Introduction
Asthma affects 25.7 million people in the US including 7 million children.1 Most patients with persistent asthma respond well to therapy; however, 2/3 of children with asthma report an attack in the last year and experience a high rate of healthcare reutilization2 underscoring the need for improved asthma management. The Global Initiative for Asthma (GINA) has recognized asthma as being a heterogeneous disease with different presentations, outcomes and underlying mechanisms. This heterogeneity contributes to the difficulty in managing asthma, especially for difficult-to-treat (DTT), which is characterized by ongoing symptoms and healthcare utilization despite high dose medications. As DTT accounts for >50% of all asthma-related expenditures,3 our goal was to develop a personalized treatment model for patients with DTT asthma. We expect that DTT asthma is heterogenous, but that existing biomarkers and other factors can be used to formulate molecular sub-classifications that contribute to variability in asthma outcomes. These molecular endotypes can then be used to develop personalized treatment strategies.4 To date, clinical phenotypes of asthma have been defined and related to a few biomarkers; however, no previous study has systematically integrated multiple asthma-relevant biomarkers into a treatment algorithm and studied the clinical impact of applying such an algorithm. The purpose of this study is to identify DTT asthma endotypes and evaluate endotype driven management strategies. Our hypothesis is that personalized treatment algorithms based on molecular sub-classification of DTT asthma are feasible and lead to improved asthma outcomes. We describe development of a biomarker-based treatment algorithm designed to augment current asthma treatment guidelines. We then conduct a proof-of-concept pilot study to test the feasibility of this treatment by endotype approach by delivering a personalized algorithm in a cohort of children with DTT asthma.
Methods
Study Population
Children aged 6–17 were identified by Cincinnati Public School nurses, community primary care clinics, or Cincinnati Children’s Hospital Medical Center clinics in Cincinnati, Ohio. All children were diagnosed with DTT asthma, defined as either moderate persistent asthma (National Asthma Education and Prevention Program (NAEPP) step 3–4 identified by the Asthma TreatSmart program5 (see below) and ≥1 of the following in the past 12 months: 1)two Childhood Asthma Control Test (c-ACT)6 or adult Asthma Control Test (ACT)7 scores <20, 2)one or more asthma-related emergency department (ED) visits or hospitalizations, and/or 3)two or more asthma-related corticosteroid bursts) or severe persistent asthma (NAEPP step 5–6). Enrollment began in March of 2018 and the study visits were complete in October of 2019. The study was reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board. Written consent was obtained from a parent or legal guardian and assent for children ≥ 11 years of age at the initial visit.
Study Visits
At V1, participants underwent a full history and physical examination and were started on guideline care8, 9 for their asthma. In addition, biological samples were collected for biomarker determinations (discussed below) and Propeller Health System® (Propeller Health, Madison, WI) adherence caps were placed on the child’s rescue and controller inhalers to begin baseline adherence data collection. Participants were seen 3–4 months later (V2) and a personalized treatment plan informed by their biomarker results was added on top of the child’s guideline care. Each child was followed for at least a 6-month observation period and seen for a final visit (V3). Monthly phone calls were conducted throughout the study to capture information about asthma control and exacerbations, as well as adherence. Pulmonary function testing was done at V1, V2, and V3. We compared outcomes from guideline care initiation (V1-V2) and from guideline + personalized interventions (V2-V3). Details on the study procedures at each study visit can be found in the eSupplement and eTable1.
Biomarkers and assessment of subjects
Key biomarkers associated with asthma phenotypes, asthma severity, asthma exacerbations, and/or treatment response to steroids were selected from the literature in order to classify subjects according to their individual biomarker results. Numerous studies link allergic sensitization and IgE to decreased lung function, increased asthma severity and exacerbations, especially in children.10–12 IL-17A serum levels have been associated with severe asthma, increased BHR and increased airway neutrophilic inflammation and mucus production in children.13 DNA methylation levels of CpG sites in the promotors of homebox protein OTX2 (OTX2) and L-lactate dehydrogenase C chain (LDHC) genes in nasal epithelial cells have been associated with poor corticosteroid response in children with asthma14–16. A correlation between severe asthma and the non-atopic condition has been reported in pediatric asthma.17, 18 CDHR3 serves as a receptor for human rhinovirus (HRV)-C, the CDHR3 coding variant rs6967330 increases CDHR3 protein surface expression, and is a genetic marker of rhinovirus susceptibility and the SNP confers risk for severe childhood asthma exacerbations.19, 20 Elevated interleukin-6 (IL-6) levels have been associated with asthma severity and non-allergic airway inflammation in both children and adults21, whereas no correlation has been found between IL-6 and T2 inflammation biomarkers.21, 22 25-hydroxyvitamin D (Vitamin D) insufficiency has been associated with impaired lung function, increased bronchial hyper-responsiveness (BHR), and increased risk of exacerbations and healthcare utilization in both children and adults which may be due to the immunomodulatory effects, enhancement of corticosteroid responsiveness, and anti-microbial and anti-inflammatory mechanisms attributed to Vitamin D.23, 24 Vitamin D replacement has been shown to be effective in asthma in many studies,23 but not all studies.25 Exposure to environmental tobacco smoke was assessed through urinary cotinine. Environmental tobacco smoke exposure in children has been associated with reduced lung function, wheezing symptoms, and ED visits and hospitalizations for asthma.26–28 The magnitude of skin barrier dysfunction was assessed by measurement of skin transepidermal water loss (TEWL), which correlates with atopic dermatitis (AD) disease severity and has been shown to contribute to aeroallergen sensitization and asthma in children.29, 30
Asthma Clinical Phenotype Definitions
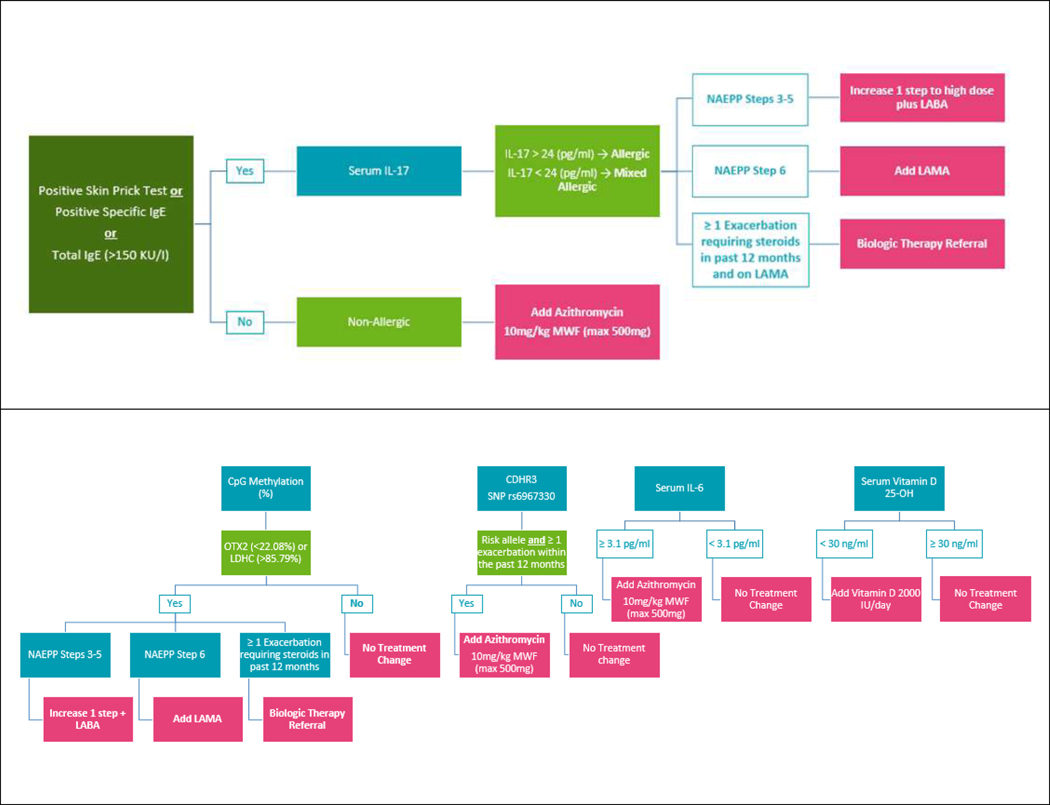
All subjects were defined into 3 allergic phenotypes based on total IgE, specific IgE, skin prick testing, and serum IL-17 level. The allergic phenotype was defined as 1) elevated serum total IgE (IgE >150 IU/mL) or at least one positive specific IgE (>0.34 IU/mL) or skin prick test AND 2) IL-17 ≤ 24 pg/ml. Elevated IL-17 (IL-17 > 24 pg/ml) in the absence of at least one elevated IgE or a positive specific IgE or skin test were defined as non-allergic (T17 inflammation). Mixed allergic (T2/T17) was defined as evidence of both which has been associated with more severe asthma and poor steroid treatment response.31–33 The personalized treatment algorithm is detailed in Figure 2 and Table 2 and described below.
Figure 2.
Biomarker Treatment Algorithm. Key biomarkers that have been associated with asthma clinical phenotypes, asthma severity, asthma exacerbations, and/or treatment response to steroids were selected from the literature in order to classify subjects according to their individual biomarker results. This was used to develop a personalized treatment algorithm. All interventions can occur simultaneously.
Table 2.
Summary of interventions included in personalized treatment algorithm and biomarkers that triggered each intervention.
| Intervention | Biomarker to Trigger Intervention | Comment |
|---|---|---|
| Medical Interventions | ||
| Increase treatment step by 1 over guideline care plus LABA | *NAEPP step 3–5 LDHC or OXT2 CpG methylation indicating poor steroid treatment responder OR *NAEPP step 3–5 and allergic or mixed allergic inflammation based on allergic status definition |
Blood IL-17 and allergic status were completely redundant with CpG methylation Figure 3A |
| Add LAMA | *NAEPP step 6 and LDHC or OXT2 CpG methylation indicating poor steroid treatment responder OR *NAEPP step 6 and allergic or mixed allergic inflammation based on allergic status definition |
|
| Biologic therapy referral (mepolizumab, omalizumab, dupilumab or benralizumab) |
*LDHC or OXT2 CpG methylation indicating poor steroid treatment responder > 1 exacerbation requiring steroid in past 12 months and on LAMA OR *Allergic or mixed allergic inflammation based on allergic status definition and > 1 exacerbation requiring steroid in past 12 months and on LAMA OR *High total IgE and SPT+ |
|
| Azithromycin | *Non-allergic asthma phenotype OR *Presence of 1 or more CDHR3 rs6967330 risk alleles and ≥1 exacerbation in the past 12 months OR *Elevated serum IL-6 |
All 3 markers were non-redundant Figure 3B |
| Cetirizine | High Total IgE | |
| Cetirizine + Nasal Steroid | ≥1 SPT positive | |
| Vitamin D | Deficient in Vitamin D | |
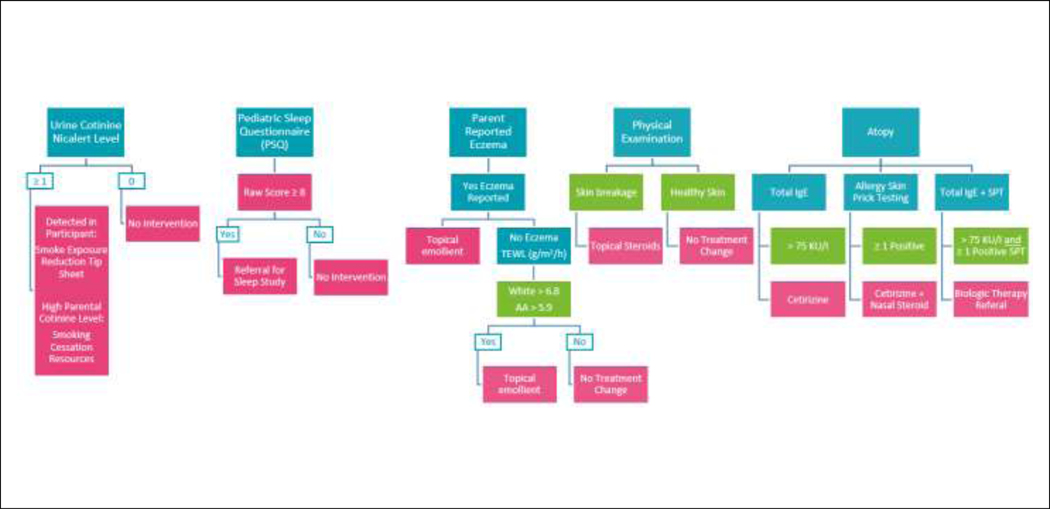
| Emollient therapy | *AD present OR *Elevated TEWL |
|
| Non-medical Interventions | ||
| Smoking exposure reduction tip sheet | Participant defined as exposed to secondhand smoke via NicAlert™test | |
| Smoking cessation referral | Parent defined as active smoker via NicAlert™ test | |
| Sleep study referral | High score on PSQ | |
| Text messaging for adherence | Baseline adherence level 50–80% | |
| Telehealth for adherence | Baseline adherence level <50% |
Outcome Measures
The primary outcome of this study was asthma control as determined by the c-ACT (age<12 years) and ACT (age ≥ 12 years) scores. Secondary outcomes included: reduction in yearly rates of asthma-related ED visits, systemic corticosteroid courses, and forced expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC)% predicted at each study visit. Clinical outcomes were assessed at baseline and throughout the study periods extending for 6 months after the personalized treatment algorithm was assigned. Lung function was obtained at clinic visits based on American Thoracic Society (ATS) criteria and Wang reference values.34, 35 Monthly telephone calls to participant families were conducted throughout the 12 month study to obtain a 2-week recall of symptoms, ED visits and/or hospitalizations, as well as the c-ACT and ACT scores.36
Details on data analysis can be found in the eSupplement.
Results
Population Demographics and Study Overview
We screened 92 participants and 21 were enrolled in the study (eFigure1). Three participants did not complete the study as they were lost to follow-up (eFigure1). Fifty percent of participants were enrolled from community sources and 50% from CCHMC. The primary reasons for ineligibility were participants had mild asthma or a chronic medical condition apart from allergy or asthma. Eighty-one percent of participants completed all 3 medical visits and 86% completed at least 2 medical visits. Ninety-one percent completed all four adherence self-management visits. Fifty-two percent (11/21) of participants had a total of 15 damaged, lost or defective monitoring sensors replaced over the yearlong study period which was under the 3 sensors per participant that was budgeted. Participant and family feedback have been very positive. At the conclusion of the study, 100% of families who completed the study reported satisfaction of with the study and 100% of families would recommend the study to others.
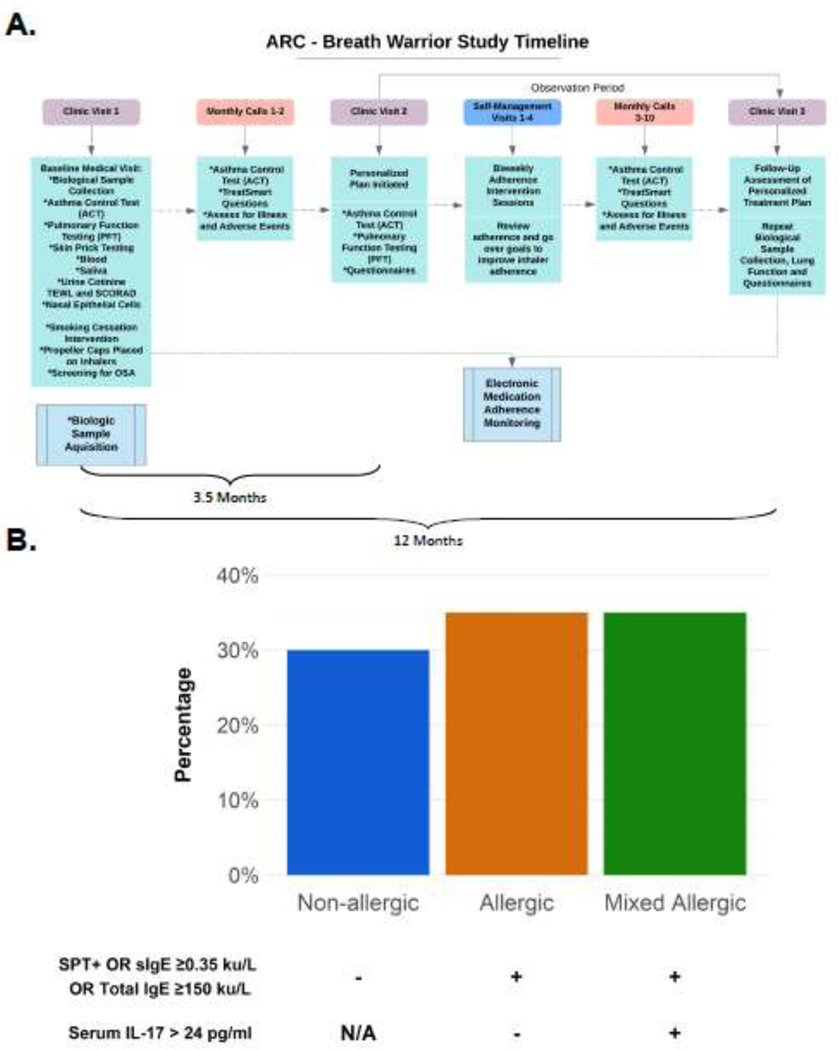
The average age was 12.4 years and 70% self-reported as Black (Table 1). The enrolled cohort had poor baseline asthma control; 40% required an asthma-related ED visit or systemic corticosteroids course in the prior year and 15% were hospitalized in the prior year (Table 1). A study timeline is shown in Figure 1A and detailed in the Online Supplement. In this DTT asthma population, there was equal distribution among the 3 clinical phenotypes: allergic, mixed allergic, and non-allergic (Figure 1B).
Table 1.
Baseline Demographics and Baseline Characteristics Obtained at Medical Visit 1.
| Baseline Demographics and Characteristics | All (n = 20*) | Subjects with Complete Data (n = 17) |
|---|---|---|
| Age, mean (SD) | 12.4 (2.8) | 12.4 (3.0) |
| Sex, n (%) Female: Male: |
9 (45.0) 11(55.0) |
(47.1) (52.9) |
| Self-reported race, n (%) Black: Non-Black: |
14 (70.0) 6(30.0) |
11 (64.7) 6 (35.3) |
| Self-reported ethnicity, nn(%) Hispanic/Latino: Not Hispanic/Latino: |
0 (0) 20 (100) |
0 (0) 17 (100) |
| Insurance, n (%) Public: Non-Public: |
13 (65.0) 7 (35.0) |
10 (58.8) 7 (41.2) |
| Eczema diagnosis Yes: No: |
9 (45.0) 11 (55.0) |
7 (41.2) 10 (58.8) |
| Age of asthma diagnosis, mean (SD) | 3.4 (2.1), n = 18 | 3.4 (2.2), n = 16 |
| BMI percentile, median | 87.7 | 89.4 |
| NAEPP Treatment Step at V1 (based on TreatSmart algorithm) 3: 5: 6: |
2 (10.0) 2 (10.0) 16 (80.0) |
2 (11.8) 1 (5.9) 14 (82.3) |
| Parental history of asthma Yes: No: |
10 (50.0) 10 (50.0) |
9 (52.9) 8 (47.1) |
| Parental report of allergy Yes: No: |
14 (82.4) 3 (17.6) |
14 (87.5) 2 (12.5) |
| In the 12 months prior to V1: | ||
| Systemic Corticosteroid use Yes: No: |
12 (60.0) 8 (40.0) |
11 (64.7) 6 (35.3) |
| ED encounter Yes: No: |
12 (60.0) 8 (40.0) |
10 (58.8) 7 (41.2) |
| Hospital admission Yes: No: |
3 (15.0) 17 (85.0) |
2 (11.8) 15 (88.2) |
excludes the participant that was lost to follow-up before the personalized algorithm was initiated.
Figure 1.
A. Study timeline. B. Distribution of allergic, mixed allergic and non-allergic clinical phenotypes. N/A: Il-17 level was not informative for this group.
Development of biomarker-informed personalized treatment algorithm
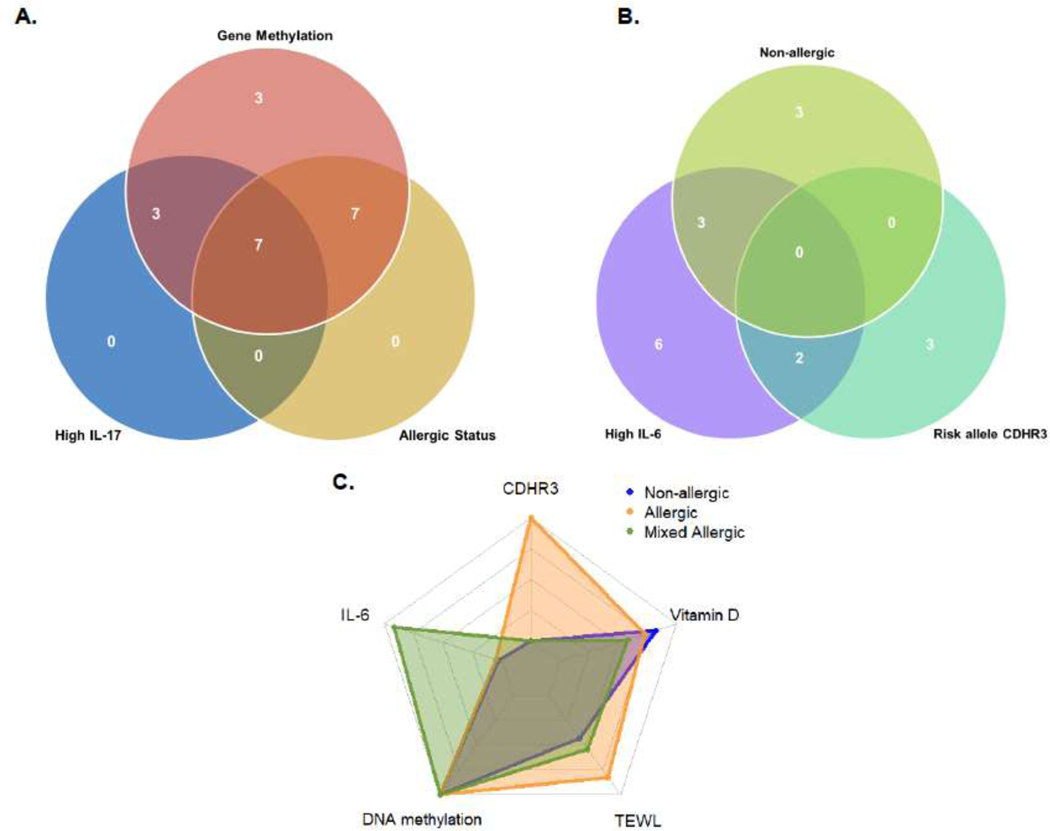
An a priori personalized treatment algorithm was developed (Figure 2, Table 2). The biomarkers were chosen from the literature and the cutoff values used for interpretation are included in eTable2. Cutoff values are from pediatric populations, with the exception of IL-6. Table 2 summarizes the biomarkers included and the conditions that triggered each intervention. The minimal biomarker subset that was necessary to inform the personalized treatment algorithm was identified. This included LDHC or OTX2 CpG methylation, CDHR3 rs6967330 genotype, clinical allergic phenotype definition, serum IL-6, TEWL, secondhand smoke exposure, and Vitamin D status. The markers triggering increase in treatment step (blood IL-17 and allergic status) were completely redundant with CpG methylation level (Figure 3A), while non-allergic asthma phenotype, CDHR3 rs6967330 genotype and elevated IL-6 all independently contributed to the addition of azithromycin (Figure 3B).
Figure 3.
Identification of key non-redundant biomarkers to inform the personalized treatment by endotype algorithm. A. The mixed allergic clinical phenotype and IL-17 were redundant with LDHC or OTX2 CpG methylation, which indicates poor steroid treatment responsiveness. B. Initiation of azithromycin treatment was informed by the non-allergic asthma phenotype, presence of 1 or more CDHR3 rs6967330 risk allele(s), and elevated serum IL-6, which were non-redundant. C. A radar chart is a plot that consists of a sequence of equi-angular spokes each representing one of the variables. The data length of a spoke is proportional to the magnitude of the variable for the data point relative to the maximum magnitude of the variable across all data points. This radar chart shows the distribution of the minimal set of informative biomarkers among the allergic, mixed allergic, and non-allergic phenotypes.
Biomarkers and classification of subjects
The distribution of each biomarker among all subjects is shown in eTable2. Eighty-five percent of all subjects were Vitamin D deficient. Surprisingly, 100% of subjects had DNA methylation levels in OTX2 and/or LDHC consistent with increased risk for poor steroid treatment response. In contrast, the prevalence of poor treatment response in children hospitalized for acute asthma exacerbation in the Ohio Pediatric Asthma Repository (OPAR) study37 was 66% (p<0·001). Fifty-five percent of subjects had increased IL-6 levels and 50% of had increased IL-17 levels. Twenty-five percent of children carried at least one copy of the risk allele for CDHR3 rs6967330, with 27% for Blacks and 17% for non-Blacks which is similar to the frequencies reported in the general population (per 1000 genomes, 26·5% and 20·8%, respectively). Forty-five percent (9/20) of participants had mild-moderate AD and none of the participants had severe AD. Seventy percent (14/20) of participants had high TEWL.
Distribution of biomarkers by allergic status
The distribution of the key biomarkers among the 3 allergic phenotypes was highly variable (Figure 3C). While all children with allergic phenotypes demonstrated low Vitamin D levels and increased risk for poor steroid treatment response (OTX2 and/or LDHC DNA methylation), the allergic phenotype was also characterized by increased CDHR3 risk genotype and high TEWL. In contrast, high serum IL-6 level was most notable in the mixed allergic subgroup. The non-allergic phenotype was characterized by the lowest levels of IL-6 and lowest frequencies of the CDHR3 risk allele. Secondhand smoke exposure did not differ across the allergic phenotype groups (allergic 30% (6/20), mixed allergic 30% (6/20) and non-allergic 20% (4/20)).
Summary of Treatment Interventions at V1 (Guideline Care) and V2 (Treatment by Endotype per Algorithm)
At V1, 19% (4/21) of participants received a step up in asthma controller level at V1 based on the Asthma TreatSmart program.5. At V2, all participants received at least one additional medical intervention with an average of 5.9 interventions (range 3–9; eFigure2A). With respect to asthma specific treatments, 15% of subjects started and 85% were continued on high dose combination (inhaled corticosteroid/long-acting beta-agonist (ICS/LABA)) controller therapy, 85% initiated tiotropium, 80% initiated and 5% continued on azithromycin, and 10% recommended to start asthma biologic therapy (1 on mepolizumab and 1 on omalizumab). For ezcema, 55% started and 15% continued on topical emollient therapy, 5% initiated and 5% continued on medium dose topical corticosteroids as well as 5% initiated and 5% continued on low dose topical corticosteroids. With respect to other treatments, 30% started and 40% continued on cetirizine, 25% started and 40% continued on nasal corticosteroids and 85% started Vitamin D supplementation.
All subjects received at least one non-medical intervention with an average of 2.3 (eFigure2B). Forty-three percent (9/20) of participants had a positive PSQ screen and were referred for a sleep study. Eighty percent (16/20) of participants had secondhand smoke exposure and were given a smoke exposure reduction tip sheet intervention. In addition, 40% (8/20) of parents identified as active smokers and were provided information for the Ohio smoking cessation quit line. Smoking interventions were not associated with a change in the measured outcomes (data not shown).
Summary of Adherence Interventions
Baseline adherence (V1) was low, 19 participants had <80% adherence to controller medication at baseline; 12 had <50% adherence and received telehealth adherence intervention while 7 had 50–80% adherence and received text-messaging adherence intervention. Adherence at the end of the study (35.8% (IQR 12.9–54.6%) was not significantly different from baseline adherence 43.3% (IQR 20.8–57.1%), (S=−27, p=0.17), however, adherence during the self-management intervention was 75.9% compared to baseline adherence (IQR 59.2–83.3%; S=68, p<.0001).
Comparison of outcomes at V1 and V2
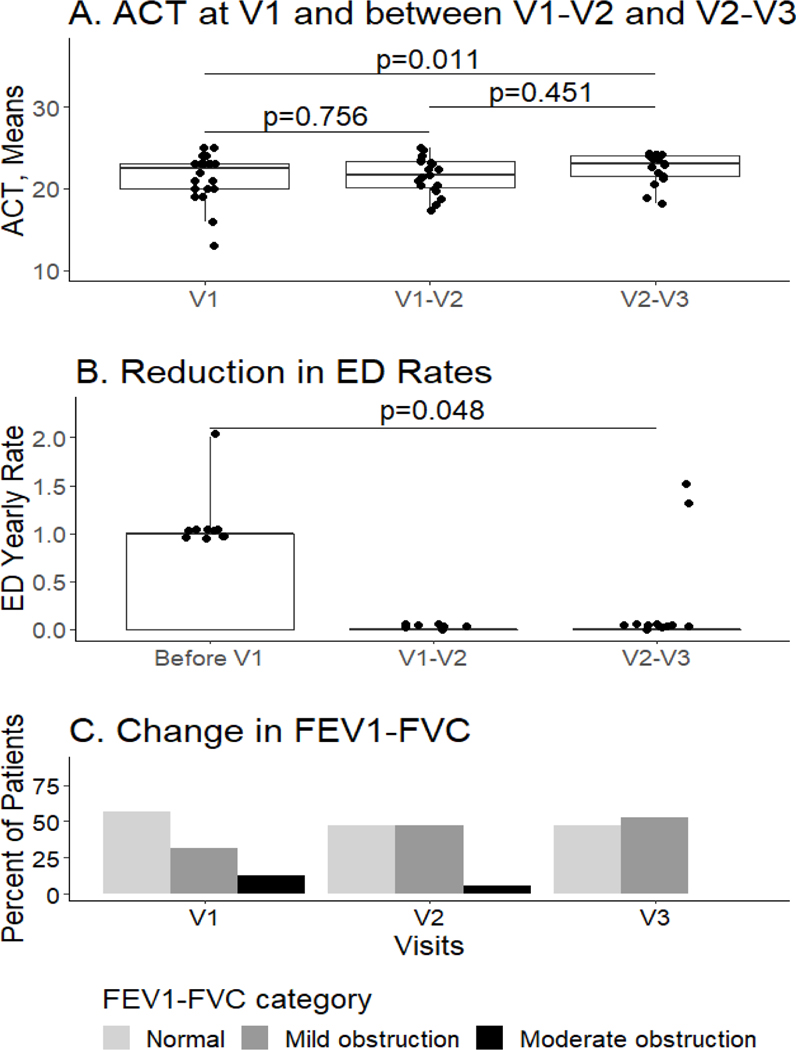
There was no significant difference in c-ACT/ACT score after initiation of treatment guideline care (V1-V2 vs. V1, median 21.7 vs 23.0, range 17.3–25.0, IQR 18.2–24.2, p=0.45), however, initiation of the personalized intervention on top of guideline care was associated with a significant improvement in the c-ACT/ACT score compared to V1 (V2-V3 vs. V1, median 23.0 vs. 22.5, range 13.0–25.0, IQR 18.2–24.2, p=0.01, Figure 4A). There were no distinct differences between allergic phenotype groups. The rate of ED visits in 12 months prior to V1 significantly decreased following the implementation of the V2 intervention (median 1, range 1–2, IQR 0 to median 0, range 0–1.5, IQR 0–1 yearly rate, p=0.048, Figure 4B). There was not a significant difference in the rate of systemic corticosteroids use (median 1 to 0, range 0–3 to 0–2.7, IQR 0–1 to 0–1.5, p=0.23, data not shown). The FEV1/FVC% predicted in the 12 months prior to V1 until V2-V3 was not significantly improved (p=0.26), however the data revealed a trend that the number of children with moderate obstruction decreased and the number with mild obstruction increased from V1 to V2 to V3 (Figure 4C). No significant associations were found between adherence and the clinical outcomes (c-ACT/ACT, ED visits, rate of systemic corticosteroid use, FEV1/FVC % predicted) (eTable3).
Figure 4:
A. Comparison of c-ACT/ACT scores at baseline (V1) to scores with guideline care alone (V1-V2) and to scores with guideline + personalized intervention (V2-V3). No distinct differences were observed between clinical phenotype groups. B. Comparison of Emergency Department (ED) utilization at baseline (V1) and following treatment with guideline + personalized intervention (V2-V3). C. Comparison of FEV1/FVC at baseline (V1), at V2 (guideline care alone), and V3 (guideline + personalized intervention) by degree of airway obstruction (normal, mild and moderate).
Discussion
Asthma is variable in presentation due to heterogeneity in both disease pathophysiology and treatment response, which contributes to the difficulty in both studying and managing asthma. The National Institutes of Health challenged investigators to develop personalized therapy defined by clinical characteristics and biomarker analyses and evaluate its efficacy.4, 18, 38, 39 We developed a personalized treatment algorithm based on integration of published biomarkers and interventions that inform asthma clinical phenotypes and endotypes including allergic status, serum IL-17 and IL-6, variation in the CDHR3 gene, steroid treatment response, vitamin D status, skin barrier function, exposure to tobacco smoke and sleep disordered breathing. IL-17 and allergic status were redundant with gene methylation. Allergic status is required to identify non-allergic asthma, but IL-17 can be removed from the minimal biomarker set. To our knowledge, this is the first proofof-concept pilot study to systematically integrate published asthma-relevant biomarkers into a personalized treatment algorithm and evaluate the feasibility of delivering the algorithm in a cohort of children with DTT asthma. Our preliminary findings suggest that personalized endotype-informed treatment may be associated with an improvement in asthma control and a decrease in ED visits. Further, our study demonstrates the feasibility of this type of intervention as our overall retention was 86%.
There was an even distribution among three clinical phenotypes: allergic (T2), mixed allergic (T2/17) and non-allergic (T17). This was an unexpected finding as other studies have suggested that childhood asthma is mostly allergic,12, 40 but the non-allergic and mixed allergic phenotypes have been associated with poor steroid responsiveness and may be overrepresented in DTT. Our biomarker-informed treatment algorithm triggered at least one intervention on top of guideline care for all patients and our findings suggest that there are several molecular endotypes which vary across DTT asthma. Patients received an average of 5.9 additional medical interventions at V2. Based on TEWL and presence of the CDHR3 variant allele, subjects with the allergic phenotype were at increased risk for rhinovirus C infections and skin barrier dysfunction and, thus, this group received azithromycin and skin emollients. In contrast, the non-allergic phenotype was treated with azithromycin, but generally did not fit criteria for treatment with skin emollients. The mixed allergic phenotype was characterized increased blood levels of IL-6 and was treated with azithromycin. All 3 allergic phenotypes were characterized by low Vitamin D levels and poor corticosteroid responsiveness.
Poor corticosteroid response in children with asthma has been associated with DNA methylation of promoter sites in OTX2 and LDHC.14, 15 Surprisingly, all DTT subjects in this study were positive for this biomarker compared to 66% in a hospitalized population of asthmatics.37 Thus, a large subset of DTT asthmatics are likely to be poor steroid responders. Vitamin D insufficiency has been associated with impaired lung function, increased bronchial hyper-responsiveness (BHR), and increased risk of exacerbations and healthcare utilization, which may be due to the immunomodulatory effects, enhancement of corticosteroid responsiveness, and anti-microbial and anti-inflammatory mechanisms attributed to Vitamin D.23
In the algorithm, azithromycin was triggered by the presence of the CDHR3 risk genotype OR high blood IL-6 OR a non-allergic asthma phenotype. While there was some overlap in these biomarkers, they were not redundant. CDHR3 serves as a receptor for human rhinovirus (HRV)-C and rs6967330 increases CDHR3 protein surface expression. Accordingly, more severe rhinovirus-C infections and childhood asthma exacerbations have been associated with rs6967330.19, 20 Thus, DTT children with the allergic asthma phenotype may be at increased risk for more severe viral infections. Our findings are consistent with previous studies that reported an endotype with elevated blood IL-6, which was associated with increased asthma severity. They found no correlation between IL-6 and T2 inflammation biomarkers suggesting that IL-6 be an important biomarker to define individuals with a low T2 asthma.16, 21, 22 In this pilot, there are not enough subjects to determine which biomarker was most predictive of improved asthma control with azithromycin treatment, but children identified by these biomarkers improved with azithromycin treatment. Treatment with azithromycin may be immunomodulatory, decrease infection rate or morbidity, or target an unrecognized pathophysiologic pathway.
Emollient therapy was given to subjects with elevated TEWL levels. Seventy percent of children in this study had high TEWL and 45% of the children had evidence of mild-severe atopic dermatitis during the visit. Thus, the majority had evidence of skin barrier dysfunction supporting the link between AD and asthma.29, 30 Treatment of children with high TEWL with targeted interventions to improve skin barrier function may impact future asthma development and control.
An average of 2.3 non-medical interventions were provided to participants based on their exposure to secondhand smoke, baseline adherence to controller medication, and PSQ score. The majority of children in this study were exposed to secondhand smoke, which is associated with increased asthma symptoms and healthcare utilization.26 They were given the smoking intervention, but the smoking intervention was not associated with a change in any of the measured outcomes. Over 40% of participants had a positive PSQ screen suggesting that a significant proportion of children with DTT asthma may have sleep disordered breathing confirming findings in other studies.41
Data from this pilot suggests that children with DTT asthma are willing to participate in objective adherence measures and interventions delivered through technology. Adherence results were encouraging for this challenging population, with significant improvements in medication adherence during the self-management intervention period. In fact, 75% of the participants demonstrated an adherence increase of greater than 20% during the adherence intervention. However, the return to low rates of adherence when behavioral self-management intervention ceased is consistent with published findings.42 Future studies should utilize booster self-management sessions to maintain adherence improvements and assess the impact of sustained improved adherence on medical outcomes.42
This proof-of-concept pilot study is innovative for several reasons. First, many endotypes were identified within the broader clinical phenotypes - allergic, mixed and non-allergic. Second, we identified the minimal number of non-redundant biomarkers that could be used in a larger study. With the minimal biomarker set, the cost of the personalized treatment intervention is considerably less than a single ED visit or hospitalization. . Finally, the biomarker-based algorithm triggered interventions on top of guideline care in all DTT children studied, suggesting there is a strong need for this type of multipronged personalized approach. The approach we developed may serve as the foundation for a practical, point-of-care, biomarker-driven standardized algorithm for practitioners which is currently lacking in asthma guideline care.9
The primary limitation of this study is the small sample size. Thus, we were unable to determine which of interventions were associated with improvements. It is important to note that our primary goal was to demonstrate feasibility of this approach and identify the minimal biomarker set that was informative. Determination of efficacy will require a large trial. Nevertheless, our study is a necessary step and provides the foundation for a definitive trial. As this was a feasibility pilot, we did not include a control group which would allow a direct comparison of the efficacy of the personalized approached compared to guideline informed care alone. However, we did implement a case-crossover design where we compared data from V1-V2 (guideline care only) with V2-V3 (guideline + personalized care) within the same individual. During the 3 months between V1 and V2, we anticipate that changes due to guideline care would already be evident. To ensure that the use of data collected with the telephone visits did not impact the results, we performed the analysis using only the clinical visit and obtained similar results to when we incorporated the telephone visits to account for the passage of time. Although statistically significant, the change in ACT score was less than the clinically minimal important difference. Finally, all children were enrolled in one clinical site which may limit the generalizability of the findings.
In summary, we developed a personalized algorithm to treat children with DTT asthma in addition to guideline care. We demonstrated the feasibility of this approach and identified biomarkers associated with steroid treatment response, inflammatory types (T2, T17 or mixed), viral susceptibility, Vitamin D status, and exposure to SHS, to be the minimal number of non-redundant biomarkers to identify key endotypes. Despite limited sample size, these data suggest that all patients may benefit from more tailored care. Larger studies are warranted to confirm these findings.
Supplementary Material
Funding
Cincinnati Children’s Hospital Medical Center Research Foundation Academic and Research Committee Fund, Ohio Department of Job and Family Services and National Heart, Lung and Blood Institute, K23HL139992. This work is also supported by the following NIH CTSA Grant and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health: UL1 TR001425.
Abbreviations
- ACT
Asthma control test
- AD
Atopic dermatitis
- BHR
Bronchial hyper-responsiveness
- c-ACT
Childhood asthma control test
- DTT asthma
Difficult-to-treat
- ED
Emergency Department
- FEV1
Forced expiratory volume in 1 sec
- FVC
Forced vital capacity
- GINA
Global Initiative for Asthma
- OTX2
Homebox protein OTX2
- IgE
Immunoglobulin E
- ICS/LABA
Inhaled corticosteroid/long-acting beta-agonist
- IL
Interleukin
- IRB
Institutional Review Board
- LDHC
L-lactate dehydrogenase C chain
- NAEPP
National Asthma Education and Prevention Program guidelines
- T2
Type 2
- PSQ
Pediatric sleep questionnaire
- TEWL
Skin transepidermal water loss
Footnotes
Conflicts of Interest: TWG reports personal fees from GSK, TEVA, and Novartis; grants from NIH and Astra-Zeneca, grants and personal fees from Sanofi/Regeneron, and Royalties from UpToDate, outside the submitted work.
JMB reports no conflicts of interest.
RRR reports no conflicts of interest.
KK reports no conflicts of interest.
KC reports no conflicts of interest.
JWK reports no conflicts of interest.
SRD reports no conflicts of interest.
MS reports no conflicts of interest.
VP reports no conflicts of interest.
LJM reports no conflicts of interest.
CMK reports no conflicts of interest.
KH reports no conflicts of interest.
GKKH reports grants from NIH and Adare.
ClinicalTrials.gov Registration: NCT04179461
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Siddiqui S, Denlinger LC, Fowler SJ, et al. Unmet Needs in Severe Asthma Subtyping and Precision Medicine Trials. Bridging Clinical and Patient Perspectives. Am J Respir Crit Care Med. 2019;199:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pakhale S, Mulpuru S, Boyd M. Optimal management of severe/refractory asthma. Clin Med Insights Circ Respir Pulm Med. 2011;5:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KF. Precision medicine in asthma: linking phenotypes to targeted treatments. Current opinion in pulmonary medicine. 2018;24:4–10. [DOI] [PubMed] [Google Scholar]
- 5.Dexheimer JW, Gu L, Guo Y, Kercsmar C. Design of the asthma treat smart system in a pediatric institution. Stud Health Technol Inform. 2013;192:1004. [PubMed] [Google Scholar]
- 6.Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–825. [DOI] [PubMed] [Google Scholar]
- 7.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. [DOI] [PubMed] [Google Scholar]
- 9.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 20202020. [Google Scholar]
- 10.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. [DOI] [PubMed] [Google Scholar]
- 11.Burrows B, Sears MR, Flannery EM, Herbison GP, Holdaway MD, Silva PA. Relation of the course of bronchial responsiveness from age 9 to age 15 to allergy. Am J Respir Crit Care Med. 1995;152:1302–1308. [DOI] [PubMed] [Google Scholar]
- 12.Howrylak JA, Fuhlbrigge AL, Strunk RC, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014;133:1289–1300, 1300 e1281–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt EB, Kovacic MB, Lee GB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudon Thibeault AA, Laprise C . Cell-Specific DNA Methylation Signatures in Asthma. Genes (Basel). 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Biagini Myers JM, Yadagiri VK, et al. Nasal DNA methylation differentiates corticosteroid treatment response in pediatric asthma: A pilot study. PLoS One. 2017;12:e0186150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson DJ, Bacharier LB, Calatroni A, et al. Serum IL-6: A biomarker in childhood asthma? J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siroux V, Oryszczyn MP, Paty E, et al. Relationships of allergic sensitization, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA Study. Clin Exp Allergy. 2003;33:746–751. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basnet S, Bochkov YA, Brockman-Schneider RA, et al. CDHR3 Asthma-Risk Genotype Affects Susceptibility of Airway Epithelium to Rhinovirus C Infections. Am J Respir Cell Mol Biol. 2019;61:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everman JL, Sajuthi S, Saef B, et al. Functional genomics of CDHR3 confirms its role in HRV-C infection and childhood asthma exacerbations. J Allergy Clin Immunol. 2019;144:962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters MC, McGrath KW, Hawkins GA, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. The lancet. Respiratory medicine. 2016;4:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jevnikar Z, Ostling J, Ax E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2019;143:577590. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Liu M, Wang C, et al. Association between vitamin D status and asthma control: A meta-analysis of randomized trials. Respir Med. 2019;150:85–94. [DOI] [PubMed] [Google Scholar]
- 24.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol. 2012;129:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forno E, Bacharier LB, Phipatanakul W, et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial. JAMA. 2020;324:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, May SM, Charoenlap S, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2015;115:396–401 e392. [DOI] [PubMed] [Google Scholar]
- 27.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect. 2015;123:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G, Zhao J, Jiang R, Song W. Rat lung response to ozone and fine particulate matter (PM2.5) exposures. Environ Toxicol. 2015;30:343–356. [DOI] [PubMed] [Google Scholar]
- 29.Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–730 e722. [DOI] [PubMed] [Google Scholar]
- 30.Hon KL, Wong KY, Leung TF, Chow CM, Ng PC. Comparison of skin hydration evaluation sites and correlations among skin hydration, transepidermal water loss, SCORAD index, Nottingham Eczema Severity Score, and quality of life in patients with atopic dermatitis. Am J Clin Dermatol. 2008;9:45–50. [DOI] [PubMed] [Google Scholar]
- 31.Al-Ramli W, Prefontaine D, Chouiali F, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. [DOI] [PubMed] [Google Scholar]
- 32.Brandt EB, Kovacic MB, Lee GB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204 e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG, Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. [DOI] [PubMed] [Google Scholar]
- 36.duRivage N, Ross M, Mayne SL, et al. Asthma Control Test. Clin Pediatr (Phila). 2017;56:341–347. [DOI] [PubMed] [Google Scholar]
- 37.Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135:271–279. [DOI] [PubMed] [Google Scholar]
- 38.Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med. 2017;195:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoratti EM, Krouse RZ, Babineau DC, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138:1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehsan Z, Kercsmar CM, Collins J, Simakajornboon N. Validation of the pediatric sleep questionnaire in children with asthma. Pediatr Pulmonol. 2017;52:382–389. [DOI] [PubMed] [Google Scholar]
- 42.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014:CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.