPE on CS+/CS- differences were modulated by REMISSION-STATUS (F(1,57) = 4.944, p = .030, η2 = 0.046; see Fig. 4D). Yet, Bonferroni-corrected post-hoc tests revealed no CS+/CS- differences between patients who later on remitted compared to those who did not in the phobia-related block (t(57) = 0.128, p = .128) and only marginal effects in the phobia-unrelated block (t(57) = 1.831, p = .072) that were also non-significant following Bonferroni correction.
Thus, while findings support that the vmPFC shows UCS-dependent associations between CS+/CS- differences and treatment outcomes, the predicted higher activity to the safety-signaling CS- compared to the CS+ in responders could not be confirmed in this cluster.
Supplementary table S1 (see SM4) summarizes the observed associations between experimental measures of fear conditioning and clinical outcome measures in reference to the hypotheses.
4. Discussion
The rationale of exposure therapy is mainly grounded in classical fear conditioning. Therefore, behavioral and neural indices of differential fear conditioning, i.e. CS+/CS- differences, were investigated as potential predictors for later treatment outcomes. Dovetailing previous behavioral fear conditioning findings (Lissek et al., 2014, Schweckendiek et al., 2011), higher fear ratings for the CS+ than CS- confirmed the effectiveness of our learning paradigm. Yet, contrary to our hypothesis, CS+/CS- differences in fear ratings were not associated with treatment outcomes at post-assessment (percentual SPQ-/BAT-reductions) or remission states at a 6-month follow-up assessment (see also Waters and Pine, 2016). However, as predicted, UCS-independent CS+/CS- differences in the left dlPFC showed a negative correlation with treatment outcome: Relatively stronger activity to the CS- (vs. the CS+) was associated with stronger percentual SPQ and BAT reductions, i.e. with better treatment outcomes. Additionally, UCS-dependent right dlPFC and left vmPFC neural clusters showed significant correlations between the neural CS+/CS- difference and the subsequent short-term treatment outcomes (percentual SPQ-/BAT-reductions). The vmPFC cluster further showed significant associations of UCS-dependent neural CS+/CS- differences and six-month follow-up remission status. In a nutshell, results suggest that individual neural differences supporting safety processing and/or fear inhibition during classical fear conditioning might be a promising, potentially even individual predictor of exposure success, which might help to identify patients with a high risk for non-response prior to treatment.
Patients who benefited more from subsequent VRET displayed higher pre-treatment neural activity to CS- compared to CS+ in brain-regions comprising the dlPFC. The dlPFC plays a cardinal role in the inhibition of fear responses and emotion regulation (Eden et al., 2015, Hermann et al., 2009, Phillips et al., 2008, Zald, 2007), in the top-down modulation of emotional attention and perceptual processes (Keuper et al., 2018, Pourtois et al., 2013, Roesmann et al., 2019) and pattern separation under safe and threat conditions (Balderston et al., 2017) as well as fear conditioning and generalization (Rehbein et al., 2015, Roesmann et al., 2020). Further, the dlPFC has been implicated in monitoring of behavior (Ray & Zald, 2012) and showed activity changes as a function of behavioral exposure (Landowska et al., 2018).
In the left hemispheric dlPFC, these associations were independent of the UCS-type and could be revealed for both outcome measures – SPQ and BAT. Regarding region, timing (i.e., covering early and late processing stages) and patterns of this activation (i.e., UCS-unrelated; CS->CS+ processing for responders), these results converge with evidence for treatment-outcome dependent fear generalization gradients in this region (Roesmann et al., 2022). Following the argument that non-reinforcement of specific CS in contexts conveying threat – i.e. the CS- in the fear acquisition phase (Haaker et al. 2015) or generalization stimuli in the generalization phase (Roesmann et al., 2022) – induces an inhibitory component to these CS, we suggest that stronger dlPFC activity to CS- versus CS+ during fear conditioning as well as fear generalization might reflect fear inhibition. Given the key role of the dlPFC in involuntary (Keuper et al., 2018, Notzon et al., 2017, Roesmann et al., 2019) and voluntary top-down control of fear responses (Hermann et al., 2009, Phillips et al., 2008), and given its cardinal function of inhibitory control in exposure therapy (Craske et al., 2008, Craske et al., 2012), we suggest that associations between the strength of fear conditioning effects and treatment-outcomes are underpinned by individual differences in neural processes of fear inhibition. Patients who exhibit stronger fear-inhibitory network activity in response to CS- already prior to treatment appear better suited to benefit from later exposure treatment. This in turn suggests that ET success involves basic associative learning processes, which rely on the recruitment of these networks.
The consistency of left dlPFC effects with two different outcome measures supports the validity of these findings. Interestingly, however, associations with SPQ and BAT reductions emerged with different temporal characteristics: While associations with the SPQ yielded significance at rather late processing stages, that have previously been shown to also be modulated by strategic emotion regulation (Hajcak & Nieuwenhuis, 2006), associations with the BAT occurred already at early to mid-latency time intervals, i.e. within the first 300ms of stimulus processing. The rather moderate associations between the SPQ and the BAT suggest that these outcome measures may capture partially independent aspects of treatment success. Speculatively, more cognitive aspects of treatment outcomes, like self-reported symptom severity assessed via the SPQ, could be reflected in relatively late conditioning effects in the dlPFC. By contrast, earlier (inhibitory?) responses to CS- might moderate more behavioral aspects of treatment outcomes, like reductions of avoidance behaviors assessed via the BAT. However, the UCS-dependent right-hemispheric dlPFC effects challenge this interpretation, as spatiotemporal characteristics of these effects were almost identical for both outcome measures and started within the first 150 ms of stimulus processing.
Right dlPFC correlations with treatment outcome were more pronounced for phobia-relevant UCS. More specifically, only if phobia-related UCS were employed, we observed stronger activation to the CS- as a function of improving treatment outcome. The direction and latency of effects are in line with findings by Rehbein and colleagues (Rehbein et al., 2015), who showed that low-anxious (versus high-anxious) healthy individuals exhibit relatively higher CS- activations in this region, resembling patients who profited from treatment. One possible explanation for the UCS-dependency of effects might be that additional top-down resources are required to inhibit fear responses to CS- if they are presented in a phobia-related context. In line with these speculations, previous research has revealed that the right dlPFC is differentially activated by (non-threat) visual stimuli that are presented in contexts conveying threat compared to safe contexts (Balderston et al., 2017, Klahn et al., 2016, Klahn et al., 2017). Additionally, these effects could be linked with adaptive and aberrant emotion regulation (Klahn et al., 2016, Klahn et al., 2017, Klinkenberg et al., 2016), interestingly already within the first 150ms after stimulus onset. Taken together, it seems plausible that the perceived threat level in the phobia-related block required resources of the right dlPFC to (1) differentiate CS+ and CS- and to (2) inhibit fear responses to the safety-signaling CS-. Notably, this dynamic interplay was associated with treatment outcomes at post-assessment.
While directions of dlPFC effects supported our hypotheses that inhibitory responses to the CS- relate to treatment outcomes, the UCS-dependency and direction of the associations of vmPFC fear conditioning effects with both outcome measures and – notably – also the remission status at FU in the vmPFC remains puzzling: On a macroscopic level, an involvement of the vmPFC in fear conditioning and treatment effects does not come as a surprise, because of its core role in fear inhibition (Milad & Quirk, 2012), as well as threat and safety learning (Battaglia et al., 2020, Fullana et al., 2016). Both, dlPFC and vmPFC activations have previously been associated with treatment outcomes to behavioral exposure (Lange et al., 2020, Lueken et al., 2013, Roesmann et al., 2022b). In contrast to the dlPFC, which plays a key role in voluntary top-down control (e.g. reappraisal), the vmPFC has especially been linked with rather automatic emotion regulatory processes (Phillips et al., 2008) including fear inhibition in response to conditioned stimuli (Myers & Davis, 2007) and safety processing (Fullana et al., 2016, Battaglia et al., 2021). In line with our findings, both structures have also previously been associated with dysfunctions during the regulation of phobia-related compared to unrelated materials in spider phobia (Hermann et al., 2009). Yet, on a microscopic level and under the assumption that vmPFC effects represent inhibitory responses to the CS-, we would have expected more negative correlations in the phobia-related rather than in the phobia-unrelated condition. More precisely, higher fear ratings of phobia-related versus unrelated UCS (see also Goossens et al., 2007) would have implied a stronger – not as observed, a weaker – need to inhibit fear responses to the CS- in the phobia-related compared to the phobia-unrelated block. Further, UCS-dependent effects stand in contrast with our previous findings of UCS-independent associations with treatment outcome during generalization (Roesmann et al., 2022). One possible explanation for these inconsistencies might lie in different functions of different subregions of the vmPFC: Only recently, Battaglia et al. (2021) outlined that posterior versus anterior vmPFC subregions might support a preparation of organisms for anticipated threat and safety-seeking during fear conditioning, respectively. To further characterize and identify the contribution of vmPFC subregions and their interplay with affect-generating subcortical brain networks in linking fear conditioning with treatment outcomes (Lueken et al., 2013), complementary evidence from fMRI with a higher spatial, though lower temporal resolution than MEG appears valuable.
Taken together, our study clearly links fear conditioning/treatment-outcome associations with frontal cortical brain structures and supports the view that exposure success relies on associative learning processes. It provides initial group-level evidence that neural CS+/CS- differences might qualify as a potential predictor for treatment success. Yet, future studies are warranted to replicate findings and to investigate their validity with different experimental design parameters and different exposure protocols: First, effects of different types of UCS should be considered. The counterbalanced assignment of phobia-related and phobia-unrelated pictures with two sounds (scream, noise) controlled for potential confounding effects of the sound. However, in our study, it cannot be ruled out that UCS were perceived as an entity in the case of phobia-unrelated (‘screaming face’) but not in the case of phobia-related UCS, where a scream might have induced an association with one's own reaction (‘me screaming, because of the spider’). Although fear ratings of the UCS and of the CS were not affected by the sound-assignment in our sample, the influence of the UCS should be disentangled in future work. Second, to induce uncertainty and thereby prolong the learning phase, we here employed a contingency rate of 33% between CS+ and UCS. It is possible, that conditioning effects in less ambiguous experimental situations (e.g., 100% contingency rate) might differ regarding their associations with treatment outcomes. Future studies on inter-individual differences in general, and on treatment response, are warranted to investigate potential experimental boundary conditions related to contingency rates (for discussion, see also Lonsdorf et al. 2017). Third, while clinical short- and long-term pre-post effects of our one-session VRET were comparable to other therapy-studies involving more than one session of in vivo or in virtuo exposure therapy, our study was not designed to investigate the effectiveness of this intervention. Due to the lack of a control group, specific contributions of the VRET cannot be disentangled from potential effects of the repetitive exposure of phobic stimuli during the assessments, e.g. during the BAT (Richter et al., 2021) and experimental tasks. Fourth, caution is also warranted when generalizing findings to exposure-based treatments in standard routine care settings, which typically are less standardized, include more sessions of exposure and include individual adjustments of exposure settings (e.g., to violate idiosyncratic expectancies). The possibility that associations between conditioning effects in the laboratory and treatment outcomes are restricted to standardized settings like ours, should be addressed by future work. As a further limitation, the variance-analytic approach of our study, testing for associations on a group level, is not suited to determine the predictive value of pre-treatment effects of fear conditioning for treatment outcomes of individual patients. In order to investigate the utility of neural fear conditioning effects as a predictive marker, future studies employing genuine predictive machine learning approaches (Leehr et al., 2021, Scheinost et al., 2019) or neuroscience-informed augmentation strategies like rTMS (Herrmann et al., 2017) would be valuable.
5. Conclusion
In conclusion, these results provide initial evidence that neural, but not behavioral CS+/CS- differences during fear conditioning are associated with later treatment response. UCS-dependent and UCS-independent neural activations in vmPFC and dlPFC regions with potentially inhibitory effects on affect generating structures link stronger activation to safety signalling CS- to better therapy response. This suggests that patients with deficient inhibitory and/or safety-associated frontal responding during affective learning, benefit to a lesser degree from subsequent therapy. Ultimately, the results of this work and follow-up studies should assist researchers and clinicians to identify patients who will benefit from exposure therapy as first-line treatment and patients needing further individualized therapy.
CRediT authorship contribution statement
Kati Roesmann: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration. Julius Toelle: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Elisabeth Johanna Leehr: Methodology, Investigation, Data curation, Writing – review & editing, Supervision, Project administration. Ida Wessing: Writing – review & editing. Joscha Böhnlein: Methodology, Investigation, Writing – review & editing, Project administration. Fabian Seeger: Methodology, Writing – review & editing. Hanna Schwarzmeier: Methodology, Writing – review & editing. Niklas Siminski: Writing – review & editing. Martin J. Herrmann: Writing – review & editing. Udo Dannlowski: Funding acquisition, Writing – review & editing. Ulrike Lueken: Funding acquisition, Writing – review & editing. Tim Klucken: Writing – review & editing. Thomas Straube: Conceptualization, Methodology, Writing – review & editing, Funding acquisition. Markus Junghöfer: Conceptualization, Methodology, Formal analysis, Data curation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was funded by the German Research Foundation (DFG) – project number 44541416 (CRC-TRR 58: Project C08 to MJ and TS, Project C09 to UD and UL, Project C07 to TS and MH) and the “Innovative Medizinische Forschung” (IMF) of the medical faculty of Münster (grant number RO211907 to KR). JT received financial support from the “Medizinerkolleg Münster” (MedK) within this research.
We would like to thank Tilman Coers, Julia Wandschura, Marielle Clerc, Hannah Casper, Sarah Hein, Karin Wilken, Andreas Wollbrink, Ute Trompeter, and Hildegard Deitermann (Institute for Biomagnetism and Biosignalanalysis), Tina Jocham, Jana Scharnagl, and Inge Gröbner (Dept. of Psychiatry, University Hospital of Würzburg); Dominik Grotegerd, Ramona Lennings, Manuel Kraft, Merle Gebauer, Sarah Thissen, Elena Wilkens, Jonathan Repple, Nina Muck, Stella Fingas, Janina Werner, Anna Kraus, and Kordula Vorspohl (Dept. of Psychiatry, University of Münster); Harald Kugel, Jochen Bauer, and Birgit Vahrenkamp (Dept. of Clinical Radiology, University of Münster), Lea Borgmann, Jaqueline Brieke, Aylin Fuchs, Carolin Heinemann, Annika Hense, Valeria Kleinitz, Kaja Loock, Johannes Lücke, and Kathrin Rüb (Institute of Medical Psychology and Systems Neuroscience), for their help and support.
The results were partially presented at the congress “EMHFC2021”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103046.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Balderston N.L., Hsiung A., Ernst M., Grillon C. Effect of threat on right dlPFC activity during behavioral pattern separation. J. Neurosci. 2017;37(38):9160–9171. doi: 10.1523/JNEUROSCI.0717-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T.M., Knapp S.E., Paulus M.P., Stein M.B. Brain activation during fear extinction predicts exposure success. Depression Anxiety. 2017;34(3):257–266. doi: 10.1002/da.22583. [DOI] [PubMed] [Google Scholar]
- Bandelow, B., Aden, I., Alpers, G., W., Benecke, A., Benecke, C., et al., 2021. Deutsche S3-Leitlinie Behandlung von Angststörungen, Version 2. AWMF online. 2021. https://www.awmf.org/leitlinien/detail/ll/051-028.html. (Accessed 5 April 2022).
- Bandelow B., Reitt M., Röver C., Michaelis S., Görlich Y., Wedekind D. Efficacy of treatments for anxiety disorders: A meta-analysis. Int. Clin. Psychopharmacol. 2015;30(4):183–192. doi: 10.1097/YIC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Battaglia S., Garofalo S., di Pellegrino G., Starita F. Revaluing the Role of vmPFC in the acquisition of Pavlovian threat conditioning in humans. J. Neurosci. 2020;40(44):8491–8500. doi: 10.1523/JNEUROSCI.0304-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia S., Harrison B.J., Fullana M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both ? A commentary on subregional contributions. Mol. Psychiatry. 2021;September:1–3. doi: 10.1038/s41380-021-01326-4. [DOI] [PubMed] [Google Scholar]
- Böhnlein J., Altegoer L., Muck N.K., Roesmann K., Redlich R., Dannlowski U., Leehr E.J. Factors influencing the success of exposure therapy for specific phobia: A systematic review. Neurosci. Biobehav. Rev. 2020;108:796–820. doi: 10.1016/j.neubiorev.2019.12.009. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., Moody E.W. Memory processes in classical conditioning. Neurosci. Biobehav. Rev. 2004;28(7):663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bradley, M. M., & Lang, P. J., 1999. International affective digitized sounds (IADS): Stimuli, instruction manual and affective ratings. Technical Report B-2. Gainesville, FL: The Center for Research in Psy- Chophysiology, University of Florida.
- Carpenter J.K., Andrews L.A., Witcraft S.M., Powers M.B., Smits J.A.J., Hofmann S.G. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depression Anxiety. 2018;35(6):502–514. doi: 10.1002/da.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M.G., Kircanski K., Zelikowsky M., Mystkowski J., Chowdhury N., Baker A. Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Liao B., Brown L., Vervliet B. Role of inhibition in exposure therapy. J. Exp. Psychopathol. 2012;3(3):322–345. doi: 10.5127/jep.026511. [DOI] [Google Scholar]
- Craske M.G., Hermans D., Vervliet B. State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philos. Trans. R. Society B: Biol. Sci. 2018;373(1742):20170025. doi: 10.1098/rstb.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P., Cath D.C., Lissek S., Hox J.J., Hamm A.O., Engelhard I.M., van den Hout M.A., Baas J.M.P. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression Anxiety. 2015;32(4):239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- Eden A.S., Schreiber J., Anwander A., Keuper K., Laeger I., Zwanzger P., Zwitserlood P., Kugel H., Dobel C. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci. 2015;35(15):6020–6027. doi: 10.1523/JNEUROSCI.3659-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa, E. B., & Kozak, M. J., 1986. Emotional Processing of Fear. Exposure to Corrective Information. Psychological Bulletin. American Psychological Association. doi:10.1037/0033-2909.99.1.20. [PubMed]
- Forcadell E., Torrents-Rodas D., Vervliet B., Leiva D., Tortella-Feliu M., Fullana M.A. Does fear extinction in the laboratory predict outcomes of exposure therapy? A treatment analog study. Int. J. Psychophysiol. 2017;121:63–71. doi: 10.1016/j.ijpsycho.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Fullana M.A., Harrison B.J., Soriano-Mas C., Vervliet B., Cardoner N., Àvila-Parcet A., Radua J. Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mol. Psychiatry. 2016;21(4):500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- Goossens L., Sunaert S., Peeters R., Griez E.J.L., Schruers K.R.J. Amygdala hyperfunction in phobic fear normalizes after exposure. Biol. Psychiatry. 2007;62(10):1119–1125. doi: 10.1016/j.biopsych.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Haaker J., Lonsdorf T.B., Schümann D., Menz M., Brassen S., Bunzeck N., Gamer M., Kalisch R. Deficient inhibitory processing in trait anxiety: evidence from context-dependent fear learning, extinction recall and renewal. Biol. Psychol. 2015;111:65–72. doi: 10.1016/j.biopsycho.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Hahn T., Kircher T., Straube B., Wittchen H.-U., Konrad C., Ströhle A., Wittmann A., Pfleiderer B., Reif A., Arolt V., Lueken U. Predicting treatment response to cognitive behavioral therapy in panic disorder with agoraphobia by integrating local neural information. JAMA Psychiatry. 2015;72(1):68. doi: 10.1001/jamapsychiatry.2014.1741. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affect. Behav. Neurosci. 2006;6(4):291–297. doi: 10.3758/CABN.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hamm A. Hogrefe; 2006. Spezifische Phobien. [Google Scholar]
- Hautzinger, M., Keller, F., & Kühner, C., 2006. Das Beck Depressionsinventar II. Deutsche Bearbeitung und Handbuch zum BDI II. Frankfurt a. M.: Harcourt Test Services.
- Hermann A., Schäfer A., Walter B., Stark R., Vaitl D., Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Social Cognit. Affective Neurosci. 2009;4(3):257–267. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M.J., Katzorke A., Busch Y., Gromer D., Polak T., Pauli P., Deckert J. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimulation. 2017;10(2):291–297. doi: 10.1016/j.brs.2016.11.007. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Kazama A., Bachevalier J., Davis M. Neuropharmacology. Pergamon; 2012. Impaired safety signal learning may be a biomarker of PTSD; pp. 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T., Tucker D.M., Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37(4):523–532. [PubMed] [Google Scholar]
- Keuper K., Terrighena E., Chan C.C.H., Junghöfer M., Lee T.M.C. How the Dorsolateral Prefrontal Cortex Controls Affective Processing in Absence of Visual Awareness – Insights from a Combined EEG-rTMS Study. Front. Hum. Neurosci. 2018;12:412. doi: 10.3389/fpls.2018.01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahn A.L., Klinkenberg I.A.G., Notzon S., Arolt V., Pantev C., Zwanzger P., Junghoefer M. Prepare for scare-Impact of threat predictability on affective visual processing in spider phobia. Behav. Brain Res. 2016;307(October):84–91. doi: 10.1016/j.bbr.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Klahn A.L., Klinkenberg I.A., Lueken U., Notzon S., Arolt V., Pantev C., Zwanzger P., Junghoefer M. Commonalities and differences in the neural substrates of threat predictability in panic disorder and specific phobia. NeuroImage: Clin. 2017;14:530–537. doi: 10.1016/j.nicl.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I.A.G., Rehbein M.A., Steinberg C., Klahn A.L., Zwanzger P., Zwitserlood P., Junghöfer M. Healthy individuals maintain adaptive stimulus evaluation under predictable and unpredictable threat. NeuroImage. 2016;136:174–185. doi: 10.1016/j.neuroimage.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Klorman R., Weerts T.C., Hastings J.E., Melamed B.G., Lang P.J. Psychometric description of some specific-fear questionnaires. Behav. Ther. 1974;5(3):401–409. http://www.sciencedirect.com/science/article/pii/S0005789474800080 Retrieved from. [Google Scholar]
- Landowska A., Roberts D., Eachus P., Barrett A. Within- and between-session prefrontal cortex response to virtual reality exposure therapy for acrophobia. Front. Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I., Goossens L., Michielse S., Bakker J., Vervliet B., Marcelis M., Wichers M., van Os J., van Amelsvoort T., Schruers K. Neural responses during extinction learning predict exposure therapy outcome in phobia: results from a randomized-controlled trial. Neuropsychopharmacology. 2020;45(3):534–541. doi: 10.1038/s41386-019-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr E.J., Roesmann K., Böhnlein J., Dannlowski U., Gathmann B., Herrmann M.J., Junghöfer M., Schwarzmeier H., Seeger F.R., Siminski N., Straube T., Lueken U., Hilbert K. Clinical predictors of treatment response towards exposure therapy in virtuo in spider phobia: a machine learning and external cross-validation approach. J. Anxiety Disord. 2021;83:102448. doi: 10.1016/j.janxdis.2021.102448. [DOI] [PubMed] [Google Scholar]
- Lissek S., Powers A.S., McClure E.B., Phelps E.A., Woldehawariat G., Grillon C., Pine D.S. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav. Res. Ther. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S., Bradford D.E., Alvarez R.P., Burton P., Espensen-Sturges T., Reynolds R.C., Grillon C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Social Cognit. Affective Neurosci. 2014;9(8):1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerinc A.G., Meuret A.E., Twohig M.P., Rosenfield D., Bluett E.J., Craske M.G. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clin. Psychol. Rev. 2015;42:72–82. doi: 10.1016/j.cpr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Lonsdorf T.B., Merz C.J. More than just noise: Inter-individual differences in fear acquisition, extinction and return of fear in humans - Biological, experiential, temperamental factors, and methodological pitfalls. Neurosci. Biobehav. Rev. 2017;80:703–728. doi: 10.1016/j.neubiorev.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Lueken U., Straube B., Konrad C., Wittchen H.-U., Ströhle A., Wittmann A., Pfleiderer B., Uhlmann C., Arolt V., Jansen A., Kircher T. Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am. J. Psychiatry. 2013;170(11):1345–1355. doi: 10.1176/appi.ajp.2013.12111484. [DOI] [PubMed] [Google Scholar]
- Lueken U., Zierhut K.C., Hahn T., Straube B., Kircher T., Reif A., Richter J., Hamm A., Wittchen H.-U., Domschke K. Neurobiological markers predicting treatment response in anxiety disorders: A systematic review and implications for clinical application. Neurosci. Biobehav. Rev. 2016;66:143–162. doi: 10.1016/j.neubiorev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63(1):129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S., Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 2008;127(3):567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Keil A. Acquired fears reflected in cortical sensory processing: A review of electrophysiological studies of human classical conditioning. Psychophysiology. 2012;49(9):1230–1241. doi: 10.1111/j.1469-8986.2012.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer O.H. On the dual nature of learning—a re-interpretation of “conditioning” and “problem-solving”. Harvard Educ. Rev. 1947;17:102–148. [Google Scholar]
- Myers K.M., Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Notzon S., Steinberg C., Zwanzger P., Junghöfer M. Modulating emotion perception – opposing effects of inhibitory and excitatory prefrontal cortex stimulation. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging. 2017;3(4):329–336. doi: 10.1016/j.bpsc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Onat S., Büchel C. The neuronal basis of fear generalization in humans. Nat. Neurosci. 2015;18(12):1811–1818. doi: 10.1038/nn.4166. [DOI] [PubMed] [Google Scholar]
- Peyk P., De Cesarei A., Junghöfer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Comput. Intelligence Neurosci. 2011;2011:861705. doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C., Drevets W.C. Automatic and volontary regulation of emotion. Mol. Psychiatry. 2008;13(9):829–857. doi: 10.1038/mp.2008.65.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G., Schettino A., Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol. Psychol. 2013;92(3):492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Raeder F., Merz C.J., Margraf J., Zlomuzica A. The association between fear extinction, the ability to accomplish exposure and exposure therapy outcome in specific phobia. Sci. Rep. 2020;10(1):4288. doi: 10.1038/s41598-020-61004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.D., Zald D.H. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehbein M.A., Steinberg C., Wessing I., Pastor M.C., Zwitserlood P., Keuper K., Junghöfer M., Antal A. Rapid plasticity in the prefrontal cortex during affective associative learning. PLoS ONE. 2014;9(10):e110720. doi: 10.1371/journal.pone.0110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehbein M.A., Wessing I., Zwitserlood P., Steinberg C., Eden A.S., Dobel C., Junghöfer M. Rapid prefrontal cortex activation towards aversively paired faces and enhanced contingency detection are observed in highly trait-anxious women under challenging conditions. Front. Behav. Neurosci. 2015;9(June):1–19. doi: 10.3389/fnbeh.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J., Pané-Farré C.A., Gerlach A.L., Gloster A.T., Wittchen H.-U., Lang T., Alpers G.W., Helbig-Lang S., Deckert J., Fydrich T., Fehm L., Ströhle A., Kircher T., Arolt V., Hamm A.O. Transfer of exposure therapy effects to a threat context not considered during treatment in patients with panic disorder and agoraphobia: Implications for potential mechanisms of change. Behav. Res. Ther. 2021;142:103886. doi: 10.1016/j.brat.2021.103886. [DOI] [PubMed] [Google Scholar]
- Rinck M., Bundschuh S., Engler S., Müller A., Wissmann J., Ellwart T., Becker E.S. Reliabilität und validität dreier instrumente zur messung von angst vor Spinnen. Diagnostica. 2002;48(3):141–149. doi: 10.1026//0012-1924.48.3.141. [DOI] [Google Scholar]
- Roesmann K., Dellert T., Junghoefer M., Kissler J., Zwitserlood P., Zwanzger P., Dobel C. The causal role of prefrontal hemispheric asymmetry in valence processing of words – Insights from a combined cTBS-MEG study. NeuroImage. 2019;191:367–379. doi: 10.1016/j.neuroimage.2019.01.057. [DOI] [PubMed] [Google Scholar]
- Roesmann K., Kroker T., Hein S., Rehbein M., Winker C., Leehr L., Klucken T., Junghoefer M. Transcranial direct current stimulation of the ventromedial prefrontal cortex modulates perceptual and neural patterns of fear generalization. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging. 2022;7(2):210–220. doi: 10.1016/j.bpsc.2021.08.001. [DOI] [PubMed] [Google Scholar]
- Roesmann K., Leehr E.J., Böhnlein J., Steinberg C., Seeger F., Schwarzmeier H., Gathmann B., Siminski N., Herrmann M.J., Dannlowski U., Lueken U., Klucken T., Hilbert T., Straube T., Junghöfer M. Behavioral and Magnetoencephalographic Correlates of Fear Generalization are Associated with Responses to Later Virtual Reality Exposure Therapy in Spider Phobia. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging. 2022;7(2):221–230. doi: 10.1016/j.bpsc.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Roesmann K., Wiens N., Winker C., Rehbein M.A., Wessing I., Junghoefer M. Fear generalization of implicit conditioned facial features – Behavioral and magnetoencephalographic correlates. NeuroImage. 2020;205 doi: 10.1016/j.neuroimage.2019.116302. [DOI] [PubMed] [Google Scholar]
- Scheinost D., Noble S., Horien C., Greene A.S., Lake E.MR., Salehi M., Gao S., Shen X., O'Connor D., Barron D.S., Yip S.W., Rosenberg M.D., Constable R.T. NeuroImage Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage. 2019;193(February):35–45. doi: 10.1016/j.neuroimage.2019.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele M.A., Reinhard J., Reif A., Domschke K., Romanos M., Deckert J., Pauli P. Developmental aspects of fear: Comparing the acquisition and generalization of conditioned fear in children and adults. Dev. Psychobiol. 2016;58(4):471–481. doi: 10.1002/dev.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmeier H., Leehr E.J., Böhnlein J., Seeger F.R., Roesmann K., Gathmann B., Herrmann M.J., Siminski N., Junghöfer M., Straube T., Grotegerd D., Dannlowski U. Theranostic markers for personalized therapy of spider phobia: Methods of a bicentric external cross-validation machine learning approach. Int. J. Methods Psychiatric Res. 2019;29(2) doi: 10.1002/mpr.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweckendiek J., Klucken T., Merz C.J., Tabbert K., Walter B., Ambach W., Vaitl D., Stark R. Weaving the (neuronal) web: Fear learning in spider phobia. NeuroImage. 2011;54(1):681–688. doi: 10.1016/j.neuroimage.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Shiban Y., Schelhorn I., Pauli P., Mühlberger A. Effect of combined multiple contexts and multiple stimuli exposure in spider phobia: A randomized clinical trial in virtual reality. Behav. Res. Ther. 2015;71:45–53. doi: 10.1016/j.brat.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Steinberg C., Bröckelmann A.-K., Rehbein M.A., Dobel C., Junghöfer M. Rapid and highly resolving associative affective learning: Convergent electro- and magnetoencephalographic evidence from vision and audition. Biol. Psychol. 2012;92(3):526–540. doi: 10.1016/j.biopsycho.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Taylor S., Abramowitz J.S., McKay D. Journal of Anxiety Disorders. Elsevier Ltd; 2012. Non-adherence and non-response in the treatment of anxiety disorders; pp. 583–589. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Pine D.S. Evaluating differences in Pavlovian fear acquisition and extinction as predictors of outcome from cognitive behavioural therapy for anxious children. J. Child Psychol. Psychiatry. 2016;57(7):869–876. doi: 10.1111/jcpp.12522. [DOI] [PubMed] [Google Scholar]
- Wiemer J., Schulz S.M., Reicherts P., Glotzbach-Schoon E., Andreatta M., Pauli P. Brain activity associated with illusory correlations in animal phobia. Social Cognit. Affective Neurosci. 2014;10(7):969–977. doi: 10.1093/scan/nsu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen, H.-U., Wunderlich, U., Gruschwitz, S., & Zaudig, M. (1997). SKID I. Strukturiertes Klinisches Interview für DSM-IV. AChse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearbeitung der amerikanischen Originalversion des SKID I. Hogrefe.
- Zald D.H. Orbital versus dorsolateral prefrontal cortex: Anatomical insights into content versus process differentiation models of the prefrontal cortex. Ann. N. Y. Acad. Sci. 2007;1121:395–406. doi: 10.1196/annals.1401.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.