Abstract
The PROSPECT study, a post-approval observational study in the U.S., showed no significant changes in lung function as measured by spirometry with clinical initiation of lumacaftor/ivacaftor. A sub-study within the PROSPECT study assessed the lung clearance index (LCI), as measured by multiple breath washout (MBW), a measure of lung function demonstrated to be sensitive among people with normal spirometry. Participants performed MBW prior to clinically initiating lumacaftor/ivacaftor therapy and for one year of follow-up. Similar to the whole PROSPECT study, this sub-study cohort (N = 49) had no significant absolute or relative changes in FEV1% predicted at any time point. LCI, however, decreased (improved) by 0.81 units or 5.3% (95% CI −9.7, −0.9%) at 1 month, 0.77 units or 5.9% at 3 months, 0.67 units or 5.9% at 6 months, and 0.55 units or 4.3% at 12 months. These results demonstrate the utility of the LCI in assessing treatment effects of relatively modest size in a heterogenous study population.
Clinical trials have shown modest efficacy of lumacaftor/ivacaftor for improving lung function, and other outcomes in subjects homozygous for F508del, the most common CF-causing mutation [1,2]. Lumacaftor/ivacaftor was approved by the U.S. Food and Drug Administration in 2015, however real-world clinical effectiveness is still under investigation. Studies out of France have shown substantial rates of treatment discontinuation in adult CF subjects due to adverse events, but among those who continued treatment, lung function improvements as measured by forced expiratory volume in 1 s (FEV1) were evident [3,4]. Recently, the PROSPECT study, a post-approval observational study of lumacaftor/ivacaftor use in the U.S., showed no significant changes in lung function with treatment [5]. This study cohort, however, had higher baseline lung function than both the previous phase III trials [6,7] and the French post-approval study [4], likely mitigating an ability to detect improvement, in addition to its smaller sample size.
There is ample evidence that spirometry can appear normal even when lung disease is progressing [8], thus limiting its ability to detect drug efficacy in those with near-normal spirometric values. The lung clearance index (LCI), as measured by multiple breath washout (MBW), has been demonstrated to be a more sensitive measure of lung function in CF subjects with normal spirometry values [9] and thus may be better suited to detect a treatment response to lumacaftor/ivacaftor, as previously demonstrated in the pediatric phase III study for lumacaftor/ivacaftor [6]. Currently, little evidence exists to support the utility of LCI to capture treatment effects in a broader age population in the post-marketing setting.
The aforementioned PROSPECT study in the U.S. included an optional sub-study in a subset of sites which performed MBW. Full details on subject population have recently been described [5]. Briefly, the study followed people with CF ≥6 years who were homozygous for the F508del mutation who were starting lumacaftor/ivacaftor clinically for one year of follow-up. Additional inclusion criteria for entry into the sub-study beyond the initial cohort included having FEV1 ≥30% predicted during the 6 months prior to drug initiation, as well as an ability to perform the testing and procedures required, as judged by the investigator. Sub-study participants performed MBW at initiation of lumacaftor/ivacaftor therapy, and at visits 1, 3, 6, and 12 months post-initiation. Other clinical measures, including spirometry, sweat chloride testing, and anthropometrics, were also collected at study visits. All spirometry measurements were standardized to percent predicted using Global Lung Initiative equations [10]. Anthropometric measures were converted to percentiles using the US Center for Disease Control (CDC) equations [11].
All variables are summarized using mean and standard deviation (SD) for normally distributed continuous outcomes, and medians with interquartile ranges (IQR) for skewed data. Changes were assessed from a stable pre-dose visit to each subsequent time point as both an absolute change and percent change. Correlations between change in LCI and other continuous variables were assessed by Pearson’s correlation coefficient (r). Predictors of response to lumacaftor/ivacaftor were assessed using multivariable linear regression.
A total of 60 participants were enrolled in the MBW sub-study of the PROSPECT study, performing MBW at 260 study visits with an overall success rate of 89%. After removing participants with fewer than 2 acceptable MBW measurements (n = 6), those without an acceptable MBW pre-dose (n = 3) and those who were unstable at the pre-dose visit (n = 2), 49 participants provided 211 MBW measurements (mean (SD) 4 (1) visits per participant) for analysis. The majority of the population were < 18 years of age (35/49, 71%), and most had a pre-dose FEV1% predicted higher than 90% (27/49, 55%). Full demographics can be found in Table 1.
Table 1.
Demographics at pre-dose visit of all included subjects (N = 49).
| Characteristic | All Included Subjects (N = 49) |
|---|---|
|
| |
| Age, median (IQR) (range) | 15.1 (12.4, 18.7) (6.7 – 50.2) |
| Female, n (%) | 26 (53%) |
| BMI percentile, median (IQR) (range) | 51.0 (34.4, 69.9) (8.3 – 94.6) |
| FEV1 % predicted, median (IQR) (range) | 91.3 (75.1, 100.1) (33.5 – 120.2) |
| LCI, median (IQR) (range) | 10.9 (8.6, 13.9) (6.6 – 25.2) |
| Sweat chloride, median (IQR) (range) | 103 (96, 108) (85 – 131) |
| Treatments | |
| Inhaled antibiotics | 22 (45%) |
| Azithromycin | 26 (53%) |
| Hypertonic saline | 37 (76%) |
| Pulmozyme | 46 (94%) |
Similar to the full PROSPECT study cohort, there was no significant absolute change in FEV1% predicted over any of the study visits in this sub-study cohort, from +1.2% predicted (95% CI −1.0, 3.4) at 1 month to +1.9% predicted (95% CI −1.1, 5.0) at 12 months. These results were also not significant when assessing percent change in FEV1, from +1.8%(95% CI −0.9, 4.5%) at 1 month to 2.5% (95% CI −1.2, 6.2%) at 12 months post lumacaftor/ivacaftor initiation (Fig. 1).
Fig. 1.
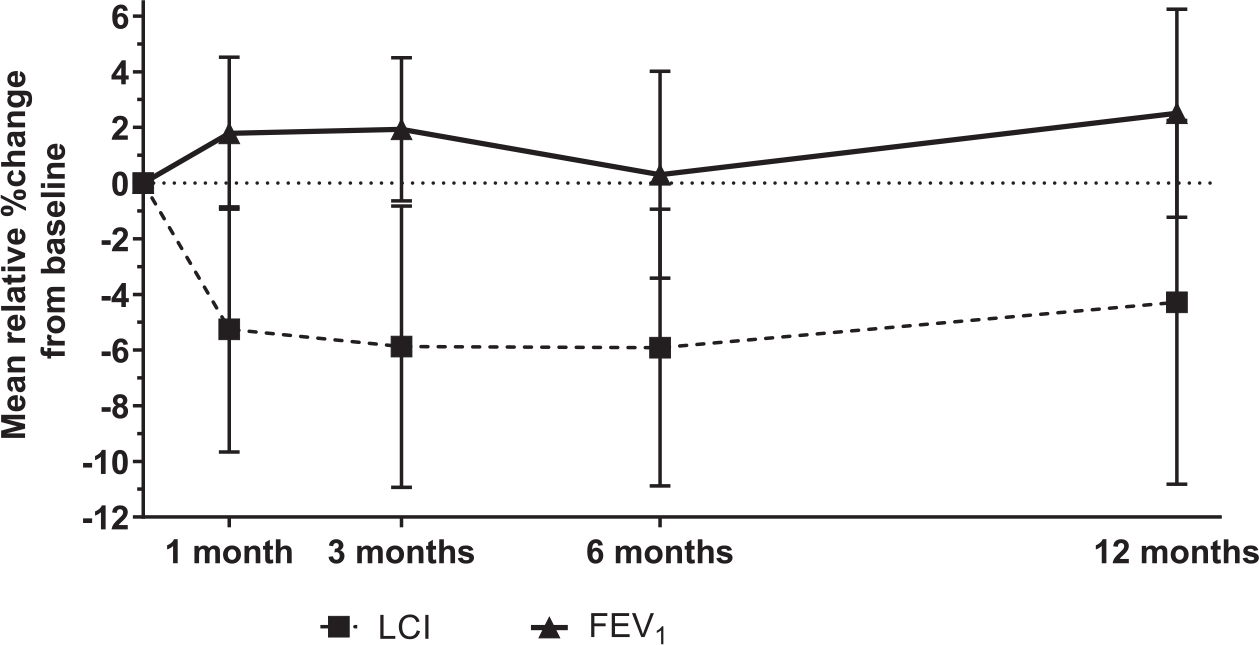
Change in lung function measurements throughout the study. Error bars represent 95% confidence intervals around the mean values at each time point.
LCI, however, decreased (improved) by 0.81 units (95% CI −1.37, −0.24; p = 0.006) at 1 month, and remained significantly lower at 3 months (Δ −0.77; 95% CI −1.37, −0.17; p = 0.01) and 6 months (Δ −0.67; 95% CI −1.23, −0.10; p = 0.02), with an attenuated effect at 12 months post lumacaftor/ivacaftor initiation (Δ −0.55; 95% CI −1.37, 0.28; p = 0.18). This represented a relative change of −5.3% (95% CI −9.7, −0.9%) at 1 month, −5.9% at 3 and 6 months, and −4.3% at 12 months(Fig. 1).
Variables independently associated with a greater percent drop (improvement) in LCI at the first on-treatment visit included having a higher (worse) baseline LCI (slope −2.2% per LCI unit; 95% CI −3.5, −0.9; p = 0.001), being male (−9.7%; 95% CI −17.5, −2.0; p = 0.02), or having a younger age (−0.8% per year; 95% CI −1.4, −0.1; p = 0.02).
The percent change in LCI was negatively associated with the percent change in FEV1% predicted, though the association was relatively weak (r = −0.44; 95% CI −0.55, −0.32; p < 0.001). While LCI was not associated with sweat chloride measurements for all visits (r = 0.01; 95% CI −0.12, 0.15; p = 0.84), the percent change in LCI was weakly associated with percent change in sweat chloride (r = 0.15; 95% CI 0.01, 0.29; p = 0.04).
Thus, these results demonstrate the utility of the LCI in assessing treatment effects of a CFTR modulator that is associated with a treatment effect of relatively modest effect size when assessed by standard spirometry. The relative change in LCI was about 3 times that of FEV1 which on the group level did not change significantly. While the ability to capture a treatment effect has been demonstrated before in the pediatric phase III studies in 6–11 year old children with preserved FEV1 [6,7], the current study included subjects over a somewhat broader range of age and lung function
In clinical trials, lumacaftor/ivacaftor has shown moderate effects overall, but it is still critical to understand how these effects translate to the clinical setting. While the LCI effect size in 6–11 year olds was over 1 LCI unit at 6 months in the phase III clinical trial for lumacaftor/ivacaftor [6] the effect size seen here was lower at −0.7 units (95% CI −1.23, −0.10) at the same timepoint. This sub-study had a smaller sample size, as only a select number of sites were equipped to perform MBW, and it did include a broader range of ages and levels of disease severity which may have affected the treatment effect. The cohort was still largely pediatric with preserved lung function, which could explain why the result was attenuated, but still discernable. Other treatments, such as the inhaled hypertonic saline study in preschoolers, have shown similar treatment sizes (−0.63 LCI units) that were also considered a clinically meaningful changes on the population-average level.
This study found that being male, having a higher baseline LCI, and being younger were each independent predictors of the initial drop in LCI upon treatment. Since factors such as adherence to the treatment regime were not well captured in this study, it is difficult to discern why these may be contributing factors; it is possible that younger children or those with more severe disease get more benefit because of stricter adherence. However, we are cautious to over-interpret the results of this multivariate analysis due to the limited sample size of this sub-study.
Clinical use of new interventions post-approval is often extended beyond the specific inclusion and exclusion criteria in the initial Phase III studies; thus, it is expected to find more modest results in real-life effectiveness studies. In both the broader PROSPECT study [5] as well as the MBW sub-study, there was no statistically significant improvement in lung function as measured by FEV1. However, LCI has again shown potential as an effective outcome measure in groups with milder disease where FEV1 may not be best suited to detect a treatment effect.
Acknowledgments
The PROSPECT study was funded by Cystic Fibrosis Foundation Therapeutics (SAGEL14K1, HAMBLE14K1, ROWE19R0) and the NIH (UL1 TR002535, DK089507, P30DK072482, R35HL135816).
Declaration of Competing Interest
MS has nothing to disclose. UK reports grants from Cystic Fibrosis Foundation, during the conduct of the study and outside the submitted work. JPC reports grants from Cystic Fibrosis Foundation, during the conduct of the study. SHD reports grants from Cystic Fibrosis Foundation, during the conduct of the study. SDS reports a grant from the Cystic Fibrosis Foundation that funds the work under consideration, and grants from the Cystic Fibrosis Foundation and National Institutes of Health funding activities outside the submitted work. SMR reports grants, contracts, and consulting services from Vertex Pharmaceuticals related to the conduct of clinical trials. FR reports grants and personal fees from Vertex, personal fees from Novartis, personal fees from Bayer, personal fees from Roche, personal fees from Genetech, outside the submitted work.
References
- [1].Boyle MP, Bell SC, Konstan MW, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2014. doi: 10.1016/S2213-2600(14)70132-8. [DOI] [PubMed] [Google Scholar]
- [2].Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N Engl J Med 2015. doi: 10.1056/NEJMoa1409547. [DOI] [PubMed] [Google Scholar]
- [3].Hubert D, Chiron R, Camara B, et al. Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J Cyst Fibros 2017. doi: 10.1016/j.jcf.2017.03.003. [DOI] [PubMed] [Google Scholar]
- [4].Burgel PR, Munck A, Durieu I, et al. Real-life safety and effectiveness of lumacaftor–ivacaftor in patients with cystic fibrosis. Am J Respir Crit Care Med 2020. doi: 10.1164/rccm.201906-1227OC. [DOI] [PubMed] [Google Scholar]
- [5].Sagel SD, Khan U, Heltsche SL, et al. Clinical effectiveness of lumacaftor/ivacaftor in cystic fibrosis patients homozygous for F508del-CFTR. Ann Am Thorac Soc 2020. Pending. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017. doi: 10.1016/S2213-2600(17)30215-1. [DOI] [PubMed] [Google Scholar]
- [7].Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M. Lumacaftor/Ivacaftor in patients aged 6–11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med 2017. doi: 10.1164/rccm.201608-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramsey KA, Rosenow T, Turkovic L, et al. Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med 2016. doi: 10.1164/rccm.201507-1409OC. [DOI] [PubMed] [Google Scholar]
- [9].Stanojevic S, Davis SD, Retsch-Bogart G, et al. Progression of lung disease in preschool patients with cystic fibrosis. Am J Respir Crit Care Med 2017;195(9):1216–25. doi: 10.1164/rccm.201610-2158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000 [PubMed] [Google Scholar]


